Abstract
Emissions of volatile and semivolatile organic compounds from various kinds of polymer sheets during thermal degradation process were determined by the passive flux sampling method. The polymer sheets used were commercial products made of: polyethylene (PE), ethylene-vinyl acetate (EVA), polypropylene (PP), polyacetal (POM), polycarbonate (PC)), and polymer sheet samples: poly (methyl methacrylate) (PMMA), polyethylene terephthalate (PET), polystyrene (PS) and four types of poly vinyl chloride (PVC) with different contents of additives; (bis(2-ethylhexyl)phthalate (DEHP)), and triphenylphosphine (TPP)). The emission fluxes from the polymer sheets were measured for up to 30 days stored under a constant temperature (25–75 °C). Emission of various kinds of chemicals were observed from PVC sheets including and products of polymer degradation, while emission of hydrocarbons were dominant from PE, PP and EVA, and the emission of an additive (DEP) only was observed from PMMA, PET, POM and PC. The TVOC (total VOC) emission rates from PVC sheets with DEHP and TPP (soft PVCs) were in the range of 30–120 mg m−2 h−1 at 50 °C, which were much higher than the TVOC emission rates from other polymers. The emission rates for these chemicals for the same sampling period increased dramatically as the temperature increased. The temperature-dependences of the emission rates from the soft PVC sheet for a given sampling period could be expressed using an Arrhenius-type equation, and the apparent emission activation energy EA, correlated well with the enthalpy of vaporization ΔHVAP by the following empirical equation.
We also found that the emission rates of chemicals changed with time with different changing characters, and the activation energy decreased with the progress of the polymer degradation.
Keywords: Atmospheric chemistry, Air quality, Environmental analysis, Waste treatment, Environmental chemical engineering, Environmental hazard, Semivolatile organic compounds (SVOCs) volatile organic compounds (VOCs) polymeric materials emissions passive flux sampling
Atmospheric chemistry; Air quality; Environmental analysis; Waste treatment; Environmental chemical engineering; Environmental hazard; Semivolatile organic compounds (SVOCs) volatile organic compounds (VOCs) polymeric materials emissions passive flux sampling.
1. Introduction
Polymeric materials are widely used in consumer products such as clothes, tableware, and building materials. Polymeric materials used in consumer products can emit volatile organic compounds (VOCs) and semivolatile organic compounds (SVOCs) during use [1, 2, 3, 4, 5, 6, 7]. Toxic VOCs and SVOCs in the atmosphere can cause adverse health effects (e.g., asthma and allergy [8, 9, 10], endocrine disruption [11], reproductive system diseases [12, 13, 14]. developmental toxicity [15, 16, 17]) in humans. Numerous studies of VOC and SVOC emissions from polymers have been published. Most have been focused on emissions of plasticizers and flame retardants that are added to polymers to improve the properties of the polymers [1, 2, 3, 4]. Emissions of phthalates (e.g., bis(2-ethylhexyl)phthalate (DEHP)), which are added to poly(vinyl chloride) (PVC) to act as plasticizers, have been studied more intensively than emissions of other species from polymers. DEHP has a relatively high boiling point (~385 °C), but DEHP emissions from PVC cannot be ignored, particularly at relatively high temperatures. It is suspected that DEHP can cause health effects, including endocrine disrupting effects, in humans (particularly infants) [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 26, 27, 28, 29].
Emissions of VOCs and SVOCs from polymers are still not fully understood despite the number of studies that have been performed. VOC and SVOC emissions will change during the lifetime of a polymer because of degradation of the polymer through, for example, thermal stress and UV irradiation, even under ambient conditions. Various chemicals can be produced when a polymer degrades. Polymer chains can degrade and generate monomer and oligomer fragments [18, 19, 20, 21, 22, 23]. These components have lower molecular weights and lower boiling points than the original polymer. Lower molecular weight compounds will have higher emission rates than higher molecular weight compounds. The monomers and oligomers released from some polymers are toxic. For example, styrene monomer can be released from polystyrene, which is recognized as highly toxic [24]. The rate at which a chemical added to a polymer is emitted will be affected by interactions between the additive and the polymer molecules. Some additives can chemically react with active chemicals (e.g., ozone and OH radicals) in the environment to form lower molecular-weight compounds that are more volatile than the original additives. These degradation products may be more toxic than the original additives and cause health effects in humans. Changes in VOC and SVOC emission rates during the lifetime of a polymer need to be investigated to determine whether they are caused by conditions within the polymer or external to the polymer. The degradation of a polymer will depend on various factors, including thermal stress (i.e., the temperature and changes in the temperature) and UV irradiation. In this study, we investigated the relationship between the temperature and VOC and SVOC emissions from polymers used in commercial products caused by chemical and physical changes within the polymers. Although the chemistry of polymer chain degradation reactions and the steady state emission characteristics of additives used in polymeric materials have been intensively studied, little information is available in the literature on the emission characteristics of the degradation products both from polymers and additives of degrading polymers. In this study, we conducted the emission measurements for various kinds of polymer sheets under different temperature conditions, i.e. thermal stress to induce thermal degradation.
2. Materials and methods
2.1. Polymers
Polymer samples used in this study were summarized in Table 1. The polymers were PVC, PE, polypropylene, ethylene-vinyl acetate, poly (methyl methacrylate), polyethylene terephthalate, polyacetal, and polycarbonate. They are categorized two types: polymers used in commercial products and polymer sheet samples provided by manufacturers. The samples of PE-1 to PE-3, EVA, PP, POM were polymers used as commercially-available consumer products, which were purchased less than one month before being used in the experiments, although the accurate production dates and history were unknown. The samples of PMMA, PET, PS, PVC-4 (hard PVC) were polymer sheets provided by Daiei Chemical Co. (Saitama, Japan). The soft PVC samples (PVC-1, 2, and 3) were provided by Achilles Co. (Tokyo, Japan), which contained DEHP as a plasticizer and triphenylphosphine (TPP) as a flame retardant at different mass ratios. The polymer samples were directly provided by the manufacturers without use history for products.
Table 1.
Polymer materials used in the volatile and semivolatile organic compound emission tests.
| ID | Material | Additives | Thickness and features |
|---|---|---|---|
| PE-1 | polyethylene | unknown | 0.08 mm, as a plastic bag∗1 |
| PE-2 | polyethylene | unknown | 2.0 mm, as foamed sheet∗1 |
| PE-3 | polyethylene | unknown | 1.0 mm, as foamed sheet∗1 |
| EVA | polyethylene/polyvinyl alcohol copolymer | unknown | 0.05 mm, as a raincoat∗1 |
| PP | polypropylene | unknown | 0.1 mm, stationary sheet∗1 |
| POM | polyacetal | unknown | 2.0 mm, foamed sheet∗1 |
| PC | polycarbonate | unknown | 2.0 mm, transparent sheet∗1 |
| PMMA | poly(methyl methacrylate) | unknown | 2.0 mm, transparent sheet∗2 |
| PET | polyethylene terephthalate | unknown | 2.0 mm, transparent sheet∗2, PET content > 80% |
| PS | polystyrene | carbon black < 10% | black sheet∗2, PS content > 89% |
| PVC-1 | soft PVC | DEHP 32.3% | 2.0 mm, transparent sheet sample∗3 |
| PVC-2 | soft PVC | DEHP 5.1%, TPP 5.1% | 1.0 mm, transparent sheet ∗3 |
| PVC-3 | soft PVC | DEHP 15.0%, TPP 8.5% | 0.5 mm, transparent sheet ∗3 |
| PVC-4 | hard PVC | unknown | 2.0 mm, transparent sheet ∗2 |
Polymer used as a consumer product.
Polymer samples provided by Daiei Chemical Co.
Polymer samples provided by Achilles Corporation.
2.2. The measurement of emission flux with the passive flux sampling method [25]
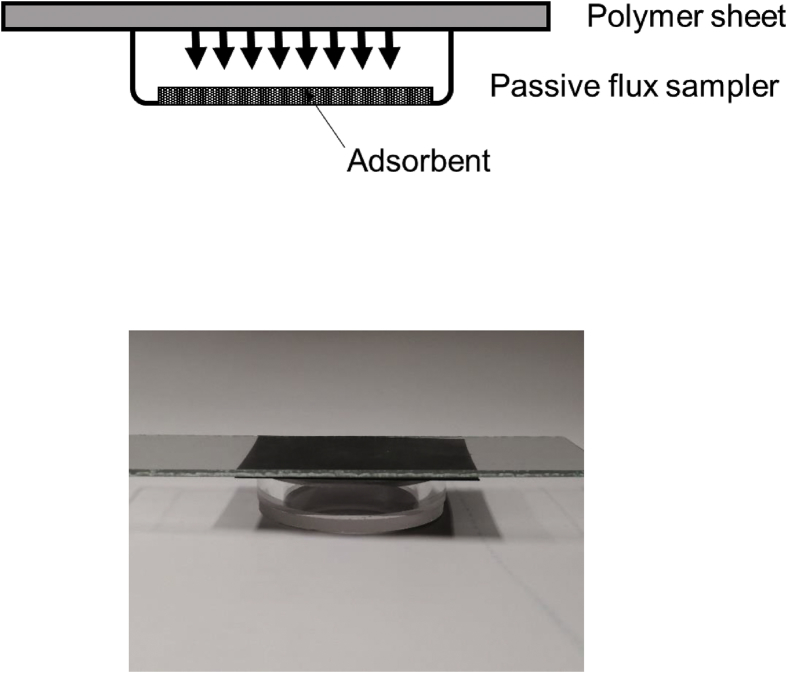
The polymer samples were used for the emission measurement without further treatment. The preparation of polymeric samples was as follows. A disk about 30 mm in diameter was cut from a polymer sheet, and set to the passive flux sampling device. The passive flux sampler is a Pyrex glass petri-dish (30 mm outer diameter, 22 mm inner diameter, 3 mm inner height) (Figure 1). A known amount of Tenax GR (GL Sciences, Tokyo, Japan) was placed in the bottom of the sampler to sorb VOCs and SVOCs emitted from a polymer disk sample, which was placed on the open top of the sampler. The sampler was placed in a temperature-controlled chamber for a specified period of between 1 and 30 d. Experiments were performed at temperatures between 25 °C (room temperature) and 75 °C. An emission measurement was made by storing a sampler for 24 h at the specified temperature (from 25 to 75 °C), then determining the VOC and SVOC concentrations in the Tenax GR (GL Sciences Co. Ltd., Tokyo, Japan) to allow the amounts of VOCs and SVOCs emitted by the polymer to be determined. The VOCs and SVOCs emitted by the polymer will have diffused through the gas phase in the sampler and been captured by the Tenax GR. The VOCs and SVOCs trapped by the Tenax GR were thermally desorbed by a desorption instrument (Turbomax ATD 650, Perkin Elmer, Waltham, Massachusetts, USA) to be analyzed by gas chromatography mass spectrometry (using an Agilent 6890 gas chromatograph coupled to an Agilent 5973 mass spectrometer; Agilent Technologies, Santa Clara, CA, USA). The analytical conditions are shown in Table 2.
Figure 1.
Schematic and image of the passive flux sampling equipment.
Table 2.
Analytical conditions for ATD/GC-MS.
| Desorption instrument | Turbomax ATD 650, Perkin Elmer |
| Primary desorption | 300 °C, 25 min, 50 mL/min, inlet split 5 mL/min |
| Secondary desorption | 5 °C → 40 °C/min → 300 °C, 10 mL/min, outlet split 9 mL/min |
| Injection ratio | 9% |
| Gas chromatograph/mass spectrometer | HP6890/HP 5973 N, Agilent Technologies |
| Column | HP-1 (60 m × 0.25 mm × 1 μm) |
| Detection | Scan mode 33–550 m/z |
| Column temperature | 40 °C (4 min) → 10 °C/min → 280 °C (25 min) |
The emission rate E was calculated, assuming that steady state had been reached, using Eq. (1).
| (1) |
In Eq. (1), Δm is the amount of a chemical collected, Δt is the sampling period, and A is the cross-sectional area of the sampler. The measurements were repeated at least three time for a condition. No contaminations from ambient air were confirmed by the emission measurement from a glass plate: no chemicals were detected.
3. Results and discussion
3.1. VOC emissions from the polymers
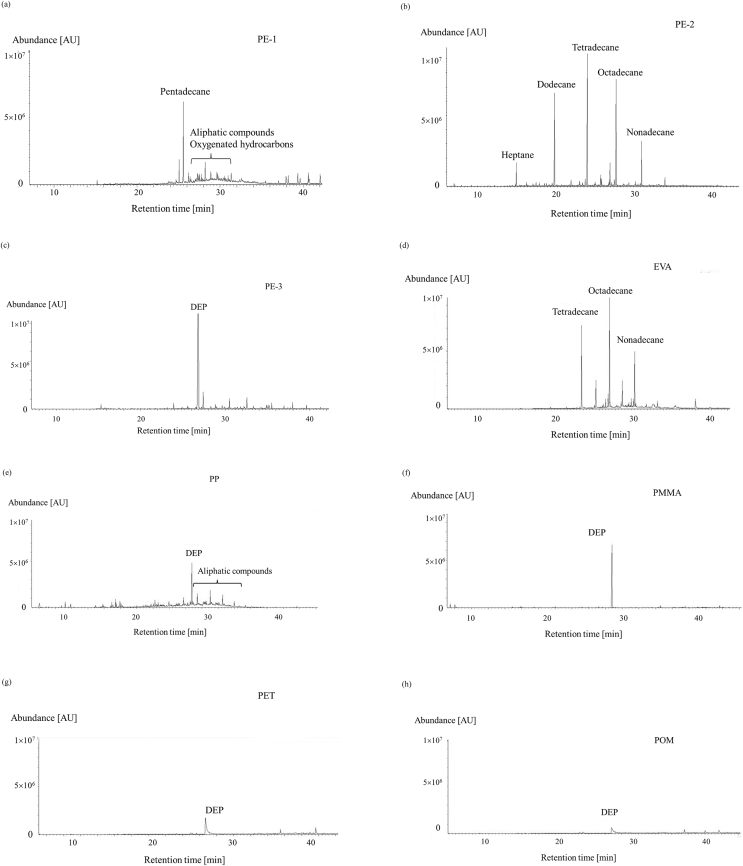
Figure 2(a)–(m) show the typical chromatograms of GC-MS for the chemicals emitted through the passive flux sampler from the polymer samples. The polymer samples were stored at 50 °C for 24 h, and the polymer samples were placed on the passive flux sampler with Tenax GR as an adsorbent at 50 °C for 24 h. The captured chemicals on the Tenax GR were desorbed by the ATD method, and injected to GC-MS, and the chromatogram was obtained.
Figure 2.
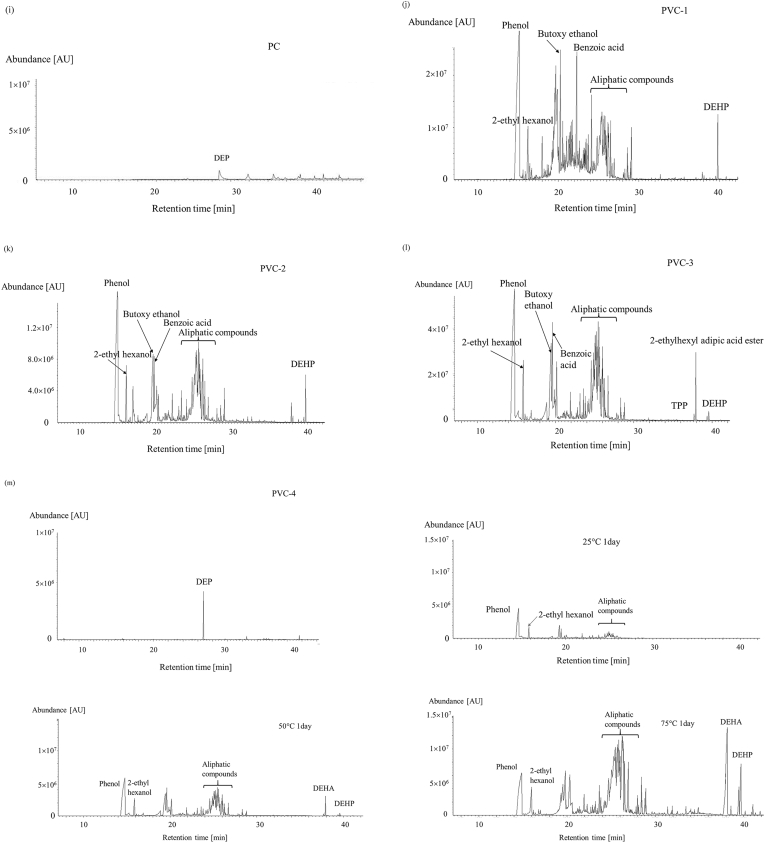
Chromatograms of the chemicals emitted by the polymer sheets during a 1 d sampling period at 50 °C. (a) polyethylene-1 (PE-1), (b) PE-2, (c) PE-3, (d) ethylene-vinyl acetate (EVA), (e) polypropylene (PP), (f) poly(methyl methacrylate) (PMMA), (g) polyethylene terephthalate (PET), (h) polyacetal (POM), (i) polycarbonate (PC), (j) poly(vinyl chloride)-1 (PVC-1), (k) PVC-2, (l) PVC-3, and (m) PVC-4. DEHP = bis(2-ethylhexyl)phthalate, DEP = diethylphosphate, TPP = triphenylphosphine.
The PE bag sample PE-1 (Figure 2(a)) emitted aliphatic compounds including pentadecane and oxygenated hydrocarbons. The PE sheet sample PE-2 (Figure 2(b)) emitted C7–C17 aliphatic hydrocarbons. These compounds would have been produced because of the polyethylene chains degrading and oxidation reactions occurring. The PE sheet sample PE-3 (Figure 2(c)) emitted aliphatic compounds (but at lower emission rates than found for samples PE-1 and PE-2) and also diethyl phosphate (DEP). The aliphatic compounds would have been produced through PE degradation, and the DEP would have been added to the PE to act as a plasticizer.
The ethylene-vinyl acetate sheet (Figure 2(d)) emitted aliphatic compounds including octadecane and tetradecane. The polypropylene sheet (intended for use as stationery) (Figure 2(e)) emitted DEP and aliphatic compounds. The poly(methyl methacrylate), polyethylene terephthalate, polyacetal, and polycarbonate samples (Figure 2(f)–(i)) emitted DEP and also some other compounds at very low emission rates.
Many more compounds were emitted by the PVC sheets than by the other polymers, as shown in Figure 2(j)–(m). The main compounds emitted by the soft PVC sheets were phenol, 2-ethylhexanol, butoxyethanol, benzoic acid, aliphatic compounds, TPP, DEHP, and 2-ethylhexyl adipic acid ester. Only the emission of DEP was observed from the hard PVC sample (PVC-4), which contained no DEHP. Aliphatic compounds such as phenol, benzoic acid, and 2-ethylhexanol may be products of the degradation of DEHP. Some aliphatic compounds would also be products of the degradation of PVC chains [18–21]. The No chemicals containing chlorine were detected, which can be expected to be produced by the polymer chain dissociations. VOCs and SVOCs emitted from the polymer sheets were clearly not only additives. The other compounds that were emitted were mainly degradation products. Thus, a variety of chemical compounds were emitted from the polymer sheet including additives and their degradation products, and hydrocarbons originated from polymer chains.
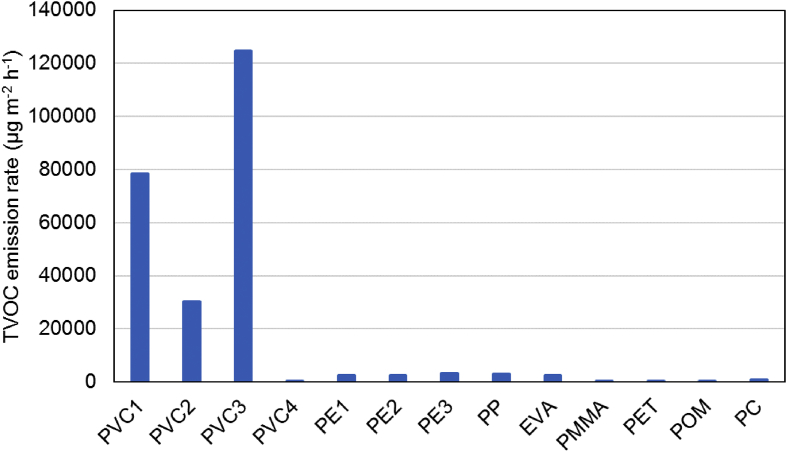
The total VOC (TVOC) emission rates for a 24 h sampling period at 50 °C are shown in Figure 3. The TVOC emission rate was defined as the sum of the amounts found of the VOCs that were analyzed normalized to the toluene emission rate. The TVOC emission rates for samples PVC-1, PVC-2, and PVC-3 were 30,000–120,000 μg m−2 h−1 (= 30–120 mg m−2 h−1), which were much higher than the TVOC emission rates for the other polymers. The highest TVOC emission rate was observed for PVC-3 among three types of soft PVC samples. Although the additive content was the highest for PVC-1 with 32.3% DEHP, and the emission rate of DEHP was the highest among three PVC samples, the emission rate of TVOC was lower than that of PVC-3. This is because the degradation products of PVC and phenol would be migrated through the polymers more quickly in PVC-3 due to the lower thickness (0.5 mm) than PVC-1 (2.0 mm). The lowest emission rate for PVC-2 was because of the lower contents of DEHP leads to the higher hardness of polymer structure and reduce the diffusion rates of chemicals through the sheet. Note DEHP is a plasticize and the higher content of DEHP would make the polymer structure more flexible. Later, we will focus on emissions from PVC-3, which contained 15.0% DEHP and 8.5% TPP and had the highest TVOC emission rate (120 mg m−2 h−1).
Figure 3.
Total volatile organic compound (TVOC) emission rates for the polymer sheets kept at 50 °C for 1 d.
3.2. Temporal changes in the emission rates for sample PVC-3
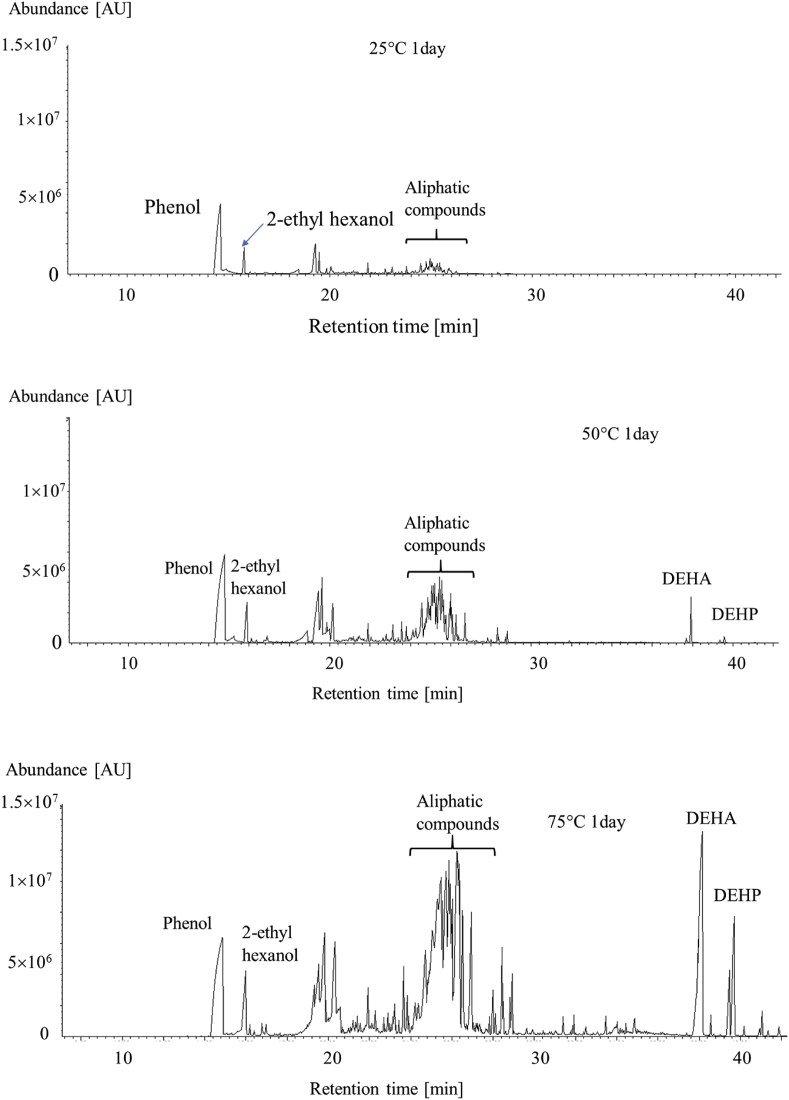
Chromatograms of the compounds emitted by sample PVC-3 kept for 1 d at between 25 and 75 °C are shown in Figure 4. Each PVC-3 sample was stored at the desired temperature for a specified time and then VOC and SVOC emissions over a 24 h period at the same temperature were measured. Only trace amounts of phenol, 2-ethylyhexanol, and hydrocarbons (e.g., octadecene) were emitted by PVC-3 at 25 °C. The emission rates of these compounds increased dramatically as the temperature increased, and TPP and DEHP were found to be emitted only at the higher temperatures.
Figure 4.
Chromatograms of the chemicals emitted by sample PVC-3 kept at 25, 50, and 75 °C for 1 d (DEHA = bis (2-ethylhexyl) adipate, DEHP = bis (2-ethylhexyl) phthalate).
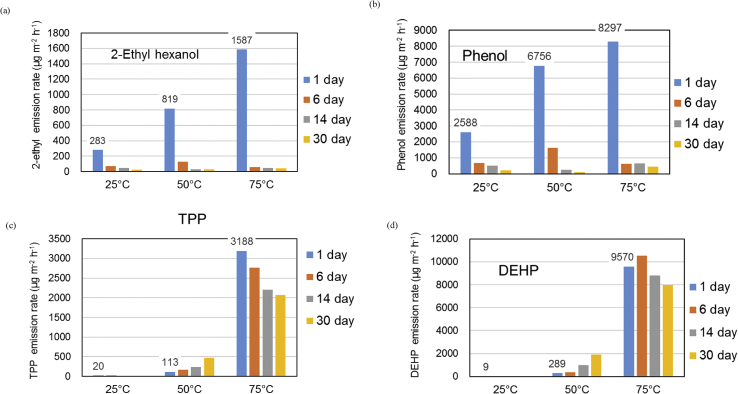
Temporal changes in the 2-ethylhexanol, phenol, TPP, and DEHP emission rates at the different test temperatures are shown in Figure 5(a)–(d). Two contrasting patterns of temporal emission rate changes were found. The 2-ethylhexanol and phenol emission rates decreased strongly after 1 d. The TPP and DEHP emission rates increased with time when the sample was kept at 50 °C but gradually decreased with time when the sample was kept at 75 °C.
Figure 5.
Emission rates for (a) 2-ethylhexanol, (b) phenol, (c) triphenylphosphine (TPP), and (d) bis(2-ethylhexyl)phthalate (DEHP) for sample PVC-3 kept at different temperatures for different periods of time.
Emissions of these compounds could be explained as described next. Phenol was presumably used as a solvent for additives such as DEHP and TPP, but would have remained in the prepared PVC sheet. Phenol would have been emitted gradually from the sheet because of its relatively low boiling point (181.7 °C). Phenol will not have been produced through degradation of the PVC, so the phenol emission rate would have decreased over time as the amount of phenol remaining in the PVC decreased. In contrast, 2-ethylhexanol (boiling point ~180 °C) would have been a product of the degradation of DEHP in the PVC, so would have been emitted for a long time (but not necessarily at a constant emission rate). The DEHP degradation rate would have been relatively low, but the amount of DEHP remaining in the PVC would have decreased over time at the experimental time scale, so the DEHP emission rate would have decreased over time. Emissions of SVOCs such as DEHP (boiling point 385 °C) and TPP (boiling point 370 °C) were not detected at 25 °C because of the high boiling points of these compounds. However, DEHP and TPP emissions were detected at higher temperatures. The SVOC emission rates increased over time when the PVC was kept at 50 °C, presumably because the compounds were relatively mobile within the polymer sheet at 50 °C and were able to be transported from the internal parts of the polymer sheet to the surface. The TPP and DEHP emission rates at 50 °C therefore increased over time. The initial DEHP and TPP emission rates were much higher (about 40 times higher for DEHP and about 30 times higher for TPP) at 75 °C than at 50 °C, but the TPP and DEHP migration rates within the PVC sheet may not have been high enough for the surface to be replenished as TPP and DEHP volatilized from the surface. The TPP and DEHP emission rates at 75 °C therefore decreased over time. The DEHP emission rate increased for the first 6 d and then gradually decreased. Sufficient DEHP must have diffused from within the PVC sheet to the surface to replenish volatilized DEHP for the first 6 d, but after 6 d the diffusion rate within the sheet must have become the dominant factor controlling the emission rate, and the DEHP emission rate decreased.
3.3. Correlation between apparent emission activation energy and enthalpy of vaporization
The temperature-dependence of the emission rate r could be described using the Arrhenius equation shown in Eq. (2).
| (2) |
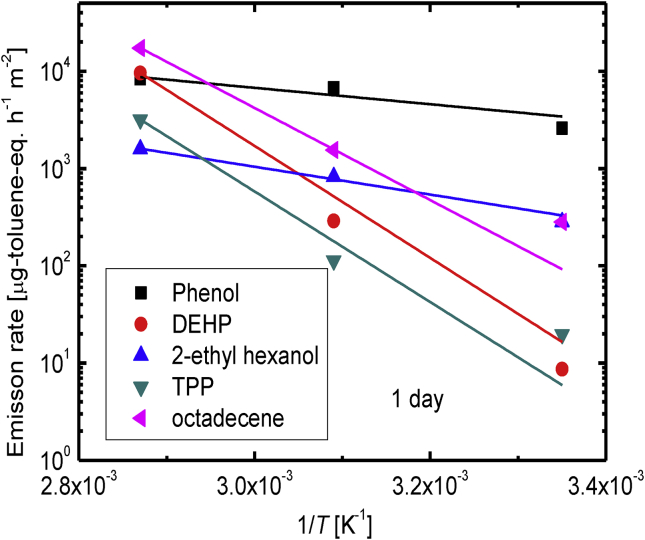
In Eq. (2), r0 is the pre-exponential constant (independent of the temperature), EA is the activation energy for emission, R is the universal gas constant, and T is the absolute temperature. The apparent emission rates for phenol, DEHP, TPP, 2-ethylhexanol, and octadecene kept for 1 d at various temperature are plotted against the reciprocal temperature in Figure 6. A linear relationship was found for each component, indicating that the temperature-dependences of the emission rates could be expressed using an Arrhenius-type equation. The apparent activation energies for emissions of the compounds of interest are shown in Table 3. The rate at which DEHP is emitted from PVC has been found to be temperature-dependent in several previous studies [[26], [27], [28], [29]]. Ekelund et al. [26] found that the DEHP emission activation energy was 89 kJ mol−1 at 100 °C, at which temperature the emission rate was controlled by DEHP volatilization from the surface. This activation energy was similar to the enthalpy of vaporization for DEHP on a glass plate (98 ± 2 kJ mol−1). Clausen et al. [27] studied the temperature-dependence of the concentration y0, defined as the DEHP concentration immediately adjacent to the PVC surface, and found that y0 was almost the same as expected from the vapor pressure of DEHP. The temperature-dependence of the vapor pressure of DEHP is described by an Arrhenius-type equation, and the vaporization enthalpy of DEHP ΔHvap was found to be 95 ± 1 kJ mol−1. Liang and Xu [28] found enthalpies of phase change between PVC and air, ΔHpa, of 123–401 kJ mol−1 from the temperature-dependences of y0 for various compounds. In this study, the apparent DEHP emission activation energy for the first 24 h was 110 kJ mol−1, which was similar to the activation energies found in previous studies.
Figure 6.
Arrhenius plots for the emission rates of phenol, bis(2-ethylhexyl)phthalate (DEHP), 2-ethylhexanol, triphenylphosphine (TPP), and octadecene emitted from sample PVC-3 for 1 d at different temperatures.
Table 3.
Emission activation energies.
| compound | Activation energy [kJ/mol] | Enthalpy of evaporation∗ [kJ/mol] |
|---|---|---|
| DEHP | 110.1 | 102.5 |
| TPP | 86.7 | 81.4 |
| Octadecane | 90.6 | 90 |
| Phenol | 16.1 | 58.8 |
| 2-Ethylhexanol | 27.0 | 65.4 |
DEHP = bis (2-ethylhexyl) phthalate, TPP = triphenylphosphine.
Data from reference [34].
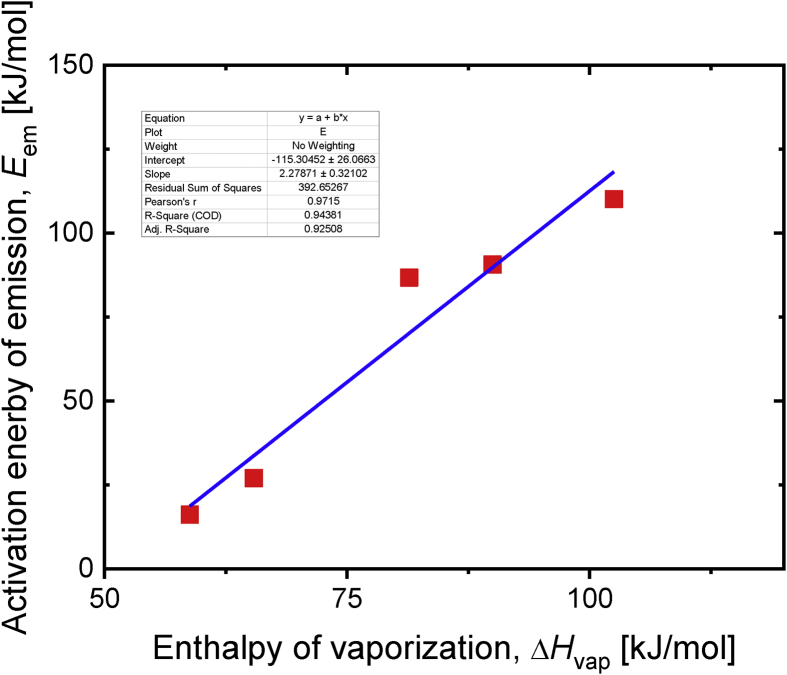
The apparent activation energies are plotted against the enthalpies of vaporization in Figure 7. The apparent activation energy and enthalpy of vaporization correlated well, and the equation describing the relationship is shown in Eq. (3).
| (3) |
Figure 7.
Apparent emission activation energy plotted against the enthalpy of vaporization.
This suggests that the VOC and SVOC emission rates are controlled by the vaporization process, i.e., transfer from the surface to the atmosphere immediately adjacent to the polymer sheet. The temperature-dependence of the emission rate of a compound with a known enthalpy of vaporization could therefore be estimated using Eq. (3).
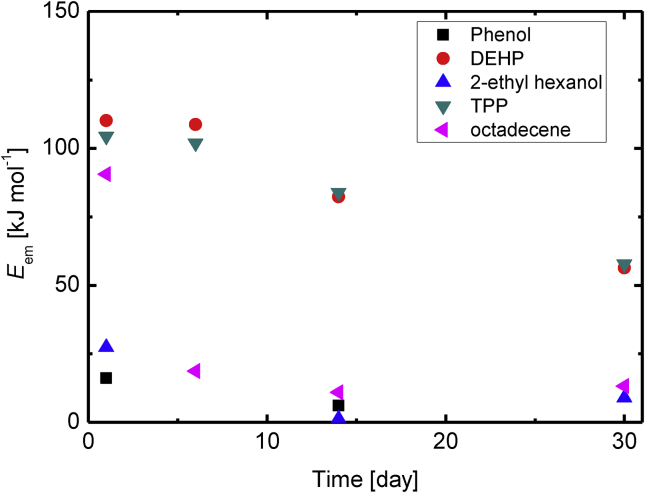
Temporal changes in the apparent activation energy (determined using the Arrhenius equation) are shown in Figure 8. The apparent activation energy gradually decreased over time for all the compounds that were studied. This would have been because diffusion of the compounds within the PVC sheet would have become an important factor controlling the emission rate. The order in which the activation energies decreased was the same at any time point. Ekelund et al. [26] found an emission activation energy for DEHP during degradation of 55 kJ mol−1, which is similar to the activation energy found after 30 d in this study. The observed values of the activation energy in this study were in the same range of the literature data. In addition, we have obtained the activation energy of the chemicals generated by the degradation process of polymers. We also found that the activation energy of these chemicals changes with time, which has not been reported elsewhere.
Figure 8.
Temporal changes in the apparent emission activation energies for phenol, bis(2-ethylhexyl)phthalate (DEHP), 2-ethylhexanol, triphenylphosphine (TPP), and octadecene.
The steady-state emission rate in a passive flux sample can be expressed as shown in Eq. (4) [15].
| (4) |
In Eq. (4), DG is the diffusion coefficient in the gas phase, L is the diffusion length, A is the cross-sectional area of the sampler, y* is the concentration in the gas phase in the vicinity of the polymer sheet, and y0 is the concentration in the gas phase in the vicinity of the sorbent on the opposite side of the sampler [[30], [31], [32]]. The sorbent strongly sorbs the compound of interest, so concentration y0 will be negligible compared to concentration y*. The emission equilibrium at the surface of the PVC sheet can be expressed as the Henry's-law-type equation
| (5) |
where KH is the Henry's law constant and C* is the concentration at the PVC sheet surface. The Henry's law constant for emission can be expressed using an exponential temperature function as
| (6) |
where KH0 is a pre-exponential factor that is not strongly temperature-dependent and ΔHem is the enthalpy for emission. The emission rate can therefore be expressed as
| (7) |
By comparing Eqs. (2) and (7) it can be seen that the apparent emission activation energy EA is equivalent to the enthalpy change for emission ΔHem. Emission of a VOC from a polymer sheet surface is similar to vaporization, so it is reasonable that the enthalpy of vaporization ΔHvap is related to the enthalpy change for emission ΔHem [[26], [27], [28]]. This explains the correlation between the apparent activation energy EA and the enthalpy of vaporization ΔHvap shown in Eq. (3). This can be extended to emissions of other VOCs from polymers. Eq. (3) will be different for different VOCs and polymers because it depends on interactions between the VOC and polymer.
4. Conclusions
Emissions of VOCs from various polymers used in commercial products were assessed at various temperatures, and temporal changes in the emission rates were investigated. Emissions of variety of chemicals, including aliphatic and aromatic hydrocarbons, oxygenated hydrocarbons, additives and their dissociation products were observed from polymers. The TVOC emission rates for soft PVC sheets containing TPP and DEHP were 30–120 mg m−2 h−1 at 50 °C after kept under 50 °C for 1 d, which were much higher than the TVOC emission rates from other polymers. The main chemicals emitted from the PVC sheets were phenol, 2-ethylhexanol, octadecene, TPP, and DEHP, and the emission rates were strongly dependent on the storage time and temperature. The apparent emission activation energy was well correlated with the enthalpy of vaporization by the following empirical equation.
The time dependences of the emission rates depended on the type of chemicals, namely, additives and degradation products.
Declarations
Author contribution statement
Akihiro Yamasaki, Miyuki Noguchi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Japanese Ministry of Economy, Trade and Industry under the program “Survey for building and strengthening basic scientific knowledge to develop and improve risk evaluation methods for chemical substances”.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Gareth Thomas, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Yu C., Crumpt D. A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ. 1998;33:357–374. [Google Scholar]
- 2.Liu Z., Yea W., Little J.C. Predicting emissions of volatile and semivolatile organic compounds from building materials: a review. Build. Environ. 2013;64:7–25. [Google Scholar]
- 3.Weschler C.J., Carslaw N. Indoor chemistry. Environ. Sci. Technol. 2018;52:2419–2428. doi: 10.1021/acs.est.7b06387. [DOI] [PubMed] [Google Scholar]
- 4.Lucattini L., Poma G., Covaci A., de Boer J., Lamoree M.H., Leonards P.E.G. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere. 2018;201:466–482. doi: 10.1016/j.chemosphere.2018.02.161. [DOI] [PubMed] [Google Scholar]
- 5.Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Jo S.-H., Lee M.-H., Kim K.-H., Kumar P. Characterization and flux assessment of airborne phthalates released from polyvinyl chloride consumer goods. Environ. Res. 2018;165:81–90. doi: 10.1016/j.envres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren T., Persson L., Andersson P.L., Haglund P. A generic emission model to predict release of organic substances from materials in consumer goods. Sci. Total Environ. 2012;437:306–314. doi: 10.1016/j.scitotenv.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Bornehag C.G., Sundell J., Weschler C.J., Sigsgaard T., Lundgren B., Hasselgren M., Hägerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ. Health Perspect. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glue C., Platzer M.H., Larsen S.T., Nielsen G.D., Skov P.S., Poulsen L.K. Phthalates potentiate the response of allergic effector cells. Basic Clin. Pharmacol. Toxicol. 2005;96:140–142. doi: 10.1111/j.1742-7843.2005.pto960208.x. [DOI] [PubMed] [Google Scholar]
- 10.Bekö, Callesen M., Weschler C.J., Toftum J., Langer S., Sigsgaard T., Høst A., Jensen T.Kold, Clausen G. Phthalate exposure through different pathways and allergic sensitization in preschool children with asthma, allergic rhinoconjunctivitis and atopic dermatitis. Environ. Res. 2015;137:432–439. doi: 10.1016/j.envres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Rudel R.A., Perovich L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009;43:170–181. doi: 10.1016/j.atmosenv.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren A., Qiu X., Jin L., Ma J., Li Z., Zhang L., Zhu H., Finnell R.H., Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Nat. Acad. Sci. USA. 2011;108:12770–12775. doi: 10.1073/pnas.1105209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., Seed J., Shea K., Tabacova S., Tyl R., Williams P., Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmentaltoxicity of di-n-butyl phthalate. Reprod. Toxicol. 2002;16:489–527. doi: 10.1016/s0890-6238(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 14.Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., Seed J., Shea K., Tabacova S., Tyl R., Williams P., Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 15.Ejaredar M., Nyanza E.C., Eycke K.T., Dewey D. Phthalate exposure and children’s neurodevelopment: a systematic review. Environ. Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar F.W., Castorina R., Maddalena R.L., Nishioka M.G., McKone T.E., Bradman A. Phthalate exposure and risk assessment in California child care facilities. Environ. Sci. Technol. 2014;48:7593–7601. doi: 10.1021/es501189t. [DOI] [PubMed] [Google Scholar]
- 17.Chiu Y.-H., Bellavia A., James-Todd T., Correia K.F., Valeri L., Messerlian C., Ford J.B., Mínguez-Alarcón L., Calafat A.M., Hauser R., Williams P.L. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: a comparison of three statistical approaches. Environ. Int. 2018;113:231–239. doi: 10.1016/j.envint.2018.02.005. for the EARTH Study Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankett J.M., Collin W.R., Chen Z. Molecular structural changes of plasticized PVC after UV light exposure. J. Phys. Chem. B. 2013;117:16336–16344. doi: 10.1021/jp409254y. [DOI] [PubMed] [Google Scholar]
- 19.Yu J., Sun L., Ma C., Qiao Y., Yao H. Thermal degradation of PVC: a review. Waste Manag. 2016;48:300–314. doi: 10.1016/j.wasman.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Royaux A., Fabre-Francke I., Balcar N., Barabant G., Bollard C., Lavedrine B., Cantin S. Aging of plasticized polyvinyl chloride in heritage collections: the impact of conditioning and cleaning treatments. Polym. Degrad. Stabil. 2017;137:109–121. [Google Scholar]
- 21.Chino S., Kato S., Seo J., Ataka Y. Study on emission of decomposed chemicals of esters contained in PVC flooring and adhesive. Build. Environ. 2009;44:1337–1342. [Google Scholar]
- 22.Ekelund M., Azhdar B., Hedenqvist M.S., Gedde U.W. Long-term performance of poly (vinyl chloride) cables, Part 2: Migration. Polym. Degrad. Stabil. 2008;93:1704–1710. [Google Scholar]
- 23.Pei J., Yin Y., Cao J., Sun Y., Liu J., Zhang Y. Time dependence of characteristic parameter for semi-volatile organic compounds (SVOCs) emitted from indoor materials. Build. Environ. 2017;125:339–347. [Google Scholar]
- 24.US EPA https://www.epa.gov/sites/production/files/2016-09/documents/styrene.pdf, accessed August 2019.
- 25.Noguchi M., Yamasaki A. Passive flux sampler measurements of emission rates of phthalates from poly (vinyl chloride) sheets. Build. Environ. 2016;100:197–202. [Google Scholar]
- 26.Ekelund M., Azhdar B., Gedde U.W. Evaporative loss kinetics of di (2-ethylhexyl) phthalate (DEHP) from pristine DEHP and plasticized PVC. Polym. Degrad. Stabil. 2010;95:1789–1793. [Google Scholar]
- 27.Clausen P.A., Liu Z., Kofoed-Sørensen V., Little J., Wolkoff P. Influence of temperature on the emission of di-(2-ethylhexyl) phthalate (DEHP) from PVC flooring in the emission cell FLEC. Environ. Sci. Technol. 2012;46:909–915. doi: 10.1021/es2035625. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y., Xu Y. Emission of phthalates and phthalate alternatives from vinyl flooring and crib mattress covers: the influence of temperature. Environ. Sci. Technol. 2014;48:14228–14237. doi: 10.1021/es504801x. [DOI] [PubMed] [Google Scholar]
- 29.Bi C., Liang Y., Xu Y. Fate and transport of phthalates in indoor environments and the influence of temperature: a case study in a test house. Environ. Sci. Technol. 2015;49:9674–9681. doi: 10.1021/acs.est.5b02787. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y., Hubal E.C., Clausen P., Little J. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: a mechanistic analysis. Environ. Sci. Technol. 2009;43:2374–2380. doi: 10.1021/es801354f. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y., Xu Y. Improved method for measuring and characterizing phthalate emissions from building materials and its application to exposure assessment. Environ. Sci. Technol. 2014;48:4475–4484. doi: 10.1021/es405809r. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y., Xie M., Cox S.S., Marr L.C., Little J.C. A simple method to measure the gas-phase SVOC concentration adjacent to a material surface. Indoor Air. 2016;26:903–912. doi: 10.1111/ina.12270. [DOI] [PubMed] [Google Scholar]
- 34.Speight J.D. seventeenth ed. McGraw-Hill; New York: 2016. Lange's Handbook of Chemistry. [Google Scholar]