Abstract
Dendritic cells are key players in regulating immunity. These cells both activate and inhibit the immune response depending on their cellular environment. Their response to hyperglycemia, a condition common amongst diabetics wherein glucose is abnormally elevated, remains to be elucidated. In this study, the phenotype and immune response of dendritic cells exposed to hyperglycemia were characterized in vitro and in vivo using the streptozotocin (STZ)-induced diabetes model. Dendritic cells were shown to be sensitive to hyperglycemia both during and after differentiation from bone marrow precursor cells. Dendritic cell behavior under hyperglycemic conditions was found to vary by phenotype, of which tolerogenic dendritic cells were particularly sensitive. Expression of the costimulatory molecule CD86 was found to reliably increase when dendritic cells were exposed to hyperglycemia. Additionally, hydrogel-based delivery of the anti-inflammatory molecule interleukin-10 (IL-10) was shown to partially inhibit these effects in vivo.
Keywords: dendritic cells, immunity, hyperglycemia, diabetes, hydrogel, interleukin-10
Introduction
One-tenth of the United States is afflicted by diabetes and nearly 2 million new cases are diagnosed each year. Diabetics have defective insulin signaling, a key regulator of glucose, lipids, and other metabolites (Saltiel & Kahn, 2001). While some diabetics lack insulin due to autoimmunity against insulin-producing beta cells, known as type-1 diabetes, the majority of diabetics lack responsiveness to insulin, known as type-2 diabetes. For both types of diabetes, exogenous insulin administration is the recommended treatment; however, monitoring glucose and reducing its levels via insulin administration remain difficult, resulting in poor glycemic control (Henske, Griffith, & Fowler, 2009).
Hyperglycemia, a condition of high glucose that is common amongst diabetic patients, has been linked to a myriad of diabetes-related complications whose origins remain unclear. Chronic high glucose is a major cause of β-cell death (Zhang et al., 2010), furthering the progression of diabetes. Dysfunction and death in endothelial cells in hyperglycemic environments (Lee et al., 2014) have been shown to lessen the extent of angiogenesis and impair vascularization (Dobler, Ahmed, Song, Eboigbodin, & Thornalley, 2006). Enhanced extracellular matrix deposition in renal fibroblasts upon hyperglycemic exposure can lead to kidney fibrosis (Zeisberg, Potenta, Sugimoto, Zeisberg, & Kalluri, 2008). Apoptosis of liver cells has been induced in diabetic rats not receiving insulin therapy (Frances et al., 2010). Neuropathy is also common in hyperglycemia and diabetes (Leppin et al., 2014). More recently, hyperglycemia has been linked to increased immune cell numbers and the inflammatory disease atherosclerosis (Nagareddy et al., 2013).
Dendritic cells activate or inhibit the immune response, depending on the environmental cues they are exposed to during differentiation and maturation (Menges et al., 2002), yet their presence and phenotype under hyperglycemia have been relatively unexplored (Lu et al., 2013). These cells are considered central figures in the immune system, interacting with T-cells, B-cells, and macrophages to dictate the immune environment (Balázs, Martin, Zhou, & Kearney, 2002; Jung et al., 2002; Ravishankar et al., 2014). In the NOD type 1 diabetes model, increased dendritic cell presence proximal to islets is well established as an initiator and progressor of the disease (Allen et al., 2009; Magnuson et al., 2015). Increased numbers of dendritic cells in organs outside of the pancreas have also been observed in this model (Gyurko et al., 2006). However, the contribution of hyperglycemia to dendritic cell behavior in diabetics remains to be elucidated due to the confounding autoimmune component of the type 1 diabetes model.
In this study, activation of dendritic cells upon exposure to hyperglycemia was investigated in vitro and in vivo. Dendritic cell phenotype was characterized using established surface markers as well as immunostimulation assays. In vitro dendritic cell regulation of immune cells in euglycemic and hyperglycemic environments were assessed for immature, tolerogenic, and mature subtypes. In vivo dendritic cell phenotype and immune cell presence were characterized in the pancreatic lymph nodes, spleen, and bone marrow of STZ-induced diabetic mice and compared to non-diabetic and naïve controls. To counteract immune cell activation in hyperglycemic animals, IL-10 was delivered using an in situ gelling material. These studies reveal the activation and plasticity of the immune state under hyperglycemic conditions and the need to incorporate immunomodulatory strategies in therapies for hyperglycemic disorders.
Materials and Methods
Flow Cytometry
To stain intracellularly, cultured cells were incubated for 4 hr at 37 °C in media with both brefeldin-A (1 μg/mL, Sigma B6542) and monensin (2 μM, Sigma M5273), then blocked for Fc-receptors (1:200, eBioscience 14–0161-86) at 4 °C for 15 min, fixed for 15 min with paraformaldehyde [2% in phosphate buffered saline (PBS), Sigma 158127] at room temperature, permeabilized for 15 min with Tween-20 (0.5%, Sigma P2287) at room temperature, and stained. Otherwise, cell suspensions (2–5 × 106 cells/mL) were blocked for Fc-receptors for 15 min and incubated with antibodies for 30 min at 4 °C. Fluorescence-activated cell sorter (FACS) wash was used for all solutions unless indicated: bovine serum albumin (BSA) (1%, Sigma A4503) and sodium azide (0.01%, Mallinckrodt 195–3-57) in PBS (Lonza, 17–516F). Antibodies were diluted 1:100 (FITC) or 1:200 (others) except where indicated (table 1 and table 2). Stained cells were analyzed for mean fluorescence intensity (MFI) using a 5-channel flow cytometry (BD LSR II Flow Cytometer, RRID:SCR_002159) scheme (supplemental figure 1). In brief, debris was excluded via a scatter gate. Then, immune cells were separated via a CD11c+/CD11b+ gate. Lastly, marker expression or presence within these quadrants was quantified. To assess regulatory cell presence, marker overlap within these quadrants was also quantified.
Table 1.
Antibodies used for immune cell characterization
| Marker | Role |
|---|---|
| CD3ε | BD Biosciences Cat# 553064, RRID:AB_394597 |
| BioLegend Cat# 100311, RRID:AB_312676 | |
| CD4 | BioLegend Cat# 100512, RRID:AB_312715 |
| BD Biosciences Cat# 553051, RRID:AB_398528 | |
| CD8a | BioLegend Cat# 100707, RRID:AB_312746 |
| CD19 | BD Biosciences Cat# 550992, RRID:AB_398483 |
| CD49b | BD Biosciences Cat# 554999, RRID:AB_395633 |
| CD49d | BD Biosciences Cat# 553157, RRID:AB_394670 |
| IL-10 | 1:100, BD Biosciences Cat# 554467, 1:100, RRID:AB_395412 |
| 1:100, BioLegend Cat# 505008, RRID:AB_315362 | |
| Ly6c | BD Biosciences Cat# 560595, RRID:AB_1727554 |
| Ly6g | BD Biosciences Cat# 551460, RRID:AB_394207 |
Table 2.
Antibodies used for dendritic cell characterization
| Marker | Role |
|---|---|
| CD11bi | BD Biosciences Cat# 562287, RRID:AB_11154216 |
| CD11c1 | 1:100, BD Biosciences Cat# 558079, RRID:AB_647251 |
| 1:100, Thermo Fisher Scientific Cat# 45–0114-82, RRID:AB_925727 | |
| CD45RAii | 1:800, BD Biosciences Cat# 553380, RRID:AB_394822 |
| CD54iii | BioLegend Cat# 116120, RRID:AB_10612936 |
| CD86iv | Thermo Fisher Scientific Cat# 17–0862-82, RRID:AB_469419 |
| BD Biosciences Cat# 553692, RRID:AB_394994 | |
| CD804 | Thermo Fisher Scientific Cat# 11–0801-86, RRID:AB_465135APC: |
| Biolegend 104714 | |
| IABv | BD Biosciences Cat# 553551, RRID:AB_394918 |
| 1:400, BD Biosciences Cat# 553552, RRID:AB_394919 | |
| Qa2vi | Thermo Fisher Scientific Cat# 11–5996-85, RRID:AB_465353 |
Identifies dendritic cell subtypes
Diao, J., Winter, E., Chen, W., Cantin, C., & Cattral, M. S. (2004). Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol, 173(3), 1826–1833.
Liu, Y. J. (2001). Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell, 106(3), 259–262.
Identifies naïve dendritic cells.
Naik, S. H., Sathe, P., Park, H. Y., Metcalf, D., Proietto, A. I., Dakic, A., … Shortman, K. (2007). Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol, 8(11), 1217–1226. doi: 10.1038/ni1522
Regulates antigen uptake.
Sheikh, N. A., & Jones, L. A. (2008). CD54 is a surrogate marker of antigen presenting cell activation. Cancer Immunol Immunother, 57(9), 1381–1390. doi: 10.1007/s00262-008-0474-9
Regulates adaptive immunity.
Fujii, S., Liu, K., Smith, C., Bonito, A. J., & Steinman, R. M. (2004). The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med, 199(12), 1607–1618. doi: 10.1084/jem.20040317
Regulates antigen presentation.
Wilson, N. S., El-Sukkari, D., & Villadangos, J. A. (2004). Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood, 103(6), 2187–2195. doi: 10.1182/blood-2003-08-2729
Regulates alternative antigen processing.
Oliveira, Cláudia C., van Veelen, Peter A., Querido, Bianca, de Ru, Arnoud, Sluijter, Marjolein, Laban, Sandra, … van Hall, Thorbald. (2010). The nonpolymorphic MHC Qa-1<sup>b</sup> mediates CD8<sup>+</sup> T cell surveillance of antigen-processing defects. The Journal of Experimental Medicine, 207(1), 207–221. doi: 10.1084/jem.20091429
Shang, Shaobin, Siddiqui, Sarah, Bian, Yao, Zhao, Jie, & Wang, Chyung-Ru. (2016). Nonclassical MHC Ib-restricted CD8+ T Cells Recognize Mycobacterium tuberculosis-Derived Protein Antigens and Contribute to Protection Against Infection. PLOS Pathogens, 12(6), e1005688. doi: 10.1371/journal.ppat.1005688
Streptozocin (STZ)-Mediated Diabetes Induction
All studies with animals were according to the Emory University and the Georgia Institute of Technology ACUC guidelines. Male mice (6–12 week old C57Bl/6, Jackson Laboratories) were injected intraperitoneally with a single dose of STZ (200 mg/kg, LKT laboratories, S7870) in DPBS 1X(Dulbecco’s Phosphate-Buffered Saline, without calcium & magnesium). Blood was extracted from the tail vein and monitored using a standard glucose meter daily. Experiments were performed 4 days after diabetes (> 250 mg/dL) was confirmed.
In Situ Delivery of IL-10
To form the hydrogel, maleimide-functionalized 4-arm poly(ethylene glycol) (PEG) monomer ( 20 kDa, Laysan Bio, PEG 4-MAL) was functionalized with RGD peptide (2.0 mM, GRGDSPC, Aapptec) for cell infiltration and crosslinked with a collagenase-sensitive peptide (GCRDVPMS↓MRGGDRCG, Aapptec) via Michael addition to form a 5.0% w/v hydrogel as described previously (Phelps, Headen, Taylor, Thule, & Garcia, 2013). Interleukin-10 protein (1.0 μg) was also incorporated without modification into the gel. Male mice (C57Bl/6, Jackson Laboratories) 6–12 week old were injected subcutaneously using an insulin (28G) syringe with 100 μL of PEG hydrogel precursor solution and allowed to polymerize in situ. Untreated mice served as a control. Immune cells were analyzed 4 days after IL-10 delivery.
Derivation of Dendritic Cells
Bone marrow cells of male mice (6–12 week old C57Bl/6, Charles River or Jackson Laboratories) were extracted and incubated in a lysis buffer for 5 min at room temperature to remove red blood cells. Animal source was maintained within experiments. Values for experimental outcomes are consistent within animal source used and may not be between experiments. After red blood cell lysing, the remaining marrow cells were cultured in 6-well plates (1× 106 cells/well) for 6 days with basal media (3ml/well) supplemented with granulocyte macrophage-colony stimulating factor (GM-CSF) (20 ng/mL, BD 554586) and interleukin-4 (IL-4) (4 ng/mL, Peprotech AF-214–14) for 6 days with half the media exchanged every 2 days to generate immature dendritic cells. To generate mature dendritic cells, lipopolysaccharide (LPS) (500 ng/mL, Sigma L4180) was added to the media on day 4, while to generate tolerogenic dendritic cells, IL-10 (10 ng/mL, eBioscience 14–8101-62) and tumor necrosis factor-α (TNF-α) (10 ng/mL, BD 554589) were added to the media from start of culture (Wakkach et al., 2003). Basal media consisted of Dulbecco’s Modified Eagle Medium (DMEM) (Gibco 11885–084, 11995–065) supplemented with fetal bovine serum (FBS) (10%, Gibco 16000–044), penicillin-streptomycin (1%, CellGro, 30–002-CI), sodium pyruvate (1%, CellGro, 25–000-CI), nonessential amino acids (1%, CellGro 25–025-CI), and beta-mercaptoethanol (0.1%, Gibco 21985–033). Lysis buffer included sodium chloride (155mM, Sigma 37653), potassium bicarbonate (10 mM, Sigma P9144), and ethylenediaminetetraacetic acid (EDTA) (0.1 mM, LifeTech 11267–028) in distilled water. Mannose (350 mg/dL, Sigma M4625) was included in the low-glucose media formulation as an osmotic control. Dendritic cells derived in low-glucose media were then cultured in basal media of varying glucose content for 3 days.
Autologous Immunostimulation Assay
Dendritic cells (5 × 105 cells) were cultured in basal media (200 μL) for 72 hr in a U-bottom 96-well plate with autologous splenocytes (5 × 105 cells). Splenocytes (5 × 106 cells/mL) were separated from red blood cells via a 5 min incubation in lysis buffer at room temperature, primed against ovalbumin, OVA257–264/323–229, (5 μg/mL each in basal media, Genscript RP10610/RP10611) antigen for 6 days, and labeled with carboxyfluorescein succinimidyl ester (CFSE) (0.5 μM in PBS, LifeTech C34554) for 15 min at 37 °C. Dendritic cells were primed to target OVA (10 μg/mL each in basal media) for 2 hr at 37 °C. Splenocyte proliferation (CFSELO) after 3 days of co-culture was measured using flow cytometry. To measure IL-10 production in the co-culture (1:1 ratio of DCs and splenocytes), the supernatant was quantified using a standard ELISA kit (R&D, M100B) that measured the concentration at 450 nm.
Phorbol 12-myristate 13-acetate (PMA)/Ionomycin-Mediated Stimulation Assay
CFSE-labeled splenocytes (5 × 105 cells) were cultured in basal media (200 μL) for 72 hr in a 96-well plate with or without PMA (5 ng/mL, Sigma P8139) and ionomycin (250 ng/mL, Calbiochem 407952) and assessed using flow cytometry for proliferation as described above.
Statistics
Comparisons were analyzed with a t-test or a one-way ANOVA with a Tukey post-hoc as appropriate. Significance was defined as p < 0.05.
Results
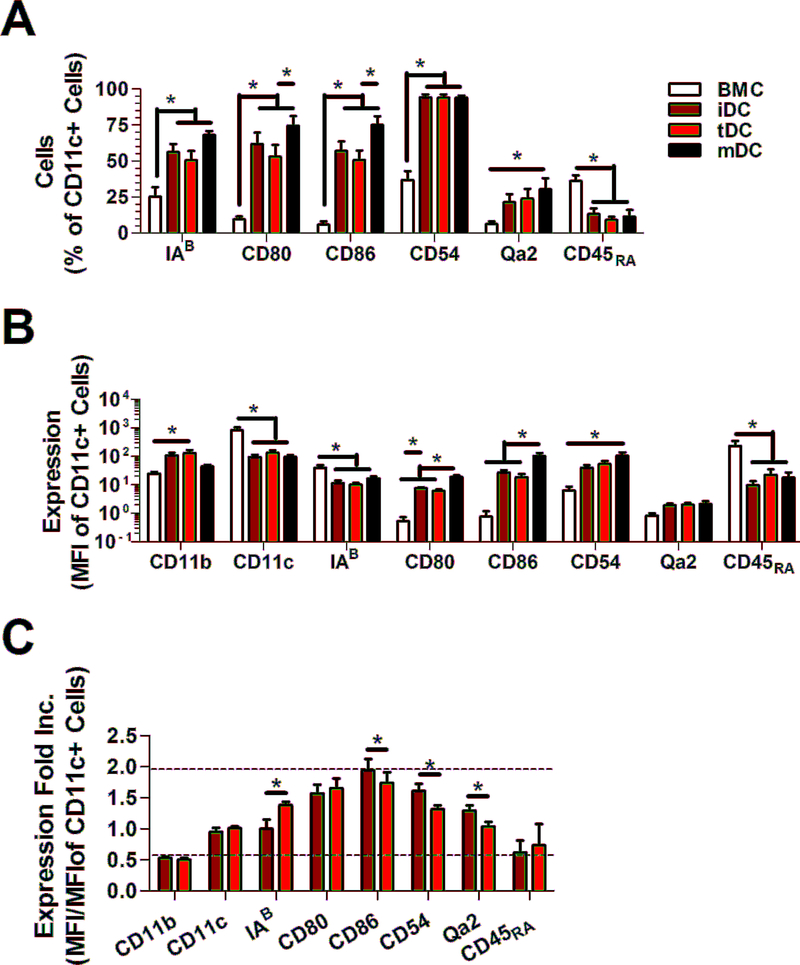
Dendritic cells were primed towards an immature, mature or tolerogenic phenotype. Phenotype was confirmed using historic surface markers CD11b, CD11c, IAB (MHC-II), CD80, CD86, CD54, Qa-2 (MHC-I), and CD45RA, which are all known to play a role in their uptake and presentation of antigens to T cells for their effective stimulation (table 2, fig. 1). These markers primarily differentiated freshly isolated and mature dendritic cells from immature and tolerogenic ones. Thus tolerogenic dendritic cells were distinguished from immature dendritic cells based on their relative stability upon LPS stimulation and their enhanced immunosuppression of T-cell and B-cell proliferation. Immunosuppression by tolerogenic dendritic cells were antigen-specific and lowered the proliferation stimulated by mature dendritic cells (suppl. fig. 2).
Figure 1.
Profiles of surface markers on dendritic cells. (a) Prevalence (%) and (b) MFI level of expression of surface markers on CD11c+ immature (iDC) and tolerogenic dendritic cells (tDC). (c) Fold increase of marker expression (MFI/MFI) upon exposure to 1 μg/mL LPS for 24 hr for iDC and tDC. Mature(mDC) dendritic cells and bone marrow cells (BMC) were assayed as controls. * indicates p < 0.05. n = 8.
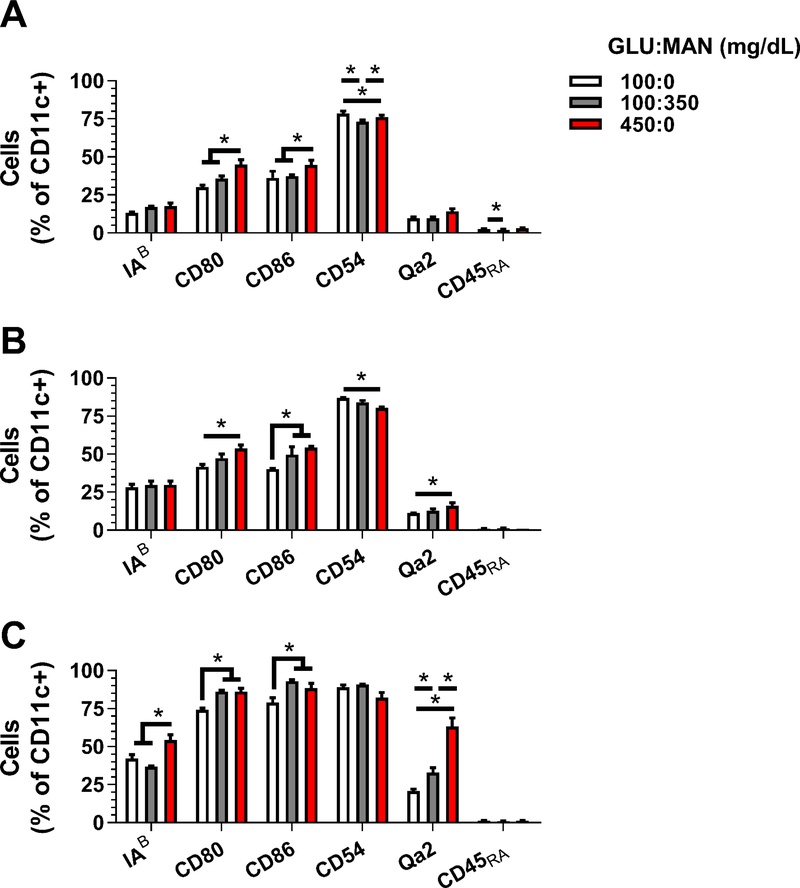
Dendritic cells were exposed in vitro to hyperglycemic (450 mg/dL) and euglycemic (100 mg/dL) media during (fig. 2) or after (fig. 3) derivation from bone marrow cells. Tonicity has been found to influence dendritic cell phenotype similar to reports of other immune cell types (Frenkel et al., 2001; Woehrle et al., 2010) and yield (suppl. fig. 3); therefore, euglycemic (100 mg/dL) media containing mannitose (350 mg/dL) was used as an osmotic control. The surface marker profile of immature dendritic cells largely did not significantly change. However, the presence of co-stimulatory molecule CD86 increased in tolerogenic dendritic cells upon exposure to hyperglycemic media (figs. 2, 3). The ability of tolerogenic dendritic cells to incorporate antigen also decreased when derived in high glucose-containing media (suppl. fig. 3). The combination correlated to a significant lessening in the immunosuppressive properties of high glucose-derived dendritic cells (fig. 4) when co-cultured with OVA-activated splenocytes. Splenocyte proliferation by mature dendritic cells also increased when derived in high glucose-containing media, and may in part be explained by the increase in MHC molecules IAB and Qa-2. Secretion of IL-10 in these co-cultures was largely unaffected by glucose (suppl. fig. 3).
Figure 2.
Prevalence (%) of surface markers on CD11c+ (a) tolerogenic (tDC), (b) immature (iDC), and (c) mature (mDC) dendritic cells cultured for 6 days days in media containing low (GLU, 100 mg/dL, 100:0) or high levels of glucose (450 mg/dL, 450:0) during derivation. Mannose (MAN, 350 mg/dL) was included in low-glucose containing media (100:350) as an osmotic control. * indicates p < 0.05. n = 6.
Figure 3.
Prevalence (%) of surface markers on CD11c+ (a) tolerogenic (tDC), (b) immature (iDC), and (c) mature (mDC) dendritic cells cultured for 3 days days in media containing low (GLU, 100 mg/dL, 100:0) or high levels of glucose (450 mg/dL, 450:0) after derivation. Mannose (MAN, 350 mg/dL) was included in low-glucose containing media (100:350) as an osmotic control. * indicates p < 0.05. n = 6.
Figure 4.
Immune cell stimulation by bone-marrow derived dendritic cells cultured in media containing low (GLU, 100 mg/dL, 100:0) or high levels of glucose (450 mg/dL, 450:0) during derivation. CFSE-labeled splenocytes were cultured with dendritic cells (1:1) and assayed (a-d) for proliferation (CFSELO) or (e-g) for markers of regulation. Mannose (MAN, 350 mg/dL) was included in low-glucose containing media (100:350) as an osmotic control. * indicates p < 0.05. n = 6.
In vivo immune cell presence and phenotype due to hyperglycemia were altered in three immunogenic organs: the pancreatic lymph nodes, the bone marrow, and the spleen after 4 days of STZ-induced hyperglycemia. T-cells (CD3ε+) (Gregori, Giarratana, Smiroldo, & Adorini, 2003), B-cells (CD19+) (DeFuria et al., 2013), dendritic cells (CD11c+) (Allen et al., 2009; Nikolic, Geutskens, van Rooijen, Drexhage, & Leenen, 2005; Rosmalen, Homo-Delarche, et al., 0000; Rosmalen, Martin, et al., 0000), and monocytes/macrophages (CD11c-/LO/CD11b+) (Homo-Delarche et al., 2006; Nikolic et al., 2005; Rosmalen, Homo-Delarche, et al., 0000; Rosmalen, Martin, et al., 0000) were assessed in these areas due to their known roles in the progression of type 1 and type 2 diabetes. The presence of natural killer cells (CD49b+) and eoisinophils (CD49d+) were also assessed at these locations. Increased numbers of T-cells were observed in the pancreas and bone marrow of hyperglycemic (STZ+/DIA+) mice compared to naïve (STZ-/DIA-) and STZ-injected euglycemic (STZ+/DIA-) controls (fig. 5), consistent with the literature (Gregori et al., 2003; Magnuson et al., 2015); however, their phenotype was largely unchanged (suppl. fig. 4 and 5). In the spleen, effector T-cells (CD4+/CD45RB-) increased in presence, consistent with reports of hyperglycemia-induced T-cell activation (Martinez et al., 2014). Dendritic cell presence was unchanged; however, dendritic cells had a more mature, activated phenotype (increased CD86 expression) in all three locations (fig. 6). These alterations corresponded to an increase in innate and PMA/ionomycin-mediated stimulation of splenic T-cell (CD4+) proliferation from hyperglycemic mice compared to controls (fig. 7) (Martinez et al., 2014). The presence (fig. 5) and phenotype (suppl. fig 5 and 6) of monocytes/macrophages and other immune cells were unchanged.
Figure 5.
Immune cell presence in mice after 4 days of streptozocin-induced hyperglycemia (STZ+/DIA+, n = 6) in the (a) pancreatic lymph nodes, (b) bone marrow, and (c) spleen. Streptozocin-injected, euglycemic (STZ+/DIA-, n = 4) and naïve (STZ-/DIA-, n = 6) mice served as a control. * indicates p < 0.05.
Figure 6.
Prevalence (%) of surface markers on CD11c+ dendritic cells after 4 days exposure to streptozotocin-induced hyperglycemia (STZ+/DIA+, n = 6) in the (a) pancreatic lymph nodes, (b) bone marrow, and (c) spleen. Streptozotocin-injected, euglycemic (STZ+/DIA-, n = 4) and naïve (STZ-/DIA-, n = 6) mice served as a control. * indicates p < 0.05.
Figure 7.
Immune cell stimulation in hyperglycemic mice (STZ-injected, STZ+/DIA+, n = 3) to PMA with ionomycin (+PI) compared to euglycemic (STZ+/DIA-, n = 4) and naïve (STZ-/DIA-, n = 3) controls. CFSE-labeled splenocytes were cultured with or without PI and assayed (a-d) for proliferation (CFSELO) or for markers of (e-g) regulation. * indicates p < 0.05.
The anti-inflammatory cytokine, IL-10, was delivered using an in situ gelling PEG hydrogel to lessen the maturation and activation of dendritic cells in vivo. Four days after implantation into the subcutaneous space, the hydrogel was fully integrated with host tissue and thus not retrievable; therefore, systemic immunomodulation was assessed in the spleen. Delivery of IL-10 resulted in a lessening in splenic dendritic cell (CD11c+) presence and a corresponding decrease in maturation of their monocytic precursors (CD11c-/LO/CD11b+, CD86) (fig. 8). T-cell phenotype in the spleen was largely unchanged (suppl. fig. 5). These alterations corresponded to an increased stimulation of regulatory T-cell (CD4+/IL-10+) and B-cell (CD19+/IL-10+) proliferation upon exposure PMA and ionomycin (fig. 9). Interestingly, we also observed an increase in CD3+/CD4-/CD8- T-cells, which has recently been suggested to potentially be a regulatory cell (Miyagawa, Okiyama, Villarroel, & Katz, 2013). Administration of IL-10 did not affect blood glucose levels, nor did it offset the splenic atrophy seen in diabetic mice (Faustman, Giesecke, Davis, Kühtreiber, & Tran, 2014) (suppl. fig. 7).
Figure 8.
Immunomodulation in the spleen of hyperglycemic mice that received hydrogel (Hyd)-delivered IL-10 (n = 4) compared to an untreated, hyperglycemic control (n = 3). (a) Immune presence and (b-d) surface marker profiles of (b) CD11c+ dendritic cells, (c) CD11b+ monocytes/macrophages, and (d) other leukocytes. * indicates p < 0.05.
Figure 9.
Immune cell stimulation in hyperglycemic mice that received hydrogel (Hyd)-delivered IL-10 (n =4) to PMA with ionomycin (+PI) compared to an untreated, hyperglycemic control (n = 3). CFSE-labeled splenocytes were cultured with or without PI and assayed assayed (a-d) for proliferation (CFSELO) or for markers of (e-g) regulation. * indicates p < 0.05. n = 4.
Discussion
Evidence has emerged recently that the immune state is altered in diabetes (Erbağci, Tarakçioğlu, Coşkun, Sivasli, & Sibel Namiduru, 2001; Magnuson et al., 2015), which may be responsible for many of the co-morbidities and medical complications associated with this disease. These changes are also highly dynamic. Acute studies (<6 weeks of hyperglycemia) have shown an increase in the activation of immune cells, corresponding to a higher incidence of inflammation-mediated disease, including atherosclerosis and periodontitis (Gregori et al., 2003; Gyurko et al., 2006; Nagareddy et al., 2013). In contrast, chronic studies revealed a lessening in immunity, resulting in higher incidences of sepsis and chronic infection (Jacob et al., 2008; Sun et al., 2012; Vallerskog, Martens, & Kornfeld, 2010). Williams et al revealed that when compared to dendritic cells of nondiabetic mice, LPS- and B.pseudomallei-induced cytokine production of diabetic mice that were hyperglycemic for 9 days and 70 days contrasted for IL-12, IL-18, and IL10 (Williams, Morris, Rush, Govan, & Ketheesan, 2011). This shift in immune responses over time may be the result of the sustained hyperactive state of immune cells in the early stages of diabetes triggering an immune shutdown in later in the disease course. This study focused on early (<4 days) changes in immunity upon exposure to hyperglycemic conditions in vivo (>350 mg/dL), which revealed immune activation primarily in the phenotype of dendritic cells. The relatively high turnover of dendritic cells and their monocytic precursors compared to T-cells, B-cells, and macrophages (Forster & Rajewsky, 1990; Kamath et al., 2000; Westera et al., 2013; Yona et al.), their role in activating these cells in other models (Balázs et al., 2002; Jung et al., 2002; Ravishankar et al., 2014), and the widespread changes in dendritic cell phenotype compared to other immune cells in this study suggest dendritic cells drive the activated immune state in hyperglycemic conditions.
In vitro studies revealed hyperglycemia affects dendritic cells differently for different subtypes. For immature dendritic cells, their surface marker profile and stimulatory capacity were unchanged. For mature dendritic cells, the expression of MHC molecules increased, which may explain their enhanced stimulation of splenocytes in hyperglycemic conditions. For tolerogenic dendritic cells, the expression of co-stimulatory molecule CD86 was enhanced, which may explain the increased proliferation of splenocytes in the presence of high glucose. The secretion of IL-10 in dendritic cell-splenocyte co-culture was largely unaffected by high glucose (450 mg/dL). In the literature, IL-10 production by derived iDCs exposed to 270 mg/dL glucose was unaffected (Whiteson, Agrawal, & Agrawal, 2017). T-cells co-cultured with these dendritic cells had similar levels of cytokine production. In well-compensated and poorly compensated diabetes, IL-10 production upon co-culture of T-cells with derived iDCs and tDCs was comparable (Danova et al., 2017). In contrast, both of these reports revealed enhanced secretion of inflammatory cytokines (IL-6, INF-γ, TNF-α) in hyperglycemic conditions. The effects of hyperglycemia on dendritic cells in vitro were recapitulated in vivo. Dendritic cells of diabetic mice at all three immunogenic sites had higher levels of CD86 expression compared to non-diabetic and naïve controls. Hyperglycemia in vivo increased the activation of PMA/ionomycin-stimulated splenocytes. Thus, presumably, the alterations of dendritic cell phenotype in vivo is primarily due to activation of tolerogenic dendritic cells, as these cells were similarly affected in vitro.
In vivo studies using STZ-mediated diabetes induction revealed immunological effects due to hyperglycemia and due to the drug itself. Diabetes induction with STZ administration is the most common model for hyperglycemia and islet transplantation. The drug is transported via GLUT2 primarily into β-cells, upon which it then alkylates DNA and donates nitric oxide to induce cell death (Szkudelski, 2001). However, this transporter is also present in major organs—liver, kidney, small intestines, and brain (astrocytes)—which are sensitive to hyperglycemia (Corpe et al., 1996; Goestemeyer, Marks, Srai, Debnam, & Unwin, 2007; Slieker et al., 1992) and STZ (Deeds et al., 2011; Imaeda, Kaneko, Aoki, Kondo, & Nagase, 2002; Kume et al., 2004). Typically, injection of the carrier without the drug is used as the control for hyperglycemia studies in vivo; however, alterations in immune cell phenotype have been observed using Alloxan (Schroder, Palinski, & Schmid-Schonbein, 1991), a drug that induces hyperglycemia with a similar mechanism. An increase in B-cell (CD19) and eoisinophil (CD49d) presence was found in the spleen and bone marrow, respectively, of STZ-injected, euglycemic mice. These findings emphasize the need to use STZ-injected euglycemic mice or similar controls to account for side effects of the drug.
Exogenous IL-10 was found to lessen the immune stimulation caused by hyperglycemia. An RGD-containing, injectable hydrogel was used to deliver the IL-10 proximal to pancreatic lymph nodes. Interestingly, both PEG (Son et al., 2013) and RGD (Acharya et al., 2010; Zaveri, Lewis, Dolgova, Clare-Salzler, & Keselowsky, 2014) has been shown in vitro to promote the activation of dendritic cells, and thus, may have counteracted some of the effects of the IL-10. In type 1, type 2 and gestational diabetes alterations in inflammatory cytokine secretion are well documented. Notably, an increase in the ratio of TNF-α to IL-10, which has been observed both in patients (Devaraj et al., 2007; Moreli et al., 2015) and in mouse models (Alleva, Pavlovich, Grant, Kaser, & Beller, 2000). The cytokines TNF-α and IL-10 have prominent and opposing roles in immunity: TNF-α matures dendritic cells (Chomarat, Dantin, Bennett, Banchereau, & Palucka, 2003), IL-10 inhibits their maturation (Allavena et al., 1998), and their combination can promote a tolerogenic phenotype in vitro (Boks et al., 2012). Four days after IL-10 delivery, the presence of mature dendritic cells (CD11c+) and the maturity of their precursors (CD11c-/LO/CD11b+) was lessened compared to mice that did not receive IL-10. Furthermore, splenic stimulation by PMA and ionomycin was lowered compared to non-treated controls. The rapid half-life of dendritic cells and their monocytic precursors in vivo, 1–2 days (Kamath et al., 2000; Yona et al.), permits rapid changes in the immune state, which may potentially explain why short-term exposure to hyperglycemia dramatically alters the profile of dendritic cells (CD11c+) in vivo and why a single dose of IL-10 counteracted those changes via inhibition of their monocytic precursors (CD11c-/LO/CD11b+).
Conclusion
In this study, changes to dendritic cell phenotype upon hyperglycemic exposure were evaluated, and the activation of these cells (increased CD86 expression) in the presence of high glucose levels was observed both in vitro and in vivo. This activation occurred rapidly (<4 days), suggesting plasticity of the immune state. Delivery of IL-10 from an injectable hydrogel counteracted some of the immunological changes in dendritic cells due to hyperglycemia. These studies revealed the role of hyperglycemia in diabetic immune dysfunction, particularly of dendritic cells, which has implications in both medical complications and transplantation-based therapies.
Supplementary Material
Acknowledgements
This work was supported by NIH (ACTSI, UL1TR000454; 1 R21 EB019166–01A1), Georgia Partner’s Regenerative Medicine (REM) seed grant, and Georgia Immunoengineering Consortium’s seed grant. The first author received support from the ILET2 training grant (1 R90 DK098981, 1 T90 DK097787). Authors would like to thank Dr. Allen D. Kirk for consultation regarding immunity in diabetes.
Footnotes
Data Availability: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare they do not have any conflicts of interest to disclose regarding this work.
References
- Acharya AP, Dolgova NV, Moore NM, Xia CQ, Clare-Salzler MJ, Becker ML, … Keselowsky BG (2010). The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials, 31(29), 7444–7454. doi: 10.1016/j.biomaterials.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, & Mantovani A (1998). IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol, 28(1), 359–369. doi:10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- Allen JS, Pang K, Skowera A, Ellis R, Rackham C, Lozanoska-Ochser B, … Peakman M (2009). Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes, 58(1), 138–145. doi: 10.2337/db08-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva DG, Pavlovich RP, Grant C, Kaser SB, & Beller DI (2000). Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes, 49(7), 1106–1115. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10909966 [DOI] [PubMed] [Google Scholar]
- Balázs M, Martin F, Zhou T, & Kearney JF (2002). Blood Dendritic Cells Interact with Splenic Marginal Zone B Cells to Initiate T-Independent Immune Responses. Immunity, 17(3), 341–352. doi: 10.1016/S1074-7613(02)00389-8 [DOI] [PubMed] [Google Scholar]
- Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, & ten Brinke A (2012). IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol, 142(3), 332–342. doi: 10.1016/j.clim.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Chomarat P, Dantin C, Bennett L, Banchereau J, & Palucka AK (2003). TNF Skews Monocyte Differentiation from Macrophages to Dendritic Cells. The Journal of Immunology, 171(5), 2262–2269. doi: 10.4049/jimmunol.171.5.2262 [DOI] [PubMed] [Google Scholar]
- Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, & Kellett GL (1996). The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch, 432(2), 192–201. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8662294 [DOI] [PubMed] [Google Scholar]
- Danova K, Grohova A, Strnadova P, Funda DP, Sumnik Z, Lebl J, … Palova-Jelinkova L (2017). Tolerogenic Dendritic Cells from Poorly Compensated Type 1 Diabetes Patients Have Decreased Ability To Induce Stable Antigen-Specific T Cell Hyporesponsiveness and Generation of Suppressive Regulatory T Cells. J Immunol, 198(2), 729–740. doi: 10.4049/jimmunol.1600676 [DOI] [PubMed] [Google Scholar]
- Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, … Kudva YC (2011). Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim, 45(3), 131–140. doi: 10.1258/la.2010.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, … Nikolajczyk BS (2013). B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences, 110(13), 5133–5138. doi: 10.1073/pnas.1215840110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, & Aoki T (2007). Evidence of Increased Inflammation and Microcirculatory Abnormalities in Patients With Type 1 Diabetes and Their Role in Microvascular Complications. Diabetes, 56(11), 2790–2796. doi: 10.2337/db07-0784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler D, Ahmed N, Song L, Eboigbodin KE, & Thornalley PJ (2006). Increased Dicarbonyl Metabolism in Endothelial Cells in Hyperglycemia Induces Anoikis and Impairs Angiogenesis by RGD and GFOGER Motif Modification. Diabetes, 55(7), 1961–1969. doi: 10.2337/db05-1634 [DOI] [PubMed] [Google Scholar]
- Erbağci AB, Tarakçioğlu M, Coşkun Y, Sivasli E, & Sibel Namiduru E (2001). Mediators of inflammation in children with type I diabetes mellitus: cytokines in type I diabetic children. Clinical Biochemistry, 34(8), 645–650. doi: 10.1016/S0009-9120(01)00275-2 [DOI] [PubMed] [Google Scholar]
- Faustman D, Giesecke C, Davis M, Kühtreiber W, & Tran S (2014). Disposable No Longer: The Spleen Holds a Reservoir of Stem Cells. J Stem Cell Res Ther, 4(219), 2. [Google Scholar]
- Forster I, & Rajewsky K (1990). The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A, 87(12), 4781–4784. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2352948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances DE, Ronco MT, Monti JA, Ingaramo PI, Pisani GB, Parody JP, … Carnovale CE (2010). Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: new insights into the insulin effect. J Endocrinol, 205(2), 187–200. doi: 10.1677/JOE-09-0462 [DOI] [PubMed] [Google Scholar]
- Frenkel O, Shani E, Ben-Bassat I, Brok-Simoni F, Shinar E, & Danon D (2001). Activation of human monocytes/macrophages by hypo-osmotic shock. Clin Exp Immunol, 124(1), 103–109. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11359448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goestemeyer AK, Marks J, Srai SK, Debnam ES, & Unwin RJ (2007). GLUT2 protein at the rat proximal tubule brush border membrane correlates with protein kinase C (PKC)-betal and plasma glucose concentration. Diabetologia, 50(10), 2209–2217. doi: 10.1007/s00125-007-0778-x [DOI] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, & Adorini L (2003). Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol, 171(8), 4040–4047. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14530324 [DOI] [PubMed] [Google Scholar]
- Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, & Van Dyke TE (2006). Chronic Hyperglycemia Predisposes to Exaggerated Inflammatory Response and Leukocyte Dysfunction in Akita Mice. The Journal of Immunology, 177(10), 7250–7256. doi: 10.4049/jimmunol.177.10.7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske JA, Griffith ML, & Fowler MJ (2009). Initiating and Titrating Insulin in Patients With Type 2 Diabetes. Clinical Diabetes, 27(2), 72–76. doi: 10.2337/diaclin.27.2.72 [DOI] [Google Scholar]
- Homo-Delarche F, Calderari S, Irminger J-C, Gangnerau M-N, Coulaud J, Rickenbach K, … Serradas P (2006). Islet Inflammation and Fibrosis in a Spontaneous Model of Type 2 Diabetes, the GK Rat. Diabetes, 55(6), 1625–1633. doi: 10.2337/db05-1526 [DOI] [PubMed] [Google Scholar]
- Imaeda A, Kaneko T, Aoki T, Kondo Y, & Nagase H (2002). DNA damage and the effect of antioxidants in streptozotocin-treated mice. Food Chem Toxicol, 40(7), 979–987. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12065220 [DOI] [PubMed] [Google Scholar]
- Jacob A, Steinberg ML, Yang J, Dong W, Ji Y, & Wang P (2008). Sepsis-induced inflammation is exacerbated in an animal model of type 2 diabetes. Int J Clin Exp Med, 1(1), 22–31. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19079684 [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G-I, De los Santos K, Sparwasser T, … Lang RA (2002). In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity, 17(2), 211–220. doi: 10.1016/S1074-7613(02)00365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AT, Pooley J, O’Keeffe MA, Vremec D, Zhan Y, Lew AM, … Shortman K (2000). The Development, Maturation, and Turnover Rate of Mouse Spleen Dendritic Cell Populations. The Journal of Immunology, 165(12), 6762–6770. doi: 10.4049/jimmunol.165.12.6762 [DOI] [PubMed] [Google Scholar]
- Kume E, Fujimura H, Matsuki N, Ito M, Aruga C, Toriumi W, … Doi K (2004). Hepatic changes in the acute phase of streptozotocin (SZ)-induced diabetes in mice. Exp Toxicol Pathol, 55(6), 467–480. doi: 10.1078/0940-2993-00351 [DOI] [PubMed] [Google Scholar]
- Lee CH, Shieh YS, Hsiao FC, Kuo FC, Lin CY, Hsieh CH, & Hung YJ (2014). High glucose induces human endothelial dysfunction through an Axl-dependent mechanism. Cardiovasc Diabetol, 13, 53. doi: 10.1186/1475-2840-13-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, … Vollmar B (2014). Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci, 55(6), 3603–3615. doi: 10.1167/iovs.14-14307 [DOI] [PubMed] [Google Scholar]
- Lu H, Yao K, Huang D, Sun A, Zou Y, Qian J, & Ge J (2013). High glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cells. Cardiovasc Diabetol, 12, 80. doi: 10.1186/1475-2840-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson AM, Thurber GM, Kohler RH, Weissleder R, Mathis D, & Benoist C (2015). Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc Natl Acad Sci U S A, 112(5), 1511–1516. doi: 10.1073/pnas.1423769112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez N, Vallerskog T, West K, Nunes-Alves C, Lee J, Martens GW, … Kornfeld H (2014). Chromatin decondensation and T cell hyperresponsiveness in diabetes-associated hyperglycemia. J Immunol, 193(9), 4457–4468. doi: 10.4049/jimmunol.1401125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, … Lutz MB (2002). Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med, 195(1), 15–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11781361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Okiyama N, Villarroel V, & Katz SI (2013). Identification of CD3+CD4-CD8- T cells as potential regulatory cells in an experimental murine model of graft-versus-host skin disease (GVHD). J Invest Dermatol, 133(11), 2538–2545. doi: 10.1038/jid.2013.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreli JB, Correa-Silva S, Damasceno DC, Sinzato YK, Lorenzon-Ojea AR, Borbely AU, … Calderon IM (2015). Changes in the TNF-alpha/IL-10 ratio in hyperglycemia-associated pregnancies. Diabetes Res Clin Pract, 107(3), 362–369. doi: 10.1016/j.diabres.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Nagareddy Prabhakara R., Murphy Andrew J., Stirzaker Roslynn A., Hu Y, Yu S, Miller Rachel G., … Goldberg Ira J. (2013). Hyperglycemia Promotes Myelopoiesis and Impairs the Resolution of Atherosclerosis. Cell Metabolism, 17(5), 695–708. doi: 10.1016/j.cmet.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, & Leenen PJM (2005). Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest, 85(4), 487–501. Retrieved from 10.1038/labinvest.3700238 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Headen DM, Taylor WR, Thule PM, & Garcia AJ (2013). Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials, 34(19), 4602–4611. doi: 10.1016/j.biomaterials.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP, … McGaha TL (2014). Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proceedings of the National Academy of Sciences, 111(11), 4215–4220. doi: 10.1073/pnas.1320924111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmalen JGM, Homo-Delarche F, Durant S, Kap M, Leenen PJM, & Drexhage HA (0000). Islet Abnormalities Associated with an Early Influx of Dendritic Cells and Macrophages in NOD and NODscid Mice. Lab Invest, 80(5), 769–777. Retrieved from 10.1038/labinvest.3780080 [DOI] [PubMed] [Google Scholar]
- Rosmalen JGM, Martin T, Dobbs C, Voerman JSA, Drexhage HA, Haskins K, & Leenen PJM (0000). Subsets of Macrophages and Dendritic Cells in Nonobese Diabetic Mouse Pancreatic Inflammatory Infiltrates: Correlation with the Development of Diabetes. Lab Invest, 80(1), 23–30. Retrieved from 10.1038/labinvest.3780004 [DOI] [PubMed] [Google Scholar]
- Saltiel AR, & Kahn CR (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 414(6865), 799–806. Retrieved from 10.1038/414799a [DOI] [PubMed] [Google Scholar]
- Schroder S, Palinski W, & Schmid-Schonbein GW (1991). Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol, 139(1), 81–100. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1713023 [PMC free article] [PubMed] [Google Scholar]
- Slieker LJ, Sundell KL, Heath WF, Osborne HE, Bue J, Manetta J, & Sportsman JR (1992). Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (Avy/a). Diabetes, 41(2), 187–193. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1733808 [DOI] [PubMed] [Google Scholar]
- Son CH, Bae JH, Shin DY, Lee HR, Choi YH, Yang K, & Park YS (2013). Enhanced maturation and function of dendritic cells using hydrogel coated plate and antigen electroporation. Immunol Invest, 42(4), 341–355. doi: 10.3109/08820139.2012.757234 [DOI] [PubMed] [Google Scholar]
- Sun C, Sun L, Ma H, Peng J, Zhen Y, Duan K, … Zhao Y (2012). The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol, 227(4), 1670–1679. doi: 10.1002/jcp.22891 [DOI] [PubMed] [Google Scholar]
- Szkudelski T (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res, 50(6), 537–546. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11829314 [PubMed] [Google Scholar]
- Vallerskog T, Martens GW, & Kornfeld H (2010). Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol, 184(11), 6275–6282. doi: 10.4049/jimmunol.1000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, & Groux H (2003). Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity, 18(5), 605–617. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12753738 [DOI] [PubMed] [Google Scholar]
- Westera L, Drylewicz J, den Braber I, Mugwagwa T, van der Maas I, Kwast L, … Borghans JAM (2013). Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood, 122(13), 2205–2212. doi: 10.1182/blood-2013-03-488411 [DOI] [PubMed] [Google Scholar]
- Whiteson K, Agrawal S, & Agrawal A (2017). Differential responses of human dendritic cells to metabolites from the oral/airway microbiome. Clin Exp Immunol, 188(3), 371–379. doi: 10.1111/cei.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NL, Morris JL, Rush C, Govan BL, & Ketheesan N (2011). Impact of streptozotocin-induced diabetes on functional responses of dendritic cells and macrophages towards Burkholderia pseudomallei. FEMS Immunol Med Microbiol, 61(2), 218–227. doi: 10.1111/j.1574-695X.2010.00767.x [DOI] [PubMed] [Google Scholar]
- Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, & Junger WG (2010). Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol, 88(6), 1181–1189. doi: 10.1189/jlb.0410211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, … Jung S Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity, 38(5), 1073–1079. doi: 10.1016/j.immuni.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, & Keselowsky BG (2014). Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials, 35(11), 3504–3515. doi: 10.1016/j.biomaterials.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, & Kalluri R (2008). Fibroblasts in Kidney Fibrosis Emerge via Endothelial-to-Mesenchymal Transition. Journal of the American Society of Nephrology, 19(12), 2282–2287. doi: 10.1681/asn.2008050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, … Stanton RC (2010). High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. The FASEB Journal, 24(5), 1497–1505. doi: 10.1096/fj.09-136572 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.