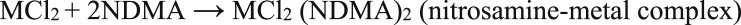Abstract
The use of ozone, chloramine and chlorine dioxide for water treatment results in the formation N-nitrosamines in the treated water. These groups of chemicals and other nitrogen-containing compounds have been described as disinfection by-products (DBPs) which are known for their toxicity. Nitrosamines are a potential source of nitric oxide (NO) which can bind with metals present in the sample matrix leading to formation of metal – nitrosyl complexes and dissolved metals have the potential to increase the total nitrosamines in water. This phenomenon has not received the desired attention and determination of metal-nitrosyl complexes lack standard analytical technique. Chromatography linked to various detectors is the commonest of the techniques for nitrosamine analysis but it is beset with reduced sensitivity as a result of inappropriate choice of the column. Incidentally, chromatographic techniques have not been really adapted for the analysis of metal-nitrosyl complexes. Therefore, there is need for the survey of existing techniques vis-à-vis metal-nitrosamine analysis and to suggest possible areas for method optimization.
Keywords: Analytical chemistry, Electrochemistry, Metals, Nitric oxide, n-nitrosodimethylamine, Column, Carcinogenic, Chromatography
Analytical chemistry; Electrochemistry; Metals; Nitric oxide; n-nitrosodimethylamine; column; carcinogenic; chromatography
1. Introduction
The occurrence of nitrosamines (NAms) as emerging disinfection by-products (EDBPs) in drinking water has been ascribed to the use of chloramine, ozone and chlorine dioxide as chemical oxidants for water treatment [1, 2]. The formation of these contaminants stems from the interaction between the residual disinfectants and the organic matters present in the water [3, 4, 5]. The ubiquity of NAms has received the attention of various authors recently [6, 7, 8]. Jurado-Sánchez et al. [9] reported 18 ng/L of total nitrosamines in drinking water treatment plant in Spain. The presence of these carcinogens has also been reported in various water sources from other parts of the world (Table 1).
Table 1.
Nitrosamine contaminants in water.
The volatile (N-nitrosodimethylamine; NDMA, N-nitrosodiethylamine; NDEA and non-volatile, (N-nitrosoproline; NPRO; N-nitrososarcosine (NSAR) nitrosamines and nitrogen containing disinfection by-products (DBPs) are known to be more toxic than the regulated DBPs [3, 15, 16]. Nitrosamines such as N-nitrosodimethylamine (NDMA) are carcinogenic in rat liver [10, 17]. The presence of nitrosamines in wastewater, source water and drinking water is an emerging issue and of health concern [3, 18]. The United State Environmental Protection Agency (US EPA) added nitrosamines to unregulated organic pollutants and as “probably carcinogenic” (Table 2) [10, 14, 19, 20]. Different permissible levels have emerged for nitrosamines in different countries. For example, 10 ng/L was the maximum permissible limit in California [21] and in Germany. Whereas in Netherland 12 ng/L was the contaminant limits for NDMA in drinking water while Ontario, accepts the maximum limit of 9 ng/L for NDMA [13, 16].
Table 2.
| Nitrosamines | Formula | US EPA MCL (ng/L) | Log Kow | Water Solubility mg∖L | US EPA Cancer classification. | Risk Level (ng/L) × 10−6 |
|---|---|---|---|---|---|---|
| NDMA | C2H6N2O | 7 | - 0.57 | 1,000,000 | B2 | 3.0 |
| NMEA | C3H8N2O | 20 | 0.04 | 300,00 | B2 | 1.5 |
| NDEA | C4H10N2O | 2 | 0.48 | 106,000 | B2 | 1.0 |
| NDPA | C6H14N2O | 50 | 1.36 | 13,000 | B2 | 5.0 |
| NDBA | C8H18N2O | 60 | 2.63 | 1,270 | B2 | 3.0 |
| NPYR | C4H8N2O | 200 | - 0.19 | 1,000,000 | 2B (IARC) | 15.0 |
| NPIP | C5H10N2O | na | 0.36 | 76,480 | B2 | 3.5 |
| NDPHA | C12H10N2O | 70,000 | 3.13 | 35 | B2 | na |
| NMOR | C4H8N2O2 | na | - 0.44 | 861,527.5 | 2B (IARC) | na |
IARC: International Agency for Research on Cancer; NA: Not available, MCL: Maximum contaminant level.
The nitrosyl group present on nitrosamines behave as electron donor (NO+), electron acceptor (NO-) as well as radical (NO*) resulting into formation of metal complexes.
The reactivity of NO radicals ensures interaction with metals present in the sample forming nitrosamine-metal complex [24, 25, 26, 27]. The analytical significance of this interaction has not been properly investigated. A survey of methodologies available for the analysis of nitrosamines in water shows that the chromatographic techniques are in the forefront of others (Table 3). The widely used method specifically is gas chromatography linked to mass spectrometric detector with carefully selected column.
Table 3.
Chromatographic methods for determination of nitrosamines.
| Matrix | Compounds | Sample Preparation | Analytical Instruments | Analytical Column | Detector | LOD | LOQ | RSD (%) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| River Water | NDMA, NDEA, NDPA, NMEA, NDBA, NDPHA NDMA–d6 |
SPE (CCC) Restek (cat. #26032). USEPA Method 521 | GC Agilent 6890 N |
Rtx 5SiL MS (30 m × 0.25 mm ID × 1.0 μm). | Agilent MS 5973. |
2.5–40.6 ng/L | 7.9–127.7 ng/L | <15 | 72.3–98.6 | [54] |
| Potable water | NDMA-d6NDMA, NDPA, NPIP, NMEA, NDEA, NPYR, NDBA, | SPE (CCC) Restek (cat. #26032). USEPA Method | GC (TQ8030 Shimadzu) | 35 m × 0.25 mm x 0.5 μm. Restek Rxi 5Sil MS | MS (TQ8030 (Shimadzu) | 1.2–9.0 ng/L | NR | <20 | 70–130 | [55] |
| Tap & River water | DMA, EMA, DEA, DPA, TMA, DMAI. DMAPI. | No preconcentration steps. Filtered through (0.22 mm) |
UFLC (Shimadzu LC-20ADXR) | ThecolumnwasaPhenomenex Polar-RPC-18 column (150× 2.0 mmI.D,4 mm particlesize, | TMS (A 4000Q, AB SCIEX, Concord) | 0.02–1 μg/L | NR | <13.8 | 88.5–116 | [56] |
| WTP | NDMA, NMEA, NPyr, NDEA, NPip, NMor, NDPA, NDBA, NDPhA, NNN, NAT, NAB, NNK, NNAL | SPE Oasis HLB cartridge/SPE absorbents LiChrolut EN (vinyl/divinyl polymer) and Ambersorb 572 (activated carbon) Sigma-Aldrich. |
HPLC (Agilent 1100) |
Kinetex C8 column (100 × 3.0 mm i.d., 2.6 μm; Phenomenex) | MS/MS | 0.01–2.7 ng/L | 0.03–8.8 ng/L | NR | 53–93 | [21] |
| Drinking Water | d6-NDMA, NDMA, NMEA, NDEA, NDPA, NPYR NMOR, NPIP, NDBA, NDPhA | SPE (CCC) (CNW Technologies) USEPA Method 521 | GC Agilent 6890 N |
DB-35MS was used (35% diphenyl/65% dimethyl polysiloxane) | Agilent QMSS 5975 |
NR | 1.5–4.9 ng/L | NR | 65–122 | [18] |
| Drinking Water | NMEA, NDEA, NDMA, NDPA, NPyr, NPip, NDBA, NDMA-d6 | SPME (57330-U, Supelco) | GC Agilent 7890 | Agilent DB-624 column (30 m_ 0.25 mm I. D. 1.4 mm) |
Agilent MS 5975. | 0.12–0.79 ng/L), | 0.1–0.8 ng/L | <10 | 77–114 | [57] |
| Finished/tap/source water | NDMA, NDEA, NMOR, NPIP, NDBA |
SPE (CCC) (Auto Trace 280, Dionex, Corp) CNW Technologie) EPA 521 method | UPLC (Agilent 1100) | A C8 (2) Capillary column (150 × 0.32 mm i.d., 5 ím) |
MS/MS (API 4000 QTrap Applied Biosystems/MDS Sciex) | 0.1–1 ng/L | NR | 1.4–5.1 | 64–116 | [16] |
| Wastewater | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, THEOB, XAN, THEOP | SPE Strata-X polymeric (100 mg/6 mL) Phenomenex/SPE (CCC) (200 mg/6 mL), Supelco | GC | 30 m × 0.25 mm i.d. × 0.25 _m SLB-IL111 (Supelco). | MS/MS A Brucker 320-MS. |
<30.6 ng/L | <48.6 ng/L | <10 | NR | [58] |
| Drinking/MilliQ water | NDMA Nmor Npyr NMEA NDEA Npip NDPA NDBA NDPhA | SPE | UPLC (Acquity HSS T3) | 50 mm × 2.1 mm, × 1.8 μm particle size. | MS Waters (Micromass Quattro Premier XE) |
<0.9 ng/L | NR | NR | 70–90 | [2] |
| Potable Water |
NDMA, NDMA-d6 Toluene-d8 |
SPE (Sep-Pak AC-2 cartridge) | GC Varian 450 |
30 m × 0.25 mm × 1.0 lm | MS Varian 300 |
<2 ng/L | NR | <11.1 | 70–120 | [59] |
| Drinking Water | NMEA, NDPA, NDEA, NDBA, NMor, NPip, NPyr. |
LLE (with dichloromethane) | GC Agilent 6890 | 40 × 0.18 mm ID and 1 μm (RTX-VMS) | MS Agilent 5973 |
0.4–2.0 ng/L | 10 ng/L | <19 | 95 | [60] |
| Sewage | NDMA, NMOR, NPYR | SPE | HPLC Agilent HP1100 /GC QP2010 Shimadzu |
NR | MS/MS (Acquity TQD) | NR | 5.0–25 ng/L /1.0 ng/L |
NR | NR | [61] |
| Bio solid | NDMA, NMEA, NDPA NDBA NPYR, NPIP, NDPhA | LLE (2 mL dichloromethane per g of biosolids) | HPLC (Shimadzu) | 130 Å, 3.5 μm, 4.6 × 150 mm. | MS/MS API 4000 (Applied Biosystems) |
0.06–5.7 ng/g | NR | NR | 90–126 | [62] |
| Meat Products (Pork Sausage) | NDMA, NMEA, NDEA, NPYR, NDPA, NPIP, NDBA | D-l-SPE | GC Varian 450 | 30 m × 0.25 mm × 1.0 l, DB5-MS. | MS (Varian 2200 | 0.01–0.12 (ng/g) | 0.03–0.36 (ng/g) | <10 | 74–105 | [51] |
| Deionized Water Water |
NDMA, DMTA | SPME (PDMS/DVB, 65 μm, Supelco) | GC Thermo TRACE. | 30 m × 0.25 mm 0.25 μm, (ZB-5ms Zebron, Phenomenex). |
MS (Thermo TRACE DSQ II). | 0.3–0.6 μM |
2.5–16 Μm | 0.2–0.8 | NR | [63] |
| Cosmetic | NDMA, NMEA, NDEA, NPYR, NDPA, NPIP, NDBA | HS-SPME (splitless liner 0.75 mm i.d. Agilent) | GC Agilent 7890B |
30 mm × 0.25 μm I.D × 0.25 μm, DB-WAX Agilent (Palo Alto) | MS Agilent 7693 |
0.46–36.54 ng/g | NR | <20 | 79 | [8] |
N-nitrosonornicotine NNN, N-nitrosoanatabine NAT N-nitrosoanabasine NAB, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol NNAL, Caffeine CAF; Theophylline THEOP, 1,7-dimethylxanthine XAN, Theobromine THEOB. Coconut charcoal cartridge (CCC). Not Registered (NR), Solid Phase Extraction (SPE), Secondary (2o), Effluent (E), Solid-phase micro extraction (SPME), high-performance liquid chromatography (HPLC), ultra-fast liquid chromatography–tandem mass spectrometry(UFLC-MS/MS), Trap mass spectrometer (TMS), Dimethylamine (DMA),Ethylmethylamine (EMA), Diethylamine (DEA), Dipropylamine (DPA), Trimethylamine (TMA), 3-(Dimethylaminomethyl)indole (DMAI), 4-Dimethylami- noantipyrine (DMAP), Electrospray Ionization (ESI), Tandem Quad (TQD) MS Technology, Water treatment plant (WTP). Qudrupole mass selective spectrometer (QMSS), (NDMA: N-nitrosodimethylamine, International Sorbent Technology (IST), Temperature: temp. Dispersive micro solid-phase extraction (D-l-SPE), Deuterated toluene (toluene-d8), Liquid-liquid extraction (LLE), Head space solid-phase micro extraction (HS-SPME).
Till date, there is a paucity of data on metal-nitrosamine complexes concentration in environmental water samples. Therefore, this review focuses on the reactions of NO functional group in NAms with certain transition metals and the applications of chromatographic techniques to the analysis of NAms and their metal complexes in the environmental waters.
2. Reactions of nitrosamines with metals
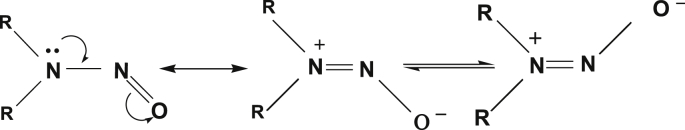
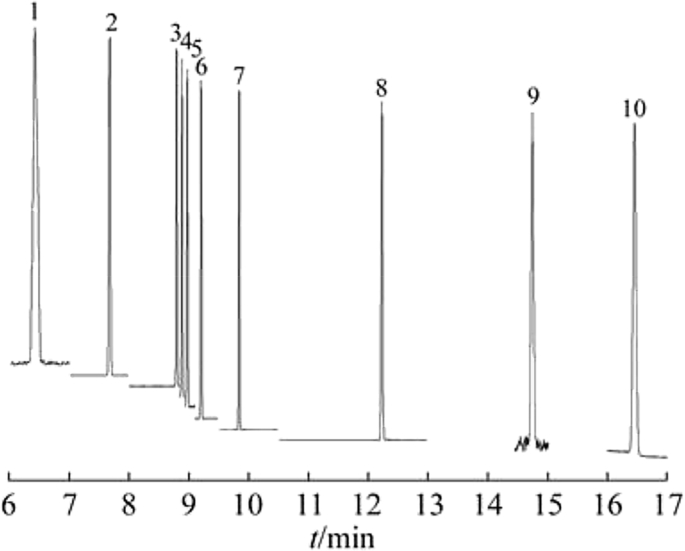
The general, resonance and tautomeric structures of N-nitrosamines are shown in Figures 1, 2, and 3 as to describe their possible reactions. Nitrosamines contain a nitrosyl group (NO) (Figure 1), the nitrogen contains five electrons in the outermost shell (valence electrons); two electrons are used to form double bonds with oxygen while three electrons left behind stay on nitrogen as one and lone pair of electrons [27, 28, 29]. Because of these features NO ligand forms structural bonding and complexes [27, 28].
Figure 1.
General structure of N-nitrosamines.
Figure 2.
Resonance structure of N-nitrosamines.
Figure 3.
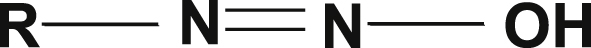
Tautomeric N-nitrosamines.
2.1. Metallic nitrosyl bond (M-NO)
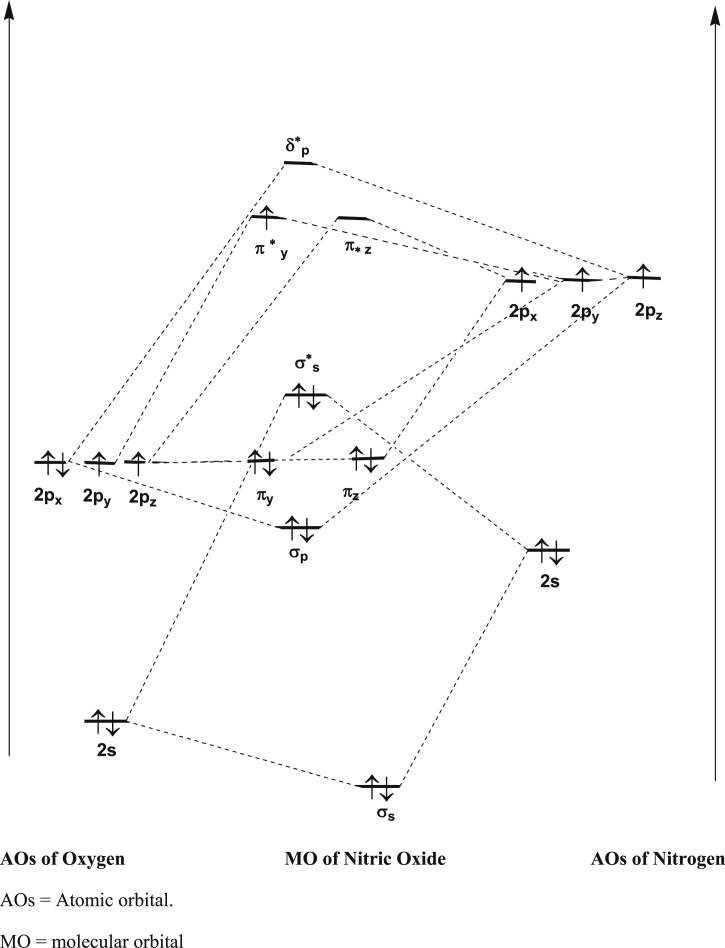
The general, resonance and tautomerization of N-nitrosamines are shown in Figures 1, 2, and 3. Also, for better understanding of metal-nitrosyls bond, the molecular orbital (MO) pattern of nitric oxide molecule is presented in Figure 4.
Figure 4.
Molecular orbital (MO) pattern of nitric oxide molecule.
The 6 and 5 electrons in the outermost shell of Oxygen and Nitrogen, respectively are used for bonding. The 11 electrons used in the formation of molecular orbital bond in NO are presented in the following order (Figure 5).
Figure 5.
The eleven electrons used in molecular orbital bond.
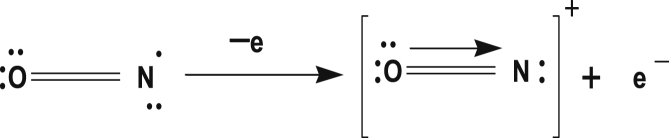
The metallic nitrosyl are as follows [27].
NO gives out an electron for formation of nitrosyl cation and oxygen releases a lone pair electron to nitrogen resulting to bond formation between oxygen and nitrogen (Figure 6).
Figure 6.
Bond formation between oxygen and nitrogen.
The unpaired electron (πy* == πz*) 1 received by the metal atom (M) changed its oxidation state from 0 to -1 (Figure 7) [27].
Figure 7.

Change in the oxidation state of metal atom (M).
The Nitrogen in the NO+ (nitrosonium ion or nitrosyl) give out a lone pair of electron to M−- for coordination (Figure 8).
Figure 8.
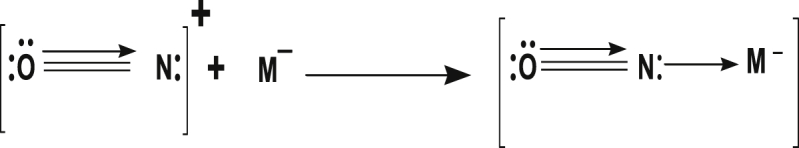
Coordinate covalent bond formation.
Nitrosyl complexes contain NO+ ligand. NO+ has three electrons, one is donated to metal ion before the donation of a lone pair of electron for coordinate covalent bond formation (Figure 8) [27, 30, 31].
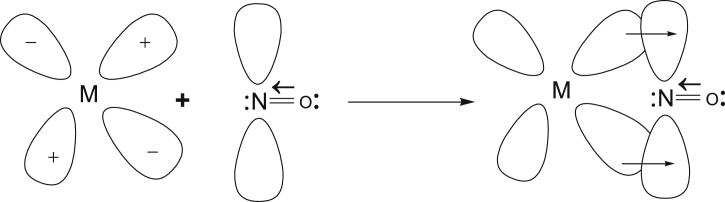
2.2. Formation of dative sigma (ϭ) bond
The dative sigma (ϭ) bond is formed due to the overlapped of the empty hybrid orbital (d, s, and p – orbital) of metal atom with the filled hybrid orbital (HOMO) of the nitrogen atom of the (NO+) ion Figures 9 and 10 [27].
Figure 9.

Movement of electron for the dative sigma bond formation.
Figure 10.
Formation of dative sigma bond.
2.3. π-Bond formation
The nitrogen in the NO+ as electron acceptor leads to formation of pie (π) bond.
The hybridized dπ or dpπ of the metal atom overlapped with empty orbital NO+ to form π-bond as shown in Figures 11 and 12 [27,32].
Figure 11.

Movement of π electron for the Formation of pie bond.
Figure 12.
Formation Pie bond.
The lowest unoccupied molecular orbitals received electrons from the filled metal orbitals leading to formation of π-bond (Figure 13) [27,[32], [33], [34]].
Figure 13.
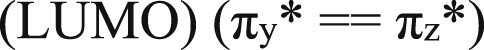
The lowest unoccupied molecular orbitals.
Therefore, there is the possibility of NO ligand present in NDMA forming complexes in Figures 14 and 15.
Figure 14.
Nitrosamine-metal complex.
Figure 15.
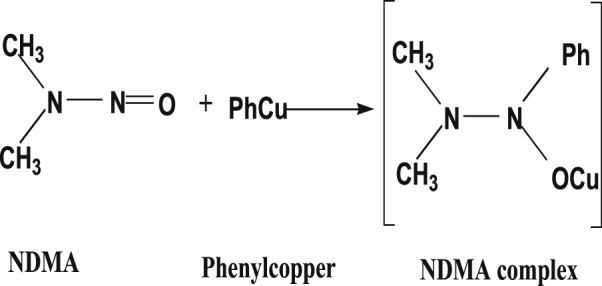
Nitrosamine-copper complex formation.
For instance, addition of NO ligand to metals lead to complexes reaction of NDMA with phenylcopper gives NDMA complex (Figure 15) [36, 37].
MCl2 = Chloride of transition metal [26, 30, 35].
The equations of the metal-nirosyl complexes formed in water samples have been reported by Anselme [36], Rose and Jurs [37] Kumar et al. [25] and Wang and Mitch, [26].
3. Application of chromatography to the analysis of nitrosamines in environmental waters
Gas chromatography (GC) coupled with various detectors have been employed for the analysis of nitrosamines and other emerging disinfection by–products (EDBPs) (Table 3). These include gas chromatography (GC) with electron capture detector (GC-ECD), nitrogen–phosphorus detector (GC/NPD), thermal energy analyzer (GC/TEA), GC with flame ionization detector (FID), GC coupled with mass spectrometry (GC-MS) [38, 39, 40, 41, 42].
The GC-ECD operates based on the ability of the organic compound to capture a thermal electron and form negatively charged ions. The electron loss is proportional to the quantity of analyte in the sample [43, 44, 45]. It is mostly used for the analysis of nitroaromatic compounds, halogen-containing compounds and conjugated compounds containing weak electrophore groups that can be improved with chemical derivatization [44, 45]. ECD is highly sensitive mostly employed for trace analysis. It has the capability to detect analyte at pictogram (10−13) levels [42, 46, 47, 48]. Similarly, Chienthavorn et al. [41] quantified four nitrosamines (NDEA, NPYR, NPIP, NMOR) with GC-FID. However, GC-ECD and FID have no library data base for the confirmation of the analyte, while GC-MS possesses. In addition, a good precision and linearity have been reported in the analysis of nitrosamines with GC-MS [49, 50, 51, 52, 53].
GC-MS technique explains the abundance of molecular composition and the amount of analyte in the sample in relation to the peak area [45, 64]. Its sensitivity is based on the mass of analyte received at the detector [64, 65]. Because of sensitivity and selectivity, it is used in the selected ion monitoring (SIM) mode for the analysis of thermally stable, semi-volatile, less polar and low molecular weight nitrosamines [23, 45, 51, 65, 66]. The study of nitrosamine has been narrowed to the US EPA eight semi-volatile nitrosamine analysis (Method 521) and less attention is given to the non-volatile nitrosamines. The non-volatile NAms (N-nornicotine, N- piperazine) and labile NDPhA are not amenable to GC-MS methods because they are highly polar and thermally unstable respectively [60, 67, 68, 69]. Also, gas chromatography–tandem mass spectrometry (GC/MS/MS) could gives better sensitivity and selectivity than GC-MS, but cannot be used for the analysis of non-volatile nitrosamines [7, 70, 71]. Analysis of nitrosamines using gas chromatography–low resolution mass spectrometry (GC/LRMS) in electrospray ionization (ESI) mode has also been applied in the analysis of nitrosamines. But its demerit is that it causes chemical interference during low molecular mass nitrosamines analysis [39, 72].
3.1. Choice of the columns in the gas chromatographic analysis of nitrosamines
The capacity of gas chromatographic separation column depends on the type of stationary phase and its polarity and the amount of the packing material used (Table 3). This increases the efficiency of the column [49, 58, 73]. A good separation is attained by the distribution of the analytes (solute) on the stationary phase (composition of the adsorbent) and a gas phase that penetrates the stationary phase [74]. Low molecular mass gases are used as mobile phase for the adequate transportation of the solute through the column [74].
A more polar stationary phase retains polar analytes better than less polar solute while a non-polar stationary phase retains any member of homologous series [49, 58]. Different types of column ranging from non-polar (HP-5MS, 5% phenyl-95% dimethylpolysiloxane; DB-5ms 5% Phenyl 95% dimethylpolysiloxane), mid-polar (DB-624, 6% cyanopropyl phenyl-94% dimethylpolysiloxane; DB-1701, 14% cyanopropylphenyl-86% dimethyl polysiloxane) and polar polar column (HP-INNOWax, Polyethylene glycol) have been reported for the analysis of nitrosamines Qiang et al. [49] used column HP-5MS, DB-624, DB-1701 and HP-INNOWax for the analysis of ten nitrosamines and they reported the best peak separation with DB-624 column. The chromatograms reported were shown in Figure 16(A–D) and Figure 17.
Figure 16.
Separation of ten volatile nitrosamines using four different gas chromatographic columns. A, HP-5MS (30 m × 0.25 mm × 0.25 μm) column; B, DB-624 (30 m × 0.25 mm × 1.40 μm) column; C, HP-1701 (30 m × 0.53 mm × 1.0 μm) column; D, HP-INNOWax (30 m × 0.25 mm × 0.25 μm) column (Qiang et al., 2011). It has been published before in Chinese Journal Analytical Chemistry and permission to reproduce the figure has been granted.
Figure 17.
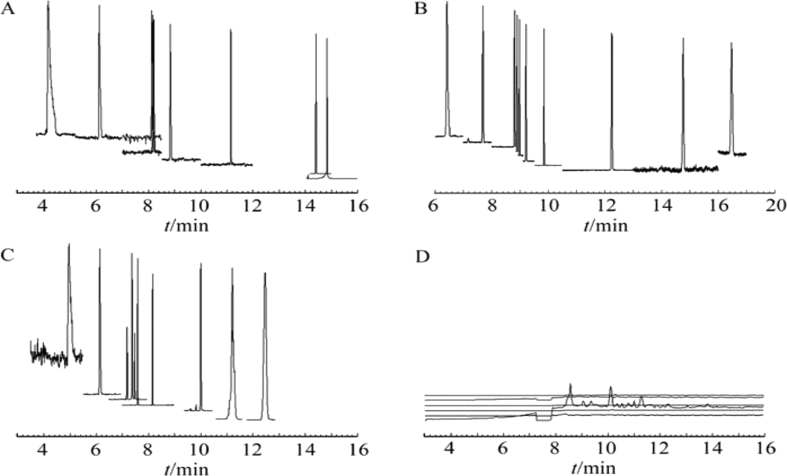
The final separation of the ten volatile nitrosamines DB-624 (30 m × 0.25 mm × 1.40 μm) column. 1. NMDA 2. NDEA 3. NDPA 4. NMOR 5. NPYR 6. NPIP 7. NDBA 8. NDPHA 9. NDCHA 10. NDBZA [49]. It has been published before in Chinese Journal Analytical Chemistry and permission to reproduce the figure has been granted.
In the non-polar column HP-5MS small background level or low signal to noise ratio, better peak resolution at the beginning and end but poor separation of NDPA, NMOR, NPYR, NPIP at the mid of the chromatogram (Figure 16A). In Figure 16B there was a negligible background noise and all peaks were well separated throughout the chromatogram. High signal to noise ratio at the beginning, analytes were well separated at the end but fairly separated at the mid of the chromatogram (Figure 16C). Figure 16D depicted poor sensitivity and an unreproducible chromatogram could be due to absence of cyanopropyl phenyl and dimethylpolysiloxane.
Similarly, a sharp peak separation with DB-624 column was also reported as shown in Figure 17 [57, 70, 75, 76]. However, there is no published literature on column with 100% dimethylpolysiloxane which could properly yield a better separation.
4. Conclusions
This survey could not find a study specifically dedicated to quantitative determination of metal-complexed nitrosamines. The reason is unknown to us. An overview of the analytical methods has shown that different methods exist for analysis of nitrosamines. However, in our view, the existing reports on total nitrosamine concentration in waters may have therefore been severely underestimated. Gas chromatography with so called mid-polar column made of cyanopropyl phenyl and dimethylpolysiloxane in the ratio 1: 16 (DB-624) and linked mass spectrometer detector is one technique adaptable to the determination of metal-complexed nitrosamines in waters in view of its reproducibility and sensitivity. Attention should focus on the high molecular weight, emerging unregulated and highly toxic nitrosamines as well as metal-nitrosyl complexes occurrence in water. Also, in order to obtain a high quality chromatogram, the chemical composition of the stationary phase used in the column should be improved.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Gratitude to Tshwane University of Technology and Rand Water Chair (Prof. J.O. Okonkwo) for their support. The authors are grateful to the Management of Covenant University for funding the article publication charges.
References
- 1.Van Huy N., Murakami M., Sakai H., Oguma K., Kosaka K., Asami M., Takizawa S. Occurrence and formation potential of N-nitrosodimethylamine in ground water and river water in Tokyo. Water Res. 2011;45:3369–3377. doi: 10.1016/j.watres.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 2.Luo Q., Wang D., Wang Z. Occurrences of nitrosamines in chlorinated and chloraminated drinking water in three representative cities, China. Sci. Total Environ. 2012;437:219–225. doi: 10.1016/j.scitotenv.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., Demarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.De Mey E., De Klerck K., De Maere H., Dewulf L. The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Sci. 2014;96:821–828. doi: 10.1016/j.meatsci.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Chen W.H., Huang T.H., Wang C.Y. Impact of pre-oxidation on nitrosamine formation from a source to drinking water: a perspective on cancer risk assessment. Process Saf. Environ. Protect. 2018;113:424–434. [Google Scholar]
- 6.Richardson S.D., Ternes T.A. Water analysis: emerging contaminants and current issues. Anal. Chem. 2011;2:4614–4648. doi: 10.1021/ac200915r. [DOI] [PubMed] [Google Scholar]
- 7.Sannino A., Bolzoni L. GC/CI-MS/MS method for the identification and quantification of volatile N-nitrosamines in meat products. Food Chem. 2013;141:3925–3930. doi: 10.1016/j.foodchem.2013.06.070. [DOI] [PubMed] [Google Scholar]
- 8.Choi N.R., Kim Y.P., Ji W.H., Hwang G.S., Ahn Y.G. Identification and quantification of seven volatile n-nitrosamines in cosmetics using gas chromatography/chemical ionization-mass spectrometry coupled with head space-solid phase microextraction. Talanta. 2016;148:69–74. doi: 10.1016/j.talanta.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Jurado-Sanchez B., Ballesteros E., Gallego M. Occurrence of aromatic amines and N-nitrosamines in the different steps of a drinking water treatment plant. Water Res. 2012;46:4543–4555. doi: 10.1016/j.watres.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Wang W., Yu J., An W., Yang M. Occurrence and profiling of multiple nitrosamines in source water and drinking water of China. Sci. Total Environ. 2016;551:489–495. doi: 10.1016/j.scitotenv.2016.01.175. [DOI] [PubMed] [Google Scholar]
- 11.Asami M., Oya M., Kosaka K. A nationwide survey of NDMA in raw and drinking water in Japan. Sci. Total Environ. 2009;407:3540–3545. doi: 10.1016/j.scitotenv.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Charrois J.W.A., Arend M.W., Froese K.L., Hrudey S.E. Detecting N-nitrosamines in drinking water at nanogram per liter levels using ammonia positive chemical ionization. Environ. Sci. Technol. 2004;38:4835–4841. doi: 10.1021/es049846j. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y.-Y., Boyd J., Hrudey S.E., Li X.-F. Characterization of new nitrosamines in drinking water using liquid chromatography tandem mass spectrometry. Environ. Sci. Technol. 2006;40:7636–7641. doi: 10.1021/es061332s. [DOI] [PubMed] [Google Scholar]
- 14.Najm I., Trussell R.R. NDMA formation in water and wastewater. Am. Water Work. Assoc. 2001;93:92–99. [Google Scholar]
- 15.Ma F., Wan Y., Yuan G., Meng L., Dong Z., Hu J. Occurrence and source of nitrosamines and secondary amines in groundwater and its adjacent jialu river basin, China. Environ. Sci. Technol. 2012;46:3236–3243. doi: 10.1021/es204520b. [DOI] [PubMed] [Google Scholar]
- 16.Bei E., Shu Y., Li S., Liao X., Wang J., Zhang X., Chen C. Occurrence of nitrosamines and their precursors in drinking water systems around mainland China. Water Res. 2016;98:168–175. doi: 10.1016/j.watres.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann S.S., Duedahl-Olesen L., Granby K. Occurrence of volatile and non- volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Contr. 2015;48:163–169. [Google Scholar]
- 18.Liu X., Cen K., Zhao L., Chen Y., Lun Z., Wu X., Tian Z. N-nitrodimethlyamine in natural and drinking water of high cancer incidence regions of Guangdong. China Appl. Geochem. 2016;74:157–164. [Google Scholar]
- 19.Zhao Y.Y., Boyd J.M., Woodbeck M. Formation of N-nitrosamines from eleven disinfection treatments of seven different surface waters. Environ. Sci. Technol. 2008;42:4857–4862. doi: 10.1021/es7031423. [DOI] [PubMed] [Google Scholar]
- 20.Kadmi Y., Favier L., Wolbert D. N-nitrosamines, emerging disinfection by- products of health concern: an overview of occurrence, mechanisms of formation, control and analysis in water. Water Sci. Technol. Water Suppl. 2015;15:11–25. [Google Scholar]
- 21.Qian Y., Wu M., Wang W., Chen B., Zheng H., Krasner S.W., Hrudey S.E., Li X. Determination of 14 nitrosamines at nanogram per liter levels in drinking water. Anal. Chem. 2015;87:1330–1336. doi: 10.1021/ac504104k. [DOI] [PubMed] [Google Scholar]
- 22.USEPA, “United States Environmental Protection Agency . UCMR2; 2010. Second Cycle of the Unregulated Contaminant Monitoring Regulation. [Google Scholar]
- 23.Wang W., Yu J., An W., Yang M. Occurrence and profiling of multiple nitrosamines in source water and drinking water of China. Sci. Total Environ. 2016;551–552:489–495. doi: 10.1016/j.scitotenv.2016.01.175. [DOI] [PubMed] [Google Scholar]
- 24.Ford P.C., Bourassa J., Miranda K., Lee B., Lorkovic I., Boggs S., Kudo S., Laverman L. Photochemistry of metal nitrosyl complexes. Delivery of nitric oxide to biological targets. Coord. Chem. Rev. 1998;171:185–202. [Google Scholar]
- 25.Kumar A., Pandey R., Gupta R.K., Ghosh K., Pandey D.S., Synthesis “. Characterization and photochemical properties of some ruthenium nitrosyl complexes. Polyhedron. 2013;52:837–843. [Google Scholar]
- 26.Wang Z., Mitch W.A. Influence of dissolved metals on N-nitrosamine formation under amine-based CO2 capture conditions. Environ. Sci. Technol. 2015;49:11974–11981. doi: 10.1021/acs.est.5b03085. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt V. Academic Press; 2016. A Simplified Approach with 3D Visuals. Essentials of Coordination Chemistry; pp. 237–257. chap. 1-9. [Google Scholar]
- 28.Doctorovich F., Bikiel D., Pellegrino J., Suárez S.A., Larsen A., Martí M.A. Nitroxyl (azanone) trapping by metalloporphyrins. Coord. Chem. Rev. 2001;255:2764–2784. [Google Scholar]
- 29.Potturi H.K., Gurung R.K., Hou Y. Nitromethane with IBX/TBAF as a nitrosating agent: synthesis of nitrosamines from secondary or tertiary amines under mild conditions. J. Org. Chem. 2012;77:626–631. doi: 10.1021/jo202276x. [DOI] [PubMed] [Google Scholar]
- 30.Cameron M., Gowenlock B.G., Vasapollo G. Coordination chemistry of C-nitroso- compounds. Chem. Soc. Rev. 1990;19:355–379. [Google Scholar]
- 31.Gowenlock B.G., Richter-Addo G.B. Preparations of C-nitroso compounds. Chem. Rev. 2004;104:3315–3340. doi: 10.1021/cr030450k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaki F., Kabasawa Y., Yanagimoto T., Umeda N., Urano Y., Nagano T., Otani Y., Ohwada T. Visible-light-triggered release of nitric oxide from N-pyramidal nitrosamines. Chem. A Eur. J. 2012;18:1127–1141. doi: 10.1002/chem.201101427. [DOI] [PubMed] [Google Scholar]
- 33.Souza M.L., RovedaJr A.C., MeloPereira C.J., Franco W.D. A Review: new perspectives on the reactions of metal nitrosyls with thiolates as nucleophiles. Coord. Chem. Rev. 2016;306:615–627. [Google Scholar]
- 34.Beaudoin D., Wuest J.D. Dimerization of aromatic C-nitroso compounds. Chem. Rev. 2016;116:258–286. doi: 10.1021/cr500520s. [DOI] [PubMed] [Google Scholar]
- 35.Coppens P., Novozhilov I., Kovalevsky A. Photoinduced linkage isomers of transition-metal nitrosyl compounds and related complexes. Chem. Rev. 2002;102:861–883. doi: 10.1021/cr000031c. [DOI] [PubMed] [Google Scholar]
- 36.Anselme J. The organic chemistry of N-nitrosamines (a brief review) Am. Chem. Soc. 1979;101:1–12. [Google Scholar]
- 37.Rose S.L., Jurs P.C. Computer-Assisted studies of structure—activity relationships of N-nitroso compounds using pattern recognition. J. Med. Chem. 1982;25:769–776. doi: 10.1021/jm00349a002. [DOI] [PubMed] [Google Scholar]
- 38.Byun M.W., Ahn H.J., Kim J.H., Lee J.W., Yook H.S., Han S.B. Determination of volatile N-nitrosamines in irradiated fermented sausage by gas chromatography coupled to a thermal energy analyser. J. Chromatogr. A. 2004;1054:403–407. [PubMed] [Google Scholar]
- 39.Grebel J.E., Young C.C., Suffet I.H. Solid-phase microextraction of N- nitrosamines. J. Chromatogr. A. 2006;1117:11–18. doi: 10.1016/j.chroma.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 40.Krasner S.W., Mitch W.A., McCurry D.L., Hanigan D., Westerhoff P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: a review. Water Res. 2013;47:4433–4450. doi: 10.1016/j.watres.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 41.Chienthavorn O., Subprasert P., Insuan W. Nitrosamines extraction from frankfurter sausages by using superheated water. Sep. Sci. Technol. 2014;49:838–846. doi: 10.1021/jf4036645. [DOI] [PubMed] [Google Scholar]
- 42.Lee N., Huang H., Zhu G. Analysis of 40 conventional and emerging disinfection by- products in fresh-cut produce wash water by modified EPA methods. Food Chem. 2018;256:319–326. doi: 10.1016/j.foodchem.2018.02.134. [DOI] [PubMed] [Google Scholar]
- 43.Wagner E.D., Chen P.H. Halonitromethane drinking water disinfection Byproducts : chemical characterization and mammalian cell cytotoxicity and genotoxicity. Env. Sci. Technol. 2004;38:62–68. doi: 10.1021/es030477l. [DOI] [PubMed] [Google Scholar]
- 44.Poole C.F. Alkylsilyl derivatives for gas chromatography. J. Chromatogr. A. 2013;1296:2–14. doi: 10.1016/j.chroma.2013.01.097. [DOI] [PubMed] [Google Scholar]
- 45.Poole C.F. Ionization-based detectors for gas chromatography. J. Chromatogr. A. 2015;1421:137–153. doi: 10.1016/j.chroma.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 46.Liang L., Singer P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol. 2003;37:2920–2928. doi: 10.1021/es026230q. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Chu W., Yao D., Yin D. ScienceDirect Control of aliphatic halogenated DBP precursors with multiple drinking water treatment processes: formation potential and integrated toxicity. J. Environ. Sci. 2017;58:322–330. doi: 10.1016/j.jes.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Schedl A., Zweckmair T., Kikul F., Bacher M., Rosenau T., Potthast A. Pushing the limits: quantification of chromophores in real-world paper samples by GC-ECD and EI-GC-MS. Talanta. 2018;179:693–699. doi: 10.1016/j.talanta.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 49.Qiang M.A., Hai-Wei X.I., Chao W., Hua B.A.I. Determination of ten volatile nitrosamines in cosmetics by gas chromatography tandem mass spectrometry. Chin. J. Anal. Chem. 2011;39:1201–1207. [Google Scholar]
- 50.Mcdonald J.A., Harden N.B., Nghiem L.D., Khan S.J. Talanta Analysis of N - nitrosamines in water by isotope dilution gas chromatography – electron ionisation tandem mass spectrometry. Talanta. 2012;99:146–154. doi: 10.1016/j.talanta.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Huang M.C., Chen H.C., Fu S.C., Ding W.H. Determination of volatile N- nitrosamines in meat products by microwave-assisted extraction coupled with dispersive micro solid-phase extraction and gas Chromatography-Chemical ionisation mass spectrometry. Food Chem. 2013;138:227–233. doi: 10.1016/j.foodchem.2012.09.119. [DOI] [PubMed] [Google Scholar]
- 52.Scheeren M.B., Sabik H., Gariépy C., Terra N.N., Arul J. Determination of N- nitrosamines in processed meats by liquid extraction combined with gas chromatography-methanol chemical ionisation/mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015;32:1436–1447. doi: 10.1080/19440049.2015.1066037. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J. Arizona State University; 2016. Investigations into the Occurrence, Formation and Fate of N- Nitrosodimethylamine (NDMA) in Air and Water. [Google Scholar]
- 54.Kim G.-A., Son H.-J., Kim C.-W., Kim S.-H. Nitrosamine occurrence at Korean surface water using an analytical method based on GC/LRMS. Environ. Monit. Assess. 2013;85:1657–1669. doi: 10.1007/s10661-012-2658-1. [DOI] [PubMed] [Google Scholar]
- 55.Carlton D., Schug K. 2014. Evaluation of Automated Solid - Phase Extraction for Nitrosamines Using US EPA Method 521. Arlington, Texas, USA. [Google Scholar]
- 56.Wu Q., Shi H., Ma Y., Adams C., Eichholz T., Timmons T., Jiang H. Determination of secondary and tertiary amines as N-nitrosamine precursors in drinking water system using ultra-fast liquid chromatography–tandem mass spectrometry. Talanta. 2015;131:736–741. doi: 10.1016/j.talanta.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Fan C.-C., Lin T.-F. N-nitrosamines in drinking water and beer: detection and risk assessment. Chemosphere. 2018;200:48–56. doi: 10.1016/j.chemosphere.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Reyes-Contreras C., Domínguez C., Bayona J. Determination of nitrosamines and caffeine metabolites in wastewaters using gas chromatography mass spectrometry and ionic liquid stationary phases. J. Chromatogr. A. 2012;1261:164–170. doi: 10.1016/j.chroma.2012.05.082. [DOI] [PubMed] [Google Scholar]
- 59.Fujioka T., Takeuchi H., Tanaka H., Nghiem L.D., Ishida K.P., Kodamatani H. A rapid and reliable technique for N-nitrosodimethylamine analysis in reclaimed water by HPLC-photochemical reaction-chemiluminescence. Chemosphere. 2016;161:104–111. doi: 10.1016/j.chemosphere.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 60.J Brisson I., Levallois P., Tremblay H., Sérode J., Deblois C., Charrois J., Taguchi V., Boyd J., Li X., Rodriguez M.J. Spatial and temporal occurrence of N-nitrosamines in seven drinking water supply systems. Environ. Monit. Assess. 2013;185:7693–7708. doi: 10.1007/s10661-013-3128-0. [DOI] [PubMed] [Google Scholar]
- 61.Kosaka K., Asami M., Ohkubo K., Iwamoto T. Identification of a New N- nitrosodimethylamine precursor in sewage containing industrial effluents. Environ. Sci. Technol. 2014;48:11243–11250. doi: 10.1021/es502284t. [DOI] [PubMed] [Google Scholar]
- 62.Venkatesan A., Pycke B., Halden R. Detection and occurrence of N - nitrosamines in archived biosolids from the targeted national sewage sludge survey of the U.S. environmental protection agency. Environ. Sci. Technol. 2014;48:5085–5092. doi: 10.1021/es5001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spahr S., Cirpka O.A., Von Gunten U., Hofstetter T.B. formation of N- nitrosodimethylamine during chloramination of secondary and tertiary amines: role of molecular oxygen and radical intermediates. Environ. Sci. Technol. 2017;51:280–290. doi: 10.1021/acs.est.6b04780. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs M.R., Hilder E.F., Shellie R.A. Applications of resistive heating in gas chromatography: a review. Anal. Chim. Acta. 2013;803:2–14. doi: 10.1016/j.aca.2013.04.063. [DOI] [PubMed] [Google Scholar]
- 65.Kirk A.T., Last T., Zimmermann S. A sensitive gas chromatography detector based on atmospheric pressure chemical ionization by a dielectric barrier discharge. J. Chromatogr. A. 2017;1483:120–126. doi: 10.1016/j.chroma.2016.12.071. [DOI] [PubMed] [Google Scholar]
- 66.Yahaya A. Method development for the identification and quantitative analysis of seven nitrosamines using gas chromatography mass spectrometry. Chem. Data Collect. 2019;21:1–12. [Google Scholar]
- 67.Bellec G., M Cauvin J., Salaun M.C., Le Calvé K. Analysis of N-nitrosamines by high- performance liquid chromatography with post-column photohydrolysis and colorimetric detection. J. Chromatogr. A. 1996;727:83–92. doi: 10.1016/0021-9673(95)01073-4. [DOI] [PubMed] [Google Scholar]
- 68.Boyd J.M., Hrudey S.E., Richardson S.D., Li X.F. Solid-phase extraction and high- performance liquid chromatography mass spectrometry analysis of nitrosamines in treated drinking water and wastewater. Trends Anal. Chem. 2011;30:1410–1421. [Google Scholar]
- 69.Zhao M., Li G., Kong W., Lu S., Xia L., Chen G., Zhao X. Convenient and sensitive HPLC method for determination of nitrosamines in foodstuffs based on pre-column fluorescence labeling. Chromatographia. 2016;79:431–439. [Google Scholar]
- 70.Holady J., Trenholm R., Snyder S. Use of automated solid-phase extraction and GC- MS/MS to evaluate nitrosamines in water environmental Nevada. Am. Lab. 2012;1:20–30. [Google Scholar]
- 71.Yoon S., Nakada N., Tanaka H. A new method for quantifying N-nitrosamines in wastewater samples by gas chromatography - triple quadrupole mass spectrometry. Talanta. 2012;97:256–261. doi: 10.1016/j.talanta.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Ventanas S., Ruiz J. On-site analysis of volatile nitrosamines in food model systems by solid-phase microextraction coupled to a direct extraction device. Talanta. 2006;70:1017–1023. doi: 10.1016/j.talanta.2006.02.031. 2006. [DOI] [PubMed] [Google Scholar]
- 73.Wang D., Chong S.L., Malik A. Sol-gel column Technology for single-step deactivation, coating, and stationary-phase immobilization in high-resolution capillary gas chromatography. Anal. Chem. 1997;69:4566–4576. [Google Scholar]
- 74.Abraham M.H., Poole C.F., Poole S.K. Classification of stationary phases and other materials by gas chromatography. J. Chromatogr. A. 1999;842:79–114. 1999. [Google Scholar]
- 75.Bei E., Liao X., Meng X., Li S., Wang J., Sheng D., Chao M. Identification of nitrosamine precursors from urban drainage during storm events: a case study in southern China. Chemosphere. 2016;160:323–331. doi: 10.1016/j.chemosphere.2016.06.081. [DOI] [PubMed] [Google Scholar]
- 76.Yanga M., Zhang X. Current trends in the analysis and identification of emerging disinfection byproducts. Trends Anal. Chem. 2016;10:24–34. [Google Scholar]