Abstract
Purpose
Genetic testing for Parkinson disease (PD) has not been widely used in clinical practice. In preparation for upcoming precision medicine–designed clinical trials for GBA and LRRK2, we evaluated movement disorders specialists’ current practice, knowledge, attitudes, and barriers to genetic testing in PD.
Methods
An anonymous questionnaire was sent to movement disorders specialists at 146 Parkinson Study Group (PSG) sites in the United States (n = 131) and Canada (n = 15) to assess their knowledge and attitudes about genetic testing for PD.
Results
One hundred seventy-eight (47.6%) PSG clinicians completed the questionnaire. Forty-one percent of respondents had not referred any PD patients for genetic testing in the last year and >80% reported referring fewer than 11 patients over the same period. Most common reasons for not referring for genetic testing included lack of insurance coverage/cost to the patient and lack of perceived utility. On a scale of 0–100, the mean level of comfort in respondents’ own ability to genetically counsel PD patients on GBA and LRRK2 was 52 (SD = 28). Sixty percent of clinicians correctly answered all questions about the inheritance and penetrance of GBA and LRRK2 variants.
Conclusions
There is an urgent need to increase knowledge and reduce practical barriers to genetic counseling and testing in PD.
Keywords: Parkinson disease, GBA, LRRK2, genetic testing, questionnaire
INTRODUCTION
Our understanding of the genetics of Parkinson disease (PD) has expanded in recent years,1 permitting a shift from observational studies, describing genotype–phenotype correlations, to interventional ones.2,3 Multiple clinical trials that will include participants who are carriers of selected genetic variants are beginning worldwide. Of all genes linked to increased PD risk, those encoding glucocerebrosidase (GBA, OMIM 606463) and leucine-rich repeat kinase-2 (LRRK2, OMIM 609007) pathogenic variants are currently the most actively targeted for clinical development, in early phase (I and II) interventional studies.2,3 Three to ten percent of all PD cases, and in selected populations up to 35–40%, carry a GBA or a LRRK2 pathogenic variant.4,5 However, despite a fairly high percentage of people with PD carrying a pathogenic variant in these genes, only a fraction of patients are referred for genetic counseling and testing in general clinical practice. Timely recruitment of people with PD into gene-specific trials of potential disease-modifying therapies will necessitate systematic testing and identification of gene pathogenic variant carriers who could potentially benefit from targeted therapy.
There is also increasing evidence that certain genetic forms of PD may have clinical features or progression rates that differ from nongenetic forms of the disease.1 More extensive testing will help to refine our current genotype–phenotype correlations, permitting a more refined prognosis for patients and understanding of genetic risk to family members. In this context, the Parkinson’s Foundation recently launched the PD GENEration study (PD GENE; ClinicalTrials.gov identifier: NCT04057794), which provides, at no cost to the participant, testing for several PD-related genes by a CLIA-certified laboratory, and genetic counseling (performed by the PD clinician in person or by a centralized genetic counseling team via telemedicine).
It is unclear if neurologists are prepared to incorporate genetic counseling and testing into the routine clinical care of Parkinson patients. In this study, we administered an anonymous questionnaire to clinician members of the Parkinson Study Group (PSG), including movement disorders specialists at 146 clinical sites in Canada and the United States, with the goal of understanding movement disorders clinicians’ practices and attitudes with respect to genetic counseling and testing to gauge where the community stands at the precipice of change.
MATERIALS AND METHODS
Participants
We invited clinicians who are members of the PSG (n = 374) to participate in an anonymous online survey. The PSG comprises specialists active in PD clinical care and research at 146 sites in the United States (131) and Canada (15). PSG-credentialed investigators possess expertise in diagnosing and treating PD with a significant percentage of their practice treating PD patients and conducting PD research. Most have had fellowship training in movement disorders. Potential study participants received an initial invitation email and weekly reminders between 22 March 2019 and 19 April 2019. A final reminder was sent on 17 May 2019. The survey was officially moved to inactive status on 29 May 2019. Participants who completed the survey were compensated with a $30 Amazon gift card. The protocol was approved by the Columbia University institutional review board (IRB); given the low-risk nature of the protocol, acceptance of the invitation and an electronic consent were accepted as a waiver for paper consent.
Questionnaire
The online questionnaire consisted of four parts:
Basic demographics of the respondent including sex, age group, country (if Canada), and geographic zone (based on time zones) in the United States (number of questions, n = 3).
Information regarding clinician’s patient populations and their genetic status (n = 15).
Questions about views, current practice, access, and utilization of genetic counseling and testing. We further asked questions about perceived current barriers to genetic testing and counseling, and whether the Parkinson’s Foundation PD GENE initiative would resolve any of these issues (n = 15).
Knowledge questions regarding PD genetics in general, and GBA andLRRK2-related PD in particular (n = 24).
The full questionnaire can be viewed in the Supplementary Material (Supplementary File 1).
Statistical analysis
Descriptive statistics (percentages, means, and SD) were used to describe the demographics of the survey responders and their report of current genetic practice, availability of counselors, attitudes toward genetic testing and counseling, and knowledge questions. To test if demographics (sex and area of practice), age (categorical value divided by decades), and clinic volume size (divided to tertiles) are associated with perceived knowledge and attitude toward genetic testing (based on the two questions “I feel that I am equipped to counsel patients adequately about the clinical relevance of both normal and abnormal genetic test results” and “I would be likely to order [PD GENE] tests on the majority of my patients,” respectively), we used Chi square statistics dichotomizing the responses to “yes” versus “no” or “unsure.” Statistical analysis was conducted using SPSS, version 24.
RESULTS
Forty-seven percent (176/374) of contacted clinicians responded to the survey (excluding an individual who chose the “do not agree to participate” response for the initial question, and two participants who consented but did not complete basic demographic questions; 148 participants requested and received the gift card). Demographics of participants were as follows: 104 (59.1%) men and 72 (40.9%) women, representing heterogeneous age groups (<40: n = 43 [24.2%]; 41–50: n = 55 [30.9%]; 51–60: n = 43 [24.2%]; >60: n = 35 [19.7%]). Fourteen participants practice in Canada and the rest in the United States. All participants are clinicians who care for people with PD (over 70,000 patients/year combined), but clinic size varied considerably. The mean n of self-reported PD patients seen per year by responders was 439, ranging from 10 to 2500.
Current genetic testing practice
The vast majority of participants (87.1%) ordered genetic testing on ten or fewer patients in the 12 months prior to completing the study. Furthermore, most participants (82.5%) reported that only ten or fewer of their patients reported undergoing direct-to-consumer testing (e.g., by 23andMe). In addition, participants reported that few of their patients received genetic testing either in research projects that returned results to patients (e.g., by the Michael J. Fox Foundation Parkinson’s Progression Markers Initiative [PPMI]) or in research projects that did not return results to patients. For either type of study design (with and without return of results), more than 80% of clinicians reported ten patients or fewer who had genetic testing. In contrast to genetic testing, DNA banking under research protocols was more prevalent. Thirty percent of respondents reported banking DNA for 11 or more patients in the past year (range 11–800). Consistent with responses citing limited genetic testing and reporting, caring for patients with known pathogenic variants was rare among survey respondents. Only 5.5% and 4.9% of responders reported providing care to more than ten known LRRK2 or GBA patients, respectively. In total, participants reported providing care for 490 known LRRK2 carriers and 402 known GBA carriers. Providing care for patients with known Parkin (OMIM 602544), PINK-1 (OMIM 608309), SNCA (OMIM 163890), and VPS35 (OMIM 614203) pathogenic variants was anecdotal (98% had two patients or fewer with these pathogenic variants).
Access to genetic counseling
Most respondents (70%) reported having access to services of a genetic counselor within their institution or in their movement disorders practice. Only 8 (4.5%) had utilized genetic counseling via telephone or telemedicine. Most reported that they would either involve a genetic counselor or recommend a genetic counseling referral (61.2%) prior to genetic testing for PD. The rest would either counsel by themselves (21.9%) or not offer genetic testing (16.9%).
The source of obstacles
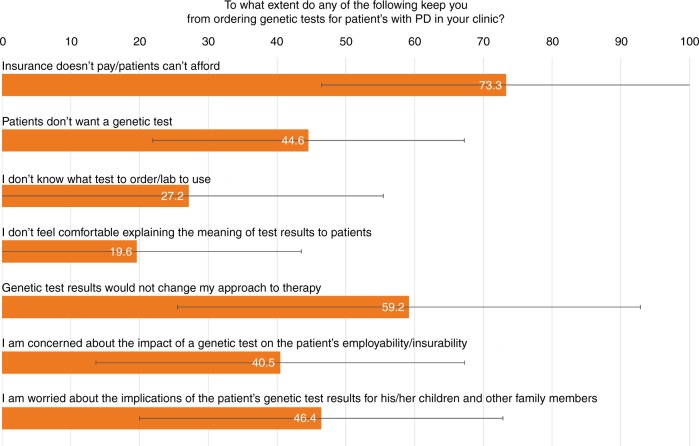
Next, we asked participants to rank the extent to which different potential obstacles keep them from ordering genetic tests for patients with PD (Fig. 1). The most common response was lack of insurance coverage/cost, followed by the perception that genetic test results would not affect care.
Fig. 1.
Responses to the question “To what extent do any of the following keep you from ordering genetic tests for patients with Parkinson disease (PD) in your clinic?” Participants graded each option on a scale from 0 to 100. Orange bars represent the mean grade for each response, and horizontal black lines represent the standard deviation. All respondents answered each question separately.
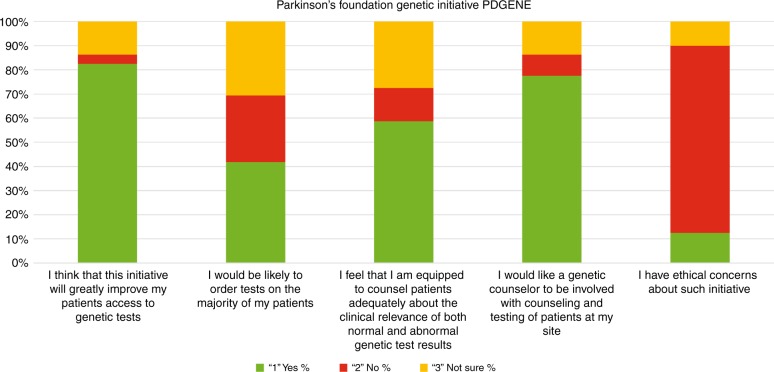
We then asked participants to evaluate the potential impact of the new Parkinson's Foundation initiative PD GENE (see “Introduction”). The response of the participants to the initiative is captured in Fig. 2. The vast majority of respondents reported that they would like to provide genetic testing and genetic counseling for their patients. Lastly, we asked participants if they have ethical concerns about such an initiative. While the majority did not have ethical concerns, those who did were invited to elaborate in free text. Concerns included the impact of the results on relatives, and the influence of the pharmaceutical industry. All free text responses are available in Supplementary File 2.
Fig. 2.
Responses from 160 participants to questions on PD GENE initiative. For each question participants could answer “yes,” “no,” or “not sure.” After the last question about ethical concerns, participants had an option to elaborate using free text.
We also tested whether sex, age, area of practice, or clinic size are associated with perceived knowledge (“I feel that I am equipped to counsel patients adequately about the clinical relevance of both normal and abnormal genetic test results”) or attitude toward genetic testing via PD GENE (“I would be likely to order [PD GENE] tests on the majority of my patients”). Age, sex, and area of practice were not associated with a positive or a negative response. While perceived knowledge was similar across practitioners of different clinic sizes, those providing care to more than 500 patients a year (upper tertile) were less likely to predict they will refer the majority of their patients to PD GENE compared with those in the lower or middle tertiles (25.6% versus 33.9% and 59.7% respectively; p = 0.001).
Knowledge and perceived knowledge
Two case scenarios and seven genetic competency questions (which were easier and designed primarily for use in a patient population) were used to evaluate knowledge. Thirty-one participants (17.6%) did not complete the knowledge questions. Responses to the case scenarios are presented in Table 1 and Table 2. The basic knowledge questions were completed correctly by almost all responders. Nevertheless, responders were not confident about their genetic knowledge. When asked if they are “confident in their knowledge of the impact of various genetic mutations on PD phenotypic manifestations,” the mean score was 47.6 (SD = 26.3) on a Likert scale from 0 to 100. Similarly, the mean score was 48.8 (SD = 28.3) on a question of whether they are “up to date on genetically targeted experimental therapeutics onGBA and LRRK2-related trials,” and 51.6 (SD = 27.5) on a question of if they are “comfortable counseling their PD patients on GBA and LRRK2 testing.”
Table 1.
Case scenario 1: Your 65-year-old male Parkinson disease (PD) patient of non-Ashkenazi heritage came to your clinic today to discuss the results of a genetic test that looked for the five most common Gaucher disease–associated GBA variants; the test showed that he has one gene with a GBA L444P variant and one that is normal for the five variants assayed (n = 145 respondents)
| Question | Correct response | Incorrect response | Uncertain response |
|---|---|---|---|
| This patient must have some Ashkenazi background but just doesn’t know it (False statement) | 100 (69.0%) | 5 (3.4%) | 40 (27.6%) |
| This patient has GBA-associated PD (True statement) | 76 (52.4%) | 19 (13.1%) | 50 (34.5%) |
| This patient does not have GBA-associated PD, because one copy of the gene is normal (False statement) | 109 (75.2%) | 8 (5.5%) | 28 (19.3%) |
| Deep brain stimulation would NOT be expected to help this patient’s motor symptoms (False statement) | 107 (73.8%) | 4 (2.8%) | 34 (23.4%) |
| Each of this patient's biological children has a 50% chance of developing Parkinson disease (False statement) | 119 (82.1%) | 9 (6.2%) | 17 (11.7%) |
| Each of this patient's biological children has a 50% chance of developing Gaucher disease (False statement) | 116 (80.0%) | 3 (2.1%) | 26 (17.9%) |
Table 2.
Case scenario 2: A 60-year-old male Parkinson disease (PD) patient of Ashkenazi background with a positive family history of Parkinson disease comes to your clinic to discuss the results of their LRRK2 genetic test, which shows a G2019S variant on one copy of the gene, with the other copy showing no variants
| Question | Correct response | Incorrect response | Uncertain response |
|---|---|---|---|
| This patient has LRRK2-associated PD (True statement) | 123 (84.8%) | 3 (2.1%) | 19 (13.1%) |
| This patient does not have LRRK2-associated PD, because one copy of the gene is normal (False statement) | 129 (89.0%) | 5 (3.4%) | 11 (7.6%) |
| This patient is more likely to have cognitive impairment sooner than a patient with idiopathic PD (False statement) | 91 (62.8%) | 20 (13.8%) | 34 (23.4%) |
| Deep brain stimulation would NOT be expected to help this patient’s motor symptoms (False statement) | 122 (84.1%) | 2 (1.4%) | 21 (14.5%) |
| Each of this patient’s biological children has a 50% chance of developing Parkinson disease (False statement) | 108 (74.5%) | 20 (13.8%) | 17 (11.7%) |
| Each of this patient's biological children has a 50% chance of developing Gaucher disease (False statement) | 133 (91.7%) | 3 (2.1%) | 9 (6.2%) |
| Testing for the G2019S variant is available through direct-to-consumer testing (True statement) | 89 (61.4%) | 1 (0.7%) | 55 (37.9%) |
DISCUSSION
Despite over 20 years since the first PD gene was reported and a growing number of newly identified variants, the results of this anonymous survey of PD clinicians clearly demonstrate that referral to PD-focused genetic counseling and testing is not a common practice in 2019. Currently, genetic testing in PD is not standard of care, but may be useful in certain cases for prognosis estimation and referral to clinical trials. Since referral to genetic testing is not a common practice, in most cases, neither patients nor their clinicians are aware of the patients’ pathogenic variant status. Specifically, based on estimations that up to 10% of all patients with PD carry GBA orLRRK2 pathogenic variants, participating practitioners in this study may care for to up to 7000 patients (of their reported total of 70,000 patients) with a pathogenic variant in one of these two genes. However, they collectively report only 490 LRRK2 and 402 GBA pathogenic variant carriers—less than 13% of the possible 7000 pathogenic variant carriers. Three top reasons for not performing genetic testing included lack of insurance coverage or cost to the patient, lack of perceived utility of genetic information in clinical practice, and concerns about the implications for family members of the patients. Thus, the obstacles to streamlined genetic testing encompass practical, knowledge-based, and ethical considerations.
Genetic testing and counseling are costly processes that demand skilled laboratories and clinicians who are comfortable in understanding and accurately explaining genetic results to people with PD and their families. PSG neurologists are skilled PD clinicians and clinical investigators, but the majority do not describe themselves as comfortable in providing counseling for PD-related genetic tests. They graded themselves a remarkably low 47.6–51.6 of 100 on the three questions about their confidence regarding the clinical genetics of PD. The number of incorrect responses to the actual genetic knowledge questions corroborated the lack of confidence in knowledge. For example, only 80.0% of respondents answered correctly that the risk for Gaucher disease in a child of a GBA pathogenic variant carrier is less than 50% (it is lower than 0.5%; Table 1). Only 74.5% responded correctly that each of a LRRK2 G2019S patient’s biological children has a less than 50% chance of developing PD (the child would have 50% chance of carrying the gene, but penetrance is controversial and ranges between 25% and 80%; Table 2). Another 17.6% of survey respondents did not attempt to answer the knowledge questions. These findings demonstrate that even among movement disorders clinicians—who are already experts in PD—there is an urgent need to educate clinicians about the analytic and clinical validity as well as the utility of genetic testing and how to properly disseminate clinical information on genetic testing in PD. These results also suggest that providing better access to genetic counselors to provide the genetic testing services directly (e.g., via telemedicine) may be a potentially useful and practical approach.
Many responding clinicians felt that genetic testing has no bearing on treatment due to lack of clinical utility. Genetic results may not change treatment plans in 2019 (though this statement has been contested),6 but can be helpful in providing patients with more accurate prediction of the estimated rate of progression of their disease. Specifically, carrying homozygous or compound heterozygous pathogenic variants in Parkin is associated with slower progression and less cognitive change.7 Mounting data from cross-sectional and longitudinal studies of LRRK2 and GBA pathogenic variant carriers also demonstrate differential progression.8–12 Furthermore, genotype determines eligibility for precision medicine clinical trials that target the metabolic pathways of GBA and LRRK2. These studies may identify novel therapies that would lead to disease modification in PD. Precision medicine clinical trials have reported recent promising results in other neurodegenerative diseases such as Huntington disease13 and amyotrophic lateral sclerosis (ALS), and trials of similar interventions for PD seem likely. Increasing awareness of genetic testing and genetic results will improve PD patients’ access to these clinical trials.
Lastly, genetic testing raises social and ethical questions. Genetic testing may raise concerns about privacy. Likewise, many clinicians raised concerns about the implications of the patients’ genetic test results for their children and other family members, and that patients do not want genetic testing. However, among the few studies assessing PD patients’ interest in genetic counseling and testing, a significant interest was reported, especially if and when it would influence treatment.14,15 Direct-to-consumer tests, specifically those that offer PD-related results, have been very successful in attracting people with PD. For example, over 10,000 people with PD are 23andMe customers.16 The level of interest in genetic counseling and testing of people with PD should be tested in follow-up studies.
Our study is the first to investigate the current practice, attitudes toward, and knowledge of genetic counseling and testing among PD clinicians. Analyzing such information from 176 clinicians who specialize in treating PD is unprecedented. However, we should interpret the results in light of the study design limitations. First, we approached a highly educated group of clinicians. Second, only 47.6% of the PSG clinician members responded. While this is a relatively high number compared with prior questionnaires,17 both limitations likely bias the results in the same direction. Our responders were likely more knowledgeable and more interested in genetic counseling and testing than the average clinician treating people with PD and were more committed to the design and conduct of clinical trials. These limitations further underscore our conclusions for the urgent need to educate clinicians about genetic testing in PD. If and when genetic testing becomes more available for people with PD, e.g., via PD GENE, study design should take into account ethical considerations. For example, some responders raised concerns about the implications of the testing to family members of PD patients and pharmaceutical industry involvement in genetic testing (see Supplementary File 2).
Improving uptake of genetic testing among PD providers may be challenging. A few barriers may be removed through a concerted effort to educate clinicians and making genetic testing financially accessible through the PD GENE effort; however, ethical and financial concerns may continue to limit genetic testing referrals as long as there is no FDA-approved intervention, which would be dependent on genotyping. If any precision medicine trial in PD were to be successful, genetic testing may be incorporated into the standard of care.
In summary, genetic interventions hold great hope in neurology, especially after the recent targeted therapy breakthroughs in spinal muscular atrophy (SMA)18 and familial (hereditary transthyretin) amyloidosis.19 Thoughtful initiatives to facilitate clinical trials for genetic-based interventions in PD are necessary. Educating clinicians, caregivers, and people with PD is a critical first step; ensuring that genetic counseling and testing are accessible to all patients is imperative. Successful implementation of widespread PD genetic education and testing will require collaboration between multiple stakeholders including clinicians, academia, professional organizations, industry developing targeted therapeutics, advocacy organizations, health-care providers, and people with PD. It will be a complex process but one that is urgently needed and essential to move the field forward.
Supplementary information
Acknowledgements
This research was funded by the Parkinson’s Foundation.
Disclosure
R.N.A. received funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS grant RM1HG007257), Michael J. Fox Foundation (MJFF), Parkinson’s Foundation (PF), and Department of Defence (DoD). He received consultation fees from Genzyme/Sanofi, Roche, and Restorbio. T.S. has served as a consultant for Acadia, Abbvie, Adamas, Anavex, Aptinyx, Allergan, Accorda, Denali, Neuroderm, Neurocrine, Revance, Sanofi, Sunovion, Teva, Takeda, Voyager, US World Meds, Parkinson’s Foundation, and the Michael J. Fox Foundation for Parkinson’s Research; has served as a speaker and received an honorarium from Acadia, Adamas, and Teva; and is on the Scientific Advisory Board for Neuroderm, Sanofi, and MJFF. She has received research funding from the NINDS, Parkinson’s Foundation, MJFF, Biogen, Roche, Neuroderm, Sanofi, and Sun Pharma. K.M. is funded by NIH grants NS100600, U24NS107168, UL1TR001873, ILM009886, and HG007257; the Parkinson’s Foundation; Michael J. Fox, Lewy Body Disease Association (LBDA); Huntington's Disease Society of America (HDSA); Cure Huntington's Disease Initiative (CHDI); and has been a site investigator for Vaccinex and Roche Genentech. A.-M.W. has received research funding from the ALS Association and the Parkinson’s Foundation; has participated in clinical trials funded by Acorda, Biogen, Bristol-Myers Squibb, Sanofi/Genzyme, Pfizer, and Abbvie; and received consultant payments from Acorda, Mitsubishi Tanabe Pharma America, and Accordant. M.A.S. recently received research funding from the NIH/NINDS, Michael J. Fox Foundation (MJFF), Parkinson’s Foundation (PF), DoD, and Farmer Family Foundation; served on a data monitoring committee for Eli Lilly & Co.; served on research advisory boards for Prevail Therapeutics, Cure Parkinson’s Trust, CBD Solutions, MJFF trust, and PF; and served on study steering committees for Acorda Therapeutics, MJFF, and PF (for the PD GENE study). M.N. receives funding from the Parkinson’s Foundation and the HDSA for Centers of Excellence; is participating in or has recently participated in clinical trials funded by Teva, Dong Pharmaceuticals, PharmaTwoB, CHDI, Impax, Theravance, and Aptinyx; serves on data monitoring committees for Roche and Uniqure; and has received consultant payments from Voyager Therapeutics and the Huntington Study Group. A.N. and J.C.B. are employees of the Parkinson’s Foundation. The other authors declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/27/2019
The affiliation for the author Anne Hall in the original version of this Article was given as Duke University, Clinical Trial Transformation Initiative (CTTI). This has now been corrected to Parkinson’s Foundation in both the PDF and HTML versions of the Article.
Supplementary information
The online version of this article (10.1038/s41436-019-0684-x) contains supplementary material, which is available to authorized users.
References
- 1.Kim CY, Alcalay RN. Genetic forms of Parkinson's disease. Semin Neurol. 2017;37:135–146. doi: 10.1055/s-0037-1601567. [DOI] [PubMed] [Google Scholar]
- 2.Ryan E, Seehra G, Sharma P, Sidransky E. GBA1-associated parkinsonism: new insights and therapeutic opportunities. Curr Opin Neurol. 2019;32:589–596. doi: 10.1097/WCO.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 3.Sardi SP, Simuni T. New era in disease modification in Parkinson's disease: review of genetically targeted therapeutics. Parkinsonism Relat Disord. 2019;59:32–38. doi: 10.1016/j.parkreldis.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 6.Giladi N, Mirelman A, Thaler A, Orr-Urtreger A. A personalized approach to Parkinson's disease patients based on founder mutation analysis. Front Neurol. 2016;7:71. doi: 10.3389/fneur.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol. 2014;71:62–67. doi: 10.1001/jamaneurol.2013.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78:1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28:1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders-Pullman R, Mirelman A, Alcalay RN, et al. Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75:312–319. doi: 10.1001/jamaneurol.2017.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cilia R, Tunesi S, Marotta G, et al. Survival and dementia in GBA-associated Parkinson's disease: the mutation matters. Ann Neurol. 2016;80:662–673. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Boot B, Locascio JJ, et al. Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's. Ann Neurol. 2016;80:674–685. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, et al. Targeting Huntingtin expression in patients with Huntington's disease. N Engl J Med. 2019;380:2307–2316. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
- 14.Sakanaka K, Waters CH, Levy OA, et al. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns. 2014;23:114–120. doi: 10.1007/s10897-013-9618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupte M, Alcalay RN, Mejia-Santana H, et al. Interest in genetic testing in Ashkenazi Jewish Parkinson's disease patients and their unaffected relatives. J Genet Couns. 2015;24:238–246. doi: 10.1007/s10897-014-9756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blauwendraat C, Heilbron K, Vallerga CL, et al. Parkinson's disease age at onset genome-wide association study: defining heritability, genetic loci, and alpha-synuclein mechanisms. Mov Disord. 2019;34:866–875. doi: 10.1002/mds.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.York M, Marsh L, Jimenez-Shahed J, Okun M, Moro E, Kumar R. Parkinson's Study Group Neurosurgical Working Group (PSG-NSWG) Deep Brain Stimulation (DBS) Non-Motor Symptoms (NMS) Survey: perceived patient and treatment team outcomes. Mov Disord. 2012;27:E12–E12. [Google Scholar]
- 18.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 19.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.