Abstract
BACKGROUND/OBJECTIVES:
This study aims to identify resident characteristics associated with being offered and subsequently shown an advance care planning (ACP) video in the Pragmatic Trial of Video Education in Nursing Homes (PROVEN) and if differences are driven by within-and/or between-facility differences.
DESIGN:
Cross-sectional study, from March 1, 2016, to May 31, 2018.
SETTING:
A total of 119 PROVEN intervention nursing homes (NHs).
PARTICIPANTS:
A total of 43 303 new NH admissions.
MEASUREMENTS:
Data came from the Minimum Data Set and an electronic record documenting whether a video was offered and shown to residents. We conduct both naive logistic regression models and hierarchical logistic models, controlling for NH fixed effects, to examine the overall differences in offer and show rate by resident characteristics.
RESULTS:
In naïve regression models, compared to white residents, black residents are 7.8 percentage point (pp) (95% confidence interval [CI] = −9.1 to −6.5 pp) less likely to be offered the video. These differences decrease to 1.3 pp (95% CI = −2.61 to −0.02 pp) when accounting for NH fixed effects. In fully adjusted models, black residents compared to white residents were 2.1 pp more likely to watch the video contingent on being offered (95% CI = 0.4–3.7 pp). Residents with cognitive impairment were less likely to be offered and shown the video.
CONCLUSIONS:
After controlling for NH fixed effects, there were smaller racial differences in being offered the video, but once offered, black residents were more likely to watch the video. This suggests that black residents are receptive to this type of ACP intervention but need to be given an opportunity to be exposed.
Keywords: advance care planning, end-of-life care, health disparities, long-term care
Approximately one-fourth of Medicare fee-for-service decedents’ site of death is the nursing home (NH), which is often associated with poorer quality care at end of life.1,2 The Institute of Medicine report on Dying in America has identified the need for improved advance care planning (ACP) at end of life.3 NHs are required to partake in ACP by asking whether the patient has an advance directive on admission, yet 30% of NH residents do not have one documented.4 Advance directives are the documentation of one’s preferences of care should one lose capacity to communicate those wishes themselves. Although the use of advance directives among older adults has increased over time,5 such use is not universal and varies across NHs.6,7
Beginning in March 2016, the Pragmatic Trial of Video Education in Nursing Homes (PROVEN) began to examine whether exposure to an ACP video in the NH reduces subsequent hospital transfers.8 PROVEN protocol dictates every new NH admission should be offered the ACP video within 7 days. However, there was substantial variation in adherence to this protocol at the NH level that was not well explained by measurable facility characteristics.9 ACP is an iterative process between multiple interested parties: the residents, their family, and their healthcare providers.10 The success of ACP within the NH setting is complex and predicated on resident-, family-, healthcare professional–, and facility-level factors.11–13 Thus, this study seeks to address the following questions: (1) What resident characteristics are associated with differences in being offered and shown the video? (2) After controlling for resident characteristics, are the remaining differences driven by within-and/or across-facility variation?
METHODS
Data
This study includes all new admissions from 119 NHs in the intervention arm in PROVEN after the intervention launched until the end of the intervention, from March 1, 2016, through May 31, 2018.8 Data on resident characteristics come from the Minimum Data Set,14 which is a federally mandated assessment completed for all NH residents for the purposes of care planning and quality measures. Data on the intervention implementation come from a video status report, a form that was embedded in each NH’s electronic medical record system to track intervention implementation. The unique resident and NH identifier along with dates of when a video was offered and, if offered, whether it was shown are recorded in the video status report (and reason for not being shown).
Outcomes
There are two outcomes for this study: being offered the video and agreeing to watch (being shown) the video, contingent on being offered. The outcomes are measured within the prescribed 7-day window after admission using the date stamps from the video status report and a positive indicator that the video was shown. If the video was offered within 7 days of admission, we considered the video to have been offered. Alternatively, if a video was offered outside of the 7-day window or not offered at all, we recorded that no video was offered.
Covariates
We use the Minimum Data Set assessment at NH admission to obtain demographic and clinical characteristics. Demographic characteristics include sex, age, race, and marital status. Clinical characteristics used in this study include the Cognitive Function Scale, which measures cognitive impairment on a four-point scale from intact to severe,15 activities of daily living (ADLs), history of falls, life-limiting illnesses (cancer, coronary artery disease, heart failure, and chronic obstructive pulmonary disease), history of falls, pain, shortness of breath, Alzheimer disease or dementia, and psychiatric mood disorders. A resident was considered ADL dependent if he/she was assessed as having total dependence on one or more of the following ADLs: bed mobility, transferring, locomotion on unit, dressing, eating, toilet use, and personal hygiene. A life-limiting illness was defined as any of the following diseases, as recorded by a checkbox: cancer, coronary artery disease, heart failure, or chronic obstructive pulmonary disease. We considered someone to have Alzheimer disease or dementia if he/she had either a checkbox diagnosis or any International Classification of Diseases, Tenth Revision (ICD-10), code. Psychiatric mood disorders were indicated by checkbox and include the following: anxiety disorder, depression, manic depression, psychotic disorder, schizophrenia, or posttraumatic stress disorder. Family member involvement was measured using section Q of the Minimum Data Set,16 dichotomized as having or not having a family member present at the care planning meeting.
Statistical Analysis
First, we describe the resident characteristics of new admissions. In supplemental analysis, we also provide frequencies of the reasons the video was not shown. To examine what resident characteristics are associated with being offered and shown the video overall, we first run naive logistic regression ignoring resident clustering within the NH. Next, we used hierarchical logistic regression models to examine the association between resident characteristics and these two outcomes, accounting for resident clustering within NH. We transformed the regression coefficients into marginal effects to interpret the coefficients as percentage point (pp) changes relative to the outcome means. Stratified analysis was conducted to examine the differences between racial subgroups within a group of residents who were cognitively intact. We calculated the intraclass correlation coefficient to measure how much of the observed variation in offer and show rates can be explained by differences between NHs. The Brown University Institutional Review Board approved the study by expedited review.
RESULTS
Table 1 presents the characteristics of the new admissions to the intervention NHs and shows that the majority of new admissions were female (57%), aged older than 65 years (76%), and not married (69%). The sample was racially diverse, with 17% black residents and 10% other racial minorities. Less than half (40%) of the residents had some level of cognitive impairment, and 21% had a diagnosis of Alzheimer disease or dementia. Overall, a little over half (56%) of the new admissions were offered the video within 7 days of being admitted and 13% watched the video (22% of those offered). The majority of refusals, 70%, were by patient or family member; and 13% indicated that staff felt it was not indicated or the patient was medically unstable (Supplementary Table SS1).
Table 1.
Characteristics of New Admissions
| Characteristics | No. | % |
|---|---|---|
| 43 303 | 100 | |
| Outcomes | ||
| Offer rate | 19 251 | 57 |
| Show rate | 5503 | 13 |
| Sex | ||
| Female | 24 666 | 57 |
| Male | 18 637 | 43 |
| Age, y | ||
| <65 | 10 340 | 24 |
| 65–74 | 12 105 | 28 |
| 75–84 | 10 963 | 25 |
| ≥85 | 9865 | 23 |
| Race | ||
| White | 31 750 | 73 |
| Black | 7448 | 17 |
| Hispanic | 1254 | 3 |
| Other | 2851 | 7 |
| Not married | 30 083 | 69 |
| Married | 13 220 | 31 |
| Not ADL dependent | 32 085 | 74 |
| ADL dependent | 11 218 | 26 |
| Cognitive function scale | ||
| Intact | 25 402 | 60 |
| Mild | 8709 | 21 |
| Moderate | 6567 | 16 |
| Severe | 1495 | 4 |
| No family involvement | 31 552 | 73 |
| Family involvement in care planning | 11 751 | 27 |
| No life-limiting illness | 20 912 | 48 |
| Life-limiting illness | 22 391 | 52 |
| No history of falls | 27 808 | 64 |
| History of fall | 15 495 | 36 |
| Pain | ||
| No pain | 22 630 | 52 |
| Intermittent | 13 395 | 31 |
| Severe | 7278 | 17 |
| No shortness of breath | 36 518 | 84 |
| Shortness of breath | 6785 | 16 |
| No Alzheimer disease/dementia | 34 330 | 79 |
| Alzheimer disease/dementia | 8973 | 21 |
| No psychiatric/mood disorder | 26 181 | 60 |
| Any psychiatric/mood disorder | 17 122 | 40 |
Note. All characteristics measured from the Minimum Data Set. ADLs include: bed mobility, transferring, locomotion on unit, dressing, eating, toilet use, and personal hygiene. ADL dependence was defined as being totally dependent on one of the seven ADLs. Life-limiting illnesses include: cancer, coronary artery disease, heart failure, and chronic obstructive pulmonary disease. Psychiatric mood disorders were indicated by checkbox and include the following: anxiety disorder, depression, manic depression, psychotic disorder, schizophrenia, and posttraumatic stress disorder.
Abbreviation: ADL, activity of daily living.
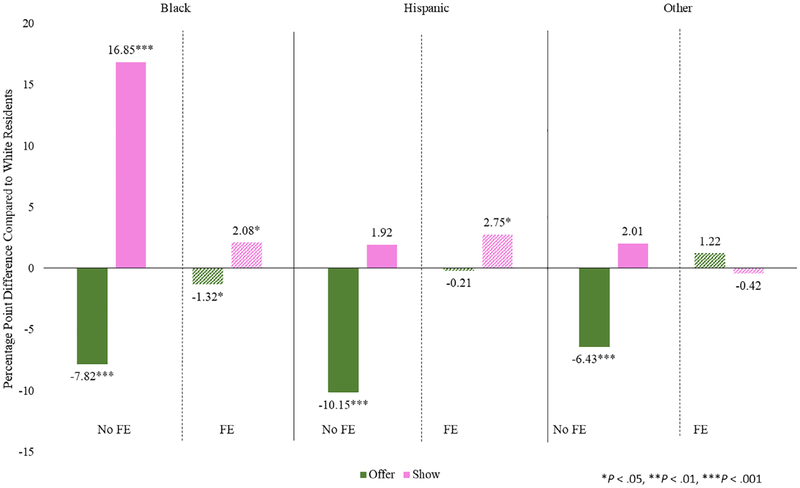
Figure 1 shows the main results by racial subgroup, presented in pp differences compared to white residents. In the model without fixed effects, compared to white residents, black residents are 7.8 pp (95% confidence interval [CI] = −9.14 to −6.50 pp) less likely to be offered the video. However, when including fixed effects, compared to white residents, black residents are 1.3 pp (95% CI = −2.61 to −0.02 pp) less likely to be offered the video. When assessing show rate differences in the model without fixed effects, black residents were 17 pp (95% CI = 15.12–18.58 pp) more likely to be shown the video, but with fixed effects, the difference decreased to 2.1 pp (95% CI = 0.43–3.74 pp). In stratified analyses with fixed effects among residents who were cognitively intact, the results were similar: black residents were 1.8 pp (95% CI = −3.56 to −0.10 pp) less likely to be offered the video and 2.6 pp less likely to be shown (95% CI = 0.46–4.69 pp).
Figure 1.
Differences in offer and show rates of advance care planning video by racial subgroups. FE indicates fixed effect.
Table 2 shows the full results examining resident characteristics associated with being offered the video. The results from model 1 (without NH fixed effects) show statistically significant differences across all resident characteristics. In model 2 (with NH fixed effects), the majority of the differences are no longer statistically significant or much smaller in magnitude. In both models, residents with mild or moderate cognitive impairment were 2 and 3 pp less likely to be offered the video. The intraclass correlation coefficient indicated 45% of the variation in offer rate was due to differences between NHs.
Table 2.
Resident Characteristics Associated With Being Offered an Advance Care Planning Video
| Characteristics | Percentage Point (95% CI) | |||
|---|---|---|---|---|
| Model 1: | Model 2: | |||
| Without NH Fixed Effects | With NH Fixed Effects | |||
| Sex | ||||
| Female | Ref. | Ref. | ||
| Male | −1.32** | (−2.31 to −0.34) | −0.63 | (−1.52 to 0.26) |
| Age, y | ||||
| <65 | −1.48* | (−2.81 to −0.16) | 0.04 | (−1.12 to 1.20) |
| 65–74 | Ref. | Ref. | ||
| 75–84 | 1.52* | (0.21 to 0.82) | 0.71 | (−0.43 to 1.84) |
| ≥85 | −3.41*** | (−4.82 to −2.00) | −0.67 | (−1.92 to 0.58) |
| Race | ||||
| White | Ref. | Ref. | ||
| Black | −7.82*** | (−9.14 to −6.50) | −1.32* | (−2.61 to −0.02) |
| Hispanic | −10.15*** | (−13.01 to −7.29) | −0.21 | (−2.80 to 2.38) |
| Other | −6.43*** | (−8.38 to −4.47) | 1.22 | (−0.75 to 3.20) |
| Not married | Ref. | Ref. | ||
| Married | 1.52** | (0.47 to 2.58) | 0.25 | (−0.60 to 1.10) |
| Not ADL dependent | Ref. | Ref. | ||
| ADL dependent | −4.08*** | (−5.18 to −2.97) | −2.84*** | (−4.42 to −1.26) |
| Cognitive Function Scale | ||||
| Intact | Ref. | Ref. | ||
| Mild | −1.88** | (−3.14 to −0.62) | −2.11*** | (−3.34 to −0.87) |
| Moderate | −3.31*** | (−4.91 to −1.72) | −3.53*** | (−5.10 to −1.97) |
| Severe | −2.02 | (−4.77 to 0.74) | −3.56* | (−6.28 to −0.85) |
| No family involvement | Ref. | Ref. | ||
| Family involvement in care planning | 0.48 | (−0.65 to 1.61) | 3.11*** | (1.48 to 4.73) |
| No life-limiting illness | Ref. | Ref. | ||
| Life-limiting illness | 1.89*** | (0.91 to 2.86) | 0.29 | (−0.96 to 1.53) |
| No history of falls | Ref. | Ref. | ||
| History of falls | 1.25* | (0.25 to 2.25) | 1.53** | (0.47 to 2.60) |
| Pain | ||||
| No pain | Ref. | Ref. | ||
| Intermittent | 0.97 | (−0.11 to 2.06) | 0.28 | (−0.85 to 1.41) |
| Severe | 1.82** | (0.46 to 3.17) | 1.33 | (−0.06 to 2.72) |
| No shortness of breath | Ref. | Ref. | ||
| Shortness of breath | −2.39*** | (−3.72 to −1.05) | 0.50 | (−0.90 to 1.90) |
| No Alzheimer disease/dementia | Ref. | Ref. | ||
| Alzheimer disease/dementia | −4.11*** | (−5.47 to −2.74) | −1.32 | (−2.68 to 0.03) |
| No psychiatric/mood disorder | Ref. | Ref. | ||
| Any psychiatric/mood disorder | −2.96*** | (−3.96 to −1.97) | −0.55 | (−1.54 to 0.44) |
| No. | 42 173 | 42 173 | ||
| Intraclass correlation coefficient | 0.45 | |||
Note. ADL dependence was defined as being totally dependent on one of the seven ADLs (bed mobility, transferring, locomotion on unit, dressing, eating, toilet use, and personal hygiene). Life-limiting illnesses include: cancer, coronary artery disease, heart failure, and chronic obstructive pulmonary disease. Psychiatric mood disorders were indicated by checkbox and include the following: anxiety disorder, depression, manic depression, psychotic disorder, schizophrenia, and posttraumatic stress disorder.
Abbreviations: ADL, activity of daily living; CI, confidence interval; NH, nursing home; Ref., reference.
P < .05.
P < .01.
P < .001.
Supplementary Table SS2 shows the results examining resident characteristics associated with being shown the video. In both models, residents with any level of cognitive impairment were less likely to be shown the video. The intraclass correlation coefficient was 0.63, indicating that 63% of the variation in show rates is attributable to differences between the NHs.
DISCUSSION
We sought to identify what resident characteristics were associated with the variation in who was offered and who viewed an ACP video and if the differences were driven by between or within NH variation. We found overall lower offer rates for black residents compared to white residents, but smaller differences in offer rate when controlling for NH fixed effects. Among those offered to view the video, black residents were more likely to have watched the video. In addition, persons with any level of cognitive impairment were less likely to be offered and shown the video. Finally, we also found high intraclass correlation coefficients, suggesting that approximately half of the variation in our outcome could be attributed to between NH differences practice variation of offering and showing the video.
There are known racial disparities in the quality of NH care.17–19 One persistent racial disparity in the NH setting is quality of end-of-life care. Black NH residents are less likely to use hospice and less likely to die in the hospital.20 Consistent with previous studies documenting that racial differences are partly driven by differences between NHs,21,22 we find that between NH practice variations can explain differential offer rates by racial group. We also found that once offered the video, black residents were more likely to watch the video compared to white residents. This finding may be indicative of differential information needs by these two racial subgroups. Consistently, studies have reported lower rates of care-limiting advance directives among black older adults compared to white older adults.5,7,23–26 One possible explanation for this disparity is that black patients are not offered optimal counseling and given the information necessary to make an informed choice.27 However, given that older black Americans tend to reside in NHs that are of lower quality and have fewer resources than NHs where their white counterparts reside,17,19 efforts to decrease ACP disparities should focus on NHs serving a high proportion of black residents to reduce differences between NHs.
Although residents with cognitive impairment are an important clinical subgroup for ACP, they were less likely to be offered and shown the video. This may reflect a reluctance from the family28 or previously having an ACP discussion with their family member prior to NH admission, which is unobservable in our data. We can, however, see that among residents with cognitive impairment, there were many reports of NH staff offering the video but recording they felt the video was not indicated at the time or the patient was medically unstable. Additionally, interviews with NH staff noted that there were some situations they thought it was inappropriate to offer the video, such as a patient being enrolled in hospice, that were not captured by our video status report.29
Our study finds a large percentage of the variation in showing and watching the videos could be attributed to the specific NH where the patient was admitted, with a 57% offer rate and a 22% show rate among those offered. This is not surprising since facility-level variation in implementation for short-stay residents varied from 0% to 100%,9 and other research has documented large variation in NH end-of-life care processes.13 Adherence to the intervention is consistent with other pragmatic trials conducted in NH settings. For example, in a pragmatic trial of Interventions to Reduce Acute Care Transfers (INTERACT), 32% of NHs began to use INTERACT regularly during the intervention window, and the positive effects of the program were concentrated in the newly adherent NHs.30 Although we do not have information on existing ACP activities at baseline, it is likely that there is some proportion of NHs that already had well-developed processes for having ACP conversations. These facilities may be less likely to offer the video. However, we would expect these underlying differences between NHs would impact the offer rate and not the show rates. Additionally, interviews with the NH staff suggest that they felt rushed to offer the video within the first week of admission given their other necessary tasks during resident intake.29
This study is not without limitations. First, the patient characteristics in this study are limited by the measurements available in the Minimum Data Set. Second, we do not know the underlying reason that the videos were offered or not. PROVEN was not designed to offer the videos only to residents with advance directives in place; rather, it was designed to offer the video to all residents. Given that black NH residents are less likely to have advance directives, the difference in viewing rate by race may be an artifact of baseline differences in advance directive use by race. If we were able to adjust for not viewing the video when an advance directive was in place, we may or may not see the same levels in viewing the video. Third, we acknowledge that there may be some measurement error in the outcome. Fourth, we focus our study population on new admissions instead of long-term residents, which likely have different patterns of implementation and willingness to watch the ACP video. Last, there are a number of NH-level characteristics, such as the interpersonal aspect of the end-of-life care process, that are unmeasured but could explain the variation between facilities.
Our study has important implications for promoting the use of ACP videos into routine use in the NH setting. There are racial disparities in the video program; black residents are less likely to be offered, but if offered are more likely to watch the video. This suggests that they are receptive to this type of ACP intervention but need to be given an opportunity to be exposed.
Supplementary Material
Supplementary Table S1:Reasons for a Video Not Being Shown for an Advance Care Planning Video Once Offered
Supplementary Table S2:Resident Characteristics Associated With Being Shown an Advance Care Planning Video
ACKNOWLEDGMENTS
Financial Disclosure: This work is supported within the National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreement UH3AG49619 from the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr Palmer was supported by grant T32AG023480, and Dr Mitchell is supported by grant K24AG033640, both from the NIA.
Sponsor’s Role: Funding sources did not have any role in the design, methods, subject recruitment, data collections, analysis, and preparation of the article.
Footnotes
Conflicts of Interest: Dr Mor is the chair of the Independent Quality Committee at HCR ManorCare, a paid consultant to NaviHealth, Inc, and chairs its Scientific Advisory Board, and the former director at PointRight, Inc. Lacey Loomer is a paid consultant to the American Health Care Association. Dr Volandes is president of the Nous Foundation Inc (http://www.ACPDecisions.org), a not-for profit (501[c]3) foundation that disseminates educational videos. Dr Volandes has a financial interest in the not for profit, which was reviewed and is managed by Massachusetts General Hospital and Partners Healthcare in accordance with their conflict of interest policies.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000–2015. JAMA. 2018; 320:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo P, Walker D, Bomba P. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: Insitute of Medicine; 2015. [Google Scholar]
- 4.Resnick HE, Schuur JD, Heineman J, Stone R, Weissman JS. Advance directives in nursing home residents aged≥ 65 years: United States 2004. Am J Hosp Palliat Med. 2009;25:476–482. [DOI] [PubMed] [Google Scholar]
- 5.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62:706–710. [DOI] [PubMed] [Google Scholar]
- 6.Levy CR, Fish R, Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005;53:2060–2068. [DOI] [PubMed] [Google Scholar]
- 7.Kiely DK, Mitchell SL, Marlow A, Murphy KM, Morris JN. Racial and state differences in the designation of advance directives in nursing home residents. J Am Geriatr Soc. 2001;49:1346–1352. [DOI] [PubMed] [Google Scholar]
- 8.Mor V, Volandes AE, Gutman R, Gatsonis C, Mitchell SL. Pragmatic trial of video education in nursing homes: the design and rationale for a pragmatic cluster randomized trial in the nursing home setting. Clin Trials. 2017;14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomer L, McCreedy E, Belanger E, et al. Nursing home characteristics associated with implementation of an advance care planning video intervention. J Am Med Dir Assoc. 2019;20:804–809. e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilissen J, Pivodic L, Gastmans C, et al. How to achieve the desired outcomes of advance care planning in nursing homes: a theory of change. BMC Geriatr. 2018;18:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilissen J, Pivodic L, Smets T, et al. Preconditions for successful advance care planning in nursing homes: a systematic review. Int J Nurs Stud. 2017;66:47–59. [DOI] [PubMed] [Google Scholar]
- 13.Temkin-Greener H, Zheng N, Norton SA, Quill T, Ladwig S, Veazie P. Measuring end-of-life care processes in nursing homes. Gerontologist. 2009;49:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services. MDS 3.0 RAI Manual v1.14. 2016. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-AssessmentInstruments/NursingHomeQualityInits/MDS30TechnicalInformationArchive.html. Accessed May 6, 2019.
- 15.Thomas KS, Dosa D, Wysocki A, Mor V. The minimum data set 3.0 cognitive function scale. Med Care. 2015;559:e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCreedy E, Loomer L, Palmer JA, Mitchell SL, Volandes A, Mor V. Representation in the care planning process for nursing home residents with dementia. J Am Med Dir Assoc. 2018;19:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mor V, Zinn J, Angelelli J, Teno JM, Miller SC. Driven to tiers: socioeconomic and racial disparities in the quality of nursing home care. Milbank Q. 2004;82:227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konetzka RT, Werner RM. Disparities in long-term care: building equity into market-based reforms. Med Care Res Rev. 2009;66:491–521. [DOI] [PubMed] [Google Scholar]
- 19.Smith DB, Feng Z, Fennell ML, Zinn JS, Mor V. Separate and unequal: racial segregation and disparities in quality across US nursing homes. Health Aff. 2007;26:1448–1458. [DOI] [PubMed] [Google Scholar]
- 20.Kwak J, Haley WE, Chiriboga DA. Racial differences in hospice use and in-hospital death among Medicare and Medicaid dual-eligible nursing home residents. Gerontologist. 2008;48:32–41. [DOI] [PubMed] [Google Scholar]
- 21.Zheng NT, Mukamel DB, Caprio T, Cai S, Temkin-Greener H. Racial disparities in in-hospital death and hospice use among nursing home residents at the end-of-life. Med Care. 2011;49:992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai S, Feng Z, Fennell ML, Mor V. Despite small improvement, black nursing home residents remain less likely than whites to receive flu vaccine. Health Aff. 2011;30:1939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degenholtz HB, Arnold RA, Meisel A, Lave JR. Persistence of racial disparities in advance care plan documents among nursing home residents. J Am Geriatr Soc. 2002;50:378–381. [DOI] [PubMed] [Google Scholar]
- 24.Frahm KA, Brown LM, Hyer K. Racial disparities in end-of-life planning and services for deceased nursing home residents. J Am Med Dir Assoc. 2012;13:819.e7–819.e11. [DOI] [PubMed] [Google Scholar]
- 25.Gerst K, Burr JA. Planning for end-of-life care: black-white differences in the completion of advance directives. Res Aging. 2008;30:428–449. [Google Scholar]
- 26.Rich SE, Gruber-Baldini AL, Quinn CC, Zimmerman SI. Discussion as a factor in racial disparity in advance directive completion at nursing home admission. J Am Geriatr Soc. 2009;57:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schickedanz AD, Schillinger D, Landefeld CS, Knight SJ, Williams BA, Sudore RLA. clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009; 57:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Steen JT, van Soest-Poortvliet MC, Hallie-Heierman M, et al. Factors associated with initiation of advance care planning in dementia: a systematic review. J Alzheimers Dis. 2014;40:743–757. [DOI] [PubMed] [Google Scholar]
- 29.Palmer JA, Mor V, Volandes AE, et al. A dynamic application of PRECIS-2 to evaluate implementation in a pragmatic, cluster randomized clinical trial in two nursing home systems. Trials. 2018;19:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huckfeldt P, Kane R, Yang Z. Degree of implementation of interact quality improvement program associated with reduced hospitalizations. J Am Geriatr Soc. 2018;66:1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1:Reasons for a Video Not Being Shown for an Advance Care Planning Video Once Offered
Supplementary Table S2:Resident Characteristics Associated With Being Shown an Advance Care Planning Video