Abstract
The widespread use of organochlorine pesticides (OCPs), essentially for the control of insects and the cultivation of food crops, has led to the pollution of ecosystems. Despite being banned several years ago in the developed world, extensive use remains ongoing on the African continent. This review summarizes the occurrence, distributions, sources, and trends of OCPs in seven environmental matrices (atmosphere, water, sediments, soils, biota, human fluids and food products) in Africa. Findings in this review revealed that α-HCH, β-HCH dichlorodiphenyltrichloroethane (DDTs), and endosulfans were the most persistent OCP residues in the African environment, particularly DDTs in breast milk samples occurring in levels above the WHO stipulated limits, thus indicating a call for concern. Also, there was paucity of data available on OCP concentrations in ambient air. Future research efforts should prioritize testing these pollutants in the atmosphere, especially in countries where they are used more frequently. While most POP analysis studies used gas chromatography coupled to electron capture detector or mass spectrometer, it is recommended that further studies should use more sensitive analytical techniques such as gas chromatography with tandem mass spectrometry (GC-MS/MS), or gas chromatography coupled to high-resolution mass spectrometry (GC-HRMS). These instruments allow for the detection of secondary and tertiary metabolites, especially those found in water, biota and food products, which are critical vectors of OCPs to human and animal bodies. Training of farmers and other domestic users on the handling of pesticides is proposed.
Keywords: Africa, Ecosystem, Environment, Occurrences, Pollutants, Occurrences. pollutants, Gas chromatograph, Analytical chemistry, Organic chemistry, Environmental science, Earth sciences, Toxicology
Africa. ecosystem. environment, Occurrences. pollutants, Gas chromatograph; Analytical chemistry; Organic chemistry; Environmental science; Earth sciences; Toxicology
1. Introduction
The adverse effects of persistent organic pollutants (POPs) in the ecosystem have been one of the major subjects of discussion all over the world. These chemicals are toxic and because of their ability to be transported long distance from the place of use and release either by water or wind, they tend to affect biota and humans in diverse ways (Buccini, 2003; EPA, 2009). Due to their ubiquitous nature, lipophilic properties, and persistence in the environment, they tend to accumulate in tissues of living organisms (Afful and Anim, 2010; Olisah et al., 2019a). Many of these compounds have been classified as endocrine disruptors, because of their tendency to reduce the efficiency of thyroid hormones and to affect the neurobehavioral development and reproductive systems in animals and humans (ATSDR, 2000). Carcinogenic cases of these compounds have been reported (El-Shahawi et al., 2010). Initially, 12 POPs classified as “Dirty Dozen” were identified as endocrine disruptors (UNEP, 2001). Chemicals such as organochlorine pesticides (OCPs) in this group, are used for pest control because of their versatility (Olisah et al., 2019b). These include insecticides such as aldrin, dieldrin, endrin, chlordane, dichlorodiphenyltrichloroethane (DDT), mirex, toxaphene, and heptachlor; fungicides - hexachlorobenzene (HCB) and industrial chemicals -polychlorinated biphenyls (PCBs). Other chemicals in this group that are unintentionally produced are polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Nine others including industrial chemicals (pentabromodiphenyl ether, octabromodiphenyl ether, chlordecone, lindane, α –hexachlorocyclohexane, β-hexachlorocyclohexane, perfluorooctane sulfonate, hexabromobiphenyl and pentachlorobenzene), classified as “Nasty Nine” were added during the meeting held in May 2009 under the treaty of the Stockholm convention (UNEP, 2009).
Population growth has led to increased use of pesticides for crop cultivation. Research have shown that pesticides are used to cultivate more than one quarter of farm products (Liu et al., 2002). A decrease in production of cereals (32%), vegetables (54%) and fruits (78%) has been reported without the use of pesticides (Cai, 2008). Apart from agriculture, the importance of pesticides in the health sector cannot be overemphasised. According to World Health Organisation (WHO), indoor residual spraying of pesticides and the use of nets treated with insecticides are the two most important measures in which humans are being protected from mosquitos and other insects capable of causing illness. This has been found in many countries in the world mostly in malaria-endemic regions (World Health Organization, 2016). Despite the benefits of using pesticides, the risk it poses to humans and animals cannot be overlooked. The improper handling of these chemicals by farmers and other end users have been responsible for the high level of pesticides found in the atmosphere, water bodies, and soils, hence posing threat to human health and the ecosystem at large. Various problems resulting from pesticides found (especially the organochlorine types) in the environment have been reported in Asia, America, Europe and Africa (Hoh and Hites, 2004; Gunnell et al., 2007; Ali et al., 2013; Elibariki and Maguta, 2017). These include DDTs, HCHs, endosulfans, HCB, Drins (aldrin, endrin, and dieldrin). As of 2009, most African countries continued usage of OCPs even though they were banned (UNEP, 2009). Most of these pesticides get into the ecosystem through runoff from agricultural farmlands, illegal disposal of waste, and by-products from chlorine combustion processes (Afful and Anim, 2010). Extremely high levels of these pollutants have been detected by researchers in Africa and other parts of the world in various matrices, including biota, sediment, soil, water and food products (Kishimba et al., 2004; Bempah et al., 2012; Kolani et al., 2016).
In the last two decades (1996–2016), several studies have been conducted to analyse environmental matrices in Africa for the presence of OCPs, although many were not documented. Based on published literature, our current knowledge of the persistence of OCPs in the African environment remains scarce (Williamson et al., 2008; Elibariki and Maguta, 2017). This article therefore summarises the levels of OCPs in environmental and biological matrices in African environment in the last two decades (1996–2016).
1.1. Pesticide use in Africa
The pesticides used in Africa from 1990 to 2016 were estimated to be 69,355.36 tons active ingredient (a.i), accounting for about 2.1% world total pesticide usage. This value is low compared to that of Asia, Americas and Europe which were reported to be 1,727,322.77 ton a.i, 956,040.22 tons a.i and 455,159.85 tons a.i respectively (http://www.fao.org/faostat/en/#data/RP/visualize). Pesticides are majorly used for large scale farming especially in the cultivation of cash crops such as cocoa, oil palm, and vegetables (Matthews et al., 2003). Their use in small scale farming was found to be almost insignificant (Nsibande and McGeoch, 1999; Ebenebe et al., 2001). Their use for large scale commercial farming pose a greater risk to the ecosystem in Africa, and studies indicate a positive correlation with poor pesticide handling (Sibanda et al., 2000; Vaissayre and Cauquil, 2000). Example of such practices include over dosage, incorrect use of equipment for pesticide application, leakage from storage tanks. This inappropriate use leads to soil, water and air contamination which in turn pose risks to humans and livestock (Conway, 2013). Some extreme cases include lack of adequate personal protective equipment, tongue tasting to verify the originality or concentration of the pesticides, the sprinkling of pesticides directly to the germinated crop and mixing of pesticides with bare hands (Sibanda et al., 2000; Dinham, 2003). Reports from the pesticides policy initiatives for various countries in Africa indicated that pesticides usage in Africa is largely influenced by various biological, economic and climatic factors (Mudimu et al., 1995; Ngowi, 2002). Hence, the levels and trends of usage vary from one country to another.
2. Occurrence of OCPs in Africa
2.1. Atmosphere
OCPs are volatile compounds that are largely found in the atmosphere after volatilising from contaminated soils and water. Very few reports have been documented on the evaluation of these contaminants in air in Africa, even though the atmosphere is considered a medium, in which they travel long distance. Karlsson et al. (2000) quantified HCHs, DDTs, chlordane and endosufan in air collected twice a week in 1997 and 1998 using polyurethane foam from Senga Bay and Laurentian Great Lake Malawi area. Samples were analysed with gas chromatograph – electron capture detector (GC-ECD). The analyses revealed that the mean concentrations of ƩDDTs, ƩHCHs, dieldrin, endrin and mirex in Senga bay were 26 ± 31 pg/m3, 40 ± 43 pg/m3, 80 ± 80 pg/m3, 1.0 ± 1.1 pg/m3, and 0.15 ± 0.44 pg/m3 respectively. In general, OCP residues ranged from 31 to 257 pg/m3 in Senga Bay while Laurentian Great Lake recorded a value ranging from 24 to 40 pg/m3. From the results, the authors inferred that excessive application of pesticides in tropical regions might be responsible for their occurrence in the atmosphere. Batterman et al. (2008) monitored three sample sites for OCPs. Two sites were industrial areas located in the southern part of Durban while the other was a residential area in the northern part. Sampling was done between the periods of 2004–2005. Air samples were collected using polyurethane foam plugs (75 mm) and analysis was done using GC-MS. p,p’–DDT and p,p’–DDD were detected at the 3 sampling locations with a mean concentration of 42 ± 27 pg/m3 and 12 ± 11 pg/m3 respectively. These values were considered to be relatively high. Other contaminants that were quantified include dieldrin, HCB aldrin, lindane, and chlordane. The authors inferred that chlordane and lindane emanated from regional or global sources as well as local sources. Data from this monitoring also revealed that this study is one of the most detailed in evaluating airborne pesticides in Africa.
2.2. Water
The aquatic systems harbour appreciable amount of OCPs through agricultural run-off, leachates from contaminated soils, spray drift from pesticides application on food crops, and careless disposal of pesticide containers. Such processes have led researchers to evaluate the occurrence of pesticides in various aquatic systems in South Africa especially in Lourens River, Hex River and Pongolo floodplain where endosulfan, DDTs and other pesticides were reported in significant concentrations (Bollmohr et al., 2007; Dabrowski et al., 2002a; Dabrowski et al., 2002b; Dalvie et al., 2003; McGregor, 1999; Naudé et al., 1998; Quinn et al., 2009; Schulz et al., 2001; Schulz, 2001a; Schulz and Peall, 2001; Thiere and Schulz, 2004). Table 1 shows the concentrations of some residues of OCP in water resources from different African countries.
Table 1.
Mean concentration (±standard deviation) and ranges of OCPs in water samples from Africa (μg/L).
| Location | Country | ƩHCHs | ƩDDTs | Lindane | ƩChlordane | Endosulfans | Dieldrin | HCBs | References |
|---|---|---|---|---|---|---|---|---|---|
| Hex River | South Africa | ND-1.79 | McGregor (1999) | ||||||
| Hex River | South Africa | BDL-26.3 | London et al. (2000) | ||||||
| El-Haram, Giza | Egypt | 3.8–290.0 | 20.7–86.2 | El-Kabbany et al. (2000) | |||||
| Akumadan | Ghana | 9.5 ± 5.2 | 124.5 | <100 | Ntow (2001) | ||||
| Lourens River | South Africa | 0.05–2.9 | Schulz (2001b) | ||||||
| Lourens River | South Africa | 0.13 ± 2.9 | Schulz (2001a) | ||||||
| Lourens River | South Africa | 0.03–0.16 | Schulz et al. (2001) | ||||||
| Lourens River | South Africa | 0.20 | Schulz and Peall (2001) | ||||||
| Lourens River | South Africa | ND-0.3 | Dabrowski et al. (2002b) | ||||||
| Lourens River | South Africa | 0.01–0.35 | Dabrowski et al. (2002a) | ||||||
| Lake Victoria | Tanzania | ND - 200 | ND -1600 | Henry and Kishimba (2003) | |||||
| Eastern Cape | South Africa | BDL-0.26 | BDL-0.12 | BDL-0.1 | BDL-0.1 | Fatoki and Awofolu (2003) | |||
| Banjul and Dakar | Senegal and Gambia | <LOQ - 0.120 | <LOQ- 0.231 | ND - 0.026 | ND – 0.0008 | Manirakiza et al. (2003) | |||
| Hex River | South Africa | 0.83 ± 1.0 | Dalvie et al. (2003) | ||||||
| Grabouw dam | South Africa | 3.16 ± 3.5 | Dalvie et al. (2003) | ||||||
| KwaZulu Natal | South Africa | BDL-0.002 | - | Sereda and Meinhardt (2003) | |||||
| Tana and Sabaki | Kenya | 5.2 ± 0.09 | 5.3 ± 0.024 | Lalah et al. (2003) | |||||
| East London | South Africa | 0.0055–0.04 | 0.0206 | 0.018 | 0.0057 | 0.015–0.16 | Fatoki and Awofolu (2004) | ||
| Port Elizabeth River | South Africa | 0.0055–0.06 | 0.0206 | 0.018 | 0.0057 | 0.01–0.07 | Fatoki and Awofolu (2004) | ||
| Buffalo River | South Africa | 0.0055–0.07 | 0.0206 | 0.018 | 0.0057 | 0.01–0.15 | Fatoki and Awofolu (2004) | ||
| Thyume River | South Africa | 0.0055–0.04 | 0.0206 | 0.018 | 0.0055 | 0.0077–0.08 | Fatoki and Awofolu (2004) | ||
| Southern Lake Victoria Basin | Tanzania | BDL-5.7 | BDL-33 | BDL-2.5 | Kishimba et al. (2004) | ||||
| KwaZulu Natal | South Africa | 0.0002–0.161 | Sereda and Meinhardt (2005) | ||||||
| Lake Volta | Ghana | 0.008 | 0.083 | Ntow (2005) | |||||
| Lake Bosumtwi | Ghana | 0.073 | 0.071 | 0.064 | Darko et al. (2008) | ||||
| Lourens | South Africa | 0.01 | 0.03 | Bollmohr et al. (2007) | |||||
| Juskei River | South Africa | 0.63–196 | 1.32–3086 | 3.82–967 | Sibali et al. (2008) | ||||
| Lagos Lagoon | Nigeria | 0.005–0.910 | 0.006–0.950 | 0.015–0.996 | 0.015–0.996 | 0.015–0.774 | Adeyemi et al. (2011) | ||
| Kilimanjaro | Tanzania | BDL-0.0552 | BDL-0.218 | - | BDL-0.012 | BDL-0.048 | Hellar-Kihampa (2011) | ||
| Vaal River Region A | South Africa | 80.8 ± 9.8 | 261 ± 67.3 | 2.5 ± 0.9 | 8.5 ± 2.2 | Wepener et al. (2011) | |||
| Vaal River Region B | South Africa | 70.1 ± 2.8 | 227 ± 56 | 1.1 ± 0.5 | 135 ± 89 | Wepener et al. (2011) | |||
| Vaal River Region C | South Africa | 10.2 ± 0.9 | 160 ± 47 | BDL | 257 ± 204 | Wepener et al. (2011) | |||
| Vaal River Region D | South Africa | 156 ± 29.6 | 468 ± 147 | 2.7 ± 0.7 | 588 ± 443 | Wepener et al. (2011) | |||
| Densu River Basin | Ghana | 0.02–1.07 | ND – 0.02 | ND – 0.12 | ND – 0.26 | ND – 0.02 | Kuranchie-Mensah et al. (2012) | ||
| Ondo State | Nigeria | ND-0.11 | ND-1.51 | Okoya et al. (2013) | |||||
| Weruweru | Tanzania | BDL-3.66 | BDL-0.81 | BDL-12.7 | - | Mohamed et al. (2014) | |||
| Lake Volta | Ghana | <LOQ – 0.669 | <LOQ – 0.031 | <LOQ - 0.009 | <LOQ -0.076 | <LOQ – 0.031 | Gbeddy et al. (2014) | ||
| Ogbesse River | Nigeria | 0.92 | 0.12 ± 0.06 | 0.54 | 0.14 | Lawrence et al. (2015) | |||
| WHO/US-EPA maximum residue limit | 1∗∗ | 2∗∗∗ | 0.03∗∗ |
BDL - below detection limit; ND – not detected; LOQ – limit of quantification; ∗∗ - (WHO, 2004); ∗∗∗ - (FAO/WHO, 2002).
Reports on OCPs from Ghana showed lindane and endosulfan sulphate as the most persistent pesticides found in over 70% of the total water samples (n = 50) collected around a farming community. Approximately 68% of waters recorded the presence of α-endosulfan with an average concentration of 62.3 ng/L. Other pesticides such as heptachlor epoxide, HCB and DDE were not detected (ND) (Ntow, 2001). In contrast, a similar study conducted in Ghana showed that p,p’-DDE and p,p’-DDT were the most prevalent, occurring in 82% and 78% of water samples from Lake Bosumtwi (Darko et al., 2008).
Surface and ground waters from the Eastern Cape province in South Africa collected during June and December 2002 were analysed for OCPs (BHC isomers, metabolites of DDTs, Drins, chlordanes, endosulfan, HCB and heptachlor) by Fatoki and Awofolu (2004). These compounds were determined using GC-ECD. In this study, the OCPs detected were lower than the maximum stipulated limit of European Union of 0.1 μg/L (Council Directives, 2008). However, an elevated level of HCB and δ-BHC was detected in water from Buffalo River; an important freshwater resource for domestic use by its host communities in the province (Fatoki and Awofolu, 2004). Similar studies carried out in this province also detected high levels of δ-BCH and heptachlor in runoff from agricultural farm land (Fatoki and Awofolu, 2003). Henry and Kishimba (2003) determined DDTs, HCHs, and endosulfan in surface waters from 9 districts in Southern Lake Victoria in Tanzania. In this study, endosulfans were not detected in all the samples while the concentrations of HCHs and DDTs were 200 μg/L and 1600 μg/L respectively. These level were higher than those reported for water bodies in Banjul and Dakar (Manirakiza et al., 2003). However, high levels of these residues were detected in the dry season compared to rainy season. This was due to the dilution in the latter season with a possible view that the kinetics of pollutants in a marine environment is controlled by advection (Hewitt, 2000). Similar seasonal variations and OCPs dominance were reported in water collected from 4 rivers in Kilimanjaro, Tanzania (Hellar-Kihampa, 2011).
Two rivers (Tana and Sabaki Rivers) largely influenced by agricultural activities in Kenya were sampled by Lalah et al. (2003) for OCP analysis. Samples were collected between 1998 and 1999 and instrumental analysis was done using GC-ECD. Despite the ban of pesticides in Kenya, notably high concentrations were detected. Among such include lindane (5.367 ± 0.024 μg/g), DDE (5.200 ± 0.092 μg/g) and aldrin (11.90 ± 0.007 μg/g) in water weed from River Sabaki. Their levels were found to be very low in all samples from rivers probably because of their hydrophobic properties. Water samples from Ingwavuma and Ubombo districts of KwaZulu Natal in South Africa collected between 2000 and 2001 were analysed for DDDs and DDEs. Sampling was planned to cover the entire duration of the malaria spraying season and instrumental analysis was conducted using GC-ECD. This study showed that over 40% of the samples in each location contained the target analytes, thus suggesting a possible source of contamination (Sereda and Meinhardt, 2005). Adeyemi et al. (2011) determined chlordane, heptachlor, methoxychlor, HCB, endosulfan, DDTs, and drins in water samples from Lagos Lagoon in Nigeria. The concentrations of all OCPs ranged up to 0.996 μg/L, while the pesticides concentration in water collected from selected rivers around a cocoa producing area in Ondo State, Nigeria ranged from ND to 1.65 μg/L (Okoya et al., 2013).
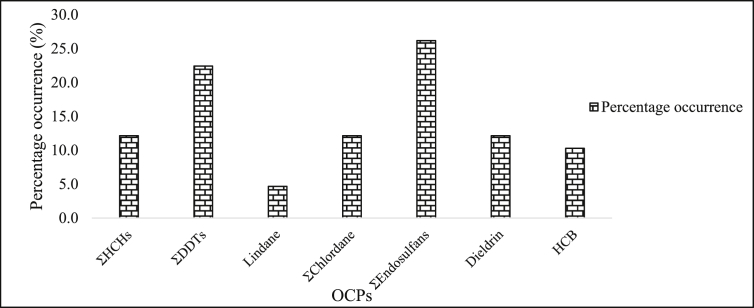
α-Chlordane, endosulfan sulfate, p,p’ –DDT, p,p’ –DDE and lindane were analysed in water samples collected from Weruweru Sub-catchment, Tanzania, in rainy and dry season of 2013 using GC-ECD (Mohamed et al., 2014). The sample sites were established based on details provided by key stakeholders and data from a 3DEM software algorithm. In most cases, pesticide residues in samples were below detection limits (BDL). Furthermore, endosulfan sulphate was the most dominant, detected in approximately 33% (in both seasons) of the total samples analysed at concentrations ranging between BDL and 12.7 μg/L. DDT metabolites levels were within the permissible limits stipulated by WHO (1 μg/L) (WHO, 2004), while lindane, detected in one of the site in dry season (3.66 μg/L), was above the WHO permissible limit (2 μg/L) (FAO/WHO, 2002). Other OCPs quantified, ranged from BDL to 45.7 μg/L in all samples. Findings from this study indicated that pesticide residues in the sample area emerged from agricultural run-off and deposits from agricultural soils. The findings summarised in this review indicates that DDTs and endosulfans were the most predominant in water samples from African environment occurring in 26.2% and 24.2% respectively (Figure 1).
Figure 1.
Percentage occurrence of OCPs in water resources from locations in Africa.
2.3. Sediments and soils
Sediments, long recognised as a reservoir for pesticides, are considered one of the environmental matrices in the monitoring of pollutants (Bhattacharya et al., 2003). The heterogeneous organic and inorganic particles found in sediments make the accumulation of contaminants possible. OCPs get into sediments through runoff from farmlands, deposition from the atmosphere through precipitation, illegal dumping of used pesticide containers in water bodies and leachate from contaminated soil and glacier deposits (Sarkar et al., 1988). These account for the varying levels of endosulfans and DDTs reported in African soils and sediments as shown in Table 2.
Table 2.
Mean concentration (±standard deviation) and ranges of OCPs in soil and sediment samples from African (μg/kg dry weight).
| Location | Country | Matrix | ƩHCHs | ƩDDTs | Lindane | Mirex | ƩChlordane | Endosulfans | Dieldrin | HCB | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KwaZulu Natal | South Africa | Sediment | 18.7–374 | Naudé et al. (1998) | |||||||
| Mpumalanga | South Africa | Sediment | 8.7–396 | Naudé et al. (1998) | |||||||
| El-Haram, Giza | Egypt | Soil | ND-16.2 | El-Kabbany et al. (2000) | |||||||
| Schulz et al., 2001 | South Africa | Sediment | 3.9–245 | Schulz et al. (2001) | |||||||
| Akumadan | Ghana | Sediment | 0.46 ± 0.24 | 3.2 ± 0.6 | 0.55 | Ntow (2001) | |||||
| Lourens River | South Africa | Suspended Particulate | ND -12082 | Schulz (2001b) | |||||||
| Lourens River | South Africa | Sediment | 9.70–273 | Dabrowski et al. (2002a) | |||||||
| Lourens River | South Africa | Sediment | ND-34.75 | Dabrowski et al. (2002b) | |||||||
| Lake Victoria | Tanzania | Soil | 5.9 × 104 | 2 × 104 | Henry and Kishimba (2003) | ||||||
| Lake Victoria | Tanzania | Sediment | 1 32 × 105 | 6.0 × 105 | Henry and Kishimba (2003) | ||||||
| Banjul and Dakar | Senegal and Gambia | Soil | 0.7–19.6 | 1.5–71.4 | ND- 0.7 | ND-88.2 | ND-23 | Manirakiza et al. (2003) | |||
| KwaZulu Natal | South Africa | Sedimenta | BDL-9.5 × 10−3 | 9 × 10-5- 2.4 × 10−3 | Sereda and Meinhardt (2003) | ||||||
| Eastern Cape | South Africa | Sediment | BDL-109 | BDL-117 | BDL-72 | BDL-60 | BDL-177 | Fatoki and Awofolu (2003) | |||
| Dar es Salaam coast | Tanzania | Sediment | BDL-2.7 | 6.4–51 | BDL-48 | Kishimba et al. (2004) | |||||
| Lake Victoria | Tanzania | Sediment | BDL-131 | BDL-705 | Kishimba et al. (2004) | ||||||
| TPC-Moshi | Tanzania | Sediment | BDL-61 | BDL-716 | BDL-7.2 | Kishimba et al. (2004) | |||||
| Zanzibar | Tanzania | Sediment | BDL-106 | BDL-20 | Kishimba et al. (2004) | ||||||
| Lourens River | South Africa | Suspended Particulate | 23–122 | Thiere and Schulz (2004) | |||||||
| Lake Volta | Ghana | Sediment | 61.3 | 2.3 | 0.74 | Ntow (2005) | |||||
| KwaZulu-Natal | South Africa | Sediment | 1 × 10−3 – 13.7 | Sereda and Meinhardt (2005) | |||||||
| Oueme River | Republic of Benin | Sedimentb | <0.1–61 | <0.1–809 | <0.1–164 | Pazou et al. (2006) | |||||
| Lourens River | South Africa | Sediment | BDL-16.4 | 0.43–12.7 | Bollmohr et al. (2007) | ||||||
| Ogba River | Nigeria | Sediment | 713 | 733 | Ize-Iyamu et al. (2007) | ||||||
| Ovia River | Nigeria | Sediment | 790 | 560 | Ize-Iyamu et al. (2007) | ||||||
| Ikoro River | Nigeria | Sediment | 600 | Ize-Iyamu et al. (2007) | |||||||
| Upper Awash | Ethiopia | Soil | <0.7–230 | <1.2–56000 | ND-2.4 | Westbom et al. (2008) | |||||
| Lake Bosomtwi | Ghana | Sediment | 12.75 | 6.755 ± 1.15 | 9.683 ± 1.76 | 0.072 ± 0.02 | Darko et al. (2008) | ||||
| Juskei River | South Africa | Sediment | 0.23–12221 | 4.62–22914 | 25.3–11462 | Sibali et al. (2008) | |||||
| Owvian River Warri | Nigeria | Sediment | 11.48 | Lawrence et al. (2009) | |||||||
| Ekakpamre River Warri | Nigeria | Sediment | 4.82 | Lawrence et al. (2009) | |||||||
| Old Korogwe | Tanzania | Soil | BDL-2.0 × 106 | Kihampa and Mato (2009) | |||||||
| Vikuge Farm | Tanzania | Soil | ND-6.3 × 107 | ND-2.8 × 108 | Kishimba and Mihale (2009) | ||||||
| Vaal River | South Africa | Soil | 3.53 | 0.09 | 4.77 | Quinn et al. (2009) | |||||
| Vaal River | South Africa | Sediment | 1.65 | 0.05 | 0.12 | Quinn et al. (2009) | |||||
| Ngarenanyuki | Tanzania | Soil | 2270 | 213 | 240 | Kihampa et al. (2010) | |||||
| Lake Victoria | Uganda | Sediment | 2.80 ± 2.00 | 4.24 ± 3.83 | 0.45 ± 0.23 | 6.00 ± 4.36 | 3.80 ± 3.55 | Wasswa et al. (2011) | |||
| Calabar River | Nigeria | Sediment | 10 | 970 | Sojinu et al. (2011) | ||||||
| Bakassi River | Nigeria | Sediment | 20 | 850 | Sojinu et al. (2011) | ||||||
| Imo River | Nigeria | Sediment | 10 | 930 | Sojinu et al. (2011) | ||||||
| Oginni River | Nigeria | Sediment | 70 | 290 | Sojinu et al. (2011) | ||||||
| Densu River Basin | Ghana | Sediment | 0.04–1.04 | 0.04–1.67 | 0.15–3.29 | 0.01–14.21 | 0.03–0.47 | - | Kuranchie-Mensah et al. (2012) | ||
| Lake Maryut | Egypt | Sediment | ND-2.20 | 0.07–105.6 | ND – 2.79 | ND-19.67 | ND-2.84 | ND-0.85 | 0.25–10.49 | Barakat et al. (2012a) | |
| Lake Manzala | Egypt | Sediment | ND-3.42 | 0.2–7.25 | ND – 0.37 | ND-2.35 | ND-0.03 | ND – 0.57 | Barakat et al. (2012b) | ||
| Ondo State | Nigeria | Sediment | ND-57400 | ND-81320 | ND-7040 | 10–8820 | ND- 10910 | Okoya et al. (2013) | |||
| Lake Qarun | Egypt | Sediment | 0.13–100.6 | ND-5.88 | 0.25–15.9 | ND-3.41 | ND-41.2 | Barakat et al. (2013) | |||
| Arusha and Mbeye region | Tanzania | Soil | 0.4–4.2 × 107 | 2.4–5.4 × 106 | ND-310 | 0.04–2.4 × 104 | Mahugija (2013) | ||||
| Tarkwa Bay Lagos Lagoon | Nigeria | Sediment | 4.5–227 | 0.5–45.7 | 0.6–80.5 | 0.6–48.8 | 9.2–176 | Williams (2013) | |||
| Nyando River | Kenya | Soil | 30.26 ± 2.09 | Abong'o et al. (2015) | |||||||
| Lake Volta | Ghana | Sediment | 2.3 | 1.23 | 0.43 | 1.34 | 0.82 | Gbeddy et al. (2014) | |||
| Ogbesse River | Nigeria | Sediment | 5210 | 850 | 540 | 940 | Lawrence et al. (2015) | ||||
| Illushi River | Nigeria | Sediment | 4890 | 970 | Ogbeide et al. (2016) | ||||||
| Ogbese River | Nigeria | Sediment | 5220 | 850 | Ogbeide et al. (2016) | ||||||
| Owan River | Nigeria | Sediment | 4900 | 290 | Ogbeide et al. (2016) | ||||||
| Agboi Creek, Lagos Lagoon | Nigeria | Sediment | 2090 | 139 | Ogbeide et al. (2016) | ||||||
| ERL | Sediment guideline | 3 | NG | 0.5 | 0.02 | MacDonald et al. (2000) | |||||
| ERM | Sediment guideline | 350 | NG | 6 | 8 | MacDonald et al. (2000) | |||||
| TEL | Sediment guideline | NG | 0.32 | 2.26 | 0.71 | CCME (1999) | |||||
| PEL | Sediment guideline | NG | 0.99 | 4.79 | 4.30 | CCME (1999) | |||||
| ISQG | Sediment guideline | NG | 0.94 | 4.5 | 2.85 | CCME (1999) | |||||
ERL - effects low range; ERM - effects median range; TEL - threshold effect level; PEL-probable effect level; ISQG - Interim fresh water sediment quality guidelines, NG - no guidelines.
ND – not detected, a wet weight, b μg/kg organic matter.
Manirakiza et al. (2003) determined 21 OCPs comprising of alachlor, mirex, heptachlor, methoxychlor, aldrin, dieldrin, endrin, heptachlor epoxide, endosulfans, HCB, HCHs and DDT metabolites in 76 soil samples. Samples were collected in 9 locations from Banjul and Dakar in Gambia and Senegal respectively. GC-μECD was used to detect analytes with lower concentration and GC-MS for analytes with higher concentration. In general, low mean concentration of OCPs in soils were observed. Levels of ƩHCHs, Ʃendosulfans, mirex, dieldrin, methoxychlor and aldrin were detected below 4 μg/kg dry weight with exception of the ƩHCHs (19.6 μg/kg)in SHG sampling site, dieldrin (12 μg/kg) in BHG site, and Ʃendosulfan in Mbao (6.7 μg/kg) and SGH (6.0 μg/kg) sites. Heptachlor, heptachlorepoxides, and alachlor were present in low concentration, except soils from SHG sites with a heptachlorepoxides concentration of 9.4 μg/kg whilst DDT and its metabolites had a concentration lower than 15 μg/kg in most of the sampling locations. In general, OCPs in this study were below that reported in Ghanaian sediments (Ntow, 2001), suggesting a very low pesticide usage in Senegal and Gambia.
Fatoki and Awofolu (2003) analysed 15 OCPs in sediments collected from Buffalo (BR), Swartkops (SKP), Thyume (TR) and Keiskamma (KR) Rivers in the Eastern Cape Province, South Africa between January and April 2002. Analysis was carried out using GC-ECD. 2,4′-DDT (trace) recorded the lowest concentration found in Buffalo River sediments while the highest was heptachlor (184 μg/kg). OCPs in the other locations recorded the following range of concentrations: 2,4′-DDT and dieldrin (trace levels) to γ-chlordane (16 μg/kg) in Keiskamma River sediments; 2,4′-DDT and dieldrin (trace levels) to 2,4′-DDD (19 μg/kg) in Thyume River sediments and 2,4′-DDT, 2,4′-DDE, dieldrin (trace levels) to β-BCH (30 μg/kg) in Swartkops River sediments. Levels of DDTs in sediments and water in this study were in line with the concentration of OCPs found in Jukskei River (Sibali et al., 2008). Probable contamination of ground water by OCPs in Vikuge Farm, Tanzania was reported when HCHs and DDTs were detected in soil samples collected from 20 cm and 50 cm depths (Kishimba and Mihale, 2009). Alarming levels of ƩDDTs [(2.8 × 108 μg/kg dry weight (dw)] and ƩHCHs (6.3 × 107 μg/kg dw) in soils were detected. These concentrations were said to be higher than the DDTs contained in commercial formulations. Their high concentrations was attributed to the illegal dumping and storage of obsolete pesticides around the study area since 1986, thus, raising concerns on the health status of people living in the area over the period of reference. These levels were higher than a similar study reported for sediments and soils from 9 districts in Tanzania where the concentrations of DDTs and HCHs were 6 × 105 μg/kg dry mass (dm) and 1.32 × 105 μg/kg dm respectively for sediments and 2 × 104 μg/kg dm and 5.9 × 104 μg/kg dm respectively for soils (Henry and Kishimba, 2003). High level of OCPs were also reported in a previous study carried out in soils and sediments collected around Vikuge farm (Elfvendahl et al., 2004; Kishimba et al., 2004) and Old Korogwe (Kihampa et al., 2010).
Kishimba et al. (2004) investigated the level of pesticides in soils and sediments collected from sugar plantations located in Arusha Chini, Kilimanjaro region, Dar es Salaam region, and Mahonda-Makoba Basin in Zanzibar, Tanzania. The targeted contaminants were analysed with GC-ECD/NPD. A lower concentration of pesticides was observed generally in all the sample matrices. For DDTs, the total concentration in soil and sediment samples were up to 700 μg/kg and 500 μg/kg, respectively whereas ƩHCHs recorded 132 μg/kg and 60 μg/kg in that order. Results of evaluation of OCPs in environmental compartments from Tanzania shows that these contaminants are still persistent in designated regions in the country despite the ban.
Pazou et al. (2006) determined 20 pesticides, including octachlorostyrene in 37 sediments collected from 9 locations from Oueme River in the Republic of Benin. OCPs in sediments ranged from BDL to 526 μg/kg organic matter. DDTs and HCHs dominated the OCP profile in sediments. Other contaminants such as dieldrin and endosulfans were present in significant concentrations. Endosulfans and HCHs were mostly detected in cotton culture areas, while locations that had maize growing farms were largely contaminated with DDTs. In general, most sites recorded pesticide concentrations above the sediment quality guidelines.
Soils from 15 sites in Uwiro village (Arusha City) in Tanzania were randomly sampled in 2009 and analysed for lindane, p,p’-DDE, p,p’-DDD, and endosulfan (Kihampa et al., 2010). Samples were collected at a depth of 0–15 cm, and analysis were done by gas chromatographic technique (GC-ECD). Results from the analysis showed that endosulfan sulphate recorded a mean concentration of 240 μg/kg dw and was the most predominant. Such high occurrence (74% in 11 soil samples) was also reported in soil samples collected from a cotton growing area in Mali by Dem et al. (2007). This indicated that endosulfan sulfate (sold as thionex) which was banned in Tanzania several years ago was still in use by a large number of local farmers as at the time of the study. Metabolites of DDT (p,p’-DDE and p,p’-DDD) were detected in 40% and 47% of soil samples with mean concentrations of 148.2 μg/kg dw and 154 μg/kg dw respectively. Thus indicating the persistence of these OCPs in Tanzania despite being banned since 1983 (Wu et al., 2008). In this study, lindane recorded the lowest occurrence of all the pesticides in the samples (33%) with a mean concentration of 212 μg/kg dw.
Westbom et al. (2008) determined 13 classes of OCPs in soils collected from Upper Awash State Farms, Ethiopia. Quantification and qualification was done using GC-μECD and GC-MS respectively. In this study, aldrin, dieldrin, endrin and heptachlor were not detectable in all samples while HCHs were detected in a very low concentration in few soils. A significant amount of DDTs and endosulfans were detected in soils while the sum total of these two classes of OCP gave up to 230 μg/kg dw and 56000 μg/kg dw respectively, signifying a potential threat to the ecosystems and the local community around the study area.
Darko et al. (2008) determined lindane, endosulfan, drins, p,p’-DDE and p,p’-DDT in sediment samples from Ghana using micro-capillary GC-ECD. These OCPs were found in all the sediments (n = 50) analysed and lindane was detected in 68% of the sediments with a total mean concentration of 6.755 ng/g. This is in agreement with a study carried out by Ntow (2005) whose total mean concentration of lindane in sediments from Lake Volta in Ghana was 6.56 μg/kg. Endosulfan (9.883 μg/kg) and p,p’-DDE (8.342 μg/kg) recorded the highest mean concentrations, occurring in 42% and 98% respectively of the total sediments analysed. The mean concentration of dieldrin (0.072 μg/kg) was lower than that of the parent compound – aldrin (0.065 μg/kg), indicating that the occurrence of the former in the sediment was not as a result of decomposition of the latter, but of the independent application of both contaminants as suggested by the authors. In general, the concentrations of OCPs in the study were in line with results obtained from two major lakes in Africa; Lake Tanganyika and Lake Victoria (Manirakiza et al., 2002; Kasozi et al., 2006). Endosulfan, aldrin, and dieldrin concentrations ranged from 0.82 to 5.62 μg/kg, 0.22–15.96 μg/kg, and 0.94–7.18 μg/kg, respectively in sediments (n = 117) collected from a depth of 0–20 cm from Lake Victoria in Uganda. More so, ƩDDTs concentrations ranged from 0.01 to 4.02 μg/kg while γ-HCH, and chlordane ranged from 0.05 to 5.48 μg/kg (Wasswa et al., 2011).
The extent of degradation of pesticide residues buried a few years ago was investigated in soil samples collected in 2009 from various depths (0–220 cm) at different sites (Tengeru, Akheri, ACU maize farm, Mbocu, and Mbimba) in Tanzania using high-resolution GC-MS (Mahugija, 2013). Of all metabolites of DDTs, 4, 4′-DDT was the most predominant with a detection frequency of 100% and concentrations ranging from 9 × 105 to 4.8 × 106 μg/kg dw in soils from Tengeru. The low ratio of (DDE + DDD)/DDT (<1) in most of the sites suggested a little or no occurrence of degradation whilst the high ratio of degradation (>1) in few samples from Mbocu, Tengeru and ACU maize signified the transformation of DDT residues to other compounds. Similarly, HCHs were detected in all the soil samples with concentrations ranging from 1.4 × 102 to 4.2 × 107 μg/kg dw. Low ratios of α-HCH/γ-HCH in most soils suggested lindane as the main cause of HCHs contamination while high ratios in few samples were related to the degradation of γ-HCH to α-HCH. Endosulfan-1 was the most consistent of all endosulfans detected in soil samples from Mbocu, Tengeru, and Akheri vegetable gardens, suggesting an insignificant degradation process. The isomers of chlordane (cis-chlordane & trans-chlordane) and heptachlor (cis-heptachloroepoxide & trans-heptachloroepoxide) were detected in samples from Akheri and Mbimba at levels that suggested ineffective degradation. Furthermore, a strong positive correlation existed among compounds from each study site, indicating a common source. The results in general showed a slow rate of degradation with OCPs concentration decreasing with an increase in depth of burial points.
Sediments from 34 sites from Lake Qarun in Egypt were analysed using GC-MS (Barakat et al., 2013). The relative level of pesticides in the samples were observed in the following order: endrin > HCHs > chlordane > aldrin > endosulfans. A significant difference occurred in the spatial variation of contaminants among the locations, which could be as a result of the rate of degradation, the texture of sediments within the locations as well as different input sources strength. Other causes of variations can be linked to desorption and preferential sedimentation (Iwata et al., 1993; Martinez et al., 2010). Except HCHs, the levels of OCPs in sediment samples collected from the north-eastern and eastern regions close to the urban areas of the Lake showed higher concentrations of the analytes. This suggests that the urban area (Faiyum city) was responsible for contamination of the Lake with OCP residues. Comparatively, it was discovered that concentration of OCPs in sediments from the study locations were lower than a similar study carried out few years in the coastal areas of Egypt (Barakat et al., 2012a, 2012b).
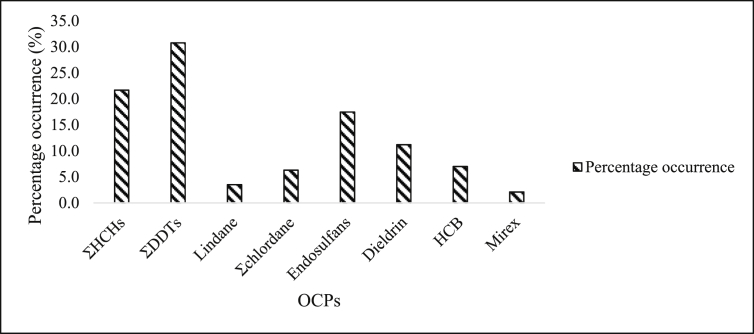
In Nigeria, water sediments collected from a cocoa producing area showed high concentrations of cis-chlordane, α-endosulfan, p.p’-DDE and dieldrin with value range of 30–6990 μg/kg, 30–6990 μg/kg, 80–19040 μg/kg and 10–7620 μg/kg respectively, thus, suggesting pesticide contamination from agricultural activities (Okoya et al., 2013). Ogbeide et al. (2016) detected significant levels of ƩHCHs and ƩDDTs in sediments from 3 water bodies (Illushi, Ogbese, and Owan River) in Niger Delta, Nigeria using GC-ECD. The average concentration of ƩHCHs at Illushi, Ogbesse and Owan River were 4089 μg/kg, 4080 μg/kg and 4900 μg/kg dw respectively, while ƩDDTs were 970 μg/kg, 1160 μg/kg and 930 μg/kg dw in that order. These concentrations were higher than those reported for rivers in Niger Delta region and Lagos Lagoon, Nigeria where a widespread distribution of OCPs in sediment was reported (Ize-Iyamu et al., 2007; Lawrence et al., 2009; Williams, 2013). From the data generated from this review, it can be deduced that the OCPs concentration in soils and sediments from Africa were in the following order: ƩDDTs>ƩHCHs> >endosulfans > dieldrins > HCB >Ʃchlordane > lindane > mirex (Figure 2).
Figure 2.
Percentage distribution of OCPs in soil and sediment from African environment.
2.4. Biota
Pesticides enter into biota through the accumulation of contaminated dust on dermal piercing, inhaling polluted air consuming contaminated food and drinking contaminated water (Winter, 1992; El-Shahawi et al., 2010). EPA (2009) reported that fish are major reservoirs of these pollutants and their concentrations in fish tissues are indicators of bioaccumulation. Significant concentration of OCPs has been detected in fish tissues from aquatic systems in Africa (Kuranchie-mensah et al., 2011; Lawrence et al., 2015). Table 3 describes the concentration of various OCP residues in biotas from different African countries.
Table 3.
Mean concentration (±standard deviation) and ranges of OCPs in biota samples from African (μg/kg).
| Location | Country | Matrix | ƩHCHs | ƩDDTs | Lindane | Toxaphene | Mirex | ƩChlordane | Endosulfans | Dieldrin | HCB | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lake Kariba | Zambia | Fisha | 7–70 | Berg and Kautsky (1997) | ||||||||
| Gamasa | Egypt | Fisha | 12.5 | 3.0 | 2.0 | Abd-Allah et al. (1998) | ||||||
| Abu-Quir | Egypt | Fisha | 7.8 | 1.4 | 3.6 | Abd-Allah et al. (1998) | ||||||
| Demiatta | Egypt | Fisha | 7.1 | 1.6 | 1.3 | Abd-Allah et al. (1998) | ||||||
| Gamasa | Egypt | Bivalve Donaxa | 38.1 | 0.9 | Abd-Allah et al. (1998) | |||||||
| Abu-Quir | Egypt | Bivalve Donaxa | 22.6 | 2.2 | 1.1 | 2.1 | Abd-Allah et al. (1998) | |||||
| Demiatta | Egypt | Bivalve Donaxa | 19.9 | 2.3 | 2.8 | 1.2 | Abd-Allah et al. (1998) | |||||
| Cross cape | Namibia | Fisha | 5–19 | 11–1115 | 10–97 | 3–159 | 4–36 | Vetter et al. (1999) | ||||
| Lake Tanganyika | Burundi | Fishb | 21.2–288.2 | 68.3–909.1 | ND-54.8 | ND-36.1 | ND-10.2 | 2.1–19.5 | Manirakiza et al. (2002) | |||
| Lake Naivasha | Kenya | Red swamp cray fisha | 9.2 | 100.5 | 21.6 | 34.6 | Gitahi et al. (2002) | |||||
| Lake Naivasha | Kenya | Black bassa | 78.6 | 2 | 2 | 2 | Gitahi et al. (2002) | |||||
| Dar es Salaam City | Tanzania | Fishb | 6.6–53.0 | BDL-31 | BDL-2.7 | Mwevura et al. (2002) | ||||||
| Southern Lake Victoria | Tanzania | Fisha | ND-30 | 20–200 | Henry and Kishimba (2006) | |||||||
| Vaal River | South Africa | Egret eggsa | 0.82 | 24 | 0.68 | 0.8 | 0.78 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | African dartar eggsa | 99 | 260 | 2 | 8.8 | 4.1 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | Reed cormorant eggsa | 3.4 | 300 | 1.5 | 2.4 | 1.7 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | Ibis eggsa | 2.3 | 68 | 0.3 | 22 | 0.9 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | Plover eggsa | 4.2 | 23 | 0.56 | 0.63 | 1.2 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | Grebe eggsa | 0.8 | 46 | 0.49 | 0.29 | 0.88 | Bouwman et al. (2008) | ||||
| Vaal River | South Africa | White fronted plover eggsa | 1.7 | 43 | 0.32 | 0.52 | 0.96 | Bouwman et al. (2008) | ||||
| Western Cape | South Africa | Kelp gull eggsa | 1.3 | 88 | 0.81 | 0.77 | 5.2 | Bouwman et al. (2008) | ||||
| Lake Bosumtwi | Ghana | Fisha | 8.877 | 0.126 ± 0.11 | 0.713 ± 0.94 | 0.035 ± 0.42 | Darko et al. (2008) | |||||
| Sharkia Province | Egypt | Animal tissue carcasses before cookinga | 96.17 ± 7.12 | 10.28 ± 0.29 | 0.66 ± 0.11 | 8.55 ± 0.36 | 0.26 ± 0.04 | (Sallam and Mohammed, 2008) | ||||
| Sharkia Province | Egypt | Animal tissue carcasses after cookinga | 57.31 ± 4.84 | 4.63 ± 0.053 | 0.38 ± 0.08 | 5.98 ± 0.23 | 1.50 ± 0.03 | (Sallam and Mohammed, 2008) | ||||
| Crocodile River | South Africa | Fisha | 10.0–30.0 | Ansara-Ross et al. (2008) | ||||||||
| KwaZulu Natal | South Africa | Fishb | 5403–5537 | McHugh et al. (2011) | ||||||||
| Volta Lake | Ghana | Fisha | 4.03–13.04 | 7.96–38.05 | 4.55–36.62 | 0.34–1.21 | Kuranchie-mensah et al. (2011) | |||||
| Lake Ziway | Ethiopia | Fisha | 0.29–5.10 | 0.9–61.9 | 0.17–4.00 | Nakayama and Ishizuka (2014) | ||||||
| Lake Awassa | Ethiopia | Fisha | 1.65–409.6 | 0.85–3.56 | ND-42.5 | Rosseland et al. (2014) | ||||||
| Roodeplat Dam | South Africa | Fisha | 288.75 ± 123.2 | 150.9 ± 87.6 | 179.0 ± 119.1 | 244.7 ± 123.6 | 239.8 ± 85.2 | Barnhoorn et al. (2015) | ||||
| Rietvlei dam | South Africa | Fisha | 336 ± 263 | 55 ± 7 | 86 ± 26 | 77 ± 36 | Barnhoorn et al. (2015) | |||||
| Harbeespoort dam | South Africa | Fisha | 79 ± 17 | 131 ± 25 | 71 ± 18 | Barnhoorn et al. (2015) | ||||||
| Lake Volta | Ghana | Fisha | 12.62–41.62 | 1.26–10.74 | 0.49–1.02 | 2.13–3.11 | 0.10–1.29 | Gbeddy et al. (2014) | ||||
| Ogbesse River | Nigeria | Fisha | 2610–4200 | 40–80 | 130–660 | 430–1040 | 80–530 | Lawrence et al. (2015) | ||||
| European commission maximum residue limitsc | 1000 | 20 | 100 | 200 | ||||||||
BDL – below detection limit; ND - not detected; a - wet weight; b - fat/lipid weight; c - EC MRLs set in Directives 2006/53, 2006/59, 2006/60, 2006/61 and 2006/62.
Fur seals (Arctocephalus pusillus pusillus) blubber from Cape Cross/Namibia were analysed for toxaphene, dieldrin, chlordane, DDTs and HCHs using GC-MS in electron capture negative and electron impact ionization modes (Vetter et al., 1999). p,p’-DDT, p,p’-DDD and p,p’-DDE were detected in all the samples with concentrations ranging from 3 to 73 μg/kg, 2–59 μg/kg and 6–1030 μg/kg respectively. p,p’-DDT recorded 80% dominance of the total DDT burden. Interestingly, α-HCH was below the detection limit in all samples. This was contrary to its level in other species of seals (Luckas et al., 1990). Toxaphene and dieldrin were detected in significant concentrations in all the samples analysed with a concentration ranges of 10–97 μg/kg and 4–36 μg/kg respectively, while chlordane ranged from 3 to159 μg/kg.
Abd-Allah et al. (1998) determined pesticide residues in fish and bivalve Donax sp collected from Abu-Quir, Demiatta and Gamasa in Egypt. Results from the samples analysed using capillary GLC showed that p,p’-DDE dominated the DDT profiles in all locations, occurring in a range of 2.0–4.1 μg/kg and 3.3–18.0 μg/kg in fish and bivalve, respectively. The endrin concentrations in fish from Gamasa and Abu-Quir were 0.6 μg/kg and 0.2 μg/kg, respectively, while that of bivalve ranged from ND to 1.5 μg/kg in that order. Heptachlor was not detected in fish samples from Abu-Quir and Demiatta and bivalve from Gamasa and Abu-Quir. The concentration of Toxaphene (9.7 μg/kg) in bivalve from Demiatta was the highest amongst all pesticides.
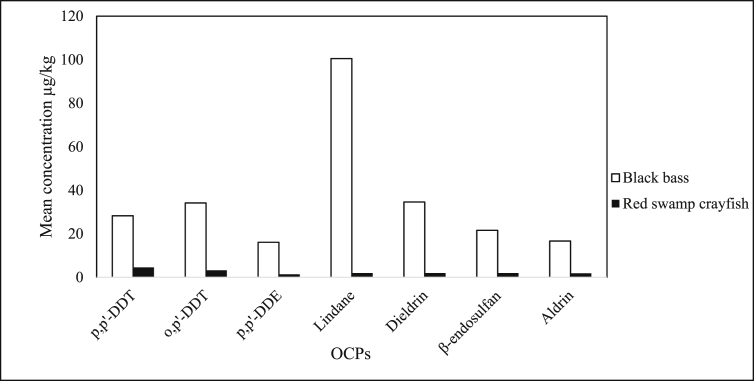
Gitahi et al. (2002) determined 7 OCPs (p,p’-DDT, o,p’-DDT, p,p’-DDE, lindane, dieldrin, β-endosulfan, and aldrin) in red swamp crayfish (Procambarus clarkii) and black bass (Micropterus salmoides) collected from Lake Naivasha in Kenya. OCP average concentrations ranged from 16.1 μg/kg (p,p’–DDE) to 100.5 μg/kg (lindane) in black bass and 1.4 μg/kg (p,p’ –DDE) to 4.6 μg/kg (o,p’ –DDT) in crayfish as shown in Figure 3. In general, the level of OCPs in black bass were higher compared to red swamp crayfish.
Figure 3.
Mean concentration (μg/kg) of OCPs in black bass and red swamp crayfish (Gitahi et al., 2002).
Manirakiza et al. (2002) determined 21 classes of OCPs using GC-μECD in 7 fish species (Boulengerochromis microlepis, n = 2; Chrysichthys sianenna, n = 1; Oreochromis niloticus, n = 10; Stolothrissa tanganikae, n = 15; Limnothrissa miodon, n = 1; Lates angustifrons, n = 1, and Lates stappersii, n = 1) from certain locations in Burundi. HCB was present in all the fish species with the highest concentration of 19.5 ± 3.7 μg/kg fat occurring in Boulengerochromis microlepis. Aldrin, α-endosulfan and endrin were present in 42% of all the samples followed by dieldrin and β-endosulfan which recorded 28% individually. Furthermore, DDTs dominated the total OCPs in all samples. Their concentrations were lower than the European Union permissible level of 1000 μg/kg fat. However, the positive correlation that existed between HCHs and DDTs in this study (R2 = 0.833) may be attributed to the similar manner of bioaccumulation of both contaminants.
Varying levels of DDT metabolites and endosulfan were detected in fish samples collected in July 1999 from 9 districts around Southern Lake Victoria, Tanzania (Henry and Kishimba, 2006). Isomers of endosulfan (α and β) were more dominant in a wide range of samples, having a ratio ranging from 0.5 to 10. The concentration of total endosulfan in each sample ranged from 400 to 42000 μg/kg lipid weight (lw), this is equivalent to 20–200 μg/kg wet weight (ww) while a high level of DDT and its metabolites were also recorded in most fish samples, suggesting a recent exposure of fish to DDT contamination. Similarly, DDT and its metabolites were the prevailing OCPs when Pazou et al. (2006) studied the occurrence of 24 OCPs in fish samples from Oueme River catchment in the Republic of Benin. Fish samples were collected from 6 locations around the catchment of Oueme River namely: Kpassa, Atchakpa-Beri, Toue, Bonou, Lowe and Houdedo areas in 2003 and instrumental analysis was carried out using GC-ECD. The results of analysis showed that α-endosulfan recorded the highest concentration of 57 μg/kg, constituting 68% of all OCPs determined in fish from Lowe area. The total OCPs concentration ranged from BDL to 1364 μg/kg lipid in all fish samples in Oueme River basin, suggesting a low intake of pesticide by people consuming fish from this water body and thus suggesting a little or no risk of exposure to these contaminants on consumption of fish from this location. More so, p,p’-DDT had the highest bioaccumulation factor ranging from 5.7 to 37 gOM/g lw followed by o,p’- DDD, p,p’-DDE, Ʃendosulfan and o,p’-DDT with values ranging from 0.9 to 22 gOM/g lw, 1.0–26 g OM/g lw, 0.6 to 11 gOM/g lipid and 0.13 to 4.2 gOM/g lw respectively. Fish samples collected from 8 locations in the coastal area of Dar es Salaam City showed that p,p’-DDE and p,p’-DDD dominated the DDT profile, occurring in all the samples with a concentration range of 3.1–53.0 μg/kg lw while dieldrin and δ-HCH ranged from BDL to 2.7 μg/kg lw and BDL to 1.0 μg/kg lw respectively (Mwevura et al., 2002).
Cormorant, darter, cattle egret, and ibis eggs from South Africa sampled between November 2004 and March 2005 were analysed for 14 OCPs using a high-resolution GC-ECD (Bouwman et al., 2008). The concentration of HCB, Ʃchlordanes, ƩDDTs, ƩHCHs and p,p’ –DDE in cattle egret eggs from Barberspan and Vaal River were 0.78 μg/kg ww, 0.80 μg/kg ww, 24 μg/kg ww, 0.82 μg/kg ww, and 24 μg/kg ww respectively. These values were lower compared to that of African darter, Reed Cormorant, and African sacred ibis. Mirex which is an unregistered OCP residue in South Africa was also detected in all the samples with the highest concentration of 2.0 μg/kg ww recorded in African darter eggs from Vaal River. The authors suggested that the occurrence of mirex in the South African environment may be attributed to its usage in various products as a flame retardant. It was recommended by the authors that monitoring of organic pollutants in the terrestrial and aquatic environment can be done using cattle egret and African darter. The residual contents of DDTs, HCHs, δ-HCH, aldrin endrin, dieldrin, HCB, toxaphene, and chlordane in the muscles, liver and kidney from carcasses of 30 Camel, 30 Cattle and 30 Sheep, slaughtered in Sharkia Province, Egypt were analysed by Sallam and Mohammed (2008). Sampling was carried out between July and September 2005 and targeted OCPs were determined using GC-ECD. The results showed that over 30% of the carcasses contained dieldrin aldrin, DDTs, lindane and HCHs while HCB, chlordane and toxaphene where only detected in 10% of the carcasses. Levels of all OCPs detected where reduced by more than 20% when samples were subjected to heat (1.5 h of boiling). Lindane recorded the highest reduction rate of 58.6 % in meat muscle. Results from this study revealed that OCPs in all samples analysed from the 3 tissues were below their respective maximum limits stipulated by local or international organisations.
In another study, p,p’-DDE and p,p’-DDT were the predominant pesticides with mean concentrations of 5.232 μg/kg and 3.645 μg/kg respectively, when 50 fish samples, collected randomly from Lake Bosumtwi, Ghana were analysed for OCPs (Darko et al., 2008). Other OCPs recorded an average concentration of 0.126 μg/kg for lindane, 0.035 μg/kg for dieldrin, 0.713 μg/kg for endosulfan, and 0.018 μg/kg for aldrin. This indicates that the level of contaminants were in the order: p,p’-DDE > p,p’-DDT > endosulfan > lindane > dieldrin > aldrin (Figure 4).
Figure 4.
Concentration (μg/kg, mean ± SD) of OCPs in fish samples from Lake Bosumtwi collected from 2006 to 2008 (Darko et al., 2008).
The possible effect of DDTs on the health status of Tigerfish (Hydrocynus vittatus) was also evaluated in fish samples collected from a low risk malaria area in KwaZulu Natal, South Africa. The location was chosen because of the usage of DDT for vector control in the area. Samples (n = 45) were collected in 2009 and analysed using GC-MS while other analytes were estimated using histological based techniques. The total DDTs analysed ranged from 5404 to 5537 μg/kg lw (McHugh et al., 2011). Levels of DDT were also evaluated in fish samples from Vaal River (Wepener et al., 2011) and it was deduced by the authors that the high level of OCPs found in fish tissues were as a result of commercial farming activities taking place within the area.
Twenty Clarias gariepinus samples were collected from 3 Dams in Gauteng South Africa for OCPs analysis (Barnhoorn et al., 2015). Sampling was done in the summer months of 2007 while instrumental analysis was carried out using GC-MS. OCPs were detected in over 15% (n = 3) of the total fish samples from Roodeplat dam (RDPD). p,p’-DDE, endosulfan, dieldrin, and lindane were the most predominant detected in the fish tissues from RDPD with a concentration range of 77.0–350 μg/kg wet mass (wm), 141.4–516 μg/kg wm, 128.5–314.5 μg/kg wm, and 108.4–514.8 μg/kg wm, respectively. p,p’- DDT, methoxychlor, and heptachlor were only detected in one of the fish sample while other samples revealed the presence of endrin aldehyde, aldrin, α-HCH, and δ-HCH. A detectable level of DDTs, endosulfans, heptachlor, endrin, and δ-HCH were observed in fish tissues from Rietvlei dam (RVD). p,p’-DDE (n = 10) and endosulfan II (n = 9) were the most prevalent contaminants while p,p’- DDE ranged from 72.4 to 426 μg/kg wm. Only p,p’- DDE, β – HCH and δ-HCH were detected in fish tissues from Hartbeespoort Dam (HBPD) with values ranging from 101.9 to 161 μg/kg wm, 60.7–111.5 μg/kg wm, and 52.4–95.9 μg/kg wm, respectively. From the results, the health risk associated with fish consumption from RDPD was mainly from dieldrin and γ-lindane while the cancer and non-cancer risk for RVD was below that of RDPD. This was due to the levels of DDTs, heptachlor, dieldrin and d-HCH whose cancer risk values were higher than the threshold value of 10−6. In view of the findings from the reviewed articles, consuming fish from Cross Cape (Namibia), Oueme River (Republic of Benin), Ogbesse River (Nigeria) and Roodeplat Dam (South Africa) may pose serious health risk since DDTs were found in concentrations above the FAO stipulated limits (FAO, 1983). Apart from the carcinogenic risk, these chemicals have been proven to have the potential of causing diabetes (Cox et al., 2007).
2.5. Blood and milk
There are possibilities for the occurrence of OCPs in human fluids due to their lipophilic nature. Various ways have been reported as possible routes in which these pollutants get into humans and animals fluids (El-Shahawi et al., 2010). Not much works have been done on evaluating this contaminants in human fluids in Africa. OCPs level in serum and milk of humans and animals are shown in Table 4. In a study carried out by Kinyamu et al. (1998), 9 OCPs were detected in 216 samples of breast milk collected from nursing mothers in a Hospital in Nairobi, Kenya. p,p’-DDE was the highest residue detected, occurring in 99.5% of the total milk samples collected while DDT and its metabolites ranged from 4 to 6321 μg/kg milk fat in all milk samples. Dieldrin was detected at a mean concentration of 19 μg/kg milk fat while α-HCH, β-HCH, and lindane were detected at average concentrations of 13 μg/kg, 83 μg/kg and 19 μg/kg milk fat respectively.
Table 4.
Mean concentration (±standard deviation) and ranges of OCPs in milk and serum samples from Africa (μg/kg).
| Location | Country | Matrix | ƩHCHs | ƩDDTs | Lindane | Endosulfans | Dieldrin | HCB | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cairo | Egypt | Human seruma | 17.6 ± 46.8 | 67 ± 109.7 | 0.5 ± 0.6 | Soliman et al. (1997) | |||
| Nairobi | Kenya | Human milkb | 96 | 4–6321 | 2–134 | 4–273 | Kinyamu et al. (1998) | ||
| Siphofaneni | Swaziland | Human milkb | ND-1930 | Okonkwo et al. (1999) | |||||
| Akumadan | Ghana | Human bloodb | 380 ± 120 | 30 ± 10 | Ntow (2001) | ||||
| Akumadan | Ghana | Human milkb | 490 ± 230 | 40 ± 20 | Ntow (2001) | ||||
| Jozini | South Africa | Human milka (μg/L) | 5.87–1537 | Bouwman et al. (2006) | |||||
| Mkuzi | South Africa | Human milka (μg/L) | 3.52–154.85 | Bouwman et al. (2006) | |||||
| Kwaliweni | South Africa | Human milka (μg/L) | 2.12–141.79 | Bouwman et al. (2006) | |||||
| Ashanti region and Kassena Nankana District | Ghana | Human milkb | 46.4 ± 5.5 | 73.3 ± 7.0 | 122.8 ± 24.8 | 4.9 ± 0.3 | Ntow et al. (2008) | ||
| Ashanti region and Kassena Nankana District | Ghana | Human serumb | 7.3 ± 7.0 | 9.1 ± 1.3 | 127.0 ± 27.2 | 5.3 ± 1.9 | Ntow et al. (2008) | ||
| Thohoyandou area | South Africa | Human milkb | ND-1930 | Okonkwo et al. (2008) | |||||
| Siphofaneni | Swaziland | Human milka | 10–8870 | ||||||
| Western cape | South Africa | Human blooda (μg/L) | 544.1 ± 82.1 | Dalvie et al. (2009) | |||||
| Tunisia | Tunisia | Human milkb | ND-189 | ND-1100 | NA-31 | Ennaceur and Driss (2013) | |||
| Asendabo | Ethiopia | Human milkb | 17170 | Gebremichael et al. (2013) | |||||
| Jimma | Ethiopia | Human milkb | 14460 | Gebremichael et al. (2013) | |||||
| Serbo | Ethiopia | Human milkb | 6420 | Gebremichael et al. (2013) | |||||
| Asendabo | Ethiopia | Cow milkb | 269 | Gebremichael et al. (2013) | |||||
| Jimma | Ethiopia | Cow milkb | 477 | Gebremichael et al. (2013) | |||||
| Serbo | Ethiopia | Cow milkb | 421 | Gebremichael et al. (2013) | |||||
| Elfaw | Sudan | Human blooda (ng/mL) | ND-7 | 56–123 | 4–43 | Elbashir et al. (2015) | |||
| Elmanagil | Sudan | Human blooda (ng/mL) | 2–21 | 30–187 | ND-43 | Elbashir et al. (2015) | |||
| ElHasahisa | Sudan | Human blooda (ng/mL) | 3–92 | 17–380 | 8–58 | Elbashir et al. (2015) | |||
| Wad Medani | Sudan | Human blooda (ng/mL) | ND-88 | 69–618 | 5–18 | Elbashir et al. (2015) | |||
| Kinana | Sudan | Human blooda (ng/mL) | ND-22 | 21–167 | 16–82 | Elbashir et al. (2015) | |||
| Gunaid | Sudan | Human blooda (ng/mL) | ND-17 | 61–13 | 10–23 | Elbashir et al. (2015) | |||
| World health organisation permissible limit | 20 | FAO/WHO (2005) | |||||||
ND - not detected; a wet weight; b fat/lipid weight.
Breast milk (n = 190) and serum (n = 115) of farmers from Ghana sampled in 2005/2006 were analysed for DDTs, HCHs, HCB, and dieldrin using GC-ECD and GC-MS (Ntow et al., 2008). Eighty-eight percent and 75% of the breast milk and serum samples respectively, were positive for DDTs. Of all the isomers of DDT, DDE had the highest mean concentration (lw) of 7.1 μg/kg in serum and 44.8 μg/kg in breast milk while the mean sum of the HCH isomers were 7.3 μg/kg lw in serum and 46.4 μg/kg in breast milk. In this study, there was a positive correlation between OCPs and the age grade of the milk donors. Furthermore, the accumulated DDTs and HCHs in breast milk in some farmers were above the daily amount of DDT recommended by Health Canada.
Okonkwo et al. (2008) analysed DDTs (using GC-MS) in human milk collected from breastfeeding mothers within the age bracket of 19 and 40 years residing in Limpopo province, South Africa. About 5–50 mL of breast milk samples were collected from 30 mothers in 10 villages (Lufule, Mangondi, Dumasi, Budeli, Tshikhudini, Tshilungoma, Gondeni, Makhuva, and Thohoyandou) within the province. Questionnaires were issued to each mother to collect data of their diet, frequency of DDT application, number of children and age. DDT and its metabolites from the study locations ranged from ND to 1930 μg/kg fat weight, the occurrence of DDTs in the study area were in the following order: ƩDDE > ƩDDD > ƩDDT. The level of DDTs were lower than those present in breast milk from lactating mothers in Siphofaneni, Swaziland (10–6470 μg/kg; Okonkwo et al., 1999).
Bouwman et al. (2006) carried out similar studies and detected a higher level of ƩDDTs (4797 μg/kg milk fat) in human breast milk from women in Jozini in KwaZulu Natal province of South Africa compared to those in Kwaliweni (799 μg/kg milk fat). This was 15 times lower than a study carried out 14 years ago in the same location (Bouwman et al., 1992).
A comparative study of the occurrence of pesticides in blood of residents performing first post spraying exercise and those who were occupationally exposed to endosulfan on a farm around the Valley Hex River in the Western Cape Province of South Africa was carried out by Dalvie et al. (2009). Analysis of endosulfan in blood was done using GC-ECD. This was performed 24 h before the first spraying season and a day after application. Information from farmers on the method of application, use of personal protective equipment, medical history, and conditions of living were taken during the testing period. Also, the job-exposure matrix was calculated using a method developed by London and Myers (1998). The baseline concentration of endosulfan of all workers had a mean concentration of 524.2 ± 49.5 μg/L while an increase of this contaminant of a better part of workers involved in post-spraying was observed (mean concentration - 544.1 ± 82.1 μg/L). The correlations between gender, drinking of alcohol, smoking and levels of endosulfan after spraying were insignificant. At the same time, there was a positive correlation between age and endosulfan concentrations in workers after spraying. This was also the case when age was correlated with high levels of DDTs, β-HCH and HCB found in human serum collected from colorectal cancer patients in Egypt during March and April 1996 (Soliman et al., 1997).
Gebremichael et al. (2013) studied OCPs in breast milk from mothers and cows residing in 3 towns in Addis Ababa. These locations were selected based on the frequent use of DDTs for mosquito control in the area. Milk samples from 101 mothers were collected in 2010, composited together for each location and analysed using GC-μECD. The average concentration of ƩDDTs in human milk from the 3 locations ranged from 6420 to 17170 μg/kg milk fat. In this study, the mean level of DDT estimated to be ingested by infant from mothers was 62.17 μg/kg. This value is said to be approximately 3 times higher than the recommended daily intake limit stipulated by WHO/FOA (20 μg/kg of body weight) and consequently a call for concern on the health status of children feeding on breast milk from mothers in the study area. Moreover, the mean concentrations of ƩDDTs in cow milk from the 3 locations ranged from 269 to 477 μg/kg lipid. The estimated daily intake (EDI) was calculated using 60 kg body weight and a cow milk consumption of 350 mL per person. It was deduced that the EDI of DDT were below the FAO/WHO recommended limit.
Most of the studies from the reviewed articles showed that DDTs were found above the stipulated limit of FAO/WHO. The rapid transformation of p,p’-DDT to p,p’-DDE may be responsible for the high concentration in human fluid since this congener has a long-lasting time in the body (Jaga and Dharmani, 2003). Infact, high ratio of p,p’-DDT to p,p’-DDE in breast milk was reported in countries where the use of DDTs have been stopped several years ago (Gebremichael et al., 2013). The high concentration in the reviewed studies may be attributed to improper handling of pesticides during farming or indoor applications of DDTs for vector control, which may result to the contamination of food stuffs, kitchen utensils and other domestic items that could easily have direct contact with the mouth.
2.6. Vegetables, fruits and food products
The use of pesticides for the control of vectors during cultivation of various agricultural produce is responsible for their varying levels in vegetables, fruits and food products in Africa (Bempah and Donkor, 2011; Darko and Acquaah, 2008; Manirakiza et al., 2003; Sojinu et al., 2012) as shown in Table 5. High levels of DDTs, endosulfan, lindane and dieldrin were detected in beans samples collected from Arusha city in Tanzania in 1992 in concentrations ranging from BDL to 99 μg/kg, BDL to 29 μg/kg, BDL to 60 μg/kg and BDL to 24 μg/kg respectively. These compounds were also found in maize and wheat samples analysed. Detected levels were above FAO/WHO permissible limits, posing a significant health risk on its consumption (Rusibamayila et al., 1998). This was disputed few years later when Ndengerio-Ndossi and Cram (2005) determined the levels of pesticides in table-ready foods (spinach, cassava leaves, stiff porridge, rice, cooking banana, beans, pigeon peas, fish varieties and beef meat) commonly eaten in Tanzania. Samples were collected from Tandale, Buguruni and Temeke in Tanzania and sample sizes were determined using the following equation:
Where n describes the desired sample size, N is the total population, σ is the standard deviation and δ is the error margin (Manly, 1992). Instrumental analysis was done by GC-ECD. p,p’-DDE was the dominant OCPs in all samples followed by δ-HCH and p,p’-DDT. The highest mean concentration of pesticides (2.89 μg/kg) was found in 72.7% of total spinach samples analysed, followed by rice (1.70 μg/kg), beef meat (0.76 μg/kg), stiff porridge (0.30 μg/kg) and beans (0.13 μg/kg) detected in 50%, 42.1%, 8.6% and 6.7% samples respectively.
Table 5.
Mean concentration (±standard deviation) and ranges of OCPs in vegetables, fruits and food products from Africa (μg/kg).
| Location | Country | Matrix | ƩHCHs | ƩDDTs | Lindane | Endosulfans | Dieldrin | Reference |
|---|---|---|---|---|---|---|---|---|
| Arusha region | Tanzania | Beansb | BDL- 99 | BDL- 60 | BDL-29 | ND-24 | Rusibamayila et al. (1998) | |
| Arusha region | Tanzania | Maizeb | BDL-105 | BDL-402 | BDL-80 | ND-23 | Rusibamayila et al. (1998) | |
| Arusha region | Tanzania | Wheatb | BDL-19 | BDL-70 | BDL-208 | ND-19 | Rusibamayila et al. (1998) | |
| South West | Nigeria | Sweet orangea | 1.2–3.4 | 1.0–8.5 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Tangerinea | 1.5–4.2 | 15–5.6 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Guavaa | 1.5–8.7 | 5.3–47.2 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Kolanuta | 2.1–10.2 | Adeyeye and Osibanjo (1999) | ||||
| South West | Nigeria | Bananaa | 1.5–21.4 | 2.0–5.6 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Plantaina | 1.2–3.3 | 1.5–3.8 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Pawpawa | 1.1–3.6 | 2.0–16 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Pineapplea | 1.5–4.2 | 2.0–4.5 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Bitter Kolaa | 30–321 | 2.8–17.6 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Amarantha | 1.4–17.4 | 1.0–50 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Water leafa | 1.6–7.2 | 1.0–33 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Leafy vegetablesa | 1.0–24.2 | 1.2–130 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Egg planta | 4.0–24.2 | 1.2–6.4 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Red peppera | 1.5–14.4 | 5.1–66.6 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Tomatoa | 2.1–7.1 | 12.2–102 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Okroa | 1.4–12.1 | 1.0–8.9 | Adeyeye and Osibanjo (1999) | |||
| South West | Nigeria | Oniona | 1.5–8.4 | - | Adeyeye and Osibanjo (1999) | |||
| Banjul and Dakar | Sene-Gambia | Sweet potatoa | 0.4–0.6 | ND-0.13 | Manirakiza et al. (2003) | |||
| Banjul and Dakar | Sene-Gambia | Egg planta | ND-0.3 | ND-5.1 | ND-1.2 | ND-2.19 | Manirakiza et al. (2003) | |
| Banjul and Dakar | Sene-Gambia | Cabbagea | ND-1.1 | ND-5.03 | ND – 2.1 | ND – 1.77 | Manirakiza et al. (2003) | |
| Banjul and Dakar | Sene-Gambia | Tomatoa | ND-0.9 | ND-1.05 | ND-0.14 | Manirakiza et al. (2003) | ||
| Banjul and Dakar | Sene-Gambia | Radisha | 0.3 | 1.44 | 0.33 | 0.49 | Manirakiza et al. (2003) | |
| Banjul and Dakar | Sene-Gambia | Sweet peppera | ND-0.1 | ND-0.16 | ND-0.14 | Manirakiza et al. (2003) | ||
| Banjul and Dakar | Sene-Gambia | Turnipa | ND-0.3 | 0.07–2.39 | ND-0.66 | ND-0.74 | Manirakiza et al. (2003) | |
| Banjul and Dakar | Sene-Gambia | Oniona | 1.7 | 4.53 | 2.68 | Manirakiza et al. (2003) | ||
| Banjul and Dakar | Sene-Gambia | Red peppera | 2.89 | Manirakiza et al. (2003) | ||||
| Banjul and Dakar | Sene-Gambia | Lettucea | ND-0.3 | 0.06–0.45 | Manirakiza et al. (2003) | |||
| Banjul and Dakar | Sene-Gambia | Carrota | ND-0.03 | 0.12–3.14 | Manirakiza et al. (2003) | |||
| Dar es Salaam City | Tanzania | Spinacha | 0.08 | 2.89 | Ndengerio-Ndossi and Cram (2005) | |||
| Dar es Salaam City | Tanzania | Cassava leavesa | 0.13 | 0.4 | Ndengerio-Ndossi and Cram (2005) | |||
| Dar es Salaam City | Tanzania | Stiff porridgea | 0.06 | 0.3 | Ndengerio-Ndossi and Cram (2005) | |||
| Dar es Salaam City | Tanzania | Ricea | 0.08 | 1.7 | Ndengerio-Ndossi and Cram (2005) | |||
| Dar es Salaam City | Tanzania | Cooked bananaa | 2.08 | Ndengerio-Ndossi and Cram (2005) | ||||
| Dar es Salaam City | Tanzania | Beansa | 0.04 | 0.13 | Ndengerio-Ndossi and Cram (2005) | |||
| Dar es Salaam City | Tanzania | Pigeon peasa | 7.6 | Ndengerio-Ndossi and Cram (2005) | ||||
| Dar es Salaam City | Tanzania | Fish varietya | 0.05 | Ndengerio-Ndossi and Cram (2005) | ||||
| Dar es Salaam City | Tanzania | Beef meata | 0.14 | 0.76 | Ndengerio-Ndossi and Cram (2005) | |||
| Vikuge farm | Tanzania | Eucalyptus sp leavesa | 15 | 818 | Marco and Kishimba (2006) | |||
| Vikuge farm | Tanzania | Cassava leavesa | ND-2 | 7–425 | Marco and Kishimba (2006) | |||
| Vikuge farm | Tanzania | Cassava rootsa | ND-5 | 191–583 | Marco and Kishimba (2006) | |||
| Vikuge farm | Tanzania | Plum leavesa | 4 | Marco and Kishimba (2006) | ||||
| Vikuge farm | Tanzania | Cashew leavesa | 16 | Marco and Kishimba (2006) | ||||
| Kumasi | Ghana | Beef fata | 663.7 | 4.04 ± 3.49 | 21.35 ± 3.85 | 5.23 ± 2.76 | Darko and Acquaah (2007) | |
| Kumasi | Ghana | Beefa | 61.7 | 2.07 ± 1.31 | 1.88 ± 0.42 | 5.92 ± 0.05 | Darko and Acquaah (2007) | |
| Buoho | Ghana | Beef fata | 435.7 | 1.79 ± 0.38 | 2.28 ± 1.74 | 6.01 ± 5.14 | Darko and Acquaah (2007) | |
| Buoho | Ghana | Beefa | 16.7 | 0.60 ± 0.38 | 0.59 ± 0.38 | 11.48 ± 4.98 | Darko and Acquaah (2007) | |
| Aboaba | Ghana | Local cheesea | 163.1 | 0.57–9.06 | 0.88–30.49 | Darko and Acquaah (2008) | ||
| Tafo | Ghana | Local cheesea | 438.7 | BDL-4.41 | 0.14–8.21 | 1.41–30.5 | Darko and Acquaah (2008) | |
| Asawasi | Ghana | Local cheesea | 73.7 | 1.29–4.88 | 1.21–3.94 | Darko and Acquaah (2008) | ||
| Ayeduasi | Ghana | Yoghurta | 10.6 | 0.02–0.12 | BDL-0.34 | Darko and Acquaah (2008) | ||
| KNUST | Ghana | Yoghurta | 4.58 | 0.01–0.05 | BDL-0.34 | 0.01–0.03 | Darko and Acquaah (2008) | |
| K-Poly | Ghana | Yoghurta | 8.07 | BDL-0.01 | 0.01–0.14 | BDL-0.03 | Darko and Acquaah (2008) | |
| Accra | Ghana | Pawpawa | 100 | 100 | Bempah and Donkor (2011) | |||
| Accra | Ghana | Tomatoa | 10 | 20 | 20 | 30 | Bempah and Donkor (2011) | |
| Accra | Ghana | Imported applea | 100 | 60 | Bempah and Donkor (2011) | |||
| Accra | Ghana | Pawpawa | BDL-60 | BDL-30 | 20–40 | BDL-30 | Bempah et al. (2011) | |
| Accra | Ghana | Tomatoa | 10–20 | BDL-10 | BDL-20 | Bempah et al. (2011) | ||
| Accra | Ghana | Imported applea | 10–20 | BDL-90 | BDL-20 | Bempah et al. (2011) | ||
| Gelemso | Ethiopia | Khata | 673.5–1372 | Daba et al. (2011) | ||||
| BadaBuna | Ethiopia | Khata | ND | Daba et al. (2011) | ||||
| Aseno | Ethiopia | Khata | 256.5–1223.8 | Daba et al. (2011) | ||||
| Accra | Ghana | Carrota | 156 | 220 | Bempah et al. (2012) | |||
| Accra | Ghana | Cabbagea | 402 | 196 | Bempah et al. (2012) | |||
| Accra | Ghana | Lettucea | 186 | 24 | Bempah et al. (2012) | |||
| Accra | Ghana | Tomatoa | 571 | 27 | 6.0 | Bempah et al. (2012) | ||
| Olomoro | Nigeria | Higher plantsb | 116.09 ± 95.12 | 6.40 ± 7.81 | 30.2 | 1.66 ± 1.91 | Sojinu et al. (2012) | |
| Oginni | Nigeria | Higher plantsb | 78.29 ± 86.54 | 1.58 ± 0.04 | 7.69 | 0.14 | Sojinu et al. (2012) | |
| Uzere | Nigeria | Higher plantsb | 70.82 ± 96.35 | 2.73 ± 2.37 | 6.47 | 0.11 | Sojinu et al. (2012) | |
| Irri and Calabar | Nigeria | Higher plantsb | 55.55 ± 22.54 | 2.65 ± 1.37 | 17.2 | 0.58 ± 0.46 | Sojinu et al. (2012) | |
| Togo | Tomatoa | BDL-11.307 | BDL- 0.78 | BDL-0.194 | Kolani et al. (2016) | |||
| Togo | Cabbagea | BDL-93.83 | BDL-1.518 | BDL-0.086 | Kolani et al. (2016) | |||
| Togo | Lettucea | 0.150–2.634 | BDL-1.236 | BDL-0.010 | Kolani et al. (2016) |
BDL – below detection limit; ND - not detected; a - fresh/wet weight; b - dry weight.
Data from this study was below the WHO/FAO Codex Alimentarius daily acceptable intake limits thus revealing that the concentration of OCPs in all food samples were not high enough to pose any significant health threat to humans. With this, the authors refuted the claims that pesticides were responsible for thousands of death in Tanzania annually.
The pattern and distribution of 11 pesticides from an obsolete storage site at Vikuge farms in Tanzania were monitored using the leaves and roots of Manihot esculenta (cassava), the leaves of Eucalyptus sp, Anacardium occidentale (cashew) and Prunus domestica (plum) (Marco and Kishimba, 2006). Choice of plant parts was based on the solubility of OCPs in the epicuticular wax found in plants (Reischl et al., 1989). Samples were collected in September 2002 from 5 locations around the point source and analysis was done using GC-ECD. A wide distribution pattern of DDTs was observed in all matrices of samples, accounting for the 100% detection of p,p’-DDT and p,p’-DDD. Furthermore, leaves of Eucalyptus sp, collected from the point source, recorded the highest mean concentration of DDTs (818 ng/g) and HCHs (9 ng/g), these levels were higher than those detected in leaves of M. esculenta and P.domestica. Meanwhile, level of DDTs in cassava roots found 4 km away from the point source were higher than the FAO/WHO limit of 200 ng/g. In addition, the ratio of DDE/DDT was very low in all samples, signifying that the presence of non-degraded DDTs. A strong positive correlation existed between concentration of ƩDDTs and ƩHCHs in all samples (p < 0.01) indicating a common source. The findings revealed that pesticide levels decreased in samples collected in points far from the point source.
Raw meat samples from 2 abattoirs in Ghana (Kumasi and Buoho), collected between 2004 and 2006 were analysed for OCPs using GC-ECD (Darko and Acquaah, 2007). Beef fat samples from Kumasi and Buoho showed a higher concentration of DDTs compared to other OCPs with values ranging from 254.26 to 905.10 μg/kg and 37–844.28 μg/kg respectively. The maximum values of ƩDDTs in beef fat from these two abattoirs were reported to be almost twice the value of WHO limit of 500 μg/kg. Also, the mean concentration of dieldrin (6.01 ± 5.14 μg/kg) in beef fat from Buoho was slightly higher than the limit stipulated by WHO (6.00 μg/kg) while other contaminants were all detected in concentrations that were slightly lower than their respective WHO recommended limits. In general, beef fat samples accounted for higher OCPs compared to beef in both abattoirs thereby making evident the lipophilic nature of chlorinated hydrocarbon. Furthermore, the authors inferred that the occurrence of OCPs in these samples may be attributed to the presence of pesticides in the Cattle's feeds or the application of pesticides on the body of Cattles for the control of parasites.
Similarly, GC-ECD was used to quantify OCPs in food crops purchased from supermarkets and open markets in Ghana in 2007 and 2008 (Bempah and Donkor, 2011). Samples of tomato (n = 110), pawpaw (n = 110) and apples (n = 110) were analysed for 16 OCP residues. Samples of pawpaw from Agbogbloshie, a popular open market dominated by rural farmers in Accra, showed a high average concentration of pesticides, notably δ-HCH (60 μg/kg), indicating the application of pesticides to food crops by farmers in rural communities. This was lower than the mean concentration of lindane reported in Nigeria food crops (Adeyeye and Osibanjo, 1999). Also, δ-HCH were detected in tomato samples collected from all locations. AT the same time, heptachlor epoxide, DDTs, γ-chlordane, alpha endosulfan and methoxychlor were only present in samples collected from the open market. The average concentration of heptachlor (20 μg/kg), γ-HCH (20 μg/kg), heptachlor epoxide (40 μg/kg) and p,p’-DDT (10 μg/kg) were below that found in tomato samples from a vegetable farming community in Akumadan, Ghana (Ntow, 2001). Also, the mean concentration of endrin aldehyde, endrin ketone, p,p’-DDE and p,p’-DDT in the imported apples were 110 μg/kg, 100 μg/kg, 10 μg/kg and 90 μg/kg respectively. These values were far lower than those reported in food crops in Shanghai, China (Nakata et al., 2002). In general, the study revealed that the OCPs detected in these food crops were lower than their respective stipulated Codex Alimentarius Commission limits (FAO/WHO, 1998).
Likewise, the quantification of pesticide residues present in 240 vegetable samples (carrot, cabbage, lettuce, and tomato) collected from different market centres in Ghana were carried out by Bempah et al. (2012). DDT and its metabolites dominated the total OCPs found in vegetables from supermarkets with the highest concentration being o,p-DDT (239 μg/kg) in tomato. Vegetables which had the highest mean concentration of pesticides in samples collected from supermarkets, roadside grocery stores and open market were in the following order: tomato > cabbage > carrot > lettuce, cabbage > tomato > lettuce > carrot and tomato > lettuce > cabbage > carrot respectively. However, only 31.48% of vegetables had pesticide residues above the European commission maximum residue levels.
A study conducted by Daba et al. (2011) showed high concentration of p.p’-DDT in khat samples from 3 study areas in Ethiopia, Galemso, Asena and Bada Buna. Amongst the three locations, Galemso and Asena had levels of p,p’-DDT ranging from 141 to 973 μg/kg and 194–990 μg/kg respectively, while the level of aldrin was below the limit of quantification. The authors inferred that the high level of p.p’-DDT might be a contributing factor to pathological diseases that exist among khat users.
Essumang et al. (2013) determined pesticides in 500 okra samples close to a watermelon farm that uses these contaminants for its cultivation process in Ghana. The OCPs’ mean concentration in okra samples ranged from 3.10 to 7.60 μg/kg. These values were below the WHO/FOA recommended residue limits. Although not existing in levels that pose a threat to life, their presence is of great concern, and pre-emptive measures must be taken to prevent further contamination. The authors inferred that since the application of pesticides affects neighbouring crops, crop mixing might not be advisable. In a recent study, Kolani et al. (2016) monitored the level of 20 pesticides in 3 types of vegetables (lettuce, cabbage, and tomato). These vegetables were chosen because of their dominance in diet among the Togolese. Analysis of OCPs was done using GC-ECD. From the results, ƩHCHs recorded the highest pesticides with a mean concentration of 5.847 μg/kg while Ʃheptachlors was the lowest with an average concentration of 0.012 μg/kg. In general, the occurrence of OCPs in all the vegetables were in the following order: ƩDDTs > Ʃchlordanes > Ʃdrins > Ʃheptachlors (Figure 5).
Figure 5.
Concentration (μg/kg, mean ± SD) of OCPs in vegetables collected from Togo (Kolani et al., 2016).
3. Discussions
Environmental samples from Vikuge Farm in Tanzania was adjudged to be the most polluted from the reviewed articles due to the accumulation of OCPs resulting from illegal dumping and storage of obsolete pesticides since 1986, thus suggesting the need for clean-up of pollutants from the area. Several countries in Africa lack information on the occurrence of these contaminants in their environment, hence making it difficult to ascertain the pollution load in the Africa ecosystem. Over 90 % of the studies employed GC-MS and GC-ECD for analyses of OCP residues. This might be due to a lack of collaboration between researchers in the African continent and other researchers in developed countries. Hence, there is need for the use of more sensitive analytical tools such as gas chromatograph/tandem mass spectrometer (GC-MS/MS), gas chromatograph/high-resolution mass spectrometer (GC-HRMS) in order to detect their secondary and tertiary metabolites, especially in water, biota, and food products since these are major media through which these pollutants get into humans and animals.
Also, most studies have been restricted to the analysis of OCPs in fish. Other aquatic animals such as Crab, Mussels, Periwinkles and Shrimps which are choice foods in Africa should attract further studies to thoroughly monitor the levels of bioaccumulation of these pesticides in aquatic systems and the ecosystem at large. There was limited data on the occurrence of OCPs in ambient air and tissues of animals and humans. There is, therefore, the need for more studies on these matrices to investigate the potential risk they pose on the health status of humans and animals and also to evaluate the levels of these chemicals in human fluids especially in countries where they are used more often. More studies have been reported on the occurrence of OCPs in Southern and Western Africa compared to other parts of the continent probably due to challenges such as lack of facilities and infrastructures as well as lack of political will and priorities of governments in such countries. Other problems may be related to lack of environmental awareness of this pollutants, the incompetence to analyse POPs among researchers, the convenience during sampling (most researchers tends to have scientific goals other than providing a geographic distribution of OCPs in the environment; therefore, sampling close to home is more convenient). To this end, the need for government and academic partnership is proposed to enable proper documentation of the occurrence, distribution, sources and health impact of OCPs (and indeed other pollutants) in their respective countries.
Generally, studies have revealed that the main challenges facing pesticide usage include inadequate enforcement of policies to checkmate the importation of these chemicals, levity in measures to stop their usage, improper pesticide handling by farmers and local users, and non-existence of training programs on the use of pesticide. These practices have led to a high concentration in environmental and biological matrices. Hence, it is advised that breastfeeding of infants by mothers living in locations where the usage of DDTs and other pollutants is rampant should be discouraged since this is an obvious medium in which these pollutants get into infants and also more models should be developed to carry out health risk assessment in infants. Furthermore, training of farmers and other domestic users on pesticides handling is highly recommended. In addition to the above-stated recommendation, the following further perspective on OCPs monitoring in the African environment should be considered.
-
•
There is a paramount need for an organised monitoring plan in the African environment, with respect to the geographical distribution of this pollutant class. This plan should be designed to cover remote and rural areas in the continent, especially where these pollutant class has been used extensively in the past.
-
•
Concerning DDTs, some reports have shown that this chemical is intentionally used to fight critical vectors, especially in malaria-endemic areas in Africa. Appropriate monitoring schemes should be established and well documented. DDT will be present in the environment for decade's therefore long term monitoring is germane in securing the environment.
-
•
Further studies in the African environment should be designed to monitor its level in the ecosystem components not only on a single matrix (sediment or water) as noticed in majority of the reviewed literature. This will provide an in-depth understanding of the mobility of this pollutant class in the African environment.
-
•
Data from the reviewed articles revealed that most researchers focus their study on “hot-spots”. This practice should be continued, and more sampling sites should be established, bearing in mind that these chemicals have the ability to transport long-range, and besides they take several years to phase out from the environment. We, therefore, recommend inventories of pesticides contamination in the African environment as well as statistical sampling design to capture newly established sites and hot-spots.
4. Conclusions
Data from published articles on the occurrence of OCPs in air, biota, water soil, sediment, vegetables, and food products in Africa environment reveals that OCPs are widely distributed in different environmental compartments in Africa, especially HCHs, DDTs, and endosulfans. Such occurrences might be due to the historical application of this pollutant class for an extended period in the region. Despite being banned several years ago, many individual OCP is still being used for various functions. From the reviewed literature, it is difficult to tell whether or not the concentration of OCPs has decreased or increased in the African environment over the years since its level of occurrence appears to be fluctuating. The current review revealed that DDTs were detected in over 90% of the biota and breast milk studied at a relatively high concentration with some samples occurring at levels above the recommended daily human intake stipulated by WHO. Such high concentrations may be attributed to runoff from agricultural farmlands, accumulated levels in the food chain and frequent use in malaria dominated areas. More so, DDTs, endosulfans, and HCHs were among the prevalent pesticides detected in water, soils, sediments, vegetation and food products, probably a consequence of their use for the control of vectors and the cultivation of food crops.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was financially supported by National Research Foundation (NRF) of South Africa for the PhD scholarship (grant number: 112780) granted to OC, the South Africa Medical Research Council (SAMRC), South Africa and the Govan Mbeki Research and Development Centre, University of Fort Hare, South Africa.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abd-Allah A.M.A., Ali H.A., El-Sebae A. Level of chlorinated hydrocarbons in a teleost fish and a bivalve from the Egyptian Mediterranean Coast and Nile Estuary. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung a-Food Research and Technology. 1998;206:25–28. [Google Scholar]
- Abong’o D.A., Wandiga S.O., Jumba I.O., Brink P.J., Nazariwo B.B., Madadi V.O., Wafula H., Kylin H. Organochlorine pesticide residue levels in soil from the Nyando river catchment, Kenya. J. Phys. Sci. 2015;2:2313–3317. [Google Scholar]
- Adeyemi D., Anyakora C., Ukpo G., Adedayo A., Darko G. Evaluation of the levels of organochlorine pesticide residues in water samples of Lagos Lagoon using solid phase extraction method. J. Environ. Chem. Ecotoxicol. 2011;3:160–166. [Google Scholar]
- Adeyeye A., Osibanjo O. Residues of organochlorine pesticides in fruits, vegetables and tubers from Nigerian markets’. Sci. Total Environ. 1999;231:227–233. doi: 10.1016/s0048-9697(99)00067-4. [DOI] [PubMed] [Google Scholar]
- Afful S., Anim A.K. Spectrum of organochlorine pesticide residues in fish samples from the Densu Basin. Res. J. Environ. Earth Sci. 2010;2:133–138. [Google Scholar]
- Ali N., Ali L., Mehdi T., Dirtu A.C., Al-Shammari F., Neels H., Covaci A. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: implication for human exposure via dust ingestion. Environ. Int. 2013;55:62–70. doi: 10.1016/j.envint.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Ansara-Ross T.M., Wepener V., Van Den Brink P.J., Ross M.J. Probabilistic risk assessment of the environmental impacts of pesticides in the Crocodile (west) Marico catchment, North-West Province. Water SA. 2008;34:637–644. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) (US Department of Health and Human Services) US Department of Health and Human Resources, Agency for Toxic Substances and Disease Registry; 2000. Toxicological Profile for Polychlorinated Biphenyls ( PCBs ) [PubMed] [Google Scholar]
- Barakat A.O., Khairy M., Aukaily I. Persistent organochlorine pesticide and PCB residues in surface sediments of Lake Qarun, a protected area of Egypt. Chemosphere. 2013;90:2467–2476. doi: 10.1016/j.chemosphere.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Barakat A.O., Mostafa A., Wade T.L., Sweet S.T., El Sayed N.B. Assessment of persistent organochlorine pollutants in sediments from Lake Manzala, Egypt. Mar. Pollut. Bull. 2012;64:1713–1720. doi: 10.1016/j.marpolbul.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Barakat A.O., Mostafa A., Wade T.L., Sweet S.T., El Sayed N.B. Spatial distribution and temporal trends of persistent organochlorine pollutants in sediments from Lake Maryut, Alexandria, Egypt. Mar. Pollut. Bull. 2012;64:395–404. doi: 10.1016/j.marpolbul.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Barnhoorn I.E.J., van Dyk J.C., Genthe B., Harding W.R., Wagenaar G.M., Bornman M.S. Organochlorine pesticide levels in Clarias gariepinus from polluted freshwater impoundments in South Africa and associated human health risks. Chemosphere. 2015;120:391–397. doi: 10.1016/j.chemosphere.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Batterman S.A., Chernyak S.M., Gounden Y., Matooane M., Naidoo R.N. Organochlorine pesticides in ambient air in Durban, South Africa. Sci. Total Environ. 2008;397:119–130. doi: 10.1016/j.scitotenv.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Bempah C.K., Buah-Kwofie A., Enimil E., Blewu B., Agyei-Martey G. Residues of organochlorine pesticides in vegetables marketed in Greater Accra Region of Ghana. Food Contr. 2012;25:537–542. [Google Scholar]
- Bempah C.K., Donkor A., Yeboah P.O., Dubey B., Osei-Fosu P. A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chem. 2011;128:1058–1065. [Google Scholar]
- Bempah C.K., Donkor A.K. Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ. Monit. Assess. 2011;175:551–561. doi: 10.1007/s10661-010-1550-0. [DOI] [PubMed] [Google Scholar]
- Berg H., Kautsky N. Persistent pollutants in Lake Kariba eco- system: a tropical man-made lake’. In: Remane K., editor. African Inland Fisheries, Aquaculture and the Environment. Fishing News Books Blackwell; Oxford: 1997. [Google Scholar]
- Bhattacharya B., Sarkar S.K., Mukherjee N. Organochlorine pesticide residues in sediments of a tropical mangrove estuary, India: implications for monitoring. Environ. Int. 2003;29:587–592. doi: 10.1016/S0160-4120(03)00016-3. [DOI] [PubMed] [Google Scholar]
- Bollmohr S., Day J.A., Schulz R. Temporal variability in particle-associated pesticide exposure in a temporarily open estuary, Western Cape, South Africa. Chemosphere. 2007;68:479–488. doi: 10.1016/j.chemosphere.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Bouwman H., Becker P.J., Cooppan R.M., Reinecke A.J. Transfer of DDT used in malaria control to infants via breast milk. Bull. World Health Organ. 1992;70:241–250. [PMC free article] [PubMed] [Google Scholar]
- Bouwman H., Polder A., Venter B., Skaare J.U. Organochlorine contaminants in cormorant, darter, egret, and ibis eggs from South Africa. Chemosphere. 2008;71:227–241. doi: 10.1016/j.chemosphere.2007.09.057. [DOI] [PubMed] [Google Scholar]
- Bouwman H., Sereda B., Meinhardt H.M. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ. Pollut. 2006;144:902–917. doi: 10.1016/j.envpol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Buccini J. The development of a global treaty on persistent organic pollutants (POPs) Handb. Environ. Chem. 2003;3:13–30. Part O Persistent Organic Pollutants, 30. [Google Scholar]
- CCME . Canadian Council of Ministers of the Environment; Winnipeg (MB), Canada: 1999. Canadian Environmental Quality Guidelines. [Google Scholar]
- Cai D.W. Understand the role of chemical pesticides and prevent misuses of pesticides. Bull. Agri. Sci. Technol. 2008;1:36–38. [Google Scholar]
- Conway G.R. CRC Press; 2013. Unwelcome Harvest: T: Agriculture and Pollution; pp. 1–676. [Google Scholar]
- Council Directives Council Directive 2008/105/EC of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union. 2008;348:84–97. [Google Scholar]
- Cox S., Niskar A.S., Venkat Narayan K.M., Marcus M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: hispanic health and nutrition examination survey, 1982-1984. Environ. Health Perspect. 2007;115:1747–1752. doi: 10.1289/ehp.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daba D., Hymete A., Bekhit A.A. Multi residue analysis of pesticides in wheat and khat collected from different regions of Ethiopia. Bull. Environ. Contam. Toxicol. 2011;86:336–341. doi: 10.1007/s00128-011-0207-1. [DOI] [PubMed] [Google Scholar]
- Dabrowski J.M., Peall S.K.C., Van Niekerk A., Reinecke A.J., Day J.A., Schulz R. Predicting runoff-induced pesticide input in agricultural sub-catchment surface waters: linking catchment variables and contamination. Water Res. 2002;36:4975–4984. doi: 10.1016/s0043-1354(02)00234-8. [DOI] [PubMed] [Google Scholar]
- Dabrowski J.M., Peall S.K.C., Reinecke a J., Liess M., Schulz R. Runo -related pesticide input into the Lourens River, South Africa: basic data for exposure assessment and risk mitigation at the catchment scale. Water Air Soil Pollut. 2002;135:265–283. [Google Scholar]
- Dalvie M.A., Cairncross E., Solomon A., London L. Contamination of rural surface and ground water by endosulfan in farming areas of the Western Cape, South Africa. Environ. Health. 2003;2:1–15. doi: 10.1186/1476-069X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie M.A., Africa A., Hassan A., Solomons A., London L., Brouwer D.H., Kromhout H. Pesticide exposure and blood endosulfan levels after first season spray amongst farm workers in the Western Cape, South Africa. J. Expo. Sci. Environ. Epidemiol. 2009;44:271–277. doi: 10.1080/03601230902728351. [DOI] [PubMed] [Google Scholar]
- Darko G., Acquaah S.O. Levels of organochlorine pesticides residues in meat. Int. J. Environ. Sci. Technol. 2007;4:521–524. [Google Scholar]
- Darko G., Acquaah S.O. Levels of organochlorine pesticides residues in dairy products in Kumasi, Ghana. Chemosphere. 2008;71:294–298. doi: 10.1016/j.chemosphere.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Darko G., Akoto O., Oppong C. Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana. Chemosphere. 2008;72:21–24. doi: 10.1016/j.chemosphere.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Dem S., Cobb J.M., Mullins D.E. Pesticide residues in soil and water from four cotton growing areas of Mali, West Africa. J. Agric. Food Chem. 2007;1:1–12. [Google Scholar]
- Dinham B. Growing vegetables in developing countries for local urban populations and export markets: problems confronting small-scale producers. Pest Manag. Sci. 2003;59:575–582. doi: 10.1002/ps.654. [DOI] [PubMed] [Google Scholar]
- Ebenebe A.A., Van Den Berg J., Van Der Linde T.C. Farm management practices and farmers’ perceptions of stalk-borers of maize and sorghum in Lesotho. Int. J. Pest Manag. 2001;47:41–48. [Google Scholar]
- El-Kabbany S., Rashed M.M., Zayed M.A. Monitoring of the pesticide levels in some water supplies and agricultural land. El-Haram, Giza (A.R.E, editors. J. Hazard Mater. 2000;72:11–21. doi: 10.1016/s0304-3894(99)00174-0. [DOI] [PubMed] [Google Scholar]
- El-Shahawi M.S., Hamza A., Bashammakh A.S., Al-Saggaf W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta. 2010;80:1587–1597. doi: 10.1016/j.talanta.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Elbashir A.B., Abdelbagi A.O., Hammad A.M.A., Elzorgani G.A., Laing M.D. Levels of organochlorine pesticides in the blood of people living in areas of intensive pesticide use in Sudan. Environ. Monit. Assess. 2015;187:1–10. doi: 10.1007/s10661-015-4269-0. [DOI] [PubMed] [Google Scholar]
- Elfvendahl S., Mihale M., Kishimba M. a, Kylin H. Ambio; 2004. Pesticide pollution remains severe after cleanup of a stockpile of obsolete pesticides at Vikuge, Tanzania. [DOI] [PubMed] [Google Scholar]
- Elibariki R., Maguta M.M. Status of pesticides pollution in Tanzania – a review. Chemosphere. 2017;178:154–164. doi: 10.1016/j.chemosphere.2017.03.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur S., Driss M.R. Time course of organochlorine pesticides and polychlorinated biphenyls in breast-feeding mothers throughout the first 10 months of lactation in Tunisia. Environ. Monit. Assess. 2013;185:1977–1984. doi: 10.1007/s10661-012-2681-2. [DOI] [PubMed] [Google Scholar]
- EPA . A Global Response - International Cooperation - US EPA; 2009. Persistent Organic Pollutants A Global Issue. [Google Scholar]
- Essumang D.K., Asare E.A., Dodoo D.K. Pesticides residues in okra (non-target crop) grown close to a watermelon farm in Ghana’. Environ. Monit. Assess. 2013;185:7617–7625. doi: 10.1007/s10661-013-3123-5. [DOI] [PubMed] [Google Scholar]
- Fatoki O.S., Awofolu O.R. Persistent organochlorine pesticide residues in freshwater systems and sediments from the Eastern Cape , South Africa’. Water SA. 2003;29:323–330. [Google Scholar]
- Fatoki O.S., Awofolu O.R. Levels of organochlorine pesticide residues in marine-, surface-, ground- and drinking waters from the Eastern Cape Province of South Africa.’. J. Environ. Sci. Health - Part B Pesticides, Food Contam. Agric. Wastes. 2004;39:101–114. doi: 10.1081/pfc-120027442. [DOI] [PubMed] [Google Scholar]
- FAO . Library; 1983. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Product. [Google Scholar]
- FAO/WHO . 2002. Lindane in Drinking Water; Background Document for Development of WHO Guidelines for Drinking Water Quality. WHO/SDE/WSH/03.04/102; pp. 1–14. [Google Scholar]
- FAO/WHO . Vol. 2. 2005. Residues in food; pp. 61–81. (Report of Joint FAO/WHO Food Standards Programm Rome, Italy). [Google Scholar]
- FAO/WHO . 2B. FAO Publishing Division; Rome, Italy: 1998. Joint FAO/WHO food standards programme. Codex Alimentarius commission; pp. 546–547. (Pesticide Residues in Food-Maximum Residue Levels). [Google Scholar]
- Gbeddy G., Glover E., Doyi I. Assessment of organochlorine pesticides in water, sediment, african cat fish and nile tilapia, consumer exposure and human health implications, Volta lake, Ghana. J. Environ. Anal. Toxicol. 2014;5:1–8. [Google Scholar]
- Gebremichael S., Birhanu T., Tessema D.A. Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere. 2013;90:1652–1657. doi: 10.1016/j.chemosphere.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Gitahi S.M., Harper D.M., Muchiri S.M., Tole M.P., Ng’ang’a R.N. Organochlorine and organophosphorus pesticide concentrations in water, sediment, and selected organisms in Lake Naivasha (Kenya) Hydrobiologia. 2002;488:123–128. [Google Scholar]
- Gunnell D., Eddleston M., Phillips M.R., Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Publ. Health. 2007;357:1–14. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellar-Kihampa H. Pesticide residues in four rivers running through an intensive agricultural area, Kilimanjaro, Tanzania. J. Appl. Sci. Environ. Manag. 2011;15:307–316. [Google Scholar]
- Henry L., Kishimba M.A. Levels of pesticide residues in water, soil and sediments from Southern Lake Victoria and its basin. Tanzan. J. Sci. 2003;29:77–90. [Google Scholar]
- Henry L., Kishimba M.A. Pesticide residues in nile tilapia (Oreochromis niloticus) and nile perch (Lates niloticus) from Southern Lake Victoria, Tanzania. Environ. Pollut. 2006;140:348–354. doi: 10.1016/j.envpol.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Hewitt A.J. Spray drift: impact of requirements to protect the environment. Crop Protect. 2000;19:623–627. [Google Scholar]
- Hoh E., Hites R.A. Sources of toxaphene and other organochlorine pesticides in North America as determined by air measurements and potential source contribution function analyses. Environ. Sci. Technol. 2004;38:4187–4194. doi: 10.1021/es0499290. [DOI] [PubMed] [Google Scholar]
- Iwata H., Tanabe S., Sakal N., Tatsukawa R. Distribution of persistent organochlorines in the oceanic air and surface seawater and the role of ocean on their global transport and fate. Environ. Sci. Technol. 1993;27:1080–1098. [Google Scholar]
- Ize-Iyamu O.K., Asia I.O., Egwakhide P.A. Concentrations of residues from organochlorine pesticide in water and fish from some rivers in Edo State Nigeria. Int. J. Phys. Sci. 2007;2:237–241. [Google Scholar]
- Jaga K., Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int. J. Occup. Med. Environ. Health. 2003;16:7–20. [PubMed] [Google Scholar]
- Karlsson H., Muir D.C.G., Teixiera C.F., Burniston D.A., Strachan W.M.J., Hecky R.E., Mwita J., Bootsma H.A., Grift N.P., Kidd K.A., Rosenberg B., Rosenberg B. Persistent chlorinated pesticides in air , water , and precipitation from the Lake Malawi area , Southern Africa. Environ. Sci. Technol. 2000;34:4490–4495. [Google Scholar]
- Kasozi G.N., Kiremire B.T., Bugenyi F.W.B., Kirsch N.H., Nkedi-Kizza P. Organochlorine residues in fish and water samples from Lake Victoria, Uganda. J. Environ. Qual. 2006;35:584–589. doi: 10.2134/jeq2005.0222. [DOI] [PubMed] [Google Scholar]
- Kihampa C., Mato R.R.A.M. Distribution of pesticide residues in soil due to point source pollution at old Korogwe , Tanzania. Int. J. Brain Cognit. Sci. 2009;3:422–430. [Google Scholar]
- Kihampa C., Ram Mato R., Mohamed H. Residues of organochlorinated pesticides in soil from tomato fields , ngarenanyuki , Tanzania. J. Appl. Sci. Environ. Manag. 2010;14:37–40. [Google Scholar]
- Kinyamu J.K., Kanja L.W., Skaare J.U., Maitho T.E. Levels of organochlorine pesticides residues in milk of urban mothers in Kenya. Bull. Environ. Contam. Toxicol. 1998;60:732–738. doi: 10.1007/s001289900687. [DOI] [PubMed] [Google Scholar]
- Kishimba M., Mihale M. Levels of pesticide residues and metabolites in soil at Vikuge farm, Kibaha district, Tanzania – a classic case of soil contamination by obsolete pesticides. Tanzan. J. Sci. 2009;30:77–86. [Google Scholar]
- Kishimba M.A., Henry L., Mwevura H., Mmochi A.J., Mihale M., Hellar H. The status of pesticide pollution in Tanzania. Talanta. 2004;64:48–53. doi: 10.1016/j.talanta.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Kolani L., Mawussi G., Sanda K. Assessment of Organochlorine Pesticide residues in vegetable samples from some agricultural areas in Togo. Am. J. Anal. Chem. 2016;7:332–341. [Google Scholar]
- Kuranchie-Mensah H., Atiemo S.M., Palm L.M.N.D., Blankson-Arthur S., Tutu A.O., Fosu P. Determination of organochlorine pesticide residue in sediment and water from the Densu River Basin, Ghana. Chemosphere. 2012;86:286–292. doi: 10.1016/j.chemosphere.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Kuranchie-mensah H., Palm L.M.N., Manukure S.A., Afful S., Adjei G., Arthur J.K. Assessment of organochlorine pesticides and polychlorinated biphenyls levels in fishes from the Volta lake , Ghana and their suitability for human consumption. Elixir Food Sci. 2011;41:5982–5990. [Google Scholar]
- Lalah J.O., Yugi P.O., Jumba I.O., Wandiga S.O. Organochlorine pesticide residues in Tana and Sabaki rivers in Kenya. Bull. Environ. Contam. Toxicol. 2003;71:298–307. doi: 10.1007/s00128-003-0164-4. [DOI] [PubMed] [Google Scholar]
- Lawrence E., Ozekeke O., Isioma T. Distribution and ecological risk assessment of pesticide residues in surface water , sediment and fish from Ogbesse River , Edo State , Nigeria. J. Environ. Chem. Ecotoxicol. 2015;7:20–30. [Google Scholar]
- Lawrence E., Thomas I., Isioma T. Distribution of propoxur in water, sediment and fish from Warri River Niger Delta, Nigeria. Turk. J. Biochem. 2009;34:121–127. [Google Scholar]
- Liu C.J., Men W.J., Liu Y.L.L. The pollution of pesticides in soils and its bioremediation. Syst. Sci. Compre. Stud. Agri. 2002;18:297. 297. [Google Scholar]
- London L., Dalvie M.A., Cairncross E., Solomons A. The quality of surface and groundwater in the rural Western Cape with regard to pesticides. Water Res. Comm. Rep. 2000;(795/1) [Google Scholar]
- London L., Myers J.E. Use of a crop and job specific exposure matrix for retrospective assessment of long-term exposure in studies of chronic neurotoxic effects of agrichemicals. Occup. Environ. Med. 1998;55:194–201. doi: 10.1136/oem.55.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckas B., Vetter W., Fischer P., Heidemann G., Plötz J. Characteristic chlorinated hydrocarbon patterns in the blubber of seals from different marine regions. Chemosphere. 1990;21:13–19. [Google Scholar]
- MacDonald D.D., Ingersoll C.G., Berger T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000;39:20–31. doi: 10.1007/s002440010075. [DOI] [PubMed] [Google Scholar]
- Mahugija J.A.M. Status and distributions of pesticides buried at five sites in Arusha and Mbeya regions , Tanzania. Afr. J. Pure Appl. Chem. 2013;7:382–393. [Google Scholar]
- Manirakiza P., Akinbamijo O., Covaci A., Pitonzo R., Schepens P. Assessment of organochlorine pesticide residues in West African city farms: Banjul and Dakar case study. Arch. Environ. Contam. Toxicol. 2003;44:171–179. doi: 10.1007/s00244-002-2006-5. [DOI] [PubMed] [Google Scholar]
- Manirakiza P., Covaci A., Nizigiymana L., Ntakimazi G., Schepens P. Persistent chlorinated pesticides and polychlorinated biphenyls in selected fish species from Lake Tanganyika, Burundi, Africa. Environ. Pollut. 2002;117:447–455. doi: 10.1016/s0269-7491(01)00188-9. [DOI] [PubMed] [Google Scholar]
- Manly B.F. Cambridge University Press; 1992. The Design and Analysis of Research Studies. [Google Scholar]
- Marco J.A.M., Kishimba M.A. Pesticides and metabolites in cassava, eucalyptus, plum and cashew leaves and roots in relation to a point source in Kibaha, Tanzania. Chemosphere. 2006;64:542–548. doi: 10.1016/j.chemosphere.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Martinez A., Norström K., Wang K., Hornbuckle K.C. Polychlorinated biphenyls in the surficial sediment of Indiana harbor and ship canal, lake Michigan. Environ. Int. 2010;36:849–854. doi: 10.1016/j.envint.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wiles T., Baleguel P. A survey of pesticide application in Cameroon. Crop Protect. 2003;22:707–714. [Google Scholar]
- McGregor F.M. University of Cape; Town, South Africa: 1999. The Mobility of Endosulfan and Chlorpyrifos in the Soil of the Hex River Valley. MSc Thesis. [Google Scholar]
- McHugh K.J., Smit N.J., Van Vuren J.H.J., Van Dyk J.C., Bervoets L., Covaci A., Wepener V. A histology-based fish health assessment of the tigerfish, Hydrocynus vittatus from a DDT-affected area. Phys. Chem. Earth. 2011;36:895–904. [Google Scholar]
- Mohamed J., Murimi S., Kihampa C. Degradation of water resources by agricultural pesticides and nutrients, Weruweru, Tanzania. Iran. J. Energy Environ. 2014;5:192–201. [Google Scholar]
- Mudimu G.D., Chigume S., Chikanda M. Pesticide use and policies in Zimbabwe issues for research. Pestic. Pol. Proj. 1995:1–30. [Google Scholar]
- Mwevura H., Othman O.C., Mhehe G.L. Organochlorine pesticide residues in edible biota from the coastal area of Dar es Salaam City. Western Indian Ocean Journal of Marine. Science. 2002;1:91–96. doi: 10.1016/s0025-326x(01)00331-9. [DOI] [PubMed] [Google Scholar]
- Nakata H., Kawazoe M., Arizono K., Abe S., Kitano T., Shimada H., Li W., Ding X. Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from China: status of contamination, historical trend, and human dietary exposure. Arch. Environ. Contam. Toxicol. 2002;43:473–480. doi: 10.1007/s00244-002-1254-8. [DOI] [PubMed] [Google Scholar]
- Nakayama S.M.M., Ishizuka M. Concentrations and human health risk assessment of organochlorine pesticides in edible fi sh species from a Rift Valley lake — Lake. Ecotoxicol. Environ. Saf. 2014;106:95–101. doi: 10.1016/j.ecoenv.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Naudé Y., De Beer W.H.J., Jooste S., Van Der Merwe L., Van Rensburg S.J. Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of DDT, DDD and DDE in sediment. Water SA. 1998;24:205–214. [Google Scholar]
- Ndengerio-Ndossi J.P., Cram G. Pesticide residues in table-ready foods in Tanzania. Int. J. Environ. Health Res. 2005;15:143–149. doi: 10.1080/09603120500061922. [DOI] [PubMed] [Google Scholar]
- Ngowi A. Health impact of exposure to pesticides in Agriculture in Tanzania. Tampereen yliopistopaino Oy Juvenes. 2002;1–2 [Google Scholar]
- Nsibande M.L., McGeoch M.A. Sweet potato, Ipomoea batatas (L), cropping practices and perceived production constraints in Swaziland: implications for pest management. Int. J. Pest Manag. 1999;45:29–33. [Google Scholar]
- Ntow W.J. Organochlorine pesticides in water, sediment, crops, and human fluids in a farming community in Ghana. Arch. Environ. Contam. Toxicol. 2001;40:557–563. doi: 10.1007/s002440010210. [DOI] [PubMed] [Google Scholar]
- Ntow W.J. Pesticide residues in Volta lake, Ghana. Lakes Reservoirs Res. Manag. 2005;10:243–248. [Google Scholar]
- Ntow W.J., Tagoe L.M., Drechsel P., Kelderman P., Gijzen H.J., Nyarko E. Accumulation of persistent organochlorine contaminants in milk and serum of farmers from Ghana. Environ. Res. 2008;106:17–26. doi: 10.1016/j.envres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Ogbeide O., Tongo I., Ezemonye L. Assessing the distribution and human health risk of organochlorine pesticide residues in sediments from selected rivers. Chemosphere. 2016;144:1319–1326. doi: 10.1016/j.chemosphere.2015.09.108. [DOI] [PubMed] [Google Scholar]
- Okonkwo J.O., Kampira L., Chingakule D.D.K. Organochlorine insecticides residues in human milk : a study of lactating mothers in Siphofaneni , Swaziland. Bull. Environ. Contam. Toxicol. 1999;63:243–247. doi: 10.1007/s001289900972. [DOI] [PubMed] [Google Scholar]
- Okonkwo J.O., Mutshatshi T.N., Botha B., Agyei N. DDT, DDE and DDD in human milk from South Africa. Bull. Environ. Contam. Toxicol. 2008;81:348–354. doi: 10.1007/s00128-008-9495-5. [DOI] [PubMed] [Google Scholar]
- Okoya A.A., Ogunfowokan A.O., Asubiojo O.I., Torto N. Organochlorine pesticide residues in sediments and waters from cocoa producing areas of Ondo State, Southwestern Nigeria. ISRN Soil Sci. 2013;2013:1–12. [Google Scholar]
- Olisah C., Okoh O.O., Okoh A.I. Global evolution of organochlorine pesticides research in biological and environmental matrices from 1992 to 2018: a bibliometric approach. Emerg. Contam. 2019;5:157–167. [Google Scholar]
- Olisah C., Adeniji A.O., Okoh O.O., Okoh A.I. Occurrence and risk evaluation of organochlorine contaminants in surface water along the course of Swartkops and sundays river estuaries, eastern Cape province, South Africa. Environ. Geochem. Health. 2019:1–25. doi: 10.1007/s10653-019-00336-0. [DOI] [PubMed] [Google Scholar]
- Pazou E.Y.A., Boko M., Van Gestel C.A., Ahissou H., Lalèyè P., Akpona S., van Hattum B., Swart K., van Straalen N.M. Organochlorine and organophosphorous pesticide residues in the Ouémé River catchment in the Republic of Bénin. Environ. Int. 2006;32:616–623. doi: 10.1016/j.envint.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Quinn L., Pieters R., Nieuwoudt C., Borgen A.R., Kylin H., Bouwman H. Distribution profiles of selected organic pollutants in soils and sediments of industrial, residential and agricultural areas of South Africa. J. Environ. Monit. 2009;11:1647–1657. doi: 10.1039/b905585a. [DOI] [PubMed] [Google Scholar]
- Reischl A., Reissinger M., Thoma H., Hutzinger O. Accumulation of organic air constituents by plant surfaces: Part IV. Plant surfaces: a sampling system for atmospheric polychlorodibenzo-p-dioxin (PCDD) and polychlorodibenzo-p-furan (PCDF) Chemosphere. 1989;18:561–568. [Google Scholar]
- Rosseland B.O., Deribe E., Rosseland B.O., Borgstrøm R., Salbu B., Dadebo E. Organochlorine pesticides and polychlorinated biphenyls in fish from Lake Awassa in the Ethiopian Rift Valley: human health riak. Bull. Environ. Contam. Toxicol. 2014;93:238–244. doi: 10.1007/s00128-014-1314-6. [DOI] [PubMed] [Google Scholar]
- Rusibamayila C.S., Ak’habuhaya J.L., Lodenius M. Determination of pesticide residues in some major food crops of northern Tanzania. J. Environ. Sci. Health B. 1998;33:399–409. doi: 10.1080/03601239809373153. [DOI] [PubMed] [Google Scholar]
- Sallam K.I., Mohammed A.E.M.A. Organochlorine pesticide residues in camel, cattle and sheep carcasses slaughtered in Sharkia Province, Egypt. Food Chem. 2008;108:154–164. [Google Scholar]
- Sarkar A., Sen Gupta R., Division C.O. DDT residues in sediments from the bay of bengal. Bull. Environ. Contam. Toxicol. 1988;41:664–669. doi: 10.1007/BF02021016. [DOI] [PubMed] [Google Scholar]
- Schulz R. Comparison of spray drift- and runoff-related input of azinphos-methyl and endosulfan from fruit orchards into the Lourens River, South Africa. Chemosphere. 2001;45:543–551. doi: 10.1016/s0045-6535(00)00601-9. [DOI] [PubMed] [Google Scholar]
- Schulz R. Rainfall-induced sediment and pesticide input from orchards into the Lourens River, Western Cape, South Africa: importance of a single event. Water Res. 2001;35:1869–1876. doi: 10.1016/s0043-1354(00)00458-9. [DOI] [PubMed] [Google Scholar]
- Schulz R., Peall S.K.C. Effectiveness of a constructed wetland for retention of nonpoint-source pesticide pollution in the Lourens River catchment, South Africa. Environ. Sci. Technol. 2001;35:422–426. doi: 10.1021/es0001198. [DOI] [PubMed] [Google Scholar]
- Schulz R., Peall S.K.C., Dabrowski J.M., Reinecke A.J. Current-use insecticides, phosphates and suspended solids in the Lourens River, Western Cape, during the first rainfall event of the wet season. Water SA. 2001;27:65–70. [Google Scholar]
- Sereda B.L., Meinhardt H.R. Contamination of the water environment in malaria endemic areas of KwaZulu-Natal, South Africa by DDT and its metabolites. Bull. Environ. Contam. Toxicol. 2005;75:538–545. doi: 10.1007/s00128-005-0785-x. [DOI] [PubMed] [Google Scholar]
- Sereda B.L., Meinhardt H.R. Water Research Commission; Pretoria: 2003. Insecticides Contamination of the Water Environment in Malaria Endemic Areas of KwaZuluNatal (SA) [Google Scholar]
- Sibali L.L., Okwonkwo J.O., McCrindle R.I. Determination of selected organochlorine pesticide (OCP) compounds from the Jukskei River catchment area in Gauteng, South Africa. Water SA. 2008;34:611–621. [Google Scholar]
- Sibanda T., Dobson H.M., Cooper -J.F., Manyangarirwa W., Chiimba W. Pest management challenges for smallholder vegetable farmers in Zimbabwe. Crop Protect. 2000;19:807–815. [Google Scholar]
- Sojinu O.S.S., Sonibare O.O., Ekundayo O.O., Zeng E.Y. Assessment of organochlorine pesticides residues in higher plants from oil exploration areas of Niger Delta, Nigeria. Sci. Total Environ. 2012;433:169–177. doi: 10.1016/j.scitotenv.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Sojinu O.S., Sonibare O.O., Ekundayo O., Zeng E.Y. Occurrence of organochlorine pesticides (OCPs) in surface sediments of the Niger Delta, Nigeria. J. Appl. Sci. Res. 2011;7:1299–1305. [Google Scholar]
- Soliman A.S., Smith M.A., Cooper S.P., Ismail K., Khaled H., Ismail S., McPherson R.S., Seifeldin I.A., Bondy M.L. Serum organochlorine pesticide levels in patients with colorectal cancer in Egypt. Arch. Environ. Health. 1997;52:409–415. doi: 10.1080/00039899709602219. [DOI] [PubMed] [Google Scholar]
- Thiere G., Schulz R. Runoff-related agricultural impact in relation to macroinvertebrate communities of the Lourens River, South Africa. Water Res. 2004;38:3092–3102. doi: 10.1016/j.watres.2004.04.045. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme (UNEP) 2009. Stockholm Convention on Persistent Organic Pollutants (POPs) in Stockholm Convention on Persistent Organic Pollutants (POPs) [Google Scholar]
- United Nations Environment Programme (UNEP) 2001. Final Act of the Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants. UNEP/POPS/CONF/4. [Google Scholar]
- Vaissayre M., Cauquil J.C. Main pests and diseases of cotton in sub-Saharan Africa, Main pests and diseases of cotton in sub-Saharan Africa. Editions Quae. 2000 [Google Scholar]
- Vetter W., Weichbrodt M., Scholz E., Luckas B., Agerà H.O. Levels of organochlorines (DDT , and HCHs) in blubber of South African Fur seals ( Arctocephalus pusillus pusillus) from Cape Cross/Namibia. Mar. Pollut. Bull. 1999;38:830–836. [Google Scholar]
- Wasswa J., Kiremire B.T., Nkedi-Kizza P., Mbabazi J., Ssebugere P. Organochlorine pesticide residues in sediments from the Uganda side of Lake Victoria. Chemosphere. 2011;82:130–136. doi: 10.1016/j.chemosphere.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Wepener V., van Dyk C., Bervoets L., O’Brien G., Covaci A., Cloete Y. An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys. Chem. Earth. 2011;36:949–962. [Google Scholar]
- Westbom R., Hussen A., Megersa N., Retta N., Mathiasson L., Björklund E. Assessment of organochlorine pesticide pollution in Upper Awash Ethiopian state farm soils using selective pressurised liquid extraction. Chemosphere. 2008;72:1181–1187. doi: 10.1016/j.chemosphere.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Williams B.A. Levels and distribution of chlorinated pesticide residues in water and sediments of Tarkwa Bay, Lagos Lagoon. J. Res. Environ. Sci. Toxicol. 2013;2:1–8. [Google Scholar]
- Williamson S., Ball A., Pretty J. Trends in pesticide use and drivers for safer pest management in four African countries. Crop Protect. 2008;27:1327–1334. [Google Scholar]
- Winter C.K. Dietary pesticide risk assessment. Rev. Environ. Contam. Toxicol. 1992:23–67. doi: 10.1007/978-1-4613-9751-9_2. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2016. World Malaria Report 2016; pp. 1–186. [Google Scholar]
- World Health Organisation . 2004. DDT and its Derivatives in Drinking Water; Background Document for Development of WHO Guidelines for Drinking Water Quality. WHO/SDE/WSH/03.04/89; pp. 1–15. [Google Scholar]
- Wu N., Zhang S., Huang H., Shan X., Christie P., Wang Y. DDT uptake by arbuscular mycorrhizal alfalfa and depletion in soil as influenced by soil application of a non-ionic surfactant. Environ. Pollut. 2008;151:569–575. doi: 10.1016/j.envpol.2007.04.005. [DOI] [PubMed] [Google Scholar]