Abstract
Objective
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is shown to as a negative-regulatory cause in osteoclasts differentiation. Cannabinoid receptor type 2 (CB2) is verified to regulate osteoclast differentiation, though with diversed results.
Methods
In current research, we studied the Nrf2 role on osteoclast differentiation regulation with the CB2-selective agonists, AM1241, or CB2-selective antagonist, AM630, in RAW 264.7 macrophages. The nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation activator was confirmed by tartrate-resistant acid phosphatase (TRAP) staining as well as the TRAP activity analysis. In addition, Nrf2 siRNA was used to characterize the function of Nrf2 during osteoclast differentiation. We analyzed HO-1 and Nrf2 proteins levels with western blotting.
Results
The results showed that AM1241 promoted, while AM630 suppressed, osteoclast differentiation in RAW 264.7 cells. Both AM1241 and AM630 increased the expressions of HO-1 and Nrf2. Nrf2 silencing promoted osteoclast differentiation and abolished the function of AM630 to inhibit osteoclast differentiation.
Conclusions
Our results suggested that Nrf2 was required for inhibiting osteoclast differentiation induced by RANKL of RAW 264.7 cells by AM630, which may provide the insights of a novel method to treat osteoclastogenic bone disease.
Keywords: Nrf2, Osteoclast differentiation, Cannabinoid receptor type 2, RANKL
1. Introduction
Osteoporosis is a bone trait marked with decreased bone mass, bone microstructure damage, as well as decreased bone strength [1]. The prevalence of osteoporosis is significantly higher in people >60 years of age, especially in women [2]. The normal bone remodeling process is mainly affected by two types of cells: osteoblasts, which promote bone formation through anabolism, and osteoclasts, which promote bone resorption through catabolism. Bone resorption mediated by osteoclasts as well as osteogenesis mediated by osteoblasts maintain a dynamic balance, which ensures the continuous renewal and repair of bone tissue after injury. When this balance is perturbed, there is more resorption than formation of bone, which results in bone loss and osteoporosis [3]. Therefore, inhibition of osteoclast formation is an important strategy to treat osteoporosis.
Investigations have verified that oxidative stress can affect bone metabolism [4]. The main manifestations include inhibiting the bone matrix secretion by osteoblasts, affecting the bone matrix mineralization, and directly participating in the degradation of bone matrix, leading to the destruction of the bone matrix; By stimulating the secretion of RANKL in osteoblasts, the formation of osteoclasts is increased, and bone resorption is increased via increasing the osteoclasts. Blocking the negative effects of oxidative stress on bone metabolism may therefore reduce or even reverse osteoporosis.
Nrf2, as a key transcription factor in antioxidant reactions, can be combined with the antioxidant response factor of the nucleus to activate the downstream target genes, playing a critical regulatory role in oxidative stress, and extensively participating in processes such as proliferation, inflammation, apoptosis, tissue regeneration, cell differentiation and metabolism [5]. A recent study has reported that Nrf2 also functions importantly in skeletal metabolism [6]. In osteoclasts, the Nrf2 absence inhibits the synthesis of downstream heme oxygenase-1 (HO-1) antioxidant enzymes, decreases ROS levels in osteoclasts, and leads to more osteoclast differentiation [7].
Reports have showed that the endocannabinoid system is very crucial in bone remodeling [8,9]. This system exerts its biological effects mainly through cannabinoid receptor type 1/2 (CB1/2) [10]. CB1 mainly exists in system of central nervous, where it regulates the release of neurotransmitters and functions importantly in pain and brain processing. CB2 is mostly expressed in immune along with hematopoietic cells, where it regulates the cytokine release and the migration of immune cells. Former reserch has validated that CB2 selective antagonists inhibit osteoclast differentiation [11], while other studies have reported that activation of CB2 protects RAW264.7 macrophages against oxidative stress [12]. Based upon the results, relationships between CB2 and oxidative stress as well as its mechanism in osteoclast differentiation need to be further confirmed.
In this investigation, we therefore characterized the CB2 and Nrf2 effects on osteoclast RANKL-induced differentiation and oxidative responses in RAW 264.7 cells.
2. Materials and methods
2.1. Cell culture and treatment
The RAW264.7 mouse macrophage cells (ATCC, Manassas, VA, USA) were culture in DMEM (Gibco BRL, MD, USA), which contained heat-inactivated FBS of 10%, 100 U/mL penicillin, 100 U/mL streptomycin and 2 mM l-glutamine under 37 °C in a 5% CO2 incubator.
We treated RAW264.7 cells by RANKL of 100 ng/mL without or with 2 μM AM1241 or 200 nM AM630 for 5 days. RANKL, AM1241, and AM630 (Sigma-Aldrich, St. Louis, USA).
2.2. Lentiviral vector construction and transfection
To knockdown Nrf2 in RAW264.7 cells, small interfering RNA targeting to Nrf2 (siNrf2) and nonsense siRNA (scramble siRNA) were purchased from Hanbio Biotechnology (Shanghai, China). The mouse Nrf2 siRNA sequence was 5′-CCACGCTGAAAGTTCAGTCTT-3′. 100 nM siNrf2 or siRNA were transfected to RAW264.7 cells via Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) for one day. Nrf2 expression was confirmed by western blotting.
2.3. Detection of tartrate-resistant acid phosphatase (TRAP) activity
TRAP activity is a particular biochemical activity marker of osteoclasts, which was determined to confirm osteoclast differentiation. Briefly, RAW264.7 cells with or without transfection were seeded in plates with 48 wells (1 × 103 cells/well). After 24 h of culturing, we cultured cells with 100 ng/mL RANKL in presence or absence of 2 μM AM1241 or 200 nM AM630 for 5 days. Then, after washing with PBS for three times, we fixed cells in 4% paraformaldehyde for 10 min. Then we stained them for TRAP through a commercial kit (Sigma-Aldrich). We classified TRAP-positive multinucleated cells (TRAP + MNCs) containing ≥ 3 nuclei for osteoclast, counted and captured them by a microscope (Olympus, Tokyo, Japan). For TRAP activity measure, the culture medium was collected, and measured with a TRAP assay kit (Sigma-Aldrich), we detected optical density at 405 nm by a microplate reader.
2.4. Western blot assays
We treated RAW264.7 cells without or with transfection with 100 ng/mL RANKL together with or without 2 μM AM1241 or 200 nM AM630 for 5 days. To extract whole cell proteins, we collected cells and lysed them with a cell lysis buffer for half of an hour on ice. We extracted total protein by protein extraction kit (Beyotime Biotech, Jiangsu, China). We resolved forty μg of protein by SDS-PAGE of 10% and transferred them onto nitrocellulose membrane (Millipore, Jaffrey, NH, USA). We blocked membrane in 5% nonfat milk for 1 h, then incubated them with antibodies of anti-Nrf2 (1:500; Abcam, MA, USA), anti–HO–1 (1:200; Santa Cruz, CA, USA), or anti-β-actin (1:1000; Abcam) overnight under 4 °C. We washed membranes for 3 times with PBS having 0.5% Tween-20, and incubated them with suitable secondary antibody (Cell Signaling Technology, MA, USA) under room temperature for 2 h. We scanned membrane by enhanced chemiluminescence detection system (Thermo Scientific, Rockford, IL, USA). We determined relative protein levels after normalization with β-actin. Densitometric of the bands were analyzed by ImageJ software.
2.5. Statistical analysis
Results were denoted by mean ± SEM from≥3 separate determinations. We analyzed data by GraphPad Prism-5 package (San Diego, CA, USA). One-way ANOVA along with Dunnett's Multiple-comparison Test were employed to test significances. p value less than 0.05 was regarded to be statistically significant.
3. Results
3.1. AM1241 promotes while AM630 inhibits osteoclast differentiation in RAW264.7 cells
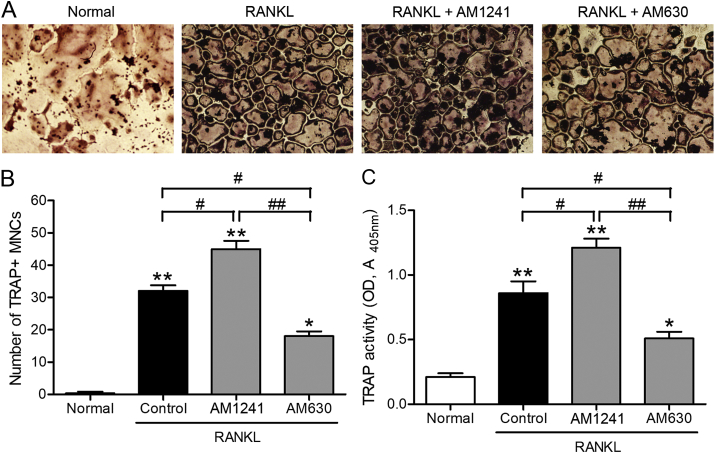
To explore the CB2 function on osteoclastogenesis, we treated RAW264.7 cells with 100 ng/mL RANKL without or with CB2-selective agonist, AM1241 (2 μM), or the CB2 selective antagonist, AM630 (200 nM), for 5 days, and performed TRAP staining. Fig. 1A shows that in the RANKL alone group, many TRAP positive multinucleated cells (TRAP + NMCs) appeared, suggesting that RAW264.7 cells differentiated into osteoclasts. Compared to RANKL alone group, the osteoclasts number were further increased after treatment with AM1241, while the maturity and number of osteoclasts significantly decreased after treatment with AM630 (Fig. 1B). Furthermore, Fig. 1C shows that AM1241 promoted TRAP activity, while AM630 inhibited TRAP activity. Together, these results indicated that AM1241 promoted RANKL-induced osteoclast differentiation while AM630 inhibited the same process.
Fig. 1.
The CB2 agonist, AM1241, promotes, while antagonist, AM630, restrains RANKL-induced osteoclast differentiation in RAW264.7 cells. The cells were treated with 100 ng/mL RANKL in the presence or absence of 2 μM AM1241 or 200 nM AM630 for 5 days. (A) The fixed cells were performed with TRAP staining and observed under a light microscope (100 × ). (B) TRAP-positive cells containing > 3 nuclei were determined as osteoclasts. (C) The cell supernatants were collected, and TRAP activity was measured by an ELISA reader (optical density at 405 nm). ∗p < 0.05, ∗∗p < 0.01 compared to normal group. #p < 0.05, ##p < 0.01 compared to indicated groups. Normal, untreated cells.
3.2. The effects of AM1241 and AM630 on the HO-1 and Nrf2 protein expressions in RAW264.7 cells
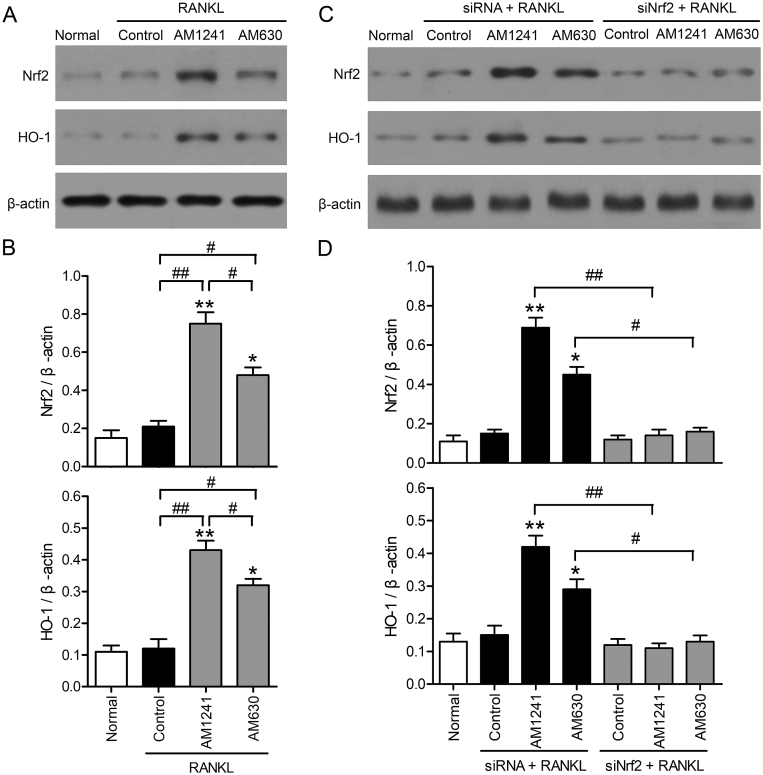
We further investigate if Nrf2/HO-1 signaling was related to the AM1241 and AM630 effects on RANKL-induced osteoclast differentiation. HO-1 and Nrf2 protein levels in RAW264.7 cells after different treatments were detected by western blotting (Fig. 2A). The data validated that both the AM1241 and AM630 treatment groups significantly upregulated the Nrf2/HO-1 protein expression comparing with the normal group and the RANKL alone treatment group. Moreover, the upregulated Nrf2/HO-1 expression after AM1241 treatment were higher than that of the AM630 treatment group (Fig. 2B). RAW264.7 cells were then transfected with siNrf2 to knockdown Nrf2 levels. Western blotting showed that Nrf2 interference successfully blocked the Nrf2/HO-1 expression in both AM1241 and AM630 treatment groups, when compared to the siRNA control group (Fig. 2C and D). Results suggested that HO-1/Nrf2 pathway participated in the AM1241 and AM630 effects during osteoclast differentiation.
Fig. 2.
The effects of AM1241 and AM630 on Nrf2/HO-1 protein level in RAW264.7 cells. (A, B) The cells were cultured with 100 ng/mL RANKL in presence or absence with 2 μM AM1241 or 200 nM AM630 for 5 days. (A) The protein level of Nrf2/HO-1 was measured by western blotting. (B) The Western blot results were normalized to β-actin. ∗p < 0.05 and ∗∗p < 0.01 compared to normal cells; #p < 0.05, ##p < 0.01 compared to the indicated groups. (C and D) RAW264.7 cells were transfected with scrambled siRNA or Nrf2 siRNA for 24 h. The cells were treated with 100 ng/mL RANKL with or without 2 μM AM1241 or 200 nM AM630 for 5 days. (C) Nrf2/HO-1 protein level was detected by western blotting. (D) Quantification of Nrf2/HO-1 protein level relative to that of β-actin. ∗p < 0.05, ∗∗p < 0.01 compared to normal cells. #p < 0.05, ##p < 0.01 compared between the indicated groups.
3.3. Nrf2 knockdown abolishes the AM630 effect on osteoclast differentiation inhibition
We further determine whether Nrf2 changes affected the AM1241 and AM630 effects on osteoclast differentiation. We treated RAW264.7 cells transfected with control siRNA or Nrf2 siRNA with RANKL of 100 ng/mL without or with 2 μM AM1241 or 200 nM AM630 for 5 days. TRAP staining showed that downregulation of Nrf2 promoted RANKL-induced osteoclast differentiation (Fig. 3A), and significantly upregulated the TRAP + NMCs number compared to the siRNA control group (Fig. 3B). In AM1241 treatment group, no significant difference was found about the TRAP + NMCs number between the cells interfering with Nrf2 and those transfected with siRNA. However, in the AM630 treatment group, interference with Nrf2 eliminated the inhibitory effect of AM630 on osteoclast differentiation comparing with siRNA cells (Fig. 3B). The results of the TRAP activity test were consistent with those of TRAP staining (Fig. 3C). Overall, the data advised that Nrf2 was a necessary factor in the osteoclast differentiation inhibition by AM630.
Fig. 3.
Inhibition of Nrf2 abolishes the effect of AM630 on osteoclast differentiation inhibition. The transfected cells were treated with as indicated for 5 days. (A) The fixed cells were performed with TRAP staining and observed by a light microscope (100 × ). (B) TRAP-positive cells containing > 3 nuclei were determined as osteoclast. (C) The TRAP activity was detected with an ELISA reader (optical density at 405 nm). ∗p < 0.05, ∗∗p < 0.01 compared to control cells. #p < 0.05 compared to siRNA transfected cells treated with RANKL and AM630.
4. Discussion
One cause of osteoporosis is the excessive activation of osteoclasts [13]. Inhibition of osteoclast formation is therefore an important step in the treatment of osteoporosis. In present research, we confirmed that the CB2 selective agonist, AM1241, promoted osteoclast differentiation and that the selective inhibitor, AM630, inhibited osteoclast differentiation. Moreover, Nrf2 was a necessary factor for AM630 to inhibit osteoclast differentiation.
Osteoclasts are derived from polynuclear terminal cells of mononuclear macrophages, which are exclusively cells with bone resorption ability in the body. Perturbation of the balance of bone metabolism is due to an increase of bone resorption by osteoclasts [14]. As an osteoclasts precursor, RAW264.7 cells differentiate into osteoclasts with the RANKL treatment [15]. The marker for osteoclast formation was TRAP positive cells, which increased, forming multinucleated cells with actin rings [16]. In our study, the results showed that in osteoclast RANKL-induced differentiation of RAW264.7 cells, the CB2 selective agonist, AM1241, enhanced TRAP positive osteoclast formation. In contrast, the CB2 selective antagonist, AM630, inhibited osteoclast formation. Sophocleous et al. [17] reported that the CB2 selective agonists, HU308 and JWH133, enhanced mouse and human breast cancer cell-induced osteoclast formation. Idris et al. [8] reported that the CB2 selective antagonist, AM630, inhibited osteoclasts activity as well as formation. However, CB2 mechanism in osteoclast differentiation is controversial. Therefore, we further studied the potential mechanism of AM1241 and AM630 in osteoclast differentiation.
Previous studies showed that the Nrf2 signaling pathway functions importantly in protecting cells from oxidative damage by promoting the antioxidant enzyme expressions in cells, which is also an attractive target in bone metabolic homeostasis [6]. Recent researches have validated that Nrf2 and its downstream HO-1 inhibit osteoclast differentiation [18,19]. Our results showed that both AM1241 and AM630 upregulated the HO-1 and Nrf2 expression, but notably, they had opposite effects on osteoclast differentiation. Giacoppo et al. [12] reported that activation of CB2 protected RAW264.7 macrophages against oxidative stress, and Kim et al. [20] found that the Nrf2/HO-1 signaling pathway activation inhibited RANKL-induced osteogenic differentiation. We therefore speculated that AM1241 affected osteoclast differentiation through other signaling pathways rather than the Nrf2/HO-1 signaling pathway.
We further investigate Nrf2 mechanism during osteoclast differentiation, and we knockdown Nrf2 in RAW264.7 cells vis transfection with siNrf2. Our results indicated that inhibiting Nrf2 enhanced osteoclast differentiation in RAW264.7 cells, which was consistent with the previous results from Hyeon et al. [7]. Downregulation of Nrf2 expression abolished the AM630 inhibition effect on osteoclast differentiation, indicating Nrf2 was an essential factor for AM630 to inhibit osteoclast differentiation. However, inhibition of Nrf2 had no effect on the effect of AM1241, which further confirmed our speculation that AM1241-affected osteoclast differentiation was not mediated through the Nrf2 signaling pathway.
5. Conclusions
In summary, our data suggested that CB2 regulated osteoclast differentiation in vitro. The CB2 agonist, AM1241, enhanced RAW264.7 cells osteoclast differentiation induced by RANKL, and the CB2 antagonist, AM630, inhibited the osteoclast differentiation in the presence of Nrf2. These results indicated that AM630 may have the potential to treat osteoporosis. However, animal model studies are needed to reassess the potential of AM630 to inhibit osteoclast formation.
Funding
The investigation was aided by funds of National Natural Science Foundation of China (Grant number: 81572221).
Authors contributions
Wan Li, Yongxin Sun.
Wan Li designed the study, performed the experiments, analyzed the data, and wrote the manuscript. And Yongxin Sun provided critical advices to the manuscript and edited it.
Declaration of Competing Interest
The authors declare no conflicts of interest in association with the present study.
Acknowledgments
Authors acknowledge the Department of Dermatology, The First Hospital Affiliated to China Medical University and Key Laboratory of Immunodermatology, Ministry of Health and Ministry of Education for permission to access experimental instruments. Authors give their heartfelt appreciation to Ph.D Ruiqun Qi (Department of Dermatology, Key Laboratory of Immunodermatology, The First Hospital Affiliated to China Medical University).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Orimo H. Bone and calcium update; diagnosis and therapy of metabolic bone disease update. Guideline for prevention and treatment of osteoporosis update. Clin Calcium. 2011;21:123–143. [PubMed] [Google Scholar]
- 2.Cano A., Chedraui P., Goulis D.G., Lopes P., Mishra G., Mueck A. Calcium in the prevention of postmenopausal osteoporosis: EMAS clinical guide. Maturitas. 2018;107:7–12. doi: 10.1016/j.maturitas.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Yang S., Duan X. Epigenetics, bone remodeling and osteoporosis. Curr Stem Cell Res Ther. 2018;13:101–109. [PubMed] [Google Scholar]
- 4.Isomura H., Fujie K., Shibata K., Inoue N., Iizuka T., Takebe G. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y.X., Xu A.H., Yang Y., Li J. Role of Nrf2 in bone metabolism. J Biomed Sci. 2015;22:101. doi: 10.1186/s12929-015-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyeon S., Lee H., Yang Y., Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Idris A.I., Sophocleous A., Landao-Bassonga E., van't Hof R.J., Ralston S.H. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619–5626. doi: 10.1210/en.2008-0150. [DOI] [PubMed] [Google Scholar]
- 9.Idris A.I., van 't Hof R.J., Greig I.R., Ridge S.A., Baker D., Ross R.A. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H.C., Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatr. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng D.C., Xu Y.Z., Yang H.L., Zhu X.S., Zhu G.M., Wang X.B. Inhibition of titanium particle-induced inflammatory osteolysis through inactivation of cannabinoid receptor 2 by AM630. J Biomed Mater Res. 2010;95:321–326. doi: 10.1002/jbm.a.32836. [DOI] [PubMed] [Google Scholar]
- 12.Giacoppo S., Gugliandolo A., Trubiani O., Pollastro F., Grassi G., Bramanti P. Cannabinoid CB2 receptors are involved in the protection of RAW264.7 macrophages against the oxidative stress: an in vitro study. Eur J Histochem : EJH. 2017;23:61. doi: 10.4081/ejh.2017.2749. 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Ono T., Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–341. doi: 10.1007/s00418-018-1636-2. [DOI] [PubMed] [Google Scholar]
- 15.Park J.H., Lee N.K., Lee S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cell. 2017;40:706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soysa N.S., Alles N. Osteoclast function and bone-resorbing activity: an overview. Biochem Biophys Res Commun. 2016;476:115–120. doi: 10.1016/j.bbrc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Sophocleous A., Marino S., Logan J.G., Mollat P., Ralston S.H., Idris A.I. Bone cell-autonomous contribution of type 2 cannabinoid receptor to breast cancer-induced osteolysis. J Biol Chem. 2015;290:22049–22060. doi: 10.1074/jbc.M115.649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florczyk-Soluch U., Jozefczuk E., Stepniewski J., Bukowska-Strakova K., Mendel M., Viscardi M. Various roles of heme oxygenase-1 in response of bone marrow macrophages to RANKL and in the early stage of osteoclastogenesis. Sci Rep. 2018;8:10797. doi: 10.1038/s41598-018-29122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thummuri D., Naidu V.G.M., Chaudhari P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-kappaB and MAPK signalling. J Mol Med. 2017;95:1065–1076. doi: 10.1007/s00109-017-1553-1. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.J., Park C., Kim G.Y., Park E.K., Jeon Y.J., Kim S. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-kappaB and activation of the Nrf2/HO-1 signaling pathway. Biosci Trends. 2018;12:257–265. doi: 10.5582/bst.2018.01107. [DOI] [PubMed] [Google Scholar]