Structured Abstract
Objectives:
The main objective of this study is to determine if chronic sound deprivation leads to poorer speech discrimination in humans.
Design:
We reviewed the audiologic profile of 240 patients presenting normal and symmetrical bone-conduction thresholds bilaterally, associated with either an acute or chronic unilateral conductive hearing loss of different etiologies.
Results:
Patients with chronic conductive impairment and a moderate, to moderately severe, hearing loss had lower speech recognition scores on the side of the pathology when compared to the healthy side. The degree of impairment was significantly correlated with the speech recognition performance, particularly in patients with a congenital malformation. Speech recognition scores were not significantly altered when the conductive impairment was acute or mild.
Conclusions:
This retrospective study shows that chronic conductive hearing loss was associated with speech intelligibility deficits in patients with normal bone-conduction thresholds. These results are as predicted by a recent animal study showing that prolonged, adult-onset conductive hearing loss causes cochlear synaptopathy.
Introduction
Otitis media (OM) is the most common group of inflammatory diseases of the middle-ear encountered in pediatric populations, many of which result from bacterial infection (Klein 1994). Up to 75% of children will experience one or more bouts before they reach five years of age, making it the most common cause for physician visits and antibiotic prescriptions in pediatric outpatients (Pennie 1998). These bouts can reoccur with a cumulative incidence of 42% by two years of age and 60% by age three (Kaur et al. 2017). Several studies of patients presenting with a unilateral chronic otitis media (COM) showed that bone conduction (BC) thresholds were significantly poorer on the affected side, suggesting that a sensorineural component had developed as well (da Costa et al. 2009; Jesic et al. 2012; Joglekar et al. 2010; Kolo et al. 2012; Luntz et al. 2013; Redaelli de Zinis et al. 2005; Yehudai et al. 2015; Yoshida et al. 2014). Others have shown long-lasting deficits in spatial hearing as well as receptive language skills that persist after the middle-ear pathology has resolved (for review, see Deggouj et al. 2012).
Sensorineural damage associated with cholesteatomas in addition to conductive hearing loss (CHL) is well documented (Rosito et al. 2016). Histopathological studies of both human and animal temporal bones suggested that penetration of bacterial toxins and inflammatory mediators into the inner ear compartment via the round window membrane can be the cause of hair cell damage and related sensorineural loss (Paparella et al., 1984; Joglekar et al. 2010; Katano et al. 2005; MacArthur, Hausman, Kempton, Choi, et al. 2013; MacArthur, Hausman, Kempton, Sautter, et al. 2013). Sensorineural damage is further increased when a fistula of the inner ear has been created by the erosive properties of the cholesteatoma. The use of ototoxic topical aminoglycosides is an additional potential cause of damage, and a surgical intervention can also contribute to sensorineural loss as cholesteatomas necessitate removal. The finding that some pathologies with a conductive component can lead to sensorineural damage, however, cannot explain why patients with single-sided congenital ear malformation (and therefore presenting conductive hearing loss) have poorer speech recognition scores in quiet and in noise on the malformed side, despite having similar BC thresholds in the normal and the affected ear (Priwin et al. 2007; Snik et al. 1994).
Animal studies on the effects of sound deprivation have shown long-lasting impact on brain and behavior. However, most studies disrupted the middle-ear during the neonatal period (e.g., Smith et al. 1983; Tucci et al. 1985, 1987) and most have evaluated its effects on the higher centers of the auditory pathways rather than in the cochlea (Clarkson et al. 2016; Dahmen et al. 2007; Grande et al. 2014; Harrison et al. 2012; Hutson et al. 2008; Kandler et al. 2005; Wang et al. 2011; Zhuang et al. 2017). Recently, we showed in mice that a chronic (one-year duration) conductive hearing loss from eardrum resection in the mature animal led to a reduction in cochlear efferent innervation and a loss of up to 30% of the afferent synapses between the cochlear nerve and the sensory cells (Liberman et al. 2015). This surprising finding revealed signs of plasticity of cochlear innervation in the fully developed ear. This type of subtotal cochlear synaptopathy will not elevate behavioral or electrophysiological thresholds until it becomes extreme (Lobarinas et al. 2013; Woellner et al. 1955), because the most vulnerable cochlear neurons in other forms of cochlear synaptopathy tend to be those with high thresholds and low spontaneous rates (SRs) (Furman et al. 2013; Schmiedt et al. 1996). However, it should degrade the signal-coding abilities of the auditory nerve and might impair performance on more complex tasks such as speech recognition.
The present study aims to determine if patients with chronic reduction in sound transmission through the middle ear show increased difficulty with word recognition tasks as predicted by the synaptopathic effects of chronic conductive hearing loss in animal models.
Materials and Methods
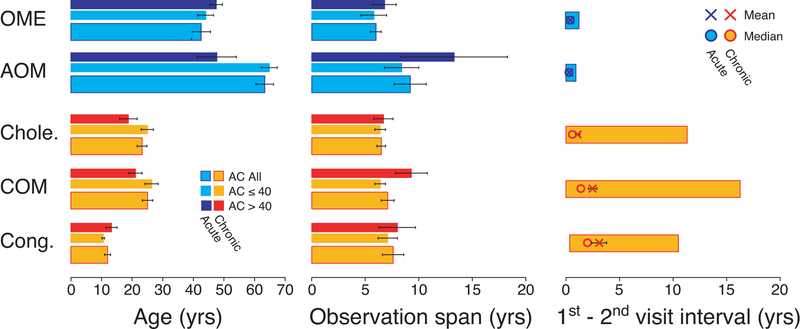
We collected audiological data from patients seen at the Massachusetts Eye & Ear Infirmary between 1993 and 2017 for otological evaluation. To be included, patient must have presented with normal (≤ 25 dB HL) and symmetrical BC thresholds bilaterally (interaural difference ≤ 10 dB from 250 Hz to 4 kHz) and a unilateral conductive hearing loss (CHL) at the first visit. A CHL was defined as ≥ 15 dB difference between the mean PTA for air-conduction (AC) and BC thresholds (air-bone gap). The CHL was defined as chronic when the air-bone gap remained ≥ 15 dB between the first and the last visit. It was defined as acute when the air-bone gap was < 15 dB at the second visit and remained so on follow-up appointments. Records spanning less than two years and patients with fewer than three hearing evaluations were excluded. Patients < 10 years of age were not considered, as hearing assessment differs in the pediatric setting. Further characteristics of patient’s profile including age, observation spans and visit intervals are described in Figure 1. With the exception of one patient, who was excluded, none of the study population wore traditional or bone-anchored hearing aids. This research study was reviewed and approved by the Institutional Review Board of the Massachusetts Eye & Ear.
Figure 1: Ages, observation spans, and visit intervals for subjects in the five groups.
Means (±SEMs) are shown for each parameter.
Audiometric thresholds were obtained using a number of different audiometers including Grason-Stadler (GS-10, GS-16), Interacoustics AC-30, Virtual 320 and Interacoustics Equinox, running under the same Harvard Audiometer Operating System (AOS; Thornton et al. 1994). Pure-tone AC thresholds were measured at standard audiometric frequencies from 0.25 kHz to 8 kHz, in octave steps using TDH39 headphones or ER-3A insert earphones. Bone-conduction thresholds were acquired from 250 Hz to 4,000 Hz with a Radioear B-71 vibrator over the mastoid. The pure-tone average (PTA) was defined as the average threshold at 500, 1000 and 2000 Hz. Hearing loss configurations were divided into 3 groups: 1) upward sloping when mean AC thresholds at 250 Hz and 500Hz were 10 dB worse than mean thresholds at 4 and 8 kHz; 2) downward sloping for patients with mean AC thresholds at 250 Hz and 500Hz 10 dB better than mean thresholds at 4 and 8 kHz; and 3) flat for all other hearing loss profiles.
Speech recognition performance was assessed using a recorded CID (Central Institute for the Deaf) W-22 phonetically balanced test, consisting of 50 CNC word lists presented with a contralateral speech-shaped noise. The Articulation Index (AI) was used to predict the performance/intensity function for speech (Pavlovic et al., 1986; Wilde and Humes, 1990) based on the audiogram, using a transfer function for CID W-22 (ANSI 1997; Sherbecoe and Studebaker 1990). This procedure was automatically generated by the Harvard AOS software as described in Halpin et al. (1994). The level at which maximal intelligibility was predicted was chosen as presentation level. If this value, however, fell below 70 dB HL, presentation level remained at 70 dB HL. All word recognition (WR) scores were obtained from native speakers of English. The scores reported here are those at the time of the initial visit, for patients with acute conductive hearing loss, and at the time of the final visit for patients with chronic conditions.
All statistical analyses were performed under the JMP statistical data analysis software (SAS Institute Inc., Cary, NC). The threshold for statistical significance was p = 0.05. Equivalent testing using the “two-one-sided t-tests (TOST)” procedure was considered to examine if interaural changes in threshold differed among groups. The non-parametric Steel-Dwass test was used to perform multiple group comparisons. A two-way ANOVA followed by a Mann-Whitney U test were performed to compare WR scores across groups. Finally, the relationship between AC or BC thresholds as a function of WR score was tested using a Spearman’s rank correlation coefficient method.
Results
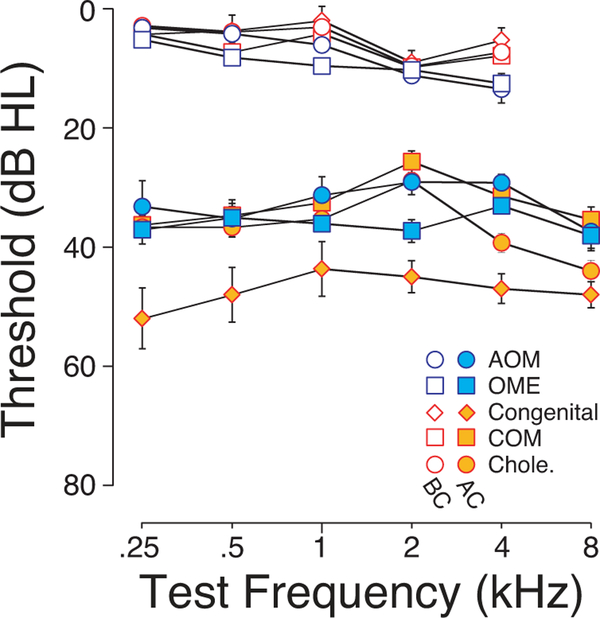
Out of 240 cases meeting our inclusion criteria, 169 cases were chronic conditions with one of three etiologies: 15 with atresia and/or a congenital middle-ear malformation, 71 with chronic otitis media (COM) and 83 with cholesteatoma. An additional 71 cases were acute conditions: 20 with acute otitis media (AOM) and 51 with otitis media with effusion (OME). Figure 2 shows the mean AC and BC thresholds of each cohort on the side of the conductive impairment. Note that whatever small intergroup differences there are, the mean BC thresholds are slightly worse in the acute groups than the chronic groups, especially at 4 kHz where the difference was statistically significant (ANOVA: F=2.15, p = 0.04).
Figure 2: Mean hearing sensitivity in the affected ear for each cohort.
Mean Air-conduction (AC) and Bone-conduction (BC) thresholds on the CHL side of each cohort, color coded according to etiologies: three chronic types of CHL (Cong.: Congenital malformations of the external/middle ear; COM: Chronic Otitis Media; Chole.: Cholesteatoma) and two acute types of CHL group (AOM: Acute Otitis Media; OME: Secretory Otitis Media). Error bars are for SEMs
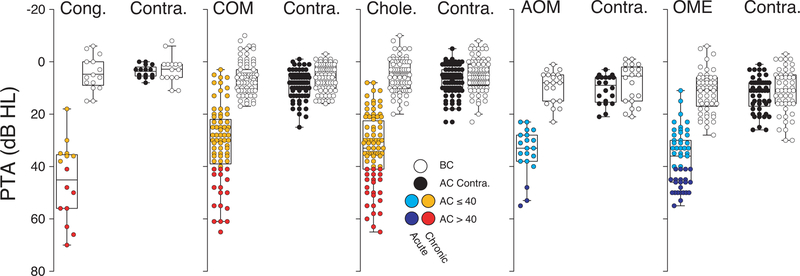
Whereas all patients presented with a mild to moderately severe CHL in one ear, in the contralateral ear there was no significant air-bone gap, and pure tone averages (PTA) for AC and BC thresholds were within normal limits (Figure 3). Patients were separated into two PTA groups, as color coded in Figure 3: mild CHL when AC threshold was ≤ 40 dB HL and moderate to moderately severe CHL for AC thresholds were between from 40 and 70 dB HL.
Figure 3: Individual PTAs, by bone and air conduction, for the affected sides vs. contralateral ears.
Box and whiskers plots of AC- and BC-threshold PTAs (500, 1000 and 200 Hz) for each subject from each group. As defined in key, two degrees of hearing loss were considered for the CHL ears: this color coding convention will be carried forward in the remaining Figures.
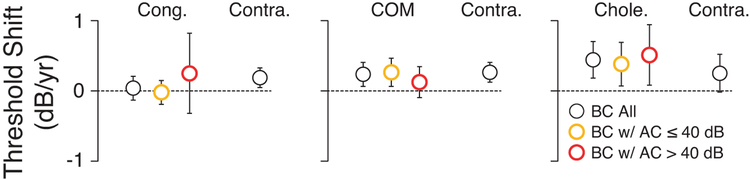
To quantify interaural differences in BC thresholds over the observation period (Figure 1) and track signs of progressive hair cell damage on the CHL side, changes in BC thresholds as a function of time were calculated for each chronic condition group in each ear (Figure 4). There were no statistically significant differences in the rate of threshold deterioration (dB/year) between the CHL ear and the contralateral ear in either PTA group, as examined with an equivalence testing approach using the TOST procedure (Congenital, p = 0.53; COM, p = 0.37; Cholesteatoma, p = 0.59; Figure 3).
Figure 4: Change in bone conduction thresholds over the obserbation span.
Rate of PTA shift in each ear was computed over the entire observation period from first to last visit. As shown in the key, an ensemble average was computed for each group (black circles) as well as separate averages for each PTA group on the affected sides. Error bars are for SEMs
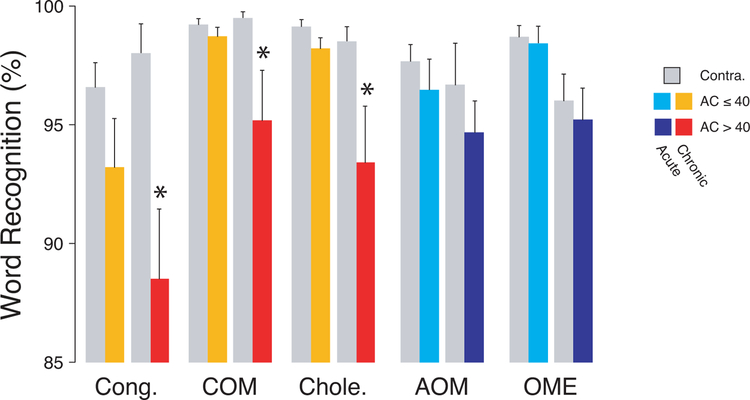
However, as shown in Figure 5, WR scores were significantly poorer on the CHL side, when the PTA was > 40 dB HL and when the condition was chronic, whether assessed by ANOVA (Congenital: F=4.70, p=0.01; COM, F=13.91, p<0.001; Cholesteatoma: F=6.21, p<0.001; AOM, F=0.54, p>0.05; OME: F=6.86, p=0.01), or by a post-hoc Steel-Dwass test for multiple comparisons (Congenital: AC≤40, Z=1.57, p>0.05 / AC>40, Z=3.03, p=0.04; COM: AC≤40, Z=0.16, p>0.05 / AC>40, Z=3.64, p=0.04; Cholesteatoma: AC≤40, Z=1.31, p>0.05 / AC>40, Z=3.72, p=0.04). Another statistical approach was to use a two-way ANOVA to show that duration (acute vs. chronic) and degree of hearing loss had significant effects on the difference in WR scores between the affected and the unaffected ear (acute vs. chronic: F = 49.7, p<0.001; degree of hearing loss: F=16.7, p<0.001), with no interaction between diagnosis and degree (p=0.48). Finally, post-hoc analysis showed a statistically significant effect of the degree of hearing loss in chronic conditions (p<0.001), but not in acute conditions (p=0.68). Similarly, we found no statistically significant difference between chronic and acute conditions in patients with mild hearing loss (p=0.99), while these differences became significant in patients with a moderate to moderately severe loss (p=0.03). Finally, there was no statistically significant effect of sex in any of the chronic groups (see Table 1).
Figure 5: Word recognition scores as a function of CHL and etiology.
For all conditions, WR scores were averaged, and error bars are for SEMs. Statistical significance of the post-hoc analysis (Steel-Dwass test for multiple comparisons) is indicated (*p<0.05).
Table 1
| Sex | Male | Female | p value |
|---|---|---|---|
| n | 7 | 8 | |
| Age | 11.1 ± 1.1 | 12.8 ± 1.6 | 0.24 |
| AC Threshold – CHL | 50.9 ± 6.3 | 40.3 ± 4.1 | 0.35 |
| WRS – CHL | 90.3 ± 2.7 | 91.3 ± 2.5 | 0.68 |
| WRS – contralateral | 97.7 ± 1.3 | 97.0 ± 1.1 | 0.59 |
| n | 42 | 29 | |
| Age | 23.4 ± 2.2 | 27.6 ± 2.6 | 0.11 |
| AC Threshold – CHL | 28.4 ± 2.2 | 34.7 ± 2.7 | 0.09 |
| WRS – CHL | 98.9 ± 0.4 | 97.0 ± 1.0 | 0.06 |
| WRS – contralateral | 99.2 ± 0.3 | 99.2 ± 0.3 | 0.38 |
| n | 51 | 32 | |
| Age | 21.1 ± 1.9 | 26.8 ± 2.9 | 0.19 |
| AC Threshold – CHL | 32.0 ± 1.8 | 35.1 ± 2.4 | 0.47 |
| WRS – CHL | 98.0 ± 0.6 | 97.4 ± 1.5 | 0.36 |
| WRS – contralateral | 99.1 ± 0.3 | 98.8 ± 0.4 | 0.56 |
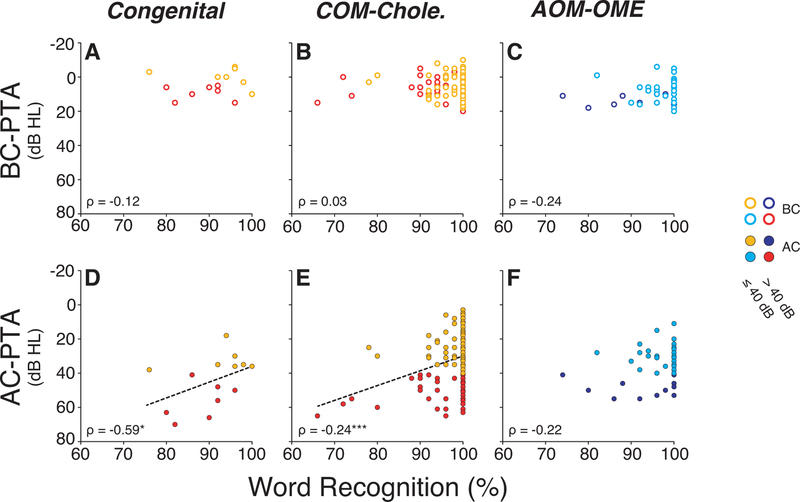
The results suggest that both degree and duration of hearing loss are relevant to the decrement in WR score. This relationship is further supported by 1) the statistically significant correlations obtained between WR score and AC-PTA thresholds in chronic conditions, as shown in Figure 6D–E (Congenital: ρ = −0.59, p = 0.02; COM-Cholesteatoma: ρ = −0.24, p = 0.001) and 2) by the absence of correlation between BC-PTA thresholds and word scores (Figure 6A–C) in the same groups of patients (Congenital: ρ = −0.12, p = 0.66; COM-Cholesteatoma: ρ = 0.03, p = 0.81; AOM-OME: ρ = −0.24, p = 0.09). Thus, inner ear threshold sensitivity, as measured with BC, is not significantly associated with WR score in these patients. Note that a higher Spearman’s rank correlation coefficient was seen for the congenital group compared to groups that included patients who experienced repeated middle-ear infections and/or cholesteatoma.
Figure 6: Predictibility of word recognition scores as a function of degree of CHL and etiology.
No statistically significant relationship was found between BC thresholds and WR score in any cohort (A-C). However, significant correlations were observed in the affected ear between AC PTAs and WR scores for all chronic CHL groups (Congenital malformations of the external/middle ear (D), Chronic Otitis Media and Cholesteatoma (E)). The same relationship did not reach statistical significance in CHL (F). The linear regression is shown when the coefficient correlation was significant. For all conditions, scores were obtained at the last visit. Statistical significance is indicated: *p<0.05; ***p<0.001.
Discussion
This study shows that patients with chronic conditions associated with at least a moderate unilateral CHL have poorer WR score on the affected side compared to the unaffected side, even if BC thresholds remain symmetrical and within normal limits bilaterally.
A number of methodological limitations intrinsic to retrospective studies need to be acknowledged. First, as a result of our inclusion criteria, cohorts with acute CHL were significantly older than patients with chronic conditions (see Figure 1). Indeed, audiometric data were collected from patients with AOM who did not repeat the condition, excluding therefore younger patients who tend to repeat ear infections (Tos 1984; Williamson et al. 1994). Similarly, we excluded patients with poor BC thresholds (> 25 dB HL). Given that BC thresholds decline with age, patients with COM and/or a cholesteatoma were relatively younger. Poorer WR scores were observed in chronic CHL cohorts, thus age is unlikely to be a significant factor detrimental to WRS in this study population.
A second limitation lies with how word recognition performance was assessed: speech material was delivered at a single presentation level, obtained from an estimate of the speech intelligibility index curve (see Methods). It is possible that the level at which maximum performance was predicted by this procedure was not optimal. However, this is unlikely, as the predicted presentation level would have to be off by more than 14 dB to produce WRs scores as poor as those observed in the chronic condition groups, as determined using the Harvard AOS software.
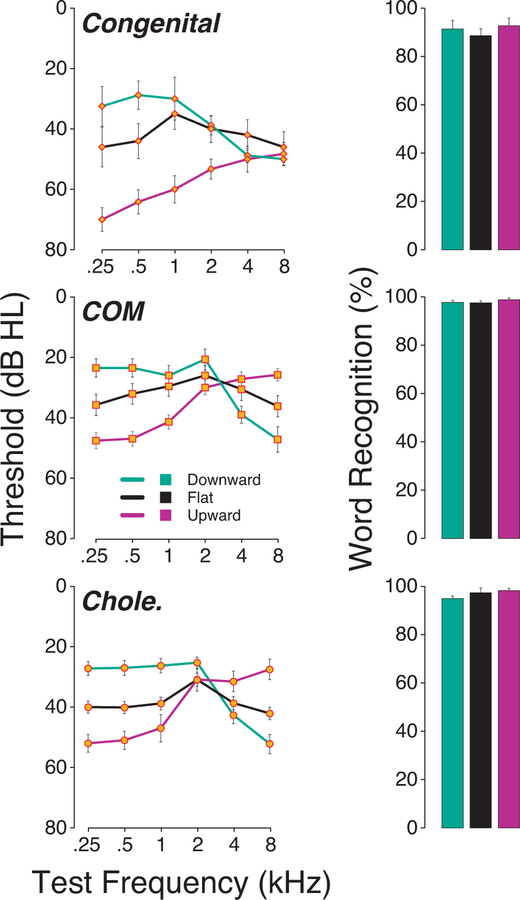
It is also possible that hearing loss configuration could alter speech perception performance by filtering out energy from the speech signal. While a majority of these chronic CHL produced “flat” audiograms as defined in Methods (74 out of 169), 41 patients presented with an upward-sloping and 54 presented with a downward-sloping configuration (Figure 7). Although the speech material was not spectrally adjusted to compensate for audiometric losses, we found no evidence that hearing loss configuration had a significant impact on WR scores (ANOVA: Congenital: F=0.63, p=0.48; COM: F=0.53, p=0.65; Cholesteatoma: F=2.47, p=0.10).
Figure 7: Effect of hearing loss configuration on word recognition scores in patients presenting a chronic CHL.
No statistically significant difference in WR scores was found from patient presenting a chronic CHL with different hearing loss configurations. U; Upward sloping, D; Downward sloping; F: Flat.
It is worth noting as well that a great majority of the chronic-CHL cohort had cholesteatoma or COM, both of which can cause inner ear damage, as documented in many investigations. Histopathological studies point at the cochlear basal turn as a target for middle-ear infections (Cook et al. 1999; Cureoglu et al. 2004; Paparella et al. 1972), and children with a history of otitis media have poorer extended high-frequency thresholds compared to controls (Hunter et al. 1996; Margolis et al. 2000). The byproducts of bacterial infections and inflammatory mediators can alter gene expression in the inner ear (Ghaheri et al. 2007; MacArthur, Hausman, Kempton, Choi, et al. 2013), including those for ion channels and transporters in the stria vascularis and spiral ligament (MacArthur, Hausman, Kempton, Sautter, et al. 2013). Such alterations could result in sensorineural hearing loss. However, here, we excluded patients with elevated BC thresholds (> 25 dB HL) to minimize the contributions of hair cell damage, strial damage or other non-neural cells in the cochlear duct to any observed degradation in speech-recognition performance on the affected side. It is possible that inflammatory byproducts of infection reach the inner ear and cause damage that is not captured by BC thresholds. Nevertheless, the WR score obtained in all groups of patients with chronic etiologies and moderate to moderately severe hearing losses were significantly lower than that predicted from the speech intelligibility curve (> 98%), and no significant correlation was observed between BC thresholds and WR score (Figure 6A–C). Thus, even if there is damage to the most basal regions of the cochlea, it should not affect speech recognition scores to the extent observed here, when words are presented at comfortable levels to patients with bilaterally normal BC thresholds. Additionally, as shown in Figure 2, acute cohorts (with the worse WR scores) actually had slightly poorer BC thresholds at 4 kHz compared to chronic cohorts. Therefore, a different mechanism likely underlies the decrement in WR score.
Evidence for a non-inflammatory etiology is provided by patients with congenital malformations (e.g., atretic canal). These patients showed the strongest correlation between AC thresholds and WR score (Figure 6). This result is consistent with the idea that a reduced acoustic drive to the inner ear is the root cause of the impairment in speech-recognition performance. Such CHL is a common form of auditory deprivation that has long-lasting deleterious effects on hearing when occurring during critical periods of development (for review, see Whitton and Polley 2011). Unilateral CHLs also alter interaural time and level differences of acoustic signals arriving at the two ears (Hall and Derlacki, 1988; Thornton et al., 2012), and therefore affect spatial hearing, particularly in the horizontal plane. The resulting degraded afferent signals when carried to brain areas during critical periods of development will impact the formation of neural circuits that mediate perception, as evidenced at the cellular level by significantly reduced cell-body diameter and dendritic arborization in regions of the cochlear nucleus and superior olivary complex (Webster and Webster, 1977, 1979; Conlee et al., 1984, 1986). CHL has also been found to disrupt temporal response properties of auditory cortical neurons in animal studies (e.g., Polley et al. 2013; Teichert and Bolz, 2017) and, more recently, in increased neural response amplitudes in humans with a chronic unilateral CHL (Parry et al. 2019). Furthermore, several studies report that sound deprivation can alter the normal development of the central auditory system even after hearing thresholds return to normal by disrupting binaural integration, by impoverishing hearing in noise (Knudsen et al. 1984, Popescu et al. 2010; Gay et al. 2014) and by disrupting normal binaural balance between the representation of sounds delivered to each ear (Clopton and Silverman, 1977; Silverman and Clopton 1977, Moore and Irvine 1981; Popescu and Polley 2010). However, normal hearing thresholds do not guarantee an absence of peripheral damage, and none of these studies looking at central effects of sound deprivation provided evidence of peripheral integrity at the neuronal level. Therefore, a peripheral involvement in the persistent perceptual impairments associated with chronic CHL in any of these prior studies cannot be ruled out.
In prior animal work, our group showed that prolonged unilateral CHL, due to resection of the eardrum, caused up to 30% loss of synapses between cochlear nerve fibers and their peripheral targets, the inner hair cells (Liberman et al. 2015). This type of cochlear synaptopathy could cause hearing impairments, especially in noisy environments (Liberman 2017), because the most vulnerable cochlear neurons to both noise and aging are those with high thresholds and low-spontaneous rates (Furman et al. 2013; Schmiedt et al. 1996). These high threshold fibers are key contributors to the coding of transient stimuli in noisy environments (Costalupes et al. 1984) despite the fact that their loss remained undetected because neural degeneration per se does not elevate behavioral or electrophysiological thresholds until it becomes extreme (Lobarinas et al. 2013; Woellner et al. 1955). Since WR score in this study were obtained in quiet, the impairment experienced by these patients may be underestimated.
As discussed above, a number of studies have documented changes in central auditory nuclei as a result of a chronic conductive impairment. Of particular interest are the changes in the superior olivary complex in animal models of neonatal CHL, where a significant abnormalities have been observed in rats (Myers et al. 2012), gerbils (Tucci et al. 2001) and guinea pigs (Potashner et al. 1997). For example, levels of oxidative enzymes, thought to reflect overall electrical activity (Wong-Riley et al. 1981), changed significantly within the lateral superior olive of adult gerbils as a result of unilateral malleus removal or cochlear ablation (Tucci et al. 2002). Given the importance of the lateral superior olive as the origin of olivocochlear feedback to the cochlea, these central changes may also lead to changes at the periphery. Our prior animal study of CHL also showed a reduction in the density of cochlear efferent fibers originating in the lateral superior olive and projecting to the dendrites of cochlear nerve fibers in the inner hair cell area (Liberman et al. 2015). The further observation that cochlear de-efferentation, per se, by surgical interruption of the fiber bundle, also leads to cochlear synaptopathy (Liberman et al. 2014), suggest that the cochlear neurodegeneration associated with CHL may be mediated by changes in the efferent feedback pathways to the inner ear. Together, these results from animal studies suggest that cochlear synaptopathy may be a contributing factor to the reduced word recognition scores observed in our cohort of human subjects with chronic CHL of a moderate to moderately-severe degree.
This study also supports the idea that amplification should be considered in the management of unilateral CHL: if hearing cannot be medically improved, patients may benefit from either conventional amplification or from an osseo-integrated device. In absence of amplification, our data suggest that speech recognition, particularly in adverse environments, may worsen on the side of the pathology, possibly also including deficits in sound localization. This speculation is further supported by a study of patients with bilateral symmetric CHL who received monaural vs. binaural amplification: speech-recognition in unaided ears was poorer than that in aided ears (Dieroff 1993). Lack of treatment for unilateral or asymmetric hearing loss can be based on the belief that the contralateral ear can compensate for the loss. Yet, children with asymmetric hearing loss have higher rates of academic, social and behavioral difficulties (Lieu et al. 2012; Wie et al. 2010). Given that cochlear synaptopathy appears to be irreversible, peripheral deficits related to cochlear neural degeneration should be considered as well, when patients report lingering deficits in auditory processing after persistent middle-ear issues are resolved.
Acknowledgements
We are grateful to William Goedicke and Dr. Barbara Herrmann for their technical help and logistic support. This work was supported by the NIH – NIDCD Grant P50 DC015857.
Footnotes
Conflicts of Interest and Source of Funding:
This research was funded by the NIH – NIDCD P50 DC015857 (SFM, Project PI).
References
- ANSI (1997). Method for the calculation of the Speech intelligibility index. ANSI S3.79–1997 In Accredited Standards Committee S3, Bioacoustics. New York: Acoustical Society of America (Melville, NY). [Google Scholar]
- Clarkson C, Antunes FM, Rubio ME (2016). Conductive Hearing Loss Has Long-Lasting Structural and Molecular Effects on Presynaptic and Postsynaptic Structures of Auditory Nerve Synapses in the Cochlear Nucleus. J Neurosci, 36, 10214–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS (1977). Plasticity of binaural interaction. II. Critical period and changes in midline response. J Neurophysiol, 40, 1275–1280. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Parks TN, Romero C, Creel DJ (1984). Auditory brainstem anomalies in albino cats: II. Neuronal atrophy in the superior olive. J Comp Neurol, 225, 141–148. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Parks TN, Creel DJ (1986). Reduced neuronal size and dendritic length in the medial superior olivarynucleus of albino rabbits. Brain Res, 363, 28–37. [DOI] [PubMed] [Google Scholar]
- Cook RD, Postma DS, Brinson GM, et al. (1999). Cytotoxic changes in hair cells secondary to pneumococcal middle-ear infection. J Otolaryngol, 28, 325–331. [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ (1984). Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol, 51, 1326–1344. [DOI] [PubMed] [Google Scholar]
- Cureoglu S, Schachern PA, Paparella MM, et al. (2004). Cochlear changes in chronic otitis media. Laryngoscope, 114, 622–626. [DOI] [PubMed] [Google Scholar]
- da Costa SS, Rosito LP, Dornelles C (2009). Sensorineural hearing loss in patients with chronic otitis media. Eur Arch Otorhinolaryngol, 266, 221–224. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ (2007). Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol, 17, 456–464. [DOI] [PubMed] [Google Scholar]
- Deggouj N, Castelein S, Gregoire A, et al. (2012). Functional consequences of chronic ENT inflammation on the development of hearing and communicative abilities. B-ENT, 8 Suppl 19, 105–115. [PubMed] [Google Scholar]
- Dieroff HG (1993). Late-onset auditory inactivity (deprivation) in persons with bilateral essentially symmetric and conductive hearing impairment. J Am Acad Audiol, 4, 347–350. [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of neurophysiology, 110, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay JD, Voytenko SV, Galazyuk AV, Rosen MJ (2014). Developmental hearing loss impairs signal detection in noise: putative central mechanisms. Front Syst Neurosci, 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaheri BA, Kempton JB, Pillers DA, et al. (2007). Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol Head Neck Surg, 137, 332–337. [DOI] [PubMed] [Google Scholar]
- Grande G, Negandhi J, Harrison RV, et al. (2014). Remodelling at the calyx of Held-MNTB synapse in mice developing with unilateral conductive hearing loss. J Physiol, 592, 1581–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW 3rd, Derlacki EL (1988). Binaural hearing after middle ear surgery. Masking-level difference for interaural time and amplitude cues. Audiology, 27,89–98. [DOI] [PubMed] [Google Scholar]
- Halpin C, Thornton A, Hasso M (1994). Low-Frequency Sensorineural Loss: Clinical Evaluation and Implications for Hearing Aid Fitting. Ear Hear, 15, 71–81. [PubMed] [Google Scholar]
- Harrison RV, Negandhi J (2012). Resting neural activity patterns in auditory brainstem and midbrain in conductive hearing loss. Acta Otolaryngol, 132, 409–414. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Margolis RH, Rykken JR, et al. (1996). High frequency hearing loss associated with otitis media. Ear Hear, 17, 1–11. [DOI] [PubMed] [Google Scholar]
- Hutson KA, Durham D, Imig T, et al. (2008). Consequences of unilateral hearing loss: cortical adjustment to unilateral deprivation. Hear Res, 237, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesic SD, Jotic AD, Babic BB (2012). Predictors for sensorineural hearing loss in patients with tubotympanic otitis, cholesteatoma, and tympanic membrane retractions. Otol Neurotol, 33, 934–940. [DOI] [PubMed] [Google Scholar]
- Joglekar S, Morita N, Cureoglu S, et al. (2010). Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol, 130, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC (2005). Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci, 28, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Iino Y, Murakami Y, et al. (2005). [Temporal bone histopathology in a patient suspected of inner ear extension of otitis media]. Nihon Jibiinkoka Gakkai Kaiho, 108, 533–536. [DOI] [PubMed] [Google Scholar]
- Kaur R, Morris M, Pichichero ME (2017). Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JO (1994). Otitis media. Clin Infect Dis, 19, 823–833. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Esterly SD, Knudsen PF (1984). Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci, 4, 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolo ES, Salisu AD, Yaro AM, et al. (2012). Sensorineural hearing loss in patients with chronic suppurative otitis media. Indian J Otolaryngol Head Neck Surg, 64, 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (2017). Noise-induced and age-related hearing loss: new perspectives and potential therapies. F1000Res, 6, 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF (2014). Efferent feedback slows cochlear aging. J Neurosci, 34, 4599–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF (2015). Chronic Conductive Hearing Loss Leads to Cochlear Degeneration. PLoS One, 10, e0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JE, Tye-Murray N, Fu Q (2012). Longitudinal study of children with unilateral hearing loss. Laryngoscope, 122, 2088–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2013). Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hearing research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz M, Yehudai N, Haifler M, et al. (2013). Risk factors for sensorineural hearing loss in chronic otitis media. Acta Otolaryngol, 133, 1173–1180. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Hausman F, Kempton JB, et al. (2013). Otitis media impacts hundreds of mouse middle and inner ear genes. PLoS One, 8, e75213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Hausman F, Kempton JB, et al. (2013). Inner ear tissue remodeling and ion homeostasis gene alteration in murine chronic otitis media. Otol Neurotol, 34, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Saly GL, Hunter LL (2000). High-frequency hearing loss and wideband middle ear impedance in children with otitis media histories. Ear Hear, 21, 206–211. [DOI] [PubMed] [Google Scholar]
- Moore DR, Irvine DR (1981). Plasticity of binaural interaction in the cat inferior colliculus. Brain Res, 208, 198–202. [DOI] [PubMed] [Google Scholar]
- Morizono T, Giebink GS, Paparella MM et al. (1985). Sensorineural hearing loss in experimental purulent otitis media due to Streptococcus pneumoniae. Arch Otolaryngol, 111, 794–798. [DOI] [PubMed] [Google Scholar]
- Myers AK, Ray J, Kulesza RJ Jr. (2012). Neonatal conductive hearing loss disrupts the development of the Cat-315 epitope on perineuronal nets in the rat superior olivary complex. Brain Res, 1465, 34–47. [DOI] [PubMed] [Google Scholar]
- Parry LV, Maslin MRD, Schaette R, Moore DR, Munro KJ (2019). Increased auditory cortex neural response amplitude in adults with chronic unilateral conductive hearing impairment. Hear Res, 372, 10–16. [DOI] [PubMed] [Google Scholar]
- Pavlovic C, Studebaker G, Sherbecoe R (1986). An articulation-index based procedure for predicting the speech recognition performance of hearing-impaired individuals. J Acoust Soc Am, 80, 50–57. [DOI] [PubMed] [Google Scholar]
- Paparella MM, Oda M, Hiraide F, et al. (1972). Pathology of sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol, 81, 632–647. [DOI] [PubMed] [Google Scholar]
- Paparella MM, Morizono T, Le CT et al. (1984) Sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol, 93, 623–629. [DOI] [PubMed] [Google Scholar]
- Pennie RA (1998). Prospective study of antibiotic prescribing for children. Can Fam Physician, 44, 1850–1856. [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Thompson JH, Guo W (2013). Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat Commun, 4, 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB (2010). Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron, 65, 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG (1997). Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol, 148, 222–235. [DOI] [PubMed] [Google Scholar]
- Priwin C, Jonsson R, Magnusson L, et al. (2007). Audiological evaluation and self-assessed hearing problems in subjects with single-sided congenital external ear malformations and associated conductive hearing loss. Int J Audiol, 46, 162–171. [DOI] [PubMed] [Google Scholar]
- Redaelli de Zinis LO, Campovecchi C, Parrinello G, et al. (2005). Predisposing factors for inner ear hearing loss association with chronic otitis media. Int J Audiol, 44, 593–598. [DOI] [PubMed] [Google Scholar]
- Rosito LS, Netto LS, Teixeira AR, et al. (2016). Sensorineural Hearing Loss in Cholesteatoma. Otol Neurotol, 37, 214–217. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA (1996). Age-related loss of activity of auditory-nerve fibers. J Neurophysiol, 76, 2799–2803. [DOI] [PubMed] [Google Scholar]
- Sherbecoe RL, Studebaker GA (1990). Regression equations for the transfer functions of ANSI S3.5–1969. J Acoust Soc Am, 88, 2482–2483. [DOI] [PubMed] [Google Scholar]
- Silverman MS, Clopton BM (1977). Plasticity of binaural interaction. I. Effect of early auditory deprivation. J Neurophysiol, 40, 1266–1274. [DOI] [PubMed] [Google Scholar]
- Smith ZD, Gray L, Rubel EW (1983). Afferent influences on brainstem auditory nuclei of the chicken: n. laminaris dendritic length following monaural conductive hearing loss. J Comp Neurol, 220, 199–205. [DOI] [PubMed] [Google Scholar]
- Snik FM, Teunissen B, Cremers WR (1994). Speech recognition in patients after successful surgery for unilateral congenital ear anomalies. Laryngoscope, 104, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Teichert M, Bolz J (2017). Data on the effect of conductive hearing loss on auditory and visual cortex activity revealed by intrinsic signal imaging. Data Brief, 14, 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A, Halpin C, Han Y, Hou Z (1994). The Harvard Audiometer Operating System [software]. Palo Alto, CA: Applitech Inc. [Google Scholar]
- Thornton JL, Chevallier KM, Koka K, et al. (2012) The conductive hearing loss due to an experimentally induced middle ear effusion alters the interaural level and time difference cues to sound location. J Assoc Res Otolaryngol, 13, 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tos M (1984). Epidemiology and natural history of secretory otitis. Am J Otol, 5, 459–462. [PubMed] [Google Scholar]
- Tucci D, Cant NB, Durham D (2002). Conductive hearing loss results in changes in cytochrome oxidase activity in gerbil central auditory system. J Assoc Res Otolaryngol, 3, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci DL, Born DE, Rubel EW (1987). Changes in spontaneous activity and CNS morphology associated with conductive and sensorineural hearing loss in chickens. Ann Otol Rhinol Laryngol, 96, 343–350. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D (2001). Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res, 155, 124–132. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Rubel EW (1985). Afferent influences on brain stem auditory nuclei of the chicken: effects of conductive and sensorineural hearing loss on n. magnocellularis. J Comp Neurol, 238, 371–381. [DOI] [PubMed] [Google Scholar]
- Wang H, Yin G, Rogers K, et al. (2011). Monaural conductive hearing loss alters the expression of the GluA3 AMPA and glycine receptor alpha1 subunits in bushy and fusiform cells of the cochlear nucleus. Neuroscience, 199, 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB, Webster M (1977). Neonatal sound deprivation affects brain stem auditory nuclei. Arch Otolaryngol, 103, 392–396. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M (1979). Effects of neonatal conductive hearing loss on brain stem nuclei. Ann Otol Rhinol Laryngol, 88, 684–688. [DOI] [PubMed] [Google Scholar]
- Whitton JP, Polley DB (2011). Evaluation the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. JARO, 12, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie OB, Pripp AH, Tvete O (2010). Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol, 119, 772–781. [PubMed] [Google Scholar]
- Wilde G, Humes L (1990). Application of the Articulation Index to the speech recognition of normal and impaired listeners wearing hearing protection. J Acoust Soc Am, 87, 1192–1199. [DOI] [PubMed] [Google Scholar]
- Williamson IG, Dunleavy J, Baine J et al. (1994). The natural history of otitis media with effusion—a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol, 108, 930–934. [DOI] [PubMed] [Google Scholar]
- Woellner RC, Schuknecht HF (1955). Hearing loss from lesions of the cochlear nerve: an experimental and clinical study. Transactions - American Academy of Ophthalmology and Otolaryngology. American Academy of Ophthalmology and Otolaryngology, 59, 147–149. [PubMed] [Google Scholar]
- Wong-Riley MT, Walsh SM, Leake-Jones PA, et al. (1981). Maintenance of neuronal activity by electrical stimulation of unilaterally deafened cats demonstrable with cytochrome oxidase technique. Ann Otol Rhinol Laryngol Suppl, 90, 30–32. [DOI] [PubMed] [Google Scholar]
- Yehudai N, Most T, Luntz M (2015). Risk factors for sensorineural hearing loss in pediatric chronic otitis media. Int J Pediatr Otorhinolaryngol, 79, 26–30. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Miyamoto I, Takahashi H (2014). Relationship between CT findings and sensorineural hearing loss in chronic otitis media. Auris Nasus Larynx, 41, 259–263. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Sun W, Xu-Friedman MA (2017). Changes in Properties of Auditory Nerve Synapses following Conductive Hearing Loss. J Neurosci, 37, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]