Abstract
Purpose
This is a retrospective case report illustrating the diagnostic and therapeutic challenges associated with a chronic rhegmatogenous retinal detachment masquerading as a severe panuveitis with intense anterior chamber inflammation. We have included clinical features, anterior segment and fundus photography, B-scan ultrasonography, fluorescein angiography, and intraoperative findings.
Observations
A 26-year-old male presented with features of unilateral panuveitis: hypotony, anterior segment inflammation (posterior synechiae and anterior chamber cell with fibrin clumping), diffuse choroidal thickening, and retinal detachment. Laboratory investigations for infectious or rheumatologic processes were negative, and empiric systemic corticosteroid therapy was unsuccessful. This prompted suspicion for an alternate primary etiology, and pars plana vitrectomy revealed small retinal breaks as the underlying cause of the retinal detachment and inflammation.
Conclusions
Rhegmatogenous retinal detachments are a known cause of intraocular inflammation. Nevertheless, it remains a challenge to recognize retinal breaks in this setting, particularly with robust anterior segment inflammation and posterior findings resembling severe exudative uveitis. Being aware of this unique presentation may prevent delays in diagnosis and have important prognostic implications.
Keywords: Rhegmatogenous retinal detachment, Exudative panuveitis, Masquerade syndrome, Anterior chamber fibrin, Hypotony
1. Introduction
Uveitis and retina specialists must routinely classify retinal detachments (RD) into rhegmatogenous or serous (exudative) processes. Due to contrasting clinical signs, typical presentations are not particularly challenging to differentiate. Rhegmatogenous RDs are associated with anterior vitreous pigmented cell and non-shifting sub-retinal fluid. In non-infectious uveitis, serous RDs may result from severe inflammation, typically at the level of the choroid, with resultant shifting subretinal fluid. RDs with overlapping clinical findings may occur and require a high index of suspicion for an atypical serous or rhegmatogenous process, particularly when there is a lack of response to directed therapy. Rhegmatogenous RDs featuring prominent inflammation have the potential to masquerade as a serous detachment with resultant diagnostic and therapeutic challenges.
2. Case report
A 26-year-old Nicaraguan male was referred to the F.I. Proctor Foundation for features of unilateral panuveitis and decreased vision in the left eye. Two weeks prior to referral, the patient noticed unilateral vision loss and tenderness to palpation in the affected eye upon awakening. Past ocular history was significant for mild myopia OU and strabismus surgery at two years of age, but was otherwise unremarkable. Review of systems was negative, medical history was unremarkable, and there was no recent travel outside of the United States. The patient presented to our uveitis clinic with hand motion visual acuity and a pressure of 0 mm Hg by applanation in the affected eye. Slit lamp biomicroscopy OS revealed conjunctival injection, diffuse corneal endothelial pigment, 4+ anterior chamber (AC) cell (which was a mixture of pigment and white blood cells as graded by the Standardization of Uveitis Nomenclature1), fibrin with clumping in the AC, and nearly 360-degrees of posterior synechiae (Fig. 1). Despite dense vitreous haze (3+), funduscopic examination revealed a macula-on bullous inferior RD, no sign of shifting subretinal fluid, and no definite corrugations or hydration lines (Fig. 2). Extensive examination by the retina service was unable to identify any breaks in the affected eye. The contralateral eye examination was notable for two tiny opercula, but was otherwise unremarkable. The unilateral presentation prompted an anterior chamber paracentesis with fluid sent for bacterial and fungal cultures, directed polymerase chain reaction for HSV, VZV, CMV, and Toxoplasmosis gondii, as well as metagenomic deep sequencing to detect less common infectious pathogens,2 all with negative results. Additionally, systemic laboratory testing for HIV, Toxoplasma antibodies, Treponemal antibody, interferon gamma release assay, HLA-B27, and chest x-ray were all negative or within normal limits. Five days after presentation, formal B-scan ultrasonography demonstrated progression of the detachment to a mobile, sub-total RD without signs of retinal breaks. Moreover, there was diffuse choroidal thickening (Fig. 3). Late phase fluorescein angiography demonstrated only disc staining and patchy hyperfluorescence of the detachment OS and was unremarkable OD, without signs of unifocal or multifocal leakage or exudation (Fig. 4). Due to the robust inflammatory response in the AC (as evidenced by the cell and fibrin) as well as hypotony, consideration for an undifferentiated unilateral panuveitis was made. The patient was subsequently started on oral prednisone at 1 mg/kg daily.
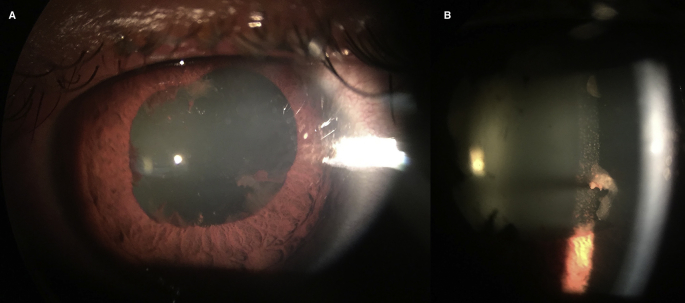
Fig. 1.
Evidence of intense anterior segment inflammation including extensive posterior synechiae (A) and fibrin clumping (B).
Fig. 2.
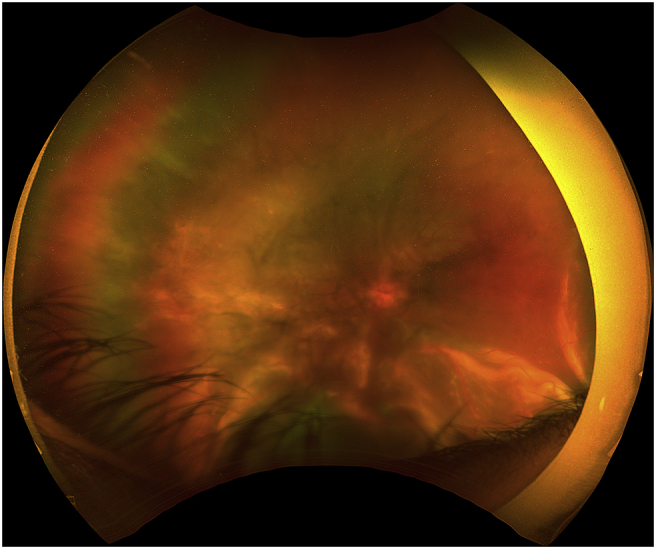
Optos fundus photography demonstrating an inferior bullous retinal detachment without signs of retinal break.
Fig. 3.
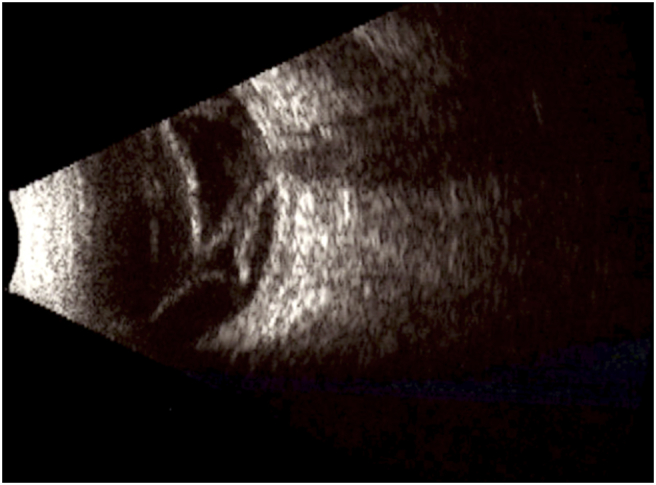
B-scan reveals sub-total retinal detachment and diffuse choroidal thickening.
Fig. 4.
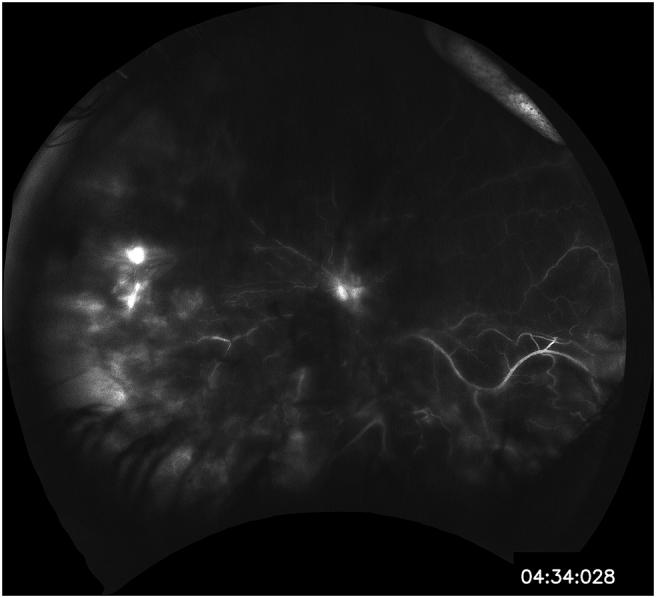
Fluorescein angiography demonstrating disc staining and nonspecific, patchy hyperfluorescence inferiorly.
After three weeks of systemic prednisone, the RD and choroidal thickening were entirely unchanged. The inflammation in the AC, however, had improved with resolution of fibrin. The lack of significant posterior segment response to high-dose systemic steroids, along with the opercula in the contralateral eye, raised suspicion for an elusive retinal break OS. A 25-gauge pars plana vitrectomy was performed and intraoperative examination revealed a cluster of pigmented areas in the inferior periphery consistent with small retinal holes (Fig. 5) as well as suspicious areas nasally and superotemporally. Indeed, during instillation of perfluorocarbon there was flattening of the retina, signaling egress of sub-retinal fluid through these retinal breaks and thereby confirming the likely chronic rhegmatogenous nature of the detachment. A partial PVD was present, and using suction on the cutting instrument, the adherent vitreous overlying the periphery was peeled to the vitreous base and then vitrectomized. Endolaser photocoagulation was applied to the area of the suspected holes and entire periphery, followed by a 16% C3F8 gas tamponade. Oral prednisone at 60 mg daily and topical corticosteroids had been re-initiated one week prior to surgery and tapered for the 2 weeks following surgery to manage the intraocular inflammation perioperatively. The patient had an uneventful recovery with retinal re-attachment and complete resolution of inflammatory findings, including choroidal thickening. Best-corrected visual acuity of the affected eye was 20/300 at five weeks post RD repair. Seven months after RD repair the patient underwent phacoemulsification with intraocular lens implant and epiretinal membrane peel, with resulting best-corrected visual acuity of 20/60.
Fig. 5.
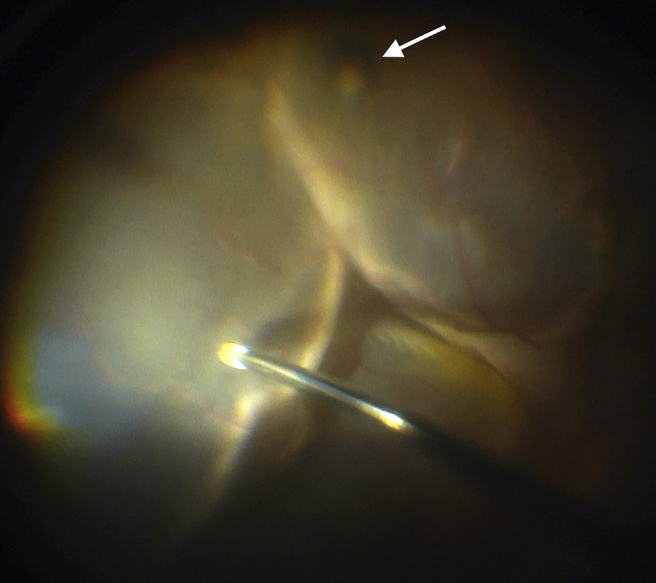
Intra-operative evaluation confirmed a retinal break (arrow).
3. Discussion
This case illustrates the potential for a chronic rhegmatogenous RD to masquerade as a serous RD while featuring panuveitis characterized by diffuse choroidal thickening, and robust anterior chamber inflammation (with fibrin and hypotony). This type of presentation is more commonly seen in Vogt-Koyanagi-Harada (VKH) and sarcoid uveitis, though these conditions are most frequently bilateral and our patient had no prodrome, neurologic, or integumentary findings consistent with VKH and no pulmonary features consistent with sarcoidosis. Moreover, the fluorescein angiogram was not consistent with the typical multiple pinpoint hyperfluorescence that typifies VKH. Infectious processes can cause a unilateral panuveitis, but our patient had no history of intraocular surgery, ocular trauma, and no systemic risk factors for an endogenous endophthalmitis. Moreover, infectious processes were further excluded by culture and molecular techniques. The lack of shifting fluid on initial clinical examination was another feature that argued against a classic serous detachment. Ultimately, the tiny opercula OD gave insight into the possible etiology of the retinal detachment OS.
Rhegmatogenous RDs are known to occasionally exhibit features of inflammation, albeit typically to a lesser extent than serous detachments. For example, Schwartz-Matsuo syndrome involves photoreceptors released from retinal breaks mimicking anterior chamber cell.3,4 Our case exhibited frank inflammatory white blood cells as well as fibrin, features that are distinctive from the Schwartz-Matsuo syndrome or the mild anterior chamber cell that can occur with rhegmatogenous RDs. In the case of Schwartz-Matsuo syndrome, there is increased intraocular pressure as the photoreceptors occlude the trabecular meshwork, and therefore inconsistent with hypotony found in our case. Additionally, the significant conjunctival injection and ocular pain are not typical of rhegmatogenous RDs and are typical signs and symptoms of uveitic entities. Release of inflammatory cytokines have also been described in association with rhegmatogenous RD and may mediate the inflammatory features in such cases.5, 6, 7, 8, 9, 10 Inflammatory changes in these studies, however, are frequently limited to choroidal detachment or vitreal membrane formation and retinal traction. In cases of rhegmatogenous RDs featuring significant inflammation including the vitreous and posterior segments, systemic corticosteroids should be considered to assist in the control of perioperative inflammation to allow for the most optimal post-surgical outcomes.
Rhegmatogenous RDs have been reported to infrequently present as a non-malignant masquerade syndrome, both in the acute and chronic setting.11, 12, 13 This unusual presentation typically occurs in elderly patients, and can involve severe intraocular inflammation, as well as hypotony secondary to choroidal detachment.12 Identifying underlying breaks in the setting of severe inflammation can be very challenging due to posterior synechiae a hazy media. While these reports share some similar findings with our own, our patient did not demonstrate obvious choroidal detachment, and instead featured a fibrinous AC reaction and diffuse choroidal thickening in a young patient, findings that are more consistent with a severe exudative panuveitis.
While a thorough investigation is generally necessary to rule out infectious or rheumatologic causes of uveitis, our case serves as an important reminder that rhegmatogenous RD may present atypically and with severe inflammation. Despite the knowledge that retinal breaks can masquerade as a uveitis, making the diagnosis remains quite difficult. Clinicians should consider retinal breaks in their differential for similar cases, especially following negative laboratory investigations and finding of contralateral retinal breaks.
Patient consent
The patient consented in writing to publication of this case and associated images.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: (AJ, RB, DP, JG).
Acknowledgements
None.
References
- 1.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of uveitis nomenclature working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doan T., Wilson M.R., Crawford E.D. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016;8(1):90. doi: 10.1186/s13073-016-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo T. Photoreceptor outer segments in aqueous humor: key to understanding a new syndrome. Surv Ophthalmol. 1994;39(3):211–233. doi: 10.1016/0039-6257(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo T., Muraoka N., Shiraga F., Matsuo N. Schwartz-Matsuo syndrome in retinal detachment with tears of the nonpigmented epithelium of the ciliary body. Acta Ophthalmol Scand. 1998;76(4):481–485. doi: 10.1034/j.1600-0420.1998.760417.x. [DOI] [PubMed] [Google Scholar]
- 5.Conart J.B., Kurun S., Ameloot F., Trechot F., Leroy B., Berrod J.P. Validity of aqueous flare measurement in predicting proliferative vitreoretinopathy in patients with rhegmatogenous retinal detachment. Acta Ophthalmol. 2017;95(4):e278–e283. doi: 10.1111/aos.13254. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Li H., Jiang P., Liu X., Xu D., Wang F. Investigating the pathological processes of rhegmatogenous retinal detachment and proliferative vitreoretinopathy with metabolomics analysis. Mol Biosyst. 2014;10(5):1055–1062. doi: 10.1039/c3mb70386j. [DOI] [PubMed] [Google Scholar]
- 7.Yu J., Peng R., Chen H., Cui C., Ba J. Elucidation of the pathogenic mechanism of rhegmatogenous retinal detachment with proliferative vitreoretinopathy by proteomic analysis. Invest Ophthalmol Vis Sci. 2012;53(13):8146–8153. doi: 10.1167/iovs.12-10079. [DOI] [PubMed] [Google Scholar]
- 8.Yui R., Kunikata H., Aizawa N., Nakazawa T. Anterior chamber aqueous flare and optic nerve microcirculation in eyes with rhegmatogenous retinal detachment. Acta Ophthalmol. 2016;94(6):e520–521. doi: 10.1111/aos.12970. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S., Adachi K., Suzuki Y., Maeno A., Nakazawa M. Profiles of inflammatory cytokines in the vitreous fluid from patients with rhegmatogenous retinal detachment and their correlations with clinical features. BioMed Res Int. 2016;2016 doi: 10.1155/2016/4256183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Y., Wu Z., Sheng H., Zhang Z., Yu M., Zhang Q. Identification of inflammatory mediators in patients with rhegmatogenous retinal detachment associated with choroidal detachment. Mol Vis. 2015;21:417–427. [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanisevic M. The natural history of untreated rhegmatogenous retinal detachment. Ophthalmologica. 1997;211(2):90–92. doi: 10.1159/000310766. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett W.H., 2nd Rhematogenous retinal detachment complicated by severe intraocular inflammation, hypotony, and choroidal detachment. Trans Am Ophthalmol Soc. 1981;79:664–683. [PMC free article] [PubMed] [Google Scholar]
- 13.Kubicka-Trzaska A., Romanowska-Dixon B. Non-malignant uveitis masquerade syndromes. Klin Oczna. 2008;110(4-6):203–206. [PubMed] [Google Scholar]