Abstract
Epidemiologic evidence indicates that regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) provides a protective effect against the development of colorectal, breast, and head and neck cancers. Genomic characterization of these cancers has lent considerable insight into the subpopulations of cancer patients who are most likely to benefit from NSAID therapy. The PIK3CA gene encodes the catalytic subunit of phosphatidylinositol 3-kinase (PI3K) and is among the most frequently mutated genes in solid tumor malignancies. Cancer-associated mutations in PIK3CA promote signaling via the PI3K pathway and stimulate tumor cell growth. In addition, activation of the PI3K pathway leads to induction of cyclooxygenase-2 (COX-2) enzyme and production of immunosuppressive prostaglandin E2 (PGE2). Notably, in both colorectal cancer and head and neck cancer the subpopulation of patients that benefit from NSAID use is restricted to those whose tumors exhibit PIK3CA genomic alterations. Preclinical studies, particularly in models of head and neck cancer, support the hypothesis that the chemopreventive impact of NSAIDs may be due, in part, to inhibition of COX-2 and reduction of PGE2 levels in the tumor microenvironment.
Keywords: Colorectal cancer, breast cancer, head and neck squamous cell carcinoma, HNSCC, PIK3CA, NSAIDs
Introduction
Members of the phosphoinositide 3-kinase (PI3K) family of intracellular kinases are activated by external stimuli and play key roles in promoting cell survival, proliferation, motility, and differentiation. PI3Ks exert their effects by phosphorylating the inositol ring structure of inositol phospholipids.
PI3K holoenzymes are comprised of two subunits, a catalytic subunit and a regulatory subunit, with the regulatory subunit acting to inhibit the kinase activity of the catalytic subunit. In humans, there are eight catalytic subunit isoforms and they are classified into 3 classes based on their specificity for different substrates (Bilanges et al., 2019; Hawkins and Stephens, 2015; Jean and Kiger, 2014; Vadas et al., 2011). Among them, Class I enzymes are involved in phosphorylation of phosphatidylinositol (4,5)-bisphosphate to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which is an important secondary messenger for activating downstream signaling components such as AKT. There are four distinct isoforms of Class I catalytic subunits (p110α, β, δ, or γ) and at least 4 distinct Class I regulatory subunits (p85α, p85β, p55γ, p101). As depicted in Figure 1, stimulation of cells with growth factors or cytokines results in the activation of membrane-spanning growth factor receptors with associated tyrosine kinase activities. This leads to recruitment of Class I PI3Ks to the receptor cytoplasmic domains and relief of the inhibition imposed by the PI3K regulatory subunit, resulting in activation of the PI3K kinase activity and generation of PIP3. The PIP3 moiety serves as a recruitment locus for proteins containing pleckstrin homology domains, including PDK1 and the serine/threonine kinase AKT. PDK1-mediated phosphorylation of AKT leads to AKT activation and subsequent activation of mTOR signaling (Dibble and Cantley, 2015). Negative regulation of PI3K/AKT signaling is mediated, in large part, by phosphatase and tensin homolog deleted on chromosome ten (PTEN), which dephosphorylates PIP3 to PIP2, shutting down AKT activation.
Figure 1. Signaling via the PI3K pathway and inhibition of PGE2 production by NSAIDs.

Activation of the PI3K/AKT pathway leads to stimulation mTORC1, as well as induction of COX enzymes. The induction of COX enzymes leads to production of immunosuppressive PGE2. NSAIDs, including aspirin, can inhibit COX-2 enzyme and reduce production of PGE2. PDK1, phosphoinositide-dependent kinase 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate.
While signaling via the PI3K/AKT/mTOR pathway is crucial in multiple cellular functions that impact growth and motility, dysregulation of this pathway also plays an important role in carcinogenesis (Fruman et al., 2017; Saxton and Sabatini, 2017). The PI3K/AKT/mTOR signaling pathway is one of the most commonly mutated pathways in many types of cancers, including colorectal cancer (CRC), breast cancer, and head and neck squamous cell carcinoma (HNSCC) (Danielsen et al., 2015; Lui et al., 2013; Noorolyai et al., 2019; Yuan and Cantley, 2008). Thus, components of this pathway have emerged as attractive targets for cancer treatment. A broad spectrum of inhibitors targeting this pathway have been developed and are being evaluated in clinical trials (Bauer et al., 2015; Cai et al., 2017; Janku, 2017; Janku et al., 2018).
Preclinical and clinical studies have shown that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) has a role in prevention and treatment of cancers such as breast cancer and CRC (Hua et al., 2019; Patrignani and Patrono, 2016; Zhang et al., 2018). Furthermore, preclinical and clinical studies in CRC have shown that the mutational status of PIK3CA (which encodes p110α) can affect tumor response to NSAIDs (Liao et al., 2012a). Recent evidence has also elucidated an important role for NSAIDs in the treatment breast cancer and HNSCC. In this review, we describe the current evidence supporting NSAID use in CRC, breast cancer, and HNSCC, with a focus on the mechanisms underlying the benefit of NSAIDs in PIK3CA-mutated cancers.
PIK3CA mutations and NSAID use in CRC and breast cancer
The PIK3CA gene is among the most commonly mutated genes in CRC and breast cancer (Cai et al., 2017). Mutations in PIK3CA have been reported in 15-32% of CRCs and 32-38% of breast cancers (Barault et al., 2008; Cancer Genome Atlas, 2012b; Ciriello et al., 2015; De Roock et al., 2010; Hamada et al., 2017; Liao et al., 2012b; Lievre et al., 2010; Mao et al., 2012). Several studies have also reported similar frequencies of PIK3CA mutations between sporadic and inherited CRC, an increased prevalence of PIK3CA mutations in women with CRC, and an increased risk of recurrence and poorer prognosis associated with PIK3CA mutations (Benvenuti et al., 2008; Kato et al., 2007; Miyaki et al., 2007). Data from The Cancer Genome Atlas (TCGA) has revealed PIK3CA mutations in 20% of sequenced CRC tumor samples, making it the fifth most common mutation found in CRC (Cancer Genome Atlas, 2012a). Over 80% of these PIK3CA mutations were “hotspot” missense mutations located in exons 9 and 20, resulting predominantly in the p110α canonical mutations E542K (5%), E545K (20%), and H1047R (12.5%). Functional studies have shown that these canonical mutations activate the kinase activity of the p110α enzyme (Kang et al., 2005; Zhao et al., 2005; Zhao and Vogt, 2010). The presence of hotspot mutations in PIK3CA is similar to what has been observed for other commonly mutated oncogenes in CRC, such as KRAS and BRAF (Cancer Genome Atlas, 2012a).
Similar findings related to PIK3CA mutation have been reported for breast cancer. Studies from the TCGA have shown that PIK3CA mutations occur in 28-47% of estrogen receptor (ER)/progesterone receptor (PR)-positive breast cancers, 25% of HER2-positive breast cancers, and 8% of triple-negative breast cancers. (Lee et al., 2015). Again, the majority of PIK3CA mutations occur in exons 9 and 20. (Lee et al., 2015) In the largest TCGA breast cancer study (977 patients), E542K, E545K, and H1047R mutations accounted for 11%, 18%, and 35%, respectively, of p110α mutations. Further studies have demonstrated an association between PIK3CA mutations and nodal metastasis, poorer survival, and increased resistance to trastuzumab (a monoclonal antibody against HER2 receptors) (Berns et al., 2007; Li et al., 2006; Saal et al., 2005).
Given the prevalence of PIK3CA activating mutations in CRC and breast cancer, numerous studies have evaluated the impact of drugs that target the PI3K/AKT/mTOR pathway, with several showing therapeutic benefit. Notably, PIK3CA activating mutations have been shown to induce the expression and cellular activity of COX-2 enzyme, leading to increased production of prostaglandin E2 (PGE2) (Di Popolo et al., 2000). Thus, COX-2 inhibitors such as NSAIDs have been used to inhibit this arm of the PI3K pathway. Approximately 85% of CRC and 72% of breast cancers have also been shown to overexpress COX-2, with overexpression being associated with larger tumor size, disease progression, and worsened survival (Eberhart et al., 1994; Gupta and Dubois, 2001; Kochel et al., 2016; Nie and Honn, 2002; Ogino et al., 2008; Shim et al., 2003; Tsujii and DuBois, 1995).
Aspirin use was first shown to be associated with a decreased incidence of CRC in 1988 (Kune et al., 1988). Since then, numerous studies have reported that regular use of aspirin has a protective effect on the development of CRC, including several observational case-control studies and 4 randomized controlled trials (RCTs), all of which supported this effect (Algra and Rothwell, 2012; Andreotti et al., 2017; Cole et al., 2009; Kune et al., 1988; Liao et al., 2012a; Patrignani and Patrono, 2016). A meta analysis of 17 case-control studies showed that regular use of aspirin was associated with a reduced risk of CRC (95% confidence interval: 0.58 – 0.67; p<0.0001) (Algra and Rothwell, 2012). Each RCT showed consistent results, with a 17-40% relative risk reduction in any adenoma recurrence and a 28% relative risk reduction in the recurrence of advanced lesions associated with 3-year treatment with an NSAID (Cole et al., 2009; Patrono and Rocca, 2008). Further post hoc analysis of these RCTs showed that daily aspirin use for 5 years reduced mortality due to CRC by 30-40% after 20 years of follow-up (Rothwell et al., 2011). This benefit was again shown in 6 primary prevention trials demonstrating that daily aspirin use led to a decrease in overall cancer incidence starting at 3 years (CI: 0.66 – 0.99; p=0.008) (Rothwell et al., 2012). Multiple large epidemiological studies have also shown a long-term decrease in CRC incidence with aspirin use (Giovannucci et al., 1995; Soriano et al., 2018). Recently the United States Preventive Services Task Force (USPSTF) issued a class B recommendation of daily aspirin use for the prevention of CRC in adults aged 50-59 years old who have a predicted life expectancy of 10 or more years.
Further analysis in CRC patients showed that the effect of aspirin on CRC patient outcomes may depend on PIK3CA mutational status. In a study of 964 CRC patients by Liao et al., aspirin use after diagnosis in PIK3CA-mutated CRC patients was associated with superior cause-specific survival (CSS) (disease stage-adjusted HR 0.18, 95% CI 0.06-0.61, p<0.001) and overall survival (OS) (disease stage-adjusted hazard ratio (HR) 0.54, 95% Confidence Interval (CI) 0.31-0.94, p=0.01), while there was no association in CRC patients with wild-type PIK3CA (Liao et al., 2012a). These results suggested that PIK3CA mutation in CRC may be predictive of response to adjuvant aspirin therapy. In addition, two recent meta-analyses also indicate a benefit to aspirin use specific to PIK3CA-mutated CRC. In one study, post-diagnosis aspirin use was associated with reduced total mortality (HR 0.71, 95% CI 0.51-0.99) and a beneficial trend in CSS (HR 0.37, 95% CI 0.11-1.32) (Paleari et al., 2016). In the other study, post-diagnosis aspirin use was associated with both a marked reduction in CSS (HR 0.45, 95% CI 0.28-0.71) and a significant reduction in total-cause mortality (HR 0.69, 95% CI 0.52-0.93) in PIK3CA-mutated CRC (Elwood et al., 2016).
Similar results have been shown with aspirin use and breast cancer. Epidemiological studies have shown that aspirin use is associated with a reduced risk of breast cancer progression (Fraser et al., 2014; Harris et al., 2003; Holmes et al., 2010; Holmes et al., 2014). To date, 3 meta-analyses have examined prospective observational studies on aspirin use and breast cancer. Each of these studies showed similar results with an approximately 30% reduced risk of death due to breast cancer associated with aspirin use (Elwood et al., 2016; Huang et al., 2015; Zhong et al., 2015). On the other hand, there is limited RCT data on NSAID use and breast cancer recurrence. Many of the RCTs of NSAID use and breast cancer have employed celecoxib and were either small RCTs or were terminated early due to cardiovascular risks associated with celecoxib (Chow et al., 2008; Falandry et al., 2009; Goss et al., 2013; Martin et al., 2010). Despite these limitations, a few of these studies have shown an increase in progression-free survival in patients taking celocoxib (Falandry et al., 2009; Martin et al., 2010).
PIK3CA mutations in HNSCC
Head and neck squamous cell carcinoma (HNSCC) is a common cancer with high morbidity and mortality. HNSCC patients who receive curative-intent therapy for a primary tumor are at high risk for the development of second primary malignancies, a leading cause of mortality (Khuri et al., 2001). As in CRC and breast cancers, mutations in the PI3K pathway are frequently observed in HNSCC tumors. Alterations in genes encoding major components of the PI3K pathway were found in 183 out of 279 HNSCC tumors from data deposited to the Cancer Genome Atlas (TCGA) (Vander Broek et al., 2015). Among them, the PIK3CA gene was the most frequently altered (altered in 34% and 56% of HPV negative and positive tumors, respectively). Genomic alterations of PIK3CA found in HNSCC include both amplification and mutation. With respect to mutation, PIK3CA was reported to be mutated in 8% of HPV-negative HNSCC tumors and 21% of HPV-positive tumors (Cancer Genome Atlas, 2015). Subsequent analysis of 504 HNSCC tumors by the TCGA indicates an overall PIK3CA mutation rate of 13.7% and an amplification rate of 16.5%, with 4.4% of tumors exhibiting both mutation and amplification. Mutation at canonical sites (E542, E545, and H1047) account for roughly 65% of PIK3CA mutations in HNSCC tumors.
Clinically, alteration of PI3K pathway has been associated with poor outcome and high risk of recurrence. PIK3CA amplification was significantly associated with shorter medium disease-specific survival time (16 months versus 23 months for wild-type patients, p = 0.037) (Brauswetter et al., 2016) and earlier recurrence in HNSCC patients without lymph node metastasis (31% with 2-year disease free status versus 90% for patients without amplification) (Suda et al., 2012). Activation of the PI3K pathway has also been shown to contribute to treatment resistance (Burris, 2013). Yes-associated protein (YAP) is a downstream effector of the Hippo pathway that controls organ size by regulating cell survival, proliferation and apoptosis (He et al., 2018). It has been proposed as a marker of poor prognosis and resistance to therapy (Segrelles et al., 2018). In a recent analysis of 243 primary HNSCC tumors from the TCGA cohort, overexpression of PIK3CA was associated with decreased expression of phosphorylated YAP (inactive) and induction of YAP-mediated gene expression (Garcia-Escudero et al., 2018). These results were confirmed with an independent cohort from the University Hospital of A Coruña (n=62). Not surprisingly, molecular targeting of the PI3K pathway has been a priority in solid tumor malignancies, including HNSCC. Multiple inhibitors of the PI3K inhibitors have been developed and are currently being evaluated in clinical trials (Cai et al., 2017; Jung et al., 2018).
NSAID use in HNSCC risk reduction
A beneficial effect of NSAID use on HNSCC was first described by Panje in 1981, where 5 cases of regression and 2 cases of stabilization of HNSCC with commonly recommended dosages of indomethacin were reported (Panje, 1981). Subsequently, several case-control studies have been conducted to investigate the impact of NSAID use in head and neck cancer. In an analysis of 3 case-control studies between 1992-2000 in Italy, 37 of 965 upper aerodigestive tract (UADT) cancer cases and 87 of 1779 controls were found to regularly use aspirin. The multivariate odds ratio for UADT decreased from 0.89 (95% CI 0.56-1.43) to 0.33 (95% CI 0.13-0.82) for aspirin users of ≥ 5 years and the reduced head and neck cancer risk was observed in all included subsites (oropharyngeal, esophageal, and laryngeal) (Bosetti et al., 2003). In the Alcohol-Related CAncers and GEnetic Susceptibility (ARCAGE) project, 235/1779 UADT SCC cases and 321/1993 controls reported regular aspirin use (Macfarlane et al., 2012). An association between aspirin use and reduced risk of head and neck cancer approached significance for cancer of the hypopharynx (Odds Ratio (OR) 0.53, 95% CI 0.28–1.02) and larynx (OR 0.74, 95% CI 0.54–1.01) (Macfarlane et al., 2012). In another case-control study, data from a comprehensive epidemiologic questionnaire of 529 patients with primary head and neck cancer and 529 healthy subjects showed an association between head and neck cancer risk reduction and aspirin use (adjusted odds ratio, 0.75; 95% CI 0.58-0.96) (Jayaprakash et al., 2006). The risk reduction was also associated with frequency and duration of aspirin use. Similar results were seen in a recent case-control study that focused on head and neck cancer patients with SCC. In 71 HNSCC patients and 71 healthy controls matched by age (within 10 years), gender, and history of tobacco use, there was significant HNSCC risk reduction with NSAID use (OR 0.25; 95% CI 0.09-0.67) and the risk reduction was associated with the frequency of NSAID use (Ahmadi et al., 2010).
Other studies have investigated the effects of different types of NSAIDs on HNSCC. In a study of data from the National Cancer Institute Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screen Trial, 151 head and neck cancer patients and 69,729 controls used aspirin (Wilson et al., 2013). Interestingly, significant reduction of head and neck cancer risk was associated with weekly and monthly aspirin use (adjusted hazard ratio (AHR) 0.69, 95% CI 0.51-0.93) but not daily aspirin use (AHR 0.85, 95% CI 0.65-1.11). Meanwhile, ibuprofen use was reported in 82 head and neck cancer patients and 41,946 controls, and there was no association with head and neck cancer risk (AHR 0.97, 95% CI 0.75-1.27). In another study, 232 daily users of COX inhibitors and 87 nonusers were identified among HNSCC patients from the cancer registry at the Ralph H. Johnson VA Medical Center (Gillespie et al., 2007). There were no significant differences between COX inhibitor users and nonusers in terms of recurrence (OR 0.94, 95% CI 0.42-2.10, p 0.88) or survival (OR 1.47, 95% CI 0.66-3.25, p 0.34). Kaplan-Meier curve analyses in this study also showed no significant differences in disease-free survival (p=0.27) or overall survival (p=0.44).
Macfarlane et al. also investigated the use of aspirin, COX-2 selective inhibitors, and other NSAIDs in a nested case–control study of UADT cancer (which included head and neck) patients using the Primary Care Clinical Informatics Unit (PCCIU) database (Macfarlane et al., 2014). In the study, 594 UADT cancer patients and 1768 controls used aspirin and aspirin use was associated with a non-significant reduction in UADT cancer risk (adjusted OR 0.90, 95% CI 0.78-1.04). Similar results were obtained when UADT cancer was stratified into head and neck cancer (adjusted OR 0.93, 95% CI 0.76-1.15) and esophageal cancer (adjusted OR 0.87, 95% CI 0.72-1.05). In the same study, the use of other NSAIDs, excluding COX-2 inhibitors, in 780 UADT cancer patients was associated with significant risk reduction for UADT cancer (adjusted OR 0.83, 95% CI 0.75-0.93) as well as head and neck cancer (adjusted OR 0.82, 95% CI 0.70-0.96). Data from these same patients were also used to investigate the effects of aspirin, COX-2 selective inhibitors, and other NSAIDs on survival (Macfarlane et al., 2015). For head and neck cancer patients, COX-2 selective inhibitor use did not improve survival but use of aspirin (AHR 0.56, 95% CI 0.44-0.71) and other NSAIDs (adjusted HR 0.74, 95% CI 0.60-0.90) did. In comparison, for esophageal cancer patients, use of aspirin (AHR 0.54, 95% CI 0.45-0.64), COX-2 selective inhibitors (AHR 0.78, 95% CI 0.62-0.98), and other NSAIDs (AHR 0.67, 95% CI 0.56-0.80) were all associated with improved survival.
While the above studies have shown that aspirin/NSAID use may reduce HNSCC risk and/or improve OS and DSS, other investigations have reported conflicting results. A study of the Danish population between 1991 and 2002 identified 75 NSAID users and 110 nonusers amongst oral cancer patients and failed to demonstrate an association between NSAID use and development of oral cancers (Friis et al., 2006). In another study of South Korean patients (n = 1392) with newly diagnosed HNSCC (between 2000 and 2013), Kim et al. defined 81 patients as low-dose aspirin users and 89 as other NSAID users (Kim et al., 2018). Even after adjusting for confounding factors in multivariate analyses, the results did not identify a significant association between use of aspirin, a different NSAID, or both aspirin and another NSAID with disease-free survival (DFS), recurrence, non-cancer survival (NCS), or OS.
Of note, the above studies did not stratify head and neck cancer into distinct subpopulations, which could impact the conclusions. For instance, the effects of aspirin and other NSAIDs may differ with HNSCC disease stage. Lumley et al. conducted a retrospective study of 584 veterans with HNSCC treated at the Washington DC Veterans Affairs Medical Center between 1995 and 2015 (Lumley et al., 2019). Among 329 patients included in the analysis, 84 had used aspirin for at least one year after HNSCC diagnosis. After a median follow-up of 40 (aspirin users) and 25 months (aspirin nonusers), aspirin users exhibited better OS (HR 0.53, 95% CI 0.38-0.75, p < 0.001) and DSS (HR 0.31, 95% CI 0.17-0.56, p < 0.001). These results may be limited by significant differences in the prevalence of stage I disease (34.5% and 13.1% for users and nonusers, respectively) and stage IV disease (28.6% and 55.5% for users and nonusers, respectively) between the aspirin and non-aspirin user groups (Lumley et al., 2019). (The study included T and N stage in multivariate regression analysis but not disease stage.) However, the authors also examined the effect of aspirin use among patients with different stages of disease. In early stage disease, aspirin remained a significant predictor of OS (p= 0.013) and DSS (p= 0.006); meanwhile, in advanced stage disease, aspirin use was significantly associated with improved DSS (p= 0.041), but only trended toward significance for OS (p= 0.072).
In addition, NSAID effects may depend on the genomic profile of HNSCC tumors, as has been shown in CRC. In a recently published study, Hedberg et al. analyzed aspirin use in HNSCC patients harboring either wild-type PIK3CA or PIK3CA altered by amplification or mutation. In patients with PIK3CA alterations they found that regular NSAID use was associated with a dramatically prolonged DSS (HR 0.23, P = 0.0032, 95% CI 0.09–0.62) and OS (HR 0.31, P = 0.0043, 95% CI 0.14–0.69) when compared to non-regular NSAID use (Hedberg et al., 2019). On the other hand, HNSCC patients whose tumors had unamplified, wild-type PIK3CA demonstrated no statistically significant change in DSS (HR 0.86, 95% CI 0.48–1.54) or OS (HR 0.98, 95% CI 0.60–1.62) with NSAID use. These results were subsequently validated in preclinical models. Specifically, mice harboring patient-derived xenografts (PDXs) endogenously expressing either wild-type PIK3CA or mutant PIK3CA were treated with saline or the NSAIDs sulindac, celecoxib, or aspirin, followed by assessment of tumor growth. NSAID treatment markedly inhibited the growth of the PIK3CA-mutated PDX tumors, but had no significant impact on the growth of PDX tumors harboring wild-type PIK3CA. Analogous results were seen in cell line models, where NSAID treatment inhibited proliferation and induced apoptosis in PIK3CA mutant cell lines, but not cell lines with wild-type PIK3CA (Hedberg et al., 2019).
Plausible mechanism of NSAID-associated risk reduction in PIK3CA-altered cancers
While the mechanisms underlying NSAID-related risk reduction in CRC, breast cancer, and HNSCC remain to be fully characterized, recent studies suggest a role for inhibition of cyclooxygenase (COX) and prostaglandin (PG) production. Aspirin is known to inhibit the two isoforms of COX enzymes, COX-1 and COX-2 (Figure 1) (Chandrasekharan and Simmons, 2004). COX-1 is constitutively expressed in various tissues and plays an important role in tissue homeostasis. While normally absent in most cells, COX-2 expression can be stimulated by an inflammatory response and is known to be associated with tumorigenesis (Echizen et al., 2018; Hashemi Goradel et al., 2019) in CRC (Liu et al., 2017; Tougeron et al., 2014) and breast cancer (Chen and Holmes, 2017). The COX enzymes play a key role in the metabolism of membrane phospholipids, following cleavage of these lipids by phospholipase A2 enzymes to release fatty acids, including arachidonic acid (Vasquez et al., 2018). Of note, COX-1 and -2 catalyze a rate-limiting step in biosynthesis of PGs from arachidonic acid (Figure 2) (Chandrasekharan and Simmons, 2004). These enzymes convert arachidonic acid to prostaglandin H2 (PGH2) and PGH2 is further converted by various enzymes to other PGs that are involved in cellular proliferation, migration, and invasion (Figure 2) (Ulrich et al., 2006). Among these PGs, prostaglandin E2 (PGE2) plays an important role in the tumor growth-promoting effects of COX-2 (Hashemi Goradel et al., 2019; Karpisheh et al., 2019; Tougeron et al., 2014).
Figure 2:
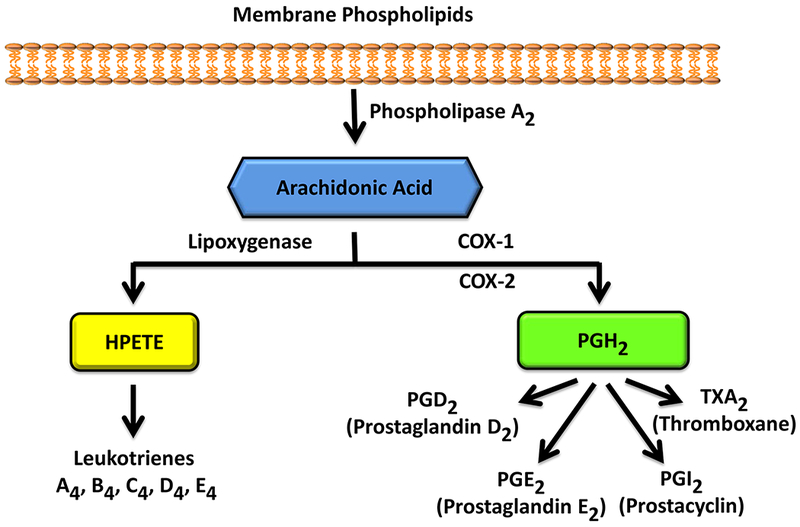
COX-mediated production of PGE2 from arachidonic acid.
Chan et al. characterized COX-2 levels in 636 paraffin-embedded CRC specimens from the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). Among them, 423 (67%) exhibited moderate or strong COX-2 expression and regular aspirin use was associated with a significant risk reduction in the cases that overexpressed COX-2 (multivariate risk ratio 0.64, 95% CI 0.52-0.78) but not cases with weak or absent expression of COX-2 (Chan et al., 2007). In addition, aspirin use in CRC patients with COX-2 overexpression was associated with lower risks of cancer-specific mortality (multivariate-adjusted HR 0.39, 95% CI 0.20-0.76) and overall mortality (multivariate-adjusted HR 0.62, 95% CI 0.42-0.93). However, these risk reductions in mortality were not seen in CRC patients who used aspirin, but whose tumors were COX-2-negative (Triadafilopoulos, 2010). These results suggest that the beneficial effects of aspirin in CRC are associated with inhibition of overexpressed COX-2.
Interaction between the PI3K/AKT/mTOR pathway and COX-2 may also underlie the NSAID-associated risk reduction in CRC and HNSCC. Some studies suggest COX-2 may activate the PI3K pathway. For instance, the PI3K pathway is activated by PGE2 in cells expressing PGE2 receptor 4 (EP4) (Fujino et al., 2002) and COX-2 activation was associated with AKT phosphorylation and poor survival in breast cancer (Glynn et al., 2010). In addition, COX-2 was found to be overexpressed in 60% of epithelial ovarian cancers and was significantly associated with activated AKT in ovarian cancer and CRC (Chang et al., 2019; Uddin et al., 2010). In hepatocellular carcinoma, COX-2 was shown to also activate the PI3K pathway by suppressing PTEN (Chen et al., 2017). Conversely, other studies suggest the PI3K pathway may activate COX-2. For example, the PI3K pathway has been shown to activate COX-2 through induction of NF-κB when PTEN is inhibited by miR - 221/222 (Li et al., 2017). Furthermore, when the PI3K pathway is inhibited by suppression of EGFR, COX-2 has been shown to be down-regulated through blockade of NF-κB (Ramu et al., 2018).
In the setting of HNSCC, work by Hedberg et al. suggests that NSAID sensitivity in PIK3CA-mutated HNSCC is mediated via inhibition of COX-2-dependent production of PGE2 (Hedberg et al., 2019). They found that mice harboring PDX tumors with mutant PIK3CA exhibited elevated levels of circulating PGE2 relative to mice harboring PDX tumors with wild-type PIK3CA. Furthermore, treatment with celecoxib, sulindac, and aspirin resulted in dramatic reduction in PGE2 levels in the mutant PIK3CA PDX group when compared to the wild-type group. Thus, taken together, the above data suggests a role for NSAIDs in PIK3CA-altered HNSCC through inhibition of COX-2 and thus lowering PGE2 levels (Figure 1). NSAID-mediated reduction of PGE2 levels in mice with PIK3CA-altered tumors may be critically important, since PGE2 is a known immunosuppressive molecule. In future studies it will be valuable to investigate whether PIK3CA-mutant tumors are characterized by a more immunosuppressive immune profile, and whether NSAID treatment serves to reverse the immunosuppressive stature of the tumor microenvironment.
Conclusions
Several studies have shown that NSAIDs can provide a protective effect against the development of CRC, breast, and head and neck cancers. First shown in CRC and breast cancer, long term NSAID use has been shown to decrease the incidence and mortality of both colorectal and breast cancer. Further analyses have indicated that this effect was most prevalent in patients with a mutated PIK3CA gene. Mutation of the PIK3CA gene leads to constitutive activation of the PI3K/AKT/mTOR pathway, upregulation of COX-2 enzyme and enhanced production of PGE2. PGE2 is a known immunosuppressive molecule and its overproduction may lead to a more immunosuppressive tumor microenvironment in CRC and breast cancers exhibiting genetic alterations of PIK3CA. As NSAIDs can be used to inhibit COX-2 activity and reduce PGE2 levels, further studies are needed to determine whether NSAIDs reverse immunosuppressive profiles in the subpopulation of CRC and breast cancer patients that harbor PIK3CA-altered tumors.
NSAID use in HNSCC has also shown potential benefit. Early epidemiological studies in HNSCC showed mixed results with some studies finding improved DSS and OS while other studies observed no differences. However, a recent retrospective study has determined that regular use of NSAIDs is associated with a dramatic improvement of DSS and OS in HNSCC. This positive benefit of NSAIDs was restricted to patients whose tumors exhibited PIK3CA alterations, and was not seen in patients with wild-type (PIK3CA) tumors. These findings support the need for prospective evaluation of NSAIDs in PIK3CA-altered HNSCC. If confirmation is obtained, NSAIDs therapy should be strongly considered as a strategy to prevent HNSCC development in patients whose initial primary tumor contained PIK3CA alteration and who are at high risk for the development of second primary tumors.
Acknowledgements
This work was supported by National Institutes of Health grants R35 CA231998 (JRG), R01 DE023685 (JRG and DEJ), and R01 DE024728 (DEJ).
Footnotes
Competing interests
JRG and DEJ are co-inventors of cyclic STAT3 decoy and have financial interests in STAT3 Therapeutics, Inc. STAT3 Therapeutics, Inc. holds an interest in a cyclic STAT3 decoy oligonucleotide. The remaining authors declare no conflicts.
References
- Ahmadi N, Goldman R, Seillier-Moiseiwitsch F, Noone AM, Kosti O, Davidson BJ, 2010. Decreased risk of squamous cell carcinoma of the head and neck in users of nonsteroidal anti-inflammatory drugs. Int J Otolaryngol 2010, 424161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algra AM, Rothwell PM, 2012. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 13(5), 518–527. [DOI] [PubMed] [Google Scholar]
- Andreotti F, De Caterina R, Crea F, 2017. Aspirin and the prevention of a common disease: Colorectal cancer. Int J Cardiol 248, 394–395. [DOI] [PubMed] [Google Scholar]
- Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, Lievre A, Cortet M, Bouvier AM, Rat P, Roignot P, Faivre J, Laurent-Puig P, Piard F, 2008. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer 122(10), 2255–2259. [DOI] [PubMed] [Google Scholar]
- Bauer TM, Patel MR, Infante JR, 2015. Targeting PI3 kinase in cancer. Pharmacol Ther 146, 53–60. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Frattini M, Arena S, Zanon C, Cappelletti V, Coradini D, Daidone MG, Pilotti S, Pierotti MA, Bardelli A, 2008. PIK3CA cancer mutations display gender and tissue specificity patterns. Hum Mutat 29(2), 284–288. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R, 2007. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4), 395–402. [DOI] [PubMed] [Google Scholar]
- Bilanges B, Posor Y, Vanhaesebroeck B, 2019. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Talamini R, Franceschi S, Negri E, Garavello W, La Vecchia C, 2003. Aspirin use and cancers of the upper aerodigestive tract. Br J Cancer 88(5), 672–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauswetter D, Danos K, Gurbi B, Felegyhazi EF, Birtalan E, Meggyeshazi N, Krenacs T, Tamas L, Petak I, 2016. Copy number gain of PIK3CA and MET is associated with poor prognosis in head and neck squamous cell carcinoma. Virchows Arch 468(5), 579–587. [DOI] [PubMed] [Google Scholar]
- Burris HA 3rd, 2013. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol 71(4), 829–842. [DOI] [PubMed] [Google Scholar]
- Cai Y, Dodhia S, Su GH, 2017. Dysregulations in the PI3K pathway and targeted therapies for head and neck squamous cell carcinoma. Oncotarget 8(13), 22203–22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas, N., 2012a. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487(7407), 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas, N., 2012b. Comprehensive molecular portraits of human breast tumours. Nature 490(7418), 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas, N., 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517(7536), 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS, 2007. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356(21), 2131–2142. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Simmons DL, 2004. The cyclooxygenases. Genome Biol 5(9), 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Tang N, Fang Q, Zhu K, Liu L, Xiong X, Zhu Z, Zhang B, Zhang M, Tao J, 2019. Inhibition of COX-2 and 5-LOX regulates the progression of colorectal cancer by promoting PTEN and suppressing PI3K/AKT pathway. Biochem Biophys Res Commun 517(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Chen H, Cai W, Chu ESH, Tang J, Wong CC, Wong SH, Sun W, Liang Q, Fang J, Sun Z, Yu J, 2017. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene 36(31), 4415–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Holmes MD, 2017. Role of Aspirin in Breast Cancer Survival. Curr Oncol Rep 19(7), 48. [DOI] [PubMed] [Google Scholar]
- Chow LW, Yip AY, Loo WT, Lam CK, Toi M, 2008. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol 111(1-2), 13–17. [DOI] [PubMed] [Google Scholar]
- Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Network TR, Perou CM, 2015. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 163(2), 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA, 2009. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101(4), 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA, 2015. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta 1855(1), 104–121. [DOI] [PubMed] [Google Scholar]
- De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S, 2010. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 11(8), 753–762. [DOI] [PubMed] [Google Scholar]
- Di Popolo A, Memoli A, Apicella A, Tuccillo C, di Palma A, Ricchi P, Acquaviva AM, Zarrilli R, 2000. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene 19(48), 5517–5524. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Cantley LC, 2015. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 25(9), 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN, 1994. Upregulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107(4), 1183–1188. [DOI] [PubMed] [Google Scholar]
- Echizen K, Oshima H, Nakayama M, Oshima M, 2018. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv Biol Regul 68, 39–45. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M, Dolwani S, 2016. Aspirin in the Treatment of Cancer: Reductions in Metastatic Spread and in Mortality: A Systematic Review and Meta-Analyses of Published Studies. PLoS One 11(4), e0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falandry C, Debled M, Bachelot T, Delozier T, Cretin J, Romestaing P, Mille D, You B, Mauriac L, Pujade-Lauraine E, Freyer G, 2009. Celecoxib and exemestane versus placebo and exemestane in postmenopausal metastatic breast cancer patients: a double-blind phase III GINECO study. Breast Cancer Res Treat 116(3), 501–508. [DOI] [PubMed] [Google Scholar]
- Fraser DM, Sullivan FM, Thompson AM, McCowan C, 2014. Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. Br J Cancer 111(3), 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis S, Poulsen A, Pedersen L, Baron JA, Sorensen HT, 2006. Use of nonsteroidal anti-inflammatory drugs and risk of oral cancer: a cohort study. Br J Cancer 95(3), 363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT, 2017. The PI3K Pathway in Human Disease. Cell 170(4), 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW, 2002. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem 277(4), 2614–2619. [DOI] [PubMed] [Google Scholar]
- Garcia-Escudero R, Segrelles C, Duenas M, Pombo M, Ballestin C, Alonso-Riano M, Nenclares P, Alvarez-Rodriguez R, Sanchez-Aniceto G, Ruiz-Alonso A, Lopez-Cedrun JL, Paramio JM, Lorz C, 2018. Overexpression of PIK3CA in head and neck squamous cell carcinoma is associated with poor outcome and activation of the YAP pathway. Oral Oncol 79, 55–63. [DOI] [PubMed] [Google Scholar]
- Gillespie MB, Moody MW, Lee FS, Poole LJ, Hornig JD, Lathers D, Young MR, Day TA, 2007. Head and neck cancer recurrence and mortality in nonselective cyclooxygenase inhibitor users. Arch Otolaryngol Head Neck Surg 133(1), 28–31. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE, 1995. Aspirin and the risk of colorectal cancer in women. N Engl J Med 333(10), 609–614. [DOI] [PubMed] [Google Scholar]
- Glynn SA, Prueitt RL, Ridnour LA, Boersma BJ, Dorsey TM, Wink DA, Goodman JE, Yfantis HG, Lee DH, Ambs S, 2010. COX-2 activation is associated with Akt phosphorylation and poor survival in ER-negative, HER2-positive breast cancer. BMC Cancer 10, 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R, D’Souza DP, Chalchal H, Spadafora S, Stearns V, Perez EA, Liedke PE, Lang I, Elliott C, Gelmon KA, Chapman JA, Shepherd LE, 2013. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol 31(11), 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN, 2001. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 1(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Hamada T, Nowak JA, Ogino S, 2017. PIK3CA mutation and colorectal cancer precision medicine. Oncotarget 8(14), 22305–22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A, Women’s Health I, 2003. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res 63(18), 6096–6101. [PubMed] [Google Scholar]
- Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K, 2019. Cyclooxygenase-2 in cancer: A review. J Cell Physiol 234(5), 5683–5699. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Stephens LR, 2015. PI3K signalling in inflammation. Biochim Biophys Acta 1851(6), 882–897. [DOI] [PubMed] [Google Scholar]
- He J, Bao Q, Yan M, Liang J, Zhu Y, Wang C, Ai D, 2018. The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br J Pharmacol 175(8), 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg ML, Peyser ND, Bauman JE, Gooding WE, Li H, Bhola NE, Zhu TR, Zeng Y, Brand TM, Kim MO, Jordan RCK, VandenBerg S, Olivas V, Bivona TG, Chiosea SI, Wang L, Mills GB, Johnson JT, Duvvuri U, Ferris RL, Ha P, Johnson DE, Grandis JR, 2019. Use of nonsteroidal anti-inflammatory drugs predicts improved patient survival for PIK3CA-altered head and neck cancer. J Exp Med 216(2), 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE, 2010. Aspirin intake and survival after breast cancer. J Clin Oncol 28(9), 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Olsson H, Pawitan Y, Holm J, Lundholm C, Andersson TM, Adami HO, Askling J, Smedby KE, 2014. Aspirin intake and breast cancer survival - a nation-wide study using prospectively recorded data in Sweden. BMC Cancer 14, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Zhang H, Kong Q, Wang J, Jiang Y, 2019. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev 39(1), 114–145. [DOI] [PubMed] [Google Scholar]
- Huang XZ, Gao P, Sun JX, Song YX, Tsai CC, Liu J, Chen XW, Chen P, Xu HM, Wang ZN, 2015. Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta-analysis. Cancer Causes Control 26(4), 589–600. [DOI] [PubMed] [Google Scholar]
- Janku F, 2017. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat Rev 59, 93–101. [DOI] [PubMed] [Google Scholar]
- Janku F, Yap TA, Meric-Bernstam F, 2018. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 15(5), 273–291. [DOI] [PubMed] [Google Scholar]
- Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, Menezes RJ, Reid ME, 2006. Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg 132(11), 1231–1236. [DOI] [PubMed] [Google Scholar]
- Jean S, Kiger AA, 2014. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci 127(Pt 5), 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Kang H, Mehra R, 2018. Targeting phosphoinositide 3-kinase (PI3K) in head and neck squamous cell carcinoma (HNSCC). Cancers Head Neck 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK, 2005. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A 102(3), 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpisheh V, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Ghalamfarsa G, Sabz G, Yousefi M, Yousefi B, Jadidi-Niaragh F, 2019. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat, 106338. [DOI] [PubMed] [Google Scholar]
- Kato S, Iida S, Higuchi T, Ishikawa T, Takagi Y, Yasuno M, Enomoto M, Uetake H, Sugihara K, 2007. PIK3CA mutation is predictive of poor survival in patients with colorectal cancer. Int J Cancer 121(8), 1771–1778. [DOI] [PubMed] [Google Scholar]
- Khuri FR, Kim ES, Lee JJ, Winn RJ, Benner SE, Lippman SM, Fu KK, Cooper JS, Vokes EE, Chamberlain RM, Williams B, Pajak TF, Goepfert H, Hong WK, 2001. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev 10(8), 823–829. [PubMed] [Google Scholar]
- Kim SA, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY, 2018. Aspirin use and head and neck cancer survival: an observational study of 11,623 person-years follow-up. Int J Clin Oncol 23(1), 52–58. [DOI] [PubMed] [Google Scholar]
- Kochel TJ, Goloubeva OG, Fulton AM, 2016. Upregulation of Cyclooxygenase-2/Prostaglandin E2 (COX-2/PGE2) Pathway Member Multiple Drug Resistance-Associated Protein 4 (MRP4) and Downregulation of Prostaglandin Transporter (PGT) and 15-Prostaglandin Dehydrogenase (15-PGDH) in Triple-Negative Breast Cancer. Breast Cancer (Auckl) 10, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kune GA, Kune S, Watson LF, 1988. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res 48(15), 4399–4404. [PubMed] [Google Scholar]
- Lee JJ, Loh K, Yap YS, 2015. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 12(4), 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lu Y, Yu L, Han X, Wang H, Mao J, Shen J, Wang B, Tang J, Li C, Song B, 2017. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-kappaB/COX-2 activation. Chem Biol Interact 277, 33–42. [DOI] [PubMed] [Google Scholar]
- Li SY, Rong M, Grieu F, Iacopetta B, 2006. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat 96(1), 91–95. [DOI] [PubMed] [Google Scholar]
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S, 2012a. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 367(17), 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, Nosho K, Qian ZR, Nishihara R, Meyerhardt JA, Fuchs CS, Ogino S, 2012b. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res 18(8), 2257–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievre A, Blons H, Laurent-Puig P, 2010. Oncogenic mutations as predictive factors in colorectal cancer. Oncogene 29(21), 3033–3043. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun H, Hu M, Zhang Y, Chen S, Tighe S, Zhu Y, 2017. The Role of Cyclooxygenase-2 in Colorectal Carcinogenesis. Clin Colorectal Cancer 16(3), 165–172. [DOI] [PubMed] [Google Scholar]
- Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, Freilino M, Sauerwein S, Peyser ND, Xiao D, Diergaarde B, Wang L, Chiosea S, Seethala R, Johnson JT, Kim S, Duvvuri U, Ferris RL, Romkes M, Nukui T, Kwok-Shing Ng P, Garraway LA, Hammerman PS, Mills GB, Grandis JR, 2013. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 3(7), 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley CJ, Kaffenberger TM, Desale S, Tefera E, Han CJ, Rafei H, Maxwell JH, 2019. Post-diagnosis aspirin use and survival in veterans with head and neck cancer. Head Neck 41(5), 1220–1226. [DOI] [PubMed] [Google Scholar]
- Macfarlane TV, Lefevre K, Watson MC, 2014. Aspirin and non-steroidal anti-inflammatory drug use and the risk of upper aerodigestive tract cancer. Br J Cancer 111(9), 1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane TV, Macfarlane GJ, Thakker NS, Benhamou S, Bouchardy C, Ahrens W, Pohlabeln H, Lagiou P, Lagiou A, Castellsague X, Agudo A, Slamova A, Plzak J, Merletti F, Richiardi L, Talamini R, Barzan L, Kjaerheim K, Canova C, Simonato L, Conway DI, McKinney PA, Thomson P, Sloan P, Znaor A, Healy CM, McCartan BE, Marron M, Brennan P, 2012. Role of medical history and medication use in the aetiology of upper aerodigestive tract cancers in Europe: the ARCAGE study. Ann Oncol 23(4), 1053–1060. [DOI] [PubMed] [Google Scholar]
- Macfarlane TV, Murchie P, Watson MC, 2015. Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer. Cancer Epidemiol 39(6), 1015–1022. [DOI] [PubMed] [Google Scholar]
- Mao C, Yang ZY, Hu XF, Chen Q, Tang JL, 2012. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol 23(6), 1518–1525. [DOI] [PubMed] [Google Scholar]
- Martin LA, Davies GL, Weigel MT, Betambeau N, Hills MJ, Salter J, Walsh G, A’Hern R, Dowsett M, 2010. Pre-surgical study of the biological effects of the selective cyclo-oxygenase-2 inhibitor celecoxib in patients with primary breast cancer. Breast Cancer Res Treat 123(3), 829–836. [DOI] [PubMed] [Google Scholar]
- Miyaki M, Iijima T, Yamaguchi T, Takahashi K, Matsumoto H, Yasutome M, Funata N, Mori T, 2007. Mutations of the PIK3CA gene in hereditary colorectal cancers. Int J Cancer 121(7), 1627–1630. [DOI] [PubMed] [Google Scholar]
- Nie D, Honn KV, 2002. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 59(5), 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B, 2019. The relation between PI3K/AKT signalling pathway and cancer. Gene 698, 120–128. [DOI] [PubMed] [Google Scholar]
- Ogino S, Kirkner GJ, Nosho K, Irahara N, Kure S, Shima K, Hazra A, Chan AT, Dehari R, Giovannucci EL, Fuchs CS, 2008. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res 14(24), 8221–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleari L, Puntoni M, Clavarezza M, DeCensi M, Cuzick J, DeCensi A, 2016. PIK3CA Mutation, Aspirin Use after Diagnosis and Survival of Colorectal Cancer. A Systematic Review and Meta-analysis of Epidemiological Studies. Clin Oncol (R Coll Radiol) 28(5), 317–326. [DOI] [PubMed] [Google Scholar]
- Panje WR, 1981. Regression of head and neck carcinoma with a prostaglandin-synthesis inhibitor. Arch Otolaryngol 107(11), 658–663. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Patrono C, 2016. Aspirin and Cancer. J Am Coll Cardiol 68(9), 967–976. [DOI] [PubMed] [Google Scholar]
- Patrono C, Rocca B, 2008. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb Vasc Biol 28(3), s25–32. [DOI] [PubMed] [Google Scholar]
- Ramu A, Kathiresan S, Ramadoss H, Nallu A, Kaliyan R, Azamuthu T, 2018. Gramine attenuates EGFR-mediated inflammation and cell proliferation in oral carcinogenesis via regulation of NF-kappaB and STAT3 signaling. Biomed Pharmacother 98, 523–530. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW, 2011. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377(9759), 31–41. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW, 2012. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379(9826), 1602–1612. [DOI] [PubMed] [Google Scholar]
- Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg A, Parsons R, 2005. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65(7), 2554–2559. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 169(2), 361–371. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Paramio JM, Lorz C, 2018. The transcriptional co-activator YAP: A new player in head and neck cancer. Oral Oncol 86, 25–32. [DOI] [PubMed] [Google Scholar]
- Shim JY, An HJ, Lee YH, Kim SK, Lee KP, Lee KS, 2003. Overexpression of cyclooxygenase-2 is associated with breast carcinoma and its poor prognostic factors. Mod Pathol 16(12), 1199–1204. [DOI] [PubMed] [Google Scholar]
- Soriano LC, Soriano-Gabarro M, Garcia Rodriguez LA, 2018. Trends in the contemporary incidence of colorectal cancer and patient characteristics in the United Kingdom: a population-based cohort study using The Health Improvement Network. BMC Cancer 18(1), 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Hama T, Kondo S, Yuza Y, Yoshikawa M, Urashima M, Kato T, Moriyama H, 2012. Copy number amplification of the PIK3CA gene is associated with poor prognosis in non-lymph node metastatic head and neck squamous cell carcinoma. BMC Cancer 12, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougeron D, Sha D, Manthravadi S, Sinicrope FA, 2014. Aspirin and colorectal cancer: back to the future. Clin Cancer Res 20(5), 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G, 2010. Aspirin use and survival following colorectal cancer diagnosis: another favorable and promising meta-effect of an old drug. Gastroenterology 138(5), 2012–2014; discussion 2014. [DOI] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN, 1995. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83(3), 493–501. [DOI] [PubMed] [Google Scholar]
- Uddin S, Ahmed M, Hussain A, Assad L, Al-Dayel F, Bavi P, Al-Kuraya KS, Munkarah A, 2010. Cyclooxygenase-2 inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian cancer. Int J Cancer 126(2), 382–394. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD, 2006. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6(2), 130–140. [DOI] [PubMed] [Google Scholar]
- Vadas O, Burke JE, Zhang X, Berndt A, Williams RL, 2011. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal 4(195), re2. [DOI] [PubMed] [Google Scholar]
- Vander Broek R, Mohan S, Eytan DF, Chen Z, Van Waes C, 2015. The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk, and therapies. Oral Dis 21(7), 815–825. [DOI] [PubMed] [Google Scholar]
- Vasquez AM, Mouchlis VD, Dennis EA, 2018. Review of four major distinct types of human phospholipase A2. Adv Biol Regul 67, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JC, Murray LJ, Hughes CM, Black A, Anderson LA, 2013. Non-steroidal anti-inflammatory drug and aspirin use and the risk of head and neck cancer. Br J Cancer 108(5), 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC, 2008. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27(41), 5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen F, Shang L, 2018. Advances in antitumor effects of NSAIDs. Cancer Manag Res 10, 4631–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM, 2005. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A 102(51), 18443–18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Vogt PK, 2010. Hot-spot mutations in p110alpha of phosphatidylinositol 3-kinase (pI3K): differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle 9(3), 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J, 2015. Association between aspirin use and mortality in breast cancer patients: a meta-analysis of observational studies. Breast Cancer Res Treat 150(1), 199–207. [DOI] [PubMed] [Google Scholar]


