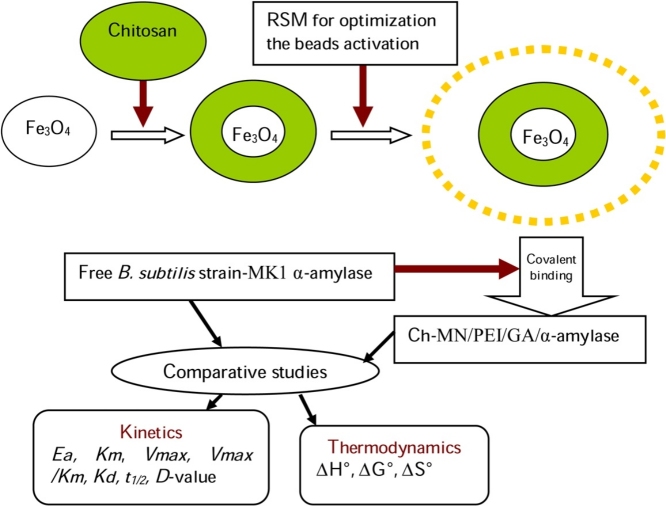
Graphical abstract
Keywords: α-amylase, Immobilization, Chitosan-magnetic nanoparticles, Catalytic, Kinetics, Thermodynamics
Highlights
-
•
α-amylase from isolated strain B. subtilis MK1 was immobilized on different carriers.
-
•
Immobilized α-amylase on Chitosan-magnetic nanoparticles had the highest immobilization yield %
-
•
Immobilization yield % was promoted using Response Surface methodology by 1.5-fold.
-
•
Chitosan-magnetic nanoparticles -Enzyme was characterized by FTIR and SEM.
-
•
Immobilization process improved the catalytic properties and stabilities of the enzyme.
Abstract
Bacillus subtilis strain-MK1 α-amylase was successfully immobilized on Chitosan-magnetic nanoparticles (Ch-MNP) that had been modified with polyethyleneimine (PEI) and glutaraldehyde (GA). Optimization of Ch-MNP/PEI/GA beads modification by Central Composite design enhanced the immobilization yield (IY %) by 1.5-fold. Ch-MNP/PEI/GA was characterized before and after modification and immobilization by FTIR and SEM. Ch-MNP/PEI/GA/Enzyme showed the same pH optima of free enzyme, while an elevation 10 °C in temperature optima was observed after its immobilization. Ch-MNP/PEI/GA/Enzyme displayed higher Km and Vmax values (2.1 and 1.2-fold) and lower Vmax/Km ratio (1.7-fold), respectively than the free enzyme. Compared to the free enzyme, Ch-MNP/PEI/GA/Enzyme exhibited lower activation energy, lower deactivation constant rate, higher D-values, higher half-life, and higher energy for denaturation. Immobilization of α-amylase increased enthalpy (4.2-fold), free energy (1.1-fold) and decreased entropy (4.6-fold) of thermal inactivation. A significant increase in pH stability of Ch-MNP/PEI/GA/Enzyme was observed especially at alkaline pH values. In addition, Ch-MNP/PEI/GA/Enzyme preserved 83.2 % of its initial activity after 15 consecutive cycles. When storing Ch-MNP/PEI/GA/Enzyme at 4 °C the residual activity was 100 and 86 %, respectively after 21 and 40 days. Finally, immobilization process improved the catalytic properties and stabilities, thus raising the suitability for industrial processes with lower cost and time.
1. Introduction
Enzymes as biocatalysts are slowly but steadily gaining importance in many industries ranging from food to pharmaceuticals. The major advantage of the enzymatic route is the low toxicity, selectivity, acceleration of mild reaction conditions with its associated high yield and exclusivity towards the desired product [1].
α-amylase catalyzes the cleavage of α-d-1,4 glucosidic bonds of starch molecule to give dextrin and other smaller polymers composed of glucose units. α-amylases have diverse applications in several industries as textile, food, baking, detergent, brewing, pharmaceutical and clinical chemistry [2,3]. In spite of the important role of amylases, they exposed to some troubles as their high sensitivity to hard conditions of industrial processes, low stability, short lifetime and difficulty in recovery which increases the production cost. To solve these problems, enzyme immobilization is considered as one of the effective techniques which not only stabilizes enzymes under operational conditions but also allows easy recovery and reusability for repeated cycles [4].
There are several methods for enzymes immobilization including covalent binding, physical adsorption, entrapment and cross linking to solid carriers. Covalent binding is considered the most effective method. The formation of covalent bonds between enzyme and carrier surface might be encouraging procedure in establishing enzymes, inhibiting their leaching out and consecutive leakage [5]. Physical adsorption is the most general, simple to perform and the oldest. The immobilized enzyme by physical adsorption shows low stability after repeated use due to the weak bonds between enzyme and carrier surface (as van der Waals, hydrogen bonding, hydrophilic/ hydrophobic and electrostatic interactions). Therefore, a method combining the advantages of physical adsorption and covalent binding was developed to enhance the immobilized enzyme stability [6].
Immobilization can involve various carriers organic and inorganic whose characteristics play an important role in the behavior of the enzyme. Nano-carrier has a large surface area, helps to decrease the diffusion barriers in the transport of the substrate and the reaction products, thereby improving the efficiency of the immobilized enzyme [7].
The magnetite (Fe3O4) nanoparticles have been considered suitable for immobilization due to their unique characteristics as their tailored surface chemistry, small size, super-paramagnetism, low toxicity, biocompatibility, biodegradability and easy separation from the reaction mixture by using external magnetic field [1,4].
For the covalent immobilization of enzymes, magnetic Fe3O4 nanoparticles need to be coated with a few layers of chemically active natural or synthetic polymers to provide functional groups for conjugation with bio-molecule [6,7].
Chitosan is a biomaterial cheap, stable and has good hydrophilic and biocompatible properties. The amino groups of chitosan are useful for conjugation with enzyme protein via cross-linking agents such as glutaldehyde [6].
Thermodynamic stability includes the resistance to thermal denaturation of a folded protein conformation. The suitability of enzymes for industrial application is judged by their thermodynamic parameters. Variation in thermodynamic parameters as enthalpy, entropy and the Gibbs free energy can provide valuable information about the enzyme behavior, activity and thermostability. In addition, the investigation of dynamic and thermodynamic properties of bio-nanocatalyst is important in designing a highly effective immobilized enzyme [8].
In the current study, B. subtilis strain-MK1 α-amylase was immobilized onto different carriers using covalent binding in order to increase its activity and stability. Modification of Chitosan- MNP/PEI/GA beads was optimized by using Central composite design to promote IY%. Finally, comparative studies including catalytic, kinetics and thermodynamics of both free and immobilized enzyme were carried out.
2. Materials and methods
2.1. Materials
Sodium alginate (Alg) was obtained from Fluka Company, Switzerland. Polyethyleneimine (PEI) and Glutaraldehyde (GA) were obtained from Sigma-Aldrich Chemie GmbH, Riedstr. 2, d-89555 Steinheim, Germany. Chitosan and κ-carrageenan were supplied from Sigma Chemical, Co., St. Louis, USA. Other chemicals were of analytical grade.
2.2. Production and Partial purification of α-amylase enzyme
α-amylase enzyme was produced under submerged fermentation from isolated strain B. subtilis strain-MK1. Cultivation of B. subtilis strain- MK1 cells (24 h old culture) was in 250-ml Erlenmeyer flasks containing 50 ml of sterile medium composed of (g/l): soluble starch 2.0, lactose 10.0, beef extract 5.0, K2HPO4 10.0, MgSO4.7H2O 5.0, FeSO4.7H2O 0.25, CaCl2 0.5, pH 7.0 and incubated at 35 °C for 72 h under shaking condition of 150 rpm. The broth media after incubation was centrifuged at 10,000×g for 15 min and the cell free filtrate was considered as source of crude enzyme. The enzyme was partially purified by using ethanol precipitation method. Each precipitate obtained from 20 to 80 % concentration (v/v) was collected by centrifugation, dried, weighed and used for α-amylase activity and protein estimation.
2.3. Enzyme assay
α-amylase activity was done according to [9]. For starting the reaction, 0.5 ml of 1% soluble starch in 0.1 M phosphate buffer (pH 7.0) was added to 0.5 ml of enzyme and was incubated for 30 min at 40 °C. The reaction was stopped by adding 1 ml of dinitrosalicysilic acid (DNS) reagent and kept on boiling water bath for 10 min [10]. Absorbance was measured at 540 nm against blank, which was devoid of enzyme. One unit of enzyme activity (U) is defined as the amount of enzyme that liberated one μmole of reducing sugar as glucose/ min under assay conditions. All the experiments were performed in triplicate and the results were expressed as mean values.
2.4. Determination of protein content
Protein estimation was done by the method of [11] using bovine serum albumin (BSA) as standard.
2.5. Carriers preparation for covalent immobilization
2.5.1. Alginate/CaCl2 (Alg/CaCl2)
Sodium alginate (Alg) was dissolved in distilled water using an overhead mechanical stirrer until complete dissolution had occurred to give a final concentration of 2 % (w/v). The alginate solution was dropped through a nozzle of 300 μm using the Innotech Encapsulator in a hardening solution containing 2.5 % (w/v) CaCl2 for 3 h [12].
2.5.2. Alginate-CMC/FeCl3 (Alg-CMC/FeCl3)
The preparation of this carrier was carried out according to [13] with some modifications. Carboxy methyl cellulose (CMC) and Alg in the ratio 3:2 were dissolved in distilled H2O using an overhead mechanical stirrer. After complete dissolution, polymer solution was dropped into 0.05 M of ferric chloride (FeCl3) by using Encapsulator to form uniform gel beads. Gel beads were hardened using 0.05 M FeCl3 for 3 h.
2.5.3. Alginate-CMC/CaCl2 (Alg-CMC/CaCl2)
For gel beads formation, solutions of Alg (2%) and CMC (3%) were mixed together using an overhead mechanical stirrer until complete dissolution had occurred. The Alg–CMC solution was dropped through a nozzle of 300 μm using the Inotech Encapsulator in a hardening solution containing 2 % (w/v) CaCl2 [14].
2.5.4. Alginate-CMC/FeCl3.CaCl2 (Alg-CMC/FeCl3.CaCl2)
For gel beads formation, solutions of Alg and CMC in a concentration of 2 and 3% (w/v), respectively, mixed together using an overhead mechanical stirrer until complete dissolution had occurred. The Alg–CMC solution was dropped through a nozzle of 300 μm using the Inotech Encapsulator in a hardening solution containing 2% (w/v) of CaCl2 and FeCl3 (1:1 ratio) [13,14].
2.5.5. CMC/FeCl3
A solution of 3 % CMC dissolved in distilled H2O using an overhead mechanical stirrer until complete dissolution had occurred. The polymer solution was dropped through a nozzle of 300 μm using the Inotech Encapsulator in a hardening solution 0.05 M FeCl3 [13].
2.5.6. Carrageenan/KCl (Car/KCl)
Polymer carrier (κ-carrageenan) 2 g was dissolved in 100 ml distilled H2O. Then, spray the polymer solution into cross-linking solution of KCl 2% (w/v), through a nozzle of 300 μm using the Inotech Encapsulator. The prepared beads were hardened in crosslinking solutions for 3 h [15].
2.5.7. Chitosan/NaOH (Ch/NaOH)
Chitosan (polymer carrier) was dissolved in distilled H2O to produce 2% (w/v) solution. Then, spray the polymer solution into 5% (w/v) cross-linking solution of NaOH through a nozzle of 300 μm using the Inotech Encapsulator. The prepared beads were hardened in crosslinking solutions for 3 h [15].
2.5.8. Alginate-Carrageenan/KCl (Alg-Car/KCl)
Sodium alginate/k-Carrageenan gel (Alg-Car) was prepared by dissolving Alg/Car (1:1 w: w) in distilled H2O to get a final concentration of 2% (w/v). The gel solution was mixed thoroughly using an overhead mechanical stirrer until complete dissolving had occurred. The polymer solution was dropped into 3% KCl by using Encapsulator with nozzle size 300 μm to form uniform gel beads. Alg-Car gel beads were hardened using KCl for 3 h [16].
2.5.9. Alginate-Carrageenan/CaCl2 (Alg-Carr/CaCl2)
Sodium alginate/k-Carrageenan gel (Alg-Car) was prepared by dissolving Alg/Car (1:1 w: w) in distilled H2O to get a final concentration of 2% (w/v). The gel solution was mixed thoroughly using an overhead mechanical stirrer until complete dissolving had occurred. The polymer solution was dropped into 2% calcium chloride (CaCl2) by using Encapsulator with nozzle size 300 μm to form uniform gel beads. The Alg-Car gel beads were hardened using CaCl2 for 3 h [16].
2.5.10. Alginate-Carrageenan/KCl.CaCl2 (Alg-Car/KCl.CaCl2)
Sodium alginate/k-Carrageenan gel (Alg-Car) was prepared by dissolving Alg/Car (1:1 w: w) in distilled H2O to get a final concentration of 2% (w/v). The gel solution was mixed thoroughly using an overhead mechanical stirrer until complete dissolving had occurred. The polymer solution was dropped into 3 % mixture solution of CaCl2 and KCl in 1:1 ratio by using Encapsulator with nozzle size 300 μm to form uniform gel beads. The Alg-Car gel beads were hardened using CaCl2 and KCl for 3 h.
2.5.11. Chitosan-Magnetic nanoparticles/NaOH (Ch-MNP/OH)
Chitosan (2 g) was dissolved in 100 ml distilled H2O and mixed with a solution of magnetic nanoparticles (Fe3O4). Then, spray the polymer solution into cross-linking solution of NaOH (5% w/v) through a nozzle of 300 μm using the Inotech Encapsulator. The prepared beads were hardened in crosslinking solutions for 3 h [15].
2.6. Beads modification (activation) for covalent immobilization
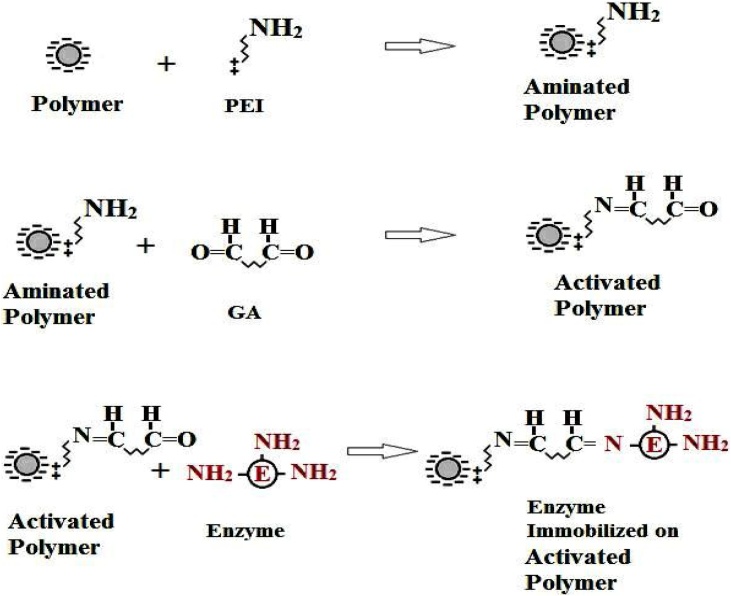
To modify the gel beads for covalent immobilization, the generated beads of all carriers were soaked in 4 % polyethyleneimine (PEI) solution (pH 9.5) for 3 h. The unreacted PEI was then removed from the beads by successive washing with distilled H2O. After washing, aminated gel beads were soaked in glutaraldehyde (GA 2.5 %) for 3 h to incorporate the new functionality aldehyde group. The gel beads were washed with distilled H2O to remove the unreacted GA. After that, the activated gel beads were ready for immobilization step as shown in Scheme 1 for covalent immobilization of enzyme. Central composite design (CCD) was used to determine the optimum level of important factors for modification of the potent carrier (PEI %, PEI activation time, GA % and GA activation time).
Scheme 1.
Mechanism of polymeric gel beads activation and enzyme immobilization.
2.7. Enzyme immobilization
α-amylase was immobilized on different polymers by covalent binding as described in Scheme 1. This was performed by mixing 2 ml of the partially purified α-amylase (200 U) with 1 g of each prepared carrier. The mixture was left overnight at 4 °C, and then the beads were washed twice with distilled H2O and were used for immobilized α-amylase assay. The best carrier that exhibited highest immobilization yield (IY %) and immobilization efficiency (IE %) was chosen and used through this study. IY (%) and IE (%) were calculated according to [17] as following:
| IY (%) = I/ (A–B) X100 | (1) |
| IE (%) = (I/A) X 100 | (2) |
Where: I is the total activity of immobilized enzyme, A is the total activity offered for immobilization and B is the total activity of unbounded enzyme.
2.8. Carrier characterization
2.8.1. Scanning electron microscope (SEM)
Morphological examinations on the surface of different carrier formulations, Ch-MNP, Ch-MNP/PEI, Ch-MNP/PEI/GA and Ch-MNP/PEI/GA/Enzyme were carried out using scanning electron microscopy (SEM, Quanta 250 FEG, accelerating voltage 200 V- 30 kV, FEI Company, Thermo Fisher Scientific). These investigations were performed in order to describe the morphological changes that happened on the surface after each reaction.
2.8.2. Fourier transforms infrared (FTIR) spectroscopy analysis
The FTIR absorption spectra of Ch-MNP, Ch-MNP/PEI, Ch-MNP/PEI/GA, Ch-MNP /PEI/GA/Enz and partially purified α-amylase were measured by FTIR spectroscopy attenuated total reflection (ATR) mode Bruker VERTEX 70/70v model using the KBr disc technique. The aim of the test was to demonstrate the presence of the new functional group and carbonyl group formed at all different formulas. To do this, 2% (w/w) of the sample was mixed with dry KBr. The mixture was ground into a fine powder using an agate mortar before it was compressed into a KBr disk under a hydraulic press at 10,000 psi. Each KBr disk was scanned over a wave number range of 400–4000 cm−1, with a resolution of 4 cm−1 and the characteristic peaks were recorded.
2.9. Characterization of free and immobilized α-amylase enzyme
2.9.1. Effect of temperature on α-amylase activity
The optimum temperature of the free and immobilized enzyme was determined by measuring the activity at different temperatures ranging from 20 to 70 °C in 0.1 M sodium phosphate buffer pH 7.0 and enzyme assay was performed. The activity of control temperature was taken as 100 % and the relative activity at each temperature was expressed comparing with the control. The activation energy (Ea) of both free and immobilized α-amylase was calculated from the slope of the Arrhenius plot according to the following equation:
| Slope = - Ea / 2.303 R | (3) |
Where: R is gas constant (8.314 KJ/mol).
2.9.2. Effect of pH on α-amylase activity
The effect of pH on enzyme activity (free and immobilized) was examined by performing the enzyme assay at different pHs ranging from 5.0 to 11.0 by using 0.1 M of the following buffer systems: citrate-phosphate buffer (pH 5.0–6.0), sodium phosphate (pH 7.0-8.0) and glycine NaOH (pH 9.0-11.0) [18]. Enzyme activity was measured at optimum assay conditions and the relative activity was determined.
2.9.3. Effect of substrate concentrations (Determination of kinetic parameters)
For estimation of optimum substrate concentration for maximum enzymatic activity, enzyme activity (free and immobilized α-amylase) was assayed at different starch concentrations (0.25- 4 %) in 0.1 M sodium phosphate buffer (pH 8.0). The enzyme kinetic parameters, Michaelis–Menten constant (Km), and maximum reaction velocity (Vmax) were determined at optimum assay conditions according to [19] method. Also catalytic efficiency (Vmax/Km) of α-amylase was determined to demonstrate the affinity of enzyme with substrate.
2.9.4. Thermal stability
Thermal stability of the free and immobilized α-amylase enzyme was determined by incubating the enzyme at various temperatures ranging from 50 to 80 °C for 60 min in the absence of substrate. Samples were taken at 15 min intervals and assayed for activity under optimized conditions. The residual activity was calculated as following by taking the enzyme activity at 0 min incubation as 100 %:
| Residual activity (RA%) = (Final activity/ Initial activity) X100 | (4) |
The thermal and thermodynamic constants were calculated as following according to [20].
Deactivation rate constant (Kd) was calculated from the Arrhenius plot of Log RA (%) against time (min) using the following equation:
| Slope = - Kd | (5) |
The enzyme half-life time (t1/2) corresponds to the time period necessary for the residual activity to decrease by 50 % of its initial value and it was calculated from the following equation:
| t1/2 = ln 2/Kd | (6) |
Decimal reduction time (D-value) was defined as the time needed to reduce the initial activity by 90 % at a specific temperature and it was calculated as follows:
| D-value = ln10/ Kd | (7) |
The activation energy (Ed) for α-amylase denaturation was determined by a plot of log denaturation rate constants (ln Kd) versus reciprocal of the absolute temperature (K) using the following equation:
| Slope = − Ed / R | (8) |
The change in enthalpy (ΔH°, kJ/mol), free energy (ΔG° kJ/mol) and entropy (ΔS°, J/mol/ K) for thermal denaturation of α-amylase were determined using the following equations:
| ΔH° = Ed – RT | (9) |
| ΔG° = −RT ln (Kd. h / kB. T) | (10) |
| ΔS°= (ΔH° − ΔG°) / T | (11) |
Where: Ed is the activation energy for denaturation (KJ/mol)), R is the gas constant (8.314 J/mol/ K), T is the absolute temperature (K), Kd is the deactivation rate constant (/min), h is the Planck constant (11.04 × 10−36 Jmin) and Kb is the Boltzman constant (1.38 × 10−23 JK).
2.9.5. pH stability
The pH stability of the free and immobilized α-amylase was determined after pre-incubating the enzyme for 60 min at 30 °C with 0.1 M buffer systems at different pH range (pH 5.0-11.0) without substrate. The enzyme activity was measured at every 30 min intervals and the pH stability was expressed as RA % considering the initial enzyme activity at 0 min incubation as 100 %.
2.9.6. Operational stability (reusability)
The reusability of immobilized α-amylase (Ch-MNP /PEI/GA/Enz) was studied for several cycles and the RA % was determined. After each cycle, the beads were washed to remove any residual substrate with sodium phosphate buffer (0.1 M, pH 8.0) and re-use to start a new cycle. The activity in the first run was taken as 100 % and the RA was expressed as a percentage of the starting operational activity.
2.9.7. Storage stability
The Immobilized enzyme was stored at 4 °C for 40 days and the residual activity was measured under the optimum conditions.
3. Results and discussion
3.1. Production and Partial purification of α-amylase enzyme
α-amylase produced by isolated bacterial strain B. subtilis MK1 (activity 141.63 U/ml and specific activity 4.8 U/mg) was partially purified by ethanol precipitation (data not shown). The fractions precipitated at 60 % ethanol possessed the highest α-amylase specific activity (15 U/mg) which gave 3.1-fold purification. Therefore it was used for the preparation of the immobilized enzyme.
3.2. Enzyme immobilization
A novel method combining the advantages of physical adsorption and covalent binding methods was developed [17]. In this method, the enzyme was first adsorbed onto supports and the adsorbed enzyme was covalently cross-linked to the carriers.
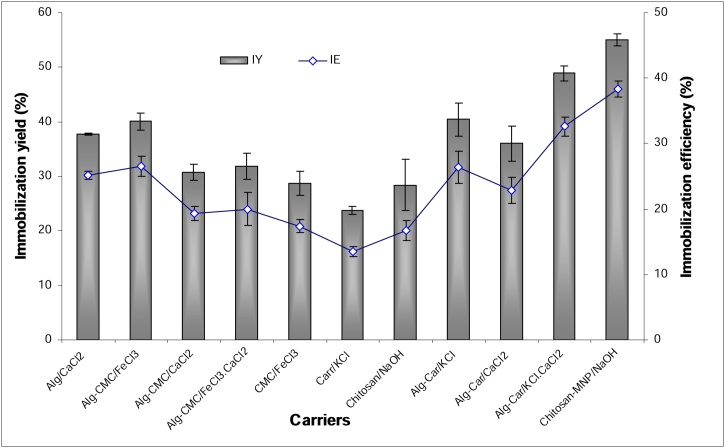
The catalytic behavior of immobilized enzymes depends on the properties of their carriers. So, α-amylase was immobilized on different carriers by covalent binding. The activated beads (carriers) reacted with the enzyme as shown in Scheme 1. The reaction happened between the free C = O group located on GA and the NH2 group found in the enzyme forming C = N– bond as reported by [15]. According to results introduced in Fig. 1, the most suitable carrier for α-amylase immobilization was Ch-MNP/PEI/GA as it exhibited the highest IY of 55 %.
Fig. 1.
Immobilization of B. subtilis strain- MK1 α-amylase on different carriers using covalent binding.
Polymeric nanocarrier can be fabricated easily in nanometer scale, in the range of 30 - 500 nm with a large surface area [21]. In addition they are attractive due to their simple synthesis, uniform size distribution, high stability, and abundance of functional groups for enzyme immobilization. The highest IY (81.6 %) using CCD was obtained at optimized conditions of 6% PEI, 4 h PEI activation time, 4% GA and 2 h GA activation time (data not shown). Optimization of beads modifications enhanced the IY by 1.5-fold compared with non-optimized.
3.3. Carrier characterization
3.3.1. Scanning electron microscope (SEM) characterization
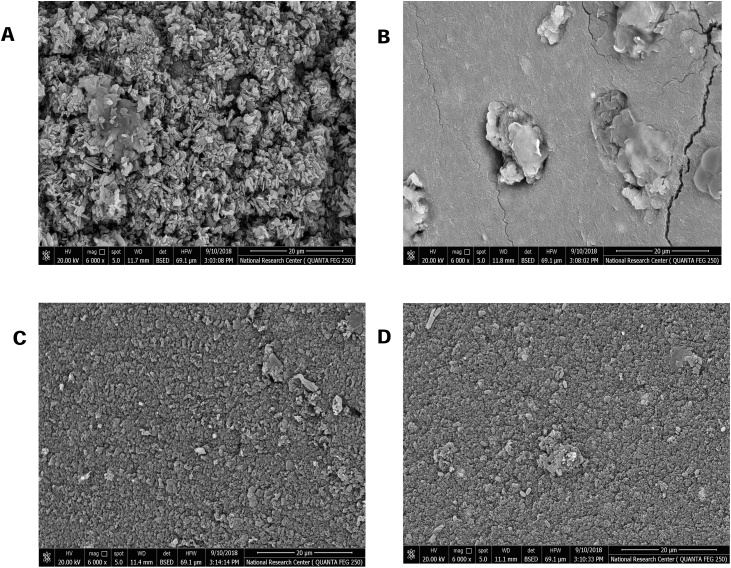
Scanning electron microscopy showed the modification which occurred during gel beads formation, activation and immobilization (Fig. 2). As shown, the Ch-MNP beads has rough surface that means high surface area available for immobilization. Noticeable changes were recognized along the steps of gel beads formation and accumulation of enzyme on the gel beads surface after immobilization. Moreover, a remarkable change in pore size can be recognized as the gel beads exhibited pores before treatment with PEI and GA. While after immobilization there was an accumulation of enzyme particles on the bead surfaces and the pores were decreased in size and tend to disappeared.
Fig. 2.
Scanning electron microscope (SEM) showed the surface of different gel formulations of Ch-MNP beads (A), Ch-MNP/PEI (B), Ch-MNP/PEI/GA (C), Ch-MNP /PEI/GA/Enzyme (D).
3.3.2. FT-IR characterization
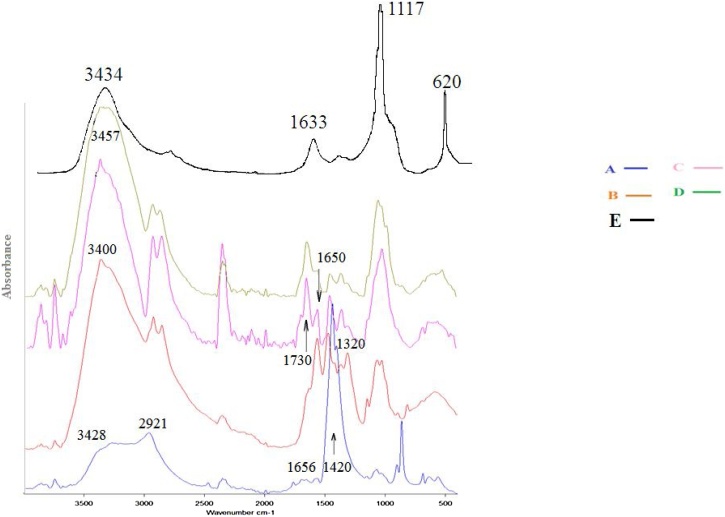
FT-IR spectroscopic analysis of Ch-MNP beads (A), aminated beads (Ch-MNP/PEI) (B), activated beads (Ch-MNP/PEI/GA) (C), immobilized beads (Ch-MNP/PEI/GA/Enz) (D) and partial purified enzyme (E) were carried out in a range varied from 400 – 4000 cm-1 (Fig. 3). In this figure the five typical characteristic absorption bands of chitosan in accordance with the literature were appeared at 3428 cm−1 (O-H and N-H stretching vibrations), 2921 cm−1 (C-H stretching vibrations), 1656 cm−1 (N-H bending vibrations) and 1073 cm−1 (C-O-C stretching vibrations). The bands at 1320–1420 cm−1 (C-N stretching vibrations) sharply increase in the spectrum of magnetic nanoparticles prepared in the presence of chitosan these characteristic bands indicate the obtaining of magnetic nanoparticles coated by chitosan (curve A). New broad beak at 3400 cm−1 was appeared that corresponding to NH2 group, indicating the presence of amino group on the surface of beads as a result of amination process (curve B); on the other hand, the activated beads showed two new beaks. One of them at 1730 cm-1 corresponding to the (C=O) group of GA free end, and the second one is at 1650 cm−1 corresponding to the (C = N-) group that produced from the reaction with GA (curve C). Moreover, immobilized beads give broader beak at 3457 cm−1, pointed to increasing NH2 group’s concentration (curve D). When comparing with partial purified enzyme (curve E) we can notice that, the characteristic peaks of enzyme, at 3434 cm−1 and at 1117 cm−1, are found in curve (D). From those data, it was revealed that the processes of amination, activation, and immobilization were successful. These results were in agreement with the results obtained by other published results [15].
Fig. 3.
FT-IR spectroscopic analysis of Ch-MNP beads (A), Ch-MNP/PEI (B), Ch-MNP/PEI/GA (C), Ch-MNP /PEI/GA/Enzyme (D), Partially purified enzyme (E).
3.4. Characterization of free and immobilized (Ch-MNP/PEI/GA/Enz) α-amylase
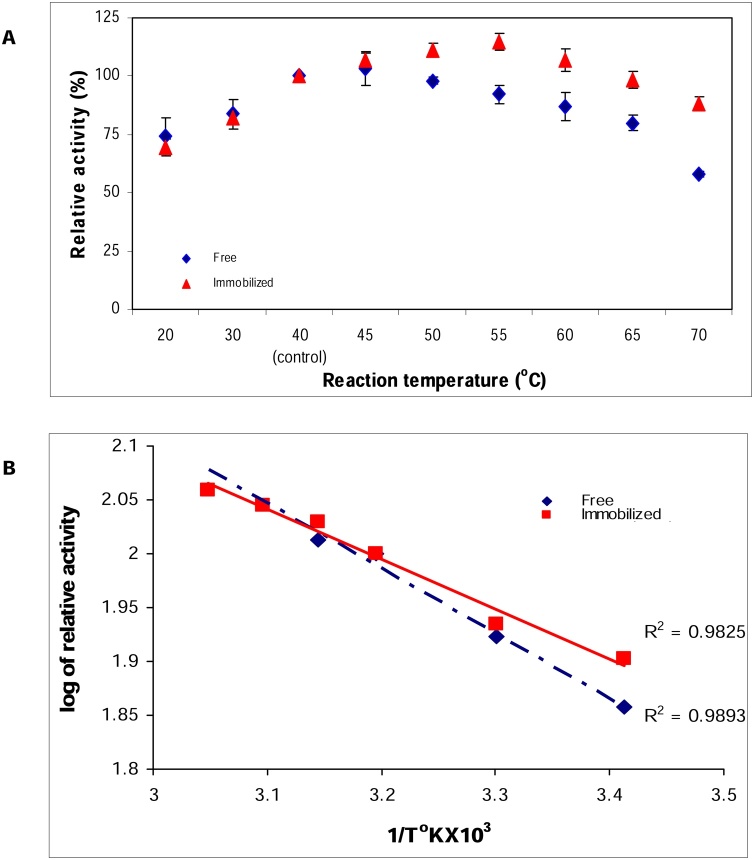
3.4.1. Effect of temperature on α-amylase activity
According to the data observed from Fig.4A, the optimum temperature was 45 and 55 °C, respectively for free and Ch-MNP/PEI/GA/Enz. Higher temperature (at 70 °C) decreased the activity by 42 and 11 %, respectively for free and Ch-MNP/PEI/GA/Enz. It is probably that binding of the carrier increased the rigidity, limited the conformational mobility of the enzyme molecules at high temperature, protecting it from denaturation [17,22]. In addition, it was thus proven that the immobilized enzyme could maintain its active structure even at a higher temperature [23]. Increased optimum temperature of immobilized α-amylase makes it useful in biotechnological and industrial applications [24].
Fig. 4.
Effect of different temperatures on α-amylase activity (A) and Arrhenius plot for temperature dependence of the activity of free and immobilized α-amylase (B).
From the Arrhenius plot (Fig.4B) the activation energy (Ea) for the Ch-MNP/PEI/GA/Enz was 3.85 kJ/mol while that for free enzyme was 5.03 kJ/mol. This result suggested that, the immobilization promoted the enzyme catalytic efficiency by reducing the Ea wanted for the formation of activated enzyme-substrate complex [22].
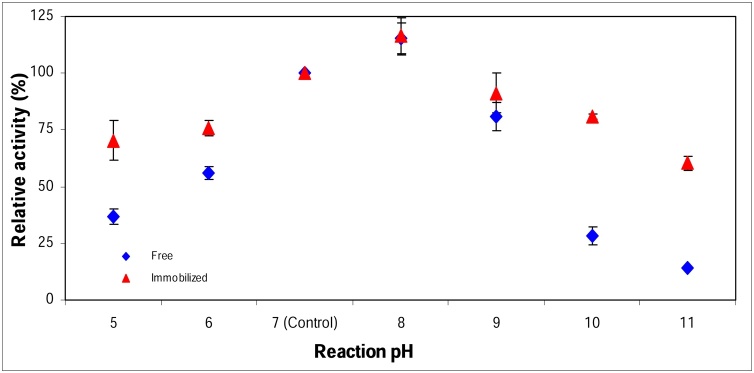
3.4.2. Effect of pH on α-amylase activity
The effect of pH on the activity of free and Ch-MNP/PEI/GA/Enz was illustrated in Fig.5. The results showed that, there was an increase in α-amylase activity with increase in pH value up to 8.0 for free and Ch-MNP/PEI/GA/Enz. However, the maximum activity of the free and Ch-MNP/PEI/GA/Enz occurs at the same pH, the Ch-MNP/PEI/GA/Enz exhibited a broader range of pH value (5.0-11.0). This report indicated that, the effective rate of the immobilized α-amylase becomes less sensitive and has better adaptation to pH changes than the free enzyme. [25] reported that a detergent enzyme must withstand to harsh conditions such as high pH values (8.0–10.5). The higher relative activities might be attributed to the multipoint attachment of enzyme with Fe3O4 at chitosan nanoparticles, which buffered variability of enzyme molecules when the pH was changed [17].
Fig. 5.
Effect of pH on α-amylase activity.
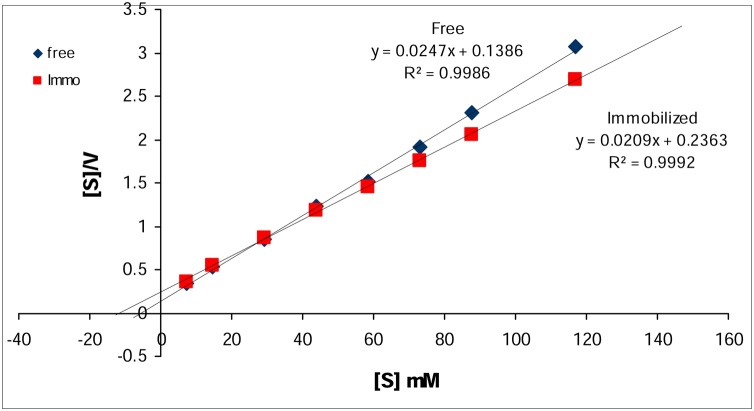
3.4.3. Effect of substrate concentration on α-amylase activity
The activity of free and Ch-MNP/PEI/GA/Enz was estimated at various starch concentrations. The enzyme activity increased with increasing starch concentration up to 2 % for free enzyme and 2.5 % for Ch-MNP/PEI/GA/Enz (data not shown). The reaction kinetics of the free and Ch-MNP/PEI/GA/Enz was estimated from Hanes - Woolf plots under optimal conditions (Fig. 6). The results indicated that the calculated Km value of the Ch-MNP/PEI/GA/Enz was 2.1-fold higher than the free form. In addition, the Vmax of the Ch-MNP/PEI/GA/Enz was higher than the value of the free form by 1.2-fold. These effects might be a consequence of the changes in enzyme configuration upon immobilization. This pointed to increase the conversion rate of substrate to product but the affinity for substrate reduced which due to change in the microenvironment of the enzyme active site by decreasing structural flexibility of immobilized form. The Vmax/Km ratio for Ch-MNP/PEI/GA/Enz was 1.7-fold lower than the free enzyme. The results indicated that immobilized enzyme have less specificity to the substrate. [22] reported that a reduction in enzyme kinetic parameters after immobilization can be related to conformational changes in its structure.
Fig. 6.
Hanes - Woolf Plot of the free and immobilized α-amylase.
3.4.4. Thermal stability
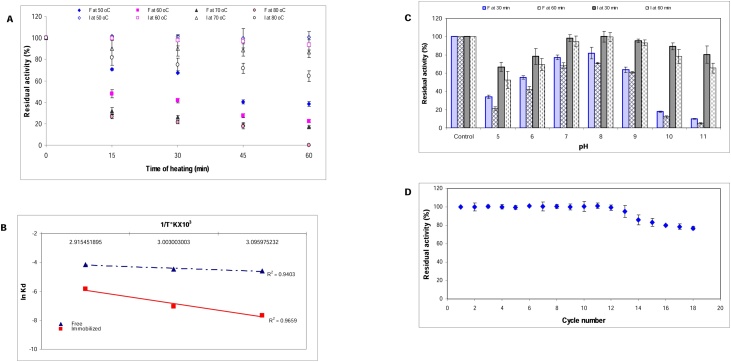
The data presented in (Fig. 7A) explained the importance of immobilization process in enhancing the thermal stability of enzyme. The residual activity at 60 °C after 30 min and 60 min for the free enzyme was 41.5 % and 22.5 %, respectively and for the Ch-MNP/PEI/GA/Enz was 97.58 % and 93.54 %, respectively. At higher temperatures and longer times, the residual activity of the free enzyme declined dramatically. Obviously, increasing the thermal stability of Ch-MNP/PEI/GA/Enz demonstrated the successful role of immobilization method in protecting the enzyme against thermal inactivation and increasing its suitability for several industrial applications. This stability comes from the formation of high amount of covalent bonds has been presented to reduce enzyme mobility and conformational flexibility which in turn inhibit aggregation and unfolding of enzyme protein [20].
Fig. 7.
Thermal stability (A), Arrhenius plot for energy of denaturation (Ed) (B), pH stability (C) and operational stability (D) of α-amylase enzyme.
The heat inactivation profile of free and Ch-MNP/PEI/GA/Enz provides a relationship between the enzyme structure and function at a certain temperature (Table 1). Deactivation rate constant (Kd) is an important parameter for the promote of an economic bioprocess at industrial level. With increasing temperature, the t½ decreased, the D-value decreased and the Kd increased (Table 1). At 60 °C, a 21.8-fold increase in t½ value of α-amylase after immobilization has been observed. In addition, at 70 °C, D-value of Ch-MNP/PEI/GA/Enz calculated as 2642.1 min, which is higher than its free form by 13.3-fold. Increasing the t½ value and D-value confirm its improved thermal stability after immobilization [22].
Table 1.
Thermal inactivation kinetics and thermodynamic parameters of free and Ch-MNP /PEI/GA/Enzym.
| Enzyme | Ed (KJ/mol) | Thermal inactivation parameters |
Temperature (°C) |
Thermodynamic parameters |
Temperature (°C) |
||
|---|---|---|---|---|---|---|---|
| 60 | 70 | 60 | 70 | ||||
| Free | 23.42 | Kd (min−1) | 10.25 × 10−3 | 11.55 × 10−3 | ΔH° (kJ/mol) | 20.65 | 20.57 |
| t1/2 (min) | 67.60 | 59.99 | ΔG° (kJ/mol) | 92.56 | 95.09 | ||
| D-value (min) | 224.56 | 199.30 | ΔS° (J/mol/°K) | −0.22 | −0.22 | ||
| Immobilized | 88.98 | Kd (min−1) | 0.47 × 10−3 | 0.87 × 10−3 | ΔH° (kJ/mol) | 86.21 | 86.13 |
| t1/2 (min) | 1472.98 | 795.34 | ΔG° (kJ/mol) | 100.91 | 102.31 | ||
| D-value (min) | 4893.15 | 2642.05 | ΔS° (J/mol/°K) | −0.05 | −0.05 | ||
The activation energy for denaturation (Ed) is the lowest amount of energy wanted to initiate the denaturation process of enzyme (Fig. 7B). The results indicated that the required energy to denature Ch-MNP/PEI/GA/Enz was 3.8-fold higher than that for free enzyme.
The thermodynamic parameters as enthalpy (ΔH°), Gibbs free energy (ΔG°), and entropy (ΔS°) were determined for free α-amylase and Ch-MNP/PEI/GA/Enz. As seen from the results (Table 1), with increasing temperature there was a gradual decrease in ΔH° for both forms of enzyme. The ΔH° is the total amount of energy wanted to deactivate the enzyme. At 80 °C, ΔH° for the Ch-MNP/PEI/GA/Enz was higher than that of the free form by 56.6 KJ/mol. [26] reported that a positive and higher value of Ed and ΔH° gives an indication of high thermal stability of the enzyme.
Gibbs free energy is known as the index of the energy wanted to cross the activation energy barrier of reaction. ΔG° value for the immobilized enzyme is more than the free enzyme indicating that immobilized enzyme requires more energy than free enzyme for thermal inactivation [8]. As shown in Table 1, there was an increase in the ΔG° value by 8.4 and 7.2 KJ/mol for Ch-MNP/PEI/GA/Enz over free enzyme at 60 and 70 °C, respectively.
The Entropy (ΔS°) is the alteration in structural disorder upon protein denaturing and is directly related to enzyme stability [20]. The obtained ΔS° values were negative, meaning that the randomness or disorder decreasing in the transition from the native state to the denatured state of the free and immobilized enzyme [27] Zaboli et al. [8]. reported that the negative ΔS° values for the immobilized enzyme indicate the lack of enzyme aggregation during thermal inactivation. Decreasing ΔS° after immobilization points to high difference in entropy between the native state and the transition state may be due to the stabilization of enzyme conformation [28].
3.4.5. pH stability
The pH stability for the free and Ch-MNP/PEI/GA/Enz was studied and displayed in (Fig. 7C). As seen, Ch-MNP/PEI/GA/Enz was significantly more stable than the free enzyme at all tested pH values especially at alkaline range. The Ch-MNP/PEI/GA/Enz exhibited great stability and kept 93.2 % and 78.3 % of its initial activity after 60 min at pH 9 and 10 while, the free enzyme preserved 61 % and 12 %, respectively. Furthermore, the residual activity of Ch-MNP/PEI/GA/Enz at pH 11 was 65.6 % after 60 min whereas the free enzyme lost 95 % of its activity. These results explained that, the immobilization process enhanced the stability and resistance of α-amylase in both acidic and alkaline pHs. [21] reported that immobilization of enzymes on nano-carriers results in stabilization of active site conformation.
3.4.6. Operational stability of Ch-MNP/PEI/GA/α-amylase
Immobilization processes are fundamental keys for managing reuse of the enzyme over a long period. The Ch-MNP/PEI/GA/Enz beads can be easily separated from its products and reused many times for hydrolysis of starch. As shown in Fig. 7D the Ch-MNP/PEI/GA/Enz could be reused for 15 consecutive cycles with 83.2 % residual activity. The loss in enzyme activity may be related to repetitive encountering of substrate to the active site of immobilized enzyme that affects the binding strength between carrier and immobilized α-amylase [29]. Furthermore, the reuse of immobilized enzyme in the reaction might be cause inactivation and denaturation of the enzyme. Our result is superior to that obtained by [29] where immobilized α-amylase was reused for 10 cycles with residual activity 50 %.
3.4.7. Storage stability
The main driving force for enzyme immobilization is stabilization. Storage Ch-MNP/PEI/GA/Enz at 4 °C for 21 days retained approximately 100 % of its initial activity (data not shown). Moreover, after 40 days Ch-MNP/PEI/GA/Enz retained 86.4 %, such observations show the improved stability of the immobilized biocatalysts for a long time. [8] suggested that the multipoint attachment of the carrier surface to the enzyme may enhance stabilization. In addition Misson et al. [21], reported that the nano-environment surrounding enzyme molecules may prevent enzyme from deactivation.
4. Conclusion
The study describes a thermostable α-amylase from isolated strain Bacillus subtilis strain-MK1. The enzyme was covalently bonded to different polymer supports. α-amylase was successfully immobilized on Chitosan-magnetic nanoparticles (Ch-MNP/PEI/GA) with highest IY%. SEM and FTIR studies confirm the linkage between Ch-MNP/PEI/GA and the enzyme. Using statistical analysis (CCD) for beads modification enhanced the IY by 1.5-fold. Based on the results, Ch-MNP/PEI/GA/Enz showed higher optimum temperature by 10 °C than the free one. Immobilization improved the quality of α-amylase by decreasing the activation energy by 1.31-fold. Also, Ch-MNP/PEI/GA/Enz was 21.79 and 13.26 times more stable than the free one at 60 and 70 °C, respectively. The higher value of Ed for Ch-MNP/PEI/GA/Enz (3.8-fold) compared with free indicates that more energy is required to enzyme thermal denaturation. Compared to the free enzyme, the Ch-MNP/PEI/GA/Enz exhibited lower Ea, lower Kd, higher t1/2 and higher D-values. The calculated thermodynamic parameters ΔH°, ΔG° and ΔS° demonstrated that covalent binding between enzyme and Ch-MNP/PEI/GA increased its thermal stability. Moreover, Ch-MNP/PEI/GA/Enz showed higher pH stability at different pH values (5-11) compared with free enzyme. The Ch-MNP/PEI/GA/Enz was successfully used in batch mode for 12 cycles for effective degradation of starch with about 100 % residual activity. In addition, Ch-MNP/PEI/GA/Enz retained 86.4 % after 40 days storage at 4 °C. The results demonstrate that immobilized biocatalyst has the potential for different applications of industrial processes under harsh conditions.
Author agreement
The author agrees to publish the manuscript in Biotechnology Reports.
Funding
This work was supported by the National Research Centre, Egypt.
Declaration of Competing Interest
The authors declare no competing financial interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00443.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Sohrabi N., Rasouli N., Torkzadeh M. Enhanced stability and catalytic activity of immobilized a-amylase on modified Fe3O4 nanoparticles. Chem. Engin. J. 2014;240:426–433. [Google Scholar]
- 2.Tambekar D.H., Tambekar S.D., Rajgire A.V., A.S Jadhav, K.K Sawale. Isolation and characterization of amylase from Lysinibacillus xylanilyticus from alkaline environment. Int. J. Resea. Stud. Biosci. 2016;4:1–4. [Google Scholar]
- 3.Pandey G., Munguambe D., Tharmavaram M., Rawtani D., Agrawal Y. Halloysite nanotube -an efficient ‘nano-support’ for the immobilization of α-amylase. Appl. Clay Sci. 2017;136:184–191. [Google Scholar]
- 4.Sojitra U.V., Nadar S.S., Rathod V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles bymacromolecular cross-linker. Carbohyd. Polym. 2017;157:677–685. doi: 10.1016/j.carbpol.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Eskandarloo H., Abbaspourrad A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018;251:115–124. doi: 10.1016/j.foodchem.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 6.Kuo C.-H., Liu Y.-C., Chang C.-M.J., Chen J.-H., Chang C., Shieh C.-J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 Nanoparticles. Carbohyd. Polym. 2012;87:2538–2545. [Google Scholar]
- 7.Bindu V.U., Shanty A.A., Mohanan P.V. Parameters affecting the improvement of properties and stabilities of immobilized α-amylase on Chitosan-metal oxide composites. Int. J. Biochem. Biophys. 2018;6:44–57. [Google Scholar]
- 8.Zaboli M., Raissi H., Zaboli M., Farzad F., Torkzadeh-Mahani M. Stabilization of D-lactate dehydrogenase diagnostic enzyme via immobilization on pristine and carboxyl-functionalized carbon nanotubes, a combined experimental and molecular dynamics simulation study. Arch. Biochem. Biophys. 2019;661:178–186. doi: 10.1016/j.abb.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Sajjad M., Choudhry S. Effect of starch containing organic substrates on alpha amylase production in Bacillus strains. Afr. J. Microbiol. Res. 2012;6:7285–7291. [Google Scholar]
- 10.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analy. Chem. 1959;31:426–428. [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Awad G.E.A., Abd El Aty A.A., Shehata A.N., Hassan M.E., Elnashar M.M. Covalent immobilization of microbial naringinase using novel thermally stable biopolymer for hydrolysis of naringin. 3 Biotech. 2016:6–14. doi: 10.1007/s13205-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akalin G.O., Pulat M. Preparation and characterization of nanoporous sodium carboxymethyl cellulose hydrogel beads, Article ID 9676949. J. Nanomat. 2018;2018:1–12. [Google Scholar]
- 14.Awad G.E.A., Wehaidy H.R., Abd El Aty A.A., Hassan M.E. A novel alginate–CMC gel beads for efficient covalent inulinase immobilization. Colloid. Polym. Sci. 2017;295:495–506. [Google Scholar]
- 15.Yuan Y., Luan X., Rana X., Hassan M.E., Dou D. Covalent immobilization of cellulase in application of biotransformation of ginsenoside Rb1. J. Molec. Cat. B: Enzy. 2016;133:S525–S532. [Google Scholar]
- 16.Karam E.A., Hassan M.E., Moharam M.E., Kansoh A.L. Immobilization of Inulinase Produced by Rhizopus oligosporus NRRL 2549 for continuous fructose production. J. Mater. Environ. Sci. 2018;9:2315–2321. [Google Scholar]
- 17.X.-Y Wang, Jiang X.-P., Li Y., Zeng S., Zhang Y.-W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biologi. Macromol. 2015;75:44–50. doi: 10.1016/j.ijbiomac.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Singh R.N., Bahuguna A., Chauhan P., Sharma V.K., Kaur S., Singh S.K., Khan A. Production, purification and characterization of thermostable α-amylase from soil isolate Bacillus sp. Strain B-10. J. BioSci. Biotech. 2016;5:37–43. [Google Scholar]
- 19.Hanes C.S. Studies on plant amylases: the effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem. J. 1932;26:1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedade D.K., Muley A.B., Singhal R.S. Magnetic cross-linked enzyme aggregates of acrylamidase from Cupriavidus oxalaticus ICTDB921 for biodegradation of acrylamide from industrial waste water. Bioresour. Technol. Rep. 2019;272:137–145. doi: 10.1016/j.biortech.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Misson M., Zhang H., Jin B. Nanobiocatalyst advancements and bioprocessing applications. J. Roy. Soci. Interf. 2015;12:1–20. doi: 10.1098/rsif.2014.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R.S., Chauhan K., Kennedy J.F. Fructose production from inulin using fungal inulinase immobilized on 3-aminopropyl-triethoxysilane functionalized multiwalled carbon nanotubes. Int. J. Biol. Macromol. 2019;125:41–52. doi: 10.1016/j.ijbiomac.2018.11.281. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang M.-Y., Jiang X.-P., Ling X.-M., Xu M.-Q., Zhu Y.-H., Zhang Y.-W. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J. Chem. Technol. Biotech. 2018;93:2351–2358. [Google Scholar]
- 24.Ahmed S.A., Mostafa F.A., Ouis M.A. Enhancement stability and catalytic activity of immobilized α-amylase using bioactive phospho-silicate glass as a novel inorganic support. Int. J. Biol. Macromol. 2018;112:371–382. doi: 10.1016/j.ijbiomac.2018.01.162. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S.A., Abdel Wahab W.A., Abdel-Hameed S.A.M. Comparative study in kinetics and thermodynamic characteristics of immobilized caseinase on novel support from basalt by physical adsorption and covalent binding. Biocat. Agricult. Biotech. 2019;18:101028. [Google Scholar]
- 26.Mohapatra B.R. Kinetic and thermodynamic properties of alginate lyase and cellulaseco-produced by Exiguobacterium sp. Alg-S5. Int. J. Biol. Macromol. 2017;98:103–110. doi: 10.1016/j.ijbiomac.2017.01.091. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira M.M., Santiago F.L.B., daSilva N.A.G., Luiz J.H.H., Fernandéz-Lafuente R., Mendes A.A., Hirata D.B. Different strategies to immobilize lipase from Geotrichum candidum: Kinetic and thermodynamic studies. Process Biochem. 2018;67:55–63. [Google Scholar]
- 28.Wehaidy H.R., Abdel-Naby M.A., Shousha W.G., Elmallah M.I.Y., Shawky M.M. Improving the catalytic, kinetic and thermodynamic properties of Bacillus subtilis KU710517 milk clotting enzyme via conjugation with polyethylene glycol. Int. J. Biol. Macromol. 2018;111:296–301. doi: 10.1016/j.ijbiomac.2017.12.125. [DOI] [PubMed] [Google Scholar]
- 29.Defaei M., Kafrani A.T., Miroliaei M., Yaghmaei P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018;113:354–360. doi: 10.1016/j.ijbiomac.2018.02.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.