Abstract
Objective:
To compare oncologic and perioperative outcomes in patients who underwent minimally invasive surgery (MIS) compared to laparotomy for newly diagnosed early-stage cervical carcinoma.
Methods:
We retrospectively identified patients who underwent radical hysterectomy for stage IA1 with lymphovascular invasion (LVI), IA2, or IB1 cervical carcinoma at our institution from 1/2007–12/2017. Clinicopathologic characteristics and surgical and oncologic survival outcomes were compared using appropriate statistical testing. Multivariable Cox regression analysis was used to control for potential confounders.
Results:
We identified 196 evaluable cases—117 MIS (106 robotic [90.6%]) and 79 laparotomy cases. Cohorts had similar age, BMI, substage, histologic subtype, clinical and pathologic tumor size, positive margins, and presence of LVI. The MIS group had more cases with no residual tumor in the hysterectomy (24.8% vs. 10.1%, P=0.01). The laparotomy group had more cases with positive nodes (29.1% vs. 17.1%, P=0.046) and more patients who received adjuvant therapy (53.2% vs. 33.3%, P=0.006). Median follow-up was ~4 years. Five-year disease-free survival (DFS) rates were 87.0% in the MIS group and 86.6% in the laparotomy group (P=0.92); 5-year disease-specific survival (DSS) rates were 96.5% and 93.9%, respectively (P=0.93); and 5-year overall survival (OS) rates were 96.5% and 87.4%, respectively (P=0.15). MIS was not associated with DFS, DSS, or OS on multivariable regression analysis. The rate of postoperative complications was significantly lower in the MIS cohort (11.1% vs. 20.3%; P=0.04).
Conclusions:
MIS radical hysterectomy for cervical carcinoma did not confer worse oncologic outcomes in our single-center and concurrent series of patients with early-stage cervical carcinoma.
Keywords: Cervical cancer, radical hysterectomy, minimal invasive surgery, laparoscopy, robotic surgery, laparotomy
Introduction
Approximately 13,170 new cases of cervical cancer will be diagnosed in the US in 2019, and an estimated 4,250 women will die from this disease.1 Most patients in the US are diagnosed at an early stage and have a favorable prognosis.2 Worldwide, however, there are nearly 530,000 new cases diagnosed annually (third most common female cancer), and more than 275,000 women will die from this disease (fourth leading cause of cancer death in women).3
The standard management for early-stage cervical cancer in those who do not wish for fertility preservation is a radical hysterectomy with pelvic lymphadenectomy.4–6 With advancements in surgical technologies and techniques and the introduction of laparoscopic and robotic-assisted radical hysterectomy, the minimally invasive approach has been increasingly used as an alternative to open radical hysterectomy.7–9 The adoption of minimally invasive surgery (MIS) for radical hysterectomy continues to gain popularity, mainly due to the operative and perioperative benefits of MIS over laparotomy, including less blood loss, fewer surgical complications, and shorter length of hospital stay.10,11
Over the last 2 decades, multiple retrospective studies comparing the oncologic outcomes of MIS to those of open radical hysterectomy for the treatment of early-stage cervical cancer have reported similar rates of recurrence, death, disease-free survival (DFS), and overall survival (OS).9–27 However, two recent publications reported worse survival outcomes with MIS compared with laparotomy, raising concerns over the MIS approach. In a large epidemiologic retrospective national cancer database (NCDB) study in the US, Melamed et al. reported shorter OS with MIS over open radical hysterectomy among patients with early-stage cervical cancer.28 Similarly, Ramirez et al. reported the results of the Laparoscopic Approach to Cervical Cancer (LACC) trial, the first randomized controlled trial (RCT) to compare MIS to open radical hysterectomy for the treatment of early-stage cervical cancer. In this multicenter, international noninferiority phase 3 trial, MIS was associated with worse 4.5-year DFS and OS compared with laparotomy.29 These data provoked profound controversy and led to a change in the treatment guidelines for early-stage cervical cancer by the National Comprehensive Cancer Network (NCCN) and the European Society of Gynaecological Oncology (ESGO).6,30
In light of the ongoing debate and emerging cervical cancer surgical treatment paradigm shift, we sought to evaluate the survival outcomes at our institution. Our primary objective was to compare disease-free survival (DFS) between patients who underwent MIS and those who underwent laparotomy for newly diagnosed early-stage cervical carcinoma. Our secondary objectives were to compare the patterns of recurrence, disease-specific survival (DSS), OS, and perioperative outcomes.
Methods
After Institutional Review Board approval, we retrospectively identified all consecutive and concurrent patients who underwent radical hysterectomy with lymph node assessment for 2009 International Federation of Gynecology and Obstetrics (FIGO) stage IA1 with lymphovascular invasion (LVI), IA2, or IB1 cervical cancer at Memorial Sloan Kettering Cancer Center (MSK) from January 2007 through December 2017. We included only patients with squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma histologic subtypes on final pathologic assessment after hysterectomy. Patients who received any preoperative chemotherapy, radiation therapy, or chemoradiation therapy were excluded. We also excluded patients who underwent a planned radical trachelectomy but in whom a completion hysterectomy was deemed necessary intraoperatively or postoperatively.
The surgical procedures of the study population were performed by a team of 11 fellowship-trained gynecologic oncologists. Surgical approach (MIS vs. laparotomy) was determined at the discretion of the surgeon. All patients underwent radical hysterectomy and pelvic lymph node assessment, with or without para-aortic lymph node assessment, by either complete lymphadenectomy or limited sentinel lymph node (SLN) dissection. The decision to proceed with a bilateral salpingo-oophorectomy was based on patient age and other risk factors.
The use of postoperative adjuvant therapy (radiation therapy with or without chemotherapy) was determined according to accepted standard pathological risk factors, using Sedlis’ “intermediate-risk” criteria31 or Peter’s “high-risk” criteria32 and in accordance with NCCN guidelines.6 All patients were followed similarly after surgery and postoperative therapy, if given, irrespective of surgical approach using accepted NCCN guidelines.
Medical records were reviewed for clinicopathological, operative and survival data, including: age, body mass index (BMI), 2009 FIGO stage, tumor histologic subtype, pathologic and clinical tumor size, positive lymph nodes, positive surgical margins, positive LVI, parametria area, residual tumor in the hysterectomy specimen, planned surgical approach, conversion to laparotomy, use of uterine trans-cervical manipulator, type of colpotomy (intracorporeal vs. vaginal), estimated blood loss (EBL), transfusion status, intra- and postoperative complications, skin-to-skin operative time, inpatient length of hospital stay, length of indwelling Foley catheter, use of adjuvant therapy, time from surgery to last follow-up or death, status at last follow-up, time from surgery to recurrence, and site of recurrence.
Pathological tumor size was defined as the greatest diameter of the cervical lesion measured by the pathologist either microscopically or macroscopically. Intraoperative complications were those occurring during surgery, and postoperative complications were those occurring up to 30 days after surgery. Disease recurrence was determined clinically, radiographically, and/or histologically. Deaths were ascertained and attributed to disease based on outcomes occurring at our institution or death notices received by our institutional registry. Deaths that could not be attributed to a specific cause were deemed as death of unknown cause and not included in the DFS but the OS analysis.
Clinicopathologic characteristics and oncologic survival outcomes were compared. Association statistical tests between the two groups and other demographic and clinical factors were performed using the chi square or Fisher exact tests for categorical variables and the Mann-Whitney-U or Kruskal-Wallis test for continuous variables. DFS, disease-specific survival (DSS), and OS were calculated according to the Kaplan-Meier method. OS was defined as the time from surgery to the time of death of any cause, and patients who were alive were censored at the time of last follow-up. DSS was defined as the time from surgery until the time of death from cervical cancer or its complications, and patients who were alive or dead from a non–disease-related or an unknown cause were censored at the time of last follow-up. DFS was defined as the time from surgery until the time of first recurrence, and patients without recurrence were censored at the time of last follow-up or time of non–disease-related death. Cox regression analysis was performed to create multivariable models for DFS, DDS, and OS to account for potential confounders. All statistical analyses were performed on IBM SPSS Statistics version 24.0 (IBM SPSS Statistics for Windows 2013, Armonk, NY).
Results
Of 286 radical hysterectomy cases, 196 met our inclusion criteria. Patients were excluded due to the following: other histology and/or stage (n=62), use of neoadjuvant therapy (n=9), planned radical trachelectomy converted to radical hysterectomy (n=11), and presence of synchronous malignancy (n=2). Of the 196 included patients, 117 (59.7%) underwent MIS and 79 (40.3%) underwent laparotomy. Within the MIS group, 106 patients (90.6%) underwent robotic-assisted total laparoscopic radical hysterectomy and 9 patients (7.7%) underwent laparoscopic-assisted radical hysterectomy (5 total laparoscopic radical hysterectomies and 4 laparoscopic-assisted vaginal radical hysterectomies). Two (1.7%) patients in the MIS group were converted to laparotomy. The rate of MIS has increased at our institution over the study period from 13% in 2007 when the robotic platform was first introduced to 60–80% in recent years (Supplemental Figure 1).
Baseline clinicopathologic characteristics are shown in Table 1. There was no difference between the MIS and laparotomy cohorts in terms of median age (46 years [range, 28–71 years] vs. 44 years [range, 23–85 years], respectively, P=0.59) and median BMI (27.2 kg/m2 [range, 17.7–51.5 kg/m2] vs. 25.9 kg/m2 [range, 17.9–45 kg/m2], P=0.46). There was also no difference with regard to FIGO substage, histologic subtype, clinical tumor size, pathologic tumor size, positive margins, presence of LVI, or the size of parametrial area resection. Most patients in both groups had FIGO stage IB1 (88% in the MIS group and 94.9% in the laparotomy group, P=0.2) and adenocarcinoma histologic subtype disease (55.6% vs. 63.3%, respectively, P=0.52). The MIS group had a higher rate of cases with no residual tumor in the hysterectomy specimen compared with the laparotomy group (24.8% vs. 10.1%, P=0.01). The laparotomy group had a higher rate of positive lymph nodes compared with the MIS group (29.1% vs. 17.1%, P=0.046), as well as a higher rate of patients receiving adjuvant therapy (53.2% vs. 33.3%, P=0.006).
Table 1.
Baseline clinicopathologic characteristics of MIS versus open radical hysterectomy
| Variable | MIS N (%) | OPEN N (%) | P |
|---|---|---|---|
| N | 117 (59.7) | 79 (40.3) | |
| Approach | |||
| Robotic | 106 (90.6) | - | |
| Laparoscopic | 9 (7.7) | - | |
| Converted to open | 2 (1.7) | - | |
| Age, years Median (range) | 46 (28–71) | 44 (23–85) | 0.59 |
| BMI, kg/m2 Median (range) | 27.2 (17.7–51.5) | 25.9 (17.9–45) | 0.46 |
| FIGO 2009 Stage | 0.24 | ||
| IA1 with LVI | 2 (1.7) | 1 (1.3) | |
| IA2 | 12 (10.3) | 3 (3.8) | |
| IB1 | 103 (88) | 75 (94.9) | |
| Histology | 0.52 | ||
| SCC | 48 (41) | 26 (32.9) | |
| Adenocarcinoma | 65 (55.6) | 50 (63.3) | |
| Adenosquamous | 4 (3.4) | 3 (3.8) | |
| Clinical tumor size, cm Median (range) | 0 (0–4) | 0 (0–4) | 0.16 |
| Path tumor size, cm Median (range) | 1.6 (0.2–7.5) | 1.9 (0.4–8.6) | 0.17 |
| Tumor size | |||
| <=2 cm | 80 (74.1) | 52 (69.3) | 0.7 |
| >2 cm | 28 (25.9) | 23 (30.7) | |
| Margin positive | 2 (1.7) | 2(2.5) | 1.0 |
| LVI | 0.33 | ||
| Yes | 41 (35) | 35 (44.3) | |
| Suspicious | 6 (5.1) | 2(2.5) | |
| Node positive | 20 (17.1) | 23 (29.1) | 0.046 |
| Rt parametria area, cm2 Median (range) | 7.0 (0.44–34.20) | 6.5 (0.9–25.0) | 0.39 |
| Lt parametria area, cm2 Median (range) | 7.5 (0.84–22.75) | 7.3 (0.9–24.5) | 0.67 |
| Adjuvant therapy | 39 (33.3%) | 42 (53.2%) | 0.006 |
| No residual in hysterectomy | 29 (24.8%) | 8 (10.1%) | 0.01 |
MIS, minimally invasive surgery; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular invasion; SCC, squamous cell carcinoma
Median follow-up time was similar between the groups, as they were both from a concurrent study period (46.2 months [range, 0.4–131.4] for the MIS and 46.9 months [range, 0.2–146.7] for the laparotomy group, P=0.79). There was no difference in the recurrence or death rates between the groups (Table 2). There were 20 recurrences (10.2%) in the entire cohort at the time of analysis. Recurrences occurred in 12 (10.3%) of 117 MIS patients and 8 (10.1%) of 79 laparotomy patients (P=0.98). One of the 12 recurrences in the MIS cohort was an isolated trocar site recurrence 29 months after initial surgery. This was resected without any additional therapy, and the patient remains alive and without disease 91 months after initial surgery. Interestingly, 1 of the 8 recurrences in the laparotomy cohort also involved a vertical incisional recurrence 17 months after initial surgery. This patient also had a vaginal recurrence at the same time. She was treated with chemotherapy and died of disease 41 months after initial surgery. There were 13 deaths of any or unknown cause (6.6%) in the entire cohort—5 (4.3%) in the MIS group and 8 (10.1%) in the laparotomy group (P=0.11). Of these patients, 4 (3.4%) in the MIS group and 3 (3.8%) in the laparotomy group died of cervical cancer or its complications (P=1.0).
Table 2.
Oncologic survival outcomes of MIS versus open radical hysterectomy
| Variable | MIS N (%) | OPEN N (%) | P |
|---|---|---|---|
| N | 117 (59.7) | 79 (40.3) | |
| Follow-up, months Median (range) | 46.2 (0.4–131.4) | 46.9 (0.2–146.7) | 0.79 |
| Recurred | 12 (10.3) | 8 (10.1) | 0.98 |
| Deaths | |||
| Died of disease | 4 (3.4) | 3 (3.8) | 1.0 |
| Died of any or unknown | 5 (4.3) | 8 (10.1) | 0.11 |
| 5-yr DFS rate | 87.0 (+/−3.6) | 86.6 (+/−4.5) | 0.92 |
| 5-yr DSS rate | 96.5 (+/−2.0) | 93.9 (+/−3.5) | 0.93 |
| 5-yr OS rate | 96.5 (+/−2.0) | 87.4 (+/−4.9) | 0.15 |
| Tumor ≤2 cm | |||
| N | 74 (61) | 47 (39) | |
| 5-yr DFS rate | 87.1 (+/−4.5) | 93.0 (+/−4.8) | 0.26 |
| 5-yr DSS rate | 98.0 (+/−2.0) | 100 (NE) | 0.25 |
| 5-yr OS rate | 98.0 (+/−2.0) | 92.1 (+/−5.3) | 0.41 |
| Tumor >2 cm | |||
| N | 42 (58) | 30 (42) | |
| 5-yr DFS rate | 86.3 (+/−5.8) | 76.9 (+/−8.4) | 0.44 |
| 5-yr DSS rate | 93.7 (+/−4.4) | 83.8 (+/−8.8) | 0.43 |
| 5-yr OS rate | 93.7 (+/−4.4) | 79.3 (+/−9.4) | 0.23 |
| No adjuvant therapy | |||
| N | 78 (68) | 37 (32) | |
| 5-yr DFS rate | 89.7 (+/−4.2) | 96.2 (+/−3.8) | 0.26 |
| 5-yr DSS rate | 98.0 (+/−2.0) | 95.7 (+/−4.3) | 0.95 |
| 5-yr OS rate | 98.0 (+/−2.0) | 86.7 (+/−7.1) | 0.31 |
| Adjuvant therapy | |||
| N | 39 (48) | 42 (52) | |
| 5-yr DFS rate | 81.6 (+/−6.9) | 77.4 (+/−7.9) | 0.85 |
| 5-yr DSS rate | 93.4 (+/−4.5) | 92.7 (+/−5.1) | 0.93 |
| 5-yr OS rate | 93.4 (+/−4.5) | 89.2 (+/−5.9) | 0.46 |
| IB1 with residual in Hyst | |||
| N | 82 (54) | 69 (46) | |
| 5-yr DFS rate | 82.1 (+/−4.8) | 85.0 (+/−5.0) | 0.55 |
| 5-yr DSS rate | 95.2 (+/−2.7) | 93.2 (+/−3.8) | 0.88 |
| 5-yr OS rate | 95.2 (+/−2.7) | 86.1 (+/−5.3) | 0.28 |
MIS, minimally invasive surgery; DFS, disease-free survival; DSS, disease-specific survival; OS, overall survival.
DFS, DSS, OS presented as percent and standard error.
There was no difference in survival rates between the groups (Figure 1 and Table 2). Five-year DFS rates were 87.0% (SE 3.6%) for the MIS and 86.6% (SE 4.5%) for the laparotomy group (P=0.92). Five-year DSS rates were 96.5% (SE 2.0%) and 93.9% (SE 3.5%), respectively (P=0.93). Five-year OS rates were 96.5% (SE 2.0%) and 87.4% (SE 4.9%), respectively (P=0.15; Table 2).
Figure 1.
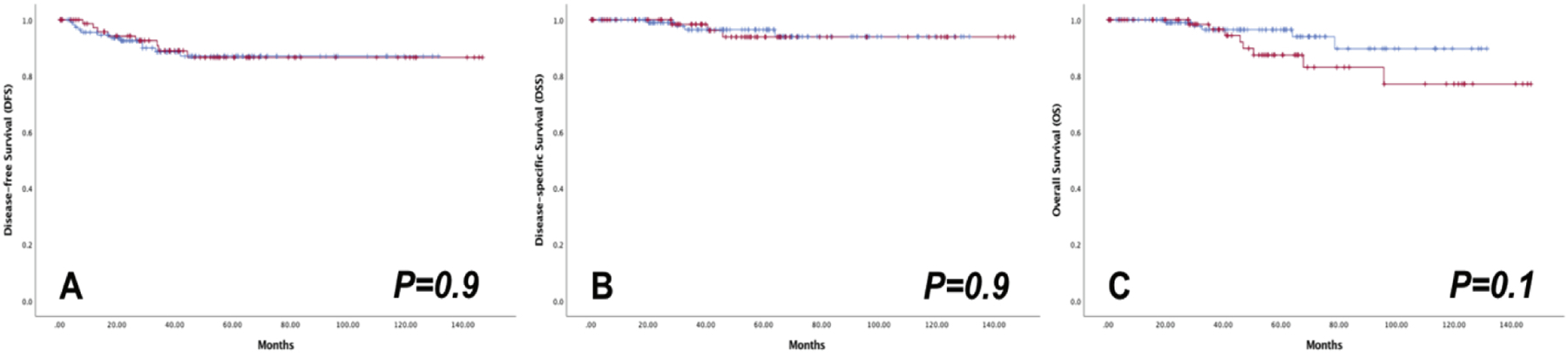
Oncologic outcomes of MIS ( ) versus OPEN (
) versus OPEN ( ) radical hysterectomy in the entire cohort
) radical hysterectomy in the entire cohort
All recurrences occurred in FIGO stage IB1 cases with residual tumor in the hysterectomy specimen. MIS recurrences occurred evenly between locoregional and distant sites; recurrences in the laparotomy group occurred most frequently in retroperitoneal nodes (Table 3). The sites of first recurrence for the MIS and laparotomy groups were as follows: vaginal cuff (16.7% vs. 12.5%), pelvis (8.3% vs. 0%), nodal only (16.7% vs. 50%), abdomen (16.7% vs. 0%), extra-abdominal (liver, lung, bone; 33.3% vs. 12.5%), and multiple sites (8.3% vs. 25%), respectively (P=0.35). Eighty-three percent of the patients who recurred in the laparotomy group received adjuvant treatment, compared with a third in the MIS group. Of note, 1 patient in the MIS group declined adjuvant therapy, recurred, and died of disease.
Table 3.
Sites of recurrence
| Recurrence site | MIS (N=12) N (%) | OPEN (N=8) N (%) | P |
|---|---|---|---|
| Vaginal cuff alone | 2 (16.7) | 1 (12.5) | 0.35 |
| RP LNs | 2 (16.7) | 4 (50) | |
| Pelvis non-cuff | 1 (8.3) | 0 (0) | |
| Abdomen | 2 (16.7) | 0 (0) | |
| Multiple | 1 (8.3) | 2 (25) | |
| Distant (liver, lung, bone) | 4 (33.3) | 1 (12.5) |
MIS, minimally invasive surgery; RP LN, retroperitoneal lymph nodes
There were no recurrences in patients with FIGO 2009 stage IA disease (n=18) or patients with no residual tumor, of any stage, in the hysterectomy specimen (n=37) (Supplemental Table 1). Stage distribution in patients with no residual disease in the hysterectomy specimen was as follows: stage IA1 with LVSI, 2 (5.4%); stage IA2, 8 (21.6%); and stage IB1, 27 (73%). The survival outcomes for patients with FIGO 2009 stage IB1 disease who also had residual tumor in the hysterectomy specimen did not differ between the two groups (5-year DFS rates of 82.1% and 85.0%, P=0.55; 5-year DSS rates of 95.2% and 93.2%, P= 0.88; and 5-year OS rates of 95.2% and 86.1%, P=0.28; Table 2 and Figure 2).
Figure 2.
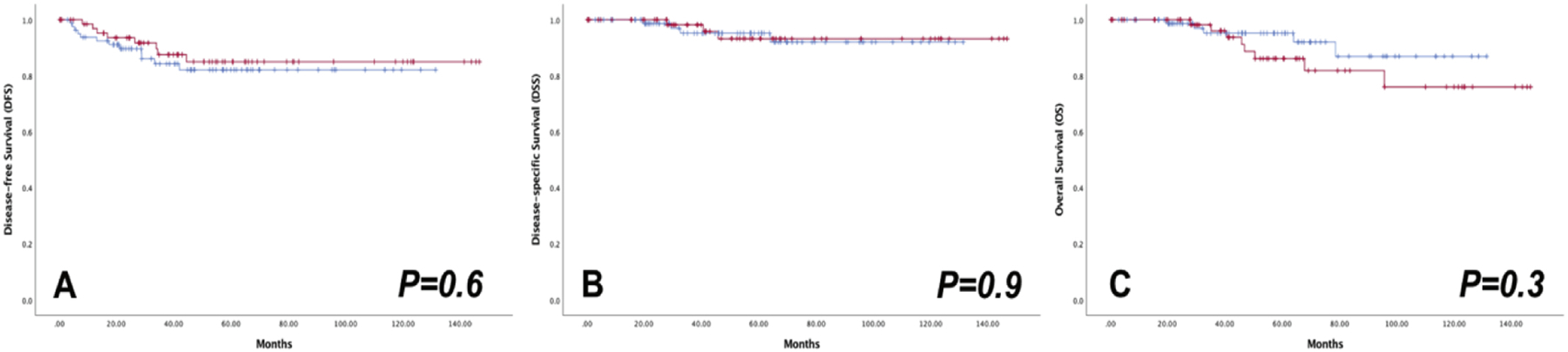
Oncologic outcomes of MIS ( ) versus OPEN (
) versus OPEN ( ) radical hysterectomy in patients with stage IB1 and residual in hysterectomy specimen
) radical hysterectomy in patients with stage IB1 and residual in hysterectomy specimen
Among all cases, 5-year DFS and DSS rates were lower for patients with tumor >2 cm, but this was not statistically significant (Supplemental Table 1). There was no difference in 5-year DFS, DSS, or OS rates between the MIS and laparotomy cohorts when stratified by tumor size ≤2 cm versus >2 cm (Table 2). Figure 3 depicts the Kaplan-Meier estimates in patients with tumors >2 cm.
Figure 3.
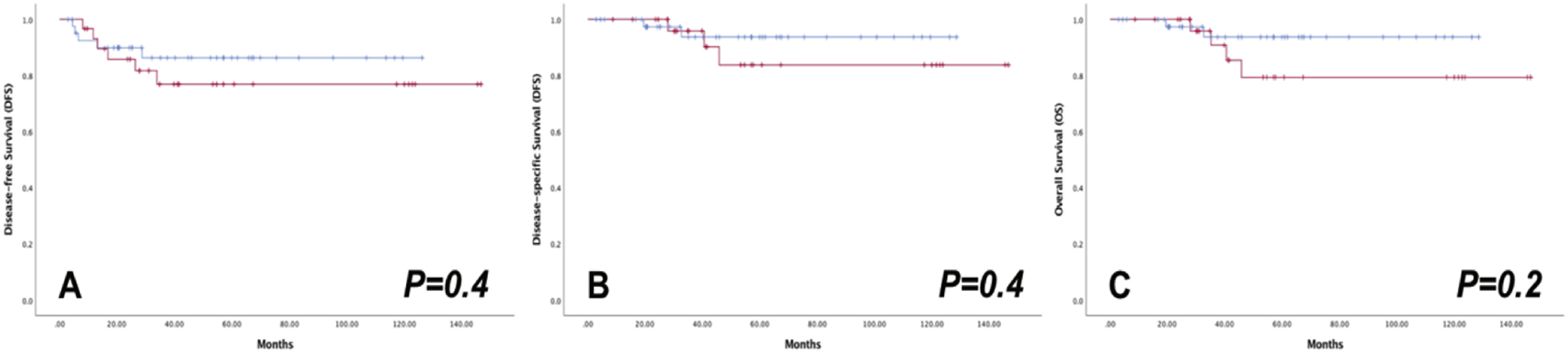
Oncologic outcomes of MIS ( ) versus OPEN (
) versus OPEN ( ) radical hysterectomy in patients with tumor size >2 cm
) radical hysterectomy in patients with tumor size >2 cm
Among all patients, those who received adjuvant therapy (a marker for intermediate- and high-risk patients) had a worse 5-year DFS rate compared with those who did not receive adjuvant therapy. The two groups had excellent and similar 5-year DSS rates (Supplemental Table 1). There were no differences in the DFS, DSS, or OS rates between the MIS and laparotomy groups regardless of adjuvant therapy use (Table 2).
Multivariable modelling was performed only in stage IB1 cases with residual tumor in the hysterectomy specimen (Supplemental Table 4). Stage IA cases and those without residual tumor were not included, because there were no events of progression or death in these cases. Based on the differences noted between the groups, we included node positivity (yes/no) as well as adjuvant therapy use (yes/no) in the model, in addition to surgical approach (MIS/laparotomy). We also included tumor size (≤2/>2 cm) in the model, as we felt it was an important potential clinical confounder. The adjusted HR for recurrence, DFS, (MIS/laparotomy) was 1.36 (95% CI: 0.55–3.39; P=0.51). The HR for cervical cancer related death, DSS, (MIS/laparotomy) was 0.79 (95% CI: 0.16–4.04; P=0.78). The HR for all-cause mortality, OS, (MIS/laparotomy) was 0.36 (95% CI: 0.10–1.24; P=0.1).
Perioperative outcomes are summarized in Supplemental Table 2. A longer, but not statistically significant, median operative time was noted in the MIS group compared with the laparotomy group (237 minutes [range, 75–562 minutes] vs. 203 minutes [range, 120–381 minutes], respectively, P=0.08). The median length of inpatient hospital stay was significantly shorter for the MIS group (1 day [range, 0–6 days] vs. 3 days [range, 2–15 days], respectively, P<0.001), as was the time with indwelling Foley catheter. The median EBL was lower in the MIS group (75 mL [range, 10–1250 mL] vs. 300 mL [range, 50–2200 mL], P<0.001), which translated into a significantly lower transfusion rate (0.9% vs. 8.9%, respectively; P=0.008). There was no difference in the overall rate of complications between the groups (P=0.1). However, 13 (11.1%) MIS cases compared with 16 (20.3%) laparotomy cases developed a postoperative complication (P=0.04)
Within the MIS group, most colpotomies (95%) were intracorporeal; 6 (5%) were vaginal, of which 1 (16.7%) recurred. A transcervical manipulator was used in most MIS cases. In 14 (12%) patients, no manipulator was used or a colpo-probe only was used, and 3 (21.4%) of these patients recurred. The small number of patient subsets did not permit a reliable statistical analysis in terms of colpotomy approach or use of transcervical manipulation.
Supplemental Table 3 describes the number of cases per surgeon and the rate of recurrence and cervical cancer deaths by surgeon. The recurrence rates ranged from 0–25% for MIS and 0–43% for laparotomy per surgeon. Robust statistical analysis was not deemed possible.
Discussion
MIS had gained acceptance as a feasible alternative to open surgery since the first report of a total laparoscopic radical hysterectomy with lymph node dissection by Nezhat et al. in 19927 and the first robotic-assisted total laparoscopic radical hysterectomy by Sert et al. in 2006.8 MIS has become increasingly popular due to its reported benefits, including less blood loss, shorter hospital stay, fewer postoperative complications, and less analgesic need.9–11 At our institution, many surgeons have adopted the MIS radical hysterectomy as their preferred approach for select cases, and the MIS rate has increased over the study period from 13% in 2007 to 60–80% in 2015–2017. Standard laparoscopic instrumentation was considered less than optimal and limited to properly perform a radical hysterectomy; these limitations were addressed by the robotic platform, which was introduced at our institution in 2007. This transition has prompted the need for quality control in terms of oncologic outcomes.
In this study, we investigated the oncologic and perioperative outcomes of MIS compared with open radical hysterectomy for the treatment of early-stage cervical cancer at our institution. We found comparable survival outcomes between the groups in terms of DFS, DSS, and OS but with the obvious limitations of a single-center retrospective analysis. Our findings are consistent with the results of previously published retrospective studies9–27 but contradict the results of the recently published LACC trial29 and NCDB analysis,28 which showed worse survival outcomes with MIS.
The LACC trial, published by Ramirez et al., is the only RCT to compare the survival outcomes of MIS versus open radical hysterectomy. It was designed as a non-inferiority international, multicenter trial, and demonstrated worse survival outcomes in the MIS group compared with the open group, with 4.5-year DFS rates of 86.0% and 96.5%, respectively (between-group difference, −10.6 percentage points; 95% CI: −16.4 to −4.7; P=0.87 for noninferiority) and 3-year OS rates of 93.8% and. 99.0% (HR for death from any cause, 6.00; 95% CI: 1.77 to 20.30).29 These unexpected results have led the NCCN and ESGO to change their consideration of surgical approach for early-stage cervical cancer and have influenced many centers to change their practice patterns and abandon the MIS approach.6,30
We used the same “inclusion” criteria as those in the LACC trial for our study. Our patients were also similar in terms of age, BMI, and FIGO substage. One difference is that the included histologies in our series were based on final pathologic assessment and not preoperative biopsies, as was the case in the LACC trial. In our study, the most common histologic subtype was adenocarcinoma, whereas in the LACC trial, it was squamous cell carcinoma. Another notable difference between the study populations is the proportion of robotically assisted cases within the MIS group. In our cohort, 91% of MIS cases were performed using the robotic platform compared with only 14% in the LACC trial. This raises the question of whether the LACC trial results apply to MIS cases in which the robotic platform is used. Retrospective studies comparing robotic to open radical hysterectomy have reported conflicting results. A sub-analysis of the NCBD study by Melamed et al., in which nearly 80% of cases in the MIS cohort were performed robotically, found that robotic radical hysterectomy was associated with a higher risk of death compared with open surgery (HR=1.61; 95% CI: 1.18–2.21).28 National database studies themselves have significant limitations, especially when assessing surgical interventions. Other studies, however, have shown comparable oncologic outcomes between robotic and open radical hysterectomy,21–24 including a large meta-analysis by Zhang et al., which showed no difference in recurrence rates between the groups (OR=0.85; 95% CI: 0.58–1.27, P=0.43).33
In our overall cohort, the laparotomy group had a higher rate of node metastases, lower rate of residual disease in the hysterectomy specimen, and a higher rate of adjuvant therapy use. All suggest that the patients in the laparotomy group were higher risk. There is no current practice for selecting patients for MIS based on risk factors among our surgeons. Some surgeons perform MIS for all cases, and others perform laparotomy for all cases, without selection. For Surgeons 1 (laparotomy only) and 5 (MIS only; Table 6), the rates of recurrence were 6.5% and 8.7%, respectively, with only 1 death with laparotomy only. The slight difference in some of the baseline characteristics among the two MIS and laparotomy groups is possibly related to the surgeons who newly adopted robotics in their practice during the time period. We attempted to account for these baseline characteristic differences with multivariable analysis. We did not include stage IA or cases with no residual in the hysterectomy, as no events occurred in these cases. MIS was not associated with worsened DFS, DSS or OS after multivariable modelling.
Patients in our MIS cohort had improved perioperative and postoperative outcomes. EBL, transfusion rates, and length of hospital stay were all significantly lower. The rate of intraoperative complications was the same and low in both groups. This is expected considering we maintain the same surgical and oncologic technique in all approaches. The rate of postoperative complications after MIS was nearly half that after laparotomy. These findings are in line with the vast majority of both retrospective and prospective data assessing MIS in gynecology, in both benign and malignant cases.
RCTs are the gold standard in hypothesis testing. The LACC trial was an internally valid trial. However, its external validity is questionable. Drug RCTs use a defined and reliable drug with dosing that is reproducible, irrespective of the physician delivering it. This is more externally valid as long as applied to the same patient type as the cohort enrolled in the RCT. Surgery is not a drug, and variation in technique, expertise, and outcomes among surgeons is well known. We noted this variation within our own institution. This creates an issue regarding the external validity of surgical RCTs, especially one involving a complex procedure and one in which the suboptimal outcomes occurred in only 13 of the 33 enrolling sites.
Surgical outcomes for various cancer procedures vary significantly between institutions.34 Cancer outcomes in general will also vary significantly by the type of institution providing the care. Pfister and colleagues reported significant differences in outcomes for patients with various malignancies between PPS-exempt institutions, NCI-designated comprehensive cancer centers, academic medical centers, and community centers.35 An analysis of Japanese Gynecologic Oncology Group (JGOG) sites noted a significant and clinically important difference in survival among patients undergoing radical hysterectomy at low-, mid-, and high-volume centers.36 Therefore, we must be cautious in the uniform and widespread application of results from one RCT in surgery to every single patient, surgeon, and center worldwide. Considering the wide variation in surgical outcomes, combining all the data among multiple surgeons brings the results to the mean/median and incorrectly classifies outcomes among individual institutions and surgeons – both better and worse. Real-world data captures all patients, unlike RCTs that enroll only a fraction, and can complement RCT findings.37 Real-world data can also be more reflective of individual outcomes if done correctly. We feel there is enough rationale to explain why our findings differ from those of the LACC trial, and it is plausible that we do have different outcomes.
A recent analysis of the Swedish Quality Register of Gynaecologic Cancer (SQRGC), which captures all women in Sweden who are treated at only 7 specialized centers to which cervical cancer patients are centralized, noted absolutely no difference between patients who underwent MIS compared to laparotomy.38 All MIS cases were performed using the robotic platform. Matsuo and colleagues also recently analyzed oncologic outcomes for patients undergoing radical trachelectomy using the NCDB.39 The MIS group seemed to have “higher risk” features, yet the OS was the same, with a trend in favor of MIS. These two studies provide some interesting insights. Patients being evaluated for radical trachelectomy undergo an extensive and thorough workup, and pelvic MRI is a requirement for better tumor assessment. Additionally, these procedures are often performed only by experienced surgeons in the US. Outcomes at specialized centers, with careful preoperative assessment of tumor beyond just a pelvic exam, may be different.
A strength of our study is that it was undertaken at a single institution, with centralized pathology review, standardized preoperative assessment and selection for surgery, and postoperative oncologic management. Additionally, we captured every single patient during a concurrent time period, with the same median follow-up time in both groups. RCTs do not include every single eligible patient for many reasons. Almost none of our cases are lost to follow-up, with only 7 cases not having follow-up beyond the initial postoperative visit.
We recognize that the current study has significant limitations. It is retrospective and subject to the same limitations as any other such study. There was no randomization, and baseline differences in the overall groups were noted, which we tried to account for through multivariable model and subgroup analyses. There is a growing trend and “in vogue” approach to use propensity scored matching to account for confounding instead of multivariable regression analysis in non-randomized retrospective datasets. While this is a valid method, it is not novel, being first described in 1976, nor superior to multivariable regression analysis, and both have their own merits and limitations.40 Compared to LACC and other studies, our total number of cases is low, but we hope it contributes somewhat to our ongoing discussions regarding this topic. The concept of post-hoc power analyses in completed retrospective studies is also another common critique and request. However, retrospective studies can only analyze what data are available and are hypothesis generating, not definitive. Power calculations are most useful in prospective trial planning and not as useful as thought to be after completion of a retrospective analysis.41
MIS approach in our single-center experience, with retrospective analyses, did not seem to compromise oncologic outcomes in patients who underwent radical hysterectomy for early-stage cervical carcinoma. Similar to those of the LACC trial, our results may not be applicable to all surgeons or centers. We should learn from the LACC trial and assess our outcomes and see where modification is needed. We understand our data are limited, but we hope do contribute to the growing body of literature as we await further randomized trials. The basis of scientific discovery is the principle of repeated testing of the hypothesis. Additional RCTs, addressing the concerns of the LACC trial, are warranted, justified with equipoise, and already underway.
Supplementary Material
Research Highlights.
Minimally invasive surgery (MIS) is an appropriate option in early-stage cervical carcinoma
MIS confers similar survival outcomes compared with laparotomy for the management of early-stage cervical carcinoma
MIS is associated with fewer postoperative complications compared with laparotomy in the management of these patients
Acknowledgements
Funding: This study was supported in part by the National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Dr. Leitao is an ad hoc consultant and proctor for Intuitive Surgical, Inc. Outside the submitted work, Dr. Abu-Rustum reports grants from Stryker/Novadaq, Olympus, and GRAIL; Dr. Chi reports personal fees from Bovie Medical Co., Verthermia Inc. (now Apyx Medical Corp.), C Surgeries, and Biom ‘Up; and Dr. Jewell reports personal fees from Covidien/Medtronic. The other authors have no potential conflicts to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Ca Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 4.Bansal N, Herzog TJ, Shaw RE, et al. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol 2009;201(5):485.e1–9. [DOI] [PubMed] [Google Scholar]
- 5.Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/ European Society for Radiotherapy and Oncology/ European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol 2018;127(3):404–416. [DOI] [PubMed] [Google Scholar]
- 6.Koh WJ, Abu-Rustum NR, Bean S, et al. J Natl Compr Canc Netw. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology 2019;17(1):64–84. [DOI] [PubMed] [Google Scholar]
- 7.Nezhat CR, Burrell MO, Nezhat FR, et al. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol 1992;166:864–865. [DOI] [PubMed] [Google Scholar]
- 8.Sert BM, Abeler VM. Robotic-assisted laparoscopic radical hysterectomy (Piver type III) with pelvic node dissection: case report. Eur J Gynaecol Oncol 2006; 27:531–533. [PubMed] [Google Scholar]
- 9.Medlin EE, Kushner DM, Barroilhet L. Robotic surgery for early stage cervical cancer: Evolution and current trends. J Surg Oncol 2015;112(7):772–81. [DOI] [PubMed] [Google Scholar]
- 10.Wang YZ, Deng L, Xu HC, et al. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer 2015;15:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shazly SA, Murad MH, Dowdy SC, et al. Robotic radical hysterectomy in early stage cervical cancer: A systematic review and meta-analysis. Gynecol Oncol 2015;138(2):457–71. [DOI] [PubMed] [Google Scholar]
- 12.Steed H, Rosen B, Murphy J, et al. A comparison of laparascopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol 2004;93(3):588–93. [DOI] [PubMed] [Google Scholar]
- 13.Jackson KS, Das N, Naik R, et al. Laparoscopically assisted radical vaginal hysterectomy vs. radical abdominal hysterectomy for cervical cancer: a match controlled study. Gynecol Oncol 2004;95(3):655–61. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Yan X, Shang H, Wang G, et al. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical cancer. Gynecol Oncol 2007;105(1):176–80. [DOI] [PubMed] [Google Scholar]
- 15.Pahisa J, Martínez-Román S, Torné A, et al. Comparative study of laparoscopically assisted radical vaginal hysterectomy and open Wertheim-Meigs in patients with early-stage cervical cancer: eleven years of experience. Int J Gynecol Cancer 2010;20(1):173–178. [DOI] [PubMed] [Google Scholar]
- 16.Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol 2012;23(4):903–11. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Kim DY, Kim JH, et al. Laparoscopic versus open radical hysterectomy in patients with stage IB2 and IIA2 cervical cancer. J Surg Oncol 2013;108(1):63–9. [DOI] [PubMed] [Google Scholar]
- 18.Xiao M, Zhang Z. Total Laparoscopic Versus Laparotomic Radical Hysterectomy and Lymphadenectomy in Cervical Cancer: An Observational Study of 13-Year Experience. Medicine (Baltimore) 2015;94(30):e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Chu HJ, Shang CL, et al. Long-Term Oncological Outcomes After Laparoscopic Versus Abdominal Radical Hysterectomy in Stage IA2 to IIA2 Cervical Cancer: A Matched Cohort Study. Int J Gynecol Cancer 2016;26(7):1264–73. [DOI] [PubMed] [Google Scholar]
- 20.Laterza RM, Uccella S, Casarin J, et al. Recurrence of Early Stage Cervical Cancer After Laparoscopic Versus Open Radical Surgery. Int J Gynecol Cancer 2016;26(3):547–52. [DOI] [PubMed] [Google Scholar]
- 21.Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: A multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol 2016;42(4):513–22. [DOI] [PubMed] [Google Scholar]
- 22.Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: A five year experience. Surg Oncol 2016;25(1):66–71. [DOI] [PubMed] [Google Scholar]
- 23.Diver E, Hinchcliff E, Gockley A, et al. Minimally Invasive Radical Hysterectomy for Cervical Cancer Is Associated With Reduced Morbidity and Similar Survival Outcomes Compared With Laparotomy. J Minim Invasive Gynecol 2017;24(3):402–406. [DOI] [PubMed] [Google Scholar]
- 24.Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol 2017;28(6):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu T, Chen X, Zhu J, et al. Surgical and Pathological Outcomes of Laparoscopic Versus Abdominal Radical Hysterectomy With Pelvic Lymphadenectomy and/or Para-aortic Lymph Node Sampling for Bulky Early-Stage Cervical Cancer. Int J Gynecol Cancer 2017;27(6):1222–1227. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Kim K, Park SJ, et al. Comparative Effectiveness of Abdominal versus Laparoscopic Radical Hysterectomy for Cervical Cancer in the Postdissemination Era. Cancer Res Treat 2019;51(2):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matanes E, Abitbol J, Kessous R, et al. Oncologic and Surgical Outcomes of Robotic Versus Open Radical Hysterectomy for Cervical Cancer. J Obstet Gynaecol Can 2019;41(4):450–458. [DOI] [PubMed] [Google Scholar]
- 28.Melamed A, Margul DJ, Chen L, et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med 2018;379(20):1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med 2018;379(20):1895–1904. [DOI] [PubMed] [Google Scholar]
- 30.Querleu D, Cibula D, Concin N, et al. Laparoscopic radical hysterectomy: an ESGO statement. https://www.esgo.org/explore/council/esgo-statement-laparoscopic-radical-hysterectomy, posted to the SEER web site, May 2019. [Google Scholar]
- 31.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–183. [DOI] [PubMed] [Google Scholar]
- 32.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–1613. [DOI] [PubMed] [Google Scholar]
- 33.Zhang SS, Ding T, Cui ZH, et al. Efficacy of robotic radical hysterectomy for cervical cancer compared with that of open and laparoscopic surgery: A separate meta-analysis of high-quality studies. Medicine (Baltimore). 2019. January;98(4):e14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoag JR, Resio BJ, Monsalve AF, Chiu AS, Brown LB, Herrin J, Blasberg JD, Kim AW, Boffa DJ. Differential safety between top-ranked cancer hospitals and their affiliates for complex cancer surgery. JAMA Network Open 2019;2(4):e191912.doi: 10.1001/jamanetworkopen.2019.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfister DG, Rubin DM, Elkin EB, Neill US, Duck E, Radzyner M, Bach PB. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol 2015;1:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuo K, Shimada M, Yamaguchi S, Matoda M, Nakanishi T, Kikkawa F, Ohmichi M, Okamoto A, Sugiyama T, Mikami M. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol 2019;133:1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth CM, Karim S, Mackillop WJ. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol 2019;16:312–325. [DOI] [PubMed] [Google Scholar]
- 38.Alfonzo E, Wallin E, Ekdahl L, Staf C, Radestad AF, Reynisson P, Stalberg K, Falconer H, Persson J, Dahm-Kahler P. No survival difference between robotic and open radical hysterectomy for women with early-stage cervical cancer: results from a nationwide population-based cohort study. Eur J Cancer 2019;116:169–177. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo K, Chen L, Mandelbaum RS, Melamed A, Roman LD, Wright JD. Trachelectomy for reproductive-aged women with early-stage cervical cancer: minimally invasive surgery versus laparotomy. Am J Obstet Gynecol 2019;220(5):469.e1–469.e13.doi: 10.106/j.ajog.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin PharmacolToxicol 2006;98:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiroutek MR, Turner JR. Why it is nonsensical to use retrospective power analyses to conduct a postmortem on your study. J Clin Hypertens 2018;20:408–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


