Abstract
Background
Data on long-term outcomes in children who have received renal replacement therapy (RRT) for end-stage renal disease are limited.
Methods
We studied long-term survival and incidence of fatal and nonfatal cardiovascular disease (CVD) events and determinants of these outcomes in children who initiated RRT between 1961 and 2013 using data from the Scottish Renal Registry (SRR). Linkage to morbidity records was available from 1981.
Results
A total of 477 children of whom 55% were boys, almost 50% had congenital urinary tract disease (CAKUT), 10% received a transplant as the first mode of RRT and almost 60% were over 11 years of age at start of RRT were followed for a median of 17.8 years (interquartile range (IQR) 8.7–26.6 years). Survival was 87.3% (95% confidence interval (CI) 84.0–90.1) at 10 years and 77.6% (95% CI 73.3–81.7) at 20 years. During a median follow-up of 14.96 years (IQR 7.1–22.9), 20.9% of the 381 patients with morbidity data available had an incident of CVD event. Age < 2 years at start of RRT, receiving dialysis rather than a kidney transplant and primary renal disease (PRD) other than CAKUT or glomerulonephritis (GN), were associated with a higher risk of all-cause mortality. Male sex, receiving dialysis rather than a kidney transplant and PRD other than CAKUT or GN, was associated with a higher risk of CVD incidence.
Conclusions
Mortality and CVD incidence among children receiving RRT are high. PRD and RRT modality were associated with increased risk of both all-cause mortality and CVD incidence.
Keywords: End-stage renal disease, Dialysis, Transplant, Children, Mortality, Cardiovascular disease
Introduction
End-stage renal disease (ESRD) in children is rare with median global incidence of renal replacement therapy (RRT) estimated to be nine children per million aged 4–18 years in 2008 [1]. Nevertheless, it is a serious healthcare problem requiring RRT in the form of dialysis or kidney transplantation to sustain life [2]. Recent data from the European Renal Registry (ESPN/ERA-EDTA) show that the incidence of RRT among children aged 0–14 years is around six per million each year [3]. Mortality rates in children receiving RRT are much higher than in the age and sex-matched general population [4]. Cardiovascular disease (CVD) is the leading cause of death in these children, responsible for 23–60% of all deaths [4–7]. Several factors are known to be associated with mortality risk. These include modality at start of RRT, with children who started RRT with dialysis having an almost seven times higher risk of mortality compared to those who received a pre-emptive kidney transplant (HR 6.6, 95% CI 2.9–14.8) [8]. Younger age at start of RRT [4–11], female sex [5] and primary renal disease (PRD) other than congenital anomalies of kidney and urinary tract (CAKUT) [4, 5, 12] have also been found to be associated with increased risk of mortality among children receiving RRT. Studies so far have generally reported 5-year survival [6, 8, 13]. Only limited data exist about longer-term survival [4, 14, 15] and incidence of nonfatal CVD events [9, 11] in individuals who start RRT in childhood. The aims of this study were to describe long-term survival and incidence of fatal and nonfatal CVD events in children after starting RRT and to describe the association between age at initiation of RRT, sex, PRD and RRT modality and these outcomes.
Materials and methods
Study population
We included all children (aged <18 years) from the Scottish Renal Registry (SRR) who started RRT between January 1, 1961 and December 31, 2013. The SRR is a national registry that collects individual patient data on date of birth, sex, PRD, start date of RRT, treatment modality at start of RRT, any subsequent changes in treatment modality and vital status for all people starting RRT in Scotland [16].
Data linkage
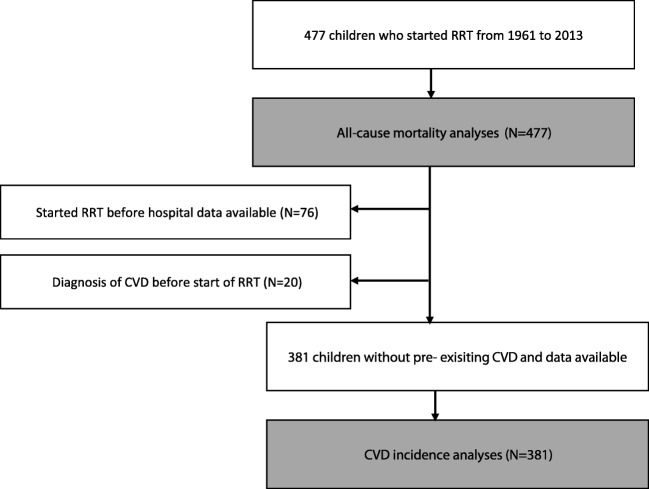
To describe mortality and CVD incidence, we linked data from National Records of Scotland (NRS) death records and the Scottish Morbidity Records (SMR01) database from Information Services Division (ISD) Scotland with SRR data. The SMR01 database holds data on inpatient and day-case hospital discharges in Scotland from 1981 onwards [17]. Therefore, we selected a sub-cohort of the SRR patients who started RRT from 1981 and 2013 to describe the incidence of fatal and nonfatal CVD events (Fig. 1). We obtained approval for the data linkage and analysis from the Public Benefit and Privacy Panel for Health and Social Care (PBPP) [18] and the SRR steering group. We stored and accessed all datasets according to ISD information governance rules and processes.
Fig 1.
Flow chart describing the study population of children who started renal replacement therapy in Scotland
Follow-up period
We followed patients from the start of RRT until the outcome of interest, the end of observation period (December 31, 2015) or loss to follow-up, whichever came first.
Outcome variables
All-cause mortality and CVD incidence were the outcomes of interest. We defined CVD incidence using the first CVD event after the start of RRT in any position listed in the SMR01 database or in NRS death records. We defined fatal CVD events from the NRS death records from International Classification of Disease (ICD) codes for any circulatory disease. We categorised fatal CVD events as ischaemic heart disease, cerebrovascular disease, heart failure, cardiac arrest/arrhythmias, cardiomyopathy and other diseases of the circulatory system. We did not include codes for other diseases of the circulatory system in the definition of nonfatal CVD events (Table 1).
Table 1.
ICD-9 and ICD-10 codes used for fatal and nonfatal CVD events
| CVD events | Nonfatal CVD events | Fatal CVD events | ||
|---|---|---|---|---|
| ICD-9 | ICD-10 | ICD-9 | ICD-10 | |
| Ischaemic heart disease | 410–414 | I20–I25 | 410–414 | I20–I25 |
| Cerebrovascular disease | 430–438 | I60–I69 | 430–438 | I60–I69 |
| Heart failure | 428 | I50 | 428 | I50 |
| Cardiac arrest/arrhythmias | 427 | I46–I49 | 427 | I46–I49 |
| Cardiomyopathy | 425 | I42, I43 | 425 | I42, I43 |
| Other diseases of circulatory system | - | - | 390-405, 415-417, 420-424, 426, 429, 440-459 | I00–I15, I26–I28, I30–I41, I44, I45, I51, I52, I70–I99 |
CVD; cardiovascular disease, ICD; international classification of diseases -9 indicates ninth revision used to classify death records and hospital records until 1999, -10 indicates tenth revision used after 1999 to classify death records and hospital records
We categorised patients in accordance with previous publications into the following four age groups at start of RRT: 0–<2 years old, 2–<6 years old, 6–<12 years old and 12–<18 years old [19, 20]. We classified PRD according to the ERA-EDTA coding system [21] and grouped it as CAKUT, glomerulonephritis (GN) and other. The other category includes cystic kidney disease, hereditary nephropathy, ischaemic renal failure, haemolytic-uraemic syndrome (HUS), metabolic disorders, vasculitis and miscellaneous. We categorised initial type of RRT as haemodialysis (HD), peritoneal dialysis (PD) and pre-emptively transplanted (pre-Tx). We classified RRT patterns during follow-up into four categories: started on HD and not transplanted during follow-up (HD + Tx-), started on PD and not transplanted during follow-up (PD + Tx-), pre-Tx and transplanted ever after starting RRT on dialysis (D + Tx+).
Statistical methods
We calculated crude all-cause mortality and CVD incidence rates per 100 person years of follow-up. We used unadjusted cumulative incidence competing risk (CICR) analysis to estimate the risk of mortality and cardiovascular disease by initial RRT modality [22] and accompanying 95% confidence intervals [23]. Cause-specific Cox proportional hazard regression analyses were used to describe survival probabilities and risk of outcomes by age at start of RRT, sex, PRD and RRT modality. Patients were censored after experiencing the outcome of interest as only first occurring events are taken into account. We evaluated the proportional hazard assumption graphically using log minus log plots. We adjusted the estimates of associations from Cox proportional hazard models for possible confounding factors including age at start of RRT, sex, PRD, initial RRT modality and period of start of RRT (1961–1990, 1991–2000 and 2001–2013). Data were complete for all variables included in the Cox regression analyses.
Sensitivity analysis
Since children who receive pre-emptive transplants (i.e. those who do not receive dialysis prior to transplant) generally have better survival than children who initially receive dialysis, we performed a sensitivity analysis excluding children who received a pre-emptive transplant from the Cox regression analysis. We compared the results of this sensitivity analysis with the results of the main analyses.
Results
Patient characteristics
Table 2 presents the demographic characteristics of the 477 children included in the all-cause mortality analyses and the 381 children included in the analyses of CVD incidence. The characteristics of both cohorts show that the proportion of boys was higher than girls and the majority of the patients started RRT when they were older than 12 years of age. Furthermore, CAKUT was the most common underlying PRD, and almost 90% of patients started RRT on dialysis, and only 10% of children received a pre-emptive transplant.
Table 2.
Characteristics of patients included in the analyses
| All-cause mortality cohort | CVD incidence cohort | |
|---|---|---|
| Characteristics | N (%) | N (%) |
| N included | 477 | 381 |
| Sex | ||
| Male | 264 (55.3) | 218 (57.2) |
| Age at start of RRT (years) | ||
| 0–<2 | 30 (6.3) | 29 (7.6) |
| 2–<6 | 50 (10.5) | 47 (12.3) |
| 6–<12 | 113 (23.7) | 90 (23.6) |
| 12–<18 | 284 (59.5) | 215 (56.4) |
| PRD | ||
| CAKUT | 229 (48.0) | 180 (48.6) |
| GN | 80 (16.8) | 59 (15.5) |
| Other | 168 (35.2) | 137 (36.0) |
| Initial RRT modality | ||
| HD | 220 (46.1) | 155 (40.7) |
| PD | 207 (43.4) | 180 (47.2) |
| Tx | 50 (10.5) | 46 (12.1) |
| RRT modality during follow-up | ||
| D + Tx+ | 371 (77.8) | 289 (75.9) |
| Pre-Tx | 50 (10.5) | 46 (12.1) |
| HD + Tx- | 30 (6.3) | 24 (6.3) |
| PD + Tx- | 26 (5.5) | 22 (5.8) |
CVD cardiovascular disease, RRT renal replacement therapy, PRD primary renal disease, CAKUT congenital anomalies of kidney and urinary tract, GN glomerulonephritis, HD haemodialysis, PD peritoneal dialysis, Tx transplanted. PD + Tx started on PD and not transplanted during follow-up, HD + Tx started on HD and not transplanted during follow-up, pre-Tx pre-emptive transplant, D + Tx + started on dialysis (PD or HD) and received a transplant during follow-up
Survival after the start of renal replacement therapy
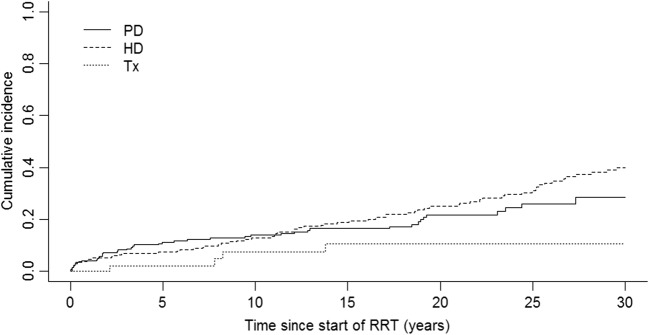
In total, 477 children were followed for a median of 17.8 years (IQR 8.7–26.6 years), giving a total follow-up time of 8710 person years. Median follow-up time was longest for patients starting RRT on HD (21.5 year), followed by PD (17.2 years), and pre-emptive transplant patients had the shortest median follow-up time of 14.2 years. During the total study period, 125 patients died resulting in a crude all-cause mortality rate of 1.44 (95% CI 1.19–1.70) per 100 person years. Of these deaths, 36.5% were due to a known cardiovascular cause, 11.2% of the deaths were due to infections, 5.6% due to malignancies, and 2.4% caused by haemorrhages. After 10 years of follow-up, the overall survival after the start of RRT was 87.3% (95% CI 84.0–90.1), and 337 were still alive with follow-up available, after 20 years overall survival was 77.6% (95% CI 73.3–81.7), and 209 children were still followed-up. Mortality was lower in children who received a pre-emptive transplant compared to children who received HD as their first RRT (0.55, 95% CI 0.01–1.09 per 100 person years and 1.68, 95% CI 1.31–2.06 per 100 person years, respectively) or children who started with PD (1.29, 95% CI 0.91–1.67 per 100 person years) although a statistically significant difference was only observed for the comparison between children receiving a pre-emptive transplant and those receiving HD as their first RRT. Figure 2 presents cumulative incidence curves based on unadjusted CICR analyses. This figure shows that after 10 and 20 years, follow-up survival among patients who started on HD was 87.1% (95% CI 82.2–91.3) and 75.0% (95% CI 68.6–81.0); on PD these figures were 86.1% (95% CI 80.9–90.5) and 78.3% (95% CI 71.6–84.4). Patients who received a pre-emptive transplant showed higher survival (after 10 years 92.5% (95% CI 81.4–98.1) and 20 years 89.4% (95% CI 76.9–96.8)).
Fig 2.
Cumulative incidence curves for mortality in Scottish children after start of renal replacement therapy between 1961 and 2013 by initial renal replacement therapy modality
Cardiovascular disease incidence after the start of renal replacement therapy
The cohort included in the CVD incidence analyses of 381 children was followed for a median of 14.96 (IQR 7.05–22.87) years with a total follow-up of 5739 person years. The mean age at the first CVD event was 22.1 (standard deviation ± 9.4) years. In total 80 (20.9%) children experienced a CVD event of which 20 had a fatal event. The most common types of CVD were cerebrovascular disease (N = 29) followed by heart failure (N = 13), cardiac arrest/arrhythmias (N = 13) and other (N = 12 of which 55% were due to cardiomegaly and 27% were classified as endocarditis), ischaemic heart disease (N = 11) and cardiomyopathy (N = 2).
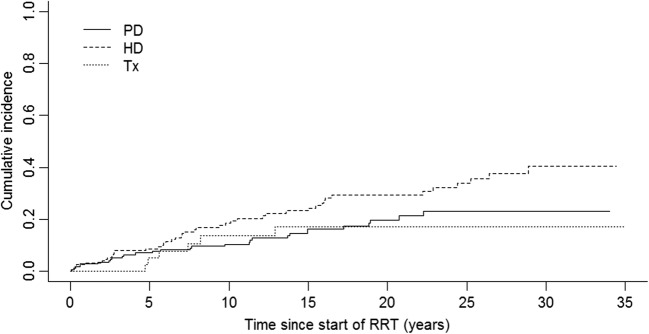
The overall CVD incidence was 1.39 (95% CI 1.09–1.70) per 100 person years. CVD incidence was lowest among pre-Tx children (0.93, 95% CI 0.19–1.67 per 100 person years) compared with children who started with HD (1.87, 95% CI 1.33–2.43) per 100 person years) or PD (1.08, 95% CI 0.68–1.47 per 100 person years) (Fig. 3), but these differences were not statistically significant. Figure 3 shows the cumulative incidence curves based on unadjusted CICR analyses.
Fig 3.
Cumulative incidence curves for cardiovascular disease in Scottish children after start of renal replacement therapy between 1981 and 2013 by initial renal replacement therapy modality
Associations between determinants and all-cause mortality and cardiovascular disease incidence
Table 3 presents the results of the cause-specific Cox proportional hazard analyses showing associations between age at start of RRT, sex, PRD and RRT modality with all-cause mortality and CVD incidence. Starting RRT at a very young age (0–2 years), PRD other than CAKUT or GN and receiving dialysis rather than a kidney transplantation as the initial RRT modality are significantly associated with increased risk of all-cause mortality. Furthermore, these results show that patients who never received a transplant have an increased mortality risk compared with patients who are pre-emptively transplanted or who received a transplant during follow-up. Sex was not associated with all-cause mortality, but boys/men had an increased risk of CVD compared to girls/women. Other factors associated with increased risk of CVD were PRD other than CAKUT or GN and receiving no transplant during follow-up. Similar results were obtained in a sensitivity analysis excluding 50 children who received a pre-emptive transplant .
Table 3.
Crude and adjusted hazard ratios for associations between determinants and all-cause mortality and cardiovascular disease incidence
| Variable | All-cause mortality | CVD incidence | ||
|---|---|---|---|---|
| Crude HR (95% CI) |
Adjusted HR (95% CI) |
Crude HR (95% CI) |
Adjusted HR (95% CI) |
|
| Age at start of RRTa | ||||
| 0–<2 | 2.41 (1.27–4.57) | 2.87 (1.42–5.82) | 0.73 (0.27–2.02) | 0.89 (0.31–2.59) |
| 2–<6 | 0.93 (0.60–1.44) | 1.39 (0.67–2.67) | 0.54 (0.23–1.25) | 0.77 (0.32–1.91) |
| 6–<12 | 0.97 (0.50–1.89) | 1.04 (0.66–1.64) | 0.67 (0.38–1.17) | 0.79 (0.44–1.41) |
| 12–18 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sexb | ||||
| Males | 1.27 (0.89–1.82) | 1.31 (0.92–1.88) | 1.51 (0.96–2.38) | 1.76 (1.11–2.79) |
| Females | 1.00 | 1.00 | 1.00 | 1.00 |
| PRDc | ||||
| GN | 0.99 (0.61–1.65) | 1.01 (0.61–1.69) | 1.17 (0.63–2.18) | 1.12 (0.59–2.19) |
| Other | 1.45 (0.98–2.13) | 1.50 (1.01–2.22) | 1.46 (0.90–2.37) | 1.67 (1.01–2.75) |
| CAKUT | 1.00 | 1.00 | 1.00 | 1.00 |
| Initial RRT modalityd | ||||
| HD | 2.97 (1.08–8.07) | 2.57 (0.92–7.18) | 2.04 (0.86–4.78) | 1.73 (0.73–4.11) |
| PD | 2.41 (0.87–6.70) | 2.06 (0.73–5.85) | 1.17 (0.49–2.82) | 1.38 (0.56–3.41) |
| Pre-Tx | 1.00 | 1.00 | 1.00 | 1.00 |
| RRT modality during follow-upe | ||||
| HD + Tx- | 14.4 (8.01–25.77) | 20.03 (10.77–37.25) | 5.51 (2.14–14.16) | 6.20 (2.29–16.80) |
| PD + Tx- | 17.3 (9.71–30.76) | 20.69 (10.73–39.89) | 5.50 (2.41–12.55) | 5.81 (2.50–13.50) |
| Pre-Tx | 0.55 (0.20–1.51) | 0.68 (0.25–1.86) | 0.74 (0.32–1.71) | 0.69 (0.30–1.61) |
| D + Tx+ | 1.00 | 1.00 | 1.00 | 1.00 |
CVD cardiovascular disease; HR hazard ratio, CI confidence interval; RRT renal replacement therapy; PRD primary renal disease; GN glomerulonephritis, CAKUT congenital anomalies of kidney and urinary tract; HD haemodialysis; PD peritoneal dialysis; pre-Tx pre-emptively transplanted; HD + Tx started on HD and not transplanted during follow-up, PD + Tx started on PD and not transplanted during follow-up, D + Tx + transplanted ever after starting on dialysis. Only patients with complete data included in unadjusted and adjusted analyses; adjusted for a sex, PRD, type of RRT at start and period of start of RRT; b age at start of RRT, PRD, type of RRT at start and period of start of RRT; cage at start of RRT, sex, type of RRT at start and period of start of RRT; d age at start of RRT, sex, PRD and period of start of RRT; e age at start of RRT, sex, PRD and period of start of RRT
Discussion
Summary of principal findings
In this study, we described survival and CVD incidence in children who started RRT after 1961 in Scotland and investigated how key patient and treatment factors were associated with these outcomes. Of the 477 children who started RRT in Scotland between 1961 and 2013, 125 died during a total of 8.710 person years of follow-up. Unadjusted cumulative survival for the cohort was 87.3% at 10 years and 77.6% at 20 years. Survival was highest in patients who received a pre-emptive transplant and lowest in patients who started RRT on HD and did not receive a transplant during follow-up. Other factors associated with poorer survival were underlying PRD other than CAKUT or GN and starting RRT at a very young age. During a median follow-up of almost 15 years, 21% of the patients developed incident CVD with the most common type being cerebrovascular disease. CVD incidence was not significantly associated with initial mode of RRT. Boys/men had an increased risk of CVD incidence compared to girls/women. PRD other than CAKUT or GN and receiving dialysis rather than a kidney transplantation were associated with increased risk of CVD.
Comparison of all-cause mortality with previous studies
Our findings were similar to the 86% cumulative 10-year survival reported from a Canadian cohort including 843 children starting RRT between 1992 and 2007 [15]. However, McDonald and Craig reported slightly lower survival figures based on older data from the Australian and New Zealand (ANZDATA) registries collected between 1963 and 2002 [4], and the ERA/EDTA has published higher overall long-term cumulative survival figures based on recent data from Europe [24]. The variation of these survival rates can at least partly be explained by differences in study period included in the analyses. For example, data from ANZDATA showed increased survival [4] due to availability of effective treatments [24].
Several other reports have studied the association between key patient and treatment factors and all-cause mortality. Consistent with the findings of previous studies, we found that the youngest age group (<2 years at start of RRT) had the highest mortality [5, 15, 24].
Unsurprisingly, we found the highest survival among children who received a pre-emptive transplants and lowest survival among patients who never received a transplant [25]. This finding is in line with earlier studies [26]. Although this finding supports the likely benefit of transplant, there is also the strong possibility that failure to receive a transplant may also be a marker of high risk of mortality due to coexistent conditions that preclude listing for transplantation.
We found the highest all-cause mortality in children with an underlying PRD other than CAKUT or GN which is consistent with reports from a cohort including Canadian infants [15] and reports based on USRDS data [5].
We found that boys had an increased risk of all-cause mortality compared with girls; however this effect was not statistically significant. In contrast a study using USRDS data including both dialysis and transplant patients showed a higher mortality among girls after a median follow-up of 7 years (HR 1.36 (95% CI 1.25–1.50)). Sex disparities in access to (pre-emptive) transplantation, health insurance and underlying PRD only partly explained the difference in survival [27]. A few other studies have shown higher mortality among girls [5, 28], but a short follow-up or only including dialysis patients makes comparison with our results difficult.
Comparison of cardiovascular outcomes with previous studies
It is well-known that similar to results from the adult RRT population, the proportion of deaths in the paediatric RRT population attributed to CVD is high [7, 8, 14, 27, 29]. However, there are only limited data available on CV outcomes including CV morbidity. A few studies have reported on fatal and nonfatal CVD, but these studies were limited to a short follow-up, a selected population and lacking detailed specification of the type of CV outcomes [9, 29].
One of the few sources reporting the incidence of CVD events is the USRDS. Data from this source showed that first-year CVD hospitalisation are common (rates of 63 per 1000 patient years from 2005 to 2009 and 42 from 2010 to 2014). The highest risk of CVD hospitalisation occurred among children on dialysis which is in agreement with our results. Chavers et al. reported that around a third of the patients who started dialysis between 1990 and 1997 developed a cardiac-related event. Of these events, the majority were arrhythmias, valvular heart disease, cardiomyopathy and cardiac arrests. Children in the oldest age group (15–19 years), blacks and girls, had the highest rates of cardiac-related events [11]. Different inclusion criteria and follow-up make it difficult to directly compare these findings with our results. A possible explanation for the overall lower incidence of CVD events we found (21% compared with 30% based on USRDS data) might be the fact that our population is predominantly Caucasian. Previous studies have reported a high prevalence of a variety of intermediate CV outcomes and CV abnormalities such as left ventricular hypertrophy, coronary calcifications, arrhythmias and significantly increased carotid intima media thickness in children and young adults who started RRT in childhood [30–35]. These results are in agreement with our findings of a high incidence of CVD after long-term follow-up during young adult age (mean age of first CVD event of 21 years) and increased CVD risk among both dialysis and transplant patients compared with general population.
Furthermore, high prevalence of traditional CV risk factors and newer risk factors such as C-reactive protein among dialysis and transplanted patients is reported [27]. For example, a cross-sectional analysis of prevalent RRT patients in the United Kingdom has shown that 75% of patients younger than 18 years old have one or more CV risk factor (obesity, hypertension, hypercholesterolaemia), and one in ten have all three risk factors [36]. These findings raise the expectation of high incidence of fatal and nonfatal CVD incidence in this population.
Strengths and limitations of the study
In our study, data from all incident paediatric RRT patients in Scotland are included. A novel data linkage with death and hospital admission data offered the opportunity to describe fatal and nonfatal incident CVD events as well as all-cause mortality during follow-up period into adulthood. The study cohort is extremely heterogeneous because all patients who started RRT in Scotland in a certain period are included. This has resulted in a cohort which covers several decades and includes both dialysis and transplant patients (pre-emptively and transplanted during follow-up), a variety of PRDs, infants, children and adolescents resulting in a diverse case mix. We were therefore able to investigate the effect of these diverse characteristics on mortality and CVD incidence and found that the sensitivity analysis excluding children who received a pre-emptive transplant (N = 50) found similar results to the main analysis.
A key limitation is the relatively small sample size despite this study being a population wide study including all incident RRT patients in Scotland over a 50-year calendar period. The small sample size has resulted in low statistical power and precision and limited ability to perform potentially interesting analyses in subgroups of the cohort. Future studies including a larger cohort will offer opportunities to look at analyses stratified by underlying PRD and the impact of time (different types) of RRT on cardiovascular risk in children after initiating RRT. Although important possible confounding variables are taken into account, information on existing comorbidities is not available.
A final potential limitation is that data on causes of death and hospital admissions before 1981 are not available, and therefore it might be possible that a small proportion of the children currently classified as having new onset CVD had an earlier CVD event prior to 1981.
Conclusion and clinical implications
In summary, our study shows that 87% and 78% of children who started RRT in Scotland between 1961 and 2013 were still alive after 10 and 20 years of follow-up. Long-term survival was highest in patients who receive a pre-emptive transplant, who have CAKUT or GN as PRD and who start RRT at older age. After a median follow-up of 13 years, a fifth of the children developed incident CVD. Male sex, dialysis as treatment modality and PRD other than CAKUT or GN were associated with higher risk of CVD. It is important that physicians providing care to young adults who started RRT during childhood are aware of the implications of childhood illness upon cardiovascular risk. Furthermore, our results showed that patients who received a pre-emptive transplant had a lower risk of all-cause mortality and CVD outcomes. Recent data from the UK Renal Registry showed that less than a quarter of paediatric RRT patients received a pre-emptive transplant [33]. To prevent early mortality and CVD pre-emptive transplantation should be promoted.
Our results strongly suggest that the effectiveness of monitoring and treating CV risk factors children on RRT and young adults who started RRT during childhood should be assessed. The results of the Dutch LERIC cohort have shown promising results of intensified antihypertensive and antidyslipidaemic therapy in young adults who started RRT in childhood. In this cohort of 249 patients who started RRT in childhood and survived into adulthood (follow-up of 25.5 years), a clear decrease in CV mortality along with strict CV management and reversal of CV risk factors was observed [30, 31]. A review published in 2017 describes the under-recognised and under-treated burden of hypertension in transplant patients [37]. In addition another study has reported on contradicting guidelines and differences in policies and treatment of hypertension in Dutch paediatric transplant patients [38]. This evidence together with our reported increased and ongoing CVD risk in paediatric patients underlines the importance of further research to provide evidence to develop specific guidelines for optimal treatment of paediatric RRT patients.
Acknowledgements
D.B.G. is supported by a Bolashak International scholarship from the president of Kazakhstan. N.H is supported by an Intermediate Basic Science Fellowship from the British Heart Foundation (fellowship number FS/16/36/32205)
Author contributions
D.B.G., S.H.W., C.A.J. and N.H have conceived and designed the study. D.G. and N.H. conducted the data analysis. D.G., S.H.W., C.A.J. and N.H drafted the manuscript. All authors interpreted the data, provided intellectual content, revised the drafts and approved the final version.
Data availability
The datasets generated and analysed during the current study are not publicly available due to the identifiable nature of the data. All data is stored and analysed in a ‘Safe Haven’ environment.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5:2172–2179. doi: 10.2215/CJN.07851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ESPN/ERA-EDTA Registry ESPN/ERA-EDTA Annual Report 2015.
- 4.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 5.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA. 2013;309:1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesnaye NC, Schaefer F, Groothoff JW, Bonthuis M, Reusz G, Heaf JG, Lewis M, Maurer E, Paripovic D, Zagozdzon I, van Stralen KJ, Jager KJ. Mortality risk in European children with end-stage renal disease on dialysis. Kidney Int. 2016;89:1355–1362. doi: 10.1016/j.kint.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–197. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 8.Chesnaye N, Bonthuis M, Schaefer F, Groothoff JW, Verrina E, Heaf JG, Jankauskiene A, Lukosiene V, Molchanova EA, Mota C, Peco-Antic A, Ratsch IM, Bjerre A, Roussinov DL, Sukalo A, Topaloglu R, Van Hoeck K, Zagozdzon I, Jager KJ, Van Stralen KJ. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol. 2014;29:2403–2410. doi: 10.1007/s00467-014-2884-6. [DOI] [PubMed] [Google Scholar]
- 9.Koshy SM, Guttmann A, Hebert D, Parkes RK, Logan AG. Incidence and risk factors for cardiovascular events and death in pediatric renal transplant patients: a single center long-term outcome study. Pediatr Transplant. 2009;13:1027–1033. doi: 10.1111/j.1399-3046.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, Ball A, Stehman-Breen CO. Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int. 2002;61:630–637. doi: 10.1046/j.1523-1755.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 11.Chavers BM, Li S, Collins AJ, Herzog CA. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 2002;62:648–653. doi: 10.1046/j.1523-1755.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexander RT, Foster BJ, Tonelli MA, Soo A, Nettel-Aguirre A, Hemmelgarn BR, Samuel SM. Survival and transplantation outcomes of children less than 2 years of age with end-stage renal disease. Pediatr Nephrol. 2012;27:1975–1983. doi: 10.1007/s00467-012-2195-8. [DOI] [PubMed] [Google Scholar]
- 13.Pruthi R, O'Brien C, Casula A, Braddon F, Lewis M, Maxwell H, Tse Y, Inward C, Sinha MD. UK Renal Registry 15th annual report: Chapter 4 demography of the UK paediatric renal replacement therapy population in 2011. Nephron Clin Pract. 2013;123(Suppl 1):81–92. doi: 10.1159/000353323. [DOI] [PubMed] [Google Scholar]
- 14.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61:621–629. doi: 10.1046/j.1523-1755.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Samuel SM, Tonelli MA, Foster BJ, Alexander RT, Nettel-Aguirre A, Soo A, Hemmelgarn BR. Survival in pediatric dialysis and transplant patients. Clin J Am Soc Nephrol. 2011;6:1094–1099. doi: 10.2215/CJN.04920610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Information Services Division (ISD) The Scottish Renal Registry
- 17.Information Services Division (ISD) https://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Datasets/SMR01-General-Acute-Inpatient-and-Day-Case.
- 18.NHS Scotland Public Benefit and Privacy Panel for Health and Social Care.
- 19.Bonthuis M, van Stralen KJ, Jager KJ, Baiko S, Jahnukainen T, Laube GF, Podracka L, Seeman T, Tyerman K, Ulinski T, Groothoff JW, Schaefer F, Verrina E. Dyslipidaemia in children on renal replacement therapy. Nephrol Dial Transplant. 2014;29:594–603. doi: 10.1093/ndt/gft429. [DOI] [PubMed] [Google Scholar]
- 20.Bonthuis M, van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar A, Edefonti A, Fischbach M, Mendes P, Molchanova EA, Paripovic D, Peco-Antic A, Printza N, Rees L, Rubik J, Stefanidis CJ, Sinha MD, Zagozdzon I, Jager KJ, Schaefer F. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant. 2013;28(Suppl 4):iv195–iv204. doi: 10.1093/ndt/gft259. [DOI] [PubMed] [Google Scholar]
- 21.ERA-EDTA Registry ERA-EDTA Registry Annual Report 2015.
- 22.Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med. 2002;21:1129–1144. doi: 10.1002/sim.1070. [DOI] [PubMed] [Google Scholar]
- 24.Chesnaye NC, van Stralen KJ, Bonthuis M, Harambat J, Groothoff JW, Jager KJ. Survival in children requiring chronic renal replacement therapy. Pediatr Nephrol. 2018;33:585–594. doi: 10.1007/s00467-017-3681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harambat J, Bonthuis M, Groothoff JW, Schaefer F, Tizard EJ, Verrina E, van Stralen KJ, Jager KJ. Lessons learned from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2016;31:2055–2064. doi: 10.1007/s00467-015-3238-8. [DOI] [PubMed] [Google Scholar]
- 26.Parekh RS, Gidding SS. Cardiovascular complications in pediatric end-stage renal disease. Pediatr Nephrol. 2005;20:125–131. doi: 10.1007/s00467-004-1664-0. [DOI] [PubMed] [Google Scholar]
- 27.Splinter A, Tjaden LA, Haverman L, Adams B, Collard L, Cransberg K, van Dyck M, Van Hoeck KJ, Hoppe B, Koster-Kamphuis L, Lilien MR, Raes A, Taylan C, Grootenhuis MA, Groothoff JW (2018) Children on dialysis as well as renal transplanted children report severely impaired health-related quality of life. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 27:1445-1454. [DOI] [PMC free article] [PubMed]
- 28.Chavers BM, Molony JT, Solid CA, Rheault MN, Collins AJ. One-year mortality rates in US children with end-stage renal disease. Am J Nephrol. 2015;41:121–128. doi: 10.1159/000380828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Renal Data System USRDS Annual Report 2013.
- 30.Vogelzang JL, Heestermans LW, van Stralen KJ, Jager KJ, Groothoff JW. Simultaneous reversal of risk factors for cardiac death and intensified therapy in long-term survivors of paediatric end-stage renal disease over the last 10 years. Nephrol Dial Transplant. 2013;28:2545–2552. doi: 10.1093/ndt/gft257. [DOI] [PubMed] [Google Scholar]
- 31.Vogelzang JL, van Stralen KJ, Jager KJ, Groothoff JW. Trend from cardiovascular to non-cardiovascular late mortality in patients with renal replacement therapy since childhood. Nephrol Dial Transplant. 2013;28:2082–2089. doi: 10.1093/ndt/gft048. [DOI] [PubMed] [Google Scholar]
- 32.Gruppen MP, Groothoff JW, Prins M, van der Wouw P, Offringa M, Bos WJ, Davin JC, Heymans HS. Cardiac disease in young adult patients with end-stage renal disease since childhood: a Dutch cohort study. Kidney Int. 2003;63:1058–1065. doi: 10.1046/j.1523-1755.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- 33.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 34.Scharer K, Schmidt KG, Soergel M. Cardiac function and structure in patients with chronic renal failure. Pediatr Nephrol. 1999;13:951–965. doi: 10.1007/s004670050737. [DOI] [PubMed] [Google Scholar]
- 35.Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, Strife CF. Severe left ventricular hypertrophy in pediatric dialysis: prevalence and predictors. Pediatr Nephrol. 2000;14:898–902. doi: 10.1007/s004670000303. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton AJ, Braddon F, Casula A, Inward C, Lewis M, Mallett T, Maxwell H, O'Brien C, Tse Y, Sinha MD. UK Renal Registry 18th Annual Report: Chapter 10 Clinical, Haematological and Biochemical Parameters in Patients Receiving Renal Replacement Therapy in Paediatric Centres in the UK in 2014: National and Centre-specific Analyses. Nephron. 2016;132(Suppl 1):237–252. doi: 10.1159/000444824. [DOI] [PubMed] [Google Scholar]
- 37.Charnaya O, Moudgil A. Hypertension in the pediatric kidney transplant recipient. Front Pediatr. 2017;5:86. doi: 10.3389/fped.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrowolski LC, van Huis M, van der Lee JH, Peters Sengers H, Lilien MR, Cransberg K, Cornelissen M, Bouts AH, de Fijter JW, Berger SP, van Zuilen A, Nurmohamed SA, Betjes MH, Hilbrands L, Hoitsma AJ, Bemelman FJ, Krediet CTP, Groothoff JW. Epidemiology and management of hypertension in paediatric and young adult kidney transplant recipients in The Netherlands. Nephrol Dial Transplant. 2016;31:1947–1956. doi: 10.1093/ndt/gfw225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to the identifiable nature of the data. All data is stored and analysed in a ‘Safe Haven’ environment.