Abstract
Brain-computer interfaces (BCIs) are technologies that provide the user with an alternative way of communication. A BCI measures brain activity (e.g. EEG) and converts it into output commands. Motor imagery (MI), the mental simulation of movements, can be used as a BCI paradigm, where the movement intention of the user can be translated into a real movement, helping patients in motor recovery rehabilitation. One of the main limitations for the broad use of such devices is the high cost associated with the high-quality equipment used for capturing the biomedical signals. Different low-cost consumer-grade alternatives have emerged with the objective of bringing these systems closer to the final users. The quality of the signals obtained with such equipments has already been evaluated and found to be competitive with those obtained with well-known clinical-grade devices. However, how these consumer-grade technologies can be integrated and used for practical MI-BCIs has not yet been explored. In this work, we provide a detailed description of the advantages and disadvantages of using OpenBCI boards, low-cost sensors and open-source software for constructing an entirely consumer-grade MI-BCI system. An analysis of the quality of the signals acquired and the MI detection ability is performed. Even though communication between the computer and the OpenBCI board is not always stable and the signal quality is sometimes affected by ambient noise, we find that by means of a filter-bank based method, similar classification performances can be achieved with an MI-BCI built under low-cost consumer-grade devices as compared to when clinical-grade systems are used. By means of this work we share with the BCI community our experience on working with emerging low-cost technologies, providing evidence that an entirely low-cost MI-BCI can be built. We believe that if communication stability and artifact rejection are improved, these technologies will become a valuable alternative to clinical-grade devices.
Keywords: Biomedical engineering, Brain-computer interfaces, Consumer-grade EEG, Motor imagery, Open-source software
Biomedical engineering; Brain-computer interfaces; Consumer-grade EEG; Motor imagery; Open-source software
1. Introduction
A brain-computer interface (BCI) provides an alternative way of communication between the brain of a person and the outside world. More specifically, a BCI measures the brain activity and converts it into an artificial output which is able to replace, restore, enhance, supplement or improve the natural central nervous system outputs used by a person to communicate with or control his/her external or internal environment [1]. For improving motor loss functions, BCIs based on motor imagery (MI), i.e. the mental simulation of movement, can be used as a complement to facilitate neurorehabilitation after neurological injures [2].
As a communication system, a BCI treats the measured brain activity as input. Although different non-invasive brain imaging technologies can be used to measure neuronal activity, electroencephalography (EEG) is one of the predominant tools in the BCI field to safely acquire electric brain signals with high temporal resolution [3]. For a correct interpretation of the registered brain activity, a good signal quality is required. Most of the existing works make their EEG registration in controlled environments with prepared shielded labs, where the subject is free of any visual or auditory external noise. The electrode montage is generally a multi-channel setup with high quality gel-based electrodes connected to a clinical-grade amplifier. In addition, the EEG amplifier is usually wire-connected to a computer, where the signals are saved and processed to finally perform the BCI communication. These conditions, are obviously highly impractical for most of the typical BCI end-users, who not only spend most of their time in noisy environments, but also might feel their mobility restricted due to the wire-connections [4]. In addition, due to the high cost of such amplifiers, the usability of EEG-based BCI systems is limited to research and clinical environments, reducing BCI applications outside the lab.
Wolpaw defines the “perfect” BCI as a safe and affordable system which works all the time, does not require the permanent assistance of a technician or a scientist, restores communication at “normal” speed, is aesthetically acceptable, is reliable and, for the same function, does not require more concentration for a patient user than what it does for an able-bodied person [1]. Despite recent development aimed at improving the current state of BCI systems, there are still several challenges that must be overcome before building an usable and effective BCI, like for example, the high cost associated with the required EEG recording system.
Different affordable consumer-grade EEG devices have appeared in both Academia (e.g. [5], [6], [7], [8]) and the market (e.g. B-Alert X10, NeuroSky, OpenBCI, Emotiv), offering different alternatives from easy-to-mount electrode systems to low-cost amplifiers. Considering the rapid development and advancement of such devices there is a growing need for exploring the feasibility of using low-cost EEG amplifiers for possible motor therapy in natural environments. Several authors have studied the advantages and disadvantages of both types of devices for BCI applications, from electrode-skin contact interface issues (dry, gel-based or saline-based electrodes) to signal quality matters [7], [9], [10], [11], [12], as well as from the user's comfort perspective [4], [13], [14]. As a drawback, most of the existing low-cost consumer EEG systems present a low electrode count with fixed localization (generally not covering the whole sensorimotor cortex of interest for MI detection) and they are all-in-one black box devices depriving of a user-friendly interplay and precluding the addition for further development tools. OpenBCI1 is an open-source, versatile and affordable biosensing system which can be used to acquire not only EEG signals but also to measure electrical activity of muscle (EMG) and heart (ECG). All OpenBCI boards are based on the open-source electronic platform Arduino with wireless connection to the computer. OpenBCI offers a variety of low-cost amplifiers (boards), electrode systems (e.g. 3D-printed headware) and a software for viewing and recording the biosignals (OpenBCI GUI).2
The use of OpenBCI for affordable BCI applications has already been studied. While some authors have shown that low-cost BCIs could be built [15], [16], others have compared the quality of the acquired signals by the OpenBCI board with those obtained by research-grade amplifiers [17], [18]. However, for the BCI community it is of interest to know how to integrate and use these devices for constructing a complete consumer-grade low-cost BCI. In addition, there is a need for reporting the accuracy and reliability of these systems for in-home measurements [12]. In this work, we aim at evaluating the applicability and feasibility of the OpenBCI devices in informal real-world environments for intended home MI-BCI use. Thus, following the aforementioned Wolpaw's definition and taking advantages of the current affordable OpenBCI boards, we have designed, implemented and evaluated a consumer-grade low-cost open-source robust MI-BCI system in an uncontrolled-environment, encouraging motor function rehabilitation at home. By means of this work we share with the BCI community our experience in the construction of a consumer-grade BCI system, being of highly relevance for affordable BCI use. A critical evaluation of OpenBCI systems from the user's point of view, as well as in regard to communication stability and MI detection performance is made. For a better contribution to the BCI community, the raw data, the syntax and settings used for acquisition and post-processing will be publicly available together with the final low-cost EEG dataset at https://github.com/vpeterson/MI-OpenBCI.
The organization of this paper is as follows. In Section 2 we give detail on how the database was built. A brief review of the low-cost hardware and open-source software used is made in Subsection 2.1. Subsection 2.2 describes the protocol and experimental design. Experiments and results are detailed in Section 3, while discussions and concluding remarks are presented in sections 4 and 5, respectively.
2. An MI-BCI dataset based on consumer-grade systems
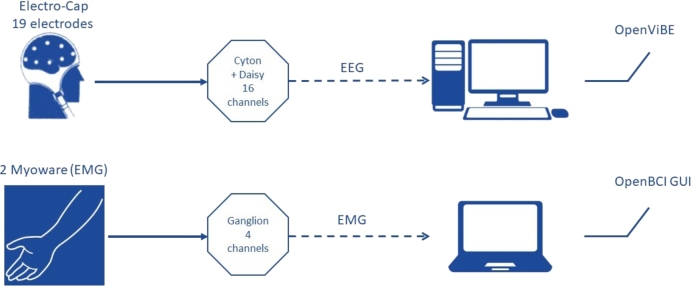
Is it possible to construct a complete low-cost BCI? Can we use it for MI detection in real-time? Which are the advantages and disadvantages of using consumer-grade devices? Is the signal quality good enough to establish communication in uncontrolled environments? For answering these and other related questions, we developed a complete consumer-grade MI-BCI system based on low-cost devices and free multi-platform software. In particular, the OpenBCI Cyton + Daisy Module (OpenBCI, USA) together with the Electrocap System II (Electrocap, USA) were used for the EEG signal recording. For EMG monitoring the OpenBCI Ganglion board (OpenBCI, USA) connected to the Myoware sensors (Advancer Technologies, USA) were used. The EEG and EMG data recording was made by the OpenViBE and the OpenBCI GUI software, respectively, on different computers, the former one running on a Linux based-platform and the latter one on a Window based computer. Fig. 1 summarizes the consumer-grade devices and free software used for both EEG and EMG acquisition.
Figure 1.
Schematic representation of the hardware and software used for EEG (top) and EMG (bottom) acquisition, respectively.
2.1. Hardware and software description
2.1.1. The OpenBCI boards
OpenBCI is an open-source, low-cost and programmable platform based on the ADS1299 Texas Instrument micro-controller. OpenBCI is specifically designed for biopotential measurements, mainly oriented to make BCI affordable to everyone. Two different battery-powered boards (amplifiers), named Cyton and Ganglion, have been developed by the OpenBCI team. Both biosensing amplifiers can be used to measure cardiac, muscle and brain electrical activity. They wirelessly connect to PCs, laptops, smartphones and to any bluetooth compatible device.
Multi-channel acquisition is possible by both OpenBCI boards. In particular, the Cyton board allows a maximum of 8 electrodes with a sampling frequency of 250 Hz. By using the expansion Daisy Module (Cyton + Daisy) 16 channels can be recorded at 125 Hz. On the other hand, the Ganglion board is built to record with up to 4 channels at a sampling frequency of 200 Hz. If for some reason, a higher sampling frequency is desired, a WiFi shield can be added to the boards in order to increase the communication rate of the devices.
The electrode connection of the OpenBCI boards is quite simple. They can be used either with the OpenBCI 3D-printed headset (Ultracortex Mark IV) or with any traditional gold cup electrode system. In addition, Myoware Muscle Sensor,3 Pulse Sensor4 and the Electro-Cap International cap5 are also compatible with these boards.
2.1.2. Low-cost sensors
The Ultracortex Mark IV is a 3D printable headset developed by OpenBCI team that has dry electrodes attached at fixed locations. For acquiring good quality signal, the electrodes must be in contact with the scalp. This might produce some degree of discomfort and pain after several minutes of usage as well as information loss due to bad electrode-skin contact. In addition, this headset has only 8 electrodes localized over the sensorimotor area, and since for MI the more covered the sensorimotor cortex the better, we decided to look for another low-cost alternative. Electro-Cap International Inc. offers low-cost electro-caps made of an elastic spandex-type fabric with recessed, pure tin electrodes attached to the fabric (see Fig. 2a). The standard cap comprises 20 electrodes positioned following the International 10-20 electrode placement. In particular, the Electro-Cap System II, provides two electro-caps with all the needed supplies (electro-gel, ear electrodes, disposable sponge disks and electrode board adapter).
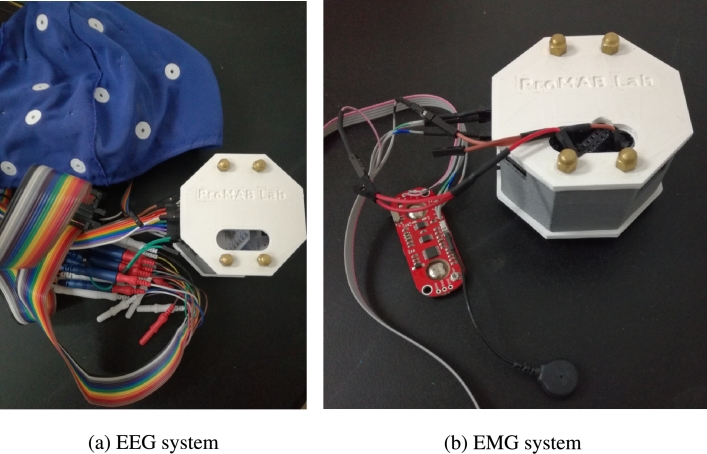
Figure 2.
Amplifier and electrodes systems used for measuring brain (a) and muscle (b) activity. A 3D-printed case was built for protecting the boards.
The MyoWare are Arduino-powered all-in-one EMG sensors. These sensors provide the raw EMG signal as well as the filtered and rectified electrical activity of a muscle as output. The system is built with embedded electrode connectors and since it is powered by the board being used, it does not require of any external energy supply, leading to a more wearable EMG acquisition.
2.1.3. Free software
The OpenBCI team has also developed its own multi-platform software called OpenBCI GUI. This software can be used for visualizing, recording and streaming data from the different OpenBCI boards. As a drawback, this GUI does not provide the possibility of acquiring data under a particular BCI paradigm nor does it allow for the on-line process of the biosignals.
For exogenous and synchronized BCI paradigms, the mental task should be performed after a cue is presented to the subject. In this regard, different neurofeedback software alternatives can be used to present and acquire the signal of interest in real-time. OpenViBE6 is a free open-source multi-platform software which is very easy to use and it does not require any technical programming knowledge, making it accessible to almost everyone. The OpenViBE Acquisition Server acquires data from an EEG device and sends them to the other OpenViBE client on the network. In the OpenViBE Designer application, the acquisition client is the main block of any OpenViBE scenario, where the signal can, for example, be visualized, filtered, on-line processed and sent to other program at the same time that the stimulus are presented and the data, with mark time stamps, are being recorded. Given that OpenViBE supports the OpenBCI drivers and has compatibility with other high-level programming languages (e.g. Matlab and Python), we decided to use OpenViBE for EEG acquisition.
In summary, for the present work, brain and muscle activity have been measured by using low-cost devices and free multi-platform software. In particular, for the EEG acquisition the OpenBCI Cyton + Daisy board together with wet EEG electrodes attached to the Electro-Cap were used. A dedicated computer with the OpenViBE software was utilized for signal acquisition, visualization and stimulation protocol presentation. In the case of the EMG signal, measured only for MI-task control purposes (more information below), the OpenBCI Ganglion board with two attached MyoWare sensors were used. The EMG signal was streamed and saved to another computer (Laptop) by using the OpenBCI GUI software. Fig. 2 shows both the amplifier and their corresponding sensor systems for EEG and EMG data recording, respectively. A 3D-printed cover was built for protecting the boards.
2.2. Experimental paradigm
The experiment was approved in Feb. 2018 by the “Comité Asesor de Ética y Seguridad en el Trabajo Experimental” (CEySTE, CCT-CONICET, Santa Fe, Argentina).7 Twelve healthy subjects (four females, right-handed, mean age ± SD = 25.9 ± 3.7 years) without any previous BCI experience participated in the experiment and gave their informed consent. The study was conducted in a non-shielded office, with a room divider between the experimenters and the participant. Each subject participated in one single session, of about 1.5 hours.
The EEG signal was acquired by using the Electro-Cap connected to the OpenBCI Cyton + Daisy board. Fifteen (15) electrodes covering the sensorimotor cortex (Fz, F3, F4, F7, F8, Cz, C3, C4, T3, T4, Pz, P3, P4, T5, T6), were selected. The reference and ground electrodes were placed at the left and right ear lobes, respectively (see Fig. 3). The sampling frequency of the OpenBCI amplifier was 125 Hz. The OpenBCI board was wirelessly connected to a dedicated PC (OS Linux, Intel® CoreTM i7-6700K CPU @ 4.00 GHz × 8) by the USB Dongle. The OpenViBE platform, acquisition server and designer, was used for the protocol presentation, visualization and storage of both the EEG signals and the time mark stamps. During acquisition, the EEG signals were filtered between 0.5 and 45 Hz with a 3rd order Butterworth bandpass-filter. In order to ensure that the subject was not making any hand movement during the MI trial, surface EMG was also acquired during the experiment. For the EMG signal recording the OpenBCI Ganglion board was set at 200 Hz. It was connected to two MyoWare sensors located at two forearm antagonist muscles, compromised in finger flexion and extension movement (flexor digitorum superficialis and extensor digitorum). Disposable pre-gelled Ag-AgCl foam electrodes were employed. Due to stability problems for simultaneous acquisition of both EEG and EMG, the Ganglion board was wirelessly connected to another dedicated laptop (OS Windows 10, Intel® CoreTM i7-6500U CPU @ 2.50 GHz × 2 ), where the OpenBCI GUI was running for visualization and storage of the EMG signals. Care was taken in manually synchronizing the EMG and EEG data recording.
Figure 3.
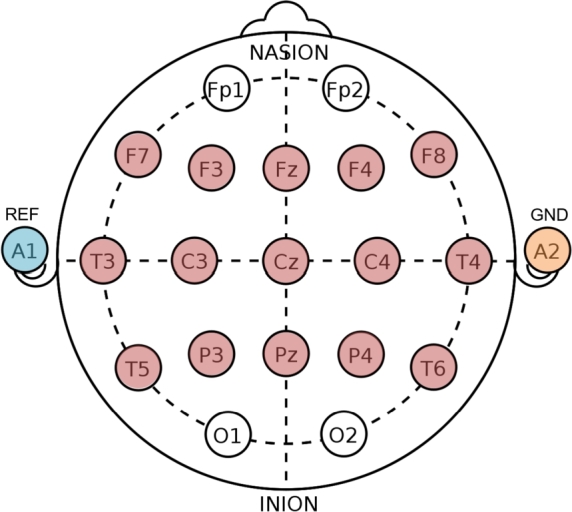
Electrode configuration of the Electro-cap. Red colored circles illustrate the location of the 15 electrodes used for acquisition. The A1 (left ear lobe) and the A2 (right ear lobe) electrodes were used as reference and ground, respectively.
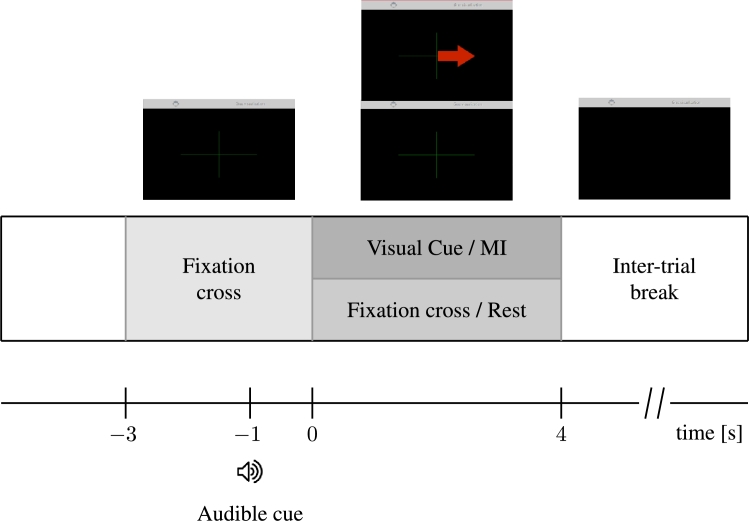
The BCI protocol consisted of two conditions, namely the kinesthetic imagination of grasping movement of the dominant hand and rest/idle condition. The session comprised four (4) runs of 40 trials (20 trials per class, randomly presented), yielding a total of 160 trials at the end of the session. A modified version of the Graz protocol [19] was used for the EEG data acquisition. Before the stimulation protocol began, 20 seconds of baseline EEG were registered. Each trial of the stimulation protocol began with a fixation cross ( s), followed by an audible beep cue two seconds later ( s). At s, the subject was asked to imagine either grasping movements of the dominant hand or just to relax for a period of 4 s. The visual cue, red arrow pointing to the right, was presented only for the MI trials. Between trials, a break of randomly selected duration (between 2.5 and 4.5 s) followed. At the end of each run, the subject could distend and relax for a longer period of time (>2 min). The stimulation protocol used is schematically depicted in Fig. 4.
Figure 4.
Stimulation protocol used, with timing references, in seconds.
At the beginning of the session each subject was clearly instructed about the tasks. To assess motor imagery ability, five kinesthetic mental exercises of the KVIQ-10 questionnaire [20] were performed. The KVIQ-10 is a fast questionnaire which aims to assess the intensity of MI sensation on a five-point ordinal scale, ranging from 1 (“no kinesthetic sensation”) to 5 (“as clear as executing the action”). At the end of the questionnaire the ability of each subject to perform MI was evaluated according to his/her final score value (maximal value: KVIQ-10=25).
Before the experiment started, the EMG rest signal was acquired for a period of 20 seconds. During the experiment, the subject was comfortably seated in front of a computer screen with both arms resting on a desk. In order to ensure kinesthetic (and no visual) MI, the dominant hand was placed inside a cardboard box, as shown in Fig. 5. Five experimental protocol runs were made. The first one (called RUN0), was used for better explaining the protocol to the subject. In addition, in this run the subject was asked to actually perform the grasping movement, to focus on the sensation of such movement in order to further try to invoke those feelings in the following MI runs. Fig. 6 summarized the experiment's stages in a block diagram design.
Figure 5.

Experiment set-up. The subject seated in front of a computer screen, where the stimulation protocol was being presented. The EEG cap was connected to the OpenBCI Cyton + Daisy board and the EMG Myoware electrodes were connected to the OpenBCI Ganglion board. Each board was wirelessly connected to a dedicated PC.
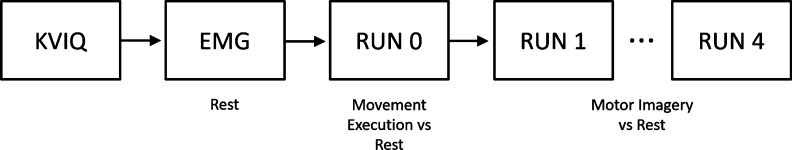
Figure 6.
Schematic representation of the experiment in a block diagram. The experiment started with the KVIQ questionnaire. After the electrode setting, the EMG rest signal was acquired. Five protocol runs were asked to be performed by the user. The first run, called RUN0, involved real grasping movement in order to better explain the protocol and to help the subject to focus on the sensation of making the movement. The rest of the runs (RUN1-RUN4) were equal, consisting of MI vs. Rest conditions.
3. Data analysis and results
3.1. Detection of corrupted EEG trials with actual muscle activity
The database comprised EEG and EMG signals of 12 healthy subjects under the MI-BCI paradigm. The EMG signals were acquired in order to determine if any EEG trial was corrupted by real hand movement. The most commonly used method for detecting EMG activation is based on the single-threshold method, where the envelope of an EMG signal is compared to a fixed threshold based on the envelope of a rest EMG period (no activity) [21]. Since during the EMG acquisition no filter was used, a digital IIR Notch filter was applied for the 50 Hz power-noise, and then a high-pass 5th order Butterworth with 10 Hz cutoff frequency was implemented. The envelope of the EMG signal was estimated by means of a low-pass 3rd order zero-phase Butterworth filter with 40 Hz cut-off frequency applied to the zero-mean rectified EMG signals. Since we were interested in detecting muscle activation within an MI trial, we extracted forty (40) EMG segments of 5 s length in correspondence with each EEG trial. Given that EEG and EMG acquisition was manually synchronized, each EMG segment was extracted starting 0.5 s before, and ending 0.5 s after, each EEG trial began and ended, respectively. The onset activity threshold was calculated as the standard deviation of the envelope of a rest segment of 0.25 s extracted from RUN0. A sliding window of 0.05 s was considered as having EMG activity if its mean value was greater than 5 times the prescribed threshold. An EEG trial was considered contaminated with EMG activity if 50% or more of the sliding windows detected muscle activity. For one of the subjects, more than 50% of the trials were contaminated with EMG activations, reason for which this subject was not taken into account in the subsequent analyses. In addition to this, due to a bad wire welding in one Myoware, for one subject the EMG data information was lost in the middle of the session. Thus, our OpenBCI dataset finally includes EEG recording coming from 10 subjects.
3.2. Temporal and frequency information
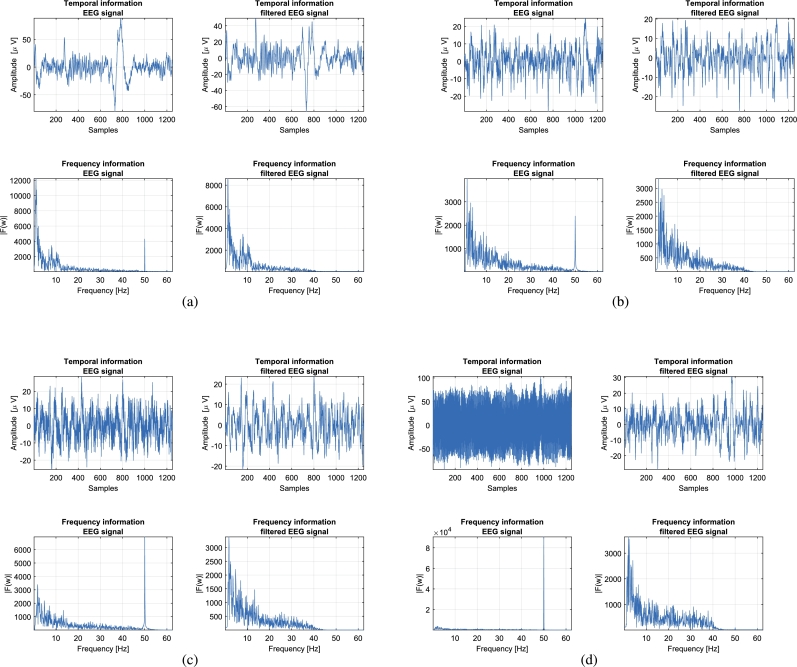
The signal quality of the EEG data can be compromised by physiological and non-physiological noise. In most hospitals and laboratories, the use of electromagnetic shields can reduce such noise level. If such equipment is not available, it is highly recommended to reduce as much as possible the electrode-to-skin contact resistance [22]. In our experiments, data were recorded in a non-shielded office. Before signal recording, the electrode impedance was checked to be below 5 KΩ. During acquisition a digital bandpass filter (implemented in OpenViBE) was applied. Despite all these cautions regarding signal quality acquisition, in some situations the electromagnetic artifacts were high enough to wrap the ongoing EEG signal. Fig. 7 shows the temporal and frequency domain information of four selected EEG data segments in four different signal-to-noise conditions. The plots are ordered from lower (a) to higher (d) 50 Hz amplitude noise component (AC frequency in Argentina). In order to get rid of such noise, in a post-processing step a backward-forward bandpass 5th order Butterworth filter between 1 and 40 Hz was applied to the acquired signal. Note how after filtering, the signal quality in the temporal domain improves and the 50 Hz frequency component is discarded, as expected.
Figure 7.
Time and frequency domain information of the EEG acquired signals corresponding to four different subjects, showing four different levels of noise artifacts during acquisition.
3.3. MI detection performance
The most commonly and widely used method for MI detection is the well-known common spatial patterns (CSP) algorithm. The CSP method performs feature extraction based on learned spatial filters. Given a set of band-pass filtered EEG trials , let be the matrix whose ith row is , where represents each one of the two considered MI conditions. The p spatial filters , are optimized by maximizing the variance for one class while minimizing it for the other class:
where “′” denotes transpose. The projections of the bandpass filtered EEG signals into the spatial filter space are called “spatial patterns”, and they can be easily computed by . Since W is non-singular, X can be obtained as , where each column , of the matrix is a spatial pattern.
As shown in Fig. 8 the spatial patterns inherit the variance separation property of the spatial filters. Given that the variance of a bandpass filtered signal is similar to its bandpower, the CSP features are obtained as the variance of a small number of pairs of spatial patterns [23]. Since motor imagery, as well as motor execution, produces amplitude increments (event related synchronization, ERS) and decrements (event related desynchronization, ERD) on the ongoing EEG signal, namely in the mu (8-12 Hz) and beta (13-30 Hz) bands [24], CSP constitutes an easy to implement tool for distinguishing between two MI conditions in terms of their corresponding bandpowers [25]. Unfortunately, the success in detecting MI highly depends on both the prescribed time segment and frequency band used to process the EEG signal. In this direction, the penalized time-frequency band common spatial pattern (PTFBCSP) method [26] has been proposed in order to improve MI detection. Roughly speaking, by means of PTFBCSP, multichannel EEG data are decomposed into predefined T temporal and F frequency bands. Then, pairs of CSP features are extracted at each t-f band. The selection and classification of the most discriminative features are simultaneously made by means of the generalized sparse discriminant analysis (GSDA) algorithm [27], a fast procedure which allows the inclusion of a-priori discriminative information into the model.
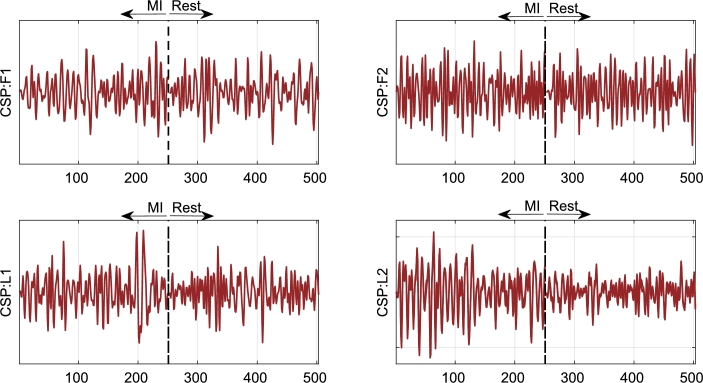
Figure 8.
Spatial patterns obtained by projection of the first two (CSP:F1 and CSP:F2) and the last two (CSP:L1 and CSP:L2) spatial filters which discriminate between hand MI and rest condition. The graphs show two band-pass filtered EEG segments after applying CSP. The spatial patterns associated to CSP:F1 and CSP:F2 present larger variances for the rest condition, while CSP:L1 and CSP:L2 have larger variances for the MI EEG segment.
In order to answer whether it is possible to detect MI by using low-cost EEG acquisition devices, we off-line evaluated the classification performance of each subject by using both the traditional CSP method and PTFBCSP in two configurations: by using a single-time window (called PFBCSP) and multiple-time windows. For the single-time window approach, EEG segments were extracted from 0.5 to 2.5 s after each stimulus onset. In the case of the CSP method, each EEG segment was bandpass filtered between 8 and 30 Hz with a 5th order Butterworth filter. The number of spatial filters in CSP was set to 3, as recommended in [28]. On the other hand, for PFBCSP, 17 sub-bands () between 4 and 40 Hz with 4 Hz bandwith with overlapping of 2 Hz were extracted ( Hz, Hz ,…, Hz). Here only one CSP pair of spatial filters was used for feature extraction. For the multiple time-windows approach, PTFBCSP was implemented following the methodology used in [26]. Thus, five time segments of 2 s length and overlapping of 1.5 s were extracted. At each time segment the same filter-bank and feature extraction procedure implemented in PFBCSP was followed. For the CSP implementation the Matlab RCSP8 toolbox was used [28].
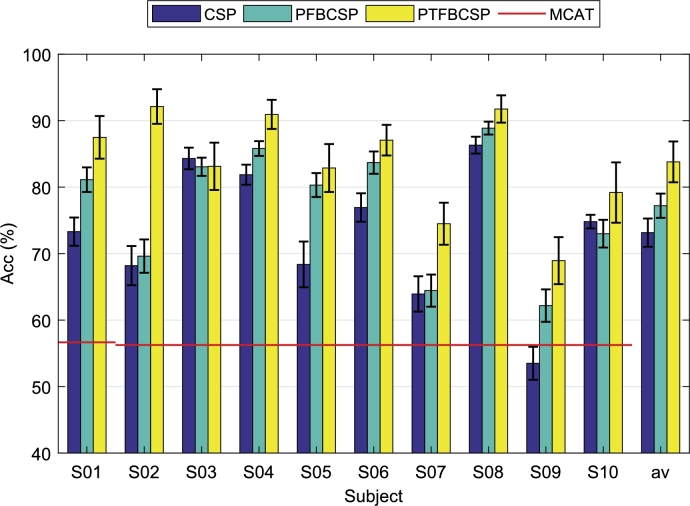
The binomial cumulative distribution function (BCDF) can be used to provide the minimum classification accuracy threshold (MCAT) needed for BCI control [29], [30]. For a balanced binary classification problem, the percentage of theoretical chance level of classification is 50%. This threshold only holds for an infinite number of samples. In practice, the minimum chance level depends on the available number of samples. For Subject 1, due to communication problems between the boards and OpenViBE, the 4th run (RUN4) presents only 30 trials instead of 40. Thus, for this subject we have 150 trials to compute the MCAT, and 160 trials for all the other subjects. By using BCDF with a confidence level of 95%, and based on the number of trials performed by each subject, the minimum threshold is found to be 56.66% for Subject 1 and 56.25% for all the other subjects.
The algorithms were implemented by 10 × 10-fold cross-validation. The accuracy achieved for each subject and each tested method is presented in Fig. 9. In addition, the minimum classification accuracy thresholds are plotted as horizontal red lines. The last columns show the average performance over all subjects yielded by each method considered.
Figure 9.
Mean and standard deviation of the classification performance (accuracy %) for each subject and each method considered. Horizontal red lines represent the minimum accuracy level for confident MI-BCI control for each subject.
3.4. Topographical maps
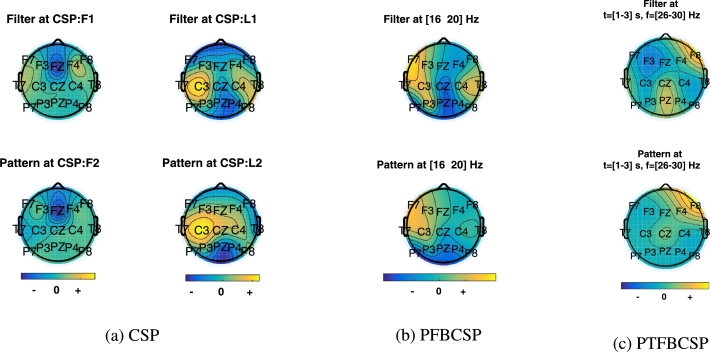
The topographical maps of the most relevant spatial filters and spatial patterns learned by CSP allow for a neurophysiological interpretation of the solution [31]. In fact, while the spatial filter maps may resemble the brain patterns associated to a specific MI task, the spatial pattern maps illustrate how the presumed brain sources are projected into the scalp [25]. Fig. 10 shows the most significant spatial filter and pattern (associated to the most relevant feature) for a randomly selected trial of Subject 2 for each method considered.
Figure 10.
Topographical maps of the most relevant spatial filter and pattern corresponding to the most significant feature for a right-handed subject. a) CSP, b) PFBCSP, c) PTFBCSP.
3.5. Motor imagery assessment via KVIQ results
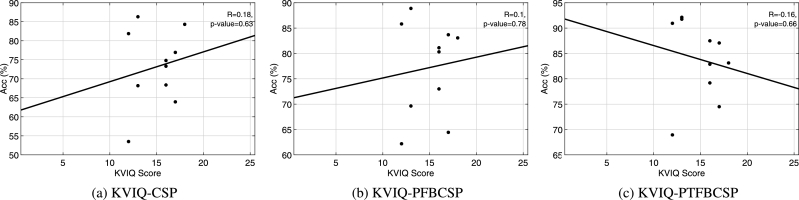
The ability of each subject in performing MI was measured by five kinesthetic items classified from “poor” (between 5 and 10) to “very good” (from 20 to 25) MI ability. In order to investigate whether correlation exists between classification accuracy and the reported MI ability, a pairwise correlation analysis was performed between the mean accuracy value reached by CSP, PFBCSP and PTFBCSP for each subject (over the 10×10 cross-validation) and the corresponding KVIQ-10 score (sum of the 5 items considered). The pairwise correlation, or Pearson's correlation, is a measure of the strength and direction of association between two variables. The method attempts to draw the line that best fit the two data variables and the associated Pearson correlation coefficient R indicates how far away all the data points are from this line of best fit. In our analyses, as presented in Fig. 11, no statistically significant correlation was found between the KVIQ-10 score and either one of the three methods considered.
Figure 11.
Correlation analysis between KVIQ score and classification accuracy yielded by CSP (a), PFBCSP (b) and PTFBCSP (c). The R-correlation coefficient and the associated p-value appear on the upper right corner of each subfigure.
4. Discussion
In this work a complete consumer-grade low-cost BCI system was built and evaluated. A detailed description of the materials and methods used for constructing an MI-BCI dataset acquired in an uncontrolled environment was made. Two biomedical signals were registered using low-cost devices and open-source software. The muscle activity, measured by the MyoWare sensors connected to the OpenBCI Ganglion board, was used for protocol controlling reasons. Thus, we were only interested in detecting muscle movement within an MI trial. In the case of the EEG signal, the OpenBCI Cyton + Daisy board connected to the Electro-Cap were used. Both temporal and frequency information of the EEG signals were shown in Fig. 7. Although efforts were made in order to reduce at a minimum level the noise artifacts, we noticed that during acquisition, in some situations (sessions, subjects) the electromagnetic noise amplitude (50 Hz) was from 2 to 4 times higher than the ongoing EEG (e.g. Figs. 7 c and d). This can be explained by the fact that the electronic components of the boards are not physically isolated from the outside, being in direct contact with the air. It is well-known that certain climate conditions (humidity, temperature and pressure) affect the behavior of the electronic components and their connections. Although a 3D-printed case for the boards was built (see Fig. 2), those boxes cannot be considered as electromagnetic isolations but only as handle protectors. In spite of the fact that this is far beyond the scope of this manuscript, an analysis of the impact of humidity-room conditions on the OpenBCI boards functionality might better explain this issue.
Although by eye inspection the quality of the EEG signal was not always good enough, after a proper post-processing filtering step the signal quality improved by discarding the high frequency noise components (mainly 50 Hz), as shown in Fig. 7. In addition, the reached average classification levels are comparable to those reported in the literature for commercial BCI systems. In fact, in a previous work [26] a 10-subject MI-BCI dataset was acquired following exactly the same protocol used here, but using clinical-grade devices. In particular, this dataset was recorded by using a 64-channel EEG system (eego™rt Ant Neuro, Netherlands) at 512 Hz. A bandpass 3rd order Butterworth filter from 0.5 and 45 Hz was also applied within acquisition by using also OpenViBE designer. At the processing stage, a bandpass filter was applied between 0.5 to 40 Hz and the signals were downsampled at 128 Hz. An analysis of the classification results provided on average by this high-quality system, allows us to compare (from a user concern point of view) the MI detection ability of the OpenBCI system. Thus, for fair comparison purposes, following the methodology used here, CSP, PFBCSP and PTFBCSP were evaluated over this Ant Neuro MI dataset by using the 15 electrode positions employed for the OpenBCI MI dataset. For this clinical-grade dataset the average accuracy reached by each method was , and , respectively, while for the OpenBCI dataset those values were found to be , and , respectively (see Fig. 9). This high accuracy values were possible in spite of the noisy signals due to the fact that the MI detecting algorithms are based on filtered EEG data segments within the frequency bands of interest. In fact, from the classifier point of view, no differences were found when using the clean signals or the noisy ones. Although by performing artifact detection due to eye movement and heart beats better signal-to-noise ratio can be obtained, this pre-processing step could lead to signal deviation and information loss if data is not clean enough and the electrode count is small, such as in the presented dataset.
By analyzing the topographical maps of the filter and spatial patterns, a strong correlation between the MI mental task and the motor brain area is observed for the single time window methods (CSP and PFBCSP). For a right-handed subject, changes over the contralateral sensorimotor brain area (around electrode C3) should emerge while performing MI of the dominant hand. On the contrary, for the rest/relax condition the brain associated patterns may vary between subjects, which it is expected to be better characterized by the multiple time-windows approach (PTFBCSP) [26]. In this sense, the maps presented in Fig. 10 increase the credibility and confidence of the acquired signals for MI-BCI control.
By observing the classification performances presented in Fig. 9, we see that Subject 9 does not reach the minimum classification accuracy threshold (MCAT) when CSP is used for MI detection. This may be a case of BCI-illiteracy [32]. For naive BCI users, it may be difficult at first to learn how to modulate their brain signals for controlling a BCI system. Several works ([32], [33], [34], to name a few) have tried to address this issue by finding a neurophysiological marker to predict the MI-BCI performance of the participants. On the other hand, the KVIQ-10 questionnaire was built to assess MI ability and help to detect “bad” BCI users to avoid frustrating training procedures. Although the authors in [20] claim that this type of questionnaires present good psychometric properties and can be reliably used for assessing the subject's MI ability, in this work, no statistical significant correlation was found between the KVIQ score and the classification accuracy reached by the three methods considered, raising the question if KVIQ is a reliable MI assessment tool for MI-BCI control.
The accuracy levels reached by the traditional CSP and our two filter bank-based methods encourage the use of the OpenBCI technology for MI detection. In fact, from the product-cost point of view, we show that it is possible to construct a complete BCI (with simultaneous EEG and EMG recording) by spending between 10 and 20 percent of the cost of a clinical-grade device. By no means we are saying that by these low-cost technologies the commercial equipment can entirely be replaced. Actually, one limitation for the use of such consumer-grade devices is that they do not possess any certification, and thus medical applications are not possible. In addition to this, and based on our own experience, the user may find the use of these technologies in long-term applications not always practical. This is so because during the experiments, several communication problems between the boards and the computer appeared. Besides the fact we were using a Linux OS computer, which may affect communication due to driver dependences, there was always the need to reboot the board for reestablishing the wireless communication. Although in the end the communication could always be established and the experiment was always successfully conducted, this problem hindered the acquisition of the signals by adding delays to the experiment and stress to the practitioners. In addition to this, simultaneous acquisition of both EEG and EMG was not possible. Several configurations of OpenViBE were designed for acquiring the EEG and EMG in a synchronized manner. These configurations not only occasionally frozen the computer, but also added delays to the streaming of the signals, reason for which we decided to use two different computers to make the recordings. Nevertheless, this could also be another consequence of using a computer running Linux OS. Besides these software issues, another limitation regarding the boards is the care that must be taken to handle the devices. For instance, we had problems during EMG acquisition due to a welding disconnection, which produced data loss in one session.
A clear limitation of this work is that we were not able to test the consumer-grade device with a clinical-grade device neither simultaneously (one electrode, two amplifiers) nor with the same control group. In addition, a quantified analysis of the noise level of the non-shielded office should be performed to detect the sources of the main artifact. On the other hand, no feedback was presented to the users during the experiments, thus we cannot predict how accurate the user-interface communication will be in on-line settings. Future works include the analysis of on-line communication, feedback strategies, multi-class MI-BCI applications and the study of wavelet transform for performing the filter-bank analysis, among others.
Anyhow, even with those limitations in mind, this work provides strong evidence that the OpenBCI technology constitutes a good alternative to traditional EEG amplifiers for BCI control, yielding similar accuracy levels for MI detection in off-line experiment. In addition, with an improvement in the communication stability and artifact rejection, we strongly believe that these technologies will become a valuable alternative to clinical-grade devices, spreading the realm of applications and increasing the number of potential BCI users.
5. Conclusions
Low-cost, easy-to-use systems make BCIs more accessible to not only researchers, but also to clinicians and their final users: patients. In this regard, as the system becomes more portable and practical it increases the number of possible applications in which a BCI can be used. In this work a complete low-cost consumer-grade BCI was built. Although in some situations noise level was high enough to mask the ongoing EEG, good classification performances were achieved () even with traditional CSP, rising up to 83% when multiple time-frequency bands were used for MI detection. Despite of the communication problems perceived by the practitioners during acquisition, the OpenBCI boards offer user-friendly interfaces, allowing to non-EEG experimented researchers to acquire their own biosignals in non-controlled environments. In this work we have shown that it is possible to successfully construct an entirely low-cost BCI system for MI detection. We strongly believe that by this research, practitioners of all over the world will feel invited to use this technologies in their class-rooms, laboratories and/or any other research activities. In order to contribute to the BCI community, the acquired dataset will be made publicly available.
Declarations
Author contribution statement
V. Peterson: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
C. M. Galván, H. S. U. Hernández: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported in part by Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET, Argentina, through PIP 2014-2016 No. 11220130100216-CO, the Air Force Office of Scientific Research, AFOSR/SOARD, through Grant FA9550-14-1-0130, by Universidad Nacional del Litoral, UNL, through CAI+D-UNL 2016 PIC No. 50420150100036LI and by Universidad Nacional de Entre Ríos through PID NOVEL 6215.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
Visit http://docs.openbci.com/FAQ/02-HowProductsGoTogether for a better understanding of the OpenBCI offers and products.
References
- 1.Wolpaw J., Wolpaw E.W. Oxford University Press; USA: 2012. Brain-Computer Interfaces: Principles and Practice. [Google Scholar]
- 2.Millán J.d.R., Rupp R., Mueller-Putz G., Murray-Smith R., Giugliemma C., Tangermann M., Vidaurre C., Cincotti F., Kubler A., Leeb R. Combining brain-computer interfaces and assistive technologies: state-of-the-art and challenges. Front. Neurosci. 2010;4:161. doi: 10.3389/fnins.2010.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zander T.O., Kothe C., Jatzev S., Gaertner M. Brain-Computer Interfaces. Springer; 2010. Enhancing human-computer interaction with input from active and passive brain-computer interfaces; pp. 181–199. [Google Scholar]
- 4.Radüntz T. Signal quality evaluation of emerging EEG devices. Front. Physiol. 2018;9:98. doi: 10.3389/fphys.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao L.-D., Chen C.-Y., Wang I.-J., Chen S.-F., Li S.-Y., Chen B.-W., Chang J.-Y., Lin C.-T. Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. J. NeuroEng. Rehabil. 2012;9(1):5. doi: 10.1186/1743-0003-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vos M., Kroesen M., Emkes R., Debener S. P300 speller BCI with a mobile EEG system: comparison to a traditional amplifier. J. Neural Eng. 2014;11(3) doi: 10.1088/1741-2560/11/3/036008. [DOI] [PubMed] [Google Scholar]
- 7.McCrimmon C.M., Fu J.L., Wang M., Lopes L.S., Wang P.T., Karimi-Bidhendi A., Liu C.Y., Heydari P., Nenadic Z., Do A.H. Performance assessment of a custom, portable, and low-cost brain–computer interface platform. IEEE Trans. Biomed. Eng. 2017;64(10):2313–2320. doi: 10.1109/TBME.2017.2667579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uktveris T., Jusas V. Development of a modular board for EEG signal acquisition. Sensors. 2018;18(7) doi: 10.3390/s18072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathirana S., Asirvatham D., Johar G. 2018 2nd International Conference on BioSignal Analysis, Processing and Systems (ICBAPS) IEEE; 2018. A critical evaluation on low-cost consumer-grade electroencephalographic devices; pp. 160–165. [Google Scholar]
- 10.Hairston W.D., Whitaker K.W., Ries A.J., Vettel J.M., Bradford J.C., Kerick S.E., McDowell K. Usability of four commercially-oriented EEG systems. J. Neural Eng. 2014;11(4) doi: 10.1088/1741-2560/11/4/046018. [DOI] [PubMed] [Google Scholar]
- 11.Nijboer F., Van De Laar B., Gerritsen S., Nijholt A., Poel M. Usability of three electroencephalogram headsets for brain–computer interfaces: a within subject comparison. Interact. Comput. 2015;27(5):500–511. [Google Scholar]
- 12.Ratti E., Waninger S., Berka C., Ruffini G., Verma A. Comparison of medical and consumer wireless EEG systems for use in clinical trials. Front. Human Neurosci. 2017;11:398. doi: 10.3389/fnhum.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacko D., Vleugels J., Fransen E., Huysmans T., De Bruyne G., Van Hulle M.M., Sijbers J., Verwulgen S. Ergonomic design of an EEG headset using 3D anthropometry. Appl. Ergon. 2017;58:128–136. doi: 10.1016/j.apergo.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Verwulgen S., Lacko D., Justine H., Kustermans S., Moons S., Thys F., Zelck S., Vaes K., Huysmans T., Vleugels J. International Conference on Applied Human Factors and Ergonomics. Springer; 2018. Determining comfortable pressure ranges for wearable EEG headsets; pp. 11–19. [Google Scholar]
- 15.Han Y., Ma Y., Zhu L., Zhang Y., Li L., Zheng W., Guo J., Che Y. 2018 2nd International Conference on Applied Mathematics, Modelling and Statistics Application (AMMSA 2018) Atlantis Press; 2018. Study on mind controlled robotic arms by collecting and analyzing brain alpha waves. [Google Scholar]
- 16.Spicer R., Anglin J., Krum D.M., Liew S.-L. 2017 IEEE Virtual Reality (VR) IEEE; 2017. REINVENT: a low-cost, virtual reality brain-computer interface for severe stroke upper limb motor recovery; pp. 385–386. [Google Scholar]
- 17.Frey J. Comparison of an open-hardware electroencephalography amplifier with medical grade device in brain-computer interface applications. 2016. arXiv:1606.02438 preprint.
- 18.Rashid U., Niazi I., Signal N., Taylor D. An EEG experimental study evaluating the performance of texas instruments ADS1299. Sensors. 2018;18(11) doi: 10.3390/s18113721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfurtscheller G., Neuper C. Motor imagery and direct brain-computer communication. Proc. IEEE. 2001;89(7):1123–1134. [Google Scholar]
- 20.Malouin F., Richards C.L., Jackson P.L., Lafleur M.F., Durand A., Doyon J. The kinesthetic and visual imagery questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J. Neuro. Phys. Ther. 2007;31(1):20–29. doi: 10.1097/01.npt.0000260567.24122.64. [DOI] [PubMed] [Google Scholar]
- 21.Reaz M.B.I., Hussain M., Mohd-Yasin F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online. 2006;8(1):11. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teplan M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002;2(2):1–11. [Google Scholar]
- 23.Ramoser H., Muller-Gerking J., Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans. Rehabil. Eng. 2000;8(4):441–446. doi: 10.1109/86.895946. [DOI] [PubMed] [Google Scholar]
- 24.Rao R.P. Cambridge University Press; 2013. Brain-Computer Interfacing: An Introduction. [Google Scholar]
- 25.Blankertz B., Tomioka R., Lemm S., Kawanabe M., Muller K.-R. Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Process. Mag. 2008;25(1):41–56. [Google Scholar]
- 26.Peterson V., Wyser D., Lambercy O., Spies R., Gassert R. A penalized time-frequency band feature selection and classification procedure for improved motor intention decoding in multichannel EEG. J. Neural Eng. 2019;16(1) doi: 10.1088/1741-2552/aaf046. [DOI] [PubMed] [Google Scholar]
- 27.Peterson V., Rufiner H.L., Spies R.D. Generalized sparse discriminant analysis for event-related potential classification. Biomed. Signal Process. Control. 2017;35:70–78. [Google Scholar]
- 28.Lotte F., Guan C. Regularizing common spatial patterns to improve BCI designs: unified theory and new algorithms. IEEE Trans. Biomed. Eng. 2011;58(2):355–362. doi: 10.1109/TBME.2010.2082539. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Putz G., Scherer R., Brunner C., Leeb R., Pfurtscheller G. Better than random: a closer look on BCI results. Int. J. Bioelectromagn. 2008;10(1):52–55. [Google Scholar]
- 30.Combrisson E., Jerbi K. Exceeding chance level by chance: the caveat of theoretical chance levels in brain signal classification and statistical assessment of decoding accuracy. J. Neurosci. Methods. 2015;250:126–136. doi: 10.1016/j.jneumeth.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Haufe S., Meinecke F., Görgen K., Dähne S., Haynes J.-D., Blankertz B., Bießmann F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage. 2014;87:96–110. doi: 10.1016/j.neuroimage.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 32.Blankertz B., Sannelli C., Halder S., Hammer E.M., Kübler A., Müller K.-R., Curio G., Dickhaus T. Neurophysiological predictor of SMR-based BCI performance. NeuroImage. 2010;51(4):1303–1309. doi: 10.1016/j.neuroimage.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Jeunet C., N'Kaoua B., Subramanian S., Hachet M., Lotte F. Predicting mental imagery-based BCI performance from personality, cognitive profile and neurophysiological patterns. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0143962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn M., Cho H., Ahn S., Jun S.C. High theta and low alpha powers may be indicative of BCI-illiteracy in motor imagery. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0080886. [DOI] [PMC free article] [PubMed] [Google Scholar]