Abstract
Women who have bilateral oophorectomies prior to the age of natural menopause are at increased risk of developing mild cognitive decline, dementia, anxiety, and depressive type disorders. Clinical and animal studies indicate angiotensin type 1 receptor (AT1R) blockers (ARBs) have blood pressure (BP)-independent neuroprotective effects. To investigate the potential use of ARBs in normotensive women at increased risk of developing neurocognitive problems, we studied a rat model of bilateral oophorectomy. Long Evans rats were sham-operated (Sham) or ovariectomized (Ovx) at 3 months of age and immediately treated continuously with vehicle (Veh) or the ARB losartan (Los) for the duration of the experiment. In contrast to many hypertensive rat models, ovariectomy did not increase mean arterial pressure (MAP) in these normotensive rats. Ovariectomized rats spent less time in the open arms of the elevated plus maze (EPM) [(% total time): Veh, 34.1 ± 5.1 vs. Ovx, 18.7 ± 4.4; p < 0.05] and in the center of the open field (OF) [(s): Veh, 11.1 ± 1.7 vs. Ovx, 6.64 ± 1.1; p < 0.05]. They also had worse performance in the novel object recognition (NOR) test as evidenced by a reduction in the recognition index [Veh, 0.62 ± 0.04 vs. Ovx, 0.45 ± 0.03; p < 0.05]. These adverse effects of ovariectomy were prevented by Los. Losartan also reduced plasma corticosterone in Ovx rats compared to Veh treatment [(ng/mL): Ovx–Veh, 238 ± 20 vs. Ovx–Los, 119 ± 42; p < 0.05]. Ovariectomy increased AT1R mRNA expression in the CA3 region of the hippocampus (Hc) [(copies x 106/µg RNA): Sham–Veh, 7.15 ± 0.87 vs. Ovx–Veh, 9.86 ± 1.7; p < 0.05]. These findings suggest the neuroprotective effects of this ARB in normotensive Ovx rats involve reduction of plasma corticosterone and blockade of increased AT1R activity in the hippocampus. These data suggest ARBs have therapeutic potential for normotensive women at increased risk of developing cognitive and behavioral dysfunction due to bilateral oophorectomy prior to the natural age of menopause.
Keywords: Cognition, Memory, Postmenopausal, Premature ovarian failure
Introduction
The Mayo Clinic Cohort Study of Oophorectomy and Aging showed that the risk of mild cognitive impairment, dementia, anxiety, and depression nearly doubled in women who underwent bilateral oophorectomy prior to the age of natural menopause, regardless of the indication for oophorectomy (e.g., benign ovarian condition or for cancer prophylaxis) (Rocca et al. 2007, 2008). These first large-scale studies of cognition and mood disorders with long-term follow-up also showed that the risk of these neurocognitive disorders increased the younger the age at oophorectomy. These findings from women living in the United States were confirmed in a Danish historical cohort study, published in 2010, that queried national disease registries (Phung et al. 2010). The Danish study showed that the risk of dementia with onset before the age of 50, increased in women who underwent bilateral oophorectomy prior to the age of natural menopause, again, with the risk increasing the younger the age at surgery. In 2014, a study of two large and well-characterized cohorts in the United States further documented the positive association between bilateral oophorectomy prior to the age of natural menopause and cognitive decline including Alzheimer’s disease (AD) neuropathology (Rocca et al. 2016). In addition, the Religious Orders Study and Rush Memory and Aging Project found a faster rate of global cognitive decline, especially episodic memory and semantic memory, the earlier the age of surgical menopause whereas no associations were observed in women who had natural menopause (Bove et al. 2014).
Bilateral oophorectomy before the onset of natural menopause causes an abrupt cessation of estrogen production with a consequent drop in circulating levels of 17β-estradiol (E2). Controversy, however, over the harm versus benefit of estrogen replacement therapy (ERT) for neurologic diseases continues (O’Hagan et al. 2012). The Women’s Health Initiative showed that women who initiated ERT alone or in combination with a progestin in the late postmenopausal stage (ages 65–79 years) experienced an increased risk of dementia and cognitive decline regardless of the presence or absence of progestin (Shumaker et al. 2004). Three observational studies, however, suggest that the neuroprotective versus harmful effects of estrogen depend on the age at time of treatment initiation and on the stage of menopause (Rocca et al. 2010). Furthermore, a randomized, double-blind, placebo-controlled trial in healthy women showed that ERT for 5 years did not benefit or harm verbal episodic memory, executive function, or global cognition regardless of time since menopause (Henderson et al. 2016). In contrast, the Mayo Clinic Cohort Study of Oophorectomy reported a failure of ERT to prevent the risk of anxiety and depressive outcomes (Rocca et al. 2008). Even if ERT is proven to provide neurocognitive benefit, it is contraindicated in some women (e.g., because of active thrombosis, endometriosis, or history of breast cancer) and others may not elect ERT for other reasons. Thus, it is imperative to develop preventive therapeutic strategies for women at increased risk of neurocognitive disorders due to premenopausal bilateral oophorectomy.
Several clinical studies have shown angiotensin receptor blockers (ARBs) have neuroprotective effects. Patients within the UK general practice database with antihypertensive prescription data and who were diagnosed with dementia (1214) or AD (5797) had fewer prescriptions for ARBs compared to other antihypertensive medications (Davies et al. 2011). In a Department of Veterans Affairs population of over 370,000 individuals who had diabetes, the risk of developing dementia was reduced in those treated with ARBs compared to other antihypertensive therapy (Johnson et al. 2012). In another large (> 810,000) US Veteran cohort, the hazard rates for incident dementia was smallest in the ARB treatment group compared to other antihypertensives and in patients with pre-existing AD, there was a lower risk of admission to a nursing home in the ARB treatment group compared to other antihypertensive medications (Li et al. 2010).
Studies in animals support these clinical findings. ARBs were shown to protect cognition (Pelisch et al. 2011) and reduce anxiety- and depressive-like behaviors (Saavedra et al. 2006) from hypertension and stroke (Faure et al. 2006), traumatic brain injury (Timaru-Kast et al. 2012) and isolation stress (Saavedra et al. 2006). However, little is known regarding the potential of ARBs to exert neurocognitive protection in women who had bilateral oophorectomies prior to the natural age of menopause or in animal models of sudden ovarian hormone loss. In this study, we investigated the effect of ovariectomy on cognitive function and anxiety- and depressive-like behavior in Long Evans rats as well as the ability of the ARB losartan (Los) to prevent neurocognitive impairments induced by ovariectomy.
Methods
Animals
Female Long Evans rats were purchased from Harlan/Envigo (Indianapolis, USA) at 3 months of age. They were housed in pairs in a temperature and humidity-controlled room under a light/dark cycle of 12 h. Food and water were provided ad libitum. Body weight, food, and water intake were measured weekly.
Surgery
Sham and ovariectomy surgeries were conducted in 3 month old rats. The surgery was performed under 2.5% isoflurane anesthesia (Patterson Veterinary, Greeley, CO) at 1 L/min oxygen (Roberts Oxygen Company Inc, Rockville, MD). Bilateral flank incisions were made. In the Ovx group, the ovaries were removed, while in the Sham group, the ovaries were exposed without excising them. Then, the muscle layers and skin were individually sutured. All rats received the analgesic carprofen (5 mg/kg (Rimadyl); Zoetis; Parsippany, NJ) subcutaneously after surgery and up to 72 h when needed.
Drug Treatment
Immediately after surgery, the Sham and Ovx rats were randomized and half were given vehicle (drinking water; filtered tap water) while the other half were treated with Los (Sigma, St. Louis, MO) dissolved in the drinking water. The dose of losartan (10 mg/kg/day) remained constant until the day of euthanasia by adjusting the concentration of drug in the drinking water based on daily water consumption and BW, as we previously described (Ji et al. 2007).
Experimental Design
Five weeks after surgery and in the presence of continuous drug treatment, the animals were exposed to a battery of behavioral tests with at least a two-day interval between each test, as illustrated in Fig. 1. In order to habituate and reduce the stress response due to manipulation of the experimenter, the animals were gently handled for 5 min a day for one week prior to the first behavioral test. Animals were transported from the animal facility to the testing room at least 30 min before initiating a test. All apparatuses were cleaned with a 70% ethanol solution between each behavioral test session. After all testing was complete, the rats were anesthetized with isoflurane and MAP was measured. Then, the animals were euthanized for plasma and tissue collection.
Fig. 1.
Experimental timeline of behavioral testing. At 3 months of age, animals were sham-operated (Sham) or ovariectomized (Ovx) and immediately treated continuously with vehicle (Veh) or losartan (Los) throughout the duration of the experimental protocol. Five weeks after the surgeries were completed, behavioral testing was initiated. Thereafter, mean arterial pressure (MAP) was measured and tissues were collected and used for further analyses. mo months, EPM elevated plus maze, OF open field, NOR novel object recognition, LVS looming visual stimuli, SP sucrose preference
Elevated Plus Maze
The EPM test was conducted as previously described (Pellow et al. 1985). The apparatus consists of two opposed open arms and two perpendicular arms enclosed by walls (50 cm × 10 cm × 40 cm; Bioseb In Vivo Research Instruments, Pinellas Park, FL) raised 50 cm above the floor. The test was performed under 115 lx illumination and was scored by EthoVision XT software (Noldus Information Technology, Leesburg, VA). The distance traveled during the testing was used as an assessment of spontaneous locomotor activity. Anxiety-like behavior was assessed by measuring the number of entries and percentage of time spent exploring the open arms over a 5-min period.
Open Field
The OF test was conducted as previously described (Prut and Belzung 2003). The apparatus consists of a square arena (90 cm × 90 cm × 40 cm; Bioseb In Vivo Research Instruments). The test was performed under 115 lx illumination and was scored by EthoVision XT software (Noldus Information Technology). The distance traveled during the testing was used as an assessment of spontaneous locomotor activity. Anxiety-like behavior was determined by measuring the number of entries and amount of time spent exploring the center portion of the field (45 cm × 45 cm) over a 5-min period.
Novel Object Recognition
The NOR test was adapted from Drumond et al. and Zanini et al. (Drumond et al. 2012; Zanini et al. 2017). This test uses the same apparatus and software (EthoVision XT; Noldus Information Technology) as for the OF test. Rats spent 10 min/day for 3 days exploring the apparatus without any stimulus in order to become habituated. On the test day, rats were placed in the center of the arena and allowed to explore two identical objects for 10 min (training phase). After 60 min, one of the objects was replaced by a new one and the animals were allowed to explore for another 5 min (test phase). The time spent exploring the objects in the training and test phases were scored, and the recognition index was obtained by the following equation: time exploring the new object/total exploration time of the two objects in the test phase. Values above 0.5 indicate preference for the new object. Exploratory behavior was defined as the amount of time spent smelling and touching the object and when the animal’s nose approached the object within 2 cm.
Looming Visual Stimuli
The LVS test was conducted as previously described (Aguilar et al. 2018). The test uses a transparent cylindrical chamber (43.5 cm × 18.5 cm) with a computer screen located above the chamber (Dell, Round Rock, TX). All testing was conducted under 20 lx red light. Initially, the computer screen was completely gray for a 2 min baseline period. During the stimulus period, the screen displayed a black dot that rapidly expanded from 2° to 20° of visual angle over 250 ms. After reaching maximum size, the dot remained stable for 250 ms before disappearing. The expanding dot was presented consecutively 15 times over 22 s, mimicking the visual stimulus of a predator attack (Wei et al. 2015). After stimulus presentation, the gray screen was again presented until the experiment ended at 3 min (poststimulus period). Videos were truncated into equivalent length periods (22 s each) and manually scored for freezing behavior by a blinded observer using ANYMaze software (Stoelting Co., Wood Dale, IL). Freezing was defined as ceasing all activity, maintaining an attentive attitude with head raised, eyes open, and body remaining in the same position.
Sucrose Preference
The SP test was conducted as previously described (Aguilar et al. 2018; Prut and Belzung 2003). The preference for sweet solutions over tap water was assessed by measuring the intake of sucrose solution (1% sucrose—w/v) and tap water over 5 days as follows: 4 consecutive days of a 2-h exposure period to the choice of sugar water vs plain. On the last day of the test, rats were water restricted for 2 h followed by a 2-h exposure period to the preference test. During the test phase, animals were housed in individual cages with water and sucrose solution bottles (200 mL each) placed in random positions. Once the test phase was completed, the animals were returned to their original cages. Sucrose preference was based on the following equation: total sucrose consumption (mL)/[total sucrose consumption (mL) + total water consumption (mL)].
Mean Arterial Pressure
Terminal BP measurements were determined as we previously described (Zheng et al. 2008). Briefly, animals were anesthetized with 2.5% isoflurane at 1 L/min oxygen. A polyethylene catheter was inserted into the femoral artery and arterial pressures were collected every minute for 40 min using a BP Analyzer (Digi-Med, Louisville, KY).
Plasma and Tissue Collection
After BP measurements were completed, each animal was euthanized and then blood was collected by cardiac puncture most often in the morning. Plasma was isolated from blood by centrifugation and stored at − 80 °C until further use. The uterus was collected and weighed and the brain was rapidly removed and placed in dry ice for fast cooling and then stored at − 80 °C until further processing. Subsequently, 300-μm-thick coronal brain sections were obtained by using a cryostat (Leica CM 1850, Leica Biosystems Inc., Buffalo Grove, IL). The sections were placed on histological slides kept in contact with dry ice. Biopsy punches of the basolateral amygdala (BLA) and CA1 and CA3 regions of the hippocampus (Hc) were collected and stored at -80 °C until mRNA was isolated from these tissues.
Angiotensin Type 1 Receptor mRNA Expression
AT1R mRNA was measured by real-time PCR. Total RNA from the CA1 & CA3 regions of the Hc and the BLA were extracted using an RNAqueous™-Micro Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA). First-strand cDNA was made from 500 ng of total RNA using a iScript cDNA synthesis kit (BioRad, Hercules, CA) with Moloney Murine Leukemia Virus (MMLV) RNase H + reverse transcriptase, oligo(dT) and random hexamers. RNA concentrations were measured on a NanoDrop 2000 (Thermo Scientific). Quantitation of specific mRNAs was performed by real-time PCR using the ABI StepOnePlus real-time PCR (Applied Biosystems Inc., Foster City, CA). The real-time PCR mixture consisted of RNase-free water, TaqMan Fast Advance Master Mix (Thermo Fisher Scientific), and the following specific primers (300 nM) and probe (10 µM): forward primer: rAT1R-CR-330F 5′-CAACCTCTACGCCAGTGTGTTC-3′; reverse primer: rAT1R-CR-470R:5′-CCAGCCATCAGCCAGATGA-3′; and probe: rAT1aR-CR-382T: Fam-CTGGCCATCGTCCACCCAATGAAGT-Tamra and cDNA samples. PCRs without reverse transcription and no template were included to control for genomic DNA contamination. The standard curve with rAT1aR plasmid DNA was used to determine the DNA copy number in the Hc and BLA samples. The data were expressed in DNA copy numbers × 106 and normalized to µg of total RNA in each sample.
Plasma Corticosterone
Corticosterone was determined in the plasma using the corticosterone ELISA Kit (Neogen, Lexington, KY). Briefly, plasma samples were diluted and added to the microplate and incubated with an enzyme conjugate for 1 hour at room temperature. The plate was then washed to remove unbound material. The bound enzyme conjugate was detected by the addition of substrate, which generated an optimal color after 30 min. Quantitative test results were obtained by measuring and comparing the absorbance reading of the sample wells to the standards using a FLUOstar Omega plate reader (BMG LABTECH Inc., Cary, NC) at 650 nm.
Statistical Analysis
Statistical comparisons were assessed using GraphPad Prism software (version 8, GraphPad Inc., La Jolla, CA). The effect of ovariectomy was analyzed by Student’s t test in both the Veh and Los treatment groups. Two-way ANOVA was used to analyze the effects of ovariectomy and Los treatment. In the NOR and LVS tests, each phase was analyzed individually. Data were expressed as mean ± standard error of the means. Significance was defined as p < 0.05.
Results
Effect of Ovariectomy and Losartan on Uterine Wet Weight and Body Weight
The rodent uterotrophic bioassay is a well-validated test for E2 deficiency (Kleinstreuer et al. 2016). Thus, to confirm that the bilateral ovariectomies were successful, we measured the uterine wet weight (WW) at the end of the behavioral testing. Ten weeks after ovariectomy, uterine WWs decreased by 80% regardless of treatment (p < 0.0001) (Table 1). Initial BWs did not differ across all four groups; however, ovariectomy increased BW gain in both the Veh- and Los-treated groups ten weeks after ovariectomy (p < 0.0001) (Table 1). Losartan had no effect on uterine WW or BW in either the Sham or Ovx animals.
Table 1.
Effect of ovariectomy and Los on uterine WW, BW, MAP, and plasma corticosterone
| Animal group | Uterine WW (g) | Initial BW (g) | Final BW (g) | BW gain (final/initial) | MAP (mm Hg) | Plasma corticosterone (ng/mL) |
|---|---|---|---|---|---|---|
| Sham–Veh | 0.15 ± 0.01 | 259 ± 5.1 | 290 ± 6.6 | 1.12 ± 0.01 | 103 ± 4.2 | 180 ± 32 |
| Ovx–Veh | 0.03 ± 0.002* | 265 ± 4.8 | 338 ± 7.6* | 1.28 ± 0.02* | 98.1 ± 2.0 | 238 ± 20 |
| Sham–Los | 0.14 ± 0.008 | 252 ± 2.6 | 280 ± 3.3 | 1.11 ± 0.01 | 90.2 ± 1.7# | 186 ± 45 |
| Ovx–Los | 0.03 ± 0.001* | 264 ± 1.9 | 328 ± 4.3* | 1.24 ± 0.01* | 88.3 ± 1.1# | 119 ± 42* |
Rats were sham-operated (Sham) or ovariectomized (Ovx) at 3 months of age and treated with vehicle (Veh) or losartan (Los) for 10 weeks before the animals were euthanized and uterine WW, BW, MAP, and plasma corticosterone were determined. For uterine WW and BW: Sham–Veh (n = 14), Ovx–Veh (n = 12), Sham–Los (n = 14), and Ovx–Los (n = 12). For MAP: Sham–Veh (n = 6), Ovx–Veh (n = 6), Sham–Los (n = 12), and Ovx–Los (n = 12). For plasma corticosterone: Sham–Veh (n = 10), Ovx–Veh (n = 10), Sham–Los (n = 10), and Ovx–Los (n = 10). *p < 0.05 vs Sham, same treatment (Student’s t test); #p < 0.05 vs. vehicle, same surgery (Student’s t test). Regardless of treatment, ovariectomy decreased WW (p < 0.0001 by two-way ANOVA (surgery, treatment) F = 248; DFn = 1; DFd = 48) and increased BW gain (p < 0.0001 by two-way ANOVA (surgery, treatment), F = 131; DFn = 1; DFd = 56). Losartan reduced the MAP in both Sham and Ovx rats (p < 0.0001 by two-way ANOVA (surgery, treatment) F = 30.1; DFn = 1; DFd = 32)
Effect of Ovariectomy and Losartan on Mean Arterial Pressure
MAP measured by indwelling catheter in anesthetized Long Evans rats did not increase ten weeks after ovariectomy (Table 1). The animals remained normotensive. Thus, we were able to use this rat strain to study the effects of ovariectomy on behavior independently of BP. Losartan treatment reduced the MAP in both Sham and Ovx rats (p < 0.0001) (Table 1); however, this reduction in MAP did not cause hypotension (≈ MAP < 80 mm Hg) and basal MAP was considered within normal limits.
Effect of Ovariectomy and Losartan on Elevated Plus Maze Behavior
Five weeks after ovariectomy, anxiety-like behavior was assessed in the EPM, which is based on natural rodent behaviors including exploration and avoidance of open areas and height (Korte and De Boer 2003). Ovariectomy had no effect on the distance traveled in the EPM (Fig. 2a) indicating there were no impairments in spontaneous motor activity. Ovariectomy reduced the number of entries (p < 0.05) (Fig. 2b) and percentage of time spent (p < 0.05) (Fig. 2c) in the open arms. These anxiety-like behavioral effects of ovariectomy were prevented by Los treatment.
Fig. 2.
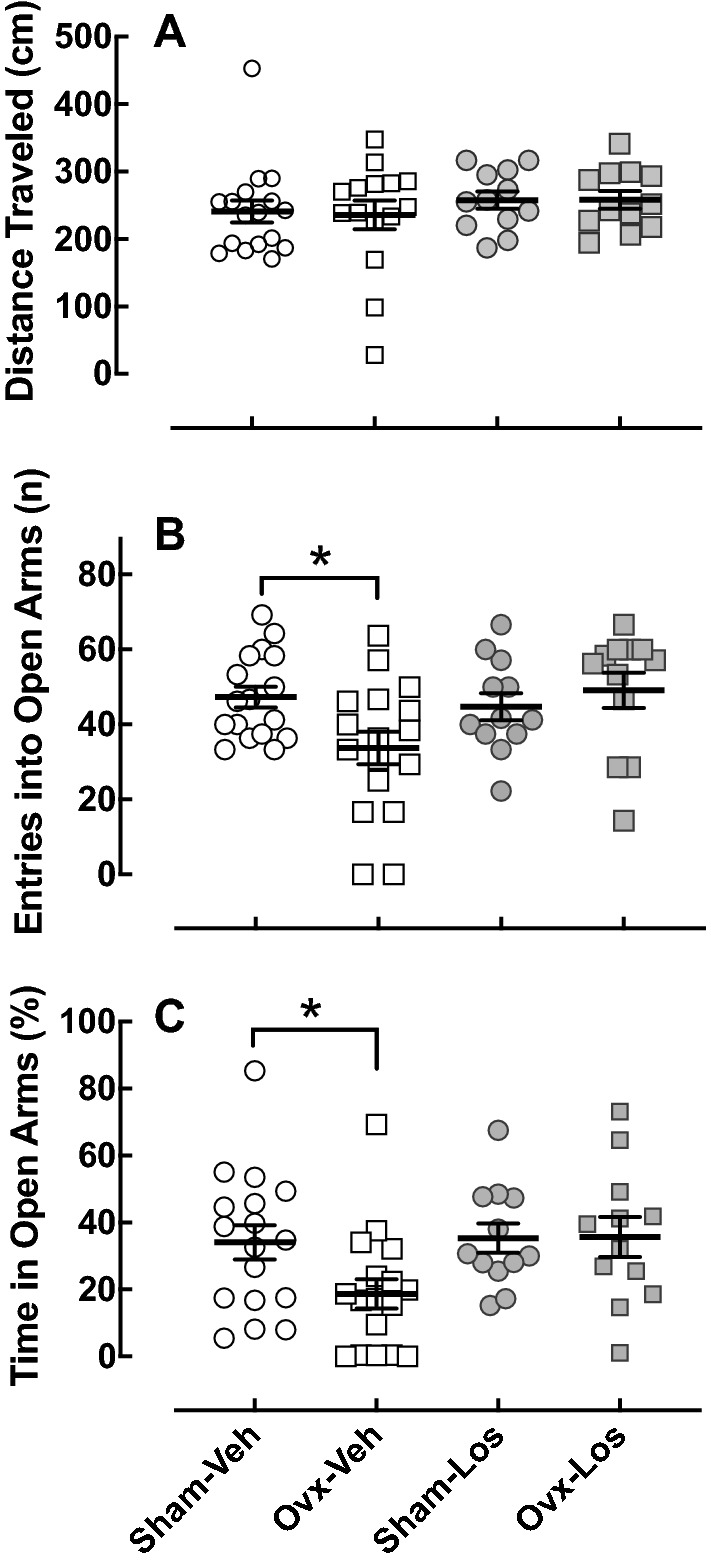
Effect of ovariectomy and losartan on behavior in the elevated plus maze. EPM was assessed in Sham–Veh (n = 17), Ovx–Veh (n = 17), Sham–Los (n = 12), and Ovx–Los (n = 12) animal groups; a distance traveled during the EPM test. b Number of entries into the open arms; Ovariectomy reduced the number of entries into the open arms in Veh-treated rats; *p < 0.05 versus Sham, same treatment (Student’s t test). c Amount of time spent in the open arms; Ovariectomy reduced the amount of time spent in the open arms in Veh-treated rats; *p < 0.05 versus Sham, same treatment (Student’s t test)
Effect of Ovariectomy and Losartan on Open Field Test Behavior
Five and a half weeks after ovariectomy, anxiety was assessed in the OF test, which is based on natural rodent behaviors including exploration and avoidance of open areas (Korte and De Boer 2003). Ovariectomy had no effect on the distance traveled in the OF (Fig. 3a) indicating there were no impairments in spontaneous motor activity. Although there was no effect of ovariectomy on the number of entries into the center field in the Veh-treated group (Fig. 3b), ovariectomy did reduce the time spent in the center of the field (p < 0.05) (Fig. 3c). This anxiety-like behavioral effect of ovariectomy was prevented in the Los-treated animals.
Fig. 3.

Effect of ovariectomy and losartan on behavior in the open field test. The OF test was assessed in Sham–Veh (n = 12), Ovx–Veh (n = 11), Sham–Los (n = 12), and Ovx–Los (n = 12) animal groups; a Distance traveled during the EPM test. b Number of entries into the center field. c Amount of time spent in the center field; Ovariectomy reduced the time spent in the center field in Veh-treated rats; *p < 0.05 vs. Sham, same treatment (Student’s t test)
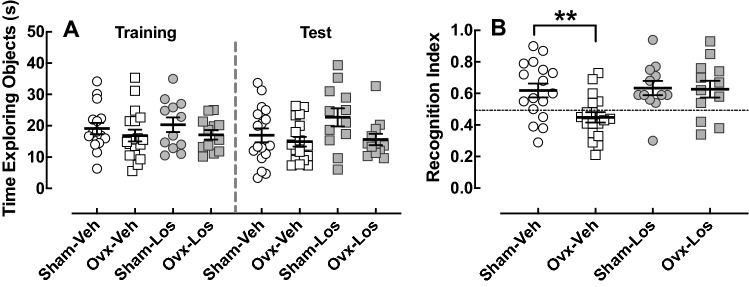
Effect of Ovariectomy and Losartan on the Novel Object Recognition Test
Six weeks after ovariectomy, cognition was assessed by the NOR test, which is based on the natural behavior of rodents to explore new objects compared to familiar ones and is widely used to assess cognitive impairments in rodent models of disease (Grayson et al. 2015). The longer time spent investigating the novel object in comparison with the familiar object indicates short-term memory recognition. There was no effect of ovariectomy on the percentage of total time spent exploring the objects during the training and testing phase (p < 0.05) (Fig. 4a) indicating there were no impairments in spontaneous motor activity or interest in exploring objects. However, ovariectomy reduced the recognition index in the Veh-treated animals to values below 0.5 (p < 0.05) (Fig. 4b), suggesting they did not recognize the familiar object. This impaired cognitive effect of ovariectomy was prevented in the Los-treated animals.
Fig. 4.
Effect of ovariectomy and losartan on novel object recognition. The NOR test was assessed in Sham–Veh (n = 17), Ovx–Veh (n = 18), Sham–Los (n = 12), and Ovx–Los (n = 12) animal groups. a Amount of time spent exploring objects during the training and test periods. b Recognition index for the amount of time spent with the familiar versus novel object. Ovariectomy reduced the recognition index in Veh-treated rats; **p < 0.01 versus Sham, same treatment (Student’s t test)
Effect of Ovariectomy and Losartan on the Looming Visual Stimuli Test
Seven and a half weeks after ovariectomy, defensive responses were assessed in the LVS test, which is based on a rodent’s reflexive behavior in response to potential threat conditions and includes freezing (total absence of movement of the body, attentive attitude, head raised, arched back, retraction of the ears, eyes open, and piloerection) (Yilmaz and Meister 2013). No differences were observed between the Sham and Ovx animals in freezing behavior (Fig. 5a) or number of jumps (Fig. 5b) during the pre-test, test, and post-test periods. During the poststimulus phase, Los treatment extinguished the freezing behavior more rapidly than the Veh-treated animals (p < 0.02) (Fig. 5a).
Fig. 5.
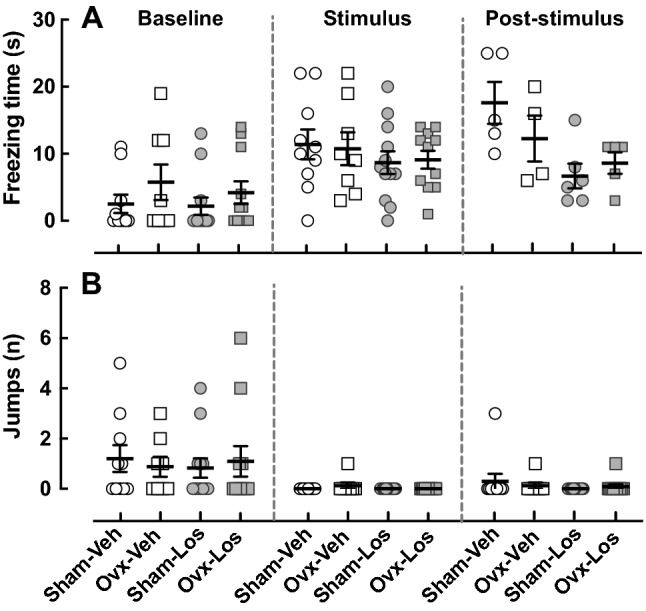
Effect of ovariectomy and losartan on behavior in the looming visual stimuli test. The LVS test was assessed in Sham–Veh (n = 10), Ovx–Veh (n = 8), Sham–Los (n = 12), and Ovx–Los (n = 11) animal groups. a Freezing behavior time during the pre-test, test, and post-test phases. Los treatment extinguished the freezing behavior more rapidly than the Veh-treated animals regardless of surgery in the poststimulus period; p < 0.02 by two-way ANOVA (surgery, treatment) F = 8.53; DFn = 1; DFd = 16. b Number of jumps during the pre-test, test, and post-test phases
Effect of Ovariectomy and Losartan on Sucrose Preference
Eight and a half weeks after ovariectomy, depression-like behavior was assessed in the SP test, which is based on a healthy rodent’s preference for drinking sweet solutions when given the choice over plain water (Sobrian et al. 2003). The absence of this preference is characteristic of anhedonic-like behavior. There was no effect of ovariectomy in the Veh-treated animals on sugar water (Fig. 6a) or tap water (Fig. 6b) intake or sucrose preference (Fig. 6c). While Los had no effect on sugar water intake or sucrose preference, there was a slight increase in tap water intake (p < 0.02) (Fig. 6b).
Fig. 6.
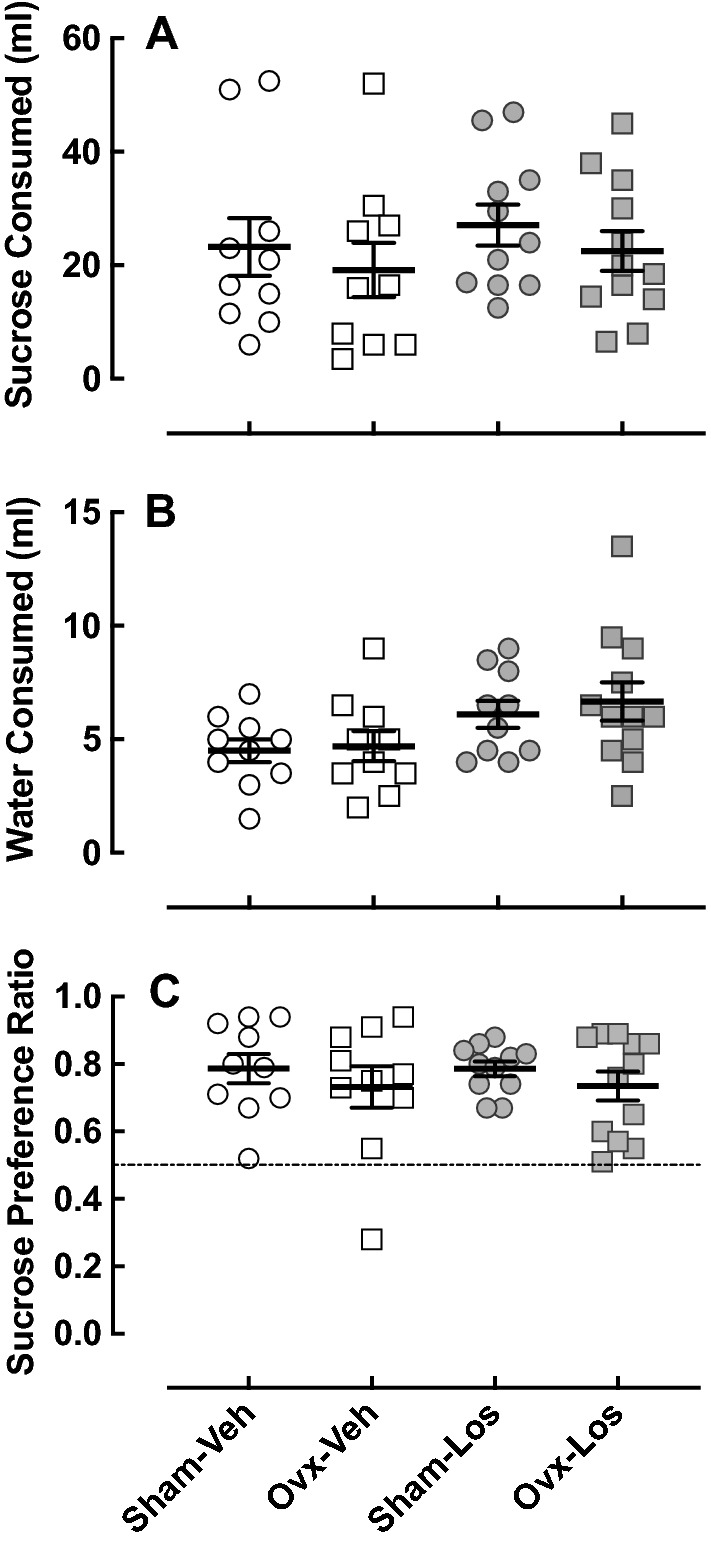
Effect of ovariectomy and losartan on sucrose preference. The sucrose preference test was assessed in Sham–Veh (n = 10), Ovx–Veh (n = 10), Sham–Los (n = 11), and Ovx–Los (n = 12) animal groups. a Daily amount of sugar water consumed. b Daily amount of tap water consumed. Los treatment caused an increase in tap water intake regardless of surgery; p < 0.02 by two-way ANOVA (surgery, treatment), F = 6.70; DFn = 1; DFd = 38. c Ratio of sugar water to tap water consumed
Effect of Losartan on Plasma Corticosterone
Corticosterone is the primary adrenal steroid associated with stress in rodents because they lack the enzyme that converts corticosterone to cortisol (Gallo-Payet and Battista 2014). Thus, corticosterone was determined in the plasma 10 weeks after ovariectomy. There was a trend for ovariectomy to increase plasma corticosterone (Table 1). Furthermore, while Los treatment had no effect on the Sham animals, this ARB markedly reduced plasma corticosterone in the Ovx animals (p < 0.05) (Table 1).
Effect of Ovariectomy and Losartan on Angiotensin Type 1 Receptor Expression in the Amygdala and Hippocampus
The amygdala contributes to anxiety-like behavior in the EPM and OF tests (Tye et al. 2011) while the hippocampus is involved in the NOR task (Cohen and Stackman 2015). Thus, we investigated the effects of ovariectomy on AT1R expression in these brain regions. There was no effect of ovariectomy on AT1R mRNA expression in the amygdala (Fig. 7a) or CA1 region of the hippocampus (Fig. 7b). In contrast, ovariectomy increased AT1R expression in the CA3 region (p < 0.05) (Fig. 7c). Losartan had no effect on AT1 mRNA expression in the amygdala or CA3 region of the Hc; however, Los increased AT1R mRNA in the CA1 region of the Hc (p < 0.05).
Fig. 7.
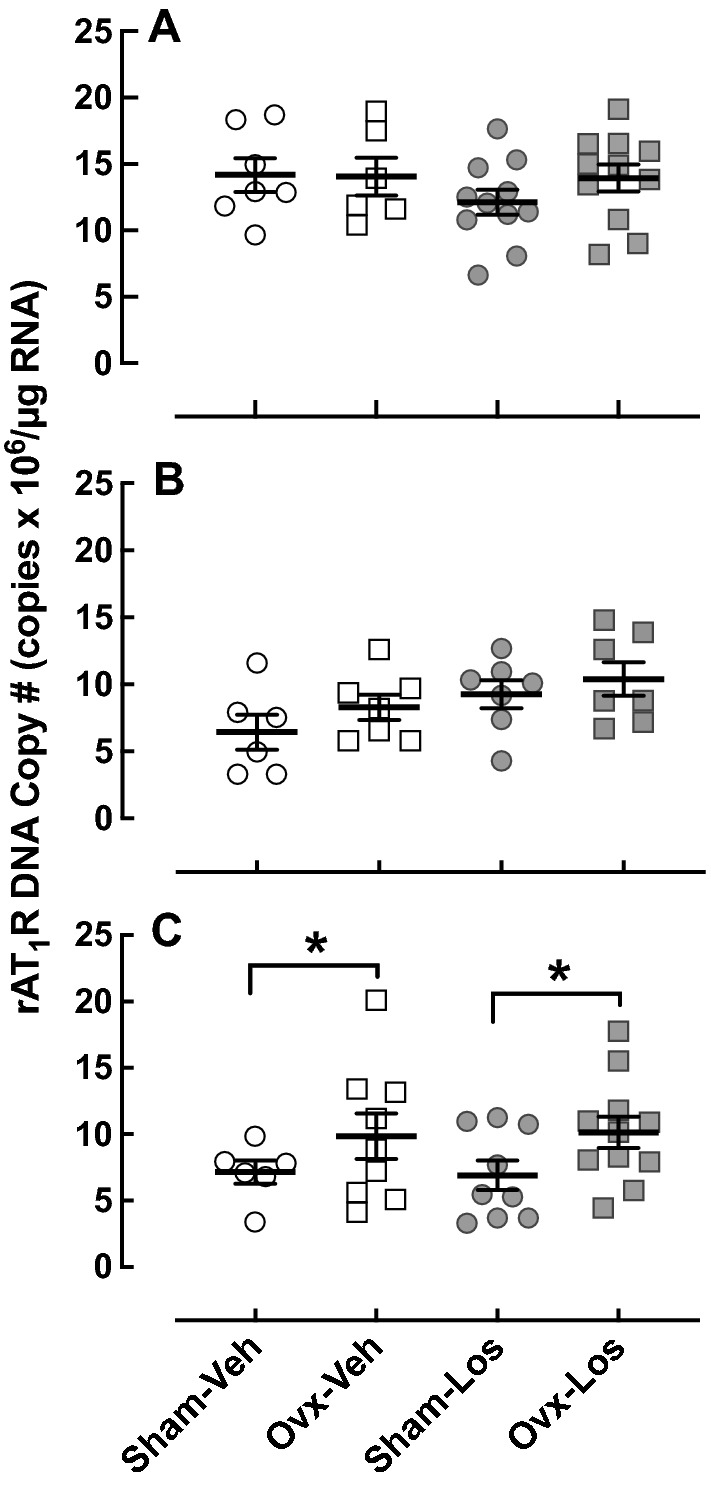
Effect of ovariectomy and losartan on AT1R mRNA expression in the BLA and HC. AT1R mRNA expression was determined by real-time PCR. a BLA [Sham–Veh (n = 7), Ovx–Veh (n = 6), Sham–Los (n = 11), and Ovx–Los (n = 11). b Hc CA1 [Sham–Veh (n = 6), Ovx–Veh (n = 7), Sham–Los (n = 7), and Ovx–Los (n = 7). Los increased AT1R mRNA regardless of surgery; p < 0.05 by two-way ANOVA (surgery, treatment) F = 4.74; DFn = 1; DFd = 23. c Hc CA3 [Sham–Veh (n = 6), Ovx–Veh (n = 9), Sham–Los (n = 9), and Ovx–Los (n = 11)]. Ovariectomy increased AT1R mRNA regardless of treatment; *p < 0.05 vs Sham, same treatment (Student’s t test); p < 0.05 by two-way ANOVA (surgery, treatment) F = 4.79; DFn = 1; DFd = 31
Discussion
A major observation in this study was that Long Evans rats exhibited increased anxiety-like behavior in the EPM and OF tests after ovariectomy. Similar anxiety-like behaviors were shown in rats in the EPM (Zimmerberg and Farley 1993; Azizi-Malekabadi et al. 2015; Fedotova et al. 2017b), OF (Azizi-Malekabadi et al. 2015; Fedotova et al. 2017b) and light–dark exploration (Patki et al. 2013; Fedotova et al. 2017b) tests 4 weeks after ovariectomy. Furthermore, Schoenrock et al. (2016) investigated the behavior of 37 strains of inbred mice in the OF test and noted an overall increase in anxiety-like behavior 2 weeks after ovariectomy although they found the behaviors depended upon the genetic background.
A second major observation was that ovariectomized Long Evans rats were unable to discriminate the new from the previously viewed object in the NOR test. Similar cognitive dysfunction was observed in ovariectomized Sprague–Dawley (Gibbs and Johnson 2008; Wallace et al. 2006; Gogos et al. 2018; Qu et al. 2013; Kim et al. 2016) and Wistar (Patki et al. 2013) rats. Ovariectomy caused impairments in the 12-arm radial maze (Gibbs and Johnson 2008), NOR test (Wallace et al. 2006; Gogos et al. 2018), Morris water maze (Qu et al. 2013; Kim et al. 2016; Patki et al. 2013) and passive avoidance tests (Gogos et al. 2018). Ovariectomy also impaired the cognitive performance of mice in NOR (Fonseca et al. 2013; Bastos et al. 2015; Monthakantirat et al. 2014), Morris water maze (Blair et al. 2016; Monthakantirat et al. 2014), step-through passive avoidance (Cai et al. 2013), and Y maze (Cai et al. 2013; Monthakantirat et al. 2014) tests.
These impairments in anxiety-like behavior and cognitive function produced in different rodent species (rats and mice) and strains (e.g., Long Evans and Sprague–Dawley rats and C57/Bl6, C58/Jj, SJL/J mice) and under various experimental conditions including the age of ovariectomy (8 weeks–9 months), the duration of ovariectomy (1 week to 15 months) and the types of tests assessing anxiety-like disorders (OF, EPM, Y maze, dark–light paradigm) and cognitive performance (NOR, Morris water maze, 12-arm radial maze, passive avoidance) indicates neurocognitive dysfunction in rodents is a widely observed response to sudden ovarian hormone loss-induced by ovariectomy. Thus, the ovariectomized rodent is a useful model for investigating therapeutic strategies for preventing neurocognitive dysfunction that is associated with bilateral oophorectomy in women. Moreover, strain-dependent differences in the degree of dysfunction reflect the human population and can be exploited when designing studies investigating susceptibility and resilience to ovariectomy-induced neurocognitive disorders.
Ovariectomy did not show any effects in the LVS test. To our knowledge, this is the first time that ovariectomized rodents have been assessed in the LVS test. The lack of effect may be due to a ceiling effect since the intact animals stayed frozen during the entire test period and for most of the poststimuli period. Aguilar et al. (Aguilar et al. 2018) showed intact Sprague–Dawley rats under the same LVS protocol exhibited a freezing response profile that was similar to our findings in the Long Evans rats. We cannot rule out the possibility that other tests of fear conditioning might reveal an inhibitory effect of ovariectomy on fear extinction in these normotensive rats.
We did not find an effect of ovariectomy on the SP test. Both Sham and Ovx animals showed a clear preference for drinking sweet water over tap water. Our results are supported by previous reports in rodents showing ovariectomy had no effect on the SP test unless the rodents were subjected to forced swimming (Gogos et al. 2018; Bastos et al. 2015), tail suspension (Bastos et al. 2015), or chronic mild stress (Rygula et al. 2008; Romano-Torres and Fernandez-Guasti 2010; Huang et al. 2015). These findings suggest ovariectomy in the absence of a stressor is insufficient to cause anhedonic-like behavior in young adult rats. However, it is also possible that another behavioral test of anhedonic-like behavior would detect depressive-like symptoms induced by ovariectomy in these rats.
Not all women develop hypertension after bilateral oophorectomy. Similarly, while ovarian hormone loss is often associated with increased BP, the effect on BP depends upon the animal model (Sandberg and Ji 2012). Ovariectomy was shown to increase BP in the spontaneous hypertensive rat, Dahl salt-sensitive rat, and mRen2-Lewis rat, among others (Sandberg and Ji 2012). In contrast, ovariectomy did not increase BP in Dahl salt-resistant rats (Zheng et al. 2008; Pai et al. 2018). In this study, we found Long Evans rats also remained normotensive and their MAP was not elevated compared to sham-operated animals 6 weeks after ovariectomy. Therefore, the Long Evans rat is a valuable normotensive model in which to study anxiety-like behavior and cognitive dysfunction induced by ovarian hormone loss in the absence of BP modulation by ovariectomy.
The major finding of our study was that Los prevented anxiety-like behavior and cognitive impairments in a normotensive animal. These observations support previous studies indicating ARBs have BP-independent neuroprotective effects. Much literature points to a positive correlation between hypertension and neurocognitive disorders. Individuals with hypertension performed worse on tasks that measured speed of information processing, short-term memory, and reaction time and they reported higher levels of anxiety than their normotensive counterparts (Blumenthal et al. 1993). Furthermore, an early placebo-controlled study of systolic hypertension in Europe found there was a lower incidence of dementia in individuals on antihypertensive treatment compared to placebo controls (Forette et al. 1998). These clinical studies suggest the neuroprotective effects of ARBs are in part due to their antihypertensive effects. However, the magnitude of ARB neuroprotection varied independently of their effectiveness for lowering BP (Yasar et al. 2013; Davies et al. 2011; Johnson et al. 2012; Li et al. 2010). Thus, clinical findings suggest that ARBs exert neuroprotection via BP-dependent and BP-independent pathways. Studies in animals support these findings. Treatment with an ARB improved age-associated memory dysfunction in a passive avoidance test in male rats under conditions in which BP was not reduced (Hirawa et al. 1999). Similarly, ARBs attenuated hypertension-associated cognitive dysfunction in male rats at doses that did not alter BP (Pelisch et al. 2011). ARBs also exerted neuroprotective effects on cognition independently of BP alterations in a male model of postoperative cognitive dysfunction (Li et al. 2014).
Bilateral oophorectomy is strongly associated with BW gain in humans and animals (Roepke 2009) and is a widely reported effect of E2 deficiency (Gonzalez-Garcia et al. 2017). Accordingly, we found Ovx rats gained more than twice as much weight compared to Sham animals over the 10-week experiment. Excessive BW is a risk factor for mood disorders (Rocca et al. 2009) and cognitive impairment (Zanini et al. 2017) in humans and animals (Zanini et al. 2017). However, it has been difficult to tease apart BW gain from ovarian hormone loss in the mechanisms contributing to Ovx-induced neurocognitive dysfunction. Ovx-induced BW gain can be prevented with E2 replacement (Hinojosa-Laborde et al. 2004; Roesch 2006) and E2 treatment is cognitively protective (Fonseca et al. 2013). Exercise, which can reduce body weight and alter body composition, also has neurocognitive protective effects (Kim et al. 2016). Therefore, our finding that Los prevents anxiety-like behavior and impairment of novel object recognition without reducing the Ovx-induced BW gain indicates the neuroprotective effects of this AT1R antagonist are either independent of neurocognitive dysfunction induced by excessive BW gain or the neuroprotective mechanisms of Los are downstream from those causing BW gain.
Our finding that plasma corticosterone levels were lower in Ovx rats treated with Los compared to Veh-treated Ovx animals and that Los also attenuated the freezing behavior during the poststimulus period of the LVS test suggests blocking AT1Rs reduces stress pathways and facilitates fear extinction. These observations support previous studies in male rats showing that blockade of central AT1Rs with the ARB candesartan decreased corticotrophin releasing factor (CRF) responses to stress (Armando et al. 2001, 2007). These studies showed candesartan reduced the expression of CRF and circulating corticosterone.
One explanation for our finding that Los treatment reduced plasma corticosterone in Ovx but not in the Sham animals is that Los selectively reduced hypothalamic CRF production induced by AT1Rs activated by ovariectomy. Activation of AT1Rs is known to induce the synthesis and secretion of hypothalamic CRF leading to corticosterone release (Raasch et al. 2006). Thus, increased activity of Hc CA3 AT1Rs contributes to stress-induced stimulation of the hypothalamic pituitary axis. In addition, studies have shown increased levels of corticosterone is associated with impaired learning and memory in rodents and humans (Joels et al. 2018). Therefore, inhibition of the corticosterone stress response may also contribute to the ability of Los to prevent cognitive deficits in the NOR test.
The observation that ovariectomy increased AT1R expression in the CA3 region of the Hc extends previous reports showing ovariectomy upregulates AT1R expression in other angiotensin II target tissues (Hinojosa-Laborde et al. 2004; Owonikoko et al. 2004). Activation of AT1Rs is known to increase oxidative stress, which in turn can induce cell death (Jackson et al. 2018). Ovariectomy was shown to increase markers of oxidative stress and apoptotic injury in primary rat hippocampal neurons (Yazgan and Naziroglu 2017) and to increase the number of apoptotic cells in CA3 (Sales et al. 2010; Peng et al. 2012). The CA3 region of the Hc is known to be involved in memory and cognition (Rolls 2013). Thus, the ovariectomy-induced increase in AT1R expression in the CA3 region of the Hc could contribute to cognitive impairment through increased oxidative stress and cell death. Taken together, these observations suggest the ability of this ARB to protect novel object recognition in the NOR test involves blockade of increased Hc CA3 AT1R activity.
Every year approximately 600,000 women elect bilateral oophorectomy in the USA (Rocca et al. 2009). These women are most often younger than the natural age of menopause (Armstrong et al. 2004). Our findings that ARBs prevent both anxiety-like behavior and cognitive decline induced by ovariectomy have immediate translational value for women who have bilateral oophorectomies. Thus, it will be worthwhile to investigate the neuroprotective effects of ARBs in controlled clinical trials in women who undergo surgical menopause prior to the natural age of menopause and to delve into the mechanisms underlying Los neurocognitive protection.
The neuroprotective effects of ARBs may also extrapolate to other populations of women who are ovarian hormone deficient. Premature ovarian failure is a syndrome defined as a non-physiological cessation of ovarian function before 40 years of age in contrast to 51, the average age of menopause (de Moraes-Ruehsen and Jones 1967). The hormonal profile of these women is similar to what occurs during the early physiological postmenopausal phase and is characterized by low levels of estrogen and high levels of follicle-stimulating hormone (Jankowska 2017; Barrett et al. 2019). Although women with premature ovarian failure do not have the sudden drop in ovarian hormones that women who undergo bilateral oophorectomies experience, studies in rats and mice suggest symptoms of anxiety are worse the longer the duration of ovarian hormone deficiency (Lagunas et al. 2010; de Chaves et al. 2009; Citraro et al. 2015). Daendee et al. (Daendee et al. 2013) conducted a time course of the effects of ovariectomy duration and showed that the percentage of rats exhibiting anxiety-like behavior in the elevated T maze increased from ~ 30 to ~ 60% and 90%, 2, 3, and 4 weeks after ovariectomy, respectively. Thus, ARBs may also be neuroprotective in women with premature ovarian failure given that their life-long exposure to ovarian hormone deficiency is increased compared to women who experience normal menopause.
While many drugs are used to treat anxiety including benzodiazepines, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, tricyclic antidepressants, or monoamine oxidase inhibitors (Fedotova et al. 2017a), they are often associated with adverse side effects that negatively impact one’s basic activities and general quality of life (Koen and Stein 2011; Trindade et al. 1998). Unfortunately, there are no particularly efficacious drugs on the market for preventing cognitive decline in individuals with mild cognitive impairment or AD (Atri 2019). Thus, a great advantage of using ARBs as a new therapeutic strategy for neuropsychiatric disorders of anxiety and cognitive impairment is the low incidence of side effects, which makes these drugs generally well tolerated; adverse effects of ARBs occur in less than 10% of patients (Barreras and Gurk-Turner 2003).
In conclusion, our findings showed that the ARB Los prevented anxiety-like behavior and memory impairments induced by ovariectomy in Long Evans rats. These neuroprotective effects of Los occurred under normotensive conditions and were independent of ovariectomy-induced BW gain. The ability of Los to lower circulating levels of corticosterone in the Ovx rat suggests the neurocognitive protective effects of this ARB are in part due to reducing activation of stress pathways. Furthermore, the ovariectomy-induced increases in AT1R expression in the CA3 region of the Hc suggests blocking the activity of increased AT1Rs in this region contributes to the neuroprotective effects of Los. This study can inform the design of controlled clinical trials aimed at testing the neurocognitive protective effects of ARB therapy in normotensive women who are at higher risk of developing neuropsychiatric dysfunction because they had bilateral oophorectomies prior to natural age of menopause.
Acknowledgements
The authors thank Drs. Sylvana Noronha and Deoclécio Chianca Júnior for constructive feedback on the project.
Author Contributions
GVC, RCdM, PAF, AMAdS, CAW, and KS conceived and designed the research; GVC, HJ, XW, BLA, and DLL performed experiments; GVC, HJ, BLA, and DLL analyzed the data; GVC, AMAdS, BLA, and CAW interpreted the results of experiments; GVC and HJ prepared figures; GVC, HJ, DLL, and KS drafted the manuscript; GVC, AMAdS, CAW, PAF, and RCdM edited and revised the manuscript; and GVC, AMAdS, HJ, CAW, XW, DLL, BLA, PAF, RCdM, and KS approved the final version of the manuscript.
Funding
This study was funded by National Heart, Lung, and Blood Institute (Grant No. AG060730), Universidade Federal de Ouro Preto, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant No. 304628/2017-4; and Programa de Doutorado Sanduíche no Exterior—PDSE, process #88881.132512/2016-1).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures were approved by the Georgetown University Animal Care and Use Committee (#2017-0068) and conducted in accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used in this study.
Research Involving Human Participants
This article does not contain any studies with human subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar BL, Malkova L, N’Gouemo P, Forcelli PA (2018) Genetically epilepsy-prone rats display anxiety-like behaviors and neuropsychiatric comorbidities of epilepsy. Front Neurol 9:476. 10.3389/fneur.2018.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Barontini M, Ito T, Saavedra JM (2001) Candesartan decreases the sympatho-adrenal and hormonal response to isolation stress. J Renin Angiotensin Aldosterone Syst 2(1_suppl):S130–S135. 10.1177/14703203010020012301 [DOI] [PubMed] [Google Scholar]
- Armando I, Volpi S, Aguilera G, Saavedra JM (2007) Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res 1142:92–99. 10.1016/j.brainres.2007.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B (2004) Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol 22(6):1045–1054. 10.1200/JCO.2004.06.090 [DOI] [PubMed] [Google Scholar]
- Atri A (2019) Current and future treatments in Alzheimer’s disease. Semin Neurol 39(2):227–240. 10.1055/s-0039-1678581 [DOI] [PubMed] [Google Scholar]
- Azizi-Malekabadi H, Pourganji M, Zabihi H, Saeedjalali M, Hosseini M (2015) Tamoxifen antagonizes the effects of ovarian hormones to induce anxiety and depression-like behavior in rats. Arq Neuropsiquiatr 73(2):132–139. 10.1590/0004-282X20140221 [DOI] [PubMed] [Google Scholar]
- Barreras A, Gurk-Turner C (2003) Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent) 16(1):123–126. 10.1080/08998280.2003.11927893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KE, Barman SM, Yuan J, Brooks HL (2019) Ganong’s review of medical physiology, 26th edn. McGraw-Hill Medical, New York [Google Scholar]
- Bastos CP, Pereira LM, Ferreira-Vieira TH, Drumond LE, Massensini AR, Moraes MF, Pereira GS (2015) Object recognition memory deficit and depressive-like behavior caused by chronic ovariectomy can be transitorialy recovered by the acute activation of hippocampal estrogen receptors. Psychoneuroendocrinology 57:14–25. 10.1016/j.psyneuen.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Blair JA, Palm R, Chang J, McGee H, Zhu X, Wang X, Casadesus G (2016) Luteinizing hormone downregulation but not estrogen replacement improves ovariectomy-associated cognition and spine density loss independently of treatment onset timing. Horm Behav 78:60–66. 10.1016/j.yhbeh.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Madden DJ, Pierce TW, Siegel WC, Appelbaum M (1993) Hypertension affects neurobehavioral functioning. Psychosome Med 55(1):44–50. 10.1097/00006842-199301000-00008 [DOI] [PubMed] [Google Scholar]
- Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, de Jager PL (2014) Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 82(3):222–229. 10.1212/WNL.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZL, Wang CY, Gu XY, Wang NJ, Wang JJ, Liu WX, Xiao P, Li CH (2013) Tenuigenin ameliorates learning and memory impairments induced by ovariectomy. Physiol Behav 118:112–117. 10.1016/j.physbeh.2013.05.025 [DOI] [PubMed] [Google Scholar]
- Citraro R, Gallelli L, Leo A, De Fazio P, Gallelli P, Russo E, De Sarro G (2015) Effects of chronic sodium alendronate on depression and anxiety in a menopausal experimental model. Pharmacol Biochem Behav 129:65–71. 10.1016/j.pbb.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. 10.1016/j.bbr.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daendee S, Thongsong B, Kalandakanond-Thongsong S (2013) Effects of time of estrogen deprivation on anxiety-like behavior and GABAA receptor plasticity in ovariectomized rats. Behav Brain Res 246:86–93. 10.1016/j.bbr.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM (2011) Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis 26(4):699–708. 10.3233/JAD-2011-110347 [DOI] [PubMed] [Google Scholar]
- de Chaves G, Moretti M, Castro AA, Dagostin W, da Silva GG, Boeck CR, Quevedo J, Gavioli EC (2009) Effects of long-term ovariectomy on anxiety and behavioral despair in rats. Physiol Behav 97(3–4):420–425. 10.1016/j.physbeh.2009.03.016 [DOI] [PubMed] [Google Scholar]
- de Moraes-Ruehsen M, Jones GS (1967) Premature ovarian failure. Fertil Steril 18(4):440–461. 10.1016/s0015-0282(16)36362-2 [DOI] [PubMed] [Google Scholar]
- Drumond LE, Mourao FA, Leite HR, Abreu RV, Reis HJ, Moraes MF, Pereira GS, Massensini AR (2012) Differential effects of swimming training on neuronal calcium sensor-1 expression in rat hippocampus/cortex and in object recognition memory tasks. Brain Res Bull 88(4):385–391. 10.1016/j.brainresbull.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Faure S, Oudart N, Javellaud J, Fournier A, Warnock DG, Achard JM (2006) Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J Hypertens 24(11):2255–2261. 10.1097/01.hjh.0000249704.34607.4c [DOI] [PubMed] [Google Scholar]
- Fedotova J, Kubatka P, Busselberg D, Shleikin AG, Caprnda M, Dragasek J, Rodrigo L, Pohanka M, Gasparova I, Nosal V, Opatrilova R, Qaradakhi T, Zulli A, Kruzliak P (2017a) Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed Pharmacother 95:437–446. 10.1016/j.biopha.2017.08.107 [DOI] [PubMed] [Google Scholar]
- Fedotova J, Zarembo D, Dragasek J, Caprnda M, Kruzliak P, Dudnichenko T (2017b) Modulating effects of cholecalciferol treatment on estrogen deficiency-induced anxiety-like behavior of adult female rats. Folia Med (Plovdiv) 59(2):139–158. 10.1515/folmed-2017-0022 [DOI] [PubMed] [Google Scholar]
- Fonseca CS, Gusmao ID, Raslan AC, Monteiro BM, Massensini AR, Moraes MF, Pereira GS (2013) Object recognition memory and temporal lobe activation after delayed estrogen replacement therapy. Neurobiol Learn Mem 101:19–25. 10.1016/j.nlm.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R (1998) Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 352(9137):1347–1351. 10.1016/s0140-6736(98)03086-4 [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Battista MC (2014) Steroidogenesis-adrenal cell signal transduction. Compr Physiol 4(3):889–964. 10.1002/cphy.c130050 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA (2008) Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149(6):3176–3183. 10.1210/en.2007-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, McCarthy M, Walker AJ, Udawela M, Gibbons A, Dean B, Kusljic S (2018) Differential effects of chronic 17beta-oestradiol treatment on rat behaviours relevant to depression. J Neuroendocrinol 30(11):e12652. 10.1111/jne.12652 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia I, Tena-Sempere M, Lopez M (2017) Estradiol regulation of brown adipose tissue thermogenesis. Adv Exp Med Biol 1043:315–335. 10.1007/978-3-319-70178-3_15 [DOI] [PubMed] [Google Scholar]
- Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC (2015) Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res 285:176–193. 10.1016/j.bbr.2014.10.025 [DOI] [PubMed] [Google Scholar]
- Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ (2016) Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology 87(7):699–708. 10.1212/WNL.0000000000002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K (2004) Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44(4):405–409. 10.1161/01.HYP.0000142893.08655.96 [DOI] [PubMed] [Google Scholar]
- Hirawa N, Uehara Y, Kawabata Y, Numabe A, Gomi T, Ikeda T, Suzuki T, Goto A, Toyo-oka T, Omata M (1999) Long-term inhibition of renin-angiotensin system sustains memory function in aged Dahl rats. Hypertension 34(3):496–502. 10.1161/01.hyp.34.3.496 [DOI] [PubMed] [Google Scholar]
- Huang H, Zhao J, Jiang L, Xie Y, Xia Y, Lv R, Dong L (2015) Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int J Clin Exp Med 8(4):5103–5111 [PMC free article] [PubMed] [Google Scholar]
- Jackson L, Eldahshan W, Fagan SC, Ergul A (2018) Within the brain: the renin angiotensin system. Int J Mol Sci 19(3):876. 10.3390/ijms19030876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska K (2017) Premature ovarian failure. Prz Menopauzalny 16(2):51–56. 10.5114/pm.2017.68592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Falconetti C, Roesch DM, Mulroney SE, Sandberg K (2007) 17beta-estradiol deficiency reduces potassium excretion in an angiotensin type 1 receptor-dependent manner. Am J Physiol Heart Circ Physiol 293(1):H17–H22. 10.1152/ajpheart.00950.2006 [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Sarabdjitsingh RA (2018) The stressed brain of humans and rodents. Acta Physiol (Oxf) 223(2):e13066. 10.1111/apha.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Parikh N, Kunik ME, Schulz PE, Patel JG, Chen H, Aparasu RR, Morgan RO (2012) Antihypertensive drug use and the risk of dementia in patients with diabetes mellitus. Alzheimers Dement 8(5):437–444. 10.1016/j.jalz.2011.05.2414 [DOI] [PubMed] [Google Scholar]
- Kim TW, Kim CS, Kim JY, Kim CJ, Seo JH (2016) Combined exercise ameliorates ovariectomy-induced cognitive impairment by enhancing cell proliferation and suppressing apoptosis. Menopause 23(1):18–26. 10.1097/GME.0000000000000486 [DOI] [PubMed] [Google Scholar]
- Kleinstreuer NC, Ceger PC, Allen DG, Strickland J, Chang X, Hamm JT, Casey WM (2016) A curated database of rodent uterotrophic bioactivity. Environ Health Perspect 124(5):556–562. 10.1289/ehp.1510183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen N, Stein DJ (2011) Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci 13(4):423–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, De Boer SF (2003) A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 463(1–3):163–175. 10.1016/s0014-2999(03)01279-2 [DOI] [PubMed] [Google Scholar]
- Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM (2010) Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm Behav 58(5):786–791. 10.1016/j.yhbeh.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B (2010) Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340:b5465. 10.1136/bmj.b5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cao Y, Li L, Liang Y, Tian X, Mo N, Liu Y, Li M, Chui D, Guo X (2014) Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun 449(1):74–80. 10.1016/j.bbrc.2014.04.153 [DOI] [PubMed] [Google Scholar]
- Monthakantirat O, Sukano W, Umehara K, Noguchi H, Chulikhit Y, Matsumoto K (2014) Effect of miroestrol on ovariectomy-induced cognitive impairment and lipid peroxidation in mouse brain. Phytomedicine 21(11):1249–1255. 10.1016/j.phymed.2014.06.012 [DOI] [PubMed] [Google Scholar]
- O’Hagan TS, Wharton W, Kehoe PG (2012) Interactions between oestrogen and the renin angiotensin system—potential mechanisms for gender differences in Alzheimer’s disease. Am J Neurodegener Dis 1(3):266–279 [PMC free article] [PubMed] [Google Scholar]
- Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, Ravert HT, Rauseo P, Sandberg K, Dannals RF, Szabo Z (2004) In vivo investigation of estrogen regulation of adrenal and renal angiotensin (AT1) receptor expression by PET. J Nucl Med 45(1):94–100 [PMC free article] [PubMed] [Google Scholar]
- Pai AV, West CA, de Souza AMA, Cheng X, West DA Jr, Ji H, Wu X, Baylis C, Sandberg K (2018) Salt-sensitive (Rapp) rats from Envigo spontaneously develop accelerated hypertension independent of ovariectomy on a low-sodium diet. Am J Physiol Regul Integr Comp Physiol 315(5):R915–R924. 10.1152/ajpregu.00449.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, Asghar M, Jafri F, Bohat R, Alkadhi KA, Salim S (2013) Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PLoS ONE 8(9):e74522. 10.1371/journal.pone.0074522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, Nishiyama A (2011) Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens 24(3):362–368. 10.1038/ajh.2010.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167. 10.1016/0165-0270(85)90031-7 [DOI] [PubMed] [Google Scholar]
- Peng Y, Jiang B, Wu H, Dai R, Tan L (2012) Effects of genistein on neuronal apoptosis, and expression of Bcl-2 and Bax proteins in the hippocampus of ovariectomized rats. Neural Regen Res 7(36):2874–2881. 10.3969/j.issn.1673-5374.2012.36.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G (2010) Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 30(1):43–50. 10.1159/000314681 [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1–3):3–33. 10.1016/s0014-2999(03)01272-x [DOI] [PubMed] [Google Scholar]
- Qu N, Wang L, Liu ZC, Tian Q, Zhang Q (2013) Oestrogen receptor alpha agonist improved long-term ovariectomy-induced spatial cognition deficit in young rats. Int J Neuropsychopharmacol 16(5):1071–1082. 10.1017/S1461145712000958 [DOI] [PubMed] [Google Scholar]
- Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Johren O (2006) Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinology 147(7):3539–3546. 10.1210/en.2006-0198 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ 3rd (2007) Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69(11):1074–1083. 10.1212/01.wnl.0000276984.19542.e6 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de Andrade M, Melton LJ 3rd (2008) Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 15(6):1050–1059. 10.1097/gme.0b013e318174f155 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ 3rd (2009) Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond) 5(1):39–48. 10.2217/17455057.5.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT (2010) Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis 7(1–3):163–166. 10.1159/000289229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Gazzuola-Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM (2016) Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 91(11):1577–1589. 10.1016/j.mayocp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA (2009) Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol 21(2):141–150. 10.1111/j.1365-2826.2008.01814.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch DM (2006) Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87(1):39–44. 10.1016/j.physbeh.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2013) A quantitative theory of the functions of the hippocampal CA3 network in memory. Front Cell Neurosci 7:98. 10.3389/fncel.2013.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Torres M, Fernandez-Guasti A (2010) Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress. Behav Pharmacol 21(2):104–111. 10.1097/FBP.0b013e328337bdfc [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flugge G (2008) Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol 19(3):183–196. 10.1097/FBP.0b013e3282fe8871 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E (2006) A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology 31(6):1123–1134. 10.1038/sj.npp.1300921 [DOI] [PubMed] [Google Scholar]
- Sales S, Ureshino RP, Pereira RT, Luna MS, Pires de Oliveira M, Yamanouye N, Godinho RO, Smaili SS, Porto CS, Abdalla FM (2010) Effects of 17beta-estradiol replacement on the apoptotic effects caused by ovariectomy in the rat hippocampus. Life Sci 86(21–22):832–838. 10.1016/j.lfs.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Sandberg K, Ji H (2012) Sex differences in primary hypertension. Biol Sex Differ 3(1):7. 10.1186/2042-6410-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenrock SA, Oreper D, Young N, Ervin RB, Bogue MA, Valdar W, Tarantino LM (2016) Ovariectomy results in inbred strain-specific increases in anxiety-like behavior in mice. Physiol Behav 167:404–412. 10.1016/j.physbeh.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH, Women’s Health Initiative Memory S (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291(24):2947–2958. 10.1001/jama.291.24.2947 [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Marr L, Ressman K (2003) Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry 27(3):501–518. 10.1016/S0278-5846(03)00042-3 [DOI] [PubMed] [Google Scholar]
- Timaru-Kast R, Wyschkon S, Luh C, Schaible EV, Lehmann F, Merk P, Werner C, Engelhard K, Thal SC (2012) Delayed inhibition of angiotensin II receptor type 1 reduces secondary brain damage and improves functional recovery after experimental brain trauma. Crit Care Med 40(3):935–944. 10.1097/CCM.0b013e31822f08b9 [DOI] [PubMed] [Google Scholar]
- Trindade E, Menon D, Topfer LA, Coloma C (1998) Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 159(10):1245–1252 [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471(7338):358–362. 10.1038/nature09820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126(1):176–182. 10.1016/j.brainres.2006.07.064 [DOI] [PubMed] [Google Scholar]
- Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y, Li J, Zhang Y, Zhou X, Xu L, Chen L, Bi G, Hu X, Xu F, Wang L (2015) Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun 6:6756. 10.1038/ncomms7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, DeKosky ST, Carlson MC, Ginkgo Evaluation of Memory Study I (2013) Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology 81(10):896–903. 10.1212/wnl.0b013e3182a35228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazgan Y, Naziroglu M (2017) Ovariectomy-induced mitochondrial oxidative stress, apoptosis, and calcium ion influx through TRPA1, TRPM2, and TRPV1 are prevented by 17beta-estradiol, tamoxifen, and raloxifene in the hippocampus and dorsal root ganglion of rats. Mol Neurobiol 54(10):7620–7638. 10.1007/s12035-016-0232-5 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Meister M (2013) Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol 23(20):2011–2015. 10.1016/j.cub.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini P, Arbo BD, Niches G, Czarnabay D, Benetti F, Ribeiro MF, Cecconello AL (2017) Diet-induced obesity alters memory consolidation in female rats. Physiol Behav 180:91–97. 10.1016/j.physbeh.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Zheng W, Ji H, Maric C, Wu X, Sandberg K (2008) Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 294(4):H1508–H1513. 10.1152/ajpheart.01322.2007 [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Farley MJ (1993) Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiol Behav 54(6):1119–1124. 10.1016/0031-9384(93)90335-d [DOI] [PubMed] [Google Scholar]