Abstract
Background:
Statins are associated with lower risk of aggressive prostate cancer, but lethal prostate cancer is understudied and contributing mechanisms unclear. We prospectively examined statins and lethal prostate cancer risk in the Health Professionals Follow-up Study (HPFS), tested associations with molecular subtypes, and integrated gene expression profiling to identify putative mechanisms.
Methods:
Our study included 44,126 men cancer-free in 1990, followed for prostate cancer incidence through 2014, with statin use recorded on biennial questionnaires. We used multivariable Cox regression to examine associations between statins and prostate cancer risk overall, by measures of clinically-significant disease, and by ERG and PTEN status. In exploratory analysis, age-adjusted gene set enrichment analysis identified statin-associated pathways enriched in tumor and adjacent normal prostate tissue.
Results:
During 24 years follow-up, 6,305 prostate cancers were diagnosed and 801 (13%) were lethal (metastatic at diagnosis or metastatic/fatal during follow-up). Relative to never/past use, current statin use was inversely associated with risk of lethal prostate cancer (HR 0.76; 95%CI 0.60–0.96) but not overall disease. We found a strong inverse association for risk of PTEN-null cancers (HR 0.40; 95%CI 0.19–0.87) but not PTEN-intact cancers (HR 1.18; 95%CI 0.95–1.48; p-heterogeneity=0.01). Associations did not differ by ERG. Inflammation and immune pathways were enriched in normal prostate of statin ever (n=10) versus never users (n=103).
Conclusion:
Molecular tumor classification identified PTEN and inflammation/immune activation as potential mechanisms linking statins with lower lethal prostate cancer risk. These findings support a potential causal association and could inform selection of relevant biomarkers for statin clinical trials.
Keywords: lethal prostate cancer, statin, TMPRSS2:ERG fusion, PTEN-null, inflammation, immune activation
Introduction
Mounting epidemiologic evidence supports an inverse association between statins and risk of advanced prostate cancer, particularly with longer duration of use.1,2 Relatively consistent evidence from different populations, together with laboratory data identifying mechanisms linking statins with reduced prostate tumor growth, supports a potential causal association.2
Given prostate cancer heterogeneity, studies of tumor subtypes could improve understanding of prostate cancer risk factors. In particular, tumors containing the androgen-driven TMPRSS2:ERG gene fusion, the most common subtype of primary prostate cancer, may have distinct etiology.3,4 Androgen pathway signaling brings the TMPRSS2 and ERG genomic loci into proximity, thereby favoring their fusion.5 Given the role for cholesterol in intratumor androgen synthesis,6 we hypothesized that the cholesterol-lowering statins would be associated with lower risk of TMPRSS2:ERG fusion-positive prostate cancer via reduced prostate androgen signaling in statin users. Furthermore, cholesterol metabolism and phosphoinositide 3-kinase (PI3K) signaling are tightly linked. Activation of the PI3K pathway drives intracellular cholesterol accumulation7 and cholesterol, in turn, is a positive regulator of PI3K/Akt signaling, an effect which can be offset by statin treatment of cell lines.8 Moreover, the PTEN promoter has been shown to be regulated by statins, resulting in upregulation of PTEN at the transcriptional level.9 We therefore hypothesized that statin use would be associated with lower risk of PTEN-null prostate cancer via reduced cholesterol signaling, increased PTEN expression and lower PI3K pathway activation in statin users.
Using data from the Health Professionals Follow-up Study (HPFS), we updated our previous analysis of statins and prostate cancer risk10 with over a decade of additional follow-up. This substantial increase in follow-up produced sufficient numbers of cases for our a priori focus on the clinically-relevant outcome of lethal prostate cancer. Moreover, we incorporated immunohistochemistry data for ERG (to detect TMPRSS2:ERG gene fusion) and PTEN to identify molecular subgroups of prostate cancer most impacted by statin use. In exploratory analysis, we analyzed gene expression data from tumor and adjacent normal prostate tissue to identify statin-associated biological pathways.
Materials and Methods
Study population
HPFS is an ongoing cancer epidemiology cohort of 51,529 men who enrolled by responding to a baseline questionnaire in 1986 when aged 40–75. Demographic and lifestyle factors, in addition to medical history, is collected on self-reported biennial questionnaires, with semi-quantitative food-frequency questionnaires included every four years. The overall questionnaire response rate is >90%.
To encompass the statin era we began follow-up in 1990, excluding men who died before returning the 1990 questionnaire (N=1,145) or who did not complete that questionnaire (N=2,996). We excluded 3,216 men with a cancer diagnosis (excluding non-melanoma skin cancer) before 1990, and 46 men missing birth or cancer diagnosis dates, for a final sample size of 44,126.
Institutional review boards at Harvard T.H. Chan School of Public Health and Partners Health Care approved the study. The study was conducted in accordance with the US Common Rule, and all participants provided written informed consent.
Statin use assessment
Men reported cholesterol-lowering medications every two years, beginning in 1990.10 The 2000 questionnaire was the first to ask specifically about statins, when 91% of cholesterol-lowering drugs were statins.10 We treated cholesterol-lowering drugs as statins prior to 2000, as statins likely constituted the majority of cholesterol-lowering drugs given their US prevalence.11 Duration of use was calculated by summing use across the two-year periods encompassed by the biennial questionnaires, and categorized as <5 vs. ≥5 years.10 Dose was unavailable. HPFS started asking about type of statin use in 2004. At that time, the majority of statin use was either atorvastatin (48% of statin users) or simvastatin (32% of statin users), with lower frequencies of lovastatin (5%), pravastatin (10%) and rosuvastatin use (3%).
Prostate cancer ascertainment
From 1990–2014, 6,305 incident prostate cancers were diagnosed. Cancers were initially self-reported and subsequently confirmed via medical and pathology records. Deaths were reported by family, or identified through the National Death Index, with >98% sensitivity. We retrieved archival prostate tumor tissue from approximately half of HPFS participants diagnosed with prostate cancer who underwent radical prostatectomy (RP; 95%) or transurethral resection of the prostate (TURP; 5%). For these specimens, central histopathologic review by study pathologists provided standardized tumor grading. We classified tumors as localized (stage T1a-T2b and N0M0; n=4,783) or advanced (stage T3b-T4, N1, or M1; n=485) at diagnosis (with stage 3a tumors omitted to maximize differences between localized and advanced groups); as low grade (Gleason sum ≤3+4; n=3,918) or high grade (≥4+3; n=1,421); and lethal (n=801) if distant metastases were present at diagnosis or occurred during follow-up, or if prostate cancer was the cause of death.
ERG and PTEN immunohistochemistry
We leveraged tumor ERG and PTEN immunohistochemistry data (available for 888 and 715 cases, respectively) from tissue microarrays (TMAs), constructed using at least three 0.6-mm cores per case from the primary tumor nodule or nodule with highest Gleason grade.12 The presence of ERG staining was assessed within prostate epithelial cells and staining in the vasculature endothelium served as an internal positive control. Tumors were classified as ERG positive if any TMA cores for a given case had positive ERG staining within prostate cancer epithelial cells, as previously described.12 Images of ERG staining in HPFS are shown in Flavin et al.13 ERG immunohistochemistry status is strongly associated with fusion status assessed by fluorescence in situ hybridization.14 Tumors were classified as PTEN-null if PTEN immunohistochemistry expression was markedly decreased (1+ intensity) or entirely lost (0+ intensity) across >10% of tumor cells compared with surrounding benign glands or stroma, which serve as internal positive controls for tumor PTEN expression.15 Images of PTEN staining in HPFS are shown in Ahearn et al.15 PTEN immunohistochemistry status is strongly associated with PTEN homozygous genetic deletion.15,16
Gene expression profiling
Whole genome gene expression was measured in tumor and adjacent normal prostate using whole-transcriptome amplification (WT-Ovation FFPE system, v2, NuGEN) paired with microarray technologies (Affymetrix GeneChip HumanGene 1.0ST microarray).17 We processed the data, totaling 20,254 genes from tumor (n=229, of which n=30 were ever statin users) and normal adjacent (n=113, of which n=10 were ever statin users) tissues, as previously described.18 Data are available through the Gene Expression Omnibus (GSE79021).
Statistical analysis
We compared age-standardized demographic and lifestyle factors by statin use in 2002. We selected this year to be midway through follow-up, as prevalence of statin use increased markedly over time. We counted person-years of follow-up from the 1990 questionnaire return date until date of diagnosis, death, or end of follow-up, whichever came first. We used Cox proportional hazards regression to calculate age-adjusted and multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CIs) for associations between statin use and risk of prostate cancer overall, by clinical measures of tumor aggressiveness, and by molecular tumor characteristics. Statin use was treated as time-varying, as previously described,10 where the reference group was past or never use. Models were adjusted for covariates listed in Table footnotes.
For analyses of total prostate cancer risk by ERG and PTEN status, cases lacking biomarker status were censored at diagnosis. We tested for heterogeneity in associations between statins and prostate cancer risk by subtype using likelihood ratio tests. Prostate tissue was available for cases diagnosed through July 2009; therefore we ended follow-up at that time for subtype-stratified analyses.
In sensitivity analyses, we truncated follow-up for our main analyses in 2009 to enable direct comparison of results with those from biomarker analyses. We restricted our main analyses to cases with available biomarker data and found similar associations. We excluded men diagnosed with stage T1a disease (n=263), as they tend to be diagnosed incidentally and are susceptible to detection bias, but found similar results. To investigate potential confounding by PSA testing, we restricted to men reporting high-intensity PSA testing (testing in >50% of possible time periods, lagged by one period to avoid counting diagnostic PSA tests). We explored associations between type of statin use, categorized as lipophilic (lovastatin, simvastatin, atorvastatin) and hydrophilic (pravastatin, rosuvastatin), and risk of overall, high-grade and low-grade prostate cancer, but findings did not vary by statin type and are not presented. We lacked sufficient numbers of advanced, lethal and PTEN-defined prostate cancer cases to examine these outcomes in association with type of statin use.
Gene set enrichment analysis (GSEA) was conducted19 to test the association between statin use and expression of Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets from the Molecular Signature Database (version 6.1, Broad Institute) (http://software.broadinstitute.org/gsea/index.jsp). To remove age-related confounding in gene expression, we obtained gene expression residuals from linear regression on age at diagnosis. Given the relatively small numbers of statin users included in this analysis, in addition to the lack of knowledge regarding potential confounders of the association between statin use and altered gene expression pathways in the prostate, we did not adjust the GSEA for any other variables. Genes were ranked on a signal-to-noise metric comparing ever versus never statin users. An enrichment score (ES) was calculated for each gene set using a weighted Kolmogorov-Smirnoff statistic. Significance was estimated with 1,000 phenotype-based permutations. The normalized ES and false-discovery rate (FDR) identified the top statin-associated KEGG pathways.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.1.0. All statistical tests were two-sided, with p<0.05 considered statistically significant.
Results
Participant characteristics by statin use
Midway through follow-up in 2002, 24% of men reported current statin use (Table 1). Relative to non-users, current statin users were slightly older, with higher BMI, and less likely to be physically active. Men using statins were more likely to have a history of diabetes, hypertension and high cholesterol, and more likely to have undergone PSA testing than non-users. Current statin users reported higher rates of other medication use, including aspirin (Table 1).
Table 1:
Age-standardized characteristics of statin users and nonusers in the Health Professionals Follow-up Study, mid-way through follow-up in 2002
| Statin use | ||
|---|---|---|
| Never/past | Current | |
| N | 26,845 (76%) | 8,642 (24%) |
| Mean age a, years (SD) | 68.0 (9.2) | 68.9 (8.4) |
| White race, % | 95.9 | 96.0 |
| Mean height, inches (SD) | 70.3 (2.7) | 70.0 (2.6) |
| Mean BMI, kg/m2 (SD) | 26.1 (3.8) | 26.8 (3.7) |
| Mean BMI at age 21, kg/m2 (SD) | 23.0 (2.9) | 23.1 (2.8) |
| Family history of prostate cancer, % | 12.5 | 11.6 |
| Mean physical activity, MET hours/week (SD) | 27.8 (22.4) | 26.0 (19.4) |
| Smoking status, % | ||
| Never | 39.0 | 38.3 |
| Former, quit >10 years | 31.1 | 39.1 |
| Former, quit ≤10 years | 8.4 | 9.0 |
| Current | 5.1 | 3.3 |
| Missing | 16.4 | 10.3 |
| History of diabetes, % | 8.3 | 14.4 |
| History of hypertension, % | 42.4 | 58.3 |
| History of high cholesterol, % | 43.5 | 92.6 |
| Had PSA test b, % | ||
| 1994 | 35.8 | 47.6 |
| 2000 | 72.6 | 87.8 |
| 2004 | 67.4 | 86.3 |
| Mean tomato sauce intake, servings/week (SD) | 1.1 (0.9) | 1.1 (0.8) |
| Mean coffee intake, cups/day (SD) | 1.6 (1.4) | 1.7 (1.3) |
| Mean calorie intake, kcal/day (SD) | 2001 (538) | 1929 (505) |
| Statin use duration, years (SD) | 0.3 (1.3) | 5.6 (3.5) |
| Current medication use, % | ||
| Aspirin | 33.4 | 65.5 |
| Beta-blockers | 9.3 | 27.7 |
| Calcium channel blockers | 4.8 | 11.8 |
| Diuretics | 7.8 | 16.0 |
| Other anti-hypertensives | 8.6 | 19.3 |
Abbreviations: BMI, body mass index; MET, metabolic equivalent tasks, PSA, prostate-specific antigen; SD, standard deviation
Age comparisons are not age-standardized
Reported having a PSA test in the 2 years before the questionnaire date indicated
Statins and prostate cancer risk, by clinical and pathologic tumor characteristics
In keeping with our previous findings,10 statin use was unrelated to overall, localized, or low-grade prostate cancer risk (Table 2). While long-term statin use was suggestively inversely associated with risk of advanced prostate cancer, the association was not statistically significant. However, current statin use was associated with 24% reduced risk of lethal prostate cancer, relative to non-use (HR 0.76; 95% CI 0.60–0.96). The association between statin use and risk of lethal prostate cancer was similar, though slightly attenuated, when statins were categorized as ever vs. never use (HR 0.83; 95% CI 0.66–1.04). The association was stronger in long-term statin users where, relative to never use, ≥5 years of use was associated with substantially lower risk of lethal prostate cancer (HR 0.65; 95% CI 0.47–0.90).
Table 2:
Hazard ratios and 95% confidence intervals of the association between statin use and risk of prostate cancer subgroups defined by tumor characteristics in the Health Professionals Follow-up Study, 1990–2014
| Cases, n | HRa (95% CI) | HRb (95% CI) | |
|---|---|---|---|
| All cases | 6,305 | ||
| Current statin use | |||
| Never/past | 4,807 | 1.00 (ref) | 1.00 (ref) |
| Current | 1,498 | 1.10 (1.03–1.17) | 1.00 (0.93–1.08) |
| Ever statin use | |||
| Never | 4,515 | 1.00 (ref) | 1.00 (ref) |
| Ever | 1,790 | 1.08 (1.01–1.14) | 0.99 (0.92–1.06) |
| Duration of statin use | |||
| Never | 4,515 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 914 | 1.09 (1.01–1.17) | 1.02 (0.94–1.10) |
| ≥5 years | 876 | 1.06 (0.98–1.15) | 0.95 (0.87–1.04) |
| Per year of statin use | 6,305 | 1.01 (1.00–1.02) | 1.00 (0.99–1.01) |
| Localized casesc | 4,783 | ||
| Current statin use | |||
| Never/past | 3,572 | 1.00 (ref) | 1.00 (ref) |
| Current | 1,211 | 1.14 (1.07–1.23) | 1.01 (0.93–1.09) |
| Ever statin use | |||
| Never | 3,350 | 1.00 (ref) | 1.00 (ref) |
| Ever | 1,433 | 1.11 (1.03–1.18) | 0.99 (0.91–1.07) |
| Duration of statin use | |||
| Never | 3,350 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 725 | 1.12 (1.03–1.21) | 1.02 (0.93–1.12) |
| ≥5 years | 708 | 1.10 (1.00–1.20) | 0.94 (0.85–1.04) |
| Per year of statin use | 4,783 | 1.01 (1.00–1.02) | 1.00 (0.99–1.01) |
| Advanced casesd | 485 | ||
| Current statin use | |||
| Never/past | 409 | 1.00 (ref) | 1.00 (ref) |
| Current | 76 | 0.87 (0.67–1.13) | 0.98 (0.73–1.31) |
| Ever statin use | |||
| Never | 388 | 1.00 (ref) | 1.00 (ref) |
| Ever | 97 | 0.92 (0.72–1.17) | 1.06 (0.80–1.41) |
| Duration of statin use | |||
| Never | 388 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 62 | 1.08 (0.82–1.43) | 1.24 (0.91–1.68) |
| ≥5 years | 35 | 0.69 (0.47–1.00) | 0.80 (0.53–1.20) |
| Per year of statin use | 485 | 0.97 (0.93–1.00) | 0.98 (0.94–1.02) |
| Low grade casese | 3,918 | ||
| Current statin use | |||
| Never/past | 2,948 | 1.00 (ref) | 1.00 (ref) |
| Current | 970 | 1.14 (1.05–1.23) | 1.00 (0.91–1.09) |
| Ever statin use | |||
| Never | 2,775 | 1.00 (ref) | 1.00 (ref) |
| Ever | 1,143 | 1.10 (1.02–1.18) | 0.97 (0.89–1.05) |
| Duration of statin use | |||
| Never | 2,775 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 577 | 1.08 (0.99–1.19) | 0.99 (0.89–1.09) |
| ≥5 years | 566 | 1.11 (1.01–1.23) | 0.94 (0.84–1.05) |
| Per year of statin use | 3,918 | 1.01 (1.00–1.02) | 1.00 (0.99–1.01) |
| High grade casesf | 1,421 | ||
| Current statin use | |||
| Never/past | 1,064 | 1.00 (ref) | 1.00 (ref) |
| Current | 357 | 1.07 (0.94–1.22) | 1.01 (0.87–1.16) |
| Ever statin use | |||
| Never | 989 | 1.00 (ref) | 1.00 (ref) |
| Ever | 432 | 1.06 (0.99–1.02) | 1.02 (0.88–1.17) |
| Duration of statin use | |||
| Never | 989 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 221 | 1.13 (0.97–1.31) | 1.08 (0.92–1.28) |
| ≥5 years | 211 | 0.99 (0.84–1.17) | 0.94 (0.78–1.12) |
| Per year of statin use | 1,421 | 1.00 (0.99–1.02) | 1.00 (0.98–1.01) |
| Lethal casesg | 801 | ||
| Current statin use | |||
| Never/past | 695 | 1.00 (ref) | 1.00 (ref) |
| Current | 106 | 0.71 (0.57–0.88) | 0.76 (0.60–0.96) |
| Ever statin use | |||
| Never | 662 | 1.00 (ref) | 1.00 (ref) |
| Ever | 139 | 0.76 (0.63–0.93) | 0.83 (0.66–1.04) |
| Duration of statin use | |||
| Never | 662 | 1.00 (ref) | 1.00 (ref) |
| <5 years | 88 | 0.90 (0.71–1.13) | 0.96 (0.75–1.23) |
| ≥5 years | 51 | 0.59 (0.43–0.80) | 0.65 (0.47–0.90) |
| Per year of statin use | 801 | 0.95 (0.92–0.98) | 0.96 (0.93–0.99) |
adjusted for age and calendar time
adjusted for age, calendar time, race (white, African American, Asian American, other), family history of prostate cancer in father/brother (yes, no), height (≤68, >68–70, >70–72, >72 inches), body mass index (BMI) at age 21 (<20, 20–<22.5, 22.5–<25, ≥25 kg/m2), current BMI (<21, 21–<25, 25–<30, ≥30 kg/m2), smoking (never, former/quit >10 years, former/quit ≤10 years, current), history of high cholesterol (yes, no), history of hypertension (yes, no), history of diabetes (yes, no), PSA testing in the two years prior to the questionnaire date (yes, no, lagged by one period to avoid counting diagnostic PSA tests), PSA testing in >50% of possible time periods (yes, no, lagged by one period to avoid counting diagnostic PSA tests), aspirin use (yes, no), physical activity (quintiles of metabolic equivalent hours/week), and total calories (quintiles of kcal/day)
Localized cases defined as stage T1a – T2b and N0, M0 at diagnosis
Advanced cases defined as stage T3b – T4, N1, or M1 at diagnosis
Low grade cases defined as ≤Gleason 3+4 at diagnosis
High grade defined as ≥Gleason 4+3 at diagnosis
Lethal cases defined as M1 at diagnosis or distant metastasis / fatal during follow up
Associations between statins and prostate cancer risk were similar when we ended follow-up in 2009, to match available follow-up for biomarker analyses (Supplementary Table 1). We observed similar, albeit slightly attenuated, inverse associations between statins and risk of lethal disease when restricting to men reporting high intensity PSA screening (Supplementary Table 2). Omitting PSA screening history and intensity from multivariable models produced effect estimates which lay between those from age-adjusted and fully-adjusted models (data not shown).
Statins and prostate cancer risk, by molecular tumor characteristics
Neither current nor long-term statin use was associated with risk of ERG-defined prostate cancer (Figure 1). However, relative to non-use, current statin use was associated with significantly reduced risk of PTEN-null cancer (HR 0.40; 95% CI 0.19–0.87). In contrast, current use was not significantly associated with risk of PTEN-intact disease (HR 1.18; 95% CI 0.95–1.48; p-heterogeneity=0.01). Longer duration of statin use was suggestively inversely associated with risk of PTEN-null prostate cancer (HRper year 0.88; 95% CI 0.76–1.03), but not PTEN-intact disease (HRper year 1.00; 95% CI 0.96–1.03; p-heterogeneity=0.06; Supplementary Table 3).
Figure 1:
Forest plot showing associations between current statin use and risk of prostate cancer, overall and by ERG and PTEN status. Hazard ratios are represented by the square and 95% confidence intervals by the line.
Gene expression pathways associated with statin use
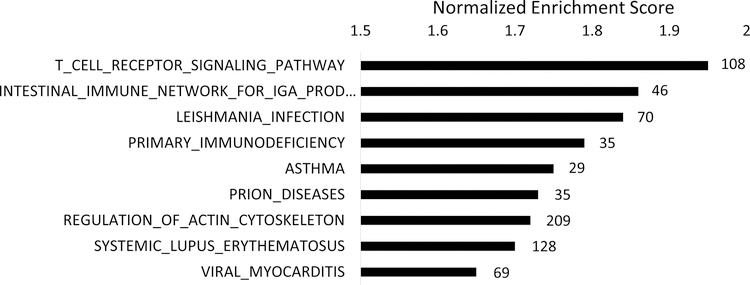
Nine gene sets were enriched in tumor-adjacent normal prostate tissue of ever vs. never statin users at FDR <0.25 (Figure 2; Supplementary Table 4). Among these top-ranking pathways, we observed enrichment for immune activation and inflammatory signaling. Four of the top-ranking gene sets (Leismania infection, asthma, systemic lupus erythematosus and immune network for IgA production) showed significant overlap of genes, further supporting inflammation and immune activation as the common underlying biologic pathways. T-cell receptor signaling was the top-ranking pathway whether statin use was categorized either as ever (n=10) vs. never (n=103), or as current (n=6) vs. never/past (n=107; Supplementary Table 5). In addition, we explored duration of statin use, categorizing length of statin use as 2 years (n=6), 4 years (n=2), 6 years (n=1) and 8 years (n=1), versus never use (n=103). T-cell receptor signaling remained the top-ranking pathway in this continuous analysis, and 6 out of 9 top-ranking pathways were identical to the results from our categorical analysis of statin ever vs. never use. Interestingly, no pathways were altered in prostate tumor tissue (all FDR >0.7; Supplementary Table 6).
Figure 2:
Age-adjusted gene set enrichment analysis showing gene sets enriched in tumor-adjacent normal prostate tissue of ever statin users (n=10) versus never users (n=103), with a false-discovery rate < 0.25. KEGG terms are ordered by the normalized enrichment score, with the number of genes enriched in each biological process indicated next to each bar.
Discussion
A little over a decade ago, HPFS findings were the first prospective data to demonstrate an association between statins and reduced risk of advanced, but not overall prostate cancer.10 This finding has now been replicated in >20 observational studies across different populations.2 Together with mechanistic data supporting anti-tumor effects of statins, this growing body of evidence has led to interest in statin trials for prostate cancer.2,20 With over 10 years additional follow-up now available in the HPFS, the number of lethal prostate cancer cases has more than tripled since our original analysis, enabling our current a priori focus on this clinically-relevant subgroup. While several previous studies among men diagnosed with prostate cancer reported lower prostate cancer-specific mortality in statin users,21–23 prospective examination of lethal prostate cancer risk in cancer-free men has been highly challenging due to the large size and long duration of study needed to ascertain sufficient numbers of cases for a well-powered analysis.10,24 Using data from a large prospective cohort of initially cancer-free men followed for 24 years, the present analysis reports a significant and substantial reduction in the risk of developing lethal prostate cancer among statin users, particularly longer duration users. Moreover, we integrate rich tissue biomarker data to elucidate potential mechanisms underlying the association, with a view to identifying potential biomarkers for use in statin clinical trials.
Prostate cancer is heterogeneous, and recent efforts have identified molecular and genetically-distinct subgroups. Common genetic aberrations include loss or inactivation of the tumor suppressor, PTEN, and presence of the TMPRSS2:ERG gene fusion.25 Several risk factors have been more strongly associated with ERG-positive disease, including height and obesity26 and lycopene.3 Formation of the TMPRSS2:ERG gene fusion can be driven by androgen signaling,5 reduced in obesity, and by free radical-induced DNA double-strand breaks,27 offset by anti-oxidant properties of lycopene, lending biologic plausibility to these associations. While tumor ERG status was previously found to modify the association between statins and prostate cancer recurrence,28 no epidemiologic studies have investigated the association between statins and risk of molecular prostate cancer subtypes. We hypothesized that lowering cholesterol, the precursor for androgen synthesis, would reduce formation of the androgen-mediated TMPRSS2:ERG gene fusion. In contrast to our hypothesis, statins were not associated with ERG status in the present analysis.
This is the first study to our knowledge to report that the risk reduction associated with statin use was specific to PTEN-null prostate cancer. If confirmed, our findings suggest that lower incidence of this poor-prognosis subgroup15 in statin users may contribute to the inverse association between statins and lethal disease. In HPFS and other cohorts, patients whose prostate tumors had higher cholesterol synthesis were more likely to develop lethal disease.29 Results from prostate cancer mouse models support a role for PI3K signaling, a key oncogenic pathway negatively regulated by PTEN, in cholesterol-driven tumor growth.8,30 In a transgenic mouse model of PTEN-null prostate cancer, serum cholesterol reduction lowered tumor androgens and slowed tumor proliferation.31 In breast cancer, PI3K signaling inhibition blocked intratumor cholesterol synthesis,32 while prostate tumor cells with PTEN loss and subsequent PI3K signaling upregulation showed enhanced cholesterol uptake and tumor growth.7 Moreover, statins upregulated PTEN transcription giving rise to higher PTEN protein levels in a dose-dependent manner in breast cancer cell lines,9,33 an effect also observed in PTEN haploinsufficient lipoma cells.34 We observed a non-significant suggestion of increased risk of PTEN-intact prostate cancer in association with statin use which should be tested in other studies. In sum, there is extensive experimental data in multiple tumor types indicating a tight interconnection between cholesterol synthesis and PTEN/PI3K/Akt signaling,32,35–37 suggesting that inhibition of cholesterol synthesis (for example with statins) may abrogate a tumor’s selective advantage from such signaling. Future studies should determine whether patients with PTEN-null tumors, potentially uniquely susceptible to cholesterol and/or PI3K pathway targeting, might benefit from post-diagnosis statin use.
In addition to cholesterol-dependent effects of statins described above, there is also evidence for cholesterol-independent statin effects. Our exploratory analyses identified inflammation/immune pathway enrichment in tumor-adjacent normal prostate tissue of statin users. Cardiovascular disease trials have demonstrated anti-inflammatory properties of statins, independent of cholesterol-lowering.38,39 A short-term randomized trial in prostate cancer reported no effect of atorvastatin on intraprostatic inflammation after approximately 1 month of treatment.40 However, observational studies have suggested that statins may influence histologic inflammation within the prostate.41,42 Immunomodulatory properties of statins have also been reported, particularly in increasing circulating levels and enhancing function of regulatory T cells.43 Of note, we observed enrichment for inflammation and immune signaling in normal, but not tumor, prostate tissue. Recent data have highlighted an important role for the prostate microenvironment in driving prostate cancer outcomes.44 As such, emerging evidence that statins could impact normal prostate biology may indeed be relevant to lethal prostate cancer prevention efforts. In support of our observation of altered immune/inflammation in the tumor-adjacent normal prostate tissue of statin users, we previously reported lower levels of histologic inflammation in benign prostate tissue from negative prostate biopsies of statin users.42 Indeed, lower prostate inflammation in statin users is thought to contribute to the observation that statin users have lower PSA levels, a finding shown among cancer-free men.45,46 Our data, in addition to those of others, suggest that statins may directly impact prostate biology. Collectively, our findings, together with these results from previous studies, point to a potential role for the tumor microenvironment in mediating biological effects of statins. Given the exploratory nature of this analysis, future studies will be needed to validate our findings.
Strengths of our study include its prospective design and large size. With 24 years follow-up for cancer incidence and mortality, we were able to evaluate lethal prostate cancer risk with considerable statistical power. We ended follow-up at prostate cancer diagnosis, and therefore did not consider post-diagnosis statin use. Therefore, this study tests the potential for statins in the prevention of lethal prostate cancer rather than in the treatment of initially localized disease. Repeated assessment of statin use as well as demographic and lifestyle characteristics throughout follow-up enabled us to consider the time-varying nature of these factors. Detailed PSA testing history allowed us to consider the potential influence of PSA screening. Another unique strength is the linkage of this cohort with a tissue biorepository, as the study of molecular tumor subtypes associated with statins may be less susceptible to screening and detection biases than studies of overall or high-grade prostate cancer risk.
The HPFS is comprised primarily of white men, potentially limiting generalizability to other racial groups. Although studies in racially-diverse cohorts are few, several have reported similar magnitudes of inverse association between statin use and risk of aggressive47,48 and fatal24 prostate cancer in white and African American men. While these data may support the generalizability of our findings, future studies in racially-diverse cohorts are needed. ERG and PTEN-stratified analyses were based on cases that underwent RP or TURP with sufficient tissue for TMA construction. However, clinical and demographic differences between cases with and without biomarker data were minimal, and therefore molecular features of tumors would also be expected to be non-differentially distributed between these groups. Moreover, previous HPFS analyses reported similar findings when differences were balanced using inverse probability weighting, indicating that the subcohort with tissue biomarker data is representative of the whole.3,26 Despite the large size of the cohort, limited numbers of cases with available data for PTEN and ERG status prevented further stratification of our analyses by tumor grade/lethality. As such, we could not formally test what proportion of the inverse association between statins and lethal prostate cancer risk may be through lower risk of poor prognosis PTEN-null disease. We hope that future cohort studies with access to tissue biomarkers will pool the results in order to investigate this hypothesis. However, our findings are the first to show a potential role for statins in preventing PTEN-null prostate cancer. Our epidemiological findings are in keeping with experimental observations that PI3K pathway activation leads to enhanced uptake and accumulation of intracellular cholesterol,7 while statin use and cholesterol reduction upregulate PTEN expression and reduce PI3K activation, respectively.8,33 Finally, analysis of statin-associated gene expression was based on a relatively small sample size and should be considered exploratory. However, the HPFS is unique in integrating detailed exposure data with gene expression on tumor and normal tissue and, to our knowledge, there are currently no appropriate validation cohorts. Combining current (n=6) with former (n=4) users may have attenuated our findings, as it is unknown whether statins could have any lasting effect on prostate biology after discontinuation. However, substantial overlap between pathways with inflammatory/immune components in the present analysis, together with published observations that statin users have lower levels of histologic prostate inflammation and evidence from the cardiovascular disease literature demonstrating a role for statins in inflammation and immune modulation, supports the biologic plausibility of our findings.
To conclude, our results from the largest prospective analysis of statins and lethal prostate cancer risk to date support a role for statins in lethal prostate cancer prevention. Of note, rather than advocating that all men start statin therapy for lethal prostate cancer prevention, our study highlights potential additional benefits of statins for existing users. The inverse association between statin use and prostate cancer risk was strongest for PTEN-null disease, a subtype with poor clinical outcomes.15 Future studies should examine the potential effect of post diagnosis statin use on prostate cancer outcomes in PTEN-null patients, potentially uniquely susceptible to cholesterol-lowering. Exploratory gene expression analysis identified inflammation and immune modulation as additional potential biologic mechanisms linking statins with lower risk of lethal disease, and future studies should attempt to further disentangle drug-specific effects of statins from their cholesterol-lowering effects. If confirmed, our findings provide support for a potential causal effect of statins on lethal prostate cancer risk and could help to inform the selection of appropriate biomarkers for use in statin clinical trials.
Supplementary Material
Statement of translational relevance:
Mounting evidence supports a role for the cholesterol-lowering statins in reducing the risk of advanced and aggressive prostate cancer. Lethal prostate cancer, arguably the most clinically-relevant endpoint, is understudied given the need for large prospective studies with long follow-up to ascertain sufficient numbers of lethal cases. Using data from the Health Professionals Follow-up Study, a large prospective cohort with 24 years follow-up, we report that statin users had a 24% reduced risk of being diagnosed with a lethal prostate cancer, with an even stronger association among longer-term statin users. Substantially lower risk of poor-prognosis PTEN-null tumors in statin users, and altered immune/inflammatory gene expression in the tumor-adjacent normal prostate tissue of statin users, provide potential mechanistic explanations for this finding. Our findings could guide the selection of relevant biomarkers to serve as endpoints for future observational studies and clinical trials testing a role for statins in lethal prostate cancer prevention.
Acknowledgements:
This work was supported by the Dana-Farber/Harvard Cancer Center Specialized Programs of Research Excellence program in prostate cancer (P50 CA090381), the National Cancer Institute (R01CA136578, U01 CA167552, to L.A. Mucci; T32 CA09001, to E.M. Ebot, T.U. Ahearn, and S.C. Markt); the NIH/NCI Cancer Center Support Grants P30 CA008748, P30 CA006516, and P30 CA006973; the Irish Cancer Society John Fitzpatrick Fellowship (to E.H. Allott); Prostate Cancer Foundation Young Investigator Awards (to K.H. Stopsack, K.M. Wilson, S.P. Finn and L.A. Mucci); the American Cancer Society – Ellison Foundation Postdoctoral Fellowship (PF-14-140-01-CCE, to T.U. Ahearn); the Office of the Assistant Secretary of Defense for Health Affairs (W81XWH-14-1-0250, to E.M. Ebot; W81XWH-18-1-0330, to K.H. Stopsack).
Footnotes
Previous presentation: This study was previously presented in part as a poster at the American Association for Cancer Research Prostate Cancer: Advances in Basic, Translational, and Clinical Research Meeting, Orlando FL (2017), at the American Urological Association Annual Meeting, San Francisco, CA (2018), and at the 4th International Molecular Pathological Epidemiology meeting, Boston MA (2018)
Disclaimers: None of the authors have any conflicts of interest
References
- 1.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PloS one. 2012;7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nature reviews Urology. 2017;14:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, et al. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. Journal of the National Cancer Institute. 2013;105:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani RS, Tomlins SA, Callahan K, Ghosh A, Nyati MK, Varambally S, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Current opinion in pharmacology. 2012;12:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell metabolism. 2014;19:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teresi RE, Planchon SM, Waite KA, Eng C. Regulation of the PTEN promoter by statins and SREBP. Human molecular genetics. 2008;17:919–928. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. Journal of the National Cancer Institute. 2006;98:1819–1825. [DOI] [PubMed] [Google Scholar]
- 11.Siegel D, Lopez J, Meier J. Use of cholesterol-lowering medications in the United States from 1991 to 1997. Am J Med. 2000;108:496–499. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavin R, Pettersson A, Hendrickson WK, Fiorentino M, Finn S, Kunz L, et al. SPINK1 protein expression and prostate cancer progression. Clin Cancer Res. 2014;20:4904–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. Journal of the National Cancer Institute. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penney KL, Sinnott JA, Tyekucheva S, Gerke T, Shui IM, Kraft P, et al. Association of prostate cancer risk variants with gene expression in normal and tumor tissue. Cancer Epidemiol Biomarkers Prev. 2015;24:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebot EM, Gerke T, Labbe DP, Sinnott JA, Zadra G, Rider JR, et al. Gene expression profiling of prostate tissue identifies chromatin regulation as a potential link between obesity and lethal prostate cancer. Cancer. 2017;123:4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton RJ. Making Sense of the Statin-Prostate Cancer Relationship: Is It Time for a Randomized Controlled Trial? Eur Urol Focus. 2017;3:221–222. [DOI] [PubMed] [Google Scholar]
- 21.Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. 2014;32:5–11. [DOI] [PubMed] [Google Scholar]
- 22.Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. The Prostate. 2013;73:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. [DOI] [PubMed] [Google Scholar]
- 24.Mondul AM, Joshu CE, Barber JR, Prizment AE, Bhavsar NA, Selvin E, et al. Longer-term Lipid-lowering Drug Use and Risk of Incident and Fatal Prostate Cancer in Black and White Men in the ARIC Study. Cancer Prev Res (Phila). 2018;11:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graff RE, Ahearn TU, Pettersson A, Ebot EM, Gerke T, Penney KL, et al. Height, Obesity, and the Risk of TMPRSS2:ERG-Defined Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani RS, Amin MA, Li X, Kalyana-Sundaram S, Veeneman BA, Wang L, et al. Inflammation-Induced Oxidative Stress Mediates Gene Fusion Formation in Prostate Cancer. Cell reports. 2016;17:2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keskivali T, Kujala P, Visakorpi T, Tammela TL, Murtola TJ. Statin use and risk of disease recurrence and death after radical prostatectomy. The Prostate. 2016;76:469–478. [DOI] [PubMed] [Google Scholar]
- 29.Stopsack KH, Gerke TA, Sinnott JA, Penney KL, Tyekucheva S, Sesso HD, et al. Cholesterol Metabolism and Prostate Cancer Lethality. Cancer Res. 2016;76:4785–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 31.Allott EH, Masko EM, Freedland AR, Macias E, Pelton K, Solomon KR, et al. Serum cholesterol levels and tumor growth in a PTEN-null transgenic mouse model of prostate cancer. Prostate cancer and prostatic diseases. 2018;21:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch JT, Polanska UM, Delpuech O, Hancox U, Trinidad AG, Michopoulos F, et al. Inhibiting PI3Kbeta with AZD8186 Regulates Key Metabolic Pathways in PTEN-Null Tumors. Clin Cancer Res. 2017;23:7584–7595. [DOI] [PubMed] [Google Scholar]
- 33.Teresi RE, Shaiu CW, Chen CS, Chatterjee VK, Waite KA, Eng C. Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer. 2006;118:2390–2398. [DOI] [PubMed] [Google Scholar]
- 34.Kassner F, Sauer T, Penke M, Richter S, Landgraf K, Korner A, et al. Simvastatin induces apoptosis in PTENhaploinsufficient lipoma cells. Int J Mol Med. 2018;41:3691–3698. [DOI] [PubMed] [Google Scholar]
- 35.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, et al. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67:6238–6246. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Wong CC, Fu L, Chen H, Zhao L, Li C, et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Science translational medicine. 2018;10. [DOI] [PubMed] [Google Scholar]
- 37.Seshacharyulu P, Rachagani S, Muniyan S, Siddiqui JA, Cruz E, Sharma S, et al. FDPS cooperates with PTEN loss to promote prostate cancer progression through modulation of small GTPases/AKT axis. Oncogene. 2019;38:5265–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. [DOI] [PubMed] [Google Scholar]
- 40.Murtola TJ, Syvala H, Tolonen T, Helminen M, Riikonen J, Koskimaki J, et al. Atorvastatin Versus Placebo for Prostate Cancer Before Radical Prostatectomy-A Randomized, Double-blind, Placebo-controlled Clinical Trial. European urology. 2018;74:697–701. [DOI] [PubMed] [Google Scholar]
- 41.Banez LL, Klink JC, Jayachandran J, Lark AL, Gerber L, Hamilton RJ, et al. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19:722–728. [DOI] [PubMed] [Google Scholar]
- 42.Allott EH, Howard LE, Vidal AC, Moreira DM, Castro-Santamaria R, Andriole GL, et al. Statin Use, Serum Lipids, and Prostate Inflammation in Men with a Negative Prostate Biopsy: Results from the REDUCE Trial. Cancer Prev Res (Phila). 2017;10:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mausner-Fainberg K, Luboshits G, Mor A, Maysel-Auslender S, Rubinstein A, Keren G, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197:829–839. [DOI] [PubMed] [Google Scholar]
- 44.Tyekucheva S, Bowden M, Bango C, Giunchi F, Huang Y, Zhou C, et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nature communications. 2017;8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. Journal of the National Cancer Institute. 2008;100:1511–1518. [DOI] [PubMed] [Google Scholar]
- 46.Chang SL, Harshman LC, Presti JC Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28:3951–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kantor ED, Lipworth L, Fowke JH, Giovannucci EL, Mucci LA, Signorello LB. Statin use and risk of prostate cancer: Results from the Southern Community Cohort Study. The Prostate. 2015;75:1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allott EH, Farnan L, Steck SE, Arab L, Su JL, Mishel M, et al. Statin use and prostate cancer aggressiveness: results from the population-based North Carolina-Louisiana Prostate Cancer Project. Cancer Epidemiol Biomarkers Prev. 2016;25:670–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.