ABSTRACT
Background
Weight loss has been associated with adaptations in energy expenditure. Identifying factors that counteract these adaptations are important for long-term weight loss and weight maintenance.
Objective
The aim of this study was to investigate whether increased protein/carbohydrate ratio would reduce adaptive thermogenesis (AT) and the expected positive energy balance (EB) during weight maintenance after weight loss in participants with prediabetes in the postobese state.
Methods
In 38 participants, the effects of 2 diets differing in protein/carbohydrate ratio on energy expenditure and respiratory quotient (RQ) were assessed during 48-h respiration chamber measurements ∼34 mo after weight loss. Participants consumed a high-protein (HP) diet (n = 20; 13 women/7 men; age: 64.0 ± 6.2 y; BMI: 28.9 ± 4.0 kg/m 2) with 25:45:30% or a moderate-protein (MP) diet (n = 18; 9 women/9 men; age: 65.1 ± 5.8 y; BMI: 29.0 ± 3.8 kg/m 2) with 15:55:30% of energy from protein:carbohydrate:fat. Predicted resting energy expenditure (REEp) was calculated based on fat-free mass and fat mass. AT was assessed by subtracting measured resting energy expenditure (REE) from REEp. The main outcomes included differences in components of energy expenditure, substrate oxidation, and AT between groups.
Results
EB (MP = 0.2 ± 0.9 MJ/d; HP = −0.5 ± 0.9 MJ/d) and RQ (MP = 0.84 ± 0.02; HP = 0.82 ± 0.02) were reduced and REE (MP: 7.3 ± 0.2 MJ/d compared with HP: 7.8 ± 0.2 MJ/d) was increased in the HP group compared with the MP group (P < 0.05). REE was not different from REEp in the HP group, whereas REE was lower than REEp in the MP group (P < 0.05). Furthermore, EB was positively related to AT (rs = 0.74; P < 0.001) and RQ (rs = 0.47; P < 0.01) in the whole group of participants.
Conclusions
In conclusion, an HP diet compared with an MP diet led to a negative EB and counteracted AT ∼34 mo after weight loss, in participants with prediabetes in the postobese state. These results indicate the relevance of compliance to an increased protein/carbohydrate ratio for long-term weight maintenance after weight loss. The trial was registered at clinicaltrials.gov as NCT01777893.
Keywords: protein, energy expenditure, adaptive thermogenesis, energy balance, weight loss, weight maintenance, obesity
Introduction
The increasing prevalence of obesity is a major problem in our modern world and has been associated with numerous comorbidities such as type 2 diabetes and cardiovascular diseases. The most straightforward remedy for obesity is weight loss, but the success of long-term weight-loss maintenance is poor (1). An important factor that prevents long-term weight-loss maintenance may be metabolic adaptation, also called adaptive thermogenesis (AT). AT leads to a reduction in energy expenditure greater than that predicted based on reductions in fat-free mass (FFM) and/or fat mass (FM) (2). Adaptations in energy expenditure have been reported lasting for multiple years after a weight-loss period, but may even persist permanently (3–6).
Therefore, reducing AT in the postobese condition after weight loss may be pivotal for long-term weight-loss maintenance and several factors have been shown to reduce AT. These factors include cold-induced brown adipose tissue activation (7), physical activity (8), capsaicinoids (9–14) and tea catechins (15–17). Another factor that may reduce AT after weight loss is high protein (HP) intake (18–21). An HP diet has been shown to increase FFM, diet-induced energy expenditure (DEE), resting energy expenditure (REE), and total energy expenditure (TEE) (22, 23). After energy restriction, an HP intake supported by a concomitant increase in physical activity was able to preserve REE despite a reduction in FM and FFM (24). Furthermore, a 12-wk HP diet (30% of energy from protein) was able to maintain sleeping metabolic rate (SMR), DEE, and TEE compared to a low-protein diet (5% of energy from protein) in weight-stable individuals (25). However, it remains unclear whether HP intake after weight loss is able to counteract AT.
In the present study, we investigated the effects of a controlled HP diet compared with a moderate protein (MP) diet on components of energy expenditure, energy balance (EB), and AT at the end of the PREVIEW study intervention in a fully controlled respiratory chamber assessment. The PREVIEW study, the PREVention of diabetes through lifestyle Intervention and population studies in Europe and around the World (FP7-KBBE-2012, no. 312,057) consisted of a 3-y weight loss and weight maintenance intervention in 4 intervention groups, differing in dietary [high-protein, low-glycemic index (HP) compared with moderate-protein, moderate-glycemic index (MP)] and physical activity guidelines (26). The primary endpoint of the PREVIEW study was the incidence of type 2 diabetes over 3 y analyzed by diet and subsequently according to physical activity and the combination of diet and physical activity. Secondary outcomes included changes in body weight, body composition, and insulin resistance. The main outcomes of the current study included differences in components of energy expenditure, substrate oxidation, and AT between the 2 diet groups. We hypothesized that a controlled HP diet would counteract AT and increase energy expenditure.
Methods
The Medical Ethical Committee of Maastricht University approved the study (clinicaltrials.gov identifier NCT01777893), and all subjects gave written informed consent.
Participants
Based on voluntary willingness to participate, 40 individuals (20 from the HP group and 20 from MP the group) were recruited from the PREVIEW population at Maastricht University in the Netherlands, of whom 2 dropped out because of lack of time (Supplemental Figure 1). For the general PREVIEW study, participants underwent screening that included anthropometric measurements as described in Fogelholm et al (26). The PREVIEW intervention study is a multicenter randomized controlled trial aimed at finding an effective lifestyle intervention to prevent the development of type 2 diabetes mellitus in individuals with prediabetes as defined by the American Diabetes Association criteria (27): fasting plasma glucose 5.6–6.9 mmol/L and/or 7.8–11.0 mmol/L at 2 h after an oral-glucose-tolerance test of 75 g glucose, with a fasting plasma glucose concentration <7.0 mmol/L.
Study design
The PREVIEW intervention consisted of 2 phases: an 8-wk weight-loss period using a low energy diet, followed by a 34-mo weight maintenance period with instructions to follow the guidelines of 1 of the 4 intervention groups: an MP, moderate-glycemic index (GI) diet or an HP, low-GI diet, combined with either moderate-intensity or high-intensity physical activity. Close to the last clinical investigation day of the weight maintenance period, participants stayed in the respiration chamber for 48 h. The 38 participants that underwent the respiration chamber measurements lost a mean of 11.1 ± 3.6 kg (11.9 ± 2.5%) during the weight-loss period and the minimum weight-loss percentage was 8.1%. The mean body weight at the time of the respiration chamber measurements was 5.5 ± 6.2 kg lower compared to baseline, corresponding with a BMI of 28.9 ± 3.9 kg/m2. There were no differences between groups regarding the changes in body weight during the PREVIEW intervention.
Respiration chamber
Subjects arrived at the Metabolic Research Unit Maastricht research facilities in the morning having fasted from 22:00 the night before. The respiration chamber session started at 09:30 and stopped 2 d later at 09:30. The respiration chamber is an airtight chamber of 14 m3 furnished with a bed, chair, desk with computer, TV, telephone, intercom, sink, and toilet. The climate inside the chamber is controlled. O2 consumption and CO2 production were continuously measured by open-circuit ventilated indirect calorimetry (28). The room was ventilated with fresh air at a rate of 70–80 L/min. Flow was measured using electronically modified dry gasmeters (G6, gasmeterfabriek Schlumberger). The concentrations of O2 and CO2 were measured with dual pairs of infrared CO2 analyzers (ABB/Hartman&Braun Uras) and paramagnetic O2 analyzers (Servomex 4100, and ABB/Hartman&Braun Magnos) (28). During each 15-min period, 6 samples of outgoing air, 1 sample of fresh air, zero gas and calibration gas were measured. The gas samples to be measured were selected by a computer that also stored and processed the data (28). Physical activity was continuously measured by use of an ActiSleep+ (ActiGraph LLC) accelerometer worn on the hip and expressed as counts-per-minute (CPM). Subjects had fixed bedtimes between 23:30 and 07:30. In the daytime, they were not allowed to sleep or to perform exercise. Meals were offered at stated times (breakfast: 09:00, lunch: 13:00, dinner: 17:45), and subjects were instructed to finish these within 30 min.
Dietary intervention
During the weight maintenance period, dietary intervention groups comprised an MP group with 15/55/30% of energy from protein/carbohydrate/fat and a moderate dietary GI (≥56), and an HP group with 25/45/30% of energy from protein/carbohydrate/fat, with a low dietary GI (≤50). Both diets were consumed ad libitum with respect to energy, but with the instruction to maintain the achieved body weight. Additional weight loss was allowed. More detailed information on the weight-loss period, dietary guidelines, and of the intervention groups has been reported before (26, 29).
In the respiration chamber, participants received an MP or HP diet, corresponding with their dietary intervention instructions during the PREVIEW study. The basis of the meals was the same between groups, combined with either carbohydrate-rich or protein-rich food items to keep menus comparable. Originally in the PREVIEW study, subjects were randomly allocated to the HP or MP diet groups by stratification on sex, age, and BMI. Protein was completely exchanged with carbohydrate, resulting in the relative fat content being similar in the 2 groups. The diets consisted of commercially available food items and were provided individually in EB. Individual daily energy requirements were calculated as the basal metabolic rate using the FFM and FM (30) multiplied by a physical activity level of 1.35 (25). Daily energy intake was divided over 3 meals, with breakfast containing 20%, and lunch and dinner 40% each. During all measurements in the respiration chamber, the meals within each condition had the same macronutrient composition. Water consumption was allowed ad libitum between the meals; no other foods or beverages were available.
Energy expenditure and respiratory quotient
TEE and the separate components of energy expenditure, SMR, DEE, and activity-induced energy expenditure (AEE) were calculated during the 48-h stay in the respiration chamber. O2 consumption and CO2 production were used to calculate TEE according to the formula of Weir (31). SMR was expressed as the lowest mean TEE during 3 consecutive hours between 00:00 and 07:00. The mean accelerometer CPM corresponding with the SMR was assumed to represent the inactive state. Simple linear regression analysis based on the relation between TEE and the corresponding accelerometer CPM was used to calculate REE (32). DEE was determined by subtracting SMR from REE. AEE was calculated by subtracting REE from TEE (33). Energy expended for thermoregulation was neglected, because subjects were staying in the respiration chamber in the thermoneutral condition. EB was determined as the difference between energy intake and TEE. Predicted resting energy expenditure (REEp) was calculated as basal metabolic rate based on the FFM and FM (30) and diet-induced thermogenesis calculated as 9.7% of the energy intake for the MP group and 11.2% for the HP group, based on median values for the thermogenic effect of separate nutrients (carbohydrate 10% EI, fat 1.5% EI, protein 25% EI) (34). AT was calculated by subtracting REE from REEp. Respiratory quotient (RQ) was calculated by dividing CO2 production by O2 consumption as a measure of substrate oxidation. The amounts of carbohydrate and fat oxidized were calculated from CO2 production, O2 consumption, and protein oxidation with the formulas of Carpenter (35). Carbohydrate and fat balances were determined as the difference between intake and oxidation.
Biomarker of protein intake
Nitrogen excretion, measured from 24-h urine collections during the respiration chamber measurements, was used as an estimate of protein intake. Urine was collected in 2-L urine bottles containing 10 mL of diluted hydrochloric acid (4 mmol/L) to prevent nitrogen loss through evaporation. The total volume of the 24-h urine was recorded. Urine was gently mixed, and samples were taken and frozen at −20°C until analysis. Nitrogen concentrations were measured with an elemental analyzer (CHN-O-Rapid, Heraeus, in the Netherlands, and Integra COBAS 400 plus, Roche Diagnostics GmbH, in the USA). Total nitrogen output was calculated as 24-h urinary nitrogen plus 10% to account for normal losses via feces and other miscellaneous losses. Nitrogen excretion was multiplied by 6.25 to determine protein oxidation. Protein balance was determined as the difference between intake and oxidation.
Body weight and body composition
Body weight and composition were determined with subjects in the fasted state before entering the respiration chamber. Body weight was measured using a calibrated scale (Life Measurement Inc.). Body composition was determined based on body density measured via air-displacement plethysmography with the BodPod system (BOD POD, Life Measurement Inc.), with use of Siri's equation for body density (36). Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm (Seca, model 222).
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, IBM Corp.) and GraphPad Prism (GraphPad Prism version 7.00 for Windows, GraphPad Software). Data are presented as mean (SD). Normality of the parameters was assessed using the Shapiro-Wilk test and outliers were detected with the use of box plots in SPSS. Sample size calculation showed that at least 16 participants per group were needed to show an interaction effect of dietary intervention and time on 24-h DEE in this parallel study. For the calculations we used an α of 0.05, a β of 0.10, and effect size of 0.4, based on a respiration chamber study by Westerterp et al. (33). In the whole group, protein oxidation, BMI, and AEE were not normally distributed. In the HP group, protein oxidation, fat oxidation, BMI, FFM, body-fat percentage, TEE, and AEE were not normally distributed. For these reasons, nonparametric tests were used for the analyses. Differences in subject characteristics, nitrogen excretion, body weight and body composition, energy expenditure and its components, and substrate oxidation between the diet groups were assessed using the Mann-Whitney U test. Differences in slopes and y-intercepts of the FFM and REE/REEp regression lines between the groups were assessed with linear regression analysis using GraphPad Prism. For the purpose of physiological comparativeness, intercepts of these regression lines were reported for the mean FFM. To assess the y-intercepts, the mean FFM was subtracted from the individual FFM values. Associations between EB and other parameters were investigated using Spearman correlation analysis.
Results
Macronutrient intake, oxidation, and balance in the 2 groups
The different macronutrient composition of the diets resulted in differences in protein and carbohydrate balance between the groups (Figure 1). Protein intake and oxidation were higher in the HP group and the protein balance was more positive compared to the MP group (P < 0.01). Carbohydrate intake and oxidation were lower and the carbohydrate balance was less positive in the HP group compared to the MP group (P < 0.05). There was a trend for a lower fat balance in the HP group compared to the MP group (P = 0.077).
FIGURE 1.
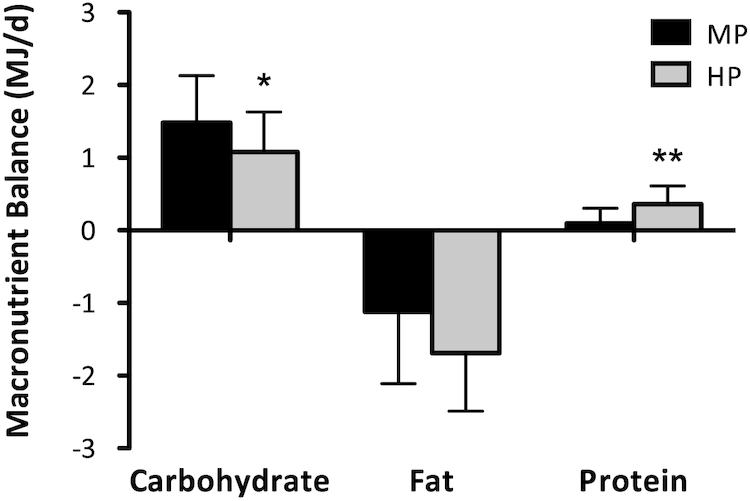
Macronutrient balances (MJ/d) assessed in participants with prediabetes in the postobese state during 48-h respiration chamber measurements. Participants received an MP (n = 18) or HP (n = 20) diet. Differences between groups were assessed by means of Mann-Whitney U tests. *Different from MP, P < 0.05; **Different from MP, P < 0.01. HP, high protein; MP, moderate protein.
Anthropometric variables, energy expenditure, and RQ
Anthropometric variables, energy expenditure, and RQ were assessed for the MP and HP groups (Table 1, Figure 2). There were no significant differences in BMI, FM, FFM, body-fat percentage, energy intake, or energy expenditure between the groups. EB was significantly lower in the HP group compared to the MP group (P = 0.015). The negative EB in the HP group was also significantly different from zero (P = 0.020). To determine AT, REE of the 2 groups and the predicted REE as functions of FFM were compared (Figure 3). For both groups, the slopes of the measured REE regression were not significantly different from the predicted regression (REEp). The intercept of the MP regression line, however, was significantly lower than the intercept of the predicted regression line (REE: 7.3 ± 0.2 MJ/d compared with REEp: 7.8 ± 0.1 MJ/d; P = 0.006). The intercept of the HP regression line was not significantly different from the intercept of the REEp regression line (REE: 7.8 ± 0.2 MJ/d compared with REEp: 8.0 ± 0.1 MJ/d; P = 0.332). The intercepts between the MP and HP regression lines were significantly different (MP: 7.3 ± 0.2 MJ/d compared with HP: 7.8 ± 0.2 MJ/d; P = 0.030). The mean RQ was lower in the HP group compared to the MP group (P = 0.004). In the whole group of participants, EB was not related to any of the anthropometric variables, but was inversely associated with DEE (rs = −0.40; P = 0.014) and positively associated with AT (rs = 0.74; P < 0.001) (Figure 4) and RQ (rs = 0.47; P = 0.003) (Figure 5).
TABLE 1.
Anthropometric variables, energy expenditure, and respiratory quotient in the MP and HP groups1
| MP (n = 18) | HP (n = 20) | P value | |
|---|---|---|---|
| Sex, f/m | 9/9 | 13/7 | |
| Age, y | 65.1 ± 5.8 | 64.0 ± 6.2 | 0.553 |
| BMI, kg/m2 | 29.0 ± 3.8 | 28.9 ± 4.0 | 0.942 |
| Fat-free mass, kg | 52.5 ± 10.9 | 50.8 ± 11.3 | 0.553 |
| Fat mass, kg | 33.9 ± 7.7 | 34.8 ± 8.8 | 0.740 |
| Body fat, % | 39.3 ± 7.4 | 40.7 ± 7.7 | 0.593 |
| Calculated EI, MJ/d | 9.4 ± 1.7 | 9.3 ± 1.6 | 0.573 |
| EB, MJ/d | 0.2 ± 0.9 | −0.5 ± 0.9 | 0.015 |
| RQ | 0.84 ± 0.02 | 0.82 ± 0.02 | 0.004 |
| Activity, CPM | 133.3 ± 34.1 | 155.0 ± 36.6 | 0.087 |
1Values are means ± SDs. Differences between groups were assessed by means of Mann-Whitney U tests. CPM, counts-per-minute; EB, energy balance; EI, energy intake; HP, high protein; MP, moderate protein; RQ, respiratory quotient.
FIGURE 2.
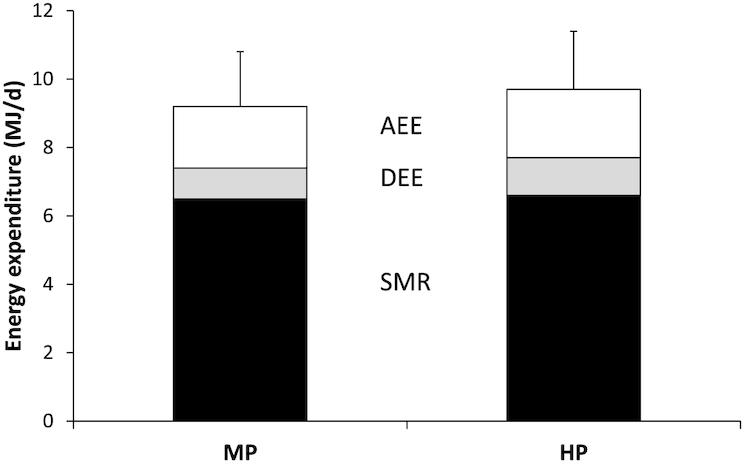
Energy expenditure assessed in participants with prediabetes in the postobese state during 48-h respiration chamber measurements. Participants received an MP (n = 18) or HP (n = 20) diet. SDs are shown for SMR + DEE + AEE. There were no significant differences between the groups. Differences between groups were assessed by means of Mann-Whitney U tests; there were none. AEE, activity-induced energy expenditure; DEE, diet-induced energy expenditure; HP, high protein; MP, moderate protein; SMR, sleeping metabolic rate.
FIGURE 3.
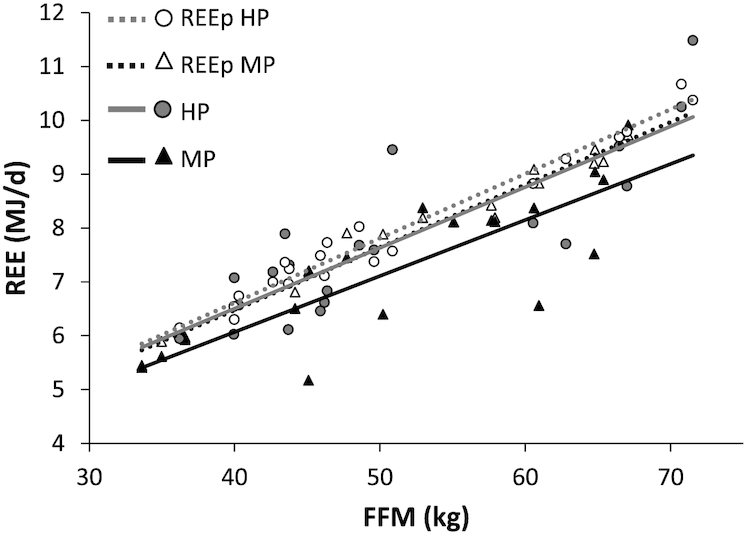
REE as a function of FFM balance assessed in participants with prediabetes in the postobese state during 48-h respiration chamber measurements. Participants received an MP (n = 18) or HP (n = 20) diet. The intercept of the HP regression line was not significantly different from the intercept of the REEp regression line (REE: 7.8 ± 0.2 MJ/d compared with REEp: 8.0 ± 0.1 MJ/d; P = 0.332). The intercepts between the MP and HP regression lines were significantly different (MP: 7.3 ± 0.2 MJ/d compared with HP: 7.8 ± 0.2 MJ/d; P = 0.030). The slopes were not different between any of the regression lines. Differences in slopes and y-intercepts of the FFM and REE/REEp regression lines between the groups were assessed with linear regression analysis using GraphPad Prism. FFM, fat-free mass; HP, high protein; MP, moderate protein; REE, resting energy expenditure; REEp, predicted resting energy expenditure.
FIGURE 4.
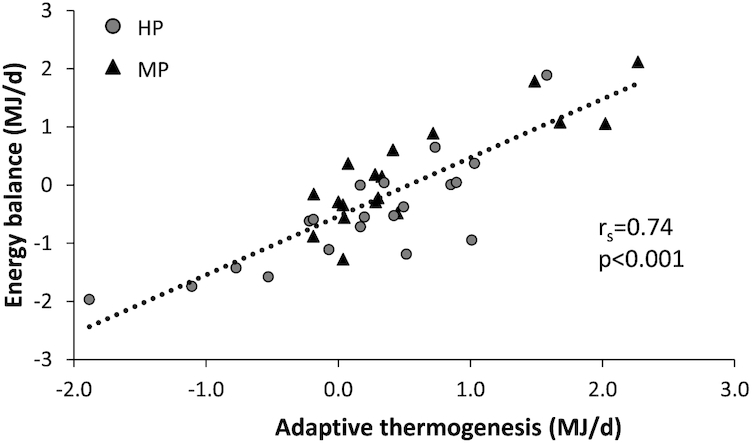
Scatter plot of adaptive thermogenesis and EB assessed in participants with prediabetes in the postobese state during 48-h respiration chamber measurements. Participants received an MP (n = 18) or HP (n = 20) diet. EB was positively associated with adaptive thermogenesis (rs = 0.74; P < 0.001). Associations were investigated using Spearman correlation analysis. EB, energy balance; HP, high protein; MP, moderate protein.
FIGURE 5.

Scatter plot of respiratory quotient and EB assessed in participants with prediabetes in the postobese state during 48-h respiration chamber measurements. Participants received an MP (n = 18) or HP (n = 20) diet. EB was positively associated with RQ (rs = 0.47; P = 0.003). Associations were investigated using Spearman correlation analysis. EB, energy balance; HP, high protein; MP, moderate protein.
Discussion
Measurements of energy expenditure showed that a 48-h HP diet compared with an MP diet induced a negative EB and increased REE. The HP diet was able to counteract AT during weight maintenance after weight loss, whereas the MP diet was not. In addition, AT was positively associated with EB, indicating its potential to reduce weight regain after weight loss and prevent weight cycling. This study shows that these effects are robust enough to hold up with a parallel study design, in overweight prediabetic participants, in the postobese state.
Although HP diets have been previously shown to acutely increase fat oxidation (37), the reduced RQ in the HP group is likely the result of the negative EB, because a negative balance results in increased fat oxidation (38, 39). Long-term negative EB leads to weight loss. This underlines the impact of increasing the protein/carbohydrate ratio to prevent AT and combat overweight/obesity by supporting body weight maintenance. HP diets are able to affect EB via increased satiety and increased thermogenesis and have favorable effects on body composition (22). The lower EB in the HP group compared to the MP group can be explained by reduced AT in the HP group.
The observed AT was 0.5 MJ/d in the MP group. These numbers are in line with results that have been described before (4, 40, 41), indicating that AT remains present even up to 34 mo after an 8-wk weight loss period during which participants lost 11.9% of their body weight on average. The HP diet was able to increase REE relative to the MP diet and the REE in the HP group was equal to the REEp. These results suggest that the HP diet was able to completely abolish the effect of the AT.
In long-term interventions it has been shown that up to 90% of weight-reduced individuals return to their previous body weight (1, 42) and weight cycling has been associated with increased mortality (43). AT has been suggested to play an important role in this process (44). Previous research has identified factors that are able to counteract AT including cold-induced brown adipose tissue activation (7), physical activity (8), capsaicinoids (9–14), and tea catechins (15–17). However, many of these factors affect AT by directly increasing energy expenditure. The counteracting effects of HP intake on AT may partly be explained by the higher thermogenic cost of protein. Because we incorporated a higher diet-induced thermogenesis for the HP group [HP: 11.2% compared with MP: 9.7% (34)] in the calculations to predict energy expenditure, the increases in REE of the HP diet compared with the MP diet exceeded the increased thermic effect of protein. The effects of protein intake on REE as a function of FFM may be mediated via upregulation of uncoupling proteins (UCP) (45), which in turn would support increased energy expenditure (46). Corroborating this cascade and in accordance with our results, a short-term study in rats showed that protein intake led to higher night-time energy expenditure, lower RQ, and higher mRNA expression values for UCP2 and UCP3 (47). Moreover, night-time energy expenditure was positively correlated with UCP mRNA expression.
In the current study, the effects of differences in protein intake on EB and AT were assessed once at the end of the PREVIEW intervention period.
In conclusion, an HP diet compared with an MP diet led to a negative EB and counteracted AT ∼34 mo after weight loss, in participants with prediabetes being in the postobese state. These results indicate the relevance of compliance to an increased protein/carbohydrate ratio for long-term weight maintenance after weight loss.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AR, MF, TCA, and MSW-P: designed research; MD, LT, and BG-C: conducted research; MD and LT: analyzed data; MD, TCA, and MSW-P: wrote the paper; AR and MSW-P: primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This study received a grant from the EU 7th Framework Programme, grant #312057 (FP7-KBBE-2012.2.2-03).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
MD and LT contributed equally to this study.
Abbreviations used: AEE, activity-induced energy expenditure; AT, adaptive thermogenesis; CPM, counts-per-minute; DEE, diet-induced energy expenditure; EB, energy balance; FFM, fat-free mass; FM, fat mass; GI, glycemic index; HP, high protein; MP, moderate protein; PREVIEW, PREVention of diabetes through lifestyle Intervention and population studies in Europe and around the World; REE, resting energy expenditure; REEp, predicted resting energy expenditure; RQ, respiratory quotient; SMR, sleeping metabolic rate; TEE, total energy expenditure; UCP, uncoupling protein.
References
- 1. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222s–5s. [DOI] [PubMed] [Google Scholar]
- 2. Muller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ et al.. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97:990–4. [DOI] [PubMed] [Google Scholar]
- 5. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–12. [DOI] [PubMed] [Google Scholar]
- 6. MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1577–88. [DOI] [PubMed] [Google Scholar]
- 7. Marlatt KL, Chen KY, Ravussin E. Is activation of human brown adipose tissue a viable target for weight management?. Am J Physiol Regul Integr Comp Physiol. 2018;315:R479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunter GR, Fisher G, Neumeier WH, Carter SJ, Plaisance EP. Exercise training and energy expenditure following weight loss. Med Sci Sports Exerc. 2015;47:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tremblay A, Arguin H, Panahi S. Capsaicinoids: a spicy solution to the management of obesity?. Int J Obes (Lond). 2016;40:1198–204. [DOI] [PubMed] [Google Scholar]
- 10. Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr. 2003;90:651–59. [DOI] [PubMed] [Google Scholar]
- 11. Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol. 2007;292:R77–85. [DOI] [PubMed] [Google Scholar]
- 12. Smeets AJ, Westerterp-Plantenga MS. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur J Nutr. 2009;48:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smeets AJ, Janssens PL, Westerterp-Plantenga MS. Addition of capsaicin and exchange of carbohydrate with protein counteract energy intake restriction effects on fullness and energy expenditure. J Nutr. 2013;143:442–7. [DOI] [PubMed] [Google Scholar]
- 14. Janssens PL, Hursel R, Martens EA, Westerterp-Plantenga MS. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One. 2013;8:e67786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13:1195–204. [DOI] [PubMed] [Google Scholar]
- 16. Hursel R, Westerterp-Plantenga MS. Thermogenic ingredients and body weight regulation. Int J Obes (Lond). 2010;34:659–69. [DOI] [PubMed] [Google Scholar]
- 17. Janssens PL, Hursel R, Westerterp-Plantenga MS. Nutraceuticals for body-weight management: the role of green tea catechins. Physiol Behav. 2016;162:83–7. [DOI] [PubMed] [Google Scholar]
- 18. Clamp LD, Hume DJ, Lambert EV, Kroff J. Successful and unsuccessful weight-loss maintainers: strategies to counteract metabolic compensation following weight loss. J Nutr Sci. 2018;7:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502. [DOI] [PubMed] [Google Scholar]
- 20. Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. [DOI] [PubMed] [Google Scholar]
- 21. Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr. 2013;143:591–6. [DOI] [PubMed] [Google Scholar]
- 22. Drummen M, Tischmann L, Gatta-Cherifi B, Adam T, Westerterp-Plantenga M. Dietary protein and energy balance in relation to obesity and co-morbidities.Front Endocrinol (Lausanne). 2018;9:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mikkelsen PB, Toubro S, Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr. 2000;72:1135–41. [DOI] [PubMed] [Google Scholar]
- 24. Pasiakos SM, Mettel JB, West K, Lofgren IE, Fernandez ML, Koo SI, Rodriguez NR. Maintenance of resting energy expenditure after weight loss in premenopausal women: potential benefits of a high-protein, reduced-calorie diet. Metabolism. 2008;57:458–64. [DOI] [PubMed] [Google Scholar]
- 25. Martens EA, Gonnissen HK, Gatta-Cherifi B, Janssens PL, Westerterp-Plantenga MS. Maintenance of energy expenditure on high-protein vs. high-carbohydrate diets at a constant body weight may prevent a positive energy balance. Clin Nutr. 2015;34:968–75. [DOI] [PubMed] [Google Scholar]
- 26. Fogelholm M, Larsen TM, Westerterp-Plantenga M, Macdonald I, Martinez JA, Boyadjieva N, Poppitt S, Schlicht W, Stratton G, Sundvall J et al.. PREVIEW: Prevention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World. Design, methods, and baseline participant description of an adult cohort enrolled into a three-year randomised clinical trial. Nutrients. 2017;9:E632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2018;41:S13–27. [DOI] [PubMed] [Google Scholar]
- 28. Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol (1985). 1997;83:2064–72. [DOI] [PubMed] [Google Scholar]
- 29. Christensen P, Meinert Larsen T, Westerterp-Plantenga M, Macdonald I, Martinez JA, Handjiev S, Poppitt S, Hansen S, Ritz C, Astrup A et al.. Men and women respond differently to rapid weight loss: metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes Metab. 2018;20:2840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westerterp KR, Donkers JH, Fredrix EW, Boekhoudt P. Energy intake, physical activity and body weight: a simulation model. Br J Nutr. 1995;73:337–47. [DOI] [PubMed] [Google Scholar]
- 31. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition. 1990;6:213–21. [PubMed] [Google Scholar]
- 32. Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr. 1995;61:1013–9. [DOI] [PubMed] [Google Scholar]
- 33. Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord. 1999;23:287–92. [DOI] [PubMed] [Google Scholar]
- 34. Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36:391–7. [DOI] [PubMed] [Google Scholar]
- 35. Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl. 1957;6:795–802. [PubMed] [Google Scholar]
- 36. Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9:480–91.; discussion, 92. [PubMed] [Google Scholar]
- 37. Stepien M, Gaudichon C, Fromentin G, Even P, Tome D, Azzout-Marniche D. Increasing protein at the expense of carbohydrate in the diet down-regulates glucose utilization as glucose sparing effect in rats. PLoS One. 2011;6:e14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Westerterp KR. Food quotient, respiratory quotient, and energy balance. Am J Clin Nutr. 1993;57:759S–64S.; discussion 64S-65S. [DOI] [PubMed] [Google Scholar]
- 39. Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr. 1991;53:430–6. [DOI] [PubMed] [Google Scholar]
- 40. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- 41. Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000;72:946–53. [DOI] [PubMed] [Google Scholar]
- 42. McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among American adults. Int J Obes Relat Metab Disord. 1999;23:1314–9. [DOI] [PubMed] [Google Scholar]
- 43. Moon JH, Lim S, Choi SH, Oh TJ, Jang HC, Park KS, Cho NH. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16-year prospective cohort study. J Clin Endocrinol Metab. 2019;104:639–46. [DOI] [PubMed] [Google Scholar]
- 44. Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes (Lond). 2007;31:204–12. [DOI] [PubMed] [Google Scholar]
- 45. Petzke KJ, Friedrich M, Metges CC, Klaus S. Long-term dietary high protein intake up-regulates tissue specific gene expression of uncoupling proteins 1 and 2 in rats. Eur J Nutr. 2005;44:414–21. [DOI] [PubMed] [Google Scholar]
- 46. Dalgaard LT, Pedersen O. Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and Type II diabetes. Diabetologia. 2001;44:946–65. [DOI] [PubMed] [Google Scholar]
- 47. Petzke KJ, Riese C, Klaus S. Short-term, increasing dietary protein and fat moderately affect energy expenditure, substrate oxidation and uncoupling protein gene expression in rats. J Nutr Biochem. 2007;18:400–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


