Abstract
Investigating the developmental sequelae of early life stress has provided researchers the opportunity to examine adaptive responses to extreme environments. A large body of work has established mechanisms by which the stressful experiences of childhood poverty, maltreatment, and institutional care can impact the brain and the distributed stress systems of the body. These mechanisms are reviewed briefly to lay the foundation upon which the current neuroimaging literature has been built. More recently, developmental cognitive neuroscientists have identified a number of the effects of early adversity, including differential behavior and brain function. Among the most consistent of these findings are differences in the processing of emotion and reward-related information. The neural correlates of emotion processing, particularly frontolimbic functional connectivity, have been well studied in early life stress samples with results indicating accelerated maturation following early adversity. Reward processing has received less attention, but here the evidence suggests a deficit in reward sensitivity. It is as yet unknown whether the accelerated maturation of emotion-regulation circuits comes at the cost of delayed development in other systems, most notably the reward system. This review addresses the early life stress neuroimaging literature that has investigated emotion and reward processing, identifying important next steps in the study of brain function following adversity.
Keywords: Early life stress, HPA axis, brain function, functional connectivity, emotion processing, reward processing
Introduction
The important influence of early experience on later development is a core tenet of developmental science. Indeed, a large proportion of the developmental psychology literature focuses on the early years of life as a period of rapid change, laying the foundation for much of an individual’s physical, emotional, and cognitive development. Given this, it is unsurprising that the experience of stress in infancy and childhood is associated with an increased probability of atypical outcomes. Indeed, adverse early environments have been suggested to contribute to up to 45% of child-onset and over 30% of adult-onset mental health disorders (Green et al., 2010; McLaughlin et al., 2010). These atypical outcomes are thought to arise in part due to adaptive responses of physiological stress systems. When individuals are exposed to stress a number of biological systems become more active, providing the resources that the body and brain need to respond appropriately. As such, many of the atypical outcomes associated with early life stress (ELS) may be adaptive in the immediate adverse environment but detrimental across the life course. Whether or not these immediately adaptive but ultimately detrimental outcomes are the result of developmental trade-offs initiated by the responses of stress-mediating systems is a crucial question for understanding the neurobiological outcomes of ELS.
Any discussion of brain development following ELS must be rooted in the underlying mechanisms by which stress “gets under the skin” (McEwen, 2012). This particular turn of phrase has been used to characterize the effects of stressful experiences in several systems associated with emotion and reward processing, particularly in the context of the neuroendocrine stress system (Carlson & Earls, 1997; Danese & McEwen, 2012; Hostinar, Sullivan, & Gunnar, 2014). Of all the systems involved in the developmental trajectories precipitated by ELS, the hypothalamic-pituitary-adrenal (HPA) axis may be the most influential and well-studied. The HPA axis acts to mobilize metabolic resource in response to external threats of sufficient intensity or specific typology (e.g. life-threatening situations or psychosocial evaluation, respectively) and shape brain systems in anticipation of future threats. Cortisol is the primary end-hormone of this system. Importantly, basal levels of cortisol are important for maintaining healthy brain development and function (McEwen et al., 2015). Repeated exposures to high levels of cortisol or its releasing hormone (corticotropin releasing hormone), however, can have negative effects throughout the brain. For example, elevated levels of cortisol exposure over periods of 48 to 72 hours can promote the formation of free radicals that are toxic to neurons themselves (Du et al., 2009). Further, animal models of chronic stress have also been related to dendritic shrinkage in the rat hippocampus; inhibited activity in the pituitary, hypothalamus, hippocampus, and amygdala; and reduced cell proliferation in subcortical structures (Hill & McEwen, 2010; McEwen et al., 2015; Treccani et al., 2014). Similar effects have been reported in humans with experiences of physical abuse, early neglect, and low socioeconomic status during childhood (Hanson, Nacewicz, et al., 2015; Hoy et al., 2012; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012). Understanding the effects of the neuroendocrine stress system on brain structure and function allows for the elucidation of one pathway by which experiences of poverty, childhood maltreatment, and institutional care shape the development of children.
Appreciating the mechanisms by which stress affects development helps to inform the focus of the current review: the effects of ELS on the central organ of stress and adaptation, the brain. Many of the physiological stress systems that are responsive to stress originate in the brain, while also feeding back on the brain to alter its structure and function. A large amount of research has been done to investigate the structure and functional activation of specific regions of interest in individuals who have experienced ELS (Bick & Nelson, 2016; Teicher, Samson, Anderson, & Ohashi, 2016). Of special interest here, however, are the changes in distributed patterns of functional activity that have been observed following ELS. Recently, neuroimaging methods have emerged that allow for the quantification of brain function at a larger scale—evaluating dynamics within- and between-systems in an effort to understand normative and nonnormative patterns of development (Bassett & Sporns, 2017). Central to these new methods is the correlation of neural activity across time in two or more brain regions, termed functional connectivity. Studies of functional connectivity have begun to elucidate the developmental sequalae of ELS on the level of neural circuits, or networks, providing a new window into the associations between early adverse experiences and psychopathology.
In addition, we will focus on the effects of ELS on two domains of interest, emotion and reward, and their underlying neural circuitry. These behaviors were selected for several reasons: (a) behavioral studies of ELS suggest that emotion- and reward-processing are impacted by early adversity, (b) there are known neurobiological mechanisms by which ELS impacts the neural systems supporting emotion processing and reward sensitivity, and (c) neural responses to emotional and rewarding stimuli are associated with the emergence of stress-related psychopathology (e.g. Bogdan, Nikolova, & Pizzagalli, 2013; Hanson, Hariri, & Williamson, 2015; Marusak, Martin, Etkin, & Thomason, 2015).
The literature reviewed here describes how functional connectivity may provide novel avenues for the study of the impact of ELS on neural function and could reveal a developmental trade-off resulting from accelerated maturation following stress. Specifically, this review will first outline the construct of ELS and how early adversity is characterized. Next, the extant functional neuroimaging literature is discussed with an emphasis on emotion and reward processing in functional connectivity-based analyses and their connection to the development of psychopathology. Finally, a synthesis of the data is offered, including a discussion of the path ahead for studies of brain connectivity following ELS.
Defining Early Life Stress: General and Specific Effects
Early life stress is the result of adversities that produce frequent or chronic activation of stress-responsive physiological systems. These adversities either deprive infants and children of the social and/or physical care needed thus creating conditions that threaten homeostasis or confront them with conditions that provoke fear and anxiety. In either case, stress-responsive physiological systems are activated with prolonged exposure to these conditions dysregulating the subsequent function of the stress systems themselves (e.g., producing allostatic load; Koss & Gunnar, 2018).
While a common element is activation of stress biology, adversity can take many forms. There is, as yet, no consensus on how to group or classify these conditions. It has been common to simply sum up the number of types of adversity experienced (i.e., cumulative risk models; Rutter, 1981; but see also adverse childhood experiences or ACEs; Dube et al., 2003; Felitti et al., 1998). Cumulative risk models grew out of recognition that often exposure to one type of adversity does not seriously impact development, but as risks accumulate, development suffers.
The most commonly studied forms of ELS are characterized by an accumulation of adversity. For example, poverty is typically a chronic, multi-dimensional stressor. In addition to insecure housing and nourishment, poverty increases the likelihood of exposure to violence and maltreatment (Evans, 2004). In one study, the proportion of childhood spent in poverty was linearly associated with decreased working memory performance in young adulthood (Evans & Schamberg, 2009). Likewise, maltreated children typically experience multiple types of abuse, but it is not only the types and frequency of abuse (Cicchetti, 2013), but the duration and age of exposure that matters (Cowell, Cicchetti, Rogosch, & Toth, 2015). Thus, for example, while maltreatment is associated with lower cognitive functioning, the magnitude of these effects is a function of chronicity (Cowell et al., 2015). Results like those of Cowell and colleagues (2015) emphasize the importance of considering the timing of stressful experiences when studying early adversity. However, due to the nature of stressful experiences it is often difficult to isolate stress in a single developmental period.
Children adopted or fostered from institutions aid investigation of developmental timing by providing a model of ELS that is typically limited to the first few years of life when neural systems are highly plastic. Institutional care often deprives infants and young children of responsive caregiving as care is regimented and occurs in an assembly line fashion. There is little one-on-one interaction and often little chance for cognitive or social stimulation (Zeanah, Smyke, Koga, & Carlson, 2005). Once adopted, children experience adequate to good care in well-resourced homes. Researchers investigating the effects of institutional care have reported smaller gray matter volumes, lower executive function skills, and atypical reward processing (Humphreys et al., 2015; Merz, Harlé, Noble, & McCall, 2016; Sheridan et al., 2012). Interestingly, a small body of literature has begun to associate the age at adoption (a measure linked to the duration of institutional care) with increased difficulty in reward-related tasks and brain volume (Hodel et al., 2015; Sheridan et al., 2018). Each of these models of ELS (poverty, maltreatment, and institutional rearing) provide important insights into the effects of adversity on brain and behavior development while also providing opportunities to assess the importance of developmental timing for outcomes following stress exposure.
As is apparent above, most of the work on ELS deals with populations exposed to multi-faceted adversities. This makes it difficult to isolate stimuli associated with particular neural or behavioral outcomes. Lately there has been a call for organizing our assessments of ELS to match the definition provided above. Specifically, assessing degrees of deprivation versus threat (McLaughlin & Sheridan, 2016). As such, investigations examining samples of individuals who have experienced childhood poverty, childhood maltreatment, or regimented care in institutional settings have begun to disentangle the specific outcomes associated with each type of stressful experience. For example, individuals exposed to abuse or violence do not exhibit strong differentiation of aversive and non-aversive cues during fear conditioning (McLaughlin et al., 2016), while individuals who have experienced institutional care exhibit hypo-responsivity to reward (Mehta et al., 2010). Although this recent work is exciting, there are, as yet, few imaging studies that have used these dimensional approaches. Thus, we will review the field in its current state, recognizing that it is challenging to know how to unpack—or even whether to unpack—specific types of adversity that lead to excessive stress system activity early in life.
Emotion Processing, Reward Sensitivity, and Brain Structure Following ELS
Behavioral work has established stark differences in emotion processing following ELS, including findings in the rodent and human literature. In one exemplary study, rat pups raised in a stressful, maternal deprivation model demonstrated altered freezing behavior in odor preference tests, suggesting altered emotion processing circuitry in early life (Moriceau, Shionoya, Jakubs, & Sullivan, 2009). Research in humans experiencing trauma during childhood or adolescence has found similar results, including increased attention toward social-threat stimuli in a dot-probe task (Dalgleish, Moradi, Taghavi, Neshat-Doost, & Yule, 2001). Further, adults with documented cases of childhood maltreatment exhibit poorer affective picture recognition, even decades following the experience of abuse (Young & Widom, 2014).
Differences in reward sensitivity have also been observed following ELS. Humphreys and colleagues (2015) reported that post-institutionalized youth were more likely to avoid losses than non-adopted youth, despite gaining less reward as a result. Further, post-institutionalized youth do not exhibit performance advantages on reward trials compared to no-reward trials in an anti-saccade task (Mueller et al., 2012). Similar effects have recently been observed in a randomized control trial of ELS, the Bucharest Early Intervention Project (Sheridan et al., 2018). In this project, children who remained in institutional care for the first 5 years of life, on average, failed to improve their performance in response to reward in a modified monetary incentive delay task at age 13, while those assigned to leave the institution for foster care by two years on average did improve. In that study, reward sensitivity mediated the relationship between the amount of time spent in institutional care and the development of depression. The behavioral effects summarized to this point represent a small portion of a substantial literature documenting the effects of ELS on the processing of emotion and reward, but demonstrate the importance of research investigating alterations in brain structure and function that may mediate the associations between ELS and these behaviors.
Turning now to brain structure, research examining the effects of ELS on brain structure has largely reported smaller brain volumes following early adversity. A wide range of brain regions have been observed to be smaller in low socioeconomic status infants and children compared to high socioeconomic status individuals, including frontal, parietal, and temporal cortex, as well as subcortical structures and total gray matter (Hair, Hanson, Wolfe, & Pollak, 2015; Hanson et al., 2013; Luby et al., 2013). Interestingly, the direction of effect in the amygdala changes in young adulthood such that higher cumulative socioeconomic risk is associated with larger amygdala volume—an effect that is similar to investigations of age at adoption in post-institutionalized samples (Evans et al., 2016; Tottenham et al., 2010). In maltreated samples, longitudinal, cross-sectional, and meta-analytic work has established reduced volume in the prefrontal cortex, amygdala, and hippocampus compared to non-maltreated individuals (e.g. Carrion, Weems, & Reiss, 2007; Chaney et al., 2014; Hoy et al., 2012; Paquola, Bennett, & Lagopoulos, 2016; Pederson et al., 2004; Sheffield, Williams, Woodward, & Heckers, 2013; among others). Finally, institutional care has also been associated with decreased brain volume in total and prefrontal gray matter as well as reduced cortical thickness compared to never institutionalized youth (Hodel et al., 2015; McLaughlin et al., 2014; Nelson, Bos, Gunnar, & Sonuga-Barke, 2011; Sheridan et al., 2012). Importantly, changes in brain structure are also associated with the behavioral differences described earlier in this section, including associations between increased anxiety an increased amygdala volume and more inattention as a function of reduced cortical thickness in post-institutionalized youth (McLaughlin et al., 2014; Tottenham et al., 2010).
The work discussed in this section emphasizes the general effects of ELS on behavior, brain structure, and their interaction. Consistent with these findings, brain function is also affected by early adversity. In the sections that follow we will focus on brain function associated with emotion processing and reward processing following ELS.
Brain Function Following ELS
Brain Function During Emotion Processing After ELS
The pattern of results in investigations of emotion-related amygdala function is largely consistent among experiences of deprivation and threat. For example, increased amygdala activation has been reported in emotional contexts following experiences of childhood poverty, maltreatment, and institutional care (Javanbakht et al., 2016; Kim et al., 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; Tottenham et al., 2011). Importantly, increased amygdala activation during emotion processing is associated with improvements in behavior including increased eye-contact during dyadic interactions. Such findings suggest that increased amygdala activation to emotional contexts is beneficial following ELS.
Unlike amygdala activity, activation in prefrontal and striatal regions differ based on the type of adversity experienced. In impoverished and post-institutionalized samples reduced activity in the dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), and nucleus accumbens are associated with ELS when participants engage in emotional reappraisal or view happy faces (Goff et al., 2013; Kim et al., 2013; Liberzon et al., 2014). Conversely, maltreated individuals tend to show increased activation in dorsal medial prefrontal cortex (mPFC) during social rejection tasks and in salience-related regions during emotion processing tasks (McLaughlin et al., 2015; Van Harmelen et al., 2014). Whether increased activation in prefrontal regions is a compensatory pattern specific to maltreated individuals in emotional situations or are the result of a more general pattern of increased activity is not yet clear. Focused investigations of functional connectivity at rest and during task states may help to interpret these results in the future.
Importantly, experiencing each type of ELS reviewed here is associated with increased amygdala activity in emotion-laden, and most notably, threatening emotion contexts. As alluded to above, this is likely an adaptive response to stressful environments early in life. Whether or not this adaptive developmental response is beneficial later in life, or in a lower stress environment, remains an open question.
Brain Function During Reward Processing After ELS
In comparison to the emotion processing circuitry reviewed above, the processing of reward following ELS has received relatively less attention. Like emotion-processing, there are consistent differences between the type of ELS experienced and neural activation in the context of reward. However, in rewarding contexts neural activation is more consistent in maltreated and post-institutionalized samples while impoverished samples exhibit different patterns of activity. For example, while the experience of childhood poverty has been associated with increased neural response to reward in the nucleus accumbens and mPFC (Gonzalez, Allen, & Coan, 2016; Romens et al., 2015) diminished activation has been observed during the anticipation, perception, and receipt of reward in maltreated and post-institutionalized youth (Boecker et al., 2014; Dillon et al., 2009; Hanson et al., 2016; Hanson, Hariri, et al., 2015; Mehta et al., 2010). Similar reductions in activity have been found in anterior cingulate cortex (ACC), middle frontal gyrus (MFG), putamen, and insula as a function of increased childhood stress (Birn, Roeber, & Pollak, 2017; Boecker-Schlier et al., 2016).
The inconsistency of reward-related brain function following ELS may be better understood when the type and timing of stressful experiences are considered. The often chronic nature of poverty (see Evans, 2004) may increase the salience of reward, helping to explain increased striatal responses in impoverished samples. Conversely, reduced responses in abused or socially deprived groups may reflect an adaptive pattern of avoiding unpredictable harm or loss. As such, it is unclear whether altered reward sensitivity is protective, reducing the likelihood of risky behavior, or a risk, leading individuals to seek larger, more potent rewards. Future research investigating longitudinal associations of ELS with reward processing and subsequent developmental outcomes could distinguish between these possibilities.
Functional Connectivity: A Promising Tool in ELS Research
Functional connectivity has been an important tool for understanding the developmental sequelae of ELS. Connectivity approaches allow for the evaluation of “circuits” within the brain, collections of regions hypothesized to function as a unit for the processing of particular stimuli (emotion processing, for example). While the application of functional connectivity to ELS research is relatively new, it is clear why the approach may be of particular interest to scientists studying the effects of stress on development.
To date, many of the investigations of brain connectivity following ELS have been restricted to the circuit level, utilizing seed-based functional connectivity methods. Two main approaches to investigating functional connectivity have been used in the ELS literature, psychophysiological interaction (PPI) and seed-based resting-state functional connectivity (rsFC). Briefly, PPI analyses examine the functional connectivity between two regions of interest whose activity has been convolved with task-related timing information. The result is an opportunity to understand the functional relationship between two regions in the context of a task. Seed-based rsFC analyses on the other hand, aim to understand the functional coherence between two regions of interest when the individual is not engaged in a task, the individual’s “resting-state.” Analyses utilizing a seed-based rsFC approach choose a single region of interest as the “seed” and correlate the time course of the seed’s functional activity with a set of regions or all other regions of the brain. Correlations with greater magnitude (both positive and negative correlations are included) are interpreted as being more functionally connected. Underlying these procedures, and all functional connectivity analysis, is the assumption that two regions demonstrating activity that is significantly correlated in time are functionally related.
In addition to PPI and seed-based analyses, graph theory has also been used to analyze rsFC and task-based fMRI data by researchers interested in understanding complex brain networks. In a graph theory analysis, each region of interest in the brain is a “node” in the network and the connections between these nodes are “edges” (Fornito, Zalesky, & Bullmore, 2016). How edges are defined varies by imaging modality, but in the case of fMRI the most common is correlation in the blood oxygen-level dependent (BOLD) signal between two nodes. The combination of nodes and edges identified from the data can then be used to create a large matrix, or graph, representing functional connections throughout the brain. Sometimes called a “connectome,” these matrices serve as the input for calculation of a number of graph metrics. Quantifying brain function across many regions through graph metrics allows for a characterization of the patterns of activity in brain networks and can be used to compare these networks across individuals or between groups. Further, graph theory analyses of fMRI data allow researchers to examine the whole-brain network, intermediate sub-networks (e.g. the limbic network), and local patterns of activity within the same empirical framework (Power et al., 2011). This flexibility is one of the strengths of graph theory as an approach for ELS research as it allows for the evaluation of the specific circuits of interest (e.g. emotion processing circuitry) while also facilitating investigations of activation patterns in a diverse set of networks within the same graph.
Functional connectivity approaches extend the levels of analysis that are possible within brain imaging data, consistent with developmental systems theory, which emphasizes evaluating development at multiple levels of analysis. In the brain, traditional approaches have investigated three levels: molecular interactions, neuronal dynamics, and the structure and function of collections of neurons (e.g. brain structures and their nuclei). The rise of connectivity-based approaches extends these levels a step further, investigating the functional dynamics between regions and the effects they have on behavior.
ELS, Functional Connectivity, and Emotion Processing
For a number of reasons, the early investigations of ELS and functional connectivity have largely focused on the connectivity between the amygdala and prefrontal cortex. Previous work has demonstrated long-term effects of maternal deprivation on frontoamygdala circuitry in rats (Callaghan & Richardson, 2011; Hofer, 1996), the vulnerability of the amygdala to early adversity in non-human primates (Sabatini et al., 2007), and a wealth of associations between adverse caregiving and altered behavior, brain structure, and function (e.g. Hodel, 2018; Nelson, Bos, Gunnar, & Sonuga-Barke, 2011). Indeed, investigations of regions often associated with high quality caregiving relationships were a natural step toward understanding the effects of ELS. Table 1 provides a brief summary of the main findings from investigations of ELS and functional connectivity in the emotion processing system.
Table 1.
Summary of emotion processing-related functional connectivity results following childhood poverty, maltreatment, and institutional care.
| ELS Type | Reference | N | Finding |
|---|---|---|---|
| Poverty | (Javanbakht et al., 2015) | 52 | Lower income-to-needs ratio is associated with diminished amygdala – PFC connectivity |
| (Kim et al., 2013) | 49 | Lower income-to-needs ratio is associated with diminished amygdala – PFC connectivity | |
| Maltreatment | (Herringa et al., 2013) | 64 | Maltreated individuals display lower amygdala- and hippocampus – PFC connectivity |
| (Kaiser et al., 2018) | 70 | Maltreated individuals display lower amygdala – PFC connectivity | |
| (Thomason et al., 2015) | 42 | Maltreated adolescents do not display negative amygdala – subgenual ACC connectivity found in comparison youth | |
| (Demers et al., 2018) | 80 | Stronger amygdala – PFC connectivity predicts more adaptive functioning regardless of maltreatment history | |
| Institutional Care | (Gee, Gabard-Durnam, et al., 2013) | 89 | More mature negative amygdala -mPFC coupling present in previously institutionalized children and adolescents during emotion-matching |
| (Silvers et al., 2016) | 89 | Significant amygdala- and hippocampus – PFC connectivity during aversive learning in post-institutionalized youth only amygdala – PFC connectivity in comparison group | |
Frontolimbic connectivity in low income samples.
Two studies with sample sizes of 49 and 52 have shown differential coupling between the amygdala and PFC as a function of childhood poverty. One of these studies employed a prospective design, prior to completing an emotion regulation task in which participants were asked to “maintain” or “reappraise” their emotional states in response to aversive or neutral pictures in the scanner at age 24 (Kim et al., 2013). The second study used a similar design, associating childhood poverty to an emotional face matching task completed in early adulthood (Javanbakht et al., 2015). In both cases, childhood poverty was significantly associated with amygdala – PFC connectivity, with task-specific differences. Results from Kim and colleagues (2013) suggest more positive coupling between the amygdala and ventrolateral PFC (vlPFC) during reappraisal of emotion responses in individuals with low childhood income-to-needs ratio. Similarly, low childhood income-to-needs ratio was associated with diminished connectivity between the left amygdala and mPFC while matching emotional faces in young adulthood in the second study (Javanbakht et al., 2015). Together, these results suggest that individuals with lower family income during childhood exhibit different patterns of frontolimbic connectivity when viewing aversive images or emotional faces. While not directly tested in these two examples, differences in frontolimbic functional connectivity such as these may result in altered emotion processing in adults with histories of childhood poverty.
Frontolimbic connectivity following childhood maltreatment.
Compared to other components of neural functioning, the effects of childhood maltreatment on later functional connectivity is much less researched (Bick & Nelson, 2016). Further, the literature that does exist has associated experiences of early maltreatment with both more positive and more negative frontolimbic functional connectivity. Two studies of rsFC have reported more negative amygdala – dlPFC connectivity as a function of increasing ELS severity assessed by retrospective report in adolescence and adulthood (Herringa et al., 2013; Kaiser et al., 2018). More negative amygdala connectivity with the subgenual anterior cingulate (sgACC) and postcentral gyrus was also associated with ELS severity—a result that was similar to hippocampal connectivity in the same sample (Herringa et al., 2013). Taken together, these results suggest a general trend toward more negative rsFC as a function of increasing stress severity. However, a third group has reported results in the opposite direction—associating more positive amygdala – sgACC connectivity with a maltreatment exposed group compared to a non-maltreated group (Thomason et al., 2015). While these results are contradictory, an important methodological difference may account for some of the inconsistency. Thomason and colleagues (2015) used three sub-regions of the amygdala as the seed region for their whole-brain analysis, reporting generally more positive connectivity between the centromedial, basolateral, and superficial amygdala. It may be that the use of a more specific amygdala seed allowed for a more granular assessment of the effects of maltreatment on frontolimbic connectivity. Conversely, the use of a dichotomous group comparison approach with smaller seeds may obscure the linear trend between connectivity and ELS severity reported in other research. Future research using continuous ELS predictors with sub-segmented amygdala seeds is needed to clarify the effects of childhood maltreatment on rsFC.
In the task-based PPI literature, two studies have evaluated functional connectivity during context-dependent emotional face processing in adolescents with and without histories of maltreatment. In both cases, more positive connectivity was reported: more positive hippocampus – vlPFC connectivity was evident in maltreated individuals during angry face encoding trials (Lambert et al., 2017) and more positive amygdala – pregenual cingulate connectivity was found in maltreated youth compared to youth without maltreatment histories during emotional conflict trials (Marusak, Martin, et al., 2015). More positive connectivity was also associated with poorer performance on the behavioral aspects of each task. In comparison to the rsFC literature, these task-related analyses show a more consistent pattern. Exposure to childhood maltreatment is related to more positive connectivity in emotional contexts—a connectivity pattern associated with poorer context memory and conflict regulation.
While differences in imaging methodology may account for some of the inconsistency in the rsFC results reported in this section, it is also important to note differences of developmental timing. Specifically, only more negative amygdala – dlPFC connectivity as a function of increasing ELS severity was reported in both adolescents and adults (Herringa et al., 2013; Kaiser et al., 2018). More widespread connectivity differences were found in the adolescent sample, including altered hippocampal connectivity. Given that the pubertal period is characterized by increased developmental plasticity in both stress physiology and brain function (DePasquale, Donzella, & Gunnar, 2018; Spear, 2013), it is possible that the older age at assessment in Kaiser et al. (2018) represents a measure of adaptation to ELS that could occur during the adolescent period. As such, longitudinal imaging studies should be used to investigate similar recalibration in functional connectivity systems during the transition into adulthood.
One such prospective study has begun to address this question in adults, though only one imaging timepoint was included. PPI analysis of an emotion-matching task was used to examine differences in functional connectivity related to child maltreatment at age 30. A group contrast revealed more positive amygdala connectivity with a number of regions including mPFC, ACC, and the posterior cingulate in the maltreatment group compared to non-maltreated individuals (Jedd et al., 2015). A follow-up investigation of these analyses related group differences in amygdala functional connectivity with adaptive functioning in adulthood, revealing a positive relationship between amygdala connectivity and adaptive functioning. Regardless of maltreatment status, stronger amygdala connectivity with the cingulate gyrus, dorsomedial PFC, and bilateral parietal cortex were associated with higher levels of adaptive functioning in adulthood (Demers et al., 2018). Interestingly, the results of Demers and colleagues indicate amygdala – PFC PPI connectivity is more strongly associated with adaptive functioning in adulthood than childhood maltreatment status. These results suggest that high functioning individuals with histories of ELS may exhibit a compensatory neural phenotype that aids in adaptive functioning later in life, further supporting the notion that adaptation may occur during the transition from adolescence into adulthood.
Frontolimbic connectivity following early institutional care.
A pair of important studies built upon a theoretical focus on frontolimbic emotion processing circuitry to establish an effect of early institutional care. Using PPI, researchers used a cross-sectional sample of individuals 4 – 22 years of age to determine the normative developmental trajectory of amygdala – prefrontal connectivity during emotional face matching. Their results indicated that during childhood, amygdala – mPFC connectivity has a positive valence but that in adolescence and adulthood the valence is negative (Gee, Humphreys, et al., 2013). Further, their results indicated that positive connectivity between the amygdala and mPFC was associated with higher separation anxiety, while those with negative connectivity exhibited less separation anxiety. In an analysis using the same imaging methodology, the same research group investigated amygdala – mPFC connectivity in a sample of post-institutionalized and non-adopted youth with an age range of approximately 6 – 17 years. Once again, positive amygdala – mPFC coupling was observed in children compared to adolescents in the comparison group. Conversely, both children and adolescents exhibited the “more mature” negative connectivity in the previously institutionalized group (Gee, Gabard-Durnam, et al., 2013). Separation anxiety was associated with connectivity such that more negative connectivity was associated with lower levels of anxiety. These results added to an existing literature that had described accelerated maturation following stressful early experiences. Previous research had implicated the HPA axis in a pathway leading to altered HPA coupling with the hypothalamic-pituitary-gonadal axis resulting in accelerated sexual maturation associated with familial disruption, parental depression, and father absence (Belsky, Steinberg, & Draper, 1991; Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011).
Beyond the work of Gee and colleagues, very little research has been done relating functional connectivity with early institutional care. In one analysis, post-institutionalized and never institutionalized adolescent’s functional connectivity were compared via PPI in an aversive learning task. Post-institutionalized youth exhibited significant hippocampus- and amygdala – PFC connectivity while the comparison group demonstrated only significant amygdala – PFC coupling (Silvers et al., 2016). Interestingly, the pattern displayed by the post-institutionalized adolescents, in which the hippocampus is recruited in addition to the amygdala, was more similar to previous studies of adult brain activation during aversive conditioning than the pattern observed in the comparison group (Knight, 2004). These results provide further evidence of a more mature frontolimbic connectivity pattern in post-institutionalized youth compared to never-institutionalized adolescents. Finally, in a study of social rejection using the Cyberball task, children with a history of foster care or adoption exhibited more negative connectivity between the dlPFC and dorsal ACC, consistent with the results of Gee and colleagues (Puetz et al., 2014). As such, the results of the limited literature investigating frontolimbic connectivity following institutional care are concordant with an early maturation framework in which individuals with ELS experience exhibit accelerated maturation in emotion processing circuits. This early maturation may represent an adaptive response to a lack of regulation by caregivers in infancy. What impact this early maturation may have on other early-developing systems, however, has not been empirically studied.
Whole-brain Patterns of rsFC and Emotion-Related Psychopathology
In recent years graph theory and other methods of whole-brain rsFC analysis have shown promise in applications to psychopathology. This is particularly important to the study of ELS as many forms of childhood adversity have been associated with increased risk for mental illness. For example, rsFC analyses have been used to distinguish the functional activation patterns of individuals with and without histories of ELS and psychopathology. Some of this research has focused specifically on major depressive disorder (MDD) and the functional connectivity patterns associated with MDD alone and in the context of childhood maltreatment. For example, in a study of 60 adults with MDD and/or ELS decreased functional connectivity was observed compared to controls (Wang et al., 2014). Specific decreases in connectivity were observed in the vmPFC in all participants with MDD, while only individuals with MDD and a history of neglect showed more widespread connectivity decreases including dlPFC, dorsal mPFC, vlPFC, insula, and amygdala. Another sample investigating similarities and differences in patterns of functional connectivity following ELS in 38 women with and without MDD diagnoses also identified the dlPFC as a region of importance. Once again, only individuals with both a history of childhood maltreatment and current diagnosis of MDD exhibited altered dlPFC connectivity (Cisler et al., 2013). Further, their results also suggested that the amygdala is relatively more important, and potentially less effectively regulated, in the brain networks of individuals who have experienced ELS and developed MDD compared to individuals with ELS histories but no psychiatric diagnoses. In combination, these results suggest an important role for functional connectivity outcomes following ELS in explaining heterogeneity observed in clinical samples. While the role of functional connectivity in the development of psychopathology following ELS is an important path for future research, both of these studies included only adults.
While rare, whole-brain graph theory approaches have been applied to the analysis of task-based fMRI data in pediatric samples. In a small sample of 20 adolescents, Cisler and colleagues (2016) found that girls with PTSD and relatively high levels of trauma exhibited more segregated processing of information and lower efficiency in information processing as compared to individuals with relatively low trauma exposure during a facial emotion processing task. Further, greater segregation of information processing (as measured by network modularity) predicted greater amygdala activation and lower connectivity between the amygdala and mPFC. Importantly, individuals who responded best to treatment were most similar to a healthy control group in brain network segregation and efficiency during the facial emotion processing task (Cisler et al., 2016). While preliminary, these findings suggest that graph theory metrics may have utility for predicting response to treatment in populations experiencing ELS. Further, increased segregation (as measured using network modularity) in individuals with histories of trauma are consistent with theoretical interpretations of the function of network segregation. In the context of childhood maltreatment, in which environments can be unpredictable and threatening, increased network segregation may serve to increase the flexibility of functional responses thereby eliminating the need to re-model the entire functional system (Sporns & Betzel, 2016). As such, Cisler and colleague’s findings demonstrate the potential of graph theory metrics for mechanistic understandings of psychiatric disorder and do so in a theoretically plausible manner.
ELS, Functional Connectivity, and Reward Processing
Reward-related connectivity following childhood poverty.
Compared to the literature investigating functional connectivity in emotion processing systems, the literature examining reward processing systems is quite limited (see Table 2 for a brief summary of results). In one study, low household income was associated with increased ventral striatum – lateral PFC connectivity while community disadvantage was associated with decreased ventral striatum – mPFC connectivity in children and adolescents (Marshall et al., 2018). These results suggest that income and socioeconomic disadvantage (e.g. neighborhood poverty levels) have unique effects on corticostriatal connectivity in late childhood and adolescence. Once again, understanding how these differential patterns of functional connectivity associated with ELS may change as individuals transition to early adulthood will be an important step to understanding the developmental trajectory of brain function following early adversity.
Table 2.
Summary of reward processing-related functional connectivity results following childhood poverty, maltreatment, and institutional care.
| ELS Type | Reference | N | Finding |
|---|---|---|---|
| Poverty | (Marshall et al., 2018) | 100 | Low household income associated with increased ventral striatum – lateral PFC connectivity |
| Maltreatment | (Marusak, Etkin, et al., 2015) | 43 | Increased salience network – insula and salience network – amygdala connectivity in trauma exposed youth compared to no trauma |
| (Marusak, Hatfield, et al., 2017) | 86 | Decreased VTA – hippocampus connectivity and increased SN – hippocampus connectivity in maltreated individuals | |
| Institutional Care | (Fareri et al., 2017) | 88 | Positive ventral striatum – mPFC connectivity in post-institutionalized youth compared to negative ventral striatum – mPFC connectivity in comparison group |
Reward-related connectivity following childhood maltreatment.
The functional connectivity of brain regions associated with reward processing has also been studied in samples with maltreatment histories. Increases in insula activity and higher salience network – insula and salience network – amygdala connectivity have been shown in trauma exposed youth compared to youth without a history of trauma (Marusak, Etkin, & Thomason, 2015). Further, salience network – insula connectivity mediated the relationship between childhood trauma and reward sensitivity. Building upon these results, a seed-based rsFC analysis of the connectivity of two dopaminergic reward regions: the ventral tegmental area (VTA) and the substantia nigra (SN) revealed decreased VTA – hippocampus and increased SN – hippocampus connectivity in trauma exposed individuals compared those without a history of trauma (Marusak, Hatfield, Thomason, & Rabinak, 2017). Importantly, both of these studies by Marusak and colleagues implicate pathways known to be important in approaching or avoiding novel stimuli as well as reward sensitivity. As reward sensitivity is a trait that has been linked to the emergence of stresss-related psychopathology (Bogdan et al., 2013), these results provide another potential pathway by which ELS increases risk for psychiatric problems.
Reward-related connectivity following institutional care.
In the institutional care literature, the functional connectivity of reward-related brain regions has gone largely unexamined. One study of post-institutionalized youth has reported a positive relationship between the ventral striatum – mPFC connectivity and social problems in children and youth (Fareri et al., 2017). Interestingly, the relationship between striatum – mPFC connectivity and social problems was moderated by age, such that functional connectivity was more likely to predict social problems in adolescents compared to children. Once again, these results highlight the importance of longitudinal investigations to elucidate the developmental trajectories that follow experiences of ELS.
Whole-brain Patterns of Functional Connectivity and Reward-Related Psychopathology
As was alluded to in the previous section, the functional connectivity of reward-related brain regions is another promising area for further understanding the links between ELS and psychopathology. For example, decreased ventral striatum – mPFC connectivity mediates the link between community disadvantage and anxiety symptomatology (Marshall et al., 2018). In children and adolescents from predominantly low income families, lower SN – nucleus accumbens connectivity has been associated with more anxiety symptoms regardless of trauma history (Marusak et al., 2017). Finally, in the context of institutional care, functional connectivity is associated with increased social problems on the Child Behavior Checklist (Fareri et al., 2017) a measure shown to be predictive of later psychopathology (Petty et al., 2008). Associations between brain and behavior such as these are important for identifying possible targets for intervention and possibly the prevention of psychopathology following ELS. More mechanistic investigations, however, are likely to be of more immediate use to researchers and clinicians.
While limited in resolution due to current imaging methods, one large study has proposed ventral striatum – mPFC connectivity as a biomarker for stress-related psychopathology. In the study, a PPI analysis revealed increased connectivity during positive feedback trials between the ventral striatum and mPFC in college-aged individuals reporting higher levels of ELS and current stress (Hanson, Knodt, Brigidi, & Hariri, 2018). This increased connectivity between the ventral striatum and mPFC was also associated with increased internalizing symptoms, perhaps reflecting the contribution of anhedonia to depressive symptomatology. A moderated mediation analysis elaborated upon this result, demonstrating that the observed ventral striatum – mPFC functional connectivity explained 10.3% of the variance between ELS and internalizing symptomatology (Hanson et al., 2018). As exemplified by this work, the whole-brain functional connectivity literature following ELS has shown promise elucidating psychopathology mechanisms via reward-related pathways. With limited exceptions, this literature has linked striatal – mPFC and striatal – salience network connectivity with internalizing symptomatology, consistent with previous behavioral investigations of individuals with experiences of deprivation and threat (Miller et al., 2018).
Conclusions, Limitations, and Future Directions
Conclusions
Experiences of early life stress have consistently been associated, on the group level, with changes in emotion- and reward-related processing. Behavioral work has established numerous effects of childhood poverty, maltreatment, and institutional care, particularly in domains known to be supported by brain regions with high sensitivity to stressful environments via a cortisol-mediated pathway. The extent to which these differences are undergirded by neural processes, however, has been investigated much more thoroughly in emotion processing than responses to reward.
Early adversity has often been associated with difficulty recalling faces presented in negative contexts, identifying negative facial expressions, and inhibiting pre-potent responses when presented with emotion-laden situations. Consistent with this behavioral literature, extensive literature has demonstrated increased amygdala activity in the context of emotion processing. The task-based literature that reports increased amygdala activity is largely built upon tasks requiring regulation and/or emotional reappraisal of faces displaying negative emotions (e.g. Kim et al., 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015). While there is some evidence to suggest blunted neural responses to positive emotions as well, less is known about emotion-processing outside of threatening of fearful stimuli. While focusing on threat stimuli following early adversity is important, additional investigations of emotion processing with neutral and positive stimuli may provide important insights into the pathways from ELS to psychopathology. Given evidence that individuals with histories of abuse struggle to differentiate threat and safety cues during fear conditioning paradigms (McLaughlin et al., 2016), individuals with ELS histories may struggle to identify safety cues in the facial expressions of others, leading to behavioral dysregulation.
Further, the vast majority of functional connectivity research investigating the developmental outcomes associated with ELS has targeted frontolimbic, emotion regulation circuitry. While the results of this research support the notion that experiences of stress in early life alter the connectivity of the emotion regulation system, the direction of effects varies across different types of stress. In samples of youth adopted from institutions the functional connectivity literature centers around the idea of accelerated maturation as indicated by more negative patterns of connectivity when viewing emotional faces (Gee, Gabard-Durnam, et al., 2013). These findings are well supported by previous research. Frontoamygdala circuitry is particularly susceptible to maternal deprivation, maternal maltreatment, and adverse caregiving experiences in both animals and humans (see Callaghan, Sullivan, Howell, & Tottenham, 2014 for a review). It is likely that the vulnerability of these systems is a byproduct of the plasticity required to respond adaptively to external stimuli in early life. In the case of early institutional care, the accelerated maturation of emotion processing systems is beneficial due to the lack of a consistent caregiver to provide external regulation.
Research in young adults with histories of maltreatment and childhood poverty has revealed more inconsistent results, with a general trend toward less effective suppression of amygdala responses to aversive stimuli. Once again, the maintenance of higher levels of amygdala activity may be adaptive in highly threatening environments. For example, physically maltreated youth discriminate between angry and fearful faces with significantly less perceptual information than non-maltreated youth, potentially due to the increased importance of identifying such facial expressions in an abusive environment (Pollak & Sinha, 2002). Though adaptive within early adverse environments, alterations in emotion processing circuitry may serve to delay the development of other systems. Understanding whether or not such a developmental trade-off exists in the functional organization of the brain will require an expansion of the circuits examined by ELS research.
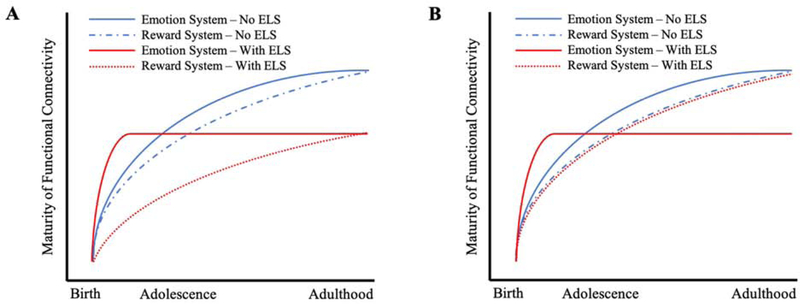
How the functional connectivity within the emotion processing system might change as development progresses is an additional important consideration following ELS. As seen in figure 1, the current literature has suggested accelerated maturation of the emotion systems following early adversity—a finding established during the adolescent period (i.e., Gee, Gabard-Durnam, et al., 2013). Other research, however, has suggested that individuals with a history of maltreatment exhibit less mature responses to emotional stimuli when tested during adulthood (i.e., Jedd et al., 2015). This difference in the developmental timing of assessment, combined with the importance of the timing of the stressful experience, plays a large role in patterns of development following ELS.
Figure 1.
Conceptual illustration of the possibility of a developmental trade-off associated with accelerated maturation of the emotion processing system following ELS (A) as compared to no developmental trade-off (B). To date, the majority of studies examining functional connectivity following ELS have focused on the development of emotion processing systems, with limited work devoted to the functional development of other systems. Behavioral work in individuals who have experienced childhood poverty, maltreatment, and institutional care suggests that the effects of early adversity extend beyond emotion processing and include difficulty processing reward-related information. As such, it may be that the accelerated maturation of emotion-related systems comes at the cost of the development of other neural systems. Due to differences in the maturational courses of neural systems it will be important for future work to evaluate the relative maturity of emotion and reward processing systems to evaluate the possibility of a developmental trade-off.
Compared to emotion processing, the behavioral and neural associations between ELS and reward processing have been less frequently studied. The literature that does exist, however, suggests that the accelerated maturation of emotion circuitry may not generalize to all neural systems. Behaviorally, previously institutionalized youth have been shown to exhibit deficits in a wide array of executive function sub-domains and tasks, including spatial working memory (Bos, Fox, Zeanah, & Nelson, 2009), backward digit span, the Tower of London task (Beckett, Castle, Rutter, & Sonuga-Barke, 2010), Stroop task (Colvert et al., 2008; Merz, McCall, Wright, & Luna, 2013), and the memory, attention, and learning tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB) and NEPSY Developmental Neuropsychological Assessment (Pollak et al., 2010). Similarly, studies targeting reward processing specifically have reported lower levels of reward sensitivity following threat-based adversity or disrupted caregiving (Boecker-Schlier et al., 2016; Humphreys et al., 2015). Further, one of the most often observed behavioral effects when studying adopted youth is increased impulsivity and difficulty inhibiting prepotent responses (Herzberg et al., 2018; Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012; McLaughlin et al., 2014). In this context, hypotheses stressing early maturation of neural systems following ELS fall short—it would be difficult to make a case in which high impulsivity is the “more mature” state following early adversity.
A small literature supports the notion that neural systems that support reward processing do not exhibit accelerated maturation after stressful early experiences. Activation in the ventral striatum has been consistently related to specific forms of ELS, as samples exposed to threat and disrupted caregiving exhibit blunted responses to the anticipation of reward while impoverished samples display increased responses to reward relative to comparison groups (Dillon et al., 2009; Mehta et al., 2010; Romens et al., 2015). Both blunted and increased responses to the anticipation of reward can lead to negative outcomes—the loss of potential gain in the case of blunting and more risk-taking to obtain reward due to higher-than-normal activity—providing a potential pathway to maladaptive behavior later in development. Consistent with this conclusion, the few extant studies of reward-related functional connectivity have identified consistent relationships between striatum – mPFC connectivity and internalizing symptomatology (Hanson et al., 2018).
Considered together, the general pattern of effects in emotion and reward processing following ELS point toward a coherent hypothesis. Given that experiencing early adversity has been associated with enhanced detection of negative emotion and impairment processing positive emotion and reward, ELS could enhance an adaptive defensive phenotype in response to threat while also leading to impaired processing of rewarding stimuli. This trade-off between risk mitigation and a loss of potential reward is an important future direction for researchers interested in the effects of ELS. Figure 1 illustrates the possibility of such a developmental trade-off.
Limitations
Studying the outcomes of early adversity is accompanied by a number of potential pitfalls that threaten the validity of results. As in many areas of developmental neuroscience, small samples continue to slow the accumulation of consistent effects following stressful early environments. To date, the longitudinal imaging work addressing these questions has had extremely limited pilot-sized samples, requiring caution when interpreting results until larger samples can be analyzed (e.g. Carrion, Weems, & Reiss, 2007). Further complicating ELS research is the possibility of genetic effects that co-occur with stressful environments. It may well be the case that some of the effects associated with poor caregiving environments are the result of intergenerational trauma or a genetic predisposition for psychopathology. One recent systematic review supports this notion suggesting that polymorphisms in a trio of genes interact with ELS and substance abuse to increase risk for bipolar disorder and schizophrenia (Misiak et al., 2018). While behavior-genetic techniques can help to account for this possibility, adequate samples for such investigations are few and far between. When possible, researchers should use genetically-informed designs when examining the developmental sequelae of early adversity.
Another limitation of the neuroimaging literature following ELS involves the variety of populations and data collection methods used. While there are likely similarities between different types of early adversity, it is equally likely that different kinds of stress affect individuals in unique ways. As such, synthesizing data from impoverished, maltreated, or institutionalized samples may lead to over-generalized theories of ELS. Additionally, the methods for collecting data about the type and intensity of early adversity vary from study to study. Some investigators have built longitudinal samples with documented cases of maltreatment and rigorous follow-up (e.g. Cowell, Cicchetti, Rogosch, & Toth, 2015) while others rely upon retrospective self-report (e.g. Cisler et al., 2013). Carefully interpreting results with an emphasis on the reliability of the ELS evaluation is important when reading the functional connectivity literature. Further complicating interpretation of results is the developmental stage of the participants included in samples with histories of early adversity. The large number of studies with neuroimaging data collected during adolescence and early adulthood should be interpreted in the context of the developmental stage of the participants to ensure that the reported results are truly a departure from developmental norms.
A final limitation of the functional connectivity literature in ELS is the overinterpretation of results. Due to the nascent state of the literature, especially the application of graph theory techniques, further study of the analytic methods employed may be necessary prior to drawing strong conclusions. For example, subcortical structures associated with emotion processing are susceptible to signal loss or signal confounding in fMRI data (Boubela et al., 2015). The possibility of signal loss or less reliable subcortical connectivity estimates may contribute to noise in the literature. Additionally, there are few studies that employ methods improving causal inference. As such, many of the mechanistic links between ELS and brain function associated with psychopathology have yet to be empirically tested. It will be important for future investigations of early adversity to be designed in ways that allow more direct testing of the links between neural functioning following ELS and the development of mental illness.
Future Directions
The diversity of neurocognitive differences between groups with histories of ELS and those without combined with the relative lack of diversity in studies investigating brain function following ELS suggest important new directions for the field. For example, the notion of a developmental trade-off in the context of ELS is worthy of direct empirical scrutiny. As is clear given the findings cited in this review, accelerated maturation of the emotion circuity following adversity is both common and adaptive. Whether this precocious development comes at the detriment of other systems is of great importance for improving the lives of individuals with histories of ELS. If the development of reward processing is delayed to allow for early emotion processing maturation, it may provide one potential avenue by which ELS increases the risk for psychopathology. In this case, prevention and intervention efforts that target improved reward processing may be appropriate for many individuals with adverse experiences. It is also possible, if less consistent with behavioral work, that there is no developmental trade-off—that the early maturation of emotion processing following ELS does not come at the cost of other neural systems. Should there be no trade-off, continued efforts toward improving early care-giving environments and otherwise alleviating the incidence of childhood poverty and maltreatment may be most appropriate.
To evaluate the possibility of a developmental trade-off, it will be critical to continue to expand the brain systems under study beyond frontolimbic emotion processing systems to fully understand the effects of ELS on brain function. At a minimum, future research should investigate attention- and reward-related circuits that may shed light on a larger subset of the behavioral differences reported to date. This approach has already been shown to be promising for better understanding psychopathology as described earlier. Further, as indicated by the results of studies investigating both emotion- and reward-related circuits in relation to psychopathology onset, investigators should place a premium on study designs that facilitate probing the interactions between these systems. This interactive approach may be especially useful during adolescence, a period in which many theories stress the immaturity of prefrontal control regions relative to subcortical emotion-related structures (Casey, 2015). It may be necessary to include the development of emotion- and reward-processing systems when attempting to understand adolescent vulnerability (Crone & Dahl, 2012). Understanding the relationships between altered emotion processing and reward sensitivity following ELS is likely a critical piece of the puzzle when investigating the negative outcomes of early adversity. The increased utilization of techniques that support whole-brain investigations of connectivity, like graph theory, may be an immediate and tractable avenue for addressing this possibility.
Studies working to identify protective factors following ELS are another important way future research can establish the mechanisms involved in the development of psychopathology following stressful childhood experiences. For example, recent work in populations of post-institutionalized youth suggests that differences in stress physiology following ELS can be recalibrated during the pubertal period to the point that they are statistically identical to nonadopted youth (DePasquale, Donzella, & Gunnar, 2019). Similar effects have also been demonstrated in youth with high levels of traumatic stress in childhood (King et al., 2017). Whether or not these changes extend to alterations in the structure and function of the brain is yet to be investigated but is a promising path for future research. Additionally, interventions targeting the caregiving relationship following ELS have reported improvements in children’s cognitive functioning (Bernard, Lee, & Dozier, 2017). Continued investigations of the specific mechanisms that give rise to this malleability in outcomes following ELS will shed additional light on the pathways from childhood adversity to the development of psychopathology. Notably, the search for protective factors and their mechanisms may also address differences between the group and individual levels of analysis. Specifically, while at the group level we see the above described sequelae of early life adversities, at the individual level there is substantial heterogeneity in the outcomes of children, youth, and adults who grow up in adverse circumstances. The literature on resilience points to both individual and environmental factors that increase resilience and reduce risk for individuals (Sapienza & Masten, 2011). Thus, as we address how adversity “gets under the skin” it will be equally important to understand how resilience does as well.
Finally, large longitudinal studies that collect data at multiple levels of analysis continue to be the strongest experimental design available to researchers interested in ELS. Combining stress physiology measurement and functional neuroimaging should continue to aid in the elucidation of the mechanisms underlying many of the negative outcomes associated with childhood adversity. Such longitudinal studies should also work to establish the effects of the timing of stress onset using ongoing assessment of negative life events. As an added benefit, large samples with a diversity of ELS experiences (e.g. abuse, neglect, deprivation, etc.) will help to improve the specificity of the conclusions that can be drawn. This has, to some degree, been possible in the context of brain structure (see Hodel, 2018 for an exemplary review) but remains an open question in the functional neuroimaging literature. While experiences of ELS may not be avoidable, longitudinal imaging and the application of new analysis methods to brain function and functional connectivity data will improve our ability to respond effectively.
Highlights.
Early life stress alters functional connectivity in emotion and reward circuits.
Early adversity may result in accelerated maturation of emotion processing systems.
Accelerated maturation of emotion processing may slow development of other systems.
Functional connectivity is a tool for studying psychopathology after early adversity.
Acknowledgements
This work was supported by NICHD Grants R01 HD075349 (“Pubertal Stress Recalibration Hypothesis”), R21 HD086312, and JPB Foundation Grant #1025: Research Network on Toxic Stress and Health (M.R.G.). Further support was provided by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants TL1R002493 and UL1TR002494 (M.P.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors would like to thank their colleagues for thoughtful discussions that shaped the ideas presented here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassett DS, & Sporns O (2017). Network neuroscience. Nature Neuroscience, 20(3), 353–364. 10.1038/nn.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett C, Castle J, Rutter M, & Sonuga-Barke EJ (2010). Institutional deprivation, specific cognitive functions, and scholastic achievement: English and Romanian Adoptee (ERA) study findings. Monographs of the Society for Research in Child Development, 75(1), 125–142. 10.1111/j.1540-5834.2010.00553.x [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, & Draper P (1991). Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development, 62(4), 647–670. 10.1111/j.1467-8624.1991.tb01558.x [DOI] [PubMed] [Google Scholar]
- Bernard K, Lee AH, & Dozier M (2017). Effects of the ABC intervention on foster children’s receptive vocabulary. Child Maltreatment, 22(2), 107755951769112. 10.1177/1077559517691126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, & Nelson CA (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–196. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Roeber BJ, & Pollak SD (2017). Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences, 114(51), 201708791. 10.1073/pnas.1708791114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker-Schlier R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Jennen-Steinmetz C, … Laucht M (2016). Interaction between COMT Val 158 Met polymorphism and childhood adversity affects reward processing in adulthood. NeuroImage, 132, 556–570. 10.1016/j.neuroimage.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, … Laucht M (2014). Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS ONE, 9(8), 1–13. 10.1371/journal.pone.0104185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, & Pizzagalli DA (2013). Neurogenetics of depression: A focus on reward processing and stress sensitivity. Neurobiology of Disease, 52, 12–23. 10.1016/j.nbd.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, & Nelson CA (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience, 3(September), 1–7. 10.3389/neuro.08.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Seidel E-M, Derntl B, Pezawas L, … Moser E (2015). fMRI measurements of amygdala activation are confounded by stimulus correlated signal fluctuation in nearby veins draining distant brain regions. Scientific Reports, 5(May), 10499 10.1038/srep10499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Richardson R (2011). Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience, 125(1), 20–28. 10.1037/a0022008 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, & Tottenham N (2014). The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits-a cross-species analysis. Developmental Psychobiology, 56(8), 1635–1650. 10.1002/dev.21260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, & Earls F (1997). Psychological and neuroendocrinological sequela of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences, 807, 419–428. Retrieved from http://ezproxy.lib.utexas.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyhref&AN=2007.06922.0030013 [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, & Reiss AL (2007). Stress predicts brain changes in children: A pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics, 119(3), 509–516. 10.1542/peds.2006-2028 [DOI] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66(1), 295–319. 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Chaney A, Carballedo A, Amico F, Fagan A, Skokauskas N, Meaney J, & Frodl T (2014). Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. Journal of Psychiatry and Neuroscience, 39(1), 50–59. 10.1503/jpn.120208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (2013). Annual research review: Resilient functioning in maltreated children - past, present, and future perspectives. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(4), 402–422. 10.1111/j.1469-7610.2012.02608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, … Kilts CD (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine, 43(03), 507–518. 10.1017/S0033291712001390 [DOI] [PubMed] [Google Scholar]
- Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, & Kilts CD (2016). Modes of large-scale brain network organization during threat processing and posttraumatic stress disorder symptom reduction during TF-CBT among adolescent girls. PLoS ONE, 11(8), 1–16. 10.1371/journal.pone.0159620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Kreppner J, Beckett C, Castle J, Groothues C, … Sonuga-Barke EJS (2008). Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: Findings from the English and Romanian adoptees study. Journal of Abnormal Child Psychology, 36(7), 1057–1068. 10.1007/s10802-008-9232-x [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, & Toth SL (2015). Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology, 27(02), 521–533. 10.1017/S0954579415000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Moradi AR, Taghavi MR, Neshat-Doost HT, & Yule W (2001). An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychological Medicine, 31(03), 541–547. 10.1017/S0033291701003567 [DOI] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology and Behavior, 106(1), 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Demers LA, McKenzie KJ, Hunt RH, Cicchetti D, Cowell RA, Rogosch FA, … Thomas KM (2018). Separable effects of childhood maltreatment and adult adaptive functioning on amygdala connectivity during emotion processing. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(2), 116–124. 10.1016/j.bpsc.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale CE, Donzella B, & Gunnar MR (2019). Pubertal recalibration of cortisol reactivity following early life stress: a cross-sectional analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines, 60(5), 566–575. 10.1111/jcpp.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, & Pizzagalli DA (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66(3), 206–213. 10.1016/j.biopsych.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, … Manji HK (2009). Dynamic regulation of mitochondrial function by glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3543–3548. 10.1073/pnas.0812671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, & Anda RF (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111(3), 564–572. 10.1542/peds.111.3.564 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, & Essex MJ (2011). Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology, 23(1), 85–99. 10.1017/S0954579410000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59(2), 77–92. 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, 106(16), 6545–6549. 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, … Liberzon I (2016). Childhood cumulative risk exposure and adult amygdala volume and function. Journal of Neuroscience Research, 94(6), 535–543. 10.1002/jnr.23681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, … Tottenham N (2017). Altered ventral striatal–medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Development and Psychopathology, 29(05), 1865–1876. 10.1017/s0954579417001456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, & Bullmore E (2016). Fundamentals of Brain Network Analysis. In Fundamentals of Human Imaging Connectomics. 10.1016/B978-0-12-407908-3.00011-X [DOI] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, & Telzer EH (2013). Early developmental emergence of human amygdala – prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15638–15643. 10.1073/pnas.1307893110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, & Tottenham N (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience, 249, 129–138. 10.1016/j.neuroscience.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MZ, Allen JP, & Coan JA (2016). Lower neighborhood quality in adolescence predicts higher mesolimbic sensitivity to reward anticipation in adulthood. Developmental Cognitive Neuroscience, 22, 48–57. 10.1016/j.dcn.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, Mclaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I. Arch Gen Psychiatry, 67(2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, & Pollak SD (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 53706(9), 1–8. 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, & Pollak SD (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84(5), 1566–1578. 10.1111/cdev.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Albert D, Iselin AMR, Carré JM, Dodge KA, & Hariri AR (2016). Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Social Cognitive and Affective Neuroscience, 11(3), 405–412. 10.1093/scan/nsv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, & Williamson DE (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. 10.1016/j.biopsych.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, & Hariri AR (2018). Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: understanding interactive effects of early and more recent stress. Psychological Medicine, 48(11), 1835–1843. 10.1017/S0033291717003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences, 110(47), 19119–19124. 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg MP, Hodel AS, Cowell RA, Hunt RH, Gunnar MR, & Thomas KM (2018). Risk taking, decision-making, and brain volume in youth adopted internationally from institutional care. Neuropsychologia, 119, 262–270. 10.1016/J.NEUROPSYCHOLOGIA.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, & McEwen BS (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(5), 791–797. 10.1016/j.pnpbp.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review, 48(December 2017), 113–144. 10.1016/j.dr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA (1996). On the nature and consequences of early loss. Psychosomatic Medicine, 58(6), 570–581. Retrieved from http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&DbFrom=pubmed&Cmd=Link&LinkName=pubmed_pubmed&LinkReadableName=RelatedArticles&IdsFromResult=8948005&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, & Gunnar MR (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, 109(Supplement_2), 17208–17212. 10.1073/pnas.1121246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1), 256–282. 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K, Barrett S, Shannon C, Campbell C, Watson D, Rushe T, … Mulholland C (2012). Childhood trauma and hippocampal and amygdalar volumes in first-episode psychosis. Schizophrenia Bulletin, 38(6), 1162–1169. 10.1093/schbul/sbr085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Lee SS, Telzer EH, Gabard-Durnam LJ, Goff B, Flannery J, & Tottenham N (2015). Exploration-exploitation strategy is dependent on early experience. Developmental Psychobiology, 57(3), 313–321. 10.1002/dev.21293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, Kim P, Swain J, Evans G, Phan K, & Liberzon I (2016). Sex-specific effects of childhood poverty on neurocircuitry of processing of emotional cues: A neuroimaging study. Behavioral Sciences, 6(4), 28 10.3390/bs6040028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, & Liberzon I (2015). Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Frontiers in Behavioral Neuroscience, 9(June), 1–8. 10.3389/fnbeh.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, … Thomas KM (2015). Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Development and Psychopathology, 27(4pt2), 1577–1589. 10.1017/S0954579415000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, … Pizzagalli DA (2018). Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychological Medicine, 48(7), 1157–1166. 10.1017/S0033291717002628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, & Gotlib IH (2017). The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology, 77, 68–74. 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC (2004). Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience, 24(1), 218–228. 10.1523/JNEUROSCI.0433-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, & Gunnar MR (2018). Annual Research Review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines, 59(4), 327–346. 10.1111/jcpp.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert HK, Sheridan MA, Sambrook KA, Rosen ML, Askren MK, & McLaughlin KA (2017). Hippocampal contribution to context encoding across development is disrupted following early-life adversity. The Journal of Neuroscience, 37(7), 1925–1934. 10.1523/JNEUROSCI.2618-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Ma ST, Okada G, Shaun Ho S, Swain JE, & Evans GW (2014). Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Social Cognitive and Affective Neuroscience, 10(11), 1596–1606. 10.1093/scan/nsv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, … Barch D (2013). The effects of poverty on childhood brain development. JAMA Pediatrics, 167(12), 1135 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NA, Marusak HA, Sala-Hamrick KJ, Crespo LM, Rabinak CA, & Thomason ME (2018). Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Human Brain Mapping, 39(5), 1982–1994. 10.1002/hbm.23978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Etkin A, & Thomason ME (2015). Clinical disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage: Clinical, 8, 516–525. 10.1016/j.nicl.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Hatfield JRB, Thomason ME, & Rabinak CA (2017). Reduced ventral tegmental area-hippocampal connectivity in children and adolescents exposed to early threat. Biol Psychiatry Cogn Neurosci Neuroimaging, 2(2), 130–137. 10.1016/j.bpsc.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, & Thomason ME (2015). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40(5), 1250–1258. 10.1038/npp.2014.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, 109(Supplement_2), 17180–17185. 10.1073/pnas.1121254109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, & Nasca C (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II. Arch Gen Psychiatry, 67(2), 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan M. a. (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child and Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25(4), 239–245. 10.1177/0963721416655883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, … Pine DS (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology, 41(8), 1956–1964. 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry, 76(8), 629–638. 10.1016/j.biopsych.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, & Sonuga-Barke E (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22(10), 2316–2325. 10.1162/jocn.2009.21394 [DOI] [PubMed] [Google Scholar]
- Merz EC, Harlé KM, Noble KG, & McCall RB (2016). Executive function in previously institutionalized children. Child Development Perspectives, 10(2), 105–110. 10.1111/cdep.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, McCall RB, Wright AJ, & Luna B (2013). Inhibitory control and working memory in post-institutionalized children. Journal of Abnormal Child Psychology, 41(6), 879–890. 10.1007/s10802-013-9737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Sheridan MA, Hanson JL, McLaughlin KA, Bates JE, Lansford JE, … Dodge KA (2018). Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. Journal of Abnormal Psychology, 127(2), 160–170. 10.1037/abn0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Stramecki F, Gawęda Ł, Prochwicz K, Sąsiadek MM, Moustafa AA, & Frydecka D (2018). Interactions between variation in candidate genes and environmental factors in the etiology of schizophrenia and bipolar disorder: A systematic review. Molecular Neurobiology, 55(6), 5075–5100. 10.1007/s12035-017-0708-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, & Sullivan RM (2009). Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. Journal of Neuroscience, 29(50), 15745–15755. 10.1523/JNEUROSCI.4106-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, … Ernst M (2012). Incentive effect on inhibitory control in adolescents with early-life stress: An antisaccade study. Child Abuse and Neglect, 36(3), 217–225. 10.1016/j.chiabu.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, & Sonuga-Barke EJS (2011). The neurobiological toll of early human deprivation. Monogaphs of the Society for Research in Child Development, 76(4), 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C, Bennett MR, & Lagopoulos J (2016). Understanding heterogeneity in grey matter research of adults with childhood maltreatment—A meta-analysis and review. Neuroscience and Biobehavioral Reviews, 69, 299–312. 10.1016/j.neubiorev.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, & Osborn RE (2004). Hippocampal volume and memory performance in a community-based sample of women with Posttraumatic Stress Disorder secondary to child abuse. Journal Trauma Stress, 17(1), 37–40. 10.1023/B:JOTS.0000014674.84517.46 [DOI] [PubMed] [Google Scholar]
- Petty CR, Rosenbaum JF, Hirshfeld-Becker DR, Henin A, Hubley S, LaCasse S, … Biederman J (2008). The child behavior checklist broad-band scales predict subsequent psychopathology: A 5-year follow-up. Journal of Anxiety Disorders, 22(3), 532–539. 10.1016/j.janxdis.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, … Gunnar MR (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81(1), 224–236. 10.1111/j.1467-8624.2009.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, & Sinha P (2002). Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology, 38(5), 784–791. 10.1037/0012-1649.38.5.784 [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schüppen A, Schultz RT, … Konrad K (2014). Neural response to social rejection in children with early separation experiences. Journal of the American Academy of Child and Adolescent Psychiatry, 53(12), 1328–1337.e8. 10.1016/j.jaac.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Romens SE, Casement MD, McAloon R, Keenan K, Hipwell AE, Guyer AE, & Forbes EE (2015). Adolescent girls’ neural response to reward mediates the relation between childhood financial disadvantage and depression. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(11), 1177–1184. 10.1111/jcpp.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M (1981). Stress, coping and development: Some issues and some questions. Journal of Child Psychology and Psychiatry, 22(4), 323–356. 10.1111/j.1469-7610.1981.tb00560.x [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, & Mirnics K (2007). Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience, 27(12), 3295–3304. 10.1523/JNEUROSCI.4765-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza JK, & Masten AS (2011). Understanding and promoting resilience in children and youth. Current Opinion in Psychiatry, 24(4), 267–273. 10.1097/YCO.0b013e32834776a8 [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Williams LE, Woodward ND, & Heckers S (2013). Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophrenia Research, 143(1), 185–191. 10.1016/j.schres.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America, 109(32), 12927–12932. 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Mclaughlin KA, Winter W, Fox N, Zeanah C, & Nelson CA (2018). Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nature Communications, 1–8. 10.1038/s41467-018-04381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, … Tottenham N (2016). Previous institutionalization is followed by broader amygdala– hippocampal–PFC network connectivity during aversive learning in human development. The Journal of Neuroscience, 36(24), 6420–6430. 10.1523/JNEUROSCI.0038-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, & Betzel RF (2016). Modular brain networks. Annual Review of Psychology, 67(1), 613–640. 10.1146/annurev-psych-122414-033634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, & Rosenberg DR (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience, 10(11), 1460–1468. 10.1093/scan/nsv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14(2), 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, … Casey BJ (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, … Popoli M (2014). Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Molecular Psychiatry, 19(4), 433–443. 10.1038/mp.2014.5 [DOI] [PubMed] [Google Scholar]
- Van Harmelen AL, Hauber K, Moor BG, Spinhoven P, Boon AE, Crone EA, & Elzinga BM (2014). Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS ONE, 9(1), 10–13. 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, … Li L (2014). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human Brain Mapping, 35(4), 1154–1166. 10.1002/hbm.22241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, & Widom CS (2014). Long-term effects of child abuse and neglect on emotion processing in adulthood. Child Abuse and Neglect, 38(8), 1369–1381. 10.1016/j.chiabu.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Koga SF, & Carlson E (2005). Attachment in institutionalized and community children in Romania. Child Development, 76(5), 1015–1028. [DOI] [PubMed] [Google Scholar]