Abstract
Unregulated stress during critical periods of development is proposed to drive deficits consistent with schizophrenia in adults. If accurate, reopening the critical period could make the adult susceptible to pathology. We evaluated the impact of early adolescent and adult stress exposure (combination of daily footshock for 10 days and 3 restraint sessions) on 1) midbrain dopamine (DA) neuron activity, 2) ventral hippocampal (vHipp) pyramidal neuron activity, and 3) the number of parvalbumin (PV) interneurons in the vHipp and their associated perineuronal nets (PNNs). Ventral tegmental area (VTA) DA neuron population activity and vHipp activity was increased 1–2 and 5–6 weeks post-adolescent stress, along with a decrease in the number of PV+, PNN+, PV+/PNN+ cells in the vHipp, which are consistent with the MAM model of schizophrenia. In contrast, adult stress decreased VTA DA neuron population activity only at 1–2 weeks post-stress, which is consistent with what has been observed in animal models of depression, without impacting vHipp activity and PV/PNN expression. Administration of valproate (VPA), which can re-instate the critical period of plasticity via histone deacetylase (HDAC) inhibition, caused adult stress to produce changes similar to those induced by adolescent stress, presumably by increasing stress vulnerability to early adolescent levels. Our findings indicate that timing of stress is a critical determinant of the pathology produced in the adult: adolescent stress led to circuit deficits that recapitulates schizophrenia, whereas adult stress induced a depression-like hypodopaminergic state. Reopening the critical period in the adult restores vulnerability to stress-induced pathology resembling schizophrenia.
Keywords: stress, psychosis, depression, parvalbumin, perineuronal nets, HDAC inhibitors
Introduction
The etiology of most major psychiatric disorders remains undetermined, but likely involves genetic and socio-environmental risk factors and their interaction. Interestingly, these disorders share both socio-environmental, including stressful events,1, 2 and genetic risk factors.3 For example, around 40% of the genetic variations observed in schizophrenia are also found in depression,4 and depression and schizophrenia tend to run in the same families.5 However, importantly, these conditions differ in the mean age of onset. Whereas schizophrenia is usually diagnosed during late adolescence/early adulthood, depression is more common in adults with the age of diagnosis reaching a peak near 32 years.6 These evidences point to an intriguing possibility; i.e., that socio-environmental factors, such as stressful events, may influence the risk of developing these disorders, with the age of exposure a critical determinant of the pathophysiology that arises in the adult.
One system impacted by stress is the parvalbumin (PV)-expressing GABAergic interneurons.7 During preadolescence, PV interneurons are highly plastic in terms of excitatory drive and neuronal activity,8 which also causes them to be particularly susceptible to damage by stressors.7 However, following adolescence PV interneurons develop perineuronal nets (PNNs), a unique extracellular matrix structure that aggregates around the soma and proximal dendrites of certain neurons. These PNNs attenuate plasticity and protects PV interneurons from stress-induced damage.7, 9
In humans, a loss of PV interneurons in the anterior limbic hippocampus, a region homologous to the ventral hippocampus (vHipp) in rodents,10 is proposed to lead to a hyperactive state of this region. This hyperactivity was found to drive the increased dopamine (DA) system activity in the ventral tegmental area (VTA) that is associated with the psychotic symptoms of schizophrenia.10, 11 Thus, our hypothesis is that exposure to stress during adolescence, which is a sensitive period in which the PV interneurons are not completely protected by the PNNs, results in PV loss in the vHipp, thus favoring the development of schizophrenia. In contrast, we propose that if the individual is “protected” during this period when PV interneurons are more vulnerable, but experiences stress later in life (in which PV interneurons have been protected by the PNNs), this could facilitate the development of affective disorders, such as depression. Thus, the age of exposure to stress could determine the possible pathophysiological consequences. If the differential developmental vulnerability of PV interneurons is a causative factor, we further predict that pharmacological tools, such as histone deacetylase inhibitors (i.e., valproate and SAHA) which are proposed to reopen the critical period of plasticity in the adult,12, 13 could recreate an adolescent phenotype of stress susceptibility.
Material and methods
Animals
Sixty-one timed-pregnant Sprague–Dawley rats (Envigo) were obtained at GD14 and housed individually in plastic breeding tubs. On postnatal day (PD) 24, litters were weaned and housed two or three per cage. Only male offspring (total of 516) were used in this study. Animals from the same litter were assigned to the different experimental groups with all the animals in the same cage devoted to the same experimental procedure. Animals were housed in a temperature (22°C)- and humidity (47%)-controlled environment (12-h light/dark cycle; lights on at 7 AM) with ad libitum access to food and water. All experiments were conducted according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh to ensure that the animals were treated humanely and did not experience needless stress beyond the experimental conditions required for the study.
Stress procedure
Adolescent rats were exposed to the combination of daily inescapable footshock (FS; from PD31–40) and three restraint stress (RS) sessions (PD31, 32, and 40), as described elsewhere.14 Similarly, changes induced by the combination of FS exposure (from PD65–74) and RS (PD65, PD66 and PD74) in adulthood were also evaluated. Briefly, rats were exposed to one session of FS per day for 10 consecutive days. In each session, animals were placed in a Plexiglas chamber fitted with a grid floor comprised of 0.48 cm stainless steel rods spaced 1.6 cm apart and housed within a soundproof box (Med Associates Inc.). Twenty-five scrambled FS (1.0 mA, 2 s) were delivered every 60±20 s using Med-PC IV software (Med Associates Inc.). On the first, second, and last day, immediately after the FS exposure, rats were submitted to RS by placing each rat in a Plexiglas cylindrical size-adjusted restraint tube. Cylinders measured 14.0 × 3.9 cm for rats at PD31–32, 20.3 × 5.1 cm for rats at PD40, and 23.0 × 6.3 cm for adult rats (length × diameter), ventilated by holes (1 cm diameter). Each RS session lasted 1 h, and immediately after the end of the RS rats were returned to their home cages. Naïve animals were left undisturbed in their home cages.
Locomotor response to amphetamine
Between 1 and 2 or 5 and 6 weeks after the stress, rats were tested in an open-field chamber (Coulbourn Instruments) in which locomotor activity was determined by beam breaks and recorded with TruScan software (Coulbourn Instruments). Basal locomotor activity was recorded for 30 min. After that, rats were injected with saline (1 mL/kg) or D-amphetamine sulfate (0.75 mg/kg, i.p.; Sigma) and their locomotor activity was recorded for another 90 min.
Extracellular recordings of DA neurons in the VTA and in the Substantia Nigra (SN).
VTA and SN recordings were carried out between 1 and 2 or 5 and 6 weeks post-stress. Briefly, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma) and mounted on a stereotaxic frame (Kopf). The stereotaxic coordinates for the VTA were 5.0 mm posterior from bregma, 0.5 mm lateral to the midline, and 6.0–8.5 mm ventral from the brain surface in animals between PD47–54 (between 1 and 2 weeks after adolescent stress), and 5.3 mm posterior from bregma, 0.6 mm lateral to the midline, and 6.5–9.0 mm ventral from the brain surface in adult animals (between 5 and 6 weeks after adolescent stress and between 1 and 2 or 5 and 6 weeks after adult stress). The stereotaxic coordinates for the SN were 5.4 mm posterior from bregma, 1.8 mm lateral to the midline, and 6.0–8.5 mm ventral from the brain surface in animals between PD47–54, and 5.5 mm posterior from bregma, 2.2 mm lateral to the midline, and 6.5–9.0 mm ventral from the brain surface in adult animals. Electrodes were lowered through six to nine vertical tracks, separated by 0.2 mm, in a predetermined pattern within either the VTA or the SN of each rat. DA neurons were identified according to well-established electrophysiological features.15, 16 The activity of each identified DA neuron was recorded for 1–3 min. Three parameters of activity were measured: (1) population activity, i.e., the number of spontaneously active DA neurons per electrode track; (2) average firing rate; and (3) the percentage of action potentials occurring in bursts. At the end of recordings, the recording sites were marked via electrophoretic ejection of Chicago Sky Blue dye from the tip of the electrode (20 μA constant negative current, 20–30 min). See Supplementary figure 1 for representative traces of recordings from DA neurons in the VTA and SN.
Extracellular recordings of pyramidal neurons in the vHipp
Under chloral hydrate anesthesia, the vHipp was sampled in a grid-like fashion via 6–9 vertical tracks with glass microelectrodes separated by 0.2 mm to encompass CA3, CA1 dentate, and vSub subregions. The stereotaxic coordinates for the vHipp were 5.3 mm posterior from bregma, 4.4 mm lateral to the midline, and 5.5–8.0 mm ventral from the brain surface in animals between PD47–54 (between 1 and 2 weeks after adolescent stress), and 5.7 mm posterior from bregma, 4.6 mm lateral to the midline, and 6.0–8.5 mm ventral from the brain surface in adult animals (5–6 weeks after adolescent stress and 1–2 or 5–6 weeks after adult stress). Recordings were made from spontaneous single pyramidal neurons identified by distinct electrophysiological characteristics for 1–3 min.17 Firing rate and bursting activity were measured. See Supplementary figure 1 representative traces of recordings from pyramidal neurons in the vHipp.
After the electrophysiological recordings, rats were euthanized by an overdose of chloral hydrate; the brains were removed, fixed for at least 24 h in 8% paraformaldehyde, cryoprotected in 25% sucrose, and sectioned for histological confirmation of the electrode sites
Immunohistochemical measures of PV and PNN-positive neurons
All immunohistochemical procedures were conducted following previously reported protocols with minor modifications.18, 19 In brief, rats were deeply anesthetized with Fatal-Plus (0.3–0.5 mL, i.p.; Vortech Pharmaceuticals), and transcardially perfused with saline followed by 4% paraformaldehyde (PFA) in phosphate buffered saline (0.1M PBS). The brains were then removed and fixed in 4% PFA for an additional 2 h, and stored in 25% sucrose. Serial 50 μm-thick coronal sections of the ventral hippocampus were collected using a cryostat (CryoStar NX50, Thermo Scientific). For each animal, five to six sections 300 μm apart spanning the rostrocaudal axis of the ventral hippocampus (within −4.8 mm to −6.3 mm from bregma) were collected and stained. Specifically, sections were incubated in a combination of 1% normal goat serum, 0. 3% Triton X-100, mouse anti-PV antibody (1:500, Sigma, catalog # P3088), and biotinylated Wisteria floribunda agglutinin (WFA; 1:2000 dilution, Vector Labs, catalog # B1355) for 48 h at 4 °C. The sections were then incubated with a mixture of 1% normal goat serum, goat anti-mouse Alexa Fluor 594 (1:500, Abcam, catalog # ab150116), and Alexa Fluor 488 conjugated to streptavidin (1:500, Life Science Technology, catalog # S32354). The sections were also processed with DAPI (1:4000, Thermo Scientifc, catalog # 62247) to visualize the border of the subiculum. PNNs were identified by staining for Wisteria floribunda agglutinin (WFA), a lectin that labels selectively residues of glycoproteins within the PNNs. While this does not show the PNN structure explicitly, nor are PNNs located exclusively around PV neurons,20 counterstaining for PV enable us to tell if the structure is indeed a perisomatic PNN encompassing PV interneurons.
For imaging, focus was set on PV-positive neurons and digital images were obtained using SimplePCI6 software (Hamamatsu Corporation). Under 10x magnification, the subiculum regions of each rostrocaudal section were imaged by three sequential 909×692 μm-images along the medial-temporal axis, using an OlympusBX51 microscope with a Hamamatsu Orca-ER camera. The border between CA1 and ventral subiculum was defined by an abrupt widening of the pyramidal layer, and border between subiculum and presubiculum was defined by a sharp reduction in PV intensity and decrease in cell size visualized by DAPI. For cell count, only the pyramidal cell layers, where most PV interneurons were located, were counted. The exposure time for PV was calibrated such that the majority of the PV-positive cells in the PD31 group were visible and within the dynamic range, and all subsequent images of the remaining age groups were taken at an identical exposure. Similar techniques were applied to PNN and DAPI to identify optimal exposure time. For each acquired images, the exposure was set based on PV-positive cells so that the majority of the PV-positive cells were in focus. The cell count was then performed using ImageJ by two individuals blind to the experimental conditions. To facilitate the identification of cells, images were magnified to 150%, and all PV- or PNN-positive cells in focus that are contained within the subicular boundaries were counted regardless of morphology or fluorescent intensity.
VPA/SAHA treatment
Sodium valproate (VPA; 300 mg/kg, i.p. in saline; MP Biomedicals) was administered to adult (PD60) male rats for 15 days, with FS+RS administered for 10 overlapping days at PD65–74. At 300 mg/kg, VPA was found to reopen the critical period of plasticity in the visual cortex and to act as anticonvulsant and mood stabilizer in animal models.13, 21, 22
In order to limit any impact of VPA-mediated mood stabilization on stress, FS+RS was delivered before 10 AM, with VPA administered after 5 PM on the same day. Also, VPA induces effects via modulation of GABA neurotransmission.23 Since we propose that VPA acts via HDAC inhibition to reopen a sensitive period of plasticity, a similar treatment protocol was applied to a different pan-HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA; 25 mg/kg, i.p. in 10% DMSO; Cayman Chemical)12, to control for the acute action of VPA on GABA neurotransmission.
Statistical analysis
Data are presented as mean ± SEM. We used t tests for two-sample comparisons and one-way or two-way ANOVA followed by Bonferroni’s post-hoc test for more than two groups. Results of statistical tests with p < 0.05 were considered significant. See supplementary information for more details.
Results
Early adolescent and adult stress induce opposite changes in VTA DA system activity
We showed previously that the exposure of rats to either 3 restraint stress (RS) sessions or daily footshock (FS) given at PD31–40 leads to a heightened anxiety state in adult rats, but only the combined stressors leads to a hyperdopaminergic state in terms of increased VTA DA neuron population activity and hyperlocomotor response to amphetamine, with the electrophysiological recordings of VTA DA neurons taking place more than 5 weeks after the end of stress.14 However, the exposure of adult rats (PD65–74) to the same combination of stressors did not change the VTA DA system activity measured in the same time frame.14 These findings underscore that adolescence is a period of particular susceptibility to long-lasting changes induced by stress. However, it is well-known that stress impacts individuals at all ages potentially creating age-dependent pathologies. In fact, the exposure of adult rats to chronic mild stress, an animal model for the study of depression, resulted in a decrease in VTA DA neuron population activity that is present between 1–2 weeks after the stress.24 Based on these findings, we decided to evaluate the impact of the combination of FS and RS during early adolescence (PD31–40) or adulthood (PD65–74) on DA system activity, recording VTA DA neurons between 1 and 2 or 5 and 6 weeks after the stress to examine the immediate actions and persistent effects of the combined stressors (Figure 1).
Figure 1 -. Impact of stress exposure during early adolescence or adulthood on DA system activity.
(a) Adolescent male rats were submitted to a combination of daily footshock (FS; through PD31–40) plus 3 restraint stress sessions (RS; PD31, 32, and 40). Extracellular recordings of VTA DA neurons and locomotor activity after saline or amphetamine (0.75 mg/kg) administration were evaluated between 1 and 2 weeks post-adolescent stress (through PD47–54). (b) Adolescent stress increased the number of spontaneously active VTA DA neurons with the recordings 1–2 weeks post-stress (naïve group: n = 8 rats, 0.87 ± 0.19 active DA neurons/track; stress group: n = 7 rats, 1.59 ± 0.15 active DA neurons/track; t13 = 2.92, p = 0.012), (c) but with no change in the firing rate (t115 = 0.79, p > 0.05) and (d) in the burst activity (t115 = 0.82, p > 0.05) of the identified spontaneously active DA neurons in the VTA (naïve group: n = 51 active DA neurons; stress group: n = 66 active DA neurons). (e) Consistent with the increased VTA DA neuron population, an increased locomotor response to amphetamine administration was also observed 1–2 weeks post-adolescent stress (n = 10 rats/group; t18 = 2.41, p = 0.03). (f) Long-term effects on DA system activity were also investigated 5 and 6 weeks post-adolescent stress (through PD75–82). (g) An increased number of spontaneously active VTA DA neurons was still present 5–6 weeks post-adolescent stress (naïve group: n = 6 rats, 0.98 ± 0.11 active DA neurons/track; stress group: n = 6 rats, 1.58 ± 0.07 active DA neurons/track; t10 = 4.63, p = 0.0009), (h) but with no change in the firing rate (t94 = 0.07, p > 0.05) or (i) in the burst activity (t94 = 1.10, p > 0.05) of active VTA DA neurons (naïve group: n = 39 active DA neurons; stress group: n = 57 active DA neurons). (j) In addition, an increased locomotor response to amphetamine administration was also observed 5–6 weeks post-adolescent stress (n = 10 rats/group; t18 = 2.42, p = 0.03). (k) Similar to the adolescent stress, adult male rats were submitted to a combination of daily footshock (FS; through PD65–74) plus 3 restraint stress sessions (RS; PD65, 66, and 74). Extracellular recordings of VTA DA neurons and locomotor activity after saline or amphetamine (0.75 mg/kg) administration were evaluated between 1 and 2 weeks post-adult stress (through PD81–88). (l) Contrary to the adolescent stress, adult stress decreased the number of spontaneously active VTA DA neurons 1–2 weeks post-stress (naïve group: n = 8 rats, 1.02 ± 0.07 active DA neurons/track; stress group: n = 10 rats, 0.69 ± 0.06 active DA neurons/track; t13 = 3.49, p = 0.003) (m) but with no change in the firing rate (t116 = 1.09, p > 0.05) and (n) burst activity (t116 = 0.12, p > 0.05) of the identified spontaneously active DA neurons in the VTA (naïve group: n = 61 active DA neurons; stress group: n = 57 active DA neurons), and (o) a previous exposure to adult stress did not exacerbate the locomotor response to amphetamine (n = 8–10 rats/group; t16 = 1.08, p > 0.05). (p) The impact of the adult stress on DA system activity was also investigated 5 and 6 weeks post-adult stress (through PD109–116). (q) No difference was found for the number of spontaneously active VTA DA neurons between naïve and stressed rat 5–6 weeks post-adult stress (naïve group: n = 7 rats, 1.12 ± 0.06 active DA neurons/track; stress group: n = 7 rats, 1.18 ± 0.09 active DA neurons/track; t12 = 0.01, p > 0.05), indicting a recovery of the hypodopaminergic state observed 1–2 weeks after the adult stress. Also, (r) no change in the firing rate (t104 = 0.08, p > 0.05) or (s) in the burst activity (t104 = 0.42, p > 0.05) of active VTA DA neurons (naïve group: n = 52 active DA neurons; stress group: n = 54 active DA neurons) was observed. Finally, (t) no change was observed in the locomotor response to amphetamine (n = 10 rats/group; t18 = 1.38, p > 0.05). Data are presented as mean ± SEM. *p < 0.05. Data are presented as mean ± SEM. *p < 0.05.
Confirming our previous findings,14 early adolescent stress increased VTA DA neuron population activity between 5 and 6 weeks after the stress(naïve: n = 6 rats/39 active DA neurons, 0.98 ± 0.11 DA neurons/track; stress: n = 6 rats/57 active DA neurons, 1.58 ± 0.07 DA neurons/track). A similar change was also found between 1 and 2 weeks after early adolescent stress, when animals were still adolescent (PD47 to PD54; naïve: n = 8 rats/51 active DA neurons, 0.87 ± 0.19 DA neurons/track; stress group: n = 7 rats/66 active DA neurons, 1.59 ± 0.15 DA neurons/track). No change in the average firing rate and burst activity of VTA DA neurons induced by early adolescent stress was found, but this is likely due to a shift in the population of neurons (i.e., activation of previously nonfiring neurons at slow rates balancing out increases in already firing neurons).25 In addition, the increased VTA DA neuron population activity induced by the early adolescent stress was accompanied by an increased locomotor response to amphetamine (n= 10 rats/group; Figure 1 and Supplementary figure 2). This hyperdopaminergic state is consistent with that observed in animal models for the study of schizophrenia.26
For the adult stress, the combination of stressors did not change VTA DA neuron population activity when recordings were performed between 5 and 6 weeks after the stress (naïve: n = 7 rats/52 active DA neurons, 1.12 ± 0.06 DA neurons/track; stress: n = 7 rats/54 active DA neurons, 1.18 ± 0.09 DA neurons/track). However, a decreased VTA DA neuron population activity was observed when the recordings were done between 1 and 2 weeks post-stress (naïve: n = 8 rats/61 active DA neurons, 1.02 ± 0.07 active DA neurons/track; stress: n = 10 rats/57 active DA neurons, 0.69 ± 0.06 DA neurons/track), which is consistent with one factor observed consistently in three animal models of depression.24, 27–29 In addition, the adult stress did not change the locomotor response to amphetamine (n =8–10 rats/group; Figure 1 and Supplementary figure 2). Furthermore, these changes were confined to the VTA, as recordings from SN DA neurons did not reveal a change in activity (Supplementary figure 3).
Greater PV interneuron susceptibility during critical periods may drive DA system hyperactivity induced by early adolescent stress
An increased activity of the vHipp is proposed to underlie the DA system overdrive in schizophrenia patients and also in animal models of the disease.30 In fact, vHipp inactivation reversed the hyperdopaminergic state found in a neurodevelopmental disruption model of schizophrenia.17 This abnormal hippocampal activity is thought to be driven by a functional loss of PV-expressing GABAergic interneurons.11 Importantly, the maturation of PV interneurons determines the onset and the duration of the critical or sensitive period of plasticity,6 which are developmental epochs with not only maximal neuroplasticity,31 but also heightened vulnerability to stress. Other mechanisms also determine the closure of the critical period which serves to restrict the influence of sensory experiences on plasticity. For example, molecular brakes such as the perineuronal nets (PNNs), which are formed mainly around PV interneurons,20 will also emerge during development to close the critical period.32
PNNs lock plasticity in place and act like a “shield” protecting PV interneurons from metabolic and oxidative damage.9 However, the formation of PNNs is not complete until early adulthood.20 Studies show that PV interneurons are more susceptible to damage in periods with immature PNNs.7 To understand the longitudinal impact of early adolescent stress, we analyzed the developmental trajectories of the expression of PV and PNNs in the ventral subiculum of the vHipp post-stress.
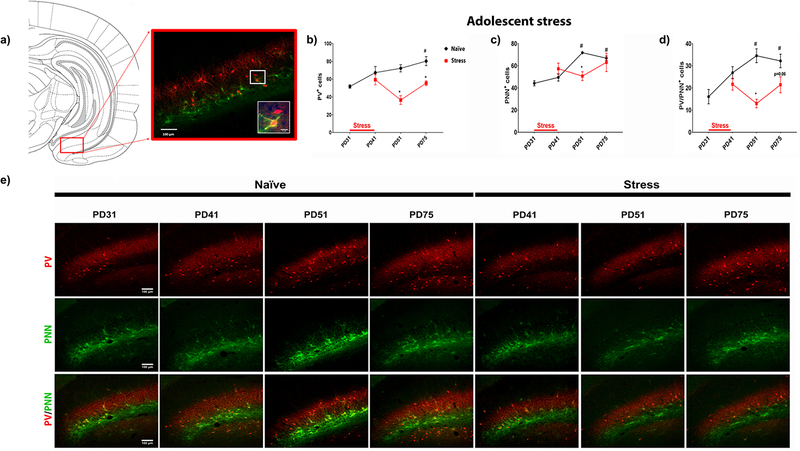
The expression of PV and PNNs was evaluated in rats at PD31 (correspondent to the first day of the adolescent stress), PD41 (1 day after terminating the stress), PD51 (between 1 and 2 weeks after the stress), and PD75 (5 weeks after the stress; n= 4–6 rats/group; Figure 2). In naïve rats, there is an increase in the number of cells stained for PV across periadolescent development, confirming the previous finding that the number of PV interneurons undergo dramatic increase during this period in the vHipp18. In addition, an increase in the number of cells surrounded by PNNs was also found across development, including the number of PV-positive cells surrounded by PNNs (Figure 2). The combined stressors during adolescence decreased the number of PV-positive cells at PD51 and 75 and also impacted the number of cells surrounded by PNNs at PD51, including the PV-positive cells surrounded by PNNs (Figure 2). However, unlike the impact of early adolescent stress on the number of PV-positive cells in late adolescence, there was a recovery at PD75 in the number of cells surrounded by PNNs, including the PV-positive cells surrounded by PNNs (Figure 2). This recovery is consistent with evidence suggesting that PNNs are completely re-formed 3 weeks after brain infusion of chondroitinase ABC, an enzyme that rapidly digests PNNs within hours.33 PV surrounded by PNNs at P75 after early adolescent stress, however, are still low (a trend p-value = 0.06). Furthermore, stress decreases the total number of cells surrounded by PNNs at PD51, but reach normal level at P75, suggesting that PNNs may increase also around cells other than PV neurons (Supplementary figure 4).
Figure 2 – Impact of early adolescent stress on the maturational trajectory of putative parvalbumin interneurons in the vHipp.
(a) The cell count on putative PV interneurons and related maturational markers, PNNs, was performed in the ventral subiculum (vSub) of vHipp at different developmental time-points to assess the effect of stress on the maturational trajectory of the region. Specifically, the vSub was sampled at PD31 (first day of the stress paradigm), PD41 (one day after the stress paradigm), PD51 (1.5 weeks post-stress) and PD75 (adulthood; 5 weeks post-stress) (n = 4–6 rats/group). Large representative image capturing part of the vSub (10x); inset image showing two putative PV-positive interneurons - the top cell is not wrapped by the PNNs, whereas the bottom cell is clearly wrapped by PNNs, especially around somal region and proximal dendrites (40x). (b) For PV+ cells, one-way ANOVA revealed significant developmental change in naïve rats (F3,12 = 6.178; p = 0.0088), with adult rats (PD75) showing increased number of PV+ cells comparing to the early-adolescent rats at PD31 (Bonferroni post-hoc test, p < 0.05). Multiple t-test revealed that adolescent stress decreased the PV+ cell count at PD51 (t6 = 5.392; p = 0.0014) and PD75 (t6 = 4.286; p = 0.0052). (c) The total number of cells wrapped by PNNs was also under developmental regulation, as an 1-way ANOVA revealed significant effect of time-points on PNN+ cell count (F3,12 = 34.6; p < 0.0001). Bonferroni post-hoc analysis revealed significant increase in PNN+ cell count at PD51 (i.e. late adolescence, p < 0.05) and PD75 (i.e. adulthood, p < 0.05), comparing to PD31 (i.e. early adolescence). Stress-induced changes were only detected at PD51 (t6 = 5.391; p = 0.002), but not at PD75 (t6 = 0.44; p = 0.6940), suggesting a potential recovery during the late-adolescence to adulthood. (d) To assess the maturational trajectory of the putative PV interneurons, cell counting of PV and PNN co-labelled neurons was performed. Again, a significant developmental change was detected in naïve rats (F3,12 = 7.295; p = 0.0048), with PD51 and PD75 rats showing increased number PV+/PNN+ cells (p < 0.05 vs. PD31). Adolescent stress resulted in a significant reduction of PV/PNN co-labelled cell count at PD51 (t6 = 5.877; p = 0.0011), and a trend of reduction at PD75 (t6 = 2.27; p = 0.0637), suggesting a delayed maturation profile of the putative PV interneurons of the region. (e) Representative figures illustrating the expression of PV and PNN in the vSub of naïve and stressed rats at different time-points. Data are presented as mean ± SEM. #p < 0.05, after 1-way ANOVA and indicating developmental cell counting changes; *p < 0.05, after multiple t test and indicating stress-induced changes.
Given that early adolescent stress decreased the number of PV-positive cells which could result in changes the inhibitory-excitatory balance, we recorded the activity of pyramidal neurons in the vHipp between 1 and 2 or 5 and 6 weeks after early adolescent stress. An increase in the firing rate of pyramidal neurons in the vHipp was observed when the electrophysiological recordings were performed between 1 and 2 weeks (naïve: n = 37 neurons from 10 rats, 0.62 ± 0.06 active neurons/track; stress: n = 36 neurons from 9 rats, 0.67 ± 0.06 active neurons/track) as well as between 5 and 6 weeks after the early adolescent stress (naïve: n = 26 neurons from 7 rats, 0.62 ± 0.09 active neurons/track; stress group: n = 48 neurons from 10 rats, 0.80 ± 0.08 active neurons/track; Figure 3). Together these findings suggest that early adolescent stress induces a PV neuron loss in the vHipp which may lead to an increase in the activity of pyramidal neurons in this brain region. This hippocampal hyperactivity is reported to drive DA system hyperactivity17 analogous to what has been proposed in schizophrenia.11
Figure 3 – Impact of early adolescent or adult stress on the activity of pyramidal neurons in the vHipp.
(a) Adolescent stress (through PD47–54) increased the firing rate of pyramidal neurons in the vHipp 1–2 weeks (naïve group: n = 37 neurons from 10 rats, 0.62 ± 0.06 active neurons/track; stress group: n = 36 neurons from 9 rats, 0.67 ± 0.06 active neurons/track) and 5–6 weeks after the stress (naïve group: n = 26 neurons from 7 rats, 0.62 ± 0.09 active neurons/track; stress group: n = 48 neurons from 10 rats, 0.80 ± 0.08 active neurons/track). A 2-way ANOVA showed a significant effect only for condition (naïve vs. stress; F1,143 = 21.34, p < 0.0001), with no effect for age of recordings (1–2 weeks vs. 5–6 weeks post-adolescent stress, F1,143 = 0.81, p > 0.05) or interaction (F1,143 = 0.13, p > 0.05). Further analysis showed that adolescent stress increased the firing rate of pyramidal neurons in the vHipp 1–2 and 5–6 weeks post-stress compared to the respective naïve rats (Bonferroni post-hoc test, p < 0.05). (b) On the other hand, adult stress (through PD65–74) did not change the firing rate of pyramidal neurons in the vHipp with the recordings 1–2 weeks (naïve group: n = 31 neurons from 6 rats, 0.86 ± 0.13 active neurons/track; stress group: n = 29 neurons from 6 rats, 0.81 ± 0.13 active neurons/track) or 5–6 weeks post-stress (naïve group: n = 27 neurons from 6 rats, 0.75 ± 0.15 active neurons/track; stress group: n = 26 neurons from 6 rats, 0.72 ± 0.12 active neurons/track). Data are presented as mean ± SEM. *p < 0.05.
We also evaluated the expression of PV and PNNs in adult animals exposed to the combined stress at PD81 and PD109 (i.e., 1 and 5 weeks post-adult stress, respectively). In contrast to the early adolescent stress, the adult stress did not change the number of PV-positive cells or cells surrounded by PNNs in the vHipp, including the PV-positive cells surrounded by PNNs (Figure 5). Additionally, no change was found in the firing rate of pyramidal neurons in the vHipp between 1 and 2 weeks (naïve: n = 31 neurons from 6 rats, 0.86 ± 0.13 active neurons/track; stress: n = 29 neurons from 6 rats, 0.81 ± 0.13 active neurons/track) or 5 and 6 weeks after the adult stress (naïve: n = 27 neurons from 6 rats, 0.75 ± 0.15 active neurons/track; stress: n = 26 neurons from 6 rats, 0.72 ± 0.12 active neurons/track; Figure 3). Thus, given that PV interneurons seem to be protected by the PNNs at adulthood,9 we believe the decreased VTA DA system activity induced by the adult stress could involve changes in other brain regions. For example, a hypodopaminergic state induced by the chronic mild stress in rats was associated with an overactivity of the basolateral amygdala-ventral pallidum system.24
Figure 5 – Impact of VPA treatment (300 mg/kg; i.p.; PD60–74) combined with adult stress (PD65–74) on histological changes in the vHipp.
Cell counting was performed on PV interneurons to assess the effect of critical period reopening on stress vulnerability. Specifically, the ventral subiculum (vSub) was sampled at 1–2 weeks and 5–6 weeks post-stress (n = 4–6 rats/group). At each time-point, a two-way ANOVA (treatment and condition as main factors) was performed on PV+, PNN+, and PV/PNN+ cells respectively, to evaluate the effect of stress and VPA co-administration. (a) For PV+ cells, only a treatment effect was detected (F3,16 = 8.798, p = 0.0091) at 1–2 weeks post-stress. At 5–6 weeks post-stress, however, significant main effects of both condition (F1,16 = 7.674, p = 0.014) and treatment (F1,16 = 12.09; p = 0.0031), as well as a significant interaction (F1,16 = 7.684, p = 0.014), were detected. Post-hoc analysis indicated a decrease in the PV+ cell count only in stress+VPA group (Bonferroni post-hoc test, p < 0.05 stress+VPA vs. all other groups), analogous to the long-term stress response in adolescent animals. (b) Adult PNNs seemed to be stable to stress and VPA treatment, as no main effect nor interaction were detected at either 1–2 weeks or 5–6 weeks post-stress. (c) In terms of PV/PNN+ cell count, which are markers of putative mature PV interneurons, treatment effect was detected at both 1–2 weeks (F1,16 = 5.132, p = 0.0377) and 5–6 weeks (F1,16 = 6.823, p = 0.0189), but condition effect was only detectable at 5–6 weeks post-stress (F1,16 = 5.998, p = 0.0262). Moreover, a significant interaction was detected only at 5–6 weeks post-stress (F1,16 = 5.94, p = 0.0268), when the stress-VPA group displayed fewer PV+/PNN+ cells (Bonferroni post-hoc test, p < 0.05, vs. all other groups), suggestive of a relatively immature status of the PV neurons. Taken together, the data suggests that adult animals treated with VPA regained adolescent-like vulnerability to stress, evident by reduced number of PV+ and PV/PNN+ cell count at 5–6 weeks post-stress. The effect of stress did not manifest in VPA-treated rats until 5–6 weeks post-stress, a phenomenon potentially attributable to a short-term effect of VPA-treatment alone on the expression of PV. (d) Representative figures illustrating the impact of adult stress and VPA treatment on the expression of PV and PNN in the vSub. Data are presented as mean ± SEM. #p < 0.05 after 2-way ANOVA, indicating a treatment effect; *p < 0.05 after 2-way ANOVA followed by a Bonferroni post-hoc test, indicating significant changes in VPA-treated stressed rats vs. controls.
Valproate treatment in the adult recreates stress susceptibility of adolescence
We posit that unregulated stress responses occurring during the critical/sensitive periods of development leads to PV loss and the emergence of circuit deficits consistent with schizophrenia in the adult. If accurate, one would predict that reopening the critical period in the adult could render it susceptible to a similar stress-induced circuit disruption. Thus, we investigated the impact of the critical period on stress susceptibility by examining whether adults with reopened critical periods regain stress-induced PV loss and DA system hyperresponsivity.
Several genetic and pharmacological manipulations have been suggested to allow the reopening of the critical period in the adult brain.32 Among these is valproate (VPA). VPA is a mood stabilizer/anticonvulsant that has been shown to re-establish the critical period of plasticity in several brain regions in the adult.34–36 We tested if combining VPA (300 mg/kg; i.p.) with adult stress would recreate an adolescent phenotype of stress susceptibility. VPA was administered to adult (PD60) rats for 15 days, with FS+RS administered for 10 overlapping days at PD65–74 (Figure 4). VPA treatment alone did not impact the VTA DA system, but instead substantially altered the impact of co-administered combined stressors. Thus, in normal, untreated adult rats 10 days of FS+RS leads to decreased VTA DA population activity that is present for 1–2 weeks after treatment. However, if rats are treated with VPA, the net effect of co-administered stressors is a persistent increase in VTA DA neuron population activity 1–2 weeks post-stress (naïve+saline: n = 8 rats/52 active DA neurons, 0.96 ± 0.04 DA neurons/track; stress+saline: n = 9 rats/32 active DA neurons, 0.60 ± 0.05 DA neurons/track; naïve+VPA: n = 10 rats/67 active DA neurons, 0.95 ± 0.11 DA neurons/track; stress+VPA: n = 11 rats/110 active DA neurons, 1.46 ± 0.08 DA neurons/track) and 5–6 weeks post-stress (naïve+saline: n = 8 rats/47 active DA neurons, 0.95 ± 0.09 DA neurons/track; stress+VPA: n = 8 rats/73 active DA neurons, 1.36 ± 0.06 DA neurons/track; t14 = 3.59, p = 0.003), analogous to that observed with early adolescent stress. No change in firing rate and burst activity of VTA DA neurons was found (Supplementary figure 5). The combination of VPA and adult stress also increased the locomotor response to amphetamine 1–2 and 5–6 weeks post-stress (n= 8 rats/group; Figure 4 and Supplementary figure 6). In addition, the combination of VPA treatment and adult stress also increased the activity of pyramidal neurons in the vHipp 1–2 post-stress (naïve+saline: n = 33 neurons from 7 rats, 0.79 ± 0.14 active neurons/track; naïve+VPA: n = 40 neurons from 8 rats, 0.83 ± 0.11 active neurons/track; stress+VPA: n = 33 neurons from 7 rats, 0.79 ± 0.12 active neurons/track) and 5–6 post-stress (naïve+saline: n = 30 neurons from 9 rats, 0.59 ± 0.09 active neurons/track; stress+VPA: n = 30 neurons from 9 rats, 0.57 ± 0.09 active neurons/track), with no change induced by the VPA treatment alone (Figure 4). Also, as previously described the adult stress alone did not change the activity of pyramidal neurons in the vHipp (Figure 3).
Figure 4 – VPA treatment in the adult causes the rat to regain stress-induced DA system hyperresponsivity and increased activity of pyramidal (Pyr) neurons in the vHipp.
(a) Adult rats were treated with VPA (300 mg/kg, i.p.) for 15 days (PD60–74) and exposed to the combined stressor (daily FS through PD65–44 and 3 RS sessions at PD65, 66, and 74). Single-cell extracellular recordings of VTA DA neurons and locomotor response to acute amphetamine (0.75 mg/kg, i.p.) were evaluated 1–2 weeks post-stress. (b) VPA treatment alone did not impact the VTA DA system when tested at adulthood (PD81–88); however, it did substantially alter the impact of co-administered FS+RS (naïve+saline: n = 8 rats, 0.96 ± 0.04 active DA neurons/track; stress+saline: n = 9 rats, 0.60 ± 0.05 active DA neurons/track; naïve+VPA: n = 10 rats, 0.95 ± 0.11 active DA neurons/track; stress+VPA: n = 11 rats, 1.46 ± 0.08 active DA neurons/track). A 2-way ANOVA showed significant effect for treatment (saline vs. VPA; F1,34 = 27.49, p < 0.0001) and interaction between treatment and condition (naïve vs. stress; F1,34 = 28.96, p < 0.0001), with no effect for condition (F1,34 = 0.92, p > 0.05). Post-hoc analysis indicated that stress exposure in adult rats led to decreased VTA DA population activity that was present for 1–2 weeks after treatment (Bonferroni post-hoc test, p < 0.05 stress+saline vs. naïve+saline). However, if rats were treated with VPA, the net effect of co-administered stressors was an increase in VTA DA neuron population activity (Bonferroni post-hoc test, p < 0.05 stress+VPA vs. all groups). (c) An increased locomotor response to amphetamine was also observed in VPA-treated rats exposed to the combined stressors (n = 8 rats/group). A 2-way ANOVA showed significant effect for treatment (saline vs. VPA; F1,28 = 9.22, p = 0.005) and for condition (naïve vs. stress; F1,28 = 15.11, p = 0.0006), with no interaction (F1,28 = 2.21, p > 0.05). Post-hoc analysis indicated an increased responsivity to amphetamine in VPA-treated stressed rats (Bonferroni post-hoc test, p < 0.05 stress+VPA vs. all groups). (d) We previously found that adult stress did not change the activity of pyramidal neurons in the vHipp. However, VPA treatment changed the response to stress, but did not induce any effect by itself. VPA combined with adult stress increased the firing rate of pyramidal neurons in the vHipp 1–2 weeks post-stress (naïve+saline: n = 33 neurons from 7 rats, 0.79 ± 0.14 active neurons/track; naïve+VPA: n = 40 neurons from 8 rats, 0.83 ± 0.11 active neurons/track; stress+VPA: n = 33 neurons from 7 rats, 0.79 ± 0.12 active neurons/track; F2,103 = 3.45, p = 0.035, 1-way ANOVA followed by Bonferroni post-hoc test, p < 0.05 stress+VPA vs. all other groups). Then, we tested (e) if changes induced by VPA co-administered with FS+RS on the DA system persisted over time (5–6 weeks). (f) An increase in VTA DA neuron population activity was also present in VPA-treated rats 5–6 weeks after the adult stress (naïve+saline: n = 8 rats, 0.95 ± 0.09 active DA neurons/track; stress+VPA: n = 8 rats, 1.36 ± 0.06 active DA neurons/track; t14 = 3.59, p = 0.003). (g) In addition, VPA-treated rats also presented an increased locomotor response after amphetamine administration 5–6 weeks post-adult stress (n = 8 rats/group; t14 = 2.82, p = 0.014). (h) The increased firing rate of pyramidal neurons in the vHipp in VPA-treated animal exposed to adult stress was also present 5–6 weeks post-stress (naïve+saline: n = 30 neurons from 9 rats, 0.59 ± 0.09 active neurons/track; stress+VPA: n = 30 neurons from 9 rats, 0.57 ± 0.09 active neurons/track; t58 = 2.71, p = 0.0088). These changes observed after VPA treatment co-administered with FS+RS at adulthood are analogous to those induced by the adolescent stress. Data are presented as mean ± SEM. *p < 0.05.
We then tested whether reopening the critical period in the adult impacted PV and PNN expression in the vHipp (n = 4–6 rats/group). No effect of the adult stress or an interaction between stress and the VPA treatment was found. But, surprisingly, there was a treatment effect, suggesting that VPA is decreasing the number of cells stained for PV in the vHipp 1 week after the stress, with no change in the number of cells surrounded by PNNs. There was also a treatment effect for the number of PV-positive cells surrounded by PNNs. These effects of VPA alone could potentially mask the electrophysiological changes in the VTA DA system induced by VPA combined with the adult stress. Interestingly, when we evaluated the expression of these markers 5 weeks post-stress, a decreased number of PV-positive cells was observed only in VPA-treated animals exposed to the combined stressors, with no change in the number of cells surrounded by PNNs. There was also a reduction in the number of neurons double stained for PV and PNNs (Figure 5). Together, these findings indicate that VPA, possibly by reopening the critical period in the adult, restores susceptibility to stress-induced pathology resembling schizophrenia. Furthermore, a greater number of non-PV cells surrounded by PNNs was found 5–6 weeks post-stress in VPA-treated rats (Supplementary figure 7).
How VPA is achieving these effects is not completely known, since this compound has several putative mechanisms of action. However, it is suggested that VPA reopens the critical period of plasticity in sensory systems possibly through histone deacetylase (HDAC) inhibition.36 In support to this hypothesis, suberoylanilide hydroxamic acid (SAHA, 25 mg/kg), a more selective HDAC inhibitor, combined with adult stress also increased VTA DA neuron population activity 5–6 weeks post-stress, with no changes in firing rate and burst activity (Supplementary figure 8). Also, SAHA by itself did not induce any effect. Together, these findings indicate that HDAC inhibition by itself did not induce short- or long-term effects on the VTA DA system.
Discussion
Stress plays a major role in susceptibility to mental disorders in general, including schizophrenia and depression. In fact, the emergence of schizophrenia, which typically manifests during late adolescence and early adulthood, is often associated with stressful events (i.e., trauma, ethnic minority status, and social disadvantage) and adolescents that are at high risk for schizophrenia experience abnormally high reactivity to stress and are more likely to develop schizophrenia if they have decreased tolerance to stress.1, 37, 38 For depression, the prevalence of the disease has been directly associated with the number of stressful events occurring before the diagnosis.39 In contrast to schizophrenia, the onset of depression is more common at adulthood.6 Since these conditions share several genetic markers and socio-environmental risk factors, such as stressful events, in common, we evaluated if timing of stress would be a critical determinant of the pathology that is present in the adult.
We observed a hyperdopaminergic state in animals exposed to early adolescent stress, indicated by an increased VTA DA neuron population activity and increased locomotor response to amphetamine, and hyperactivity in the vHipp, which is also the site of PV loss. The PV loss is thought to lead to vHipp hyperactivity which in turn results in a hyperdopaminergic state. This condition is highly consistent with clinical observations in schizophrenia. In fact, schizophrenia patients show abnormally high amphetamine-induced DA release in associative striatum,40 which can be behaviorally modelled in rodents by the observed increase in amphetamine-induced hyperlocomotion. Schizophrenia patients also show an increase in striatal fluorodopa uptake41 indicative of increased number of active terminals, which is consistent with the increased number of spontaneously active DA neurons observed in the present study. Furthermore, the observed vHipp pyramidal neuron firing induced by early adolescent stress is analogous to the increased glutamate transmission and metabolic activity in the anterior hippocampus observed in patients.42, 43 Taken together, the stress exposure during early adolescence appears to induce changes that recapitulate the circuit-level disruptions observed in schizophrenia patients. On the other hand, adult stress decreased VTA DA neuron population activity 1–2 weeks post-stress, which seems to be followed by a recovery since no differences were observed 5–6 weeks post-adult stress. This recovery is consistent with evidence showing that abnormal behaviors and plastic changes induced by the exposure to stress during adulthood revert to normal if sufficient time is given to the organism to recover.44 In addition, no change in the vHipp activity and in the content of PV in this brain region was induced by the exposure to the combined stressors during adulthood. The hypodopaminergic state observed after the adult stress is consistent with one measure proposed to be related to anhedonia/amotivation that has been consistently observed in three animal models of depression: cold stress, chronic mild stress, and learned helplessness.24, 27, 29
What could explain the opposite effects induced by early adolescent and adult stress on VTA DA system activity? Adolescence involves several age-related dynamic changes in physiological processes and social environment.45 Combined with genetically determined developmental alterations, these processes shape the neurobiological substrates that underlie maturation of the adolescent brain. For example, adolescence is a critical period for the refinement of GABAergic transmission, including the maturation of PV interneurons.8, 18 PV interneurons are fast-spiking interneurons and, likely due to their high firing rates that cause high metabolic load and generation of reactive oxygen species,9 they are particularly vulnerable to both environmental and oxidative stress.7 It is thought that PV interneurons are even more susceptible to damage by stressor during critical periods of brain plasticity, such as adolescence, in which the PV interneurons are not completely mature.7, 46 Furthermore, at this time the PNNs, which stabilize glutamatergic inputs onto the PV interneurons to end the plastic phase and also protects these interneurons from metabolic and oxidative damage,7 are not yet completely formed around the interneurons.20 Thus, these dynamics of brain maturation make the developing brain highly vulnerable to environmental factors, for example the deleterious effects of stress, that can lead to the development of psychiatric disorders.6 The loss of PV in the prefrontal cortex and hippocampus is one of the most robust findings in the postmortem brain of schizophrenia patients47, 48 and it has also been replicated in several genetic, environmental, and pharmacological rodent models used to study the disease.49 Importantly, an altered PV expression has not been found in other psychiatric disorders, such as depression and bipolar disorder.47
Our findings suggest that the maturational trajectory of PV interneurons in the vHipp is a marker of vulnerability to stress. While vHipp has been shown to be sensitive to stress throughout life, during adolescence the vHipp shows a particular high susceptibility to negative environments. This is largely attributable to the PV interneurons in the region and the massive excitatory inputs that they receive. The PV interneurons in the vHipp, especially in the ventral subiculum, are known to receive diverse yet potent excitatory inputs from regions such as CA1, amygdala, and thalamus.50 These excitatory inputs serve as important regulators to the overall plasticity of PV interneurons through altering excitation/inhibition balance, but also remain a possible source of pathology. In fact, dysregulated excitation can alter calcium dynamics, increase metabolic demand, and possibly generate excessive oxidative stress, leading to cell damage and even cell death.46, 51 This vulnerability persists until the end of the critical period, marked by the encasement of PNNs on the PV interneurons, protecting them from metabolic and oxidative damage.7 This is supported by the immunohistochemistry data in the current study. Indeed, in the ventral subiculum, PNN expression continues to increase until after PD51, and such change may drive the maturation of the PV interneurons in the region, as putative mature PV interneurons (i.e. neurons co-labelled by PV and PNNs) also continue to increase in number until late adolescence (i.e. PD51). The relative regional immaturity of the PV interneurons during mid-adolescence may underlie their particular vulnerability to stress.
Overlapping mechanisms underlying the regulation of critical period plasticity has been established from visual system and fear learning system, implicating common mechanisms underlying the closure of the critical period.32 The same principals have been repeatedly observed across brain regions,9 including the hippocampus.52 The molecules that are involved in limiting critical period plasticity in adulthood are collectively terms as molecular “brakes”. Importantly, lifting these “brakes” has been shown to reopen critical period plasticity in adulthood. Several studies have used chondroitinase ABC, an enzyme which breaks down the PNNs, to reopen critical period plasticity.31 Interestingly, it was found that dissolving PNNs in the vHipp through the local infusion of chondroitinase ABC increased the firing rate of pyramidal cells in the vHipp as well as a hyperdopaminergic state indicated by an increased VTA DA neuron population activity and locomotor response to amphetamine.53 Thus, we decided to use a more subtle and critical period-specific effect of histone deacetylase inhibitor, VPA, which is proposed to reopen the critical period of plasticity in the adult and which in our hands did not change the VTA DA system activity by itself, to test whether the observed age-dependent vulnerability to stress is indeed a critical period. Our results indicate that VPA-treated animals regained adolescent-like stress vulnerability, evident from the findings that stress in VPA-treated animals increased VTA DA neuron population activity, vHipp hyperactivity, and the reduction in numbers of PV-positive neurons. Curiously, exposure to VPA in naïve rats reduces significantly the number of PV+ and PV+/PNN+ stained cells in the vHipp at the 1–2 weeks post-treatment. This is similar to what was observed with the genetic deletion of HDAC2 specifically from PV interneurons.54 We believe that the decrease in PV induced by VPA is likely due to a decrease in synthesis without cell loss, since there is a recovery of PV and PNN and no correlative changes in vHipp or VTA activity, suggesting that vHipp inhibitory circuits are intact. But with stress the loss seems to be persistent, suggesting actual PV cell loss. Therefore, a decrease of PNN/PV would suggest vulnerability, not pathology.
VPA has multiple mechanisms of action, including the potentiation of GABA neurotransmission to enhance inhibitory function and enduring effects on gene transcription as a pan-HDAC inhibitor.55 HDAC inhibition is more likely to be of relevance to the observed action of critical period reopening. In fact, in primary visual cortex, enhancement of intercortical inhibition does not trigger plasticity in adults, but HDAC inhibition does.36 Also, adult prefrontal cortex encodes acoustic preferences established during early life and is rendered malleable again later by VPA by renewing prefrontal neuron recruitment in mice.35 Interestingly, it has been successfully applied to healthy adult humans learning absolute pitch discrimination.34
To give further support to the finding that the effect of VPA “recreating an adolescent phenotype” of stress vulnerability in adult animals is indeed due to HDAC inhibition, we utilized another pan-HDAC inhibitor, SAHA. SAHA has no documented action on inhibitory neurotransmission, and when used at the dose enhancing critical period of plasticity in the visual cortex.12 Similar to VPA, SAHA-treated adult rats recapitulated the hyperdopaminergic state induced by the early adolescent stress. Taken together, our findings indicate that, like critical periods of sensory systems, adolescence is also a critical period of hippocampal stress vulnerability, which may be reopened using shared mechanisms. Although some evidence indicates that reopening the critical period plasticity in the adult brain could be a therapeutic strategy to improve visual acuity in amblyopia56 and to erase fear memories.33, 57 our data suggest that it can come with a price by increasing the vulnerability of PV interneurons to the negative effects of stress.
In conclusion, we found that timing of stress is a critical determinant for the pathology that is present in the adult, potentially due to their distinct impacts on the DA system and its regulators. While early adolescent stress led to behavioral, physiological, and histological changes that recapitulate schizophrenia, adult stress induced a hypodopaminergic state observed in animal models of depression. Furthermore, HDAC inhibitors, which are proposed to reopen the critical period plasticity, restores susceptibility to stress-induced pathology resembling schizophrenia in adults. These findings can have strong implications for the etiology, pathophysiology, and underlying neural circuitry of developmentally related disorders, with a particular emphasis on schizophrenia and depression. Schizophrenia and depression are marked by important sex differences58, 59 and recent studies have demonstrated sex-specific response of PV neurons to early adolescent stress.60 Thus, further studies are required to investigate if the findings described here in males would be observed in females as well, and whether the time course of susceptibility correlates with postnatal age or pubertal stage. Finally, understanding how developmental factors can lead to pathological states, identifying timing of susceptibility to stressors, and precisely identifying the neural substrates underlying such susceptibility, can provide a roadmap to prevention, rather than treatment after the insult has been established. Our findings can help to elucidate evidence indicating that ultra-high risk individuals that do not develop schizophrenia are more vulnerable to depression as adults.61
Supplementary Material
Acknowledgments
We thank Niki MacMurdo and Christy Smolak for technical assistance and Kaetlyn Conner for the analysis of imaging experiments.
Financial support
National Institutes of Health (NIH MH57440) to AAG.
Conflict of interest
AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, and Takeda. FVG and XZ declare no conflict of interest.
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD et al. Schizophrenia. Nat Rev Dis Primers 2015; 1: 15067. [DOI] [PubMed] [Google Scholar]
- 2.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M et al. Major depressive disorder. Nat Rev Dis Primers 2016; 2: 16065. [DOI] [PubMed] [Google Scholar]
- 3.Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018; 359(6376): 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donovan MC, Owen MJ. The implications of the shared genetics of psychiatric disorders. Nat Med 2016; 22(11): 1214–1219. [DOI] [PubMed] [Google Scholar]
- 5.Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull 2014; 40(1): 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin O Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med 2016; 22(11): 1229–1238. [DOI] [PubMed] [Google Scholar]
- 7.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A 2013; 110(22): 9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caballero A, Tseng KY. GABAergic Function as a Limiting Factor for Prefrontal Maturation during Adolescence. Trends Neurosci 2016; 39(7): 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do KQ, Cuenod M, Hensch TK. Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull 2015; 41(4): 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 2016; 17(8): 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grace AA, Gomes FV. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr Bull 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baroncelli L, Scali M, Sansevero G, Olimpico F, Manno I, Costa M et al. Experience Affects Critical Period Plasticity in the Visual Cortex through an Epigenetic Regulation of Histone Post-Translational Modifications. J Neurosci 2016; 36(12): 3430–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci 2010; 31(12): 2185–2192. [DOI] [PubMed] [Google Scholar]
- 14.Gomes FV, Grace AA. Prefrontal Cortex Dysfunction Increases Susceptibility to Schizophrenia-Like Changes Induced by Adolescent Stress Exposure. Schizophr Bull 2017; 43(3): 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 1984; 4(11): 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 1984; 4(11): 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 2007; 27(42): 11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caballero A, Diah KC, Tseng KY. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus 2013; 23(12): 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct 2014; 219(1): 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lensjo KK, Christensen AC, Tennoe S, Fyhn M, Hafting T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro 2017; 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esneault E, Peyon G, Castagne V. Efficacy of anticonvulsant substances in the 6Hz seizure test: Comparison of two rodent species. Epilepsy Res 2017; 134: 9–15. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa FJ, Hesse B, de Almeida RB, Baretta IP, Boerngen-Lacerda R, Andreatini R. Magnesium sulfate and sodium valproate block methylphenidate-induced hyperlocomotion, an animal model of mania. Pharmacol Rep 2011; 63(1): 64–70. [DOI] [PubMed] [Google Scholar]
- 23.Biggs CS, Pearce BR, Fowler LJ, Whitton PS. The effect of sodium valproate on extracellular GABA and other amino acids in the rat ventral hippocampus: an in vivo microdialysis study. Brain Res 1992; 594(1): 138–142. [DOI] [PubMed] [Google Scholar]
- 24.Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 2014; 76(3): 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci 2008; 28(31): 7876–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes FV, Rincon-Cortes M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev 2016; 70: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology 2001; 24(4): 410–419. [DOI] [PubMed] [Google Scholar]
- 28.Moreines JL, Owrutsky ZL, Grace AA. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacology 2017; 42(4): 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 2014; 76(12): 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci 2015; 38(3): 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6(11): 877–888. [DOI] [PubMed] [Google Scholar]
- 32.Nabel EM, Morishita H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front Psychiatry 2013; 4: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee SB, Gutzeit VA, Baman J, Aoued HS, Doshi NK, Liu RC et al. Perineuronal Nets in the Adult Sensory Cortex Are Necessary for Fear Learning. Neuron 2017; 95(1): 169–179 e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gervain J, Vines BW, Chen LM, Seo RJ, Hensch TK, Werker JF et al. Valproate reopens critical-period learning of absolute pitch. Front Syst Neurosci 2013; 7: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang EJ, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci U S A 2012; 109 Suppl 2: 17213–17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennartsson A, Arner E, Fagiolini M, Saxena A, Andersson R, Takahashi H et al. Remodeling of retrotransposon elements during epigenetic induction of adult visual cortical plasticity by HDAC inhibitors. Epigenetics Chromatin 2015; 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res 2012; 135(1–3): 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry 2013; 74(6): 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slopen N, Williams DR, Fitzmaurice GM, Gilman SE. Sex, stressful life events, and adult onset depression and alcohol dependence: are men and women equally vulnerable? Soc Sci Med 2011; 73(4): 615–622. [DOI] [PubMed] [Google Scholar]
- 40.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67(3): 231–239. [DOI] [PubMed] [Google Scholar]
- 41.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry 2013; 74(2): 106–112. [DOI] [PubMed] [Google Scholar]
- 42.Stone JM, Howes OD, Egerton A, Kambeitz J, Allen P, Lythgoe DJ et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry 2010; 68(7): 599–602. [DOI] [PubMed] [Google Scholar]
- 43.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78(1): 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN et al. Mechanisms of stress in the brain. Nat Neurosci 2015; 18(10): 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24(4): 417–463. [DOI] [PubMed] [Google Scholar]
- 46.Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 2013; 73(6): 574–582. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 2002; 55(1–2): 1–10. [DOI] [PubMed] [Google Scholar]
- 48.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6(4): 312–324. [DOI] [PubMed] [Google Scholar]
- 49.Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry 2017; 22(7): 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Mara S The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat 2005; 207(3): 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 2009; 19(2): 220–230. [DOI] [PubMed] [Google Scholar]
- 52.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 2013; 504(7479): 272–276. [DOI] [PubMed] [Google Scholar]
- 53.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 2013; 3: e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nott A, Cho S, Seo J, Tsai LH. HDAC2 expression in parvalbumin interneurons regulates synaptic plasticity in the mouse visual cortex. Neuroepigenetics 2015; 1: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 2001; 276(39): 36734–36741. [DOI] [PubMed] [Google Scholar]
- 56.Hensch TK, Quinlan EM. Critical periods in amblyopia. Vis Neurosci 2018; 35: E014. [DOI] [PubMed] [Google Scholar]
- 57.Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011; 334(6063): 1731–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 2014; 35(3): 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry 2002; 59(2): 154–164. [DOI] [PubMed] [Google Scholar]
- 60.Page CE, Coutellier L. Adolescent Stress Disrupts the Maturation of Anxiety-related Behaviors and Alters the Developmental Trajectory of the Prefrontal Cortex in a Sex- and Age-specific Manner. Neuroscience 2018; 390: 265–277. [DOI] [PubMed] [Google Scholar]
- 61.Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry 2015; 172(3): 249–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.