Abstract
This study involves a detailed investigation about the effect of three elicitors, such as chitosan, jasmonic acid and salicylic acid (SA) on withaferin A and withanolide A contents of Withania somnifera (L.) Dunal (Poshita variety). Moreover, the different environmental regimes were also studied to assess and optimise the accumulation of withaferin A and Withanolide A contents. In an open environment, the total withaferin A content was found to be increased 6.3 and 5.8 times when sprayed with chitosan, 10 ppm and 50 ppm, respectively, as compared to control. Similarly, the total withanolide A content was found to be increased 4.5 and 3.6 times when sprayed with jasmonic acid (400 ppm and 200 ppm, respectively) with respect to control. In a controlled condition, the total withaferin A content was found to be increased 6 and 4.5 times when sprayed with jasmonic acid (400 ppm and 200 ppm, respectively) as compared to control. On the other hand, the total withanolide A content was found to be enhanced by 7 and 4.3 times when sprayed with jasmonic acid (400 ppm) and SA (1 ppm), respectively, as compared to control. Therefore, this study was focussed on the optimisation of enhanced accumulation of withaferin A and withanolide A contents in the aerial parts of the plant in open and controlled environment by foliar application of elicitors in minimal concentrations.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2153-2) contains supplementary material, which is available to authorized users.
Keywords: Withania somnifera, Withaferin A, Withanolide A, Elicitors, Salicylic acid, Jasmonic acid, Chitosan, Foliar spray
Introduction
Since the age of Ayurveda, the extraction and development of medicinal compounds from plants have been a traditional medicinal practice, where some compounds show the direct relationship between a local and biomedical use for curing diseases (Heinrich and Gibbons 2001). On an average, around 35,000–70,000 plant species are being used worldwide for health and medicinal purposes (Farnsworth et al. 1985).
Plants produce bioactive compounds in varied form of natural products, which are known as secondary metabolites that protect them from environmental stresses. These secondary metabolites are distributed in the plant kingdom under different taxonomic and chemotypic groups. Unlike primary metabolites, such as amino acids, nucleotides, lipids, etc., phytosterols and organic acids are the biosynthetic products known as plant secondary metabolites, which are restricted to few plant species (Collin 2001; Hussain et al. 2012).
Withania somnifera (WS) is a highly revered and widely used medicinal plant of Ayurveda. This is one of the few selected important medicinal plant, which is in great demand in the national and international market (Hassannia et al. 2019). WS has been recognised as the medicinal plant in the manufacturing of more than 100 traditional medicinal herbal formulations; hence, this plant is highly valued in pharma industries (Jain et al. 2012). The leaf and root extract of this plant were reported to contain various secondary metabolites, which have been used as medicines in different diseases, such as cancer, neurological and immune disorders with their minimal side effects (Mishra et al. 2000; Gupta and Rana 2007; Lele 2010). Among the secondary metabolites, different forms of withanolides are found in different parts of the plant (Singh et al. 2017; Pal et al. 2017). Among withanolides, withaferin A and withanolide A were quantified from the plant methanolic extracts using reverse-phase HPLC (Jain et al. 2011). Earlier we have reported that among withanolides, withaferin A and withanolide A were dominant metabolites with varietal distribution among different tissues of the plant in various concentrations (Singh et al. 2018). Researchers showed that phytoconstituents profile of the plant is mainly affected by biotic and abiotic factors. These factors modulate the defence mechanism of the plant by modulating the secondary metabolites through biosynthetic genes (Hugueney et al. 1996; Zulak et al. 2007). Elicitation is an efficient tool which induces the plant to synthesize beneficial phytochemical (Kim et al. 2007) as well as nutraceuticals (Zhao et al. 2002). Several factors, such as concentration, type of elicitor, growth stages, etc., are responsible for the enhancement of secondary metabolites. It has been reported by other researchers that the molecules inducing the production of secondary metabolites to ensure their survival in plants are known as “elicitors” (Namdeo 2007). For example, chitosan has also been reported to elicit the production of various secondary metabolites in the in vitro cell cultures (Ferri and Tassoni 2011).
Researchers had already reported about the bio-efficacy of elicitors, such as signalling molecules, methyl jasmonate (MeJA) and salicylic acid (SA) in enriching the phytoconstituents in seasonal and perennial plants (Puthusseri et al. 2012; Saini et al. 2013, 2014). The exogenous addition of elicitors as biotic and abiotic stresses was considered as promising method for the enhanced production of secondary metabolites (Radman et al. 2003). On the basis of the pharmaceutical significance of the withanolides, the tissue wide assessment and distribution of withanolides contents of the plant (Ashwagandha), Poshita (variety) are described in this study. Effect of foliar applications of elicitors on withaferin A and withanolide A contents in two environment conditions—open and controlled—are also shown in this study.
The objectives of this study were to assess the efficacies of foliar administration of selective elicitors or signalling molecules. Three different elicitors—chitosan, jasmonic acid and SA in different concentrations—were used for foliar spray. The elicitors effect was studied in two environmental regimes, that is, open and controlled conditions. Withaferin A and withanolide A contents were assessed by their quantification through HPLC to substantiate the improvement in accumulation of withanolides content in the leaf, stem and roots.
Materials and methods
The pot experiment was conducted on 1.5-year old WS (variety-Poshita). The plants were purchased from CIMAP, Pantnagar. These were grown in two different environmental regimes: one in open environment, that is, in mango garden, Dept. of Plant Physiology, G.B. Pant University of Agriculture and Technology and another in controlled environment, that is, transgenic polyhouse (light intensity of 600 µE/m2/s and a temperature of 20 °C) Dept. of Molecular Biology and Genetic Engineering, G.B. Pant University of Agriculture and Technology during March 2015 (at 25 °C). The plants were potted in autoclaved soil mixed with vermicompost. The plants were regularly watered and observed and the readings were recorded. The withaferin A and withanolide A contents were analysed in the leaf, stem and roots and their quantification was done through HPLC to substantiate the improvement in accumulation of withanolides.
Elicitor application
The experiment was set up to see the effect of elicitors in two conditions—open and controlled on the withaferin A and withanolide A contents of the plant. Three consecutive foliar applications of the different elicitors were done at regular interval of 15 days, and then after 6 months the plants were harvested. Freshly prepared different elicitors in varying concentrations were used for the experiment were chitosan (10 ppm, 50 ppm and 100 ppm); jasmonic Acid (50 ppm, 200 ppm and 400 ppm) and SA (0.5 ppm, 1 ppm and 2 ppm). The foliar application of the elicitors was done with the help of graduated atomizer or sprayer (5 ml) during the morning at 10:00 am every day. Every plant was sprayed with 5 ml of different elicitors in varying concentrations carefully. The experiment was performed as completely randomized design with three replicates of each set.
Preparation of standards
Standards of Withanolide A and Withaferin A were purchased from Natural Remedies, Bangalore. Stock solution of both withanolide (1 mg/ml) and withaferin A (1 mg/ml) was prepared with HPLC-grade methanol and kept in the refrigerator at − 20 °C. From the stock solution, working solution was prepared by dilution.
Preparation of solvent: Methanol and water were mixed in the appropriate ratio (70:30) and filtered through nylon filter membranes (0.45 μ) with the help of a vacuum pump. Then the solvent was subjected to water sonication for 10 min for the removal of air bubbles (Singh et al. 2018).
Quantification of withanolide A and withaferin A
For the quantification of withaferin A and withanolide A contents using HPLC method, we have followed the protocol suggested by Ganzera et al. (2003). The withanolide fractions were analysed using HPLC (Agilent 1120 Compact LC) equipped with C18, 5 μm (ODS34.6 × 250 mm) column. The mobile phase was a mixture of methanol and water (70:30, v/v) at a flow rate of 1 ml/min and column temperature was maintained at 30 °C. The detection wavelength was set at 254 nm. The chromatography system was equilibrated by the mobile phase. A total of 10 μl of sample with the help of Hamilton syringe was injected into the injection port of HPLC equipment. The sample was allowed to run for the pre-set run time. The retention time and peak area of the peak of interest were observed. With the help of the standard curve, the quantification of withanolides in the samples was done (Singh et al. 2018). The metabolite contents were expressed as mg/DW (mg/dry weight).
Results
The applications of different elicitors in different concentrations chitosan, jasmonic acid and SA were used for the foliar application to analyse the effect of each elicitor on the withanolide A and withaferin A contents of WS.
The HPLC peak profile obtained after foliar application of elicitor was compared with the peak profiles of standard withaferin A and withanolide A to assess the withanolides, A/withaferin A contents among the different tissues of WS (Poshita variety) in the open and controlled environment (as shown in supplementary file).
In open environment, the withaferin A content was found to be highest in the leaf, that is, 1.245 mg/g DW (dry weight) when sprayed with 50 ppm chitosan with respect to control, that is, 0.213 mg/g (DW). It was followed by root, that is, 1.108 mg/g (DW) with respect to control of 0.103 mg/g (DW) when sprayed with 10 ppm chitosan. On the other hand, withaferin A content was found to be least in stem, that is, 0.047 mg/g (DW) when sprayed with 0.5 ppm SA with respect to control, that is, 0.041 mg/g (DW) (Fig. 1; Table 1 in supplementary file). In controlled environment, the withaferin A content was found to be highest in leaf, that is, 1.785 mg/g (DW) with respect to control, that is, 0.105 mg/g (DW) when sprayed with 400 ppm jasmonic acid. It was followed by leaf, that is, 1.642 mg/g (DW) with respect to control, that is, 0.105 mg/g (DW) when sprayed with 200 ppm of jasmonic acid. On the other hand, it was found to be least in leaf, that is, 0.062 mg/g (DW) when sprayed with 50 ppm chitosan as compared to control, that is, 0.105 mg/g (DW) (Fig. 2; Table 2 in the supplementary file).
Fig. 1.
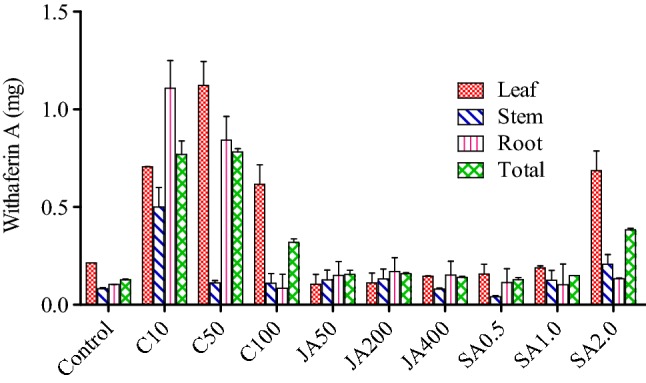
Effect of elicitors (viz., chitosan, jasmonic acid and salicylic acid) on different tissues (viz., leaf, stem, and root on Withaferin A contents in WS in open environment). *Total is the average of three tissues (leaf, stem and root); C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
Fig. 2.
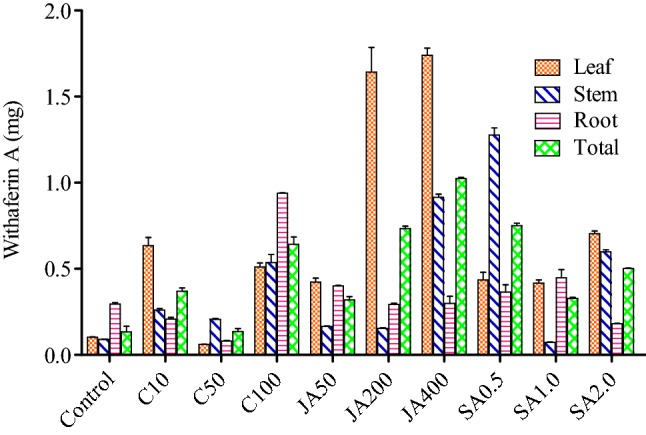
Effect of elicitors (viz., chitosan, jasmonic acid and salicyclic acid) on different tissues (viz., leaf, stem, and root on Withaferin A contents in WS in controlled environment). *Total is the average of three tissues (leaf, stem and root); C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
In open environment, withanolide A content was found to be highest in root, that is, 0.862 mg/g (DW) when sprayed with 400 ppm jasmonic acid with respect to control (0.054 mg/g (DW). It was followed by stem, that is, 0.365 mg/g (DW) when sprayed with 200 ppm jasmonic acid with respect to control, that is, 0.084 mg/g (DW). On the other hand, it was found to be least in stem, that is, 0.027 mg/g (DW) when sprayed with 50 ppm jasmonic acid with respect to control, that is, 0.084 mg/g (DW) (Fig. 3; Table 3 in the supplementary file). Furthermore, in controlled environment, withanolide A content was found to be highest in leaf, that is, 0.665 mg/g (DW) when sprayed with 400 ppm jasmonic acid with respect to control, that is, 0.026 mg/g (DW). It was followed by leaf, that is, 0.593 mg/g (DW) when sprayed with 1 ppm SA with respect to control, that is, 0.026 mg/g (DW). Moreover, it was found to be least in stem, that is, 0.030 mg/g (DW) when sprayed with 200 ppm jasmonic acid with respect to control, that is, 0.155 mg/g (DW) (Fig. 4; Table 4 in supplementary file).
Fig. 3.
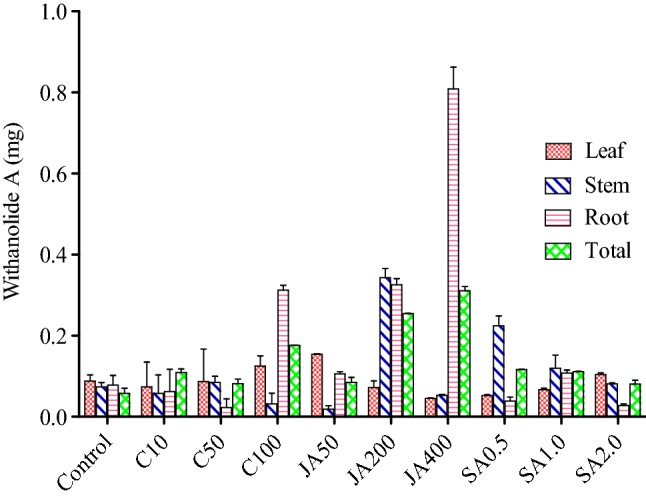
Effect of three elicitors (viz., chitosan, jasmonic acid and salicyclic acid) on different tissues (viz., leaf, stem, and root on Withanolide A contents in WS in open environment). *Total is the average of three tissues (leaf, stem and root); C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
Fig. 4.
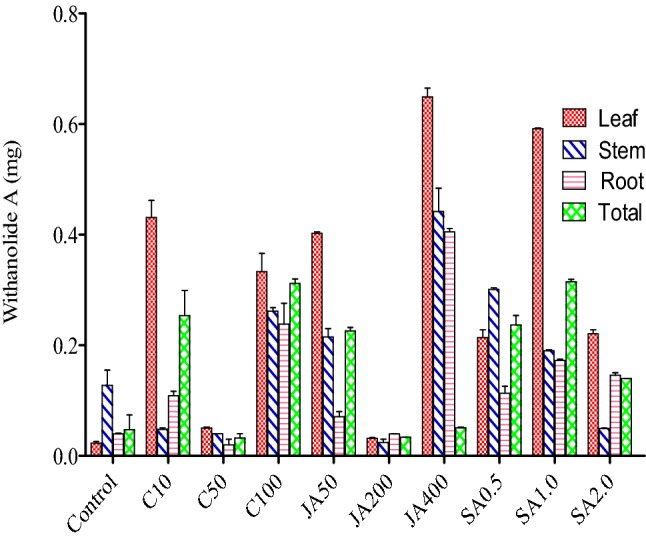
Effect of three elicitors (viz., chitosan, jasmonic acid and salicyclic acid) on different tissues (viz., leaf, stem, and root on Withanolide A contents in WS in controlled environment). *Total is the average of three tissues (leaf, stem and root); C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
The total withaferin A, in open environment was found to be highest with 0.838 mg/g (DW) when sprayed with chitosan (10 ppm), which was followed by 0.766 mg/g (DW) when sprayed with chitosan (50 ppm) with respect to control, that is, 0.131 mg/g (DW) and this resulted in 6.3 fold and 5.8 fold, respectively, higher than control (Fig. 1). Similarly, the highest withanolide A content, that is, 0.321 mg/g (DW) was recorded, when WS was sprayed with Jasmonic acid (400 ppm), followed by 0.253 mg/g when sprayed with jasmonic acid (200 ppm) with respect to control, that is, 0.070 mg/g (DW) and this resulted in 4.5 fold and 3.6 fold, respectively, higher than the control (Fig. 3). In controlled condition, the highest withaferin A contents, that is, 1.018 mg/g (DW) (Fig. 2) was recorded, when WS was sprayed with jasmonic acid (400 ppm), which was followed by 0.747 mg/g when sprayed with jasmonic acid (200 ppm) and this resulted in sixfold and 4.5 fold, respectively, higher than the control. On the other hand, the highest withanolide A contents, that is, 0.52 mg/g (DW) was recorded when sprayed with jasmonic acid (400 ppm) and was followed by 0.319 mg/g (DW) when sprayed with SA (1 ppm) with respect to control, that is, 0.074 mg/g (DW) and this resulted in seven-fold and 4.3 fold, respectively, higher than control (Fig. 4).
The mentioned elicitors were also found to enhance agronomical traits of the plant (e.g., plant height and biomass) (fresh and dry weight) (Tables 1, 2). It was observed that the withaferin A content in controlled condition was highest, that is, 29.5 mg/plant (DW) when sprayed with 400 ppm jasmonic acid (Table 2). However, in an open environment, the highest yield of withaferin A was observed to be 23.4 mg/plant (DW) when sprayed chitosan (10 ppm) (Table 1). The highest yield of withanolide A, that is, 15 mg/plant (DW) was observed in a controlled environment when 400 ppm jasmonic acid was applied, followed by 10.5 mg/plant withanolide A when sprayed with 1 ppm of SA (Table 2). The maximum yield of withaferin A, that is, 29.5 mg/plant and withanolide A, that is, 15 mg/pant was obtained when sprayed with 400 ppm jasmonic acid in the controlled condition as compared to control, that is, 2.6 mg/plant for withaferin A and 1.1 mg/plant for withanolide A.
Table 1.
Effect of elicitors (viz., chitosan, jasmonic acid and salicyclic acid) on plant biomass and yield of withaferin A and withanolide A in WS in open environment
| Elicitor | Plant height (cm) | Plant biomass, fresh weight FW (g) | Plant biomass, dry weight DW (g) | Yield (withaferin A) (mg/plant) | Yield (withanolide A) (mg/plant) |
|---|---|---|---|---|---|
| Control | 35 ± 0.02a | 28 ± 0.84ab | 17 ± 0.36ab | 2.27a | 1.19a |
| C10 | 92 ± 0.25g | 42 ± 0.2 | 28 ± 0.6h | 23.47h | 3.304c |
| C50 | 90 ± 0.0g | 39 ± 0.1g | 22 ± 0.14g | 16.85g | 2.05ab |
| C100 | 91 ± 0.3gh | 41 ± 0.0gh | 26 ± 0.7gh | 8.762e | 4.602ab |
| JA50 | 94 ± 0.2h | 41 ± 0.5gh | 24 ± 0.9gh | 4.256d | 2.344a |
| JA200 | 78 ± 0.12f | 33 ± 0.6de | 20 ± 0.1de | 3.08e | 5.07a |
| JA400 | 57 ± 0.3d | 31 ± 0.7cd | 22 ± 0.08c | 3.19c | 7.06a |
| SA0.5 | 60 ± 0.8e | 34 ± 0.6e | 26 ± 0.2e | 3.62c | 3.042a |
| SA1.0 | 57 ± 0.2d | 29 ± 0.5bc | 18 ± 0.2b | 2.69b | 2.016a |
| SA2.0 | 40 ± 0.1b | 26 ± 0.0a | 15 ± 0.10a | 4.20d | 1.075ab |
| CD at 1% | 1.9 | 2.15 | 2.8 | 1.5 | 0.05 |
| CD at 5% | 1.4 | 1.54 | 2.0 | 1.10 | 0.03 |
| CV | 4.8 | 3.17 | 3.2 | 2.2 | 2.7 |
C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
Data shown are mean ± SEm (n = 3). The genotypes with same superscript, within each assay (parameter) are not significantly different at p ≤ 0.05, according to Duncan multiple comparison procedure (ANOVA)
Table 2.
Effect of elicitors (viz., chitosan, jasmonic acid and salicylic acid) on plant biomass and yield of withaferin A and withanolide A in WS in a controlled environment
| Elicitor | Plant height (cm) | Plant biomass FW (g) | Plant biomass DW (g) | Yield (withaferin A) (mg/plant) | Yield (withanolide A) (mg/plant) |
|---|---|---|---|---|---|
| Control | 35 ± 0.02d | 25 ± 0.6a | 16 ± 0.25a | 2.661b | 1.184b |
| C10 | 53 ± 0.3d | 38 ± 0.2e | 21 ± 0.2g | 8.176e | 4.375a |
| C50 | 66 ± 0.5d | 36 ± 0.1d | 23 ± 0.2 | 2.74ab | 0.935a |
| C100 | 94 ± 0.25b | 41 ± 0.1f | 28 ± 0.1g | 19.16g | 8.49d |
| JA50 | 80 ± 0.8a | 29 ± 0.5b | 13 ± 0.1b | 4.402h | 3.01gh |
| JA200 | 68 ± 0.4b | 38 ± 0.5e | 22 ± 0.8f | 16.44a | 0.836h |
| JA400 | 82 ± 0.1d | 42 ± 0.4f | 29 ± 0.7h | 29.53j | 15.08d |
| SA0.5 | 84 ± 0.2b | 38 ± 0.4e | 26 ± 0.5g | 19.11f | 5.70d |
| SA1.0 | 94 ± 0.2d | 41 ± 0.3f | 33 ± 0.5d | 11.04i | 10.549fg |
| SA2.0 | 101 ± 0.3c | 48 ± 0.2g | 28 ± 0.6e | 14.12d | 3.901e |
| CD at 1% | 2.1 | 2.4 | 2.41 | 2.14 | 1.07 |
| CD at 5% | 1.54 | 1.72 | 1.72 | 1.52 | 0.76 |
| CV | 3.988 | 5.19 | 5.34 | 4.61 | 3.46 |
C10, C50, C100 = concentration of chitosan at 10 ppm, 50 ppm and 100 ppm; JA50, JA200, JA400 = concentration of jasmonic acid at 50 ppm, 200 ppm and 400 ppm; SA0.5, SA1.0, SA2.0 = concentration of SA at 0.5 ppm, 1 ppm and 2 ppm
Data shown are mean ± SEm (n = 3). The genotypes with same superscript, within each assay (parameter), are not significantly different at p ≤ 0.05, according to Duncan multiple comparison procedure (ANOVA)
Discussion
In Ayurvedic system of medicine, WS is regarded as an important medicinal herb (Singh et al. 2011). 'Ashwagandha' is commonly known as 'Indian ginseng', (Kulkarni and Dhir 2008). Ashwagandha has attributed immense pharmacological research interest. According to the researchers, the plant is reported to have adaptogenic, anticancerous, immunomodulatory, antioxidative properties and in treating neurological disorders (Schliebs et al. 1997; Dhuley 2001; Naidu et al. 2003). The extract of WS was reported to prevent the skin carcinomaas induced by DMBA (dimethyl benzanthracene) and croton oil (Davis and Kuttan 2001). It was also reported to treat arthritis and neurological behavioural problems (Kulkarni and Ninan 1997). Ashwagandha is one of the most commonly used sedatives in India, similar to ginseng in China (Bown 1995). It has also been reported to have rejuvenating effect on the reproductive and nervous systems, and improves the vitality and helps in early recovery after an illness (Bown 1995; Chevallier 1996).
Secondary metabolites play vital role not only in the survival of the plant but also in curing the different ailments and diseases of human. Among the active metabolites, withaferin A and withanolide A are of tremendous importance as they have the potential of curing cancer, neurological, and gastro-intestinal disorders, etc. (Durg et al. 2015). It has also been reported that the content of withanolides varied with respect to leaves and roots. This variation can be due to differential genetic factors associated with the differential pathway for the production of secondary metabolites in the different tissues of the plant (Dhar et al. 2006). Thus to optimise the conditions for accumulation and enhanced production of withanolides, different strategies can be adopted such as tissue culture techniques, use of elicitors, genetic modification, modifying the biosynthetic pathway and signal transduction pathway, through different biotic and abiotic stress (drought and high temperatures), etc. (Verma et al. 2017). It has also been reported by researchers that the temperature plays an important role in maintaining the nutritive value of Azadirachta indica (Vats 2016). In another research, it has been demonstrated that ammonium suphate induces the production of withaferin A of potted plant in controlled condition as compared to the open field (Pal et al. 2017).
In our study, we have used three different elicitors in three different concentrations. The reason behind using three concentrations was to optimise elicitor and its concentration that accumulated maximum withanolide contents. Moreover, the two different environmental regimes—open field and closed (transgenic polyhouse)—were studied to see the effect of agro-climatic factors on the withanolide A and withaferin A contents.
In an open environment, the withaferin A content is highest in the foliar spray of chitosan (10 ppm), which was 6.3 fold higher than the control. Researchers have also reported the enhancement of two-fold in the withanolide A and withaferin A contents of regenerated plantlets as compared to field-grown plants (Jain et al. 2011). It has also been reported that chitosan enhances the contents of total withanolides contents in cell suspension culture of the plant (Sivanandhan et al. 2014). Chitosan is reported to be more effective in inducing lycopene accumulation in tomato as compared to methyl ester, MeJA (Osano et al. 2017). Chitosan has also been reported to elicit the production of various secondary metabolites in in vitro cell cultures (Ferri and Tassoni 2011). On the other hand, there are many biotic and abiotic factors, such as wind, light, rainfall, humidity, temperature, soil, air, etc., in open environment, which contributes the increased production of the withanolides. It has also been reported that phytoconstituents profile of the plant is generally affected by various biotic and abiotic factors (Hugueney et al. 1996; Zulak et al. 2007). Elicitation is an efficient tool which induces the plant to synthesize beneficial phytochemical (Kim et al. 2007) as well as nutraceuticals (Zhao et al. 2002). Several factors such as concentration, type of elicitor, growth stages, etc., are responsible for the enhancement of secondary metabolites. It has been reported by other researchers that the molecules that induce the production of secondary metabolites to ensure their survival in plants are known as “elicitors” (Namdeo 2007; Sivanandhan et al. 2014). Moreover, the environmental factors, such as wind, light, rainfall, humidity, temperature, soil and air, play a key role in the biosynthesis of the bioactive metabolites with enhanced withanolide A/withaferin A contents (Mir et al. 2014).
In our study, it was found that the highest withanolide A content was recorded in WS (Poshita) after foliar spray of jasmonic acid (400 ppm). It was also recorded that withanolide A content was 4.5 fold higher than the control. Jasmonic acid (JA) and its methyl ester, MeJA, have been reported to play an important role as elicitors in the hyperproduction of various secondary metabolites (Walker et al. 2002). They have also been reported to be important in signal transduction processes that regulate defence responses in plants and have shown effective response in enhanced production of secondary metabolites in cell cultures (Zhao et al. 2010). A jasmonate-responsive MYC2 transcription factor was identified and functionally characterized from WS and it was shown that over-expression of WsMYC2 resulted in the production of withanolides and stigmasterol (Sharma et al. 2019). An important gene S-adenosyl l-methionine dependent sterol-C24-methyltransferase type 1 (SMT1) was found to be involved in WS withanolides biosynthesis predominantly through campesterol and stigmasterol routes (Pal et al. 2019). In controlled condition, the highest withaferin A contents was recorded, when sprayed with Jasmonic acid (400 ppm) which was six-fold higher than the control. MeJA and Jasmonic Acid (JA) together have been used as elicitors to increase the accumulation of secondary metabolites (viz., artemisinin) in cell suspension culture of Artemisia absinthium (Ali et al. 2015). Similarly, withanolide A content was found to be highest after foliar spray of jasmonic acid (400 ppm) and SA (1 ppm), respectively, and this resulted in the increase of seven-fold and 4.3 fold with respect to control. The increased concentration of WS-1 (withanolide A) (36.669 ± 0.59 to 257.546 ± 0.29 μg/g of DW) and WS-3 (withaferin A) (396.37 ± 0.44 to 1505.463 ± 0.41 μg/g of DW) were also reported earlier in the presence of SA (Razdan et al. 2017). Studies have shown that MeJA and SA are the most efficient elicitors for the accumulation of secondary metabolites in plant cell cultures (Ketchum et al. 1999; Bae et al. 2011; Sivanandhan et al. 2012). The application of SA and MeJA has enhanced the expression of selected genes in 6–72 h. Some genes were reported to be up-regulated upto 6 h (Agarwal et al. 2017). SA is an important elicitor in the production of plant secondary metabolite as like MeJA (Bae et al. 2011) and act as a defence role in plants against many plants (Hayat et al. 2010). Moreover, research has demonstrated that the application of nitrogen-based fertilizer not only enhances the accumulation of secondary metabolites probably through jasmonate pathway in WS but it also increases the biomass of the plant (Pal et al. 2017).
Conclusion
On the basis of these studies, the pharmaceutical significance of the secondary metabolites (withaferin A and withanolide A) of WS can be enhanced in the controlled environment as compared to the open environment. Moreover, the foliar application of elicitors at very minimal concentrations has also been found to be useful in enhanced accumulation of the withanolides. Their usage for the commercial purpose can be used as a novel approach for consistent production of these secondary metabolites. Thus, it will meet the commercial demands of pharmaceutical purposes without uprooting the whole plant or endangering the elite varieties for the extraction of bioactive metabolites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge GBPIHED, Almora for providing the financial support in carrying out this research work.
Author contributions
There is no conflict of interest of the authorship. The main work and data analysis were done by MS; the experiment was planned by SA while DS and NKP helped in writing the manuscript.
References
- Agarwal AV, Gupta P, Singh D, Dhar YV, Chanda D, Trivedi PK. Comprehensive assessment of the gene involved in withanolide biosynthesis from Withania somnifera: chemotype specific and elicitor responsive expression. Funct Integr Genom. 2017;17(4):477–490. doi: 10.1007/s10142-017-0548-x. [DOI] [PubMed] [Google Scholar]
- Ali M, Abbasi BH, Ali GS. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Culture. 2015;120(3):1099–1106. [Google Scholar]
- Bae EK, Lee H, Lee JS, Noh EW. Drought, salt and wounding stress induce the expression of the plasma membrane intrinsic protein 1 gene in popular (Populus alba × P. tremula var. glandulosa) Gene. 2011;483(1–2):43–48. doi: 10.1016/j.gene.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Bown D. The royal horticultural Society encyclopedia of herbs and their uses. London: Dorling Kindersley Limited; 1995. p. 424. [Google Scholar]
- Chevallier A. The encyclopedia of medicinal plants: a practical reference guide to over 550 key herbs and their medicinal uses. London: Dorling Kindersley; 1996. [Google Scholar]
- Collin HA. Secondary product formation in plant tissue cultures. Plant Growth Regulat. 2001;34(1):119–134. [Google Scholar]
- Davis L, Kuttan G. Effect of Withania somnifera on DMBA induced carcinogenesis. J Ethnopharmacol. 2001;75(2–3):165–168. doi: 10.1016/s0378-8741(00)00404-9. [DOI] [PubMed] [Google Scholar]
- Dhar RS, Verma V, Suri KA, Sangwan RS, Satti NK, Kumar A, Tuli R, Qazi GN. Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry. 2006;67(20):2269–2276. doi: 10.1016/j.phytochem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Dhuley JN. Nootropic-like effect of Ashawagandha (Withania somnifera L.) in mice. Phytoter Res. 2001;15:524–528. doi: 10.1002/ptr.874. [DOI] [PubMed] [Google Scholar]
- Durg S, Dhadde SB, Vandal R, Shivakumar BS, Charan CS. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: a systematic review and meta-analysis. J Pharm Pharmacol. 2015;67(7):879–899. doi: 10.1111/jphp.12398. [DOI] [PubMed] [Google Scholar]
- Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63(6):965. [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Tassoni A. Chitosan as elicitor of health beneficial secondary metabolites in in vitro plant cell cultures. In: Mackay RG, Tait JM, editors. Handbook of Chitosan research and applications. New York: Nova Science Publishers; 2011. pp. 389–413. [Google Scholar]
- Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia. 2003;74(1–2):68–76. doi: 10.1016/s0367-326x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Gupta GL, Rana AC. Withania somnifera (Ashwagandha): a review. Pharmacognosy Rev. 2007;1(1):129–136. [Google Scholar]
- Hassannia B, Logie E, Vandenabeele P, Vanden Berghe T, Vanden Berghe W. Withaferin A: from ayurvedic folk medicine to preclinical anti-cancer drug. Biochem Pharmacol. 2019;2952(19):30292–30298. doi: 10.1016/j.bcp.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Hayat Q, Hayat S, Irfan M, Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot. 2010;68(1):14–25. [Google Scholar]
- Heinrich M, Gibbons S. Ethnopharmacology in drug discovery: an analysis of its role and potential contributions. J Pharm Pharmacol. 2001;53(4):425–432. doi: 10.1211/0022357011775712. [DOI] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Quennemet J, d'Harlingue A, Camara B. Developmental and stress regulation of gene expression for plastid and cytosolic isoprenoid pathways in pepper fruits. Plant Physiol. 1996;111(2):619–626. doi: 10.1104/pp.111.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Fareed S, Ansari S, Rahman A, Iffat-Zareen A, Saeed M. Current approaches toward production of secondary plant metabolites. J Pharm Bioall Sci. 2012;4:10–20. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Sinha A, Jain D, Kachhwaha S, Kothari SL. Adventitious shoot regeneration and in vitro biosynthesis of steroidal lactones in Withania coagulans (Stocks) Dunal. Plant Cell Tissue Organ Culture. 2011;105(1):135–140. [Google Scholar]
- Jain R, Kachhwaha S, Kothari SL. Phytochemistry, pharmacology and biotechnology of Withania coagulans and Withania somnifera: a review. J Med Plant Res. 2012;69(41):5388–5399. [Google Scholar]
- Ketchum RE, Raymond EB, Gibson DM, Croteau RB, Shuler ML. The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng. 1999;62(1):97–105. doi: 10.1002/(sici)1097-0290(19990105)62:1<97::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kim OT, Bang KH, Shin YS, Lee MJ, Jung SJ, Hyun DY, Kim YC, Seong NS, Cha SW, Hwang B. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) Urban elicited by methyl jasmonate. Plant Cell Rep. 2007;26(11):1941–1949. doi: 10.1007/s00299-007-0400-1. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psych. 2008;32(5):1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kulkurni SK, Ninan I. Inhibition of morphine tolerance and dependence by Withania somnifera in mice. J Ethnopharmacol. 1997;57(3):213–217. doi: 10.1016/s0378-8741(97)00064-0. [DOI] [PubMed] [Google Scholar]
- Lele RD. Beyond reverse pharmacology: mechanism based screening of Ayurvedic drugs. J Ayurveda Integr Med. 2010;1(4):257–265. doi: 10.4103/0975-9476.74435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir BA, Mir SA, Koul S. In vitro propagation and withaferin A production in Withania ashwagandha, a rare medicinal plant of India. Physiol Mol Biol Plants. 2014;20(3):357–364. doi: 10.1007/s12298-014-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra LC, Singh SS, Dagenais S. Scientific basis for the use of Withania somnifera (Ashwagandha): a review. Altern Med Rev. 2000;5(4):334–346. [PubMed] [Google Scholar]
- Naidu PS, Singh A, Kulkarni SK. Effect of Withania somnifera root extract on haloperidol induced Orofacial Dyskinesia: possible mechanism of action. J Med Food. 2003;6(2):107–114. doi: 10.1089/109662003322233503. [DOI] [PubMed] [Google Scholar]
- Namdeo AG. Plant cell elicitation for production of secondary metabolites: a review. Pharmacognosy Rev. 2007;1(1):69–79. [Google Scholar]
- Osano A, Fultang N, Davis J. Exogenous pre-harvest treatment with methyl jasmonate and chitosan elicits lycopene biosynthesis in tomato plants. J Environ Sci Eng. 2017;6:561–568. [Google Scholar]
- Pal S, Yadav AK, Singh AK, Rastogi S, Gupta MM, Verma RK, Nagegowda DA, Pal A, Shasany AK. Nitrogen treatment enhances sterols and withaferin A through transcriptional activation of jasmonate pathway, WRKY transcription factors, and biosynthesis genes in Withania somnifera (L.) Dunal. Protoplasma. 2017;254(1):389–399. doi: 10.1007/s00709-016-0959-x. [DOI] [PubMed] [Google Scholar]
- Pal S, Rastogi S, Nagegowda DA, Gupta MM, Shasany AK, Chanotiya CS. RNAi of sterol methyl transferase1 reveals its direct role in diverting intermediates towards withanolide/phytosterol biosynthesis in Withania somnifera. Plant Cell Physiol. 2019;60(3):672–686. doi: 10.1093/pcp/pcy237. [DOI] [PubMed] [Google Scholar]
- Puthusseri B, Divya P, Lokesh V, Neelwarne B. Enhancement of folate content and its stability using food grade elicitors in coriander (Coriandrum sativum L.) Plant Foods Hum Nutr. 2012;67(2):162–170. doi: 10.1007/s11130-012-0285-1. [DOI] [PubMed] [Google Scholar]
- Radman R, Saez T, Bucke C, Keshavarz T. Elicitation of plants and microbial cell systems. Biotech Appl Biochem. 2003;37(Pt 1):91–102. doi: 10.1042/ba20020118. [DOI] [PubMed] [Google Scholar]
- Razdan S, Bhat WW, Dhar N, Rana S, Pandith AS, Wani TA, Vishwakarma R, Lattoo SK. Molecular characterization of DWF1 from Withania somnifera (L.) Dunal: its implications in withanolide biosynthesis. J Plant Biochem Biotechnol. 2017;26(1):52–63. [Google Scholar]
- Saini RK, Akithadevi MK, Giridhar P, Ravishankar GA. Augmentation of major isoflavones in Glycine max L. through the elicitor-mediated approach. Acta Bot Croat. 2013;72(2):311–322. [Google Scholar]
- Saini RK, Shetty NP, Giridhar P. Carotenoid content in vegetative and reproductive parts of commercially grown Moringa oleifera Lam. cultivars from India by LC–APCI–MS. Eur Food Res. 2014;238(6):971–978. [Google Scholar]
- Schliebs R, Liebmann A, Bhattacharya SK, Kumar GS, Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int. 1997;30(2):181–190. doi: 10.1016/s0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Sharma A, Rather GA, Misra P, Dhar MK, Lattoo SK. Jasmonate responsive transcription factor WsMYC2 regulates the biosynthesis of triterpenoid withanolides and phytosterol via key pathway genes in Withania somnifera (L.) Dunal. Plant Mol Biol. 2019;100(4–5):543–560. doi: 10.1007/s11103-019-00880-4. [DOI] [PubMed] [Google Scholar]
- Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Shah P, Punetha H, Agrawal S. Varietal comparison of withanolide contents in different tissues of Withania somnifera (L.) Dunal (Ashwagandha) Int J Life Sci Sci Res. 2018;4(3):1752–1758. [Google Scholar]
- Singh M, Shah P, Punetha H, Gaur AK, Kumar A, Agrawal S. Isolation and quantification of a potent anti cancerous compound, Withaferin A from the aerial parts of Withania somnifera (Ashwagandha) Ad Plant Sci. 2017;30(II):231–235. [Google Scholar]
- Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Dev GK, Manickavasagam M, Selvaraj N, Ganapathi A. Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotech. 2012;168(3):681–696. doi: 10.1007/s12010-012-9809-2. [DOI] [PubMed] [Google Scholar]
- Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M. Enhanced biosynthesis of withanolides by elicitation and precursor feeding in cell suspension culture of Withania somnifera (L.) Dunal in shake-flask culture and bioreactor. PLoS ONE. 2014;9(8):1–11. doi: 10.1371/journal.pone.0104005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats S. Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Appl Biochem Biotechnol. 2016;178(3):504–512. doi: 10.1007/s12010-015-1890-x. [DOI] [PubMed] [Google Scholar]
- Verma KS, Haq SU, Kachhwaha S, Kothari SL. RAPD and ISSR marker assessment of genetic diversity in Citrullus colocynthis (L.) Schrad: a unique source of germplasm highly adapted to drought and high-temperature stress. 3 Biotech. 2017;7(5):288. doi: 10.1007/s13205-017-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Vivanco JM. Jasmonic acid induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort) Phytochemistry. 2002;60(3):289–293. doi: 10.1016/s0031-9422(02)00074-2. [DOI] [PubMed] [Google Scholar]
- Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull (Tokyo) 2002;50(6):760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Zhou LG, Wu JY. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol. 2010;87:137–144. doi: 10.1007/s00253-010-2443-4. [DOI] [PubMed] [Google Scholar]
- Zulak KG, Cornish A, Daskalchuk TE, Deyholos MK, Goodenowe DB, Gordon PM, Klassen D, Pelcher LE, Sensen CW, Facchini PJ. Gene transcript and metabolite profiling of elicitor-induced opium poppy cell cultures reveals the coordinate regulation of primary and secondary metabolism. Planta. 2007;225(5):1085–1106. doi: 10.1007/s00425-006-0419-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


