Abstract
Scorpion venom heat-resistant peptide (SVHRP) is a component purified from Buthus martensii Karsch scorpion venom. Our previous studies have shown that SVHRP is neuroprotective in models of Alzheimer’s disease and Parkinson’s disease. The present study aimed to explore the potential neuroprotective effects of SVHRP on cerebral ischemia/reperfusion (I/R) injury, using a mouse model of middle cerebral artery occlusion/reperfusion (MCAO/R) and a cellular model of oxygen-glucose deprivation/reoxygenation (OGD/R). Our results showed that SVHRP treatment decreased the neurological deficit scores, edema formation, infarct volume and neuronal loss in the MCAO/R mice, and protected primary neurons against OGD/R insult. SVHRP pretreatment suppressed the alterations in protein levels of N-methyl-D-aspartate receptors (NMDARs) and phosphorylated p38 MAPK as well as some proinflammatory factors in both the animal and cellular models. These results suggest that SVHRP has neuroprotective effects against cerebral I/R injury, which might be associated with inhibition of the NMDA-MAPK-mediated excitotoxicity.
Keywords: Scorpion venom heat-resistant peptide, Cerebral ischemia/reperfusion injury, Neuroprotection, NMDARs, p38 MAPK
Introduction
Ischemic cerebrovascular disease is a common brain disorder characterized by high incidence, high disability, and high mortality worldwide. Each year, ~6 million people die of acute ischemia [1–3]. Thrombolytic therapy is often used in the treatment of ischemic disease in clinical practice. However, it has strict indications and certain risks such as post-perfusion lesions and bleeding [4]. The molecular mechanisms of cerebral ischemia and reperfusion (I/R) injury are complicated and not fully understood. Increasing evidence has suggested that excitatory amino-acid toxicity and inflammation are the predominant mechanisms underlying cerebral I/R injury [5–7]. These complex processes involve interactions among many factors that slow cerebral healing and even aggravate cerebral damage.
Scorpion venom heat-resistant peptide (SVHRP, National Invention Patent No. ZL011061669) was isolated from Buthus martensii Karsch (BmK) venom in our laboratory. Our previous studies have shown that SVHRP regulates gene expression in astrocytes, promotes neurogenesis in mice, protects dopamine neurons in a 6-hydroxydopamine rat model and protects Caenorhabditis elegans from β-amyloid toxicity [8–11]. However, it is unknown whether SVHRP is protective against cerebral I/R insult. In this study, we investigated the neuroprotective effects of SVHRP in a mouse model of middle cerebral artery occlusion/reperfusion (MCAO/R) and a cellular model of oxygen-glucose deprivation/reoxygenation (OGD/R).
Materials and Methods
Isolation of Scorpion Venom Heat-Resistant Peptide
The isolation of SVHRP from BmK venom was reported in our previous publication [8] with a patented method in our laboratory (No. 011061669). First, the crude venom was collected by electrical stimulation of the telson of BmK scorpions from Henan Province, China, and the lyophilized crude venom was dissolved in ddH2O and maintained at 100 °C for 4 h followed by centrifugation. Then the supernatant was loaded onto a Superdex Peptide 10/300GL Column (ÄKTA avant 25) and separated by fast protein liquid chromatography. Fraction I (P1) from the Superdex Peptide 10/300GL Column was collected and used for cell treatment. The results from reverse-phase HPLC using a C18 column (Zorbax SB-C18 250 x 4.6 mm, 5 μm) demonstrated that the purity of SVHRP was > 99.5%.
Experimental Animals and Drug Administration
Male C57BL/6 mice (6-8 weeks old and weighing 16–25 g) were provided by Dalian Medical University. The animals were housed under international standards. Forty-eight mice were divided randomly into four groups: normal saline (NS) + sham, SVHRP + sham, NS + MCAO/R, and SVHRP + MCAO/R groups. The mice were pretreated with SVHRP (20 μg/kg, i.p.) or NS (5 mL/kg, i.p.) 48 h and 1 h before MCAO/R [9]. The dose of SVHRP was chosen based on previous animal studies [9, 10] showing that 20–50 μg/kg of SVHRP promotes neurogenesis in mouse hippocampus and olfactory bulb and protects dopamine neurons challenged by oxidative stress.
Establishment of the MCAO/R Model
C57BL/6 mice were anaesthetized by intraperitoneal injection of 1% pentobarbital (50 mg/kg, i.p.). The MCAO/R model was established according to a published method [12] with minor modifications. Briefly, a ventral midline neck incision was made to expose the right common carotid artery, and right internal and external carotid arteries. After ligation at the proximal end of the common carotid artery, an MCAO suture (Southern Medical University, China) was inserted and positioned at the origin of the middle cerebral artery to occlude it. The same surgical procedure was performed in sham-operated animals except for occlusion of the artery. After occlusion for 2 h, the suture was removed and blood flow was restored (reperfusion) [13].
Evaluation of Neurological Deficit
The mice were returned to their cages after the suture was withdrawn and were given free access to food and water. The neurological behavior of the mice was scored 24 h after MCAO/R. A 28-point scale of neurological deficit was used to evaluate neurological behavior [14]. The scale was based on the following seven tests, each of which was scored as 0–4 points: (1) body symmetry, (2) gait, (3) climbing, (4) circling behavior, (5) forelimb symmetry, (6) compulsory circling, and (7) whisker response.
Measurement of Cerebral Infarct Volume
After neurological evaluation, the mice were euthanized with isofluriane and brains were collected for measurement of infarct volume [15]. In brief, each brain was first frozen for 20 min before being cut into 2-mm-thick coronal sections, and stained with 2% triphenyltetrazolium chloride (TTC) (Solarbio, Beijing, China) for 15 min at 37 °C followed by overnight immersion in 4% paraformaldehyde. The infarct area of each slice was demarcated and analyzed using Image Pro plus software.
Quantification of Brain Water Content
After neurological measurement, we used the wet-dry method to determine the brain water content [16]. Each hemisphere was weighed (wet weight) and left in a desiccating oven at 105 °C overnight, then the dried hemispheres were weighed again (dry weight) and the brain water content was calculated.
Nissl Staining
For this staining protocol, brain samples were collected 24 h after reperfusion. After transcardial perfusion with 50 mL of 4% paraformaldehyde under anesthesia (50 mg/kg pentobarbital, i.p.), each brain was removed and post-fixed for 24 h. The tissue was embedded in paraffin and sectioned at 10 μm. The sections were stained with 2% cresyl violet, cleaned in graded ethanols and xylene, and coverslipped with Permount mounting medium (Sigma, Markham, ON, Canada).
Cortical Neuron Culture and Establishment of the OGD/R Model
Primary cortical neurons were cultured from newborn C57BL/6 mice anesthestized with diethyl ether inhalation within 24 h using the procedures described below [17]. The cerebral cortices were isolated and digested in 0.125% trypsin for 30 min at 37 °C. Then, 500 μL of suspension was added to each well of a 24-well plate pre-coated with poly-L-lysine and cultured in Dulbecco’s modified Eagle’s medium with 10% FBS, 1% L-glutamate, and 1% penicillin-streptomycin in an incubator at 37 °C with 5% CO2 for 4 h. Then, the supernatant was replaced with 500 μL Neurobasal medium containing 1% L-glutamate, 1% penicillin-streptomycin, 1% N2, and 2% B27 and maintained for 12 days. Half of the medium was changed every other day. The purity of mature neurons (> 95%) was confirmed by microtubule-associated protein-2 staining.
OGD/R was performed using a combination of chemical hypoxia and glucose deprivation according to published methods [18, 19] with modifications. Mature cortical neurons were transiently deprived of both oxygen and glucose through incubation with a glucose-free Earle’s balanced salt solution (EBSS) containing 1 mmol/L azide (Sigma, St. Louis, MO), a deoxygenating reagent, for 3 h. Reoxygenation was induced by quickly replacing the deoxygenated and glucose-free EBSS with the pre-OGD culture medium to return the cells to normoxic conditions. The cortical neurons were divided into four groups: vehicle + control, SVHRP + control, vehicle + OGD/R, and SVHRP + OGD/R groups. Cells were cultured in the presence or absence of SVHRP (20 μg/mL) for 24 h before OGD/R. The concentration of 20 μg/mL was selected as it is the optimal concentration within the dose range (0.2–80 μg/mL) in cell culture and Caenorhabditis elegans studies, according to our previous reports [8, 9].
Assessment of Cell Viability and Cytotoxicity
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The cortical neurons were pretreated with SVHRP (20 μg/mL) or vehicle before being subjected to OGD/R insult. MTT solution was added to the culture medium to a final concentration of 0.5 mg/mL 24 h after reoxygenation and incubated for 4 h at 37 °C. The supernatant was then removed, and 100 μL dimethyl sulfoxide was added. The optical density was measured at 490 nm.
Lactic dehydrogenase (LDH) released from cells was detected using a commercial LDH kit (Roche, Basel, Switzerland) to evaluate the neurotoxicity. Absorbance was measured at 490 nm, and the relative LDH release rate was calculated.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RT-PCR was used to analyze the mRNA levels of tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 at 24 h after reperfusion in MCAO/R mice. Total RNA was extracted using an RNA extraction kit according to the manufacturer’s instructions (Takara Biotechnology, China). Reverse transcription of RNA to cDNAs was performed using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotechnology). Regular PCR was then conducted to amplify the cDNA using the following conditions: a hot start at 95 °C for 600 s, followed by 95 °C for 30 s, 58 °C for 40 s, and 72 °C for 40 s at the annealing temperature through 35 cycles for TNF-α and IL-6; a hot start at 95 °C for 180 s, followed by 94 °C for 15 s, 60 °C for 25 s, 72 °C for 20 s through 30 cycles, and then 72 °C for 300 s for β-actin. The following primers were used: for TNF-α forward 5’-CGT CAG CCG ATT TGC TAT CT-3’, reverse 5’-CGG ACT CCG CAA AGT CTA AG -3′; for IL-6 forward 5′-TCC ATC CAG TTG CCT TCT TGG-3′, reverse 5′-CCA CGA TTT CCC AGA GAA CAT G-3′; and for β-actin forward 5′-AGC CAT GTA CGT AGC CAT CC-3′, reverse 5′-GCT GTG GTG GTG AAG CTG TA-3′.
Western Blot Analysis
Samples from the ischemic penumbra of cerebral cortex and cortical neurons were homogenized in ice-cold lysis buffer and then centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatants were collected, and the protein concentrations were determined using a bicinchoninic acid (BCA) protein assay reagent kit (Pierce Biotechnology, Waltham, MA) with bovine serum albumin (BSA, Sigma) as a standard. Equal amounts of protein samples from each group were separated by 8%–10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Chemicon, Billerica, MA) for immunoblotting using an electrophoretic transfer system (Bio-Rad, Hercules, CA). The membrane was blocked at room temperature for 1 h with 5% nonfat dry milk or 5% BSA (Sigma) in TBST (Tris-buffered saline containing 0.1% Tween-20), followed by incubation overnight at 4 °C with primary antibodies for rabbit p38 (1:1000 dilution, Cell Signaling, Beverly, MA), mouse p-p38 (1:1000, Cell Signaling), rabbit NR1 (1:1000, Abcam, Cambridge, MA), mouse NR2A (1:1000, Abcam), mouse NR2B (1:1000, Abcam), mouse GABAA (1:1000, Abcam), iNOS (1:1000, Cell Signaling), β-actin (1:2000, Abcam), and β-tubulin (1:2000, Abcam). The following day, the membranes were washed three times with TBST buffer (10 min each) and incubated with horseradish peroxidase secondary antibodies for 1 h at room temperature. The bands corresponding to the proteins of interest were detected using an enhanced chemiluminescence detection system (Bio-Rad).
Statistics
Data were analyzed using the Statistical Package for the Social Sciences (IBM SPSS Statistics 19.0). Values are presented as the mean ± SEM. All data were analyzed by one-way analysis of variance (ANOVA). P < 0.05 indicated statistically significant differences.
Results
Effects of SVHRP on Neurological Deficit Scores, Infarct Volume, and Brain Water Content
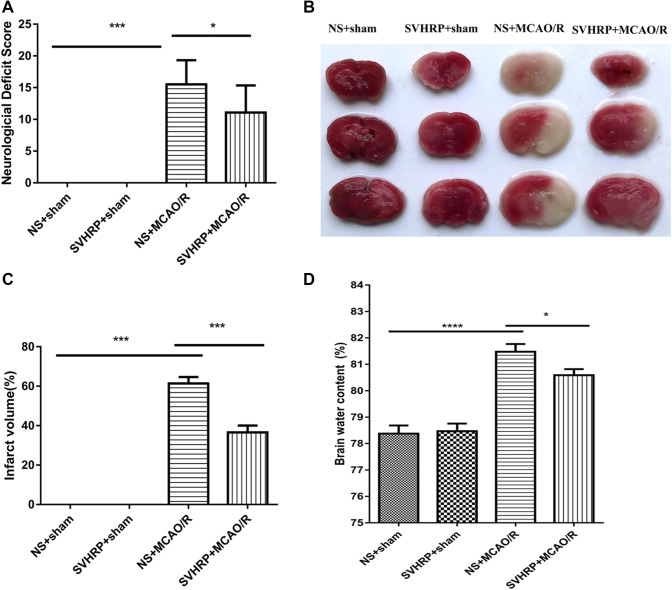
MCAO has been used extensively for modelling ischemic stroke and leads to cerebral ischemia and edema, as well as serious brain injury such as neurological impairment and even death. To test whether SVHRP has neuroprotective effect on ischemic stroke, we used MCAO in mice. After 2 h of ischemia followed by 24 h of reperfusion, MCAO/R mice showed neurologic impairment and demonstrated significantly higher neurological deficit scores compared with those in the sham group (P < 0.001, Fig. 1A). Meanwhile, in the group pretreated with SVHRP (20 μg/kg, i.p.) 48 h before MCAO/R, the total neurological score was significantly lower than that of the MCAO/R group (11.07 ± 1.14 vs 15.5 ± 0.96, P < 0.05; n = 14–16). Among the seven items, the scores for circling behavior (1.71 ± 0.19 vs 2.44 ± 0.20, P < 0.01, n = 14–16) and whisker responses (1.50 ± 0.17 vs 2.19 ± 0.23, P < 0.01, n = 14–16) were significantly reduced by SVHRP pretreatment. There was no significant difference between the NS+sham group and the SVHRP+sham group (Fig. 1A).
Fig. 1.
Effects of SVHRP (20 μg/kg, i.p.) on neurological deficit score, infarct volume, and water content in mice after MCAO/R. A Neurological deficit scores 24 h after MCAO/R. Pretreatment with SVHRP significantly reduced the scores. B Representative coronal brain slices stained with TTC. Red, healthy tissue; white, infarcted tissue. Pretreatment with SVHRP significantly reduced the infarct size. C, D Quantitative analyses of infarct volume (C) and brain water content (D). Pretreatment with SVHRP reduced brain water content after MCAO/R (mean ± SEM; n = 6–16 mice/group; *P < 0.05, ***P < 0.001, one-way ANOVA followed by the LSD post-hoc test).
MCAO leads to a prominent cerebral infarct. Mice pretreated with SVHRP (20 μg/kg, i.p.) showed significantly smaller infarct volumes (Fig. 1B) than those in the MCAO/R group (36.53% ± 3.5% vs 61.37% ± 3.3%, P < 0.001, n = 11–13, Fig. 1C). These results indicated that SVHRP pretreatment reduces the brain damage caused by I/R injury.
Cerebral I/R injury leads to cerebral edema. Brain water content was used to assess edema in the hemispheres. The brain water content was higher in the MCAO/R group than in the sham group; however, this effect was significantly reduced by SVHRP pretreatment (20 μg/kg, i.p.) (80.59% ± 0.23% vs 81.48% ± 0.29%, P < 0.05, n = 6–10, Fig. 1D). No infarction or edema formation occurred in the sham operation group.
Effects of SVHRP on the Survival of Neurons after I/R Injury
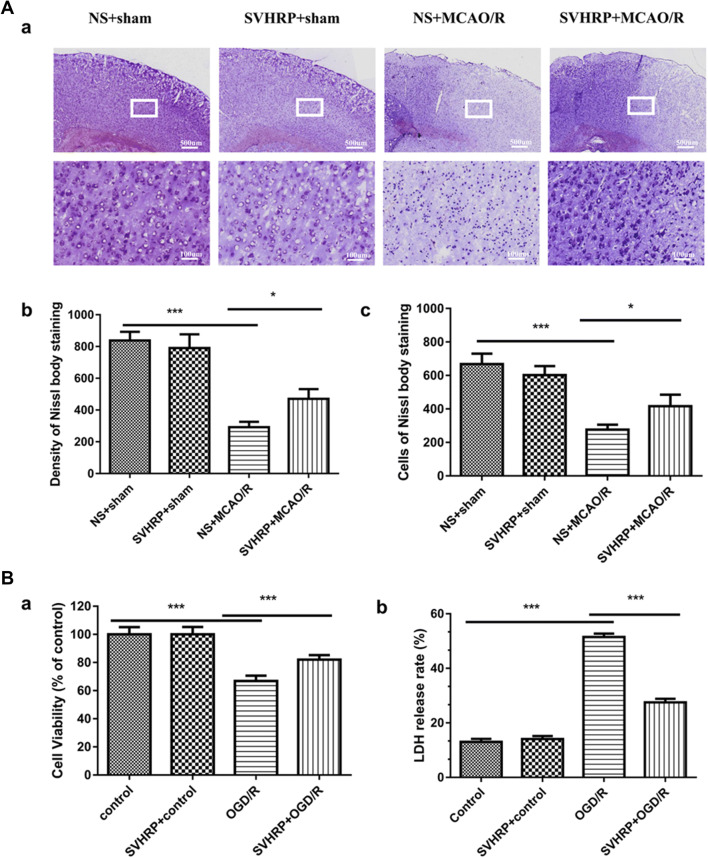
A Nissl body is a large granular body found in neurons. The Nissl staining method is used to localize the cell body—the stain can be seen in the soma and dendrites. Nissl staining was performed here to determine the numbers of neurons in the ischemic penumbra (Fig. 2Aa). Dramatic decreases in staining density and numbers of cells stained were induced in the MCAO/R group, and these were ameliorated by SVHRP pretreatment (292 ± 34.26 vs 470 ± 62.29, P < 0.05, n = 5, Fig. 2Ab; 276 ± 30.59 vs 416 ± 69.4, P < 0.05, n = 5, Fig. 2Ac). Our results revealed dramatic neuronal loss in the MCAO/R group, while SVHRP pretreatment partially prevented the neuronal loss, suggesting that SVHRP protected neurons against the I/R insult and contributed to the improved functional outcome after I/R.
Fig. 2.
Effects of SVHRP on the survival of neurons after I/R injury. A Effects of SVHRP (20 μg/kg, i.p.) on neuronal survival in the ischemic penumbra after MCAO/R. (a) Representative image of Nissl staining in cortex 24 h after MACO/R (scale bars, 500 μm for upper panel, 100 μm for lower panel). (b, c) Density (b) and cell number (c) analyzed by quantitative imaging (Proplus). SVHRP pretreatment attenuated the neuronal loss caused by MCAO/R and SVHRP alone had no effect on these histological changes (mean ± SEM; n = 5 mice/group; *P < 0.05, **P < 0.01, one-way ANOVA followed by Turkey’s test). B Effects of SVHRP (20 μg/mL) on survival of primary cortical neurons after OGD/R. (a, b) Cell viability (a) by MTT assay and LDH release rate (b) from cultured cortical neurons (mean ± SEM; data from 3 separate experiments; ***P < 0.001, one-way ANOVA followed by the LSD post-hoc test).
OGD/R is used to study I/R injury in vitro. In order to investigate the effects of SVHRP on OGD/R-induced neuronal injury, the viability/injury of neurons was assessed by the MTT assay and by measuring LDH. Studies have shown that the amount of LDH leakage is correlated with cell membrane damage [20, 21]. Viability by the MTT assay was lower and the LDH release rate was higher in the OGD/R group (P < 0.001, Fig. 2B). This indicated that OGD/R treatment led to neuronal damage and impaired cell viability. However, pretreatment with SVHRP (20 μg/mL) raised the cell viability and reduced the LDH release rate under OGD/R conditions (P < 0.001, Fig. 2B), reconfirming the neuroprotective effect of SVHRP on I/R injury (F = 261.00, Fig. 2Ba; F = 185.90, Fig. 2Bb).
Effects of SVHRP on the Expression Levels of NMDA and γ-Aminobutyric Acid (GABA) Receptors in the MCAO/R and OGD/R Models
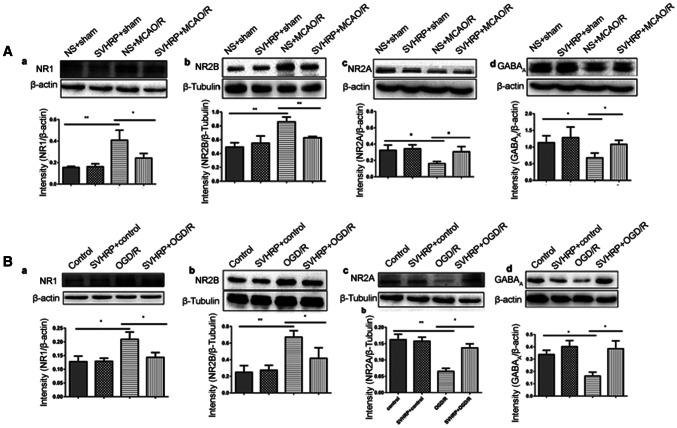
Excitatory toxicity, referring to the over-activation of NMDA receptors (NMDARs) (such as subunits NR1 and NR2B) and the consequent increase in intracellular levels of Ca2+, activates noxious signaling cascades and promotes neuronal cell death [22, 23]. To explore whether SVHRP pretreatment has a neuroprotective effect against this excitotoxicity pattern, we investigated the protein expression of NR1 and NR2B in the MCAO/R and OGD/R models by Western blotting. Compared with the sham operation groups, the protein expression levels of NR1 and NR2B were up-regulated in the MCAO/R group (P < 0.01, Fig. 3Aa, b). However, pretreatment with SVHRP inhibited the increment of expression levels for NR1 (P < 0.05, Fig. 3Aa) and NR2B (P < 0.01, Fig. 3Ab).
Fig. 3.
Effects of SVHRP on the expressions of NR1, NR2B, NR2A and GABAA receptors in the ischemic penumbra after MCAO/R and in cultured cortical neurons under OGD/R. A Pretreatment with SVHRP (20 μg/kg, i.p.) significantly decreased the protein levels of NR1 (a) and NR2B (b), and increased the NR2A (c) and GABAA (d) levels compared with the MCAO/R group. B Primary cortical neurons subjected to ODG/R injury and the effects of SVHRP (20 μg/mL) pretreatment on the protein levels of NR1 (a), NR2B (b), NR2A (c), and GABAA (d) corresponded with those in the MCAO/R mice. Data are presented as the mean ± SEM; n = 3 mice/group; *P < 0.05, **P < 0.01, one-way ANOVA followed by Turkey’s test.
The NR2A and NR2B subunits play different roles in mediating excitotoxic neuronal ischemic tolerance and cell death; increased synaptic expression of the NR2A subunit promotes the survival of neurons, whereas increased expression of NR2B promotes neuronal apoptosis [24, 25]. Here, our results showed lower expression of NR2A in the MCAO/R group than in the sham operation groups (P < 0.05, Fig. 3Ac). GABA is the principal inhibitory neurotransmitter that binds to GABAA, GABAB, and GABAC receptors. The GABAA receptor plays a major role in inhibiting excitotoxicity and ischemia-induced neuronal cell death in the central nervous system [26, 27]. Compared with the sham operation groups, MCAO/R mice also showed decreased expression of GABAA (P < 0.05, Fig. 3Ad). Moreover, compared with the MCAO/R mice, the SVHRP pretreatment group showed significantly increased expression levels of NR2A and GABAA (P < 0.05, Fig. 3Ac, d). No differences were found in the expression of NMDARs and GABAA between the NS + sham and SVHRP + sham groups (P > 0.05) (F = 14.67, Fig. 3Aa; F = 14.16, Fig. 3Ab; F = 7.39, Fig. 3Ac; F = 4.445, Fig. 3Ad).
A similar pattern of protein expression of the above receptors was detected with OGD/R. The results in the OGD/R model were consistent with those in the MCAO/R mice (Fig. 3Ba-d) (F = 11.43, Fig. 3Ba; F = 14.16, Fig. 3Bb; F = 12.33, Fig. 3Bc; F = 5.524, Fig. 3Bd). In summary, these results demonstrated that SVHRP had neuroprotective effects by inhibiting glutamate excitotoxicity.
Effects of SVHRP on the Expression of Components of the p38 MAPK Pathway in MCAO/R Mice and the OGD/R Model
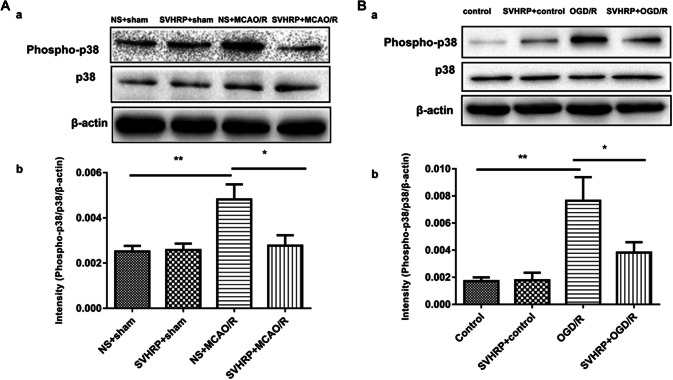
Reports have shown that the MAPK pathways are involved in NMDA-induced excitotoxicity. In addition, p38 kinases in MAPK systems are well known to be activated after several types of ischemia [28, 29]. In our study, 24 h after reperfusion, western blot analysis showed significantly higher expression of active p-p38 MAPK in the MCAO/R group than in the sham operation group (Fig. 4A). Compared with the MCAO/R group, SVHRP pretreatment significantly attenuated the increase in p-p38 MAPK expression induced by MCAO/R (P < 0.01, Fig. 4A).
Fig. 4.
Effect of SVHRP on the expression of p-p38 MAPK in MCAO/R and OGD/R models. A Representative immunoblots (a) and densitometric analysis (b) of the immunoblots demonstrated that SVHRP (20 μg/kg, i.p.) decreased the expression of p-p38 MAPK in the ischemic penumbra after MCAO/R. B SVHRP (20 μg/mL) remarkably down-regulated p-p38 MAPK expression in OGD/R-treated cortical neurons, corresponding with the effect in vivo (mean ± SEM; n = 3 mice/group; *P < 0.05, **P < 0.01, one-way ANOVA followed by Turkey’s test).
Consistent with the results in vivo, p-p38 MAPK was up-regulated in the in vitro OGD/R group (P < 0.01, Fig. 4B), and this was suppressed by SVHRP pretreatment (P < 0.05, Fig. 4B). No significant difference was found between the control and SVHRP groups (Fig. 4B) (F = 18.20, Fig. 4A; F = 23.16, Fig. 4B).
SVHRP Inhibits Pro-inflammatory Cytokines in the Ischemic Penumbra after MCAO/R
p38 MAPKs are known to play a critical role in the signaling pathways that induce a series of pro-inflammatory cytokines and iNOS in glial cells. To further elucidate the mechanisms underlying the neuroprotection by SVHRP against I/R injury, we assessed the protein expression of iNOS after I/R, and found that pretreatment with SVHRP inhibited the increment of iNOS expression (P < 0.05, Fig. 5A). In addition, we investigated the mRNA levels of TNF-α and IL-6 in the ischemic penumbra after 24 h of reperfusion using RT-PCR. We found that the mRNA levels of both were dramatically higher in the MCAO/R group than in the sham groups, and this was suppressed by SVHRP pretreatment (0.89 ± 0.11 vs 0.37 ± 0.08, P < 0.05, n = 3, Fig. 5B; 0.54 ± 0.08 vs 0.24 ± 0.07, P < 0.05, n = 3, Fig. 5C). There were no significant differences between the NS + sham and SVHRP + sham groups (F = 6.02, F = 6.80, Fig. 5B, C). These results indicated that SVHRP inhibited the activation of pro-inflammatory cytokines in the ischemic penumbra after MCAO/R.
Fig. 5.
Effects of SVHRP (20 μg/kg, i.p.) on the expression of iNOS protein and TNF-α and IL-6 mRNA in the ischemic penumbra after MCAO/R. The MCAO/R group had significantly higher expression of iNOS protein (A) and TNF-α (B), IL-6 mRNA (C) than that of the sham operation groups, and SVHRP pretreatment significantly reduced their levels. There were no significant differences between the NS+sham and SVHRP+sham groups (mean ± SEM; n = 3 mice/group; *P < 0.05, one-way ANOVA followed by Tukey’s test).
Discussion
Ischemic stroke greatly affects health, but its mortality is still at a high level, with few medications available [30, 31]. Reperfusion therapy is an important medical treatment following stroke; however, for a certain period of time, the recovery of blood flow frequently causes reperfusion injury. It is believed that cerebral I/R injury involves many complicated pathological mechanisms and induces multiple cell signaling pathways in the brain [32, 33]. Among these, excitotoxicity and inflammation are both important events [34, 35].
BmK and its venom, as well as many other natural product-based therapies, are used to treat neurological diseases [36–39]. SVHRP is isolated from BmK venom, which is highly biologically active [40–43]. Our previous studies have shown that SVHRP is neuroprotective in animal models of Alzheimer’s disease and Parkinson’s disease [10, 11]. In this study, we used models of MCAO/R in mice and OGD/R in primary cultured neurons to further investigate the neuroprotection of SVHRP against cerebral I/R injury and the potential underlying mechanisms, focusing on NMDAR-mediated p38 MAPK signaling pathways.
First, our MCAO/R in vivo study showed that SVHRP had neuroprotective effects by reducing the neurological deficit score, infarct volume, edema formation, and neuronal loss. In vitro, pretreatment with SVHRP reduced the damage to primary cultured neurons subjected to OGD/R, further suggesting the neuroprotective effects of SVHRP.
Excitotoxicity, i.e., increased extracellular glutamate concentration, is an essential etiology associated with I/R injury [44]. NMDARs, ligand-gated ion channels with a high permeability to Ca2+, are activated by glutamate [45–47]. After cerebral ischemia, excess glutamate accumulates in synapses and activates NMDARs, leading to an excessive influx of Ca2+ and thereby resulting in excitotoxicity [48]. Functional NMDARs associated with cerebral I/R injury mainly consist of the NR1 and the NR2A-D subunits [49]. The NR2A and NR2B subunits play different roles in mediating excitotoxic neuronal death and ischemic tolerance. Increased expression of synaptic NR2A promotes the survival of neurons, whereas elevated expression of NR2B promotes neuronal apoptosis and increases the sensitivity to excitotoxicity [24, 25, 50]. In the central nervous system, GABAA plays a major role in inhibiting excitotoxicity and ischemia-induced neuronal death [26, 27]. The level of GABAA on neuronal cell membranes decreases with exposure to OGD/R [51]. Here, we provide the first evidence showing that the neuroprotection conferred by SVHRP is mediated by inhibiting the expression of NR1 and NR2B and preventing the reduction in expression of NR2A and GABAA in the cortex of MCAO/R mice and in OGD/R neurons, suggesting the anti-neurotoxic action of SVHRP.
Reports have shown that the MAPK pathways are involved in NMDA-induced excitotoxicity. p38 MAPK plays an important role in pathophysiological processes, such as cell growth, cell injury, apoptosis, inflammation, and the stress response. Activated MAPK signaling cascade reactions play roles in neuronal damage [52, 53]. p38 kinase is one of the best-known MAPK effectors that is activated after various types of ischemia [28, 29]. In cerebral I/R injury, cellular stress mediated by activated p38 MAPKs is linked to inflammatory cytokine production and apoptosis via transcription factors, phosphorylating intracellular enzymes, and cytosolic proteins [54]. Inhibition of p38 MAPK activation can provide protection against brain injury. As many investigations have shown that activation of the p38 MAPK participates in the process of I/R injury, therapy with p38 MAPK inhibitors could reduce the inflammatory cytokine protein expression induced by ischemia. The suppression of p38 MAPK phosphorylation would reduce neuronal death by inhibiting NMDA-induced excitotoxicity and inflammatory mediator production [55–57]. Our results demonstrated that the phosphorylation of p38 MAPK proteins was significantly increased in the MCAO/R and OGD/R models, and this was remarkably suppressed by SVHRP. Intriguingly, SVHRP also inhibited the pro-inflammatory factors iNOS, TNF-α, and IL-6 at the protein and mRNA levels. This indicated that SVHRP inhibited the neuroinflammation induced by p38 MAPK phosphorylation. Taken together, our findings supported the hypothesis that the NMDA-mediated p38 MAPK pathways are involved in the neuroprotective and anti-inflammatory effects of SVHRP.
Traditional Chinese medicines and their active components have been widely used for thousands of years in many Asian countries, including China, Korea, and Japan. Scorpions were used as a source of medicinal material as early as the Han Dynasty (206 BC–220 AD) and are recorded in the Compendium of Materia Medica (Bencao Gangmu). However, due to the complexity of the active components and lack of scientific study, the effectiveness and safety of these natural materials have not been determined in modern medicine. Our study shows that SVHRP might be an important component that is responsible for the therapeutic effects of scorpion products in traditional Chinese medicine.
In conclusion, this study demonstrated that SVHRP, derived from scorpion venom, is neuroprotective against cerebral I/R injury both in vivo and in vitro, which might be due to a reduction of neurotoxicity. This finding suggests that natural products used in traditional medicinal practices are a good source for finding potential therapeutic agents for diseases that need more effective treatments.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81571061 and 81671061), the Scientific Study Project for Institutes of Higher Learning, Ministry of Education, Liaoning Province, China (LZ2017001), Liaoning Provincial Key R&D Program (2019JH2/10300043) and the Liaoning Revitalization Talents Program (XLYC1808031).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Xu-Gang Wang, Dan-Dan Zhu and Na Li have contributed equally to this work.
Change history
1/27/2025
A Correction to this paper has been published: 10.1007/s12264-024-01340-w
Contributor Information
Shao Li, Email: lishao89@hotmail.com, Email: lishao89@dmu.edu.cn.
Jie Zhao, Email: dlzhaoj@163.com.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014, 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology: Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Zhu L, Li F, Wang J, Wan H, Pan Y. Bone marrow stromal cells as a therapeutic treatment for ischemic stroke. Neurosci Bull 2014, 30: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 2011, 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aromugam TV, Woodruff TM, Lathia JD, Selvaraj PK, Mattson MP, Taylor SM. Neuroprotection in stroke by complement inhibition and immunoglohulin therapy. Neuroscience 2009, 158: 1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Zjpfel GJ, Choi DW. The changing landscape of ischemic brain injury mechanisms. Nature 1999, 399: A7–14. [DOI] [PubMed] [Google Scholar]
- 8.Cao Z, Wu XF, Peng Y, Zhang R, Li N, Yang JY, et al. Scorpion venom heat-resistant peptide attenuates glial fibrillary acidic protein expression via c-jun/ap-1. Cell Mol Neurobiol 2015, 35: 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Wang SW, Zhang Y, Wu XF, Peng Y, Cao Z, et al. Scorpion venom heat-resistant peptide (SVHRP) enhances neurogenesis and neurite outgrowth of immature neurons in adult mice by up-regulating brain-derived neurotrophic factor (BDNF). PLoS One 2014, 9: e109977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin SM, Zhao D, Yu DQ, Li SL, An D, Peng Y, et al. Neuroprotection by scorpion venom heat resistant peptide in 6-hydroxydopamine rat model of early-stage Parkinson’s disease. Sheng Li Xue Bao 2014, 66: 658–666. [PubMed] [Google Scholar]
- 11.Zhang XG, Wang X, Zhou TT, Wu XF, Peng Y, Zhang WQ, et al. Scorpion venom heat-resistant peptide protects transgenic Caenorhabditis elegans from β-amyloid toxicity. Front Pharmacol 2016, 7: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Tong L, Luan Q, Deng J, Li Y, Li Z, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K (ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab 2012, 32: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hossmann KA. Cerebral ischemia: models, methods and outcomes. Neuropharmacolog 2008, 55: 257–270. [DOI] [PubMed] [Google Scholar]
- 14.Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral arterv occlusion in the mouse. Neurol Res 1997, 19: 641–648. [DOI] [PubMed] [Google Scholar]
- 15.Tsubokawa T, Jadhav V, Solaroglu I, Shiokawa Y, Konishi Y, Zhang JH. Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke 2007, 38: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 16.Mdzinarishvili A, Kiewert C, Kumar V, Hillert M, Klein J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience 2007, 144: 217–222. [DOI] [PubMed] [Google Scholar]
- 17.Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J Neurochem 2007, 103: 1666–1681. [DOI] [PubMed] [Google Scholar]
- 18.Shu JC, Bradley ME, Lee TC. Chemical hypoxia triggers apoptosis of cultured neonatal rat cardiac myocytes: Modulation by calcium-regulated proteases and protein kinases. Mole Cell Bioch 1998, 178: 141–149. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg MP, Choi DW. Oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci 1993, 13: 3510-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JB, Song NN, Wei XB, Guan HS, Zhang XM. Protective effects of paeonol on cultured rat hippocampal neurons against oxygen-glucose deprivation induced injury. J Neurol Sci 2008, 264: 50–55. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Zha RP, Wang WY, Wang YP. Effects of scutellarin on PKCgamma in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharmacol Sin 2007, 28: 1573–1579. [DOI] [PubMed] [Google Scholar]
- 22.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 1996, 16: 6125–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog Neurobiol 2012, 96: 69–86. [DOI] [PubMed] [Google Scholar]
- 24.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995, 15: 961–973. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, et al. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39: 3042–3048. [DOI] [PubMed] [Google Scholar]
- 26.Liu, B, Li L, Zhang Q, Chang N, Wang D, Shan Y, et al. Preservation of GABAA receptor function by PTEN inhibition protects against neuronal death in ischemic stroke. Stroke 2010, 41: 1018–1026. [DOI] [PubMed] [Google Scholar]
- 27.Lyden PD, Jackson-Friedman C, Shin C, Hassid S. Synergistic combinatorial stroke therapy: A quantal bioassay of a GABA agonist and a glutamate antagonist. Exp Neurol 2000, 163: 477–489. [DOI] [PubMed] [Google Scholar]
- 28.Krupinski J, Slevin M, Marti E, Catena E, Rubio F, Gaffney J. Time-course phosphorylation of the mitogen activated protein (MAP) kinase group of signalling proteins and related molecules following middle cerebral artery occlusion (MCAO) in rats. Neuropathol Appl Neurobiol 2003, 29: 144–158. [DOI] [PubMed] [Google Scholar]
- 29.Piao CS, Che Y, Han PL, Lee JK. Delayed and differential induction of p38 MAPK isoforms in microglia and astrocytes in the brain after transient global ischemia. Mol Brain Res 2002, 107: 137–144. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 2008, 55: 363–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moussaddy A, Demchuk AM, Hill MD. Thrombolytic therapies for ischemic stroke: Triumphs and future challenges. Neuropharmacology 2018, 134: 272–279. [DOI] [PubMed] [Google Scholar]
- 32.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 2007, 54: 34–66. [DOI] [PubMed] [Google Scholar]
- 33.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 2008, 37: 7–38. [DOI] [PubMed] [Google Scholar]
- 34.Aromugam TV, Woodruff TM, Lathia JD, Selvaraj PK, Mattson MP, Taylor SM. Neuroprotection in stroke by complement inhibition and immunoglohulin therapy. Neuroscience 2009, 158: 1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burd I, Welling J, Kannan G, Johnston MV. Excitotoxicity as a common mechanism for fetal neuronal injury with hypoxia and intrauterine inflammation. Adv Pharmacol 2016, 76: 85–101. [DOI] [PubMed] [Google Scholar]
- 36.Howes MJ, Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 2003, 75: 513–527. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Luo X, Bai D. Progress in clinical, pharmacological, chemical and structural biological studies of huperzine A: a drug of traditional Chinese medicine origin for the treatment of Alzheimer’s disease. Curr Med Chem 2003, 10: 2231–2252. [DOI] [PubMed] [Google Scholar]
- 38.Yan H, Li L, Tang XC. Treating senile dementia with traditional Chinese medicine. Clin Interv Aging 2007, 2: 201–208. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Wang W, Shao Z, Gao B, Li J, Ma J, et al. Eukaryotic expression and purification of anti-epilepsy peptide of Buthus martensii Karsch and its protein interactions. Mol Cell Biochem 2009, 330: 97–104. [DOI] [PubMed] [Google Scholar]
- 40.Gong JP, Gwee MC, Gopalakrishnakone P. Buthus martensi karsch venom: prejunctional adrenergic activity in the rat isolated anococcygeus muscle. Toxicon 1995, 33: 1133–1139. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan KN, Nirthanan S, Sasaki T, Sato K, Cheng B, Gwee MC, et al. Functional site of bukatoxin, an alpha-type sodium channel neurotoxin from the Chinese scorpion (Buthus martensi Karsch) venom: probable role of the (52) PDKVP (56) loop. FEBS Lett 2001, 494: 145–149. [DOI] [PubMed] [Google Scholar]
- 42.Ye P, Jiao Y, Li Z, Hua L, Fu J, Jiang F, et al. Scorpion toxin BmK I directly activates Nav1.8 in primary sensory neurons to induce neuronal hyperexcitability in rats. Protein Cell 2015, 6: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Wang Z, Jin J, Pei X, Zhao Y, Wu H, et al. Parkinson’s disease-like forelimb akinesia induced by BmK I, a sodium channel modulator. Behav Brain Res 2016, 308: 166–176. [DOI] [PubMed] [Google Scholar]
- 44.Mitani A, Kataoka K. Critical levels of extracellular glutamate mediating gerbil hippocampal delayed neuronal death during hypothermia: Brain microdialysis study. Neuroscience 1991, 42: 661–670. [DOI] [PubMed] [Google Scholar]
- 45.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001, 11: 327–335. [DOI] [PubMed] [Google Scholar]
- 46.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 2007, 8: 413–426. [DOI] [PubMed] [Google Scholar]
- 47.Prybylowski K, Wenthold RJ. N-methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem 2004, 279: 9673–9676. [DOI] [PubMed] [Google Scholar]
- 48.Su F, Guo AC, Li WW, Zhao YL, Qu ZY, Wang YJ, et al. Low-dose ethanol preconditioning protects against oxygen-glucose deprivation/reoxygenation-induced neuronal injury by activating large conductance, Ca2+-activated K+ channels in vitro. Neurosci Bull 2017, 33: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prybylowski K, Wenthold RJ. N-methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem 2004, 279: 9673–9676. [DOI] [PubMed] [Google Scholar]
- 50.Chang YY, Gong XW, Gong HQ, Liang PJ, Zhang PM, Lu QC. GABAA receptor activity suppresses the transition from interictal to ictal epileptiform discharges in juvenile mouse hippocampus. Neurosci Bull 2018, 34:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mele M, Aspromonte MC, Duarte CB. Downregulation of GABAA receptor recycling mediated by HAP1 contributes to neuronal death in in vitro brain ischemia. Mol Neurobiol 2017, 54: 45–57. [DOI] [PubMed] [Google Scholar]
- 52.Kovalska M, Kovalska L, Pavlikova M, Janickova M, Mikuskova K, Adamkov M, et al. Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury. Neurochem Res 2012, 37:1568–1577. [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Liu X, Wang H, Qu M. Gastrodin attenuates pentylenetetrazole-induced seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses in mice. Neurosci Bull 2017, 33: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab 2002, 22: 631–647. [DOI] [PubMed] [Google Scholar]
- 55.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev 2001, 21: 129–145. [DOI] [PubMed] [Google Scholar]
- 56.Jiang M, Li J, Peng Q, Liu Y, Liu W, Peng J, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation 2014, 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strassburger M, Braun H, Reymann KG. Anti-inflammatory treatment with the p38 mitogen-activated protein kinase inhibitor SB239063 is neuroprotective, decreases the number of activated microglia and facilitates neurogenesis in oxygen-glucose-deprived hippocampal slice cultures. Eur J Pharmacol 2008, 592: 55–61. [DOI] [PubMed] [Google Scholar]