Abstract
Rosellinia (Xylariaceae) is a large, cosmopolitan genus comprising over 130 species that have been defined based mainly on the morphology of their sexual morphs. The genus comprises both lignicolous and saprotrophic species that are frequently isolated as endophytes from healthy host plants, and important plant pathogens. In order to evaluate the utility of molecular phylogeny and secondary metabolite profiling to achieve a better basis for their classification, a set of strains was selected for a multi-locus phylogeny inferred from a combination of the sequences of the internal transcribed spacer region (ITS), the large subunit (LSU) of the nuclear rDNA, beta-tubulin (TUB2) and the second largest subunit of the RNA polymerase II (RPB2). Concurrently, various strains were surveyed for production of secondary metabolites. Metabolite profiling relied on methods with high performance liquid chromatography with diode array and mass spectrometric detection (HPLC-DAD/MS) as well as preparative isolation of the major components after re-fermentation followed by structure elucidation using nuclear magnetic resonance (NMR) spectroscopy and high resolution mass spectrometry (HR-MS). Two new and nine known isopimarane diterpenoids were identified during our mycochemical studies of two selected Dematophora strains and the metabolites were tested for biological activity. In addition, the nematicidal cyclodepsipeptide PF1022 A was purified and identified from a culture of Rosellinia corticium, which is the first time that this endophyte-derived drug precursor has been identified unambiguously from an ascospore-derived isolate of a Rosellinia species. While the results of this first HPLC profiling were largely inconclusive regarding the utility of secondary metabolites as genus-specific chemotaxonomic markers, the phylogeny clearly showed that species featuring a dematophora-like asexual morph were included in a well-defined clade, for which the genus Dematophora is resurrected. Dematophora now comprises all previously known important plant pathogens in the genus such as D. arcuata, D. bunodes, D. necatrix and D. pepo, while Rosellinia s. str. comprises those species that are known to have a geniculosporium-like or nodulisporium-like asexual morph, or where the asexual morph remains unknown. The extensive morphological studies of L.E. Petrini served as a basis to transfer several further species from Rosellinia to Dematophora, based on the morphology of their asexual morphs. However, most species of Rosellinia and allies still need to be recollected in fresh state, cultured, and studied for their morphology and their phylogenetic affinities before the infrageneric relationships can be clarified.
Key words: Xylariaceae, Rosellinia, Dematophora, Genus resurrection, Polythetic taxonomy, PF1022A, Isopimarane diterpenoids
Taxonomic novelties: New combinations: Dematophora acutispora (Theiss.) C. Lambert, K. Wittstein & M. Stadler; Dematophora arcuata (Petch) C. Lambert, K. Wittstein & M. Stadler; Dematophora asperata (Massee ex Wakef.) Lambert, K. Wittstein & M. Stadler; Dematophora beccariana (Ces.) C. Lambert, K. Wittstein & M, Stadler; Dematophora boedijnii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora bothrina (Berk. & Broome) C. Lambert, K. Wittstein & M. Stadler; Dematophora bunodes (Berk. & Broome) C. Lambert, K. Wittstein & M. Stadler; Dematophora buxi (Fabre) C. Lambert, K. Wittstein & M. Stadler; Dematophora compacta (Takemoto) C. Lambert, K. Wittstein & M. Stadler; Dematophora francisiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora freycinetiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora gigantea (Ellis & Everh.) C. Lambert, K. Wittstein & M. Stadler; Dematophora grantii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora hsiehiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora hughesii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora javaensis (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora macdonaldii (Bres.) C. Lambert, K. Wittstein & M. Stadler; Dematophora obregonii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora obtusiostiolata (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora paraguayensis (Starbäck) C. Lambert, K. Wittstein & M. Stadler; Dematophora pepo (Pat.) C. Lambert, K. Wittstein & M. Stadler; Dematophora puiggarii (Pat.) C. Lambert, K. Wittstein & M. Stadler; Dematophora pyramidalis (Lar.N. Vassiljeva) C. Lambert, K. Wittstein & M. Stadler; Dematophora samuelsii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler; Dematophora siggersii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler
Introduction
The genus Rosellinia was erected by De Notaris (1844) based on R. aquila as type species and later synonymised with various other genera of pyrenomycetes, including Dematophora (Hartig 1883), which had been typified by D. necatrix. The sexual morphs of Rosellinia are characterised by uniperithecioid stromata usually growing from a subiculum. The ascospore morphology, as well as the conidiogenous structures and other characteristics of the asexual morphs are rather variable, even though most of the conidiogenous structures of Rosellinia spp. known can be assigned to the Geniculosporium type. In fact, only some species of the “R. thelena group” for which no molecular data are available, have been reported to form nodulisporium-like conidiophores according to the definition of Ju & Rogers (1996). Many species are only known from one or a few collections and have never been cultured or included in molecular phylogenetic analyses. Recent studies of stromatic Xylariales by Hsieh et al. (2010) and Wendt et al. (2018) included some taxa of Rosellinia and have clearly showed that the genus has affinities to the genus Xylaria and other genera of Xylariaceae sensu stricto, in particular Entoleuca, Euepixylon and Nemania. The Hypoxylon lenormandii complex, which contains some species that were previously included in Rosellinia for their superficially similar stromatal morphology (Kuhnert et al. 2015), has been shown to belong to the hypoxyloid clade of the Xylariales, which have stromatal pigments and a nodulisporium-like asexual morphs. From polythetic studies employing multi locus molecular phylogenies, this complex now resides in the Hypoxylaceae (Daranagama et al. 2018, Wendt et al. 2018), while Rosellinia is retained in the Xylariaceae, owing to its phylogenetic affinities to Xylaria. In general, the recent segregation of the stromatic Xylariales proposed in the latter papers demonstrated that ascospore morphology is often incongruent with the lineages defined by multi locus phylogeny than conidiogenous structures or secondary metabolite profiles.
The current study embarks from the comprehensive monograph by Petrini (2013), which compiled all relevant information on the taxonomy of the genus. This work was based on decades of meticulous morphological studies (Petrini, 1992, Petrini, 2003, Petrini and Petrini, 2005). The monograph classified the more than 140 currently accepted species into seven informal “Groups” rather than into formal subgeneric taxa. Notably, Petrini (2013) predicted that the genus would need to be further subdivided as data on the phylogeny of its species accumulated. Out of the seven Groups, two, viz. the R. buxi and the R. necatrix groups, are characterised by dematophora-like asexual morphs, which are also often found in nature because of the rather conspicuous synnemata. In the phylogeny of Wendt et al. (2018), Rosellinia buxi and R. necatrix formed a sister clade to a clade containing the type species, R. aquila, R. corticium and Entoleuca mammata, making Rosellinia sensu Petrini (2013) paraphyletic. This observation motived a detailed study of the phylogeny and the secondary metabolites of these fungi in an attempt to further unravel their phylogenetic affinities. The results and conclusions are presented in the current paper.
Materials and methods
Fungal material and chemicals
All scientific names of fungi are given without authorities and years of publications, in accordance with MycoBank (www.mycobank.org), with the exception of the species discussed in the taxonomic part of this manuscript. Reference cultures are deposited at the Westerdijk Fungal Biodiversity Institute (formerly known as CBS, Utrecht, The Netherlands), BCCM/MUCL Agro-food & Environmental Fungal Collection (Louvain, Belgium) and the STMA culture collection of the HZI (Braunschweig, Germany). Unless indicated otherwise solvents were obtained in analytical grade from J.T. Baker (Deventer, Netherlands) or Merck (Darmstadt, Germany) and media ingredients from Carl Roth (Karlsruhe, Germany) or Sigma Aldrich (St. Louis, US). For glucose monitoring test stripes Medi-Test glucose by Macherey-Nagel (Düren, Germany) were used. In order to study their molecular and chemical diversity 14 strains of the genus Rosellinia and closely related species were selected. For molecular phylogenetic analyses additional GenBank sequences were selected. The origin of the fungal strains and their corresponding GenBank accessions are listed in Table 1.
Table 1.
List of used taxa for chemical analysis and phylogenetic reconstruction. GenBank accession numbers, strain ID of public culture collections or herbaria (if available), origin and reference studies are given. Type specimens are labelled with HT (holotype) or ET (epitype). Strains included in the chemical study are marked in bold. ∗This strain was labelled Rosellinia britannica, but this is a later erected synonym of Rosellinia marcucciana Ces., Atti dell´Accademia di Scienze Fisiche e Matematiche Napoli (1872) 5:13 fidePetrini (2013).
| Species | Strain number | Origin | Status | GenBank accession numbers |
Reference | |||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | TUB2 | |||||
| Amphirosellinia fushanensis | HAST 91111209 | Taiwan | HT | GU339496 | N/A | GQ848339 | GQ495950 | Hsieh et al. (2010) |
| A. nigrospora | HAST 91092308 | Taiwan | HT | GU322457 | N/A | GQ848340 | GQ495951 | Hsieh et al. (2010) |
| Astrocystis mirabilis | ATCC 66432 | Taiwan | No sequence data used | Ju & Rogers (1990) | ||||
| Coniolarelia limoniispora | MUCL 29409 | Japan | MN984615 | MN984624 | MN987235 | MN987240 | This study | |
| Dematophora bunodes | CBS 123584 | Peru | MN984617 | N/A | N/A | MN987243 | This study | |
| D. bunodes | CBS 123585 | Peru | MN984618 | N/A | N/A | MN987244 | This study | |
| D. bunodes | CBS 123597 | Peru | MN984619 | MN984625 | N/A | MN987245 | This study | |
| D. bunodes | CBS 124028 | Peru | No sequence data used | This study | ||||
| D. buxi | JDR 99 | France | GU300070 | N/A | GQ844780 | GQ470228 | Hsieh et al. (2010) | |
| D. necatrix | CBS 349.36 | Argentina | AY909001 | KF719204 | KY624275 | KY624310 | Peláez et al. (2008; ITS, LSU), Wendt et al. (2018; RPB2, TUB2) | |
| D. necatrix | W 97 | Japan | DF977487 | DF977487 | DF977459 | DF977466 | Shimizu et al. (2018) | |
| D. pepo | CBS 123592 | Peru | MN984620 | N/A | N/A | MN987246 | This study | |
| Entoleuca mammata | JDR 100 | France | GU300072 | N/A | GQ844782 | GQ470230 | Hsieh et al. (2010) | |
| Euepixylon sphaeriostomum | JDR 261 | USA | GU292821 | N/A | GQ844774 | GQ470224 | Hsieh et al. (2010) | |
| Graphostroma platystomum | CBS 270.87 | France | JX658535 | DQ836906 | KY624296 | HG934108 | Stadler et al. (2014; ITS), Zhang et al. (2006; LSU), Wendt et al. (2018; RPB2), Koukol et al. (2015; TUB2) | |
| Hypoxylon fragiforme | MUCL 51264 | Germany | ET | KC477229 | KM186295 | KM186296 | KX271282 | Stadler et al. (2013; ITS), Daranagama et al. (2015; LSU, RBP2), TUB2 (Wendt et al. 2018) |
| Kretzschmaria deusta | CBS 163.93 | Germany | KC477237 | KY610458 | KY624227 | KX271251 | Stadler et al. (2013; ITS), Wendt et al (2018; LSU, RPB2, TUB2) | |
| Nemania abortiva | BISH 467 | USA | HT | GU292816 | N/A | GQ844768 | GQ470219 | Hsieh et al. (2010) |
| N. beaumontii | HAST 405 | Martinique | GU292819 | N/A | GQ844772 | GQ470222 | Hsieh et al. (2010) | |
| N. beaumontii | FL 0980 | USA | N/A | JQ760608 | KU684243 | KU684161 | U'Ren et al. (2012; LSU), U'Ren et al. (2016; RPB2, TUB2) | |
| N. bipapillata | HAST 90080610 | Taiwan | GU292818 | N/A | GQ844771 | GQ470221 | Hsieh et al. (2010) | |
| N. primolutea | HAST 91102001 | Taiwan | HT | EF026121 | N/A | GQ844767 | EF025607 | Hsieh et al. (2010) |
| Podosordaria mexicana | WSP 176 | Mexico | GU324762 | N/A | GQ853039 | GQ844840 | Hsieh et al. (2010) | |
| Poronia punctata | CBS 656.78 | Australia | KT281904 | KY610496 | KY624278 | KX271281 | Senanayake et al. (2015; ITS), Wendt et al. (2018; LSU, RPB2, TUB2) | |
| Rosellinia aquila | MUCL 51703 | France | KY610392 | KY610460 | KY624285 | KX271253 | Wendt et al. (2018) | |
| R. aquila | STMA 15208 | Germany | No sequence data used | This study | ||||
| R. marcucciana∗ | MUCL 51704 | France | MN984616 | MN984626 | MN987238 | MN987238 | This study | |
| R. corticium | MUCL 51693 | France | KY610393 | KY610461 | KY624229 | KX271254 | Wendt et al. (2018) | |
| R. corticium | STMA 13324 | Germany | MN984621 | MN984627 | MN987237 | MN987241 | This study | |
| R. corticium | STMA 12170-15209 | Germany | MN984623 | MN984629 | MN987236 | MN987242 | This study | |
| R. nectrioides | CBS 449.89 | Sweden | MN984622 | MN984628 | MN987239 | N/A | This study | |
| R. quercina | MUCL52247 | Germany | No sequence data used | This study | ||||
| Xylaria arbuscula | CBS 126415 | Germany | KY610394 | KY610463 | KY624287 | KX271257 | Wendt et al. (2018) | |
| X. hypoxylon | CBS 122620 | Germany | ET | KY204024 | KY610495 | KY624231 | KX271279 | Sir et al. (2016; ITS), Wendt et al. (2018; LSU, RPB2, TUB2) |
| X. polymorpha | MUCL 49884 | France | KY610408 | KY610464 | KY624288 | KX271280 | Wendt et al. (2018) | |
| X. bambusicola | WSP 205 | Taiwan | HT | EF026123 | N/A | GQ844802 | AY951762 | Hsieh et al. (2010) |
Taxon selection
The general strategy of taxon selection followed the recent study by Wendt et al. (2018) and a previous comprehensive study by Hsieh et al. (2010), mainly using representatives of the Xylariaceae s. str. species of Rosellinia and presumably closely related genera like Coniolariella, Entoleuca, Euepixylon and Nemania made up the bulk of the taxa. The phylogeny thus covers all relevant taxa that have ever been connected with the genus Rosellinia and of which multi locus sequence data are extant in the public domain. In case sequences of the type species or the ex-(epi-)type strains were not available, data derived from vouchers of a related species in the same genus were chosen. As outgroups, members of the Graphostromataceae (Graphostroma platystomum) and the Hypoxylaceae (Hypoxylon fragiforme) were chosen (Fig. 1G, H).
Fig. 1.
Inferred phylogenetic tree of selected Xylariaceae, Hypoxylaceae and Graphostromataceae calculated by PhyML with 1 000 bootstrap replicates from a multigene alignment of the ITS-LSU ribosomal DNA region and the TUB2 and RPB2 regions. Bootstrap values above 50% are displayed at their respective branches. Sequence information originating from type strains are highlighted in bold.
Molecular phylogenetic analysis
DNA extraction, primer selection, resulting sequence analysis and processing as well as the alignment calculations were carried out as described previously (Wendt et al., 2018, Lambert et al., 2019) with Geneious® v. 7.1.9 (http://www.geneious.com, Kearse et al. 2012). Sequences were aligned via MAFFT v. 7.017 with the G-INS-I algorithm set to default in respect to gap opening and extension penalties. Regions of phylogenetic information were filtered by processing via the Castresana Lab Gblocks Server with stringency set to low (allows smaller final blocks and gap positions within final blocks, Talavera & Castresana 2007). The resulting alignments were concatenated with Geneious and the resulting multigene alignment submitted to the PhyML Online Server (http://www.atgc-montpellier.fr/phyml/) with the PhyML v. 3.0 algorithm for phylogenetic relationship inference and preliminary determination of the best fitting substitution model via smart model selection (SMS, see Guindon et al., 2010, Lefort et al., 2017). Automatic model selection was carried out by Bayesian Information Criterion (BIC). The tree topology was optimized by subtree pruning and regrafting (SPR). Bootstrap support values (BS) were calculated from 1 000 replicates. The phylogenetic tree was rooted with Graphostroma platystomum and Hypoxylon fragiforme.
Cultivation procedures
Cultures were grown first on yeast-malt-glucose agar plates (YMG) at 23 °C in darkness. Erlenmeyer flasks with 30 mL YM medium were inoculated with four mycelial plugs (0.5 × 0.5 cm2) of well-grown plates to obtain seed cultures. For the preparation of submerged cultures flasks with 200 mL medium were inoculated with four to six mycelial plugs (0.5 × 0.5 cm2), while flasks with 400 mL or 1 000 mL medium were inoculated with well-grown seed cultures (7.5 %). The flasks were kept at 23 °C and 150 rpm in darkness and the consumption of glucose was monitored regularly using glucose test stripes (Macherey-Nagel, Germany). The following liquid media were used: YM [malt extract 10 g/L, yeast extract 4 g/L, D-glucose 4 g/L, pH 6.3], ZM/2 (Rupcic et al. 2018b) [molasses 5 g/L, oatmeal 5 g/L, sucrose 4 g/L, mannitol 4 g/L, D-glucose 1.5 g/L, CaCO3 1.5 mg/L, edamin (lactalbumin hydrolysate, LP0048 from Oxoid) 0.5 mg/L, (NH4)2SO4 0.5 mg/L, pH 7.2], Q6 [glycerol 10 g/L, cottonseed flour 5 g/L, D-glucose 2.5 g/L, pH 7.2] and CM [corn meal 20 g/L, D-glucose 4 g/L, pH 5.5]. Strains CBS 123585, CBS 123584, CBS 123597, CBS124028 (D. bunodes), CBS 123592 (D. pepo), STMA 13324, MUCL 51693 (R. corticium), MUCL 51703 (R. aquila), MUCL 51704 (R. marcucciana) and ATCC 66432 (A. mirabilis) were selected for investigating their metabolite profiles and cultivated in all four liquid media (200 mL scale, Table S2). For the isolation of compounds the corresponding culture volumes were increased up to 4 L in total (200–1 000 mL per flask). The cultivation was stopped three to four days after total consumption of glucose.
In another attempt, strains MUCL 51703, STMA 15208 (R. aquila), MUCL 51704 (R. marcucciana), STMA13324, MUCL 51693, STMA 15209/12170 (R. corticium), MUCL 52247 (R. quercina), CBS449.89 (R. nectrioides) and ATCC 66432 (A. mirabilis) were cultivated on mPDA, CSA and CMD plates at 23 °C in darkness in order to investigate their production of cyclodepsipeptides (Table S2). mPDA (modified potato dextrose agar) [ potato starch 20 g/L, D-glucose 5 g/L, yeast extract 1.5 g/L, agar 20 g/L], CSA (cotton seed agar) [maltose 40 g/L, cottonseed flour 20 g/L, CaCO3 3 mg/L, soy peptone (type II, from Marcor) 2 g/L, MgSO4 • 7H2O 2 g/L, NaCl 2 g/L, agar 20 g/L (similar to Seed agar in Harder et al. 2011)], CMD [corn meal 20 g/L, D-glucose 4 g/L, agar 20 g/L, pH 5.5]. Culture plates were extracted when at least two-thirds of the plate surfaces were covered by mycelium (14–21 d). For the isolation of cyclodepsipeptide PF1022 A, STMA13324 (R. corticium) was cultivated in liquid CS medium [maltose 40 g/L, cottonseed flour 20 g/L, CaCO3 3 mg/L, soy peptone (type II, from Marcor) 2 g/L, MgSO4 • 7H2O 2 g/L, NaCl 2 g/L] in a total volume of 3 L (200 mL per flask).
Extraction and isolation of secondary metabolites
For the extraction of submerged cultures biomass and culture medium were separated by gauze filtration. The biomass was extracted with acetone in an ultrasonic bath (2 × 30 min) and after removing the organic solvent under reduced pressure at 40 °C the residual aqueous phase was extracted twice with ethyl acetate. The combined ethyl acetate phases were dried over sodium sulphate and the solvent was removed under reduced pressure at 40 °C to yield the crude extract. The culture medium was extracted with ethyl acetate twice and processed as described above. Culture plates were cut into pieces and extracted with 30 mL ethyl acetate twice on a magnetic stirrer for 30 min. After filtration, the organic solvent was removed under reduced pressure at 40 °C to yield the crude extract. The crude extracts were subjected to analytical HPLC-UV/Vis-MS analyses. For the isolation of compounds, the crude extracts were dissolved in 0.5–4 mL methanol and applied to RP solid phase cartridges (Strata-X 33 mm, Polymeric Reversed Phase; Phenomenex, Aschaffenburg, Germany) and eluted with methanol and acetonitrile to remove highly nonpolar contaminants (e.g. fatty acids) before injection for preparative HPLC.
Following the procedures described above the cultivation of D. bunodes (CBS123585) and D. pepo (CBS123592) in CM medium (4 L) provided 1.68 g and 0.27 g of crude extract, respectively. The extracts were subjected to RP-MPLC and/or preparative HPLC (details are given in the Supplementary Information) and finally resulted in the isolation of dematophorane A (1, 21.3 mg), dematophorane B (2, 2.1 mg), libertellenone M (3, 2.4 mg), γ-lactone of libertellenone M (4, 0.3 mg), myrocin B (5, 59.6 mg), spiropolin A (6, 4.5 mg), libertellenone C (7, 1.4 mg), hymatoxin K (8, 6.5 mg), elaeicolaside C (9, 6.6 mg), 16-α-D-glucopyranosyloxyisopimar-7-en-19-oic acid (10, 2.6 mg) and 16-α-D-glucopyranosyloxyisopimar-7-en-19-oic acid (11, 15.4 mg). The cultivation of R. corticium (STMA13324) in 3 L CS medium and subsequent extraction resulted in 0.48 g of crude extract. After pre-purification via a RP solid phase cartridge (Strata-X 33 mm, Polymeric Reversed Phase; Phenomenex) it was subjected to preparative HPLC to afford PF1022A (12, 13.2 mg) (details are given in the Supplementary Information).
Analytical data of the new diterpenoids
Dematophorane A (1): [α]25D -13.0 (c 2.1, CH3CN); UV (MeOH) λmax (log ε) 228 nm (3.98), 238 nm (3.98) 334 nm (4.51); 1H NMR (500 MHz, MeOH-d4), 13C NMR (125 MHz, MeOH-d4) (see Table 2); HR-ESI-MS m/z 373.1984 [M+Na]+ (calcd for C20H30NaO5, 373.1991). (Fig. S2)
Table 2.
13C and 1H NMR data of dematophoranes A and B (1–2).
| (1) | (2) | |||
|---|---|---|---|---|
| 1 | 75.2, CH | 3.68, dd (8.6, 7.4) | 69.9, CH | 4.26, m |
| 2 | 29.7, CH2 | 1.73, m | 29.7, CH2 | 1.70, m |
| 3a | 34.9, CH2 | 1.13, dt (13.0, 3.4) | 34.3, CH2 | 1.06, m |
| 3b | 1.61, m | 1.68, m | ||
| 4 | 38.0, C | 37.8, C | ||
| 5 | 55.5, CH | 2.95, s | 48.9, CH | 3.76, s |
| 6 | 212.4, C | 4.66, m | 211.1, C | |
| 7 | 73.8, CH | 76.5, CH | 4.76, m | |
| 8 | 131.8, C | 139.65, C | ||
| 9 | 146.9, C | 75.3, C | ||
| 10 | 51.2, C | 52.4, C | ||
| 11a | 24.9, CH2 | 2.34, dt (19.4, 7.5) | 31.3, CH2 | 2.06, m |
| 11b | 2.54, ddd (19.2, 7.9, 5.5) | 2.42, td (14.7, 3.4) | ||
| 12a | 30.4, CH2 | 1.41, m | 32.2, CH2 | 1.44, dtd (14.2, 4.1,2.8) |
| 12b | 1.81, ddd (13.5, 7.9, 6.1) | 1.75, td (14.2, 3.4) | ||
| 13 | 40.2, C | 37.9, C | ||
| 14 | 72.7, CH | 3.99, s | 130.6, CH | 5.84, s |
| 15 | 145.8, CH | 6.10, dd (17.6, 11.0) | 149.4, CH | 5.86, dd (17.6, 10.6) |
| 16a | 113.3, CH2 | 5.10, dd (10.2, 1.6) | 110.9, CH2 | 4.92, dd (10.7, 1.4) |
| 16b | 5.10, dd (10.2, 1.6) | 5.00, dd (17.5, 1.4) | ||
| 17 | 22.8, CH3 | 0.95, s | 24.1, CH3 | 1.01, s |
| 18 | 18.6, CH3 | 1.24, s | 18.8, CH3 | 1.21, s |
| 19a | 70.7, CH2 | 3.08, d (10.8) | 70.6, CH2 | 3.05, m |
| 19b | 3.46, d (10.8) | 3.54, m | ||
| 20 | 18.0, CH3 | 1.01, s | 13.9, CH3 | 0.87, s |
| OH-1 | 3.41, d (5.6) | |||
| OH-7 | 3.84, d (4.3) | |||
| OH-9 | 4.35, s | |||
| OH-18 | 3.74, t (5.8) | |||
1 (125 MHz and 500 MHz, MeOH-d4), 2 (175 MHz and 700 MHz, acetone-d6),
Dematophorane B (2): [α]25D +34.1 (c 1.8, CH3CN); UV (MeOH) λmax (log ε) 227 nm (4.19), 268 nm (4.26) 342 nm (4.37); 1H NMR (700 MHz, acetone-d6), 13C NMR (175 MHz, acetone-d6) (see Table 2); HR-ESI-MS: m/z 373.1984 [M+Na]+ (calcd for C20H30NaO5, 373.1991).(Fig. S3)
Analytical HPLC-UV/Vis-MS analyses
All HPLC-MS analyses were performed on Agilent 1260 Infinity or Dionex Ultimate 3000 Systems with diode array detector and C18 Waters Acquity UPLC BEH column (2.1 × 50 mm, 1.7 μm). Solvent A: H2O + 0.1 % formic acid, solvent B: acetonitrile + 0.1 % formic acid, gradient system: 5 % B for 0.5 min increasing to 100 % B in 19.5 min, maintaining 100 % B for 5 min, flow rate = 0.6 mL/min, detection at 200−600 nm. LC-ESIMS spectra were recorded on an ion trap MS (amaZon speed, Bruker), and HR-ESIMS spectra on a time-of-flight (TOF) MS (MaXis, Bruker).
Structure elucidation
1D and 2D NMR spectra were recorded on a Bruker Avance III 700 spectrometer with a 5 mm TXI cryoprobe (1H 700 MHz, 13C 175 MHz) and a Bruker Avance III 500 (1H 500 MHz, 13C 125 MHz) spectrometer. UV-Vis spectra were recorded with a UV-2450 Shimadzu UV−Vis spectrophotometer and optical rotations were determined with a PerkinElmer 241 polarimeter.
Bioactivity assays
In vitro cytotoxic effects (IC50) against mouse fibroblast cell line L929 and human carcinoma cell line KB-3-1 of the compounds (1 mg/mL stock solutions in methanol) were determined as described by Surup et al. (2018). Minimum inhibitory concentrations (MIC) were obtained in serial dilution assays with pure substances dissolved in methanol and various bacterial and fungal test organisms (Kuephadungphan et al. 2017). Nematicidal activity was detected using an assay with Caenorhabditis elegans as described by Rupcic et al. (2018a). The nematodes were inoculated monoxenically on nematode agar (soy peptone 2 g/L, NaCl 1 g/L, agar 20 g/L, cholesterol (1 mg/mL in EtOH, 0.5 mL), 1M CaCl2 (1 mL), 1M MgSO4 (1 mL) and 40 mM potassium phosphate buffer 12.5 mL; pH 6.8) with living Escherichia coli (DSM498) at 21 °C for 4–5 d. For the assay the nematodes were taken up in M9 buffer (0.5 M KH2PO4/K2HPO4 and 0.1 M NaCl, pH 7.2) and counted using a Malassez counting chamber. A final concentration was adjusted to 500 nematodes per mL and 1 mL of this suspension was added to each well of a 24-well microtiter plate. The pure compounds were dissolved in acetonitrile or ethanol and were tested at different concentrations (100 μg/mL, 50 μg/mL, 20 μg/mL, 10 μg/mL). Ivermectin (in the same concentrations in ethanol) was the positive control. The plate was incubated on a plate shaker for 18 h at 24 °C.
Results
To infer phylogenetic relationships (Fig. 1), 32 different strains were included, viz. one representative of the Graphostromataceae (G, Graphostroma plastystomum), one representing the Hypoxylaceae (H, Hypoxylon fragiforme), two representing the coprophilous Xylariaceae (P) and 28 strains for the remaining Xylariaceae, representing nine different genera (X, A, N, D, R). From these strains, 28 new sequences were generated for their relevant DNA loci, whilst the remaining loci were complemented with 80 sequences from GenBank. The resulting multigene alignment (MGA) consisted of 4 176 characters, with 469 and 1 273 positions originating from ITS and LSU ribosomal DNA, respectively, and 1 101 and 1 333 characters derived from protein coding regions (RPB2 and TUB2). The initial alignments as well as the final inferred phylogenetic tree of the curated and concatenated single-gene alignments are available in the Supplementary Information.
The inferred phylogeny shows six supported (>50 % BS) distinct clades with the Xylariaceae rendered as strongly supported (99 %). The coprophilous Podosordaria mexicana and Poronia punctata (P) are placed as sister clade to other saprotrophic and pathogenic representatives. Additional clades include Xylaria and Kretzschmaria spp. (X); Xylaria polymorpha clustered within a weakly supported (54 %) Amphirosellinia clade (A); and the clade consisting of Nemania spp. (N) forms a well-supported sister clade (94 %) to the Rosellinia and Dematophora clades (R, D). Clade N consists of two well supported subclades (100 %) of Euepixylon sphaeriostomum and Nemania beaumontii on the one hand and the type strains of Nemania abortiva and Nemania primolutea as well as a non-type strain of Nemania bipapillata on the other hand. The sister clade of clade N consisted of two subclades, the Dematophora (D) and Rosellinia (R) clades.
Studies on secondary metabolism of Rosellinia s. lat
In parallel to the molecular phylogenetic investigations several strains were cultivated in four different liquid media (see cultivation procedures and Table S2) and their corresponding extracts were analysed by HPLC-UV/Vis-MS. After comparing the secondary metabolite production of the submerged cultures, two Dematophora strains, D. bunodes CBS 123585 and D. pepo CBS 123592, were selected for the cultivation in CM medium in larger scale for the isolation of compounds due to a variety of interesting HPLC-UV/MS profiles in their extracts and in case of CBS 123585 relatively high quantities of substances (Fig. S1). Two new (1–2) and seven known isopimarane diterpenoids, libertellenone M (3) and the γ-lactone of libertellenone M (4) (Kildgaard et al. 2017), myrocin B (5) (Hsu et al. 1988), spiropolin A (6) (Shiono et al. 2013), libertellenone C (7) (Oh et al. 2005), hymatoxin K (8) (Jossang et al. 1995) and elaeicolaside C (9) (Wang et al. 2012), were isolated from a submerged culture (4 L) of D. bunodes CBS123585 (Fig. 2, Fig. 3, Fig. 4). Further, two known isopimarane diterpene glycosides, 16-α-D-glucopyranosyloxy-isopimar-7-en-19-oic acid (10) and 16-α-D-mannopyranosyl-oxyisopimar-7-en-19-oic acid (11) (Shiono et al. 2009) were obtained from D. pepo CBS123592 (4 L) (Fig. 4). All metabolites were characterised by HRMS and NMR. For known compounds, the data were compared with literature values in order to confirm their identity. The isolated isopimarane diterpenoids (1–11) were the prevailing components within all extracts of the investigated Dematophora strains, but some of them could be also detected in extracts of species belonging to the Rosellinia clade, like R. aquila, R. corticium and R. marcucciana as well as in extracts of Astrocystis mirabilis (Fig. 5). No cytochalasins, as previously reported from D. necatrix (Aldridge et al., 1972, Kimura et al., 1989, Shimizu et al., 2018), were detected using the described cultivation conditions.
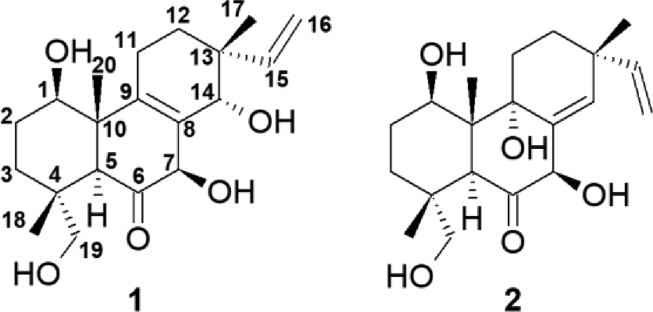
Fig. 2.
New isopimarane diterpenoids, dematophoranes A and B (1-2), isolated from Dematophora bunodes (CBS123585).
Fig. 3.
Relevant 1H,1H-COSY- (red arrows) and 1H-13C-HMBC (green arrows) correlations of dematophorane A (1).
Fig. 4.
Isolated secondary metabolites (3-12), known from different species of the orders Xylariales and Hypocreales.
Fig. 5.
HPLC-UV chromatograms (210 nm) of crude extracts from Dematophora bunodes (CBS123584), D. bunodes (CBS123597), D. pepo (CBS123592), Rosellinia corticium (STMA13324), R. aquila (STMA15208), R. marcucciana (MUCL51704), and Astrocystis mirabilis (ATTC66432) cultivated in liquid CM medium (200 mL). The detected isopimarane diterpenoids (1–3, 5–6, 8–11) are marked.
In order to investigate the production of cyclodepsipeptides of the PF1022 family (Scherkenbeck et al. 2002) by species of the genus Rosellinia, as indicated in a patent application by Harder et al. (2011), several Rosellinia strains and Astrocystis mirabilis (ATCC 66432) were cultivated on three different solid media (see cultivation procedures and Table S2). PF1022 A (12) (Fig. 4) was detected in extracts of R. corticium (STMA 15209), A. mirabilis (ATCC 66432) and in highest concentration in the extract of R. corticium (STMA 13324), (Fig. 6). The compound was isolated from submerged cultures of strain STMA 13324 in CS medium (3 L) and used as standard for the analysis of extracts obtained within this study (details are given in the Supplementary Information). Additionally, compounds with the masses of PF1022 C and PF1022 D with similar retention times to PF1022 A were observed in the CS medium extracts of R. corticium (STMA13324). After comparison with a fermentation sample of the original PF1022 producer strain, we concluded that these compounds were most likely cyclodepsipeptides PF1022 C and PF1022 D (Fig. S6).
Fig. 6.
HPLC-UV chromatograms (210 nm) of crude extracts containing PF1022A (top to bottom: pure PF1022A, Rosellinia corticium (STMA13324), R. corticium (STMA15209), Astrocystis mirabilis (ATTC66432).
Structure elucidation
Dematophorane A (1) was obtained as a colourless oil (21.3 mg) and its molecular formula was deduced as C20H30O5 by high resolution ESI-MS (Fig. S2), indicating six degrees of unsaturation. The 1H and 1H,13C-HSQC NMR spectra of (1) revealed one olefinic methine proton, one olefinic methylene group with a characteristic carbon at δC 113.3 ppm, four methines (three of them oxygenated), five methylene groups (one of them oxygenated) and three methyl groups. Additionally, the 13C NMR spectrum exhibited signals of one ketone, two olefinic quaternary carbons and three sp3 hybridized quaternary carbons. The 1H,1H-COSY correlations between the methylene groups CH2-11 and CH2-12 as well as between methine H-1, methylenes CH2-2 and CH2-3 and the analysis of the 1H,13C-HMBC correlations of (1) indicated the presence of a tricyclic diterpene scaffold. Key HMBC correlations include H-7 to Cq-6, Cq-9, Cq-8, H-5 to Cq-6, Cq-9, C-1, C-18, Cq-10, Cq-4, C-3, C-19 and H-14 to Cq-9, Cq-8, C-7, Cq-13, C-12, C-17 (Fig. 3). Further COSY correlations between olefinic methine H-15 and methylene CH2-16 and HMBC correlations of H-15 to Cq-13, C-17, C-12 and C-14 demonstrated a terminal vinyl group at Cq-13 between methylene CH2-12 and oxygenated methine H-14, a structural feature of many pimarane-type diterpenoids (Yu et al. 2018). The oxygenated methylene group at δ 3.08 (d, 1H), 3.46 (d, 1H), C 70.7 ppm was identified as hydroxylated methylene CH2-19 at Cq-4 via 1H,13C long range correlations of H-19 to C-5, Cq-4, C-3 and C-18. The relative stereochemistry of dematophorane A (1) was assigned by the analysis of ROESY data, which showed strong correlations between methyl protons H-17, methine H-14 and methylene proton H-11a as well as between methyl protons H-20, H-18 and H-11a, indicating that methyl groups CH3-17, CH3-20 and CH3-18 as well as methine H-14 are positioned at the same face of the molecule. On the opposite side, methine proton H-5 displayed strong correlations to H-7 and H-1 (Figs. S6–11). This stereochemistry is in accordance with the typical structure of an isopimarane-type diterpenoid (Wang et al. 2018).
Dematophorane B (2) was isolated as colourless film (2.1 mg) and displayed a major peak in the HR-ESIMS spectrum at m/z 373.1984 [M+Na]+, calcd. for C20H30NaO5, 373.1991), consistent with the molecular formula C20H30O5 and indicating six degrees of unsaturation (Fig. S3). The 1H and 1H,13C-HSQC NMR spectra of (2) were similar to those of (1) and showed signals which were assigned to a terminal vinyl group with CH-15 (δH 5.86 (dd, J = 17.6, 10.6), δC 149.4) and CH2-16 (δH 4.92 (dd, J = 10.7, 1.4), 5.00 (dd, J = 17.5, 1.4), δC 110.9), one olefinic methine, three further methines (two oxygenated), five methylene groups (one of them oxygenated) and three methyl groups. The 13C NMR spectrum exhibited signals of one ketone, one olefinic quaternary carbon, three sp3 hybridized quaternary carbons and one oxygenated quaternary carbon. Similar to dematophorane A (1) a pimarane-type diterpenoid scaffold was assigned due to 1H,1H COSY correlations between CH2-11 and CH2-12 as well as between OH-1, H-1, CH2-2 and CH2-3 and corresponding 1H,13C HMBC correlations. But in contrast to derivative (1) an additional olefinic methine (δH 5.84 (s), δC 130.6) and an oxygenated quaternary carbon (δC 75.3) were detected instead of a second olefinic quaternary carbon. 1H,13C HMBC correlations of this olefinic methine proton H-14 to C-15, Cq-8, C-7, Cq-9 and C-12 indicated a position of the olefin between the terminal vinyl group and hydroxyl group OH-7. HMBC correlations of H-20, H-12, H-11, H-5, H-1 and OH-9 to the quaternary carbon Cq-9 confirmed the assignment of a hydroxyl group at position C-9. The analysis of the NOESY data showed strong correlations between protons H-17, H-11a, H-12a and H-20 as well as between H-20, OH-1 and H-18. Additional NOE correlations between protons H-7, OH-9, H-12b, H-1 and protons H-5, H-7, H-1 demonstrated the same relative stereochemistry as for compound (1), (Figs. S12–17). During the purification and analysis processes, the rearrangement of dematophorane B (2) in methanol solutions at room temperature was observed. After keeping (2) in methanol at 40 °C for 19 d, approximately 70 % of the molecule converted into another major compound, which was identified as libertellenone C (7). The slightly basic conditions and/or thermal energy seem to induce a rearrangement of the alpha-hydroxyketone of (2) and a final stabilization of the molecule by dehydrogenation.
Biological activity of extracts and pure compounds
Extracts were screened for antimicrobial activity against Gram-positive/Gram-negative bacteria (Bacilus subtilis/Escherichia coli), a yeast (Candida albicans) and the filamentous fungus Mucor plumbeus as well as for nematicidal activity against Caenorhabditis elegans. The pure compounds were tested against a larger panel of bacteria and fungi (Table 3) and for their cytotoxicity on mouse fibroblast cells L929, cervix carcinoma cells KB-3-1 or primary human umbilical vein endothelial cells HUVEC (Table 3). Due to its instability the γ-lactone of libertellenone M (4) was not tested.
Table 3.
In vitro antibacterial, antifungal and cytotoxic activity of dematophoranes A-B (1-2), libertellenone M (3), myrocin B (5) and libertellenone C (7). All compounds were dissolved in methanol (1 mg/ml, test volume: 20 μl). 20 μl of methanol showed no effect on the test organisms. MIC: Minimum inhibitory concentration. Positive controls: [a] Oxytetracyclin hydrochloride, [b] Gentamycin, [c] Nystatin; [d] Epothilon B, n.i.: no inhibition.
| Test organisms | MIC [μg/mL] |
|||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (5) | (7) | Ref [a,b,c] | |
| Gram-positive bacteria | ||||||
| Bacillus subtilis DSM 10 | n.i | 67.0 | n.i | 33.3 | n.i. | 8.3 [a] |
| Micrococcus luteus DSM 1790 | 67.0∗ | 67.0 | n.i | 67.0 | n.i | 0.42 - 0.83 [a] |
| Mycobacterium smegmatis ATCC700084 | 67.0 | 67.0 | n.i | 67.0 | n.i. | 3.3 [a] |
| Staphylococcus aureus DSM 346 | 67.0 | 67.0 | n.i | 33.3 | 67.0∗ | 0.42–0.83 [a] |
| Gram-negative bacteria | ||||||
| Chromobacterium violaceum DSM 30191 | n.i | n.i | n.i | n.i | n.i | 0.83 [a] |
| Escherichia coli DSM 1116 | n.i | n.i | n.i | n.i | n.i | 3.3–6.7 [a] |
| Pseudomonas aeruginosa PA 14 | n.i | n.i | n.i | n.i | n.i | 4.2 [b] |
| Yeasts | ||||||
| Candida albicans DSM 1665 | n.i | n.i | n.i | n.i | n.i | 8.3 [c] |
| Wickerhamomyces anomalus DSM 6766 | n.i | n.i | n.i | - | n.i | 16.6 [c] |
| Rhodotorula glutinis DSM 10134 | n.i. | n.i. | n.i. | n.i | n.i | 2.1 [c] |
| Schizosaccharomyces pombe DSM 70572 | n.i | n.i | n.i | n.i | n.i | 16.6 [c] |
| Filamentous fungi | ||||||
| Mucor hiemalis DSM 2656 | n.i. | 67.0∗ | 67.0∗ | n.i | 67.0∗ | 8.3 [c] |
| Cell lines | ||||||
| Mouse fibroblast cell line L929 | 28 | 20 | 6.3 | 6.5 | 9.5 | 1.1 × 10-3 [d] |
| Cervix carcinoma cell line KB-3-1 | 6.5 | 16 | 6.1 | - | 14 | 0.06 × 10-3 [d] |
| Primary Human Umbilical Vein Endothelial Cells HUVEC | - | - | - | 0.75 | - | 0.2 × 10-3 [d] |
The cell density was adjusted to 8 × 106 cells/mL. ∗ no complete inhibition; DSMZ: German Collection of Microorganisms and Cell Cultures, Braunschweig.
Extracts from D. bunodes strains (CBS123584 – CM medium, CBS123585 – CM and Q6 medium, CBS 123597 – CM medium, CBS124028 – CM medium) showed antibiotic activity against B. subtilis (DSM10). No further antimicrobial activity was detected among the crude extracts. Nematicidal activity was observed for the extracts of R. corticium (STMA 15209 – mPDA medium, STMA 13324 – CSA medium, STMA12170 – CSA medium, MUCL 51693 – CMD medium), R. aquila (STMA 15208 – CSA and CMD medium), R. marcucciana (MUCL 51704 – CSA and CMD medium) and A. mirabilis (ATCC 66432 – CSA and CMD medium).
The pure compounds dematophorane A (1) and B (2) showed weak antibiotic activity (MIC: 67 μg/mL) against several Gram-positive test strains (B. subtilis, Micrococcus luteus, Mycobacterium smegmatis, S. aureus). Also, myrocin B (5) displayed weak to moderate activity (MIC: 67–33 μg/mL) against these Gram-positive strains. Its activity against B. subtilis was reported before by Hsu et al. 1988 and Lehr et al. 2006. For dematophorane B (2), libertellenone M (3) and libertellenone C (7) weak antifungal activity (MIC: 67 μg/mL) against Mucor hiemalis was observed. In addition, Libertellenone M (3) displayed moderate cytotoxicity on mouse fibroblast cells L929 (6.3 μg/mL) and carcinoma cells KB-3-1 (6.1 μg/mL). Dematophorane A (1) also showed cytotoxic effects on carcinoma cell line KB-3-1 (6.5 μg/mL) and myrocin B (5) on L929 cells (6.5 μg/mL) as well as on primary HUVEC cells (0.75 μg/mL) (Table 3).
Taxonomy
The phylogenetic study, in which various species of Rosellinia and allies were compared in a multi-locus phylogeny, indicated that the genus should be subdivided. While the majority of species in Rosellinia sensu Petrini (2013) remain to be incorporated into a comprehensive molecular phylogeny, we noted a strong correlation between the type of asexual morph and the results of our phylogenetic study.
Table 4 gives a summary on the crucial characteristics of the genus Rosellinia sensu Petrini (2013), summarising the most important morphological features of the seven species groups defined in the latter monograph.
Table 4.
Species groups of Rosellinia sensuPetrini (2013) and their salient morphological features.
| Species group | Conidiophore type | Ratio of ascospore length/width | Ascospore germ slit | Other characteristic features of asci/ascospores |
|---|---|---|---|---|
| Rosellinia aquila | Geniculosporum | <4 | straight, spore length | cellular appendages, slimy caps and sheath mostly present |
| R. mammaeformis | Geniculosporum | <4 | straight or diagonal, spore length to 2/3 spore length | cellular appendages, slimy caps and sheath mostly present |
| R. emergens | Geniculosporum | ≥4 | variable | cellular appendages, slimy caps and sheath mostly absent |
| R. mammoidea | Geniculosporum | <4 | straight, mostly spore length | cellular appendages, slimy caps and sheath absent; usually smaller than 16 μm |
| R. necatrix | Dematophora | ≥4 | straight, short | cellular appendages absent, slimy sheath present |
| R. buxi | Dematophora | <4 | straight, mostly spore length | cellular appendages absent, slimy caps or sheath present |
| R. thelena | Geniculosporum or Nodulisporium | <4 | straight, mostly spore length | cellular appendages, slimy caps and sheath present |
All Rosellinia spp. that are known to form a dematophora-like asexual morph are included in the “R. buxi Group” or the “R. necatrix Group”, and at the same time, their DNA sequences clustered in clade D. On the other hand, sequences of those species studied that have a geniculosporium-like asexual morph, clustered in clade R along with the type species, R. aquila. In order to make the systematics of Rosellinia more compatible with the evidence obtained from the current molecular study and congruent with the morphological evidence, in particular considering the “One Fungus-One Name” concept (see also Stadler et al. 2013 for a respective treatment of the stromatic Xylariales), we therefore propose to resurrect the genus Dematophora for the two aforementioned “Groups”. We propose the following taxonomic rearrangements:
Rosellinia De Not., G. bot. ital. 1(1): 334. 1844. Fig. 7.
Fig. 7.
Sexual and asexual morph structures of different Rosellinia species. A, L, T. R. breensis (Sir & Hladki 841-LIL). B, S. R. hyalospora (Sir & Hladki 463-LIL). C. R. megalospora (Sir & Hladki 972-LIL). D, E, J, M, P. R. longispora (Sir & Hladki 939-LIL). F–I, K. R. rickii (Sir & Hladki 062-LIL). N, O. R. canzacotoana (Sir & Hladki 198-LIL). Q, R. Rosellinia sp. (Sir & Hladki 377-LIL). A, B, D. Stromata in substrate. C. Stromata emerging from the subiculum (arrow). E. Cross section of stromata. F. Stromata and conidiophores (arrows). G–I. Conidiogenous structure in 3% KOH. J, K. Ascus in 3% KOH. L–N. Ascus apical plugs in Melzer's reagent. O, P, S, T. Ascospores showing germ slit in 3% KOH (arrows). Q, R. Ascospores showing cellular appendages in 3% KOH (arrows). Scale bars: A = 2 mm; B–F = 1 mm; G, H, L–T = 10 μm; I, J = 50 μm; K = 20 μm.
Synonyms: Amphisphaerella Henn., Hedwigia 41: 18. 1902.
Byssitheca Bonord., Abh. naturforsch. Ges. Halle 8: 82, 156. 1864.
Vrikshopama D. Rao & P. Rag. Rao, Mycopath. Mycol. appl. 23: 289. 1964.
Type species: Rosellinia aquila (Fr.) Ces. & De Not. 1844.
Typus: Sweden, “Småland”, (today Skåne) Lund, E. Fries (UPS, sub Sphaeria aquila - holotype).
Generic description (modified from Petrini 2013):
Subiculum woolly, wiry, felted, brown, white, yellow, persistent or evanescent. Stromata subglobose, mammaeform, semiglobose or conical, sessile or broadly stipitate, glabrous, ostiolate, brown, grey, black, uniperitheciate, sometimes confluent containing a few perithecia, superficial. Perithecia globose, collapsing and usually detached from stromatal wall, rarely remaining attached. Paraphyses filiform, evanescent. Asci cylindrical, long stipitate, evanescent. Ascus apical plugs cylindrical with bulge at upper rim, rarely without, amyloid, rarely not amyloid. Ascospores unicellular, asymmetrically ellipsoidal to fusoid, light to dark brown, with straight, sigmoid or spiral germ slit extending over the whole spore length or shorter, rarely absent.
Asexual morph: Geniculosporium-like, rarely nodulisporium-like.
Notes: The above generic description is identical to the one by Petrini (2013) except for the definition of asexual morph. The species that remain in Rosellinia after segregation of Dematophora cannot be segregated by morphological features of the asci, ascospores or stromata. The species that are retained in the genus Rosellinia comprise the R. aquila, R. mammaeformis, R. emergens, R. mammoidea and R. thelena “Groups” (the latter of which has been regarded as subgenus Corrugata) as defined by Petrini (2013). A lot of work remains to be done in order to clarify their affinities and the genus may possibly have to be further subdivided once more molecular data and more information about the morphology of the asexual morphs become available (see Fig. 7, Fig. 8). The segregation of Dematophora also has some practical implications, since almost all important pathogens among the rosellinoid Xylariaceae, except for Entoleuca mammata and the conifer pathogens of the R. thelena Group sensu Petrini (2013, subgenus Corrugata) are included in the genus. We hope that this taxonomic change may facilitate future work on the non-pathogenic species that are retained in Rosellinia. Even though the present study does not provide any direct evidence that the “beneficial” compound PF1022 A actually can play a role in the protection of the host plants by the saprotrophic/endophytic producers, there are only few reports on potential, weak pathogenicity of the species that were not transferred to Dematophora.
Fig. 8.
Asexual and sexual morph structures of different Dematophora species. A, B, F, O. D. necatrix (Hladki 4004-LIL). C-E, G, H, L–N, P. D. paraguayensis (Sir & Hladki 1098-LIL). I–K. D. arcuata (Sir & Hladki 1098-LIL). A, B. Stromata on substrate. D, C. Stromata and subiculum (arrows). E. Stromata and synnemata (arrows) on substrate. F. Short stipitate stroma in cross section. G. Synnema on substrate. H, I. Synnemata in 3 % KOH solution. J. Details of conidiogenous region. K. Detail of conidiogenous cell. L. Asci in Melzer’s reagent. M. Ascus apical plugs in 3 % KOH. N. Ascus apical plug in Melzer's reagent. O, P. Ascospores showing short and central germ slit in 3 % KOH (arrows). Scale bars: A = 5 mm; B, C = 2 mm; D, E = 1 mm; F, G = 500 μm; H, I = 200 μm; J, L = 50 μm; K, M, N, O, P = 10 μm.
Dematophora R. Hartig, Untersuch. Forstbot. Inst. München 3: 95, 125. 1883. Fig. 7.
Type species: Dematophora necatrix R. Hartig, Untersuch. Forstbot. Inst. München 3: 126. 1883.
Typus: Germany, Bavaria, Munich, R. Hartig (FOMU-LectotypefidePetrini 2013).
Emended generic description: Differs from Rosellinia as defined further above in the presence of characteristic synnemata of the Dematophora type, while sharing a similar morphology of stromata, asci and ascospores. The asexual morph is geniculosporium-like. Nodulisporium-like conidiogenous structures are not observed.
Notes: The genus comprises the following species (below), all of which were previously retained in Rosellinia by Petrini (2013) and are reported to possess a dematophora-like asexual morph.
Dematophora acutispora (Theiss.) C. Lambert, K. Wittstein & M. Stadler, comb. et stat. nov. MycoBank MB827530.
Basionym: Rosellinia desmazieri var. acutispora Theiss., Annls mycol. 6(4): 350. 1908.
Synonym: Rosellinia acutispora (Theiss.) L.E. Petrini, Index Fungorum 25: 1. 2013.
Dematophora arcuata (Petch) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827529.
Basionym: Rosellinia arcuata Petch, Ann. Roy. Bot. Gard. (Peradeniya) 6 (1): 175. 1916.
Dematophora asperata (Massee ex Wakef.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827531.
Basionym: Rosellinia asperata Massee ex Wakef., Bull. Misc. Inf., Kew: 209. 1918.
Dematophora beccariana (Ces.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827532.
Basionym: Rosellinia beccariana Ces., Accad. Sci. Fis. Napoli 5 (21): 12. 1872.
Dematophora boedijnii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827533.
Basionym: Rosellinia boedijnii L.E. Petrini, Index Fungorum 25: 1. 2013.
Dematophora bothrina (Berk. & Broome) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827534.
Basionym: Sphaeria bothrina Berk. & Broome, J. Linn. Soc., Bot. 14: 125. 1875.
Synonym: Rosellinia bothrina (Berk. & Broome) Sacc., Syll. Fung. 1: 257. 1882.
Dematophora bunodes (Berk. & Broome) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827535.
Basionym: Sphaeria bunodes Berk. & Broome, J. Linn. Soc., Bot. 14 (74): 125. 1873.
Synonyms: Hypoxylon bunodes (Berk. & Broome) P.M.D. Martin, S. African J. Bot. 42 (1): 72. 1976.
Rosellinia bunodes (Berk. & Broome) Sacc., Syll. Fung. 1: 254. 1882.
Rosellinia echina Massee, Bull. Misc. Inform. Kew: 155. 1901.
Rosellinia zingiberis F. Stevens & Atienza Philipp. Agric. 20 (3): 174. 1931.
Dematophora buxi (Fabre) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827546.
Basionym: Rosellinia buxi Fabre, Ann. Sci. Nat., Bot., sér. 6, 9: 78. 1879.
Dematophora compacta (Takemoto) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827536.
Basionym: Rosellinia compacta Takemoto, Mycologia 101 (1): 89. 2009.
Dematophora francisiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827547.
Basionym: Rosellinia francisiae L.E. Petrini, Index Fungorum 25: 2. 2013.
Dematophora freycinetiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827537.
Basionym: Rosellinia freycinetiae L.E. Petrini, New Zealand J. Bot. 41(1): 98. 2003.
Dematophora gigantea (Ellis & Everh.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827538.
Basionym: Rosellinia gigantea Ellis & Everh., Bull. Lab. Nat. Hist. Iowa State Univ. 2: 401. 1893.
Synonym: Hypoxylon giganteum (Ellis & Everh.) P.M.D. Martin, Jl S. African J. Bot. 42(1): 72. 1976.
Dematophora grantii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827539.
Basionym: Rosellinia grantii L.E. Petrini, Index Fungorum 25: 2. 2013.
Dematophora hsiehiae (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827548.
Basionym: Rosellinia hsiehiae L.E. Petrini, Index Fungorum 25: 3. 2013.
Dematophora hughesii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827549.
Basionym: Rosellinia hughesii L.E. Petrini, New Zealand J. Bot. 41 (1): 102. 2003.
Dematophora javaensis (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827550.
Basionym: Rosellinia javaensis L.E. Petrini, Index Fungorum 25: 3. 2013.
Dematophora macdonaldii (Bres.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827551.
Basionym: Rosellinia macdonaldii Bres. [as 'macdonaldi'], Stud. Trent. 7 (1): 66. 1926.
Dematophora obregonii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827540.
Basionym: Rosellinia obregonii L.E. Petrini, Index Fungorum 25: 3. 2013.
Dematophora obtusiostiolata (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB82754.
Basionym: Rosellinia obtusiostiolata L.E. Petrini, Index Fungorum 25: 4. 2013.
Dematophora paraguayensis (Starbäck) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827542.
Basionym: Rosellinia paraguayensis Starbäck, Ark. Bot. 2(5): 15. 1904.
Dematophora pepo (Pat.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827543.
Basionym: Rosellinia pepo Pat., Bull. Trimestriel Soc. Mycol. France 24 (1): 9. 1908.
Dematophora puiggarii (Pat.) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827544.
Basionym: Rosellinia puiggarii Pat., J. Bot. (Morot) 2: 217. 1888.
Dematophora pyramidalis (Lar.N. Vassiljeva) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827553.
Basionym: Rosellinia pyramidalis Lar.N. Vassiljeva, Nizshie Rasteniya, Griby i Mokhoobraznye Dalnego Vostoka Rossii, Griby. Tom 4. Pirenomitsety i Lokuloaskomitsety 202. 1998.
Dematophora samuelsii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827552.
Basionym: Rosellinia samuelsii L.E. Petrini, New Zealand J. Bot. 41 (1): 124. 2003.
Dematophora siggersii (L.E. Petrini) C. Lambert, K. Wittstein & M. Stadler, comb. nov. MycoBank MB827545.
Basionym: Rosellinia siggersii L.E. Petrini, Index Fungorum 25: 4. 2013.
Discussion
The secondary metabolite production of the genera Rosellinia and Dematophora is poorly investigated. So far, there are only a few reports dealing with the metabolites, including cytochalasins from D. necatrix (Chen, 1960, Chen, 1964, Kimura et al., 1989, Shimizu et al. 2018), cytochalasins from R. sanctae-cruciana (Sharma et al. 2018), sordarin and xylarin from various Rosellinia species (Vicente et al. 2009) and the producer strain of the cyclodepsipeptides “PF1022”, which was tentatively assigned to the genus Rosellinia (Sasaki et al. 1992). During our chemical analyses of the submerged cultures mainly isopimarene diterpenoids were detected and isolated, in particular from Dematophora species. But since these compounds are known to be produced by different ascomycetes, especially from Xylariales (Helaly et al. 2018), and were also detected in extracts of the Rosellinia species, they cannot be considered as specific (marker metabolites) for the genus Dematophora. All previously reported diterpenoids (3-11) were isolated from fungi of the order Xylariales or Hypocreales, but none of these metabolites have been reported from species of the genus Rosellinia (or Dematophora) before. Although compounds (1–3, 5 and 7) showed weak effects on Gram-positive bacterial strains and M. hiemalis, no significant antimicrobial activity could be observed for any of the isolated isopimarane diterpenoids. Some of them (1, 3, 5) displayed moderate cytotoxic effects on different cell lines. They remain to be tested for phytotoxic activities in order to evaluate possible natural functions as they were predominant in the extracts of the pathogenic D. bunodes and D. pepo. However, many compounds detected in the extracts of the submerged cultivations are not yet clearly identified. Further isolation campaigns, along with the modification of culture media, and in particular the inclusion of additional species will shed further light on the true potential of these ascomycetes to produce interesting metabolites, which might be also useful for the taxonomic classification. Cyclodepsipeptide PF1022 A (12), which is known for its strong nematicidal activity (Conder et al. 1995) was detected in different extracts of R. corticium (and Astrocystis mirabilis), but in some of them titres were very low (Fig. 6). A patent application by Harder et al. (2011) had already indicated that the genera Rosellinia (in particular R. aquila and R. corticium) and Coniolariella hispanica were producer organisms of PF1022 A. However, the data were derived from strains that are unavailable in public collections, and the corresponding stromata were likewise not identified by experts or deposited in a public herbarium. Furthermore, the compound was only detected by HPLC-MS. We were unable to find a corresponding publication in a peer-reviewed journal. The patent application mentioned that two strains of R. abscondita from the CBS collection were also examined for comparison, but no data were presented suggesting that those strains produced the cyclodepsipeptides. Another patent application by Harder et al. (2012) treated the isolation and identification of novel PF1022 derivatives and mentioned that various strains, including R. abscondita (strains CBS 447.89, CBS 448.89 and CBS 450.89), “R. britannica” (current valid name: R. marcucciana, strain CBS 446.89) and R. mammaeformis (strain CBS 445.89), R. nectrioides (strain CBS 449.89, all the former resulting from the study of Petrini 1992) were studied, as well as R. millegrana (strain CBS 111.75), and even strains of D. necatrix and Xylaria hypoxylon. The patent application does, however, not indicate which, if any, of the organisms studied actually yielded the known and new metabolites. In addition, even though semi-preparative fractionations of the crude extracts were described, the new compounds were only identified tentatively by their HPLC-MS characteristics and not isolated to purity. The authors also reported on “lines” they grew from the CBS cultures, which were able to overproduce the compounds (presumably by some means of classical strain optimisation), but did not state from which of the strains these “lines” were derived. Evidently, the patent application was filed in order to protect the new intellectual property on the newly detected cyclodepsipeptides, rather than to disclose information on their distribution. However confusing these data have been reported, the work gives corroborating evidence that PF1022 derivatives and other cyclodepsipeptides are widespread in Rosellinia. In any case, our present study is the first one in which PF1022 A has been isolated to purity from an ascospore-derived isolate that is derived from a taxonomically well-defined specimen and characterised by NMR spectroscopy. Nematicidal activity was observed for extracts of several Rosellinia species even though PF1022 A could not be detected in all of them or just in very small amounts, which indicated that PF1022 A is only one active principle in the produced metabolite mixtures. Other components, including derivatives described by Harder et al. (2012) may also contribute to the anthelmintic activity of extracts from these species.
Acknowledgements
This project received funding from the European Union’s Horizon 2020 research and innovation programme (RISE) under the Marie Skłodowska-Curie grant agreement No. 645701, project acronym “GoMyTri” (beneficiaries Kathrin Wittstein, Lucile Wendt and Marc Stadler. We cordially thank Silke Reinecke for technical assistance in the purification and analytical measurements of compounds, Cäcilia Bergmann and Aileen Gollasch for LC-MS measurements, Anke Skiba, Vanessa Stiller and Simone Heitkämper for the maintenance of cultures, Wera Collisi for conducting bioassays and Christel Kakoschke for NMR spectroscopic measurements. Furthermore, we thank AnalytiCon Discovery for providing a fermentation sample of the original PF1022 producer strain for comparisons. We thank Jacques Fournier, J. Gaborit and Margitta Schönfeld for the collection and/or morphological identification of specimens.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.simyco.2020.01.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Aldridge D.C., Burrows B.F., Turner W.B. The structures of the fungal metabolites cytochalasin E and F. Journal of the Chemical Society - Chemical Communications. 1972;3:148–149. [Google Scholar]
- Chen Y.S. Studies on the metabolic products of Rosellinia necatrix Berlese. Bulletin of the Agricultural Chemical Society Japan. 1960;24:372–381. [Google Scholar]
- Chen Y.S. The metabolic products of Rosellinia necatrix. II. The structure of rosellinic acid. Bulletin of the Agricultural Chemical Japan. 1964;28:431–435. [Google Scholar]
- Conder G.A., Johnson S.S., Nowakowski D.S., et al. Anthelmintic profile of the cyclodepsipeptide PF1022A in in vitro and in vivo models. Journal of Antibiotics. 1995;48:20–823. doi: 10.7164/antibiotics.48.820. [DOI] [PubMed] [Google Scholar]
- Daranagama D.A., Camporesi E., Tian Q., et al. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Diversity. 2015;73:203–238. [Google Scholar]
- Daranagama D.A., Hyde K.D., Sir E.B., et al. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Diversity. 2018;88:1–165. [Google Scholar]
- De Notaris G. Cenni sulla tribù dei Pirenomiceti sferiacei e descrizione di alcuni generi spettanti alla medesima. Giornale Botanico Italiano. 1844;1:322–355. [Google Scholar]
- Guindon S., Dufayard J.F., Lefort V., et al. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Harder A., Krieger K., Pham T.L.H., et al. 2012. Novel 24- membered cyclooctadepsipeptides from fungal strains and their use as anthelmintics or endoparasiticides. US Patent Application US 20120302496. [Google Scholar]
- Harder A., Pham T.L., Jarling R., et al. 2011. Method for producing optically active, cyclic depsipeptides comprising lactic acid and phenyl lactic and having 24 ring atoms, using fungus strains of Rosellinia type, and further species of Xylariaceae. US Patent Application US 13/133,611. [Google Scholar]
- Hartig R. Rhizomorpha (Dermatophora) necatrix n. sp. Untersuchungen aus dem Forstbotanischen Institut zu München. 1883;3:95–140. [Google Scholar]
- Helaly S.E., Thongbai B., Stadler M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Natural Products Report. 2018;35:992–1014. doi: 10.1039/c8np00010g. [DOI] [PubMed] [Google Scholar]
- Hsieh H.M., Lin C.R., Fang M.J., et al. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Molecular Phylogenetics and Evolution. 2010;54:957–969. doi: 10.1016/j.ympev.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hsu Y.M., Nakagawa M., Hirota A., et al. Structure of myrocin B, a new diterpene antibiotic produced by Myrothecium verrucaria. Agricultural and Biological Chemistry. 1988;52:1305–1307. [Google Scholar]
- Jossang A., Mbeminack B., Pinon J., et al. Hymatoxins K and L, novel phytotoxins from Hypoxylon mammatum, fungal pathogen of aspens. Natural Product Letters. 1995;6:37–42. [Google Scholar]
- Ju Y.M., Rogers J.D. Astrocystis reconsidered. Mycologia. 1990;82:342–349. [Google Scholar]
- Ju Y.M., Rogers J.D. APS Press; St. Paul: 1996. A revision of the genus Hypoxylon. Mycologia Memoir n° 20. 365 pp. [Google Scholar]
- Kearse M., Moir R., Wilson A., et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kildgaard S., Subko K., Phillips E., et al. A Dereplication and Bioguided Discovery Approach to Reveal New Compounds from a Marine-Derived Fungus Stilbella fimetaria. Marine Drugs. 2017;15:253. doi: 10.3390/md15080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Nakajima H., Hamasaki T. Structure of rosellichalasin, a new metabolite produced by Rosellinia necatrix. Agricultrual and Biological Chemistry. 1989;53:1699–1701. [Google Scholar]
- Koukol O., Kelnarová I., Černý K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. Forest Pathology. 2015;45:21–27. [Google Scholar]
- Kuephadungphan W., Helaly S.E., Daengrot C., et al. Akanthopyrones A-D, α-pyrones bearing a 4-O-methyl-β-D-glucopyranose moiety from the spider-associated ascomycete Akanthomyces novoguineensis. Molecules. 2017;22:1202. doi: 10.3390/molecules22071202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert E., Surup F., Sir E.B., et al. Lenormandins A - G, new azaphilones from Hypoxylon lenormandii and Hypoxylon jaklitschii sp. nov., recognised by chemotaxonomic data. Fungal Diversity. 2015;71:165–184. [Google Scholar]
- Lambert C., Wendt L., Hladki A.I., et al. Hypomontagnella (Hypoxylaceae): a new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycological Progress. 2019;18:187–201. [Google Scholar]
- Lefort V., Longueville J.E., Gascuel O. SMS: Smart model selection in PhyML. Molecular Biology and Evolution. 2017;34:2422–2424. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr N.A., Meffert A., Antelo L., et al. Antiamoebins, myrocinB and the basis of antifungal antibiosis in the coprophilous fungus Stilbella erythrocephala (syn.S. fimetaria) FEMS Microbiology Ecology. 2006;55:105–112. doi: 10.1111/j.1574-6941.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- Oh D.-C., Jensen P.R., Kauffman C.A., et al. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorganic & Medicinal Chemistry. 2005;13:5267–5273. doi: 10.1016/j.bmc.2005.05.068. [DOI] [PubMed] [Google Scholar]
- Peláez F., González V., Platas G., et al. Molecular phylogenetic studies within the family Xylariaceae based on ribosomal DNA sequences. Fungal Diversity. 2008;31:111–134. [Google Scholar]
- Petrini L.E. Rosellinia species of the temperate zones. Sydowia. 1992;44:169–281. [Google Scholar]
- Petrini L.E. Rosellinia and related genera in New Zealand. New Zealand Journal of Botany. 2003;41:71138. [Google Scholar]
- Petrini L.E. Rosellinia - a world monograph. Bibliotheca Mycologica. 2013;205 Stuttgart: J. Cramer. [Google Scholar]
- Petrini L.E., Petrini O. Morphological studies in Rosellinia (Xylariaceae): the first step towards a polyphasic taxonomy. Mycological Research. 2005;109:569580. doi: 10.1017/s0953756205002510. [DOI] [PubMed] [Google Scholar]
- Rupcic Z., Chepkirui C., Hernández-Restrepo M., et al. New nematicidal and antimicrobial secondary metabolites from a new species in the new genus, Pseudobambusicola thailandica. Mycokeys. 2018;33:1–23. doi: 10.3897/mycokeys.33.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupcic Z., Rascher M., Kanaki S., et al. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. International Journal of Molecular Sciences. 2018;19:740. doi: 10.3390/ijms19030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Takagi M., Yaguchi T., et al. A new anthelmintic cyclodepsipeptide, PF1022A. Journal of Antibiotics. 1992;45:692–697. doi: 10.7164/antibiotics.45.692. [and Erratum (1996) in Journal of Antibiotics 49: C-2] [DOI] [PubMed] [Google Scholar]
- Senanayake I.C., Maharachchikumbura S.S.N., Hyde K.D., et al. Towards unravelling relationships in Xylariomycetidae (Sordariomycetes) Fungal Diversity. 2015;73:73–144. [Google Scholar]
- Sharma N., Kushwaha M., Arora D., et al. New cytochalasin from Rosellinia sanctae-cruciana, an endophytic fungus of Albizia lebbeck. Journal of Applied Microbiology. 2018;125:111–120. doi: 10.1111/jam.13764. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kanematsu S., Yaegashi H. Draft Genome Sequence and Transcriptional Analysis of Rosellinia necatrix Infected with a Virulent Mycovirus. Phytopathology. 2018;108:1206–1211. doi: 10.1094/PHYTO-11-17-0365-R. [DOI] [PubMed] [Google Scholar]
- Shiono Y., Matsui N., Imaizumi T., et al. An unusual spirocyclic isopimarane diterpenoid and other isopimarane diterpenoids from fruiting bodies of Xylaria polymorpha. Phytochemistry Letters. 2013;6:439–443. [Google Scholar]
- Shiono Y., Motoki S., Koseki T., et al. Isopimarane diterpene glycosides, apoptosis inducers, obtained from fruiting bodies of the ascomycete Xylaria polymorpha. Phytochemistry. 2009;70:935–939. doi: 10.1016/j.phytochem.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Sir E.B., Lambert C., Wendt L., et al. A new species of Daldinia (Xylariaceae) from the Argentine subtropical montane forest. Mycosphere. 2016;7:596–614. [Google Scholar]
- Scherkenbeck J., Jeschke P., Harder A. PF1022A and related cyclodepsipeptides-a novel class of anthelmintics. Current Topics in Medicinal Chemistry. 2002;2:759–777. doi: 10.2174/1568026023393624. [DOI] [PubMed] [Google Scholar]
- Stadler M., Kuhnert E., Peršoh D., et al. The Xylariaceae as model example for a unified nomenclature following the “One Fungus- One Name” (1F1N) Concept. Mycology: An International Journal on Fungal Biology. 2013;4:5–21. [Google Scholar]
- Stadler M., Læssøe T., Fournier J., et al. A polyphasic taxonomy of Daldinia (Xylariaceae) Studies in Mycology. 2014;77:1–143. doi: 10.3114/sim0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surup F., Kuhnert E., Böhm A., et al. The rickiols, 20-, 22-, and 24-membered macrolides from the ascomycete Hypoxylon rickii. Chemistry - An European Journal. 2018;24:2200–2213. doi: 10.1002/chem.201704928. [DOI] [PubMed] [Google Scholar]
- Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- U’Ren J., Lutzoni F., Miadlikowska J., et al. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Mycology. 2012;99:898–914. doi: 10.3732/ajb.1100459. [DOI] [PubMed] [Google Scholar]
- U’Ren J., Miadlikowska J., Zimmerman N.B., et al. Contributions of North American endophytes to the phylogeny, ecology and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota) Molecular Phylogenetics and Evolution. 2016;98:210–232. doi: 10.1016/j.ympev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Vicente F., Basilio A., Platas G., et al. Distribution of the antifungal agents sordarins across filamentous fungi. Mycological Research. 2009;113:754–770. doi: 10.1016/j.mycres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Wang G.J., Liang W.L., Ju Y.M., et al. Inhibitory effects of terpenoids from the fermented broth of the ascomycete Stilbohypoxylon elaeicola YMJ173 on nitric oxide production in RAW264.7 macrophages. Chemistry and Biodiversity. 2012;9:131–138. doi: 10.1002/cbdv.201100025. [DOI] [PubMed] [Google Scholar]
- Wang X., Yu H., Zhang Y., et al. Bioactive pimarane-type diterpenes from marine Organisms. Chemistry and Biodiversity. 2018;15 doi: 10.1002/cbdv.201700276. [DOI] [PubMed] [Google Scholar]
- Wendt L., Sir E.B., Kuhnert E., et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycological Progress. 2018;17:115–154. [Google Scholar]
- Yu H.B., Wang X.L., Zhang Y.X., et al. Libertellenones O-S and Eutypellenones A and B, Pimarane diterpene derivatives from the arctic fungus Eutypella sp. D-1. Journal of Natural Products. 2018;81:1553–1560. doi: 10.1021/acs.jnatprod.8b00039. [DOI] [PubMed] [Google Scholar]
- Zhang N., Castlebury L., Castlebury M., et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia. 2006;98:1076–1087. doi: 10.3852/mycologia.98.6.1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.