Abstract
Proteinuria is a hallmark of kidney disease. Therefore, measurement of urine protein content plays a central role in any diagnostic work-up for kidney disease. In many cases, proteinuria analysis is restricted to the measurement of total protein content knowing that very high levels of proteinuria (nephrotic proteinuria) are characteristic of glomerular disease. Still, proteinuria can also be a manifestation of impaired tubular protein reabsorption or even be physiological. This review will discuss the physiology of renal protein handling and give guidance on a more sophisticated analysis of proteinuria differentiating albumin, low-molecular weight proteins and immunoglobulins. These non-invasive tests are available in most routine clinical laboratories and may guide the clinician in the diagnostic process before ordering far more expensive (molecular genetic testing) and/or invasive (kidney biopsy) diagnostics.
Keywords: Proteinuria , Low molecular weight proteins, Tubulointerstitial disease, Glomerular disease, Acute kidney injury, Selectivity
Introduction
Besides serum creatinine, blood pressure, and urinalysis, the measurement of urinary protein excretion plays a central role in the recognition and classification of renal disease. Even small amounts of proteinuria, i.e., microalbuminuria, are associated with dismal outcomes and are therefore included in the staging of chronic kidney disease according to the KIDGO guidelines [1]. This is even more so for nephrotic range proteinuria. As the intact glomerular filter is almost impermeable to large proteins, proteinuria is a hallmark of glomerular disease. Still, significant proteinuria can also be found in tubulointerstitial disease, which can pose a diagnostic challenge. This is illustrated in the case presented by Preston et al. [2] in this issue of Pediatric Nephrology. The paper by Beara-Lasic et al. [3] also published in this issue demonstrates that a more detailed analysis of urinary protein excretion can distinguish glomerular from tubulointerstitial disease and pure tubular proteinuria. Of note, their approach only requires measurement of α1-microglobulin on top of the standard parameters, i.e., urinary albumin, total protein, and creatinine. The present review will put their findings in a broader perspective and focus on the physiology and diagnostic potential of low-molecular weight (LMW) proteins in the urine. It will not address albuminuria in detail, a finding which has received much more attention and been extensively reviewed elsewhere [4–11].
Filtration and reabsorption of plasma proteins
Under normal circumstances, urine is almost free of protein (i.e., proteinuria < 4 mg/m2/h or protein-creatinine ratio of < 180 mg/g (20 mg/mmol)). Still, there are three situations when proteinuria may be physiological: (i) orthostatic proteinuria [12], (ii) febrile proteinuria, and (iii) exercise proteinuria [13, 14]. In all these situations, proteinuria is transient and hence must be absent when tested in a first morning urine sample collected directly after getting up, after recovery from the febrile condition, or after recovery from strenuous exercise, respectively.
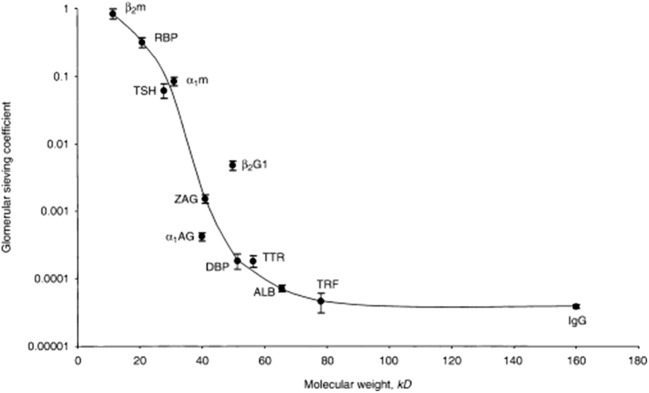
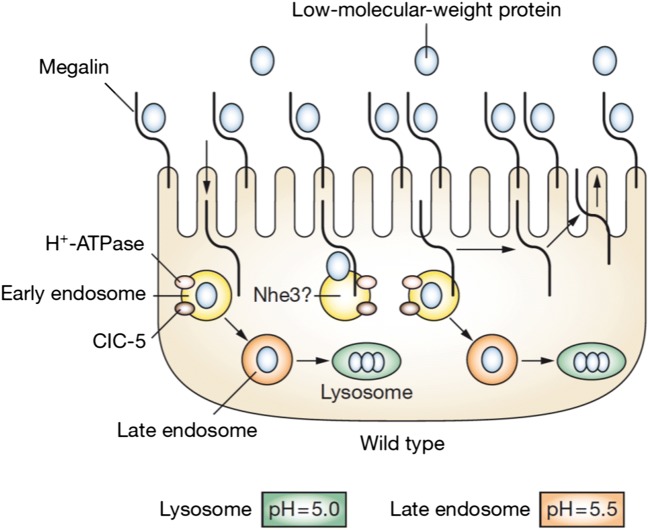
Water and small solutes up to the size of inulin (5 kDa) can pass the glomerular filter freely. For larger molecules, permeability is inversely related to molecular size. Therefore, LMW proteins with a molecular mass between 10 and 20 kDa such as α1-microglobulin, β-2 microglobulin, cystatin C, retinol-binding protein (RBP), and many other macromolecules including hormones and cytokines also pass the glomerular filter in considerable amounts (Fig. 1). Still, the final urine contains negligible amounts of LMW proteins. This is due to the extensive reabsorption of proteins in the proximal tubule by receptor-mediated multi-ligand endocytosis involving megalin and cubulin. Reabsorbed LMW proteins are digested at low pH in lysosomes in the proximal tubule and do not enter the circulation intact (Fig. 2) [16].
Fig. 1.
Estimated glomerular sieving coefficients for 12 plasma proteins versus molecular weight. Abbreviations: ß2m, ß2-microglobulin; RBP, retinol-binding protein; α1m, α1-microglobulin; TSH, thyroid-stimulating hormone; ß2GI, ß2-glycoprotein-I; ZAG, zinc-α2-globulin; α1AG, α1-acid glycoprotein; DBP, vitamin D-binding protein; TTR, transthyretin; ALB, albumin; TRF, transferrin; IgG, immunoglobulin G. From Norden et al. [15], reproduced with permission
Fig. 2.
Absorption and intra-cellular handling of LMW proteins in the proximal tubule. Megalin is present on the cell surface in high abundance. When low-molecular-weight proteins bind to megalin, this “cargo” is internalized into the early endosome. The endosome is acidified by the action of H+ATPase in concert with chloride channel 5 (CLCN5). At low pH, the cargo dissociates from megalin and passes through the late endosome and on to the lysosome where it is degraded. Megalin returns from the early endosome back to the surface of the cell, where it is available for internalization of more cargo. From Guggino [16], reproduced with permission
Although the plasma concentrations of LMW proteins are in the mg/l range, i.e., almost 1000 times lower than albumin, the higher permeability leads to some 9.6 g being filtered and reabsorbed each day in an adult [17]. This process is saturable if excessive amounts of proteins are filtered, leading to shedding of LMW proteins in the absence of tubular damage. This is exemplified when comparing urinary cystatin C excretion in minimal change nephrotic syndrome during recurrence and in remission (Fig. 3) [18]. This overflow LMW proteinuria has also been reported for other LMW proteins [19, 20].
Fig. 3.
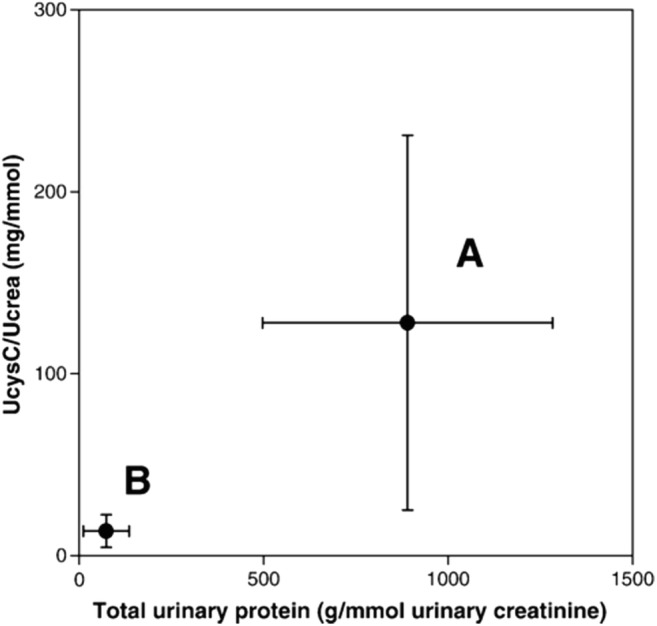
Simultaneous changes of the urinary cystatin C-creatinine ratio and the protein-creatinine ratio. Paired measurement of patients during the active phase (A) and in remission of minimal change nephropathy (B). Data presented as mean ± SD. From Herget-Rosenthal et al. [18], reproduced with permission
By contrast, the intact glomerular membrane is almost impermeable to albumin due its larger size and negative charge causing reflection of this anionic molecule [21–23]. In rat models, the sieving coefficient of albumin (i.e., the albumin-concentration in ultrafiltrate/plasma albumin-concentration) has been determined at about 0.0001 to 0.0006 [17]. This results in around 3.3 g of albumin being filtered per day in an adult [17]. Based on micropuncture studies in rats [24], 71% is reabsorbed in the proximal and 26% in the distal tubule so that albumin excretion is negligible under normal conditions (Fig. 4). More recently, much higher glomerular sieving coefficients around 0.02 have been reported for albumin and molecules of similar size. Dickson et al. propose that filtered albumin is not only reabsorbed in clathrin-coated pits on the surface of proximal tubular cells following binding to cubulin but also via fluid-phase endocytosis [25]. They hypothesize that absorbed albumin can leave the cell intact after binding to the neonatal Fc receptor (FcRn) rather than being degraded in lysosomes.
Fig. 4.
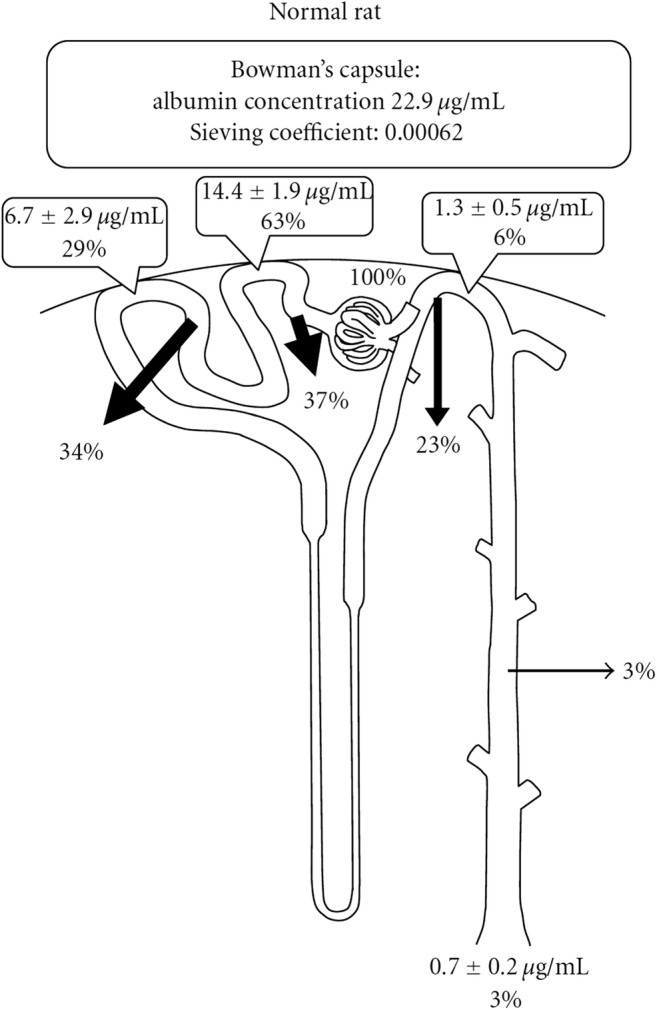
Albumin concentration along the nephron. Data calculated from a rat fractional micropuncture study. Arrows indicate the percentage of ultrafiltrated albumin which is reabsorbed in the respective nephron segment. From Tojo et al. [17], reproduced with permission
Both the classical view and Dickson’s findings imply that substantial amounts of albumin will be detected in the urine of individuals with defective tubular protein reabsorption but normal glomeruli, and does not necessarily imply a glomerular origin of albuminuria. This is illustrated by an increased urine albumin-creatinine ratio of 38 mg/mmol in patients with Dent disease, who have impaired proximal tubular protein absorption [16, 26], reported by Norden et al. [27].
Proteinuria
Pathological proteinuria may result from two principal mechanisms (or a combination of the two): (i) excessive permeability of the glomerular barrier for protein or (ii) impaired reabsorption of protein in the proximal tubule. While there is an association between nephrotic range proteinuria and glomerular disease, there is considerable overlap with non-glomerular disease which can also cause large proteinuria and albuminuria [28].
Measurement of proteins in the urine
The first screening for proteinuria is by urine dipstick. This colorimetric method is based on a change in pH in the presence of anionic proteins, i.e., albumin and transferrin, while most other proteins have much less affinity for protons. Therefore, the limits of detection vary considerably between different proteins: 150 mg/l for albumin, 200 mg/l for transferrin, 500 mg/l for IgG, 600 mg/l for ß2-microglobulin, and > 1000 mg/l for immunoglobulin light chains [29]. Urine dipsticks have good sensitivity as screening tool for macroalbuminuria (albumin-creatinine ratio > 30 mg/mmol), yet specificity is limited [30]. It should be borne in mind that the dipstick measures urine protein concentration and (anti-) diuresis therefore strongly influences sensitivity and specificity of this test.
In most clinical laboratories, total protein is measured using the colorimetric biuret [31] or a turbidimetric method [32] and related to urine creatinine to correct for urine concentration when using spot urine samples. Urine proteins can be differentiated using SDS-PAGE gel-electrophoresis and fast protein liquid chromatography discriminating between glomerular and tubular proteinuria [33]. Still, in daily routine, a selection of marker proteins is used to classify proteinuria [34, 35]. More recently, a mass spectrometry–based proteonomic analysis of urine was introduced in research settings [36].
In order to achieve high sensitivity (“microalbuminuria”), urine albumin concentrations are measured by immunoturbidimetry or nephelometry using anti-albumin antibodies [37]. This method is also used for the measurement of LMW proteins such as α1-microglobulin, ß2-microglobulin, retinol-binding protein (RBP), and cystatin C. The characteristics and upper limits of normal of the different marker proteins are summarized in Table 1. It should be borne in mind that these reference values do not apply to neonates, where higher values apply due to tubular immaturity and lower creatinine excretion [42–44]. α1-microglobulin and RBP are preferred above ß2-microglobulin because of the instability of the latter in acidic urine [45]. Branten et al. described a method for alkalizing urine by oral bicarbonate administration before urine collection to make sure that urine pH is above six [46], yet it is doubtful if this method is suitable for daily clinical practice, in particular in children. Data from Tomlinson suggest that RBP is most closely associated with histologically proven tubular abnormality and least affected by increasing albuminuria [20].
Table 1.
Characteristics of different LMW proteins in urine. Upper limits for adults are 90th centiles [38], limits for children refer to the maximum from 43 healthy controls [39]. For conversion to SI units (mg/mmol) divide by 9
| LMW protein | Molecular mass [40] | Upper reference limit | Interactions |
|---|---|---|---|
| α1-microglobulin | 26 kDa |
11.7 mg/g creatinine, adults [38] 19.8 mg/g creatinine, children [39] |
None |
| ß2-microglobulin | 12 kDa |
2.9 mg/g creatinine, adults [38] 0.37 mg/g creatinine, children [39] |
Unstable at pH < 6 |
| Cystatin C | 13 kDa | 0.77 mg/g creatinine, not specified [41] | None |
| Retinol-binding protein | 22 kDa |
0.1 mg/g creatinine, adults [38] 0.22 mg/g creatinine, children [39] |
None |
As stated above, urine protein concentration is strongly influenced by (anti-)diuresis. Therefore, proteinuria is quantified either in timed urine samples or by normalizing for urine creatinine concentration as a surrogate marker of (anti-)diuresis [47]. The former is hampered by inaccurate urine collection [47, 48] while the latter assumes normal creatinine production [49]. Conditions with increased (e.g., body building, creatinine supplements) or decreased production (e.g., neuromuscular disease, muscle wasting) will lead to falsely decreased or increased ratios, respectively. This is illustrated by data from Carter et al. who showed that intra-individual variability improved when urine concentrations were normalized for urine creatinine, while inter-individual variability did not improve or even increased [50].
Studies comparing both methods for albuminuria and total proteinuria suggest that analysis of spot urine is sufficiently accurate in clinical practice [47, 51, 52]. However, Lane et al. noted a logarithmic relationship between spot protein-creatinine ratio and 24-h protein excretion and concluded that spot urine analysis is less suitable for the follow-up of high proteinuria [51]. Hogan et al. found a relatively poor correlation between both parameters [53]. Therefore, a recent KDIGO conference on glomerular disease recommended 24-h measurements when changes in proteinuria impact therapeutic decisions [54].
The commonly used unit to express the protein-creatinine ratio is gram/gram, and this can be transformed to SI units (g/mmol) by dividing by 9.
Assessing selectivity of glomerular proteinuria
In heavy glomerular proteinuria, the selectivity index (SI) describes if urine protein is largely composed of albumin and transferrin (“selective”) or if significant amounts of very large proteins, such as IgG, are present too. The SI is calculated as the relation of IgG in blood and urine related to transferrin (uIgG × sTf / sIgG × uTf) [55]. An SI ≤ 0.10 is classified as selective proteinuria, a typical finding in minimal change disease, and bears a good prognosis. An SI between 0.11 and 0.20 is classified as moderately selective and ≥ 0.21 as unselective, often observed in steroid-resistant nephrotic syndrome with ominous prognosis of kidney function [56]. In patients with moderately selective and unselective proteinuria, an increased fractional excretion of α1-microglobulin indicates a worse prognosis reflecting additional tubulointerstitial damage (see below). McQuarrie et al. measured the fractional excretions of albumin (FEAlb) and IgG (FEIgG) [57]. In their hands, both FEAlb (hazard ratio 35.2 using a cutoff 0.0325%) and FEIgG (hazard ratio 37.1 using a cutoff 0.043%) were strong predictors of end-stage kidney disease with a median follow-up of 7 years.
Low-molecular weight proteinuria in glomerular disease
Several authors have reported LMW proteinuria in patients with documented glomerular disease [19, 56, 58–62]. Portman et al. measured the fractional excretion of ß2-microglobulin in children with tubular and glomerular disease [58]. While they observed a highly significant difference between both groups (0.104 vs. 4.27%) they noted that about one half of the patients with glomerular disease also had increased ß2-microglobulinuria. Re-assessment for the presence of tubulointerstitial lesions on renal biopsy showed that increased excretion of ß2-microglobulin separated the 13 patients with such lesions from 17 patients with isolated glomerular findings (3.76 vs. 0.063%). They suggest a cutoff of 0.36% to discriminate between isolated glomerular and glomerular disease with tubulointerstitial damage.
Van den Brand et al. studied LMW-protein markers ß2-microglobulin and α1-microglobulin as predictors of disease progression in idiopathic membranous glomerulopathy [59, 63]. They noted that the prognostic performance of either marker (area under the receiver-operating characteristic curve (AUROC)) was around 0.80 and comparable to the Toronto Risk Score, which incorporates baseline GFR, GFR-slope during 6-month follow-up, and persistent proteinuria [63]. A more detailed analysis of the risk score revealed that baseline GFR and change in GFR, rather than the severity of proteinuria, predicted deterioration of kidney function. Although not documented histologically, their findings suggest that the prognostic value of the LMW protein markers in this setting reflects tubulointerstitial damage rather than impaired reabsorption due to overflow proteinuria. Although urinary ß2-microglobulin was related to kidney function in patients with IgA nephropathy, Shin et al. did not find a correlation between urinary ß2-microglobulin and tubulointerstitial inflammation or fibrosis [62].
Several papers have addressed LMW proteinuria as a potential predictor of steroid resistance in childhood nephrotic syndrome [19, 60, 61]. Sesso et al. measured RBP and ß2-microglobulin at presentation in 37 patients with idiopathic nephrotic syndrome [61]. In their hands, both markers were much more elevated in steroid-resistant patients: a ß2-microglobulin-creatinine ratio > 3 mg/g and a RBP-creatinine ratio > 4 mg/g were 3.0 and 3.8 times more likely to come from steroid-unresponsive patients, respectively. By contrast, Valles et al. observed comparable excretion of urinary ß2-microglobulin in relapse in patients with steroid-dependent and steroid-resistant nephrotic syndrome [19]. In 11 patients with focal segmental glomerulosclerosis (FSGS), they found no significant correlation between urinary ß2-microglobulin and tubulointerstitial damage (r = 0.54, p = 0.19)
Low-molecular weight proteinuria as marker of acute kidney injury
In recent years, a number of urine markers have been identified for the prediction of imminent acute renal failure. These include neutrophil gelatinase-associated lipocalin (NGAL) [64] and kidney injury molecule-1 (KIM-1) [65], which are upregulated in damaged tubular cells. Proximal tubular dysfunction in acute kidney injury (AKI) leads to impaired reabsorption of LMW proteins and has therefore been studied as a marker/predictor of AKI. Of all LMW protein markers, cystatin C has been studied most extensively. Herget-Rosenthal et al. addressed non-oliguric AKI in an adult ICU setting [40]. In their hands, increased excretion of cystatin C and α1-microglobulin was a strong predictor (AUROC 0.92 and 0.86, respectively) for the need to initiate renal replacement therapy (RRT) within a median interval of 4 days. Cutoffs with optimal sensitivity and specificity were 9 mg/g for cystatin C and 180 mg/g for α1-microglobulin. Koyner et al. [66] studied adult patients following cardiac surgery and classified AKI using the RIFLE criteria [67]. The urinary cystatin C-creatinine ratio at the end of cardio-pulmonary bypass, on admission to the ICU and at 6 h after admission, was significantly higher in patients who developed AKI and even higher in patients requiring RRT when compared to patients with an uneventful course. In this series, the AUROC to predict AKI was 0.734.
Carter et al. found high inter- and intra-individual variability of serum and urine markers of AKI in patients with chronic kidney disease. However, the changes during AKI were high, indicating that these markers are still clinically useful if baseline values are available [50]. For urine markers, normalization to creatinine concentration reduced intra-individual variability.
A recent meta-analysis of four studies in children showed an AUROC of 0.85 (95% CI 0.81–0.88) for urinary cystatin C [68], while this marker was less accurate for the prediction of AKI in adults (AUROC 0.64, 95% CI 0.62–0.66) [69]. This meta-analysis was hampered by heterogeneity across the studies, in particular, lack of exclusion of pre-renal azotemia in many studies [69]. Assessing RRT as an outcome parameter in their meta-analysis, Klein et al. found a better predictive value for urinary cystatin C (AUROC 0.72, 95% CI 0.575–0.868), which improved to 0.790 (0.645 to 0.934) after normalization for creatinine [70], stressing the need to normalize LMW proteins for urine creatinine concentrations. Still, all meta-analyses concluded that serum cystatin C was superior to urine cystatin C.
Putting proteinuria analysis into clinical practice
As outlined above, the presence and amount of various proteins in the urine varies considerably across the spectrum of renal disease, in particular in distinguishing patients with an isolated tubulopathy from patients with chronic kidney disease involving the glomeruli and chronic kidney disease of non-glomerular origin (i.e., CAKUT). Here, a limited strategy measuring albumin, α1-microglobulin, and creatinine is able to separate these entities with high sensitivity and specificity [3]. Beara-Lasic et al. confirm that the protein-creatinine ratio does not differentiate between Dent disease and a glomerulopathy, a fact that causes much confusion and has led to unnecessary kidney biopsies [28]. Instead, an α1-microglobulin-creatinine ratio of 120 mg/g had a sensitivity of 86% and specificity of 95% to distinguish Dent disease from other forms of chronic kidney disease, even when analyzing the subgroup of tubulointerstitial disease separately. α1-microglobulin can be substituted by the albumin-total protein ratio (cutoff 0.21 g/g). Still, this reduces specificity, in particular when separating Dent disease from tubulointerstitial disease (specificity 55%). Based on these findings, the low albumin contribution of some 30% of total proteinuria in the case report by Preston et al. [2] published in this issue of Pediatric Nephrology argues against FSGS as primary diagnosis in their patient and points towards the diagnosis of Dent disease [28].
Beara-Lasic et al. did not present cutoffs to distinguish chronic kidney disease of tubulointerstitial origin from glomerular disease. These two entities can be separated using urinary albumin and total protein concentrations (AUROC 0.82), whereas the albumin-total protein ratio (AUROC 0.61) and the α1-microglobulin-creatinine ratio (AUROC 0.53) performed poorly. In this setting, α1-microglobulin should be related to total protein (AUROC 0.82) or albumin concentration (AUROC 0.82).
Smith et al. used the albumin-total protein ratio to distinguish tubular from glomerular proteinuria defined by urine protein electrophoresis and immunofixation in some 1000 urine samples [71]. In their hands, ROC analysis of the albumin-total protein ratio yielded an AUROC of 0.84 and was comparable to the ß2-microglobulin-creatinine ratio. Using a cutoff of 0.40 mg/mg for the albumin-total protein ratio, sensitivity for the diagnosis of tubular proteinuria was 75% and specificity 73%. Figure 5 shows the distribution of the albumin-total protein ratio vs. histological findings. In patients with combined glomerular and tubulointerstitial lesions, albumin-total protein ratio was inversely related to the severity of tubulointerstitial lesions, indicating increasing amounts of LMW proteins as it is unlikely that the shedding of immunoglobulins will account for this change [71]. Most patients with pure glomerular disease had values above 0.60 mg/mg. This is in line with Ohisa’s series of 579 patients (69% with kidney biopsy) where this cutoff had a sensitivity of 97% and specificity of 100% [72].
Fig. 5.
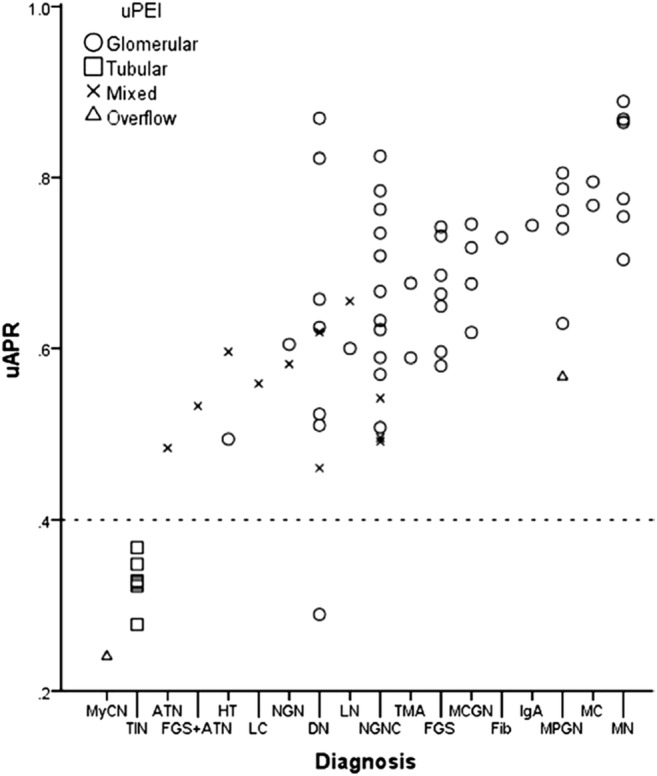
Histological diagnosis and urine albumin/protein ratio. A urine albumin/creatinine ratio (uAPR) value of 0.4 (dotted line) demonstrates a clear distinction between tubulointerstitial disorders and glomerular disorders. uPEI, urine protein electrophoresis and immunofixation; ATN, acute tubular necrosis; DN, diabetic nephropathy; Fib, fibrillary glomerulonephritis; FGS, focal segmental glomerulosclerosis; HT, hypertensive nephrosclerosis; IgA, IgA nephropathy; LC, light chain deposition disease; LN, lupus nephritis; MC, minimal change disease; MCGN, mesangiocapillary glomerulonephritis; MPGN, mesangioproliferative glomerulonephritis; MN, membranous nephropathy; MyCN, myeloma cast nephropathy; NGN, necrotizing glomerulonephritis; NGNC, necrotizing glomerulonephritis with crescents; TIN, tubulointerstitial nephritis; TMA, thrombotic microangiopathy. From Smith et al. [71], reproduced with permission
These findings can also be used in the diagnostics of macroscopic hematuria. Serum albumin constitutes about 55% of total protein in healthy persons [73]. In post-glomerular hematuria, full blood has mixed with urine, therefore the albumin total protein ratio will be about 0.55 mg/mg, whereas a higher value suggests a glomerular origin of hematuria.
Conclusion
A detailed analysis of proteinuria can provide important diagnostic and prognostic information. These tests are cheap, non-invasive, and rapidly available in most clinical laboratories and an important adjunct to renal biopsy and modern molecular genetic techniques. From a historical perspective, taking a close look at urine was the starting point of laboratory medicine more than 2000 years ago [74].
Compliance with ethical standards
Conflict of interest
The author declares that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.KDIGO (2013) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl:1–150
- 2.Preston R, Naylor RW, Stewart G, Bierzynska A, Saleem MA, Lowe M, Lennon R (2019) A role for OCRL in glomerular function and disease. Pediatr Nephrol. 10.1007/s00467-019-04317-4 [DOI] [PMC free article] [PubMed]
- 3.Beara-Lasic L, Cogal A, Mara K, Enders F, Mehta RA, Haskic Z, Furth SL, Trachtman H, Scheinman SJ, Milliner DS, Goldfarb DS, Harris PC, Lieske JC, investigators of the Rare Kidney Stone Consortium (2019) Prevalence of low molecular weight proteinuria and Dent disease 1 CLCN5 mutations in proteinuric cohorts. Pediatr Nephrol. 10.1007/s00467-019-04210-0 [DOI] [PMC free article] [PubMed]
- 4.Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, Collins JF, Gansevoort RT, Gutierrez OM, Hamano T, Heine GH, Ishikawa S, Jee SH, Kronenberg F, Landray MJ, Miura K, Nadkarni GN, Peralta CA, Rothenbacher D, Schaeffner E, Sedaghat S, Shlipak MG, Zhang LX, van Zuilen AD, Hallan SI, Kovesdy CP, Woodward M, Levin A, CKD Prognosis Consortium Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am J Kidney Dis. 2019;73:206–217. doi: 10.1053/j.ajkd.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, Chan TM, Hou FF, Lewis JB, Locatelli F, Praga M, Schena FP, Levey AS, Inker LA, Chronic Kidney Disease Epidemiology Collaboration Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128–139. doi: 10.1016/S2213-8587(18)30314-0. [DOI] [PubMed] [Google Scholar]
- 6.Gansevoort RT, Snieder H. Albuminuria as a cause of hypertension. Nat Rev Nephrol. 2019;15:6–8. doi: 10.1038/s41581-018-0073-8. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Heerspink HL, Sang YY, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, Fox CS, Inker LA, Ishani A, Ito S, Jassal S, Konta T, Polkinghorne K, Romundstad S, Solbu MD, Stempniewicz N, Stengel B, Tonelli M, Umesawa M, Waikar S, Wen CP, Wetzels JFM, Woodward M, Grams ME, Kovesdy CP, Levey AS, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–127. doi: 10.1016/S2213-8587(18)30313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrero JJ, Grams ME, Sang YY, Arnlov J, Gasparini A, Matsushita K, Qureshi AR, Evans M, Barany P, Lindholm B, Ballew SH, Levey AS, Gansevoort RT, Elinder CG, Coresh J. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91:244–251. doi: 10.1016/j.kint.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerspink HJL, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: The Pro View. Clin J Am Soc Nephrol. 2015;10:1079–1088. doi: 10.2215/CJN.11511114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grams ME, Sang YY, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, Oien C, Smith DH, Coresh J, Sarnak MJ, Stengel B, Tonelli M, CKD Prognosis Consortium A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66:591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauta FL, Scheven L, Meijer E, van Oeveren W, de Jong PE, Bakker SJL, Gansevoort RT. Glomerular and tubular damage markers in individuals with progressive albuminuria. Clin J Am Soc Nephrol. 2013;8:1106–1114. doi: 10.2215/CJN.04510512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rytand DA, Spreiter S. Prognosis in postural (orthostatic) proteinuria: forty to fifty-year follow-up of six patients after diagnosis by Thomas Addis. N Engl J Med. 1981;305:618–621. doi: 10.1056/NEJM198109103051105. [DOI] [PubMed] [Google Scholar]
- 13.Poortmans JR. Postexercise proteinuria in humans. Facts and mechanisms. JAMA. 1985;253:236–240. [PubMed] [Google Scholar]
- 14.Poortmans JR, Brauman H, Staroukine M, Verniory A, Decaestecker C, Leclercq R. Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Phys. 1988;254:F277–F283. doi: 10.1152/ajprenal.1988.254.2.F277. [DOI] [PubMed] [Google Scholar]
- 15.Norden AGW, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FWK, Thakker RV, Unwin RJ, Wrong O. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001;60:1885–1892. doi: 10.1046/j.1523-1755.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 16.Guggino SE. Mechanisms of disease: what can mouse models tell us about the molecular processes underlying Dent disease? Nat Clin Pract Nephrol. 2007;3:449–455. doi: 10.1038/ncpneph0541. [DOI] [PubMed] [Google Scholar]
- 17.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol. 2012;2012:481520. doi: 10.1155/2012/481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herget-Rosenthal S, van Wijk JA, Brocker-Preuss M, Bokenkamp A. Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem. 2007;40:946–951. doi: 10.1016/j.clinbiochem.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Valles P, Peralta M, Carrizo L, Martin L, Principi I, Gonzalez A, Manucha W. Follow-up of steroid-resistant nephrotic syndrome: tubular proteinuria and enzymuria. Pediatr Nephrol. 2000;15:252–258. doi: 10.1007/s004670000472. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson PA, Dalton RN, Hartley B, Haycock GB, Chantler C. Low molecular weight protein excretion in glomerular disease: a comparative analysis. Pediatr Nephrol. 1997;11:285–290. doi: 10.1007/s004670050278. [DOI] [PubMed] [Google Scholar]
- 21.Ciarimboli G, Schurek HJ, Zeh M, Flohr H, Bokenkamp A, Fels LM, Kilian I, Stolte H. Role of albumin and glomerular capillary wall charge distribution on glomerular permselectivity: studies on the perfused-fixed rat kidney model. Pflugers Arch. 1999;438:883–891. doi: 10.1007/s004249900120. [DOI] [PubMed] [Google Scholar]
- 22.Bertolatus JA, Hunsicker LG. Glomerular sieving of anionic and neutral bovine albumins in proteinuric rats. Kidney Int. 1985;28:467–476. doi: 10.1038/ki.1985.153. [DOI] [PubMed] [Google Scholar]
- 23.Ciarimboli G, Hjalmarsson C, Bokenkamp A, Schurek HJ, Haraldsson B. Dynamic alterations of glomerular charge density in fixed rat kidneys suggest involvement of endothelial cell coat. Am J Physiol Ren Physiol. 2003;285:F722–F730. doi: 10.1152/ajprenal.00227.2001. [DOI] [PubMed] [Google Scholar]
- 24.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Phys. 1992;263:F601–F606. doi: 10.1152/ajprenal.1992.263.4.F601. [DOI] [PubMed] [Google Scholar]
- 25.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really! J Am Soc Nephrol. 2014;25:443–453. doi: 10.1681/ASN.2013090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig Michael, Levtchenko Elena, Bökenkamp Arend. Clinical utility gene card for: Dent disease (Dent-1 and Dent-2) European Journal of Human Genetics. 2014;22(11):1338–1338. doi: 10.1038/ejhg.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norden AG, Lapsley M, Igarashi T, Kelleher CL, Lee PJ, Matsuyama T, Scheinman SJ, Shiraga H, Sundin DP, Thakker RV, Unwin RJ, Verroust P, Moestrup SK. Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J Am Soc Nephrol. 2002;13:125–133. doi: 10.1681/ASN.V131125. [DOI] [PubMed] [Google Scholar]
- 28.van Berkel Y, Ludwig M, van Wijk JAE, Bokenkamp A. Proteinuria in Dent disease: a review of the literature. Pediatr Nephrol. 2017;32:1851–1859. doi: 10.1007/s00467-016-3499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boege F, Luther A. Gesamtproteinbestimmung im Urin: Adaptierung einer nephelometrischen Methode zur Erfassung typischer Leitproteine und Bence-Jones-Proteine. Lab Med. 1989;13:14–19. [Google Scholar]
- 30.Collier G, Greenan MC, Brady JJ, Murray B, Cunningham SK. A study of the relationship between albuminuria, proteinuria and urinary reagent strips. Ann Clin Biochem. 2009;46:247–249. doi: 10.1258/acb.2009.008189. [DOI] [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Luxton RW, Patel P, Keir G, Thompson EJ. A micro-method for measuring total protein in cerebrospinal fluid by using benzethonium chloride in microtiter plate wells. Clin Chem. 1989;35:1731–1734. [PubMed] [Google Scholar]
- 33.Brocklebank T, Cooper EH, Richmond K. Sodium dodecyl sulphate polyacrylamide gel electrophoresis patterns of proteinuria in various renal diseases of childhood. Pediatr Nephrol. 1991;5:371–375. doi: 10.1007/BF01453654. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann W, Ehrich JHH, Guder WG, Keller F, Scherberich JE, Working Group Diagnostic Pathways of the German United Society for Clinical Chemistry and Laboratory Medicine; Society of Nephrology Diagnostic pathways for exclusion and diagnosis of kidney diseases. Clin Lab. 2012;58:871–889. [PubMed] [Google Scholar]
- 35.Guder WG, Ivandic M, Hofmann W. Physiopathology of proteinuria and laboratory diagnostic strategy based on single protein analysis. Clin Chem Lab Med. 1998;36:935–939. doi: 10.1515/CCLM.1998.162. [DOI] [PubMed] [Google Scholar]
- 36.Jia LL, Zhang L, Shao C, Song EL, Sun W, Li MX, Gao YH. An attempt to understand kidney’s protein handling function by comparing plasma and urine proteomes. PLoS One. 2009;4:e5146. doi: 10.1371/journal.pone.0005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florvall G, Basu S, Helmersson J, Larsson A. Microalbuminuria measured by three different methods, blood pressure and cardiovascular risk factors in elderly Swedish males. Anal Chem Insights. 2008;3:69–74. [PMC free article] [PubMed] [Google Scholar]
- 38.Norden AG, Scheinman SJ, Deschodt-Lanckman MM, Lapsley M, Nortier JL, Thakker RV, Unwin RJ, Wrong O. Tubular proteinuria defined by a study of Dent’s (CLCN5 mutation) and other tubular diseases. Kidney Int. 2000;57:240–249. doi: 10.1046/j.1523-1755.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson PA, Dalton RN, Turner C, Chantler C. Measurement of beta 2-microglobulin, retinol-binding protein, alpha 1-microglobulin and urine protein 1 in healthy children using enzyme-linked immunosorbent assay. Clin Chim Acta. 1990;192:99–106. doi: 10.1016/0009-8981(90)90073-2. [DOI] [PubMed] [Google Scholar]
- 40.Herget-Rosenthal S, Poppen D, Husing J, Marggraf G, Pietruck F, Jakob HG, Philipp T, Kribben A. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–558. doi: 10.1373/clinchem.2003.027763. [DOI] [PubMed] [Google Scholar]
- 41.Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta. 2002;323:121–128. doi: 10.1016/s0009-8981(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 42.Saeidi B, Koralkar R, Griffin RL, Halloran B, Ambalavanan N, Askenazi DJ. Impact of gestational age, sex, and postnatal age on urine biomarkers in premature neonates. Pediatr Nephrol. 2015;30:2037–2044. doi: 10.1007/s00467-015-3129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFreitas MJ, Seeherunvong W, Katsoufis CP, RamachandraRao S, Duara S, Yasin S, Zilleruelo G, Rodriguez MM, Abitbol CL. Longitudinal patterns of urine biomarkers in infants across gestational ages. Pediatr Nephrol. 2016;31:1179–1188. doi: 10.1007/s00467-016-3327-3. [DOI] [PubMed] [Google Scholar]
- 44.Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res. 2011;70:302–306. doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernard AM, Moreau D, Lauwerys R. Comparison of retinol-binding protein and beta 2-microglobulin determination in urine for the early detection of tubular proteinuria. Clin Chim Acta. 1982;126:1–7. doi: 10.1016/0009-8981(82)90356-4. [DOI] [PubMed] [Google Scholar]
- 46.Branten AJW, du Buf-Vereijken PW, Klasen IS, Bosch FH, Feith GW, Hollander DA, Wetzels JF. Urinary excretion of beta 2-microglobulin and IgG predict prognosis in idiopathic membranous nephropathy: a validation study. J Am Soc Nephrol. 2005;16:169–174. doi: 10.1681/ASN.2004040287. [DOI] [PubMed] [Google Scholar]
- 47.Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005;51:1577–1586. doi: 10.1373/clinchem.2005.049742. [DOI] [PubMed] [Google Scholar]
- 48.Westland R, Abraham Y, Bokenkamp A, Stoffel-Wagner B, Schreuder MF, van Wijk JA. Precision of estimating equations for GFR in children with a solitary functioning kidney: the KIMONO study. Clin J Am Soc Nephrol. 2013;8:764–772. doi: 10.2215/CJN.07870812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.den Bakker E, Gemke R, Bokenkamp A. Endogenous markers for kidney function in children: a review. Crit Rev Clin Lab Sci. 2018;55:163–183. doi: 10.1080/10408363.2018.1427041. [DOI] [PubMed] [Google Scholar]
- 50.Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, Lamb EJ. Biological variation of plasma and urinary markers of acute kidney injury in patients with chronic kidney disease. Clin Chem. 2016;62:876–883. doi: 10.1373/clinchem.2015.250993. [DOI] [PubMed] [Google Scholar]
- 51.Lane C, Brown M, Dunsmuir W, Kelly J, Mangos G. Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? Nephrology (Carlton) 2006;11:245–249. doi: 10.1111/j.1440-1797.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 52.Guy M, Borzomato JK, Newall RG, Kalra PA, Price CP. Protein and albumin-to-creatinine ratios in random urines accurately predict 24 h protein and albumin loss in patients with kidney disease. Ann Clin Biochem. 2009;46:468–476. doi: 10.1258/acb.2009.009001. [DOI] [PubMed] [Google Scholar]
- 53.Hogan MC, Reich HN, Nelson PJ, Adler SG, Cattran DC, Appel GB, Gipson DS, Kretzler M, Troost JP, Lieske JC. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int. 2016;90:1080–1089. doi: 10.1016/j.kint.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Floege J, Barbour SJ, Cattran DC, Hogan JJ, Nachman PH, Tang SCW, Wetzels JFM, Cheung M, Wheeler DC, Winkelmayer WC, Rovin BH, Conference Participants Management and treatment of glomerular diseases (part 1): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Cameron JS, Blandford G. The simple assessment of selectivity in heavy proteinuria. Lancet. 1966;2:242–247. doi: 10.1016/s0140-6736(66)92539-6. [DOI] [PubMed] [Google Scholar]
- 56.Bazzi C, Petrini C, Rizza V, Arrigo G, D'Amico G. A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int. 2000;58:1732–1741. doi: 10.1046/j.1523-1755.2000.00334.x. [DOI] [PubMed] [Google Scholar]
- 57.McQuarrie EP, Shakerdi L, Jardine AG, Fox JG, Mackinnon B. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant. 2011;26:1563–1569. doi: 10.1093/ndt/gfq605. [DOI] [PubMed] [Google Scholar]
- 58.Portman RJ, Kissane JM, Robson AM. Use of beta 2 microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986;30:91–98. doi: 10.1038/ki.1986.156. [DOI] [PubMed] [Google Scholar]
- 59.van den Brand JA, Hofstra JM, Wetzels JF. Low-molecular-weight proteins as prognostic markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:2846–2853. doi: 10.2215/CJN.04020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khurana M, Traum AZ, Aivado M, Wells MP, Guerrero M, Grall F, Libermann TA, Schachter AD. Urine proteomic profiling of pediatric nephrotic syndrome. Pediatr Nephrol. 2006;21:1257–1265. doi: 10.1007/s00467-006-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sesso R, Santos AP, Nishida SK, Klag MJ, Carvalhaes JT, Ajzen H, Ramos OL, Pereira AB. Prediction of steroid responsiveness in the idiopathic nephrotic syndrome using urinary retinol-binding protein and beta-2-microglobulin. Ann Intern Med. 1992;116:905–909. doi: 10.7326/0003-4819-116-11-905. [DOI] [PubMed] [Google Scholar]
- 62.Shin JR, Kim SM, Yoo JS, Park JY, Kim SK, Cho JH, Jeong KH, Lee TW, Ihm CG. Urinary excretion of beta2-microglobulin as a prognostic marker in immunoglobulin A nephropathy. Korean J Intern Med. 2014;29:334–340. doi: 10.3904/kjim.2014.29.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Brand JA, Hofstra JM, Wetzels JF. Prognostic value of risk score and urinary markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:1242–1248. doi: 10.2215/CJN.00670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 65.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 66.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, Connor MFO, Konczal DJ, Trevino S, Devarajan P, Murray PT. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakhjavan-Shahraki B, Yousefifard M, Ataei N, Baikpour M, Ataei F, Bazargani B, Abbasi A, Ghelichkhani P, Javidilarijani F, Hosseini M. Accuracy of cystatin C in prediction of acute kidney injury in children; serum or urine levels: which one works better? A systematic review and meta-analysis. BMC Nephrol. 2017;18:120. doi: 10.1186/s12882-017-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 70.Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, Joannidis M. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018;44:323–336. doi: 10.1007/s00134-018-5126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant. 2012;27:1534–1541. doi: 10.1093/ndt/gfr708. [DOI] [PubMed] [Google Scholar]
- 72.Ohisa N, Yoshida K, Matsuki R, Suzuki H, Miura H, Ohisa Y, Murayama N, Kaku M, Sato H. A comparison of urinary albumin-total protein ratio to phase-contrast microscopic examination of urine sediment for differentiating glomerular and nonglomerular bleeding. Am J Kidney Dis. 2008;52:235–241. doi: 10.1053/j.ajkd.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Vavricka SR, Burri E, Beglinger C, Degen L, Manz M. Serum protein electrophoresis: an underused but very useful test. Digestion. 2009;79:203–210. doi: 10.1159/000212077. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong JA. Urinalysis in Western culture: a brief history. Kidney Int. 2007;71:384–387. doi: 10.1038/sj.ki.5002057. [DOI] [PubMed] [Google Scholar]