Abstract
Long-term diabetic complications are exacerbated by post-prandial hyperglycemia which could be ameliorated by α-glucosidase inhibitor including oxyresveratrol. Puag-Haad is an aqueous extract from Artocarpus lakoocha Roxb. containing ~65% oxyresveratrol. Oxyresveratrol is an inhibitor of isolated yeast α-glucosidase enzyme but has not been tested on intact gut enterocytes where the enzyme is membrane-bound. Accordingly, differentiated Caco-2 cells that contain the native enzyme were used to test maltose hydrolysis in the present study. The results demonstrated that purified yeast α-glucosidase was non-competitively inhibited by oxyresveratrol (Ki 54.4 ± 0.7 μg/mL) and Puag-Haad (2.7 ± 0.1 μg/mL) compared to 153.8 ± 4.3 μg/mL acarbose, an anti-diabetic drug. In differentiated Caco-2 cells, both oxyresveratrol and Puag-Haad inhibited maltose hydrolysis with lesser potency compared to acarbose. Thus, although weaker than acarbose, oxyresveratrol and Puag-Haad do not inhibit pancreatic amylase which might be a therapeutic asset in preventing fermentation of unabsorbed carbohydrate causes abdominal bloating, flatulence, or diarrhea. Oxyresveratrol and Puag-Haad may help control postprandial hyperglycemia with low risk of gastrointestinal side effects.
Keywords: Biochemistry, Cell biology, Digestive system, Pharmacology, Alternative medicine, Artocarpus lakoocha Roxb, Oxyresveratrol, α-glucosidase inhibitors, Caco-2 cells
Biochemistry; Cell biology; Digestive system; Pharmacology; Alternative medicine; Artocarpus lakoocha Roxb; Oxyresveratrol; α-glucosidase inhibitors; Caco-2 cells.
1. Introduction
Diabetes mellitus (DM) is a global public health problem that afflicts 366 million patients and will continue to rise (Whiting et al., 2011). Postprandial hyperglycemic peaks are particularly important risks for promoting the cardiovascular co-morbidities particularly during the early phases of the disease (pre-diabetes) (Baron, 1998; Patel, 2016). Therefore, controlling postprandial hyperglycemia may provide an effective strategy to prevent diabetes onset. Among anti-diabetic agents, α-glucosidase inhibitors act predominantly by reducing postprandial plasma glucose levels by delaying intestinal absorption of dietary carbohydrate hydrolysis to glucose, frucose, galactose, and other monosaccharides (Patel, 2016). α-Glucosidase inhibitors that currently licensed are acarbose, miglitol, and voglibose. In addition, many natural products/plants have been shown to be strong α-glucosidase inhibitors (Benalla et al., 2010).
Oxyresveratrol (trans-3,5,2′,4′-tetrahydroxystilbene) exhibits many biological properties including tyrosinase inhibition (Kim et al., 2002), photoprotective (Hu et al., 2015), antihyperlipidemic (Jo et al., 2014), anti-inflammatory (Chung et al., 2003), antioxidative (Choi et al., 2016), and neuroprotective (Andrabi et al., 2004; Weber et al., 2012).
Oxyresveratrol is present in high content of the heartwood of Artocarpus lakoocha Roxb., especially in “Puag-Haad” which comprises 70–80% oxyresveratrol (Maneechai et al., 2009). Puag-Haad is a dried aqueous extract prepared by boiling heartwood of A. lakoocha with water. Its traditional use is as an antihelminthic (Charoenlarp et al., 1989; Saowakon et al., 2009). Due to its high oxyresveratrol content and low toxicity (LD50 > 5 g/kg bodyweight in rat) (Nilvises et al., 1985), Puag-Haad is a good candidate for rational pharmacological treatments.
Oxyresveratrol is also an α-glucosidase inhibitor (He and Lu, 2013) having a higher potency than acarbose using an in vitro cell-free enzymatic assay using yeast α-glucosidase and (p-nitrophenyl-α-D-glucopyranoside, PNPG) as the substrate. The properties of α-glucosidases vary according to their origin (Oki et al., 1999) and whether tested in the free or membrane-bound form (Oki et al., 2000) and according the species (Chiba, 1997). Accordingly, the present study aimed to test the inhibitory of oxyresveratrol and Puag-Haad on α-glucosidase activity of intact differentiated intestinal epithelial cells (human Caco-2 cells) using maltose as the substrate and compare this with free commercial yeast enzyme by cell-free assay and pancreatic α-amylase with starch as the substrate.
2. Materials and methods
2.1. Chemicals and reagents
Fetal bovine serum (FBS), trypsin/EDTA (0.25%) and 1% penicillin–streptomycin solution were purchased from Gibco (Grand Island, NY). Oxyresveratrol, Saccharomyces cerevisiae α-glucosidase (catalog number G5003), p-nitrophenyl α-D-glucopyranoside (PNPG), Dulbecco's modified Eagle's medium and Ham's F12 medium (DMEM/F12), gallic acid, quercetin, porcine pancreatic α-amylase, starch, dinitrosalicylic acid (DNS), acarbose, 4-nitrophenol, Folin and Ciocalteu's phenol reagent were purchased from Sigma–Aldrich (St. Louis, MO). Maltose and sucrose were obtained from Ajax Finechem Pty Ltd (Taren Point, NSW, Australia). 3-[4,5-dimethylthiazol-2-yl]-2,3-diphenyl tetrazolium bromide (MTT) was purchased from Amresco (Solon, OH), and glucose liquicolor complete test kit (Cat. No. 10260) was purchased from Human GmbH (Wiesbaden, Germany). Puag-Haad was obtained from Origin Plant Co., Ltd., (Samutprakan, Thailand), briefly, it was prepared by water boiling the A. lakoocha wood chips, collecting the bubble foam, and finally drying collected material. The study was conducted in accordance with the Basic & Clinical Pharmacology &Toxicology policy for experimental and clinical studies (Tveden-Nyborg et al., 2018).
2.2. Quantitative phytochemical analysis
2.2.1. Total phenolic content
Total phenolic content was determined using Folin-Ciocalteu method (Lin and Tang, 2007). Puag-Haad in distilled water (20 μL) was mixed with Folin-Ciocalteu reagent (100 μL) and 15 g/L sodium carbonate (80 μL) in 96-well plates. The mixtures were incubated at 50 °C for 5 min and subsequently at room temperature for 30 min before measuring the absorbance at 750 nm. Gallic acid was used as the standard phenolic compound. Total phenolic content was calculated and expressed as gallic acid equivalents (GAE).
2.2.2. Total flavonoid contents
Total flavonoid contents were determined using the aluminum chloride colorimetric method (Lin and Tang, 2007). Puag-Haad (20 μL), 95% ethanol (60 μL), 4% AlCl3 (10 μL), 0.4 M CH3COOK (10 μL) and distilled water (100 μL) were mixed in 96-well plates and incubated at room temperature for 40 min before measuring its absorbance at 415 nm. Quercetin was used as a standard flavonoid. Total flavonoid content was calculated and expressed as quercetin equivalent (QE).
2.2.3. High-performance liquid chromatography (HPLC) analysis
Oxyresveratrol content in Puag-Haad was determined by HPLC with some modification (Maneechai et al., 2009). Briefly, the separation was performed using a Shimadzu LC-20AT liquid chromatograph equipped with a SPD-20A UV detector, an Ultra HPLC columns (250 × 4.60 mm), with C18 column packing, 5 μm particle size. A 20 μL sample solution was injected using 35% methanol as mobile phase flowing at 0.8 mL/min. The oxyresveratrol was measured by absorption at 254 nm at peaks integrated by the associated software. Puag-Haad was assigned by a retention time of 17min for oxyresveratrol. Calibration curves for oxyresveratrol were constructed using peak areas of 7 concentrations (2.5–250 μg/mL).
2.3. α-Glucosidase inhibitory activity and kinetic analysis
Inhibition of α-glucosidase was assessed by the method of He and Lu (2013) with some modifications by measuring the release of p-nitrophenol from PNPG. Briefly, 125 mU/mL of soluble purified α-glucosidase in phosphate-buffer saline (PBS) at pH 6.8 (20 μL) was mixed with different concentrations of Puag-Haad or oxyresveratrol (80 μL) in 96-well plates. The mixtures were incubated at 37 °C for 10 min, then 0.2 mM PNPG (100 μL) added, and further incubated for 30 min before measuring the absorbance at 405 nm using a microplate reader. Acarbose was prepared in distilled water at various concentrations and used as positive controls. Half-maximal inhibitory concentrations (IC50) were calculated from dose-response curves of tested compounds.
For the enzyme kinetic analysis, Lineweaver-Burk and Dixon plots were performed based on previous reports (Yoshino, 1987; Yoshino and Murakami, 2009). Various concentrations of enzyme inhibitor (tested compounds) and substrate (PNPG) were used in the reactions, the initial rate (over 10 min) of reaction (v) based on p-nitrophenol release rate were calculated and displayed as Lineweaver-Burk (1/v versus 1/[substrate]) or Dixon plots (1/v versus [inhibitor]).
2.4. Disaccharide digestion, maltase and sucrase inhibitory activity
This assay was similar to that for α-glucosidase inhibitory activity but natural disaccharides (maltose and sucrose) were substrates instead of PNPG. The methodology was modified from Zhang et al. (2013). Maltose or sucrose (50 mM, 100 μL) were mixed with 2000 mU/mL α-glucosidase (50 μL) alone or with various concentrations tested compounds (50 μL). The liberated glucose product was measured by glucose oxidase (glucose liquicolor complete test kit) absorbance at 510 nm using microplate reader.
2.5. α-Amylase inhibitory activity
The α-amylase assay followed the method of Sabiu et al. (2016) with some modification and the product was determined by colorimetry with glucose as the standard. Briefly, various concentrations of oxyresveratrol, Puag-Haad or acarbose (50 μL) were incubated with starch (12.5 mg/mL, 100 μL) in PBS at 37 °C for 5 min, further incubated with α-amylase (1.5 unit/mL, 100 μL) for 10 min, and 50 μL mixture was pipetted to react with 100 μL of 1% 3,5-dinitrosalicylic acid (DNS). The mixtures were heated at 100 °C for 5 min, cooled, and finally measured the absorbance at 540 nm.
2.6. Membrane bound α-glucosidase inhibitory activity in cell culture
2.6.1. Caco-2 cell culture and preparation
Caco-2 cells were obtained from the American Type Culture Collection (ATCC). Cells were grown in DMEM/F12 containing 10% FBS and 1% of the penicillin–streptomycin solution and maintained at 37 °C under 95% air and 5% CO2, 100% humidity. Caco-2 cells were seeded on 96-well plates for the viability test and 24-well plates for determining inhibition of α-glucosidase. After confluence, cells underwent differentiation and expressed lactase sucrose-isomaltase and alkaline phosphatase within 2–3 weeks (Van Beers et al., 1995). During this period cells were fed with fresh medium every 2 days and the cells thereafter (18–21days) used for most experiments.
2.6.2. Determination of cell viability
Cell viability was assessed by MTT conversion to the insoluble purple formazan dye by mitochondrial dehydrogenases in viable cells, which was measured spectrophotometrically. No conversion occurs in dead cells. Caco-2 cells were treated with various concentrations of Puag-Haad, oxyresveratrol, or acarbose for 24 h and 5 mg/mL MTT (10 μL) was added at 2 h before the end of the treatment. Then the medium was removed, the formazan solubilized with DMSO:EtOH (1:1; v/v), and absorbance read at 595 nm by a microplate reader.
2.6.3. α-Glucosidase inhibitory activity in Caco-2 cells
Membrane bound α-glucosidase activity in Caco-2 cells was modified from previous study (Zhang et al., 2013). Briefly, Caco-2 cell were seeded on 24-well plate at 100,000 cells/well and cultured for 18–21 days to allow differentiation in DMEM/F12 containing 10% FBS and 1% of the penicillin–streptomycin solution. The culture medium was removed and cells were washed with 1 mL of PBS and incubated overnight with serum- and glucose-free medium. Cells in 160 μL culture medium were incubated with oxyresveratrol, Puag-Haad, or acarbose (20 μL, various concentrations) for 15 min, and α-glucosidase substrate (20 μL, 250 mM maltose) for 4 h. Culture medium (20 μL) were collected to measure glucose product by glucose assay kit. The experiments were conducted in triplicate and the α-glucosidase/maltase inhibitory activities expressed as %inhibition.
2.7. Statistical analysis
Results are expressed as the mean ± SEM of n experiments. Half-maximal inhibitory concentration (IC50) values were calculated using the Prism program (GraphPad Software Inc). The data were analyzed by analysis of variance (ANOVA) with a LSD test between groups. Comparisons between two groups used student's t-test and differences considered to be significant when p ≤ 0.05.
3. Results
3.1. Phytochemicals of Puag-Haad
Phytochemical analysis of Puag-Haad, a dried powder of aqueous extract from heartwood of A. laxoocha, is presented in Table 1. Puag-Haad contained 5.67% phenolic compounds and traces of flavonoids while the bulk of Puag-Haad appeared to be oxyresveratrol by HPLC analysis.
Table 1.
Total phenolic, flavonoid, and oxyresveratrol contents of Puag-Haad.
| Puag-Haad | Content (% w/w) |
|---|---|
| Total phenolic (GAE) | 5.7 ± 0.2 |
| Total flavonoid (QE) | 0.031 ± 0.004 |
| Oxyresveratrol | 64.9 ± 5.6 |
Values are expressed as means ± SD of three experiments.
GAE = gallic acid equivalent, QE = quercetin equivalent.
3.2. α–glucosidase inhibitory activities
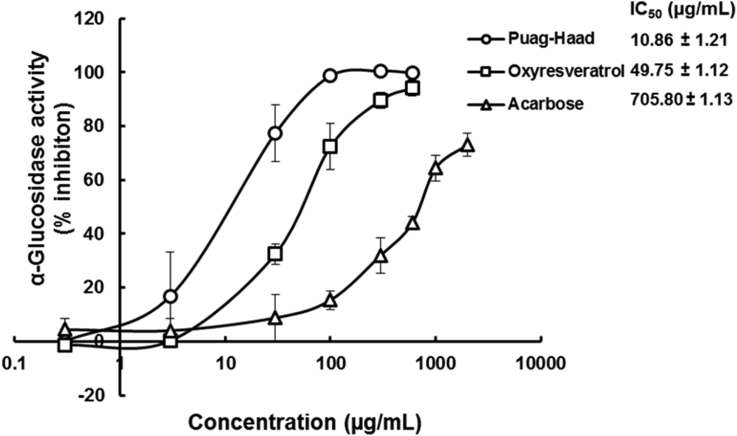
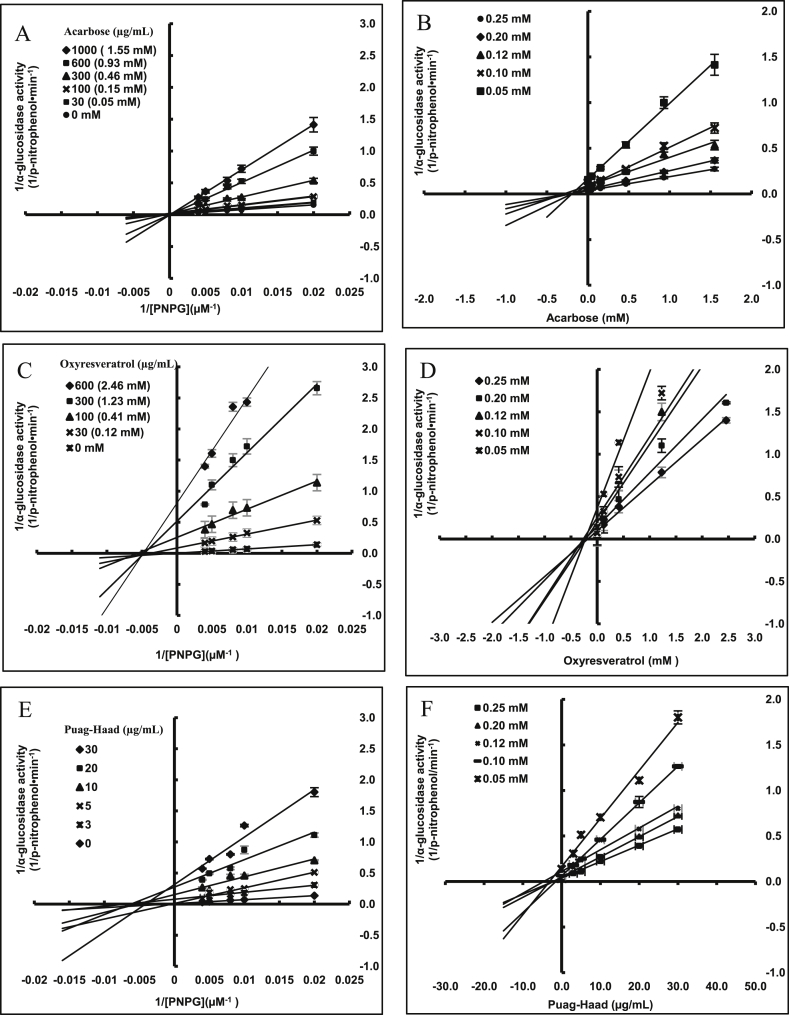
Oxyresveratrol, Puag-Haad, and acarbose inhibited α–glucosidase dose-dependently and Puag-Haad and oxyresveratrol showed higher potency when compared with acarbose (Figure 1). To investigate the mechanisms of α–glucosidase inhibition of these compounds, data was displayed as Lineweaver-Burk and Dixon plots (Figure 2). Lineweaver-Burk plots indicated that acarbose was an α–glucosidase competitive inhibitor (Figure 2A) whereas oxyresveratrol and Puag-Haad inhibited α–glucosidase by noncompetitively model (Figure 2C and E). From the Dixon plots (Figure 2B, D, F), the inhibitor constants (Ki) of Puag-Haad had the lowest Ki, highest potency and affinity for the α–glucosidase (Table 2).
Figure 1.
Dose-inhibition curves for acarbose, oxyresveratrol, and Puag-Haad on yeast α–glucosidase in cell-free assays. Each value represents the mean ± S.D. of 4 experiments.
Figure 2.
Inhibition of yeast α–glucosidase in cell-free assays by acarbose (A, B), oxyresveratrol (C, D) and Puag-Haad (E, F). Data are displayed as double reciprocal (Lineweaver-Burk A, B, C) and single reciprocal (B, D, F) Dixon plots of inhibitory activity at different concentrations of substrate and inhibitors.
Table 2.
Inhibition of oxyresveratrol, Puag-Haad, and acarbose on α-glucosidase hydrolysis of PNPG.
| Compound | Ki (μg/mL)a | Mode of Inhibitionb |
|---|---|---|
| Acarbose | 153.8 ± 4.3 (228.96 ± 4.02 μM) | Competitive |
| Oxyresveratrol | 54.5 ± 0.8 (223.28 ± 3.04 μM) | Non-competitive |
| Puag-Haad | 2.7 ± 0.1 | Non-competitive |
Values are expressed as means ± SEM of five experiments.
Determined by Dixon plots.
Determined by Lineweaver-Burk plots.
3.3. Inhibition of disaccharide digestion by maltase and sucrase
Here, we used the physiological substrates of α-glucosidase (maltose and sucrose). Clearly, acarbose was the weakest inhibitor from all three substrates (Table 3). Where either disaccharide was the substrate, oxyresveratrol or Puag-Haad appeared to be much more potent (~1 μg/mL) than acarbose (~450–1,200 μg/mL) (Table 3).
Table 3.
Inhibition by oxyresveratrol, Puag-Haad, and acarbose of yeast α-glucosidase hydrolysis of physiological substrates.
| Substrate | IC50 (μg/mL) |
||
|---|---|---|---|
| Acarbose | Oxyresveratrol | Puag-Haad | |
| PNPG | 705.8 ± 1.1 (1093.2 ± 1.7 μM) | 49.7 ± 1.1 (203.7 ± 4.6 μM) | 10.9 ± 1.2 (28.9 ± 3.2 μM)a |
| Sucrose | 445.6 ± 1.6 (690.2 ± 2.4 μM) | 1.2 ± 1.3 (4.8 ± 5.4 μM) | 1.5 ± 1.3 (4.1 ± 3.5 μM)a |
| Maltose | 1210 ± 2.5 (1874.2 ± 3.8 μM) | 0.8 ± 1.6 (3.2 ± 6.4 μM) | 0.4 ± 1.7 (0.9 ± 4.5 μM)a |
Values are expressed as means ± SD of 3 experiments.
Expressed as oxyresveratrol content in Puag-Haad.
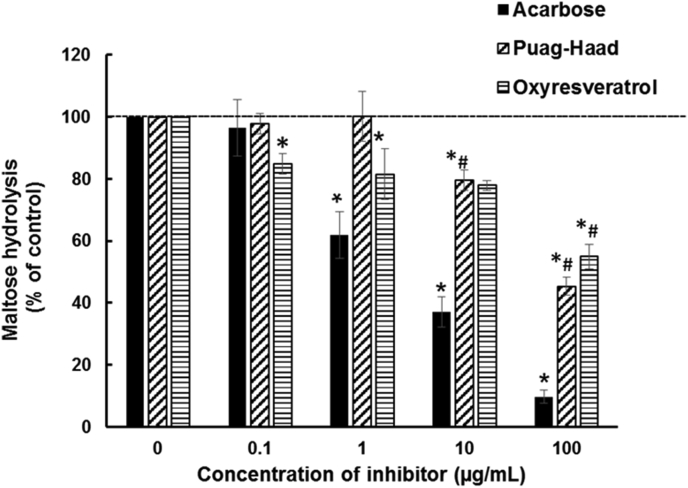
3.4. Membrane bound α–glucosidase inhibitory activities
Intestinal α-glucosidases are bound to the luminal membrane and these are expressed in differentiated Caco-2 cells (human enterocytes). In contrast to the free yeast enzyme in earlier experiments, membrane-bound human α-glucosidases with maltose as the substrate was far more susceptible to acarbose compared to oxyresveratrol and Puag-Haad (Figure 3). Where sucrose was the substrate, the hydrolysis rates were below detectability (data not shown). None of the substrates or inhibitors showed any toxicity to Caco-2 cells test by MTT assay (data not shown).
Figure 3.
Inhibition of hydrolysis of 25 mM maltose by membrane-bound α–glucosidases on differentiated Caco-2 cells by acarbose, oxyresveratrol or Puag-Haad at 0.1, 1, 10, 100 μg/ml for 4 h. Values represent mean ± SEM (n = 3). ∗p ≤ 0.05 compared to untreated cell (control) #p ≤ 0.05 compared to acarbose.
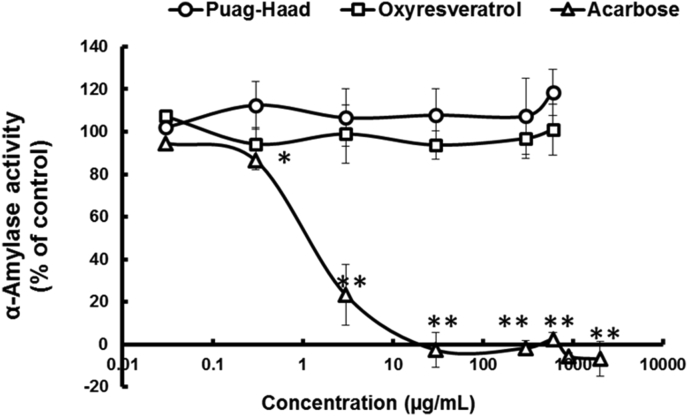
3.5. Inhibition of α-amylase
Another source of post-prandial blood glucose comes from starch hydrolysis by α-amylase and the next experiments sought to assess our inhibitors on this enzyme. Using porcine pancreatic α-amylase, acarbose was also an effective inhibitor of starch digestion (IC50 1.3 ± 1.3 μg/mL, Figure 4). In contrast, oxyresveratrol or Puag-Haad showed no detectable inhibition up to 600 μg/mL (Figure 4).
Figure 4.
Inhibitory activity of oxyresveratrol, Puag-Haad, and acarbose on porcine pancreatic α-amylase. Each value represents the mean ± SD of four experiments. ∗p ≤ 0.05 and ∗∗p ≤ 0.001 compared to control.
4. Discussion
Both oxyresveratrol and Puag-Haad non-competitively inhibited soluble yeast α-glucosidase when PNPG was the substrate with higher potency than acarbose. These differences were even more marked where disaccharides were the substrates. However, these two natural compounds had much lesser efficacy than acarbose to inhibit disaccharide hydrolysis by membrane-bound α-glucosidase of differentiated Caco-2 cells. In contrast to acarbose, oxyresveratrol and Puag-Haad showed no inhibitory effects against amylase enzyme.
Thus, while acarbose can reduce glucose release and post prandial hyperglycemia, Puag-Haad and oxyresveratrol could reduce sources of glucose from yeast action. Whether or not this could extend to bacterial fermentation by gut bacteriodes spp is unclear.
This property of oxyresveratrol and Puag-Haad may be benefit due to less chance of fermentation of undigested starch and subsequently low gastrointestinal side effect compared to acarbose. Oxyresveratrol and Puag-Haad may be helpful to control postprandial glucose level in pre-diabetic and/or risk groups with low unfavorable side effect of α-glucosidase inhibitory agents.
In agreement with previous report (He and Lu, 2013), acarbose (competitive) and oxyresveratrol (non-competitive) had different mechanisms of actions. Acarbose and other commercial drugs are glycoside derivatives acting as competitive inhibitors, whereas oxyresveratrol and other bioactive compounds belong to non-competitive inhibitor where their binding sites are not at catalytic domain (He and Lu, 2013). Ligand-enzyme binding possibly via hydrophobic or hydrogen bonding reduced the surface hydrophobicity which then hindered the active center in the enzyme molecules (He and Lu, 2013; Liu et al., 2011). Many in vitro enzymatic assays use Saccharomyces cerevisiae yeast α-glucosidase, which may not correspond with human enzyme. Yeast α-glucosidase (S. cerevisiae) has very little sequence homology with mammal α-glucosidase including the catalytic region (Chiba, 1997). Other sources of α-glucosidases may also be irrelevant to humans (Oki et al., 1999).
The subcellular location of the enzyme also influences the efficacy of α-glucosidase inhibitors (Oki et al., 2000). To replicate enzyme expression on the luminal membrane has been tested using rat α-glucosidase assay system immobilized on to CNBr-activated Sepharose 4B and data from this system better corresponded with anti-hyperglycemic effect in rats in some studies (Matsui et al., 2004, 2007) but not in another (Matsui et al., 2002). Differentiated Caco-2 cells offer yet another test system which form a monolayer expressing the human form of the enzyme which is anchored to the external surface of the plasma membrane. Thus this micro-anatomical disposition of the enzyme more closely replicates the in vivo situation in human small intestine. Accordingly, acarbose exhibited superior α-glucosidase potency compared to oxyresveratrol and Puag-Haad (Figure 3).
In membrane-bound α-glucosidase assay system, most data was obtained using maltose [α-(1,4) linkage] as the substrate whereas sucrose [α-(1,2) linkage] failed to demonstrate the efficacy of tested compounds in the present study. This could be because sucrase on expressed on enterocytes has lower hydrolytic activity than maltase and lesser distributed among four catalytic subunits of intestinal enzymes (Sim et al., 2008). In the brush-border of the human small intestine, sucrase activity arises from a pair of heterodimeric proteins [maltase-glucoamylase (MGAM) and sucrase−isomaltase (SI)] (Sim et al., 2008). Most subunits display α-(1,4) linkage catalytic activity, so that α-(1,4) glucose oligomers and maltose seem to more effectively hydrolyzed than α-(1,2) linkage as sucrose. So these differences between the sucrase and maltase subunits accounts for the apparent selective inhibition of oxyresveratrol towards the two substrates.
The α-glucosidase inhibitors currently used clinically (acarbose, miglitol and voglibose) commonly cause flatulence and abdominal bloating (Derosa and Maffioli, 2012; Neuser et al., 2005). Such adverse effects arise from fermentation of undigested carbohydrate by pathogenic bacteria in the colon, dysbiosis, and risk of gut wall inflammation (Zhang et al., 2015), results from its inhibition of pancreatic α-amylase activity (Dehghan-Kooshkghazi and Mathers, 2004; Samulitis et al., 1987). With no α-amylase inhibition, oxyresveratrol and Puag-Haad would benefit due to low undigested starch fermentation and subsequently low gastrointestinal side effect. Oxyresveratrol and Puag-Haad demonstrates a different selectivity towards starch digestion and may be considered as one of several pharmacological tools by selectively inhibiting carbohydrate digestion by intestinal yeasts. Oxyresveratrol also ameliorates several tissue pathologies associated with obesity (Ahn et al., 2017; Tan et al., 2017) thus augmenting its clinical usefulness in treating metabolic disease. Oxyresverstrol demonstrates low oral bioavailability in rats (Chen et al., 2016) and a high re-export rate from Caco-2 cells (Ahn et al., 2017; Mei et al., 2012). Thus, these multiplicity of oxyresveratrol effects are likely to be confined to the gastrointestinal tract.
Declarations
Author contribution statement
Matusorn Wongon: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nanteetip Limpeanchob: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Naresuan University (Grant number P2560B037), Thailand.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dr. Norman Scholfield for manuscript preparation.
References
- Ahn E., Lee J., Jeon Y.-H., Choi S.-W., Kim E. Anti-diabetic effects of mulberry (Morus alba L.) branches and oxyresveratrol in streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2017;26(6):1693–1702. doi: 10.1007/s10068-017-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi S.A., Spina M.G., Lorenz P., Ebmeyer U., Wolf G., Horn T.F. Oxyresveratrol (trans-2,3',4,5'-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004;1017(1-2):98–107. doi: 10.1016/j.brainres.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Baron A.D. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998;40(Suppl):S51–55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Benalla W., Bellahcen S., Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr. Diabetes Rev. 2010;6(4):247–254. doi: 10.2174/157339910791658826. [DOI] [PubMed] [Google Scholar]
- Charoenlarp P., Radomyos P., Bunnag D. The optimum dose of Puag-Haad in the treatment of taeniasis. J. Med. Assoc. Thai. 1989;72(2):71–73. [PubMed] [Google Scholar]
- Chen W., Yeo S.C.M., Elhennawy M.G.A.A., Lin H.-S. Oxyresveratrol: a bioavailable dietary polyphenol. J. Funct. Foods. 2016;22:122–131. [Google Scholar]
- Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997;61(8):1233–1239. doi: 10.1271/bbb.61.1233. [DOI] [PubMed] [Google Scholar]
- Choi H.Y., Lee J.H., Jegal K.H., Cho I.J., Kim Y.W., Kim S.C. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem. Biol. Interact. 2016;245:110–121. doi: 10.1016/j.cbi.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Chung K.O., Kim B.Y., Lee M.H., Kim Y.R., Chung H.Y., Park J.H., Moon J.O. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003;55(12):1695–1700. doi: 10.1211/0022357022313. [DOI] [PubMed] [Google Scholar]
- Dehghan-Kooshkghazi M., Mathers J.C. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the alpha-glucosidase inhibitor acarbose. Br. J. Nutr. 2004;91(3):357–365. doi: 10.1079/BJN20031063. [DOI] [PubMed] [Google Scholar]
- Derosa G., Maffioli P. Alpha-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012;8(5):899–906. doi: 10.5114/aoms.2012.31621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Lu Y.H. Comparison of inhibitory activities and mechanisms of five mulberry plant bioactive components against alpha-glucosidase. J. Agric. Food Chem. 2013;61(34):8110–8119. doi: 10.1021/jf4019323. [DOI] [PubMed] [Google Scholar]
- Hu S., Chen F., Wang M. Photoprotective effects of oxyresveratrol and Kuwanon O on DNA damage induced by UVA in human epidermal keratinocytes. Chem. Res. Toxicol. 2015;28(3):541–548. doi: 10.1021/tx500497u. [DOI] [PubMed] [Google Scholar]
- Jo S.P., Kim J.K., Lim Y.H. Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem. Toxicol. 2014;65:213–218. doi: 10.1016/j.fct.2013.12.040. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Yun J., Lee C.K., Lee H., Min K.R., Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002;277(18):16340–16344. doi: 10.1074/jbc.M200678200. [DOI] [PubMed] [Google Scholar]
- Lin J.-Y., Tang C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. [Google Scholar]
- Liu M., Zhang W., Qiu L., Lin X. Synthesis of butyl-isobutyl-phthalate and its interaction with alpha-glucosidase in vitro. J. Biochem. 2011;149(1):27–33. doi: 10.1093/jb/mvq110. [DOI] [PubMed] [Google Scholar]
- Maneechai S., Likhitwitayawuid K., Sritularak B., Palanuvej C., Ruangrungsi N., Sirisa-Ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and 'Puag-Haad'. Med. Princ. Pract. 2009;18(3):223–227. doi: 10.1159/000204354. [DOI] [PubMed] [Google Scholar]
- Matsui T., Ebuchi S., Fukui K., Matsugano K., Terahara N., Matsumoto K. Caffeoylsophorose, a new natural alpha-glucosidase inhibitor, from red vinegar by fermented purple-fleshed sweet potato. Biosci. Biotechnol. Biochem. 2004;68(11):2239–2246. doi: 10.1271/bbb.68.2239. [DOI] [PubMed] [Google Scholar]
- Matsui T., Kobayashi M., Hayashida S., Matsumoto K. Luteolin, a flavone, does not suppress postprandial glucose absorption through an inhibition of alpha-glucosidase action. Biosci. Biotechnol. Biochem. 2002;66(3):689–692. doi: 10.1271/bbb.66.689. [DOI] [PubMed] [Google Scholar]
- Matsui T., Tanaka T., Tamura S., Toshima A., Tamaya K., Miyata Y., Tanaka K., Matsumoto K. Alpha-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007;55(1):99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- Mei M., Ruan J.Q., Wu W.J., Zhou R.N., Lei J.P., Zhao H.Y., Yan R., Wang Y.T. In vitro pharmacokinetic characterization of mulberroside A, the main polyhydroxylated stilbene in mulberry (Morus alba L.), and its bacterial metabolite oxyresveratrol in traditional oral use. J. Agric. Food Chem. 2012;60(9):2299–2308. doi: 10.1021/jf204495t. [DOI] [PubMed] [Google Scholar]
- Neuser D., Benson A., Bruckner A., Goldberg R.B., Hoogwerf B.J., Petzinna D. Safety and tolerability of acarbose in the treatment of type 1 and type 2 diabetes mellitus. Clin. Drug Invest. 2005;25(9):579–587. doi: 10.2165/00044011-200525090-00003. [DOI] [PubMed] [Google Scholar]
- Nilvises N., Panyathanya R., Wamanutajinda W. Toxicity test of Puag Haad (Artocarpus lakoocha) Bull. Dept. Med. Sci. 1985;(27):49–55. [Google Scholar]
- Oki T., Matsui T., Matsumoto K. Evaluation of alpha-glucosidase inhibition by using an immobilized assay system. Biol. Pharm. Bull. 2000;23(9):1084–1087. doi: 10.1248/bpb.23.1084. [DOI] [PubMed] [Google Scholar]
- Oki T., Matsui T., Osajima Y. Inhibitory effect of alpha-glucosidase inhibitors varies according to its origin. J. Agric. Food Chem. 1999;47(2):550–553. doi: 10.1021/jf980788t. [DOI] [PubMed] [Google Scholar]
- Patel S.S. Cerebrovascular complications of diabetes: alpha glucosidase inhibitor as potential therapy. Horm. Metab. Res. 2016;48(2):83–91. doi: 10.1055/s-0035-1565181. [DOI] [PubMed] [Google Scholar]
- Sabiu S., O'Neill F.H., Ashafa A.O. Kinetics of alpha-amylase and alpha-glucosidase inhibitory potential of Zea mays Linnaeus (Poaceae), Stigma maydis aqueous extract: an in vitro assessment. J. Ethnopharmacol. 2016;183:1–8. doi: 10.1016/j.jep.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Samulitis B.K., Goda T., Lee S.M., Koldovsky O. Inhibitory mechanism of acarbose and 1-deoxynojirimycin derivatives on carbohydrases in rat small intestine. Drugs Exp. Clin. Res. 1987;13(8):517–524. [PubMed] [Google Scholar]
- Saowakon N., Tansatit T., Wanichanon C., Chanakul W., Reutrakul V., Sobhon P. Fasciola gigantica: anthelmintic effect of the aqueous extract of Artocarpus lakoocha. Exp. Parasitol. 2009;122(4):289–298. doi: 10.1016/j.exppara.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Sim L., Quezada-Calvillo R., Sterchi E.E., Nichols B.L., Rose D.R. Human intestinal maltase-glucoamylase: crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. J. Mol. Biol. 2008;375(3):782–792. doi: 10.1016/j.jmb.2007.10.069. [DOI] [PubMed] [Google Scholar]
- Tan H.Y., Tse I.M., Li E.T., Wang M. Oxyresveratrol supplementation to C57bl/6 mice fed with a high-fat diet ameliorates obesity-associated symptoms. Nutrients. 2017;9(2) doi: 10.3390/nu9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveden-Nyborg P., Bergmann T.K., Lykkesfeldt J. Basic & clinical Pharmacology & toxicology policy for experimental and clinical studies. Basic Clin. Pharmacol. Toxicol. 2018;123(3):233–235. doi: 10.1111/bcpt.13059. [DOI] [PubMed] [Google Scholar]
- Van Beers E.H., Al R.H., Rings E.H., Einerhand A.W., Dekker J., Buller H.A. Lactase and sucrase-isomaltase gene expression during Caco-2 cell differentiation. Biochem. J. 1995;308(Pt 3):769–775. doi: 10.1042/bj3080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.T., Lamont M., Chibrikova L., Fekkes D., Vlug A.S., Lorenz P., Kreutzmann P., Slemmer J.E. Potential neuroprotective effects of oxyresveratrol against traumatic injury. Eur. J. Pharmacol. 2012;680(1-3):55–62. doi: 10.1016/j.ejphar.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Yoshino M. A graphical method for determining inhibition parameters for partial and complete inhibitors. Biochem. J. 1987;248(3):815–820. doi: 10.1042/bj2480815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M., Murakami K. A graphical method for determining inhibition constants. J. Enzym. Inhib. Med. Chem. 2009;24(6):1288–1290. doi: 10.3109/14756360902829766. [DOI] [PubMed] [Google Scholar]
- Zhang Y.J., Li S., Gan R.Y., Zhou T., Xu D.P., Li H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015;16(4):7493–7519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Luo A., Zhong K., Huang Y., Gao Y., Zhang J., Gao H., Xu Z., Gao X. α-Glucosidase inhibitory activity by the flower buds of Lonicera japonica Thunb. J. Funct. Foods. 2013;5(3):1253–1259. [Google Scholar]