Abstract
Objective
To characterize the most relevant changes in the lipidome of endometrial fluid aspirate (EFA) in non-implantative cycles.
Design
Lipidomics in a prospective cohort study.
Settings
Reproductive unit of a university hospital.
Patients
Twenty-nine women undergoing an IVF cycle. Fifteen achieved pregnancy and 14 did not.
Intervention
Endometrial fluid aspiration immediately before performing embryo transfer.
Main outcome measures
Clinical pregnancy rate and lipidomic profiles obtained on an ultra-high performance liquid chromatography coupled to time-of-flight mass spectrometry (UHPLC-ToF-MS)-based analytical platform.
Results
The comparative analysis of the lipidomic patterns of endometrial fluid in implantative and non-implantative IVF cycles revealed eight altered metabolites: seven glycerophospholipids and an omega-6 polyunsaturated fatty acid. Then, women with a non-implantative cycle were accurately classified with a support vector machine algorithm including these eight lipid metabolites. The diagnostic performances of the algorithm showed an area under the receiver operating characteristic curve, sensitivity, specificity, and accuracy of 0.893 ± 0.07, 85.7%, 80.0%, and 82.8%, respectively.
Conclusion
A predictive lipidomic signature linked to the implantative status of the endometrial fluid has been found.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01670-z) contains supplementary material, which is available to authorized users.
Keywords: Endometrial fluid, Implantation, Lipidomics, In vitro fertilization, Pregnancy, Assisted reproduction, Machine learning algorithms
Introduction
Implantation is one of the most inefficient steps in assisted reproduction techniques (ART) [1]. In recent years, the endometrial side of implantation has been receiving increased attention. Most studies have been based on an endometrial biopsy performed in a previous cycle, since performing an endometrial biopsy in the cycle in which embryo transfer is performed could have a detrimental effect on implantation [2, 3]. If the results of the aforementioned biopsy meet certain requirements, the endometrium is called “receptive,” implying that the transfer of a good quality embryo should result in pregnancy [4, 5]. Nonetheless, it is well known that pregnancy is not always achieved in cases of “receptive endometrium.” On the other hand, in fresh embryo in vitro fertilization (IVF) transfers, advanced endometrial maturation may happen [6, 7]. An advancement of endometrial maturation of 2.5 days has been observed in antagonist cycles at oocyte retrieval [7]. Endometrial advancement exceeding 3 days never resulted in an ongoing clinical pregnancy [7]. Apparently, it is this aberrant maturation of the endometrium that leads to dyssynchrony with the developing embryo causing reduced success rates after embryo transfer in the same cycle when compared with embryo transfer in frozen or donor oocyte recipient cycles [8].
Therefore, we have focused our research in what we have called “implantative” cycles, that is, cycles in which implantation takes place. It has been demonstrated that performing an aspiration of endometrial fluid in the very same cycle as embryo transfer has no detrimental effect on implantation [2, 9]. We have found a different pattern of protein expression in implantative cycles [2, 10], making it evident that endometrial/endometrial fluid protein status and protein metabolism are very different in implantative and non-implantative cycles.
Metabolomics and a branch thereof, lipidomics, are attracting increasing attention in the clinical arena, including pregnancy-related diseases [3, 5, 11]. This interest is founded on the close association between the metabolites and the phenotype, which implies that metabolomics may serve as a powerful tool for the real-time assessment of a given metabolic state. In recent years, several metabolomic profiling studies have linked specific metabolic patterns with endometriosis [12–14], endometrial cancer [15], endometrial receptivity [3], and good prognosis follicular fluid [16], among other conditions. Endometrial fluid during normal ovarian cycles has also been studied revealing an increase in prostaglandins during the window of implantation in normal ovarian cycles [17]. In implantative freeze-thawed cycles, a different lipidomic pattern in endometrial fluid has been reported, employing MALDI-TOF technology [18].
Here we studied the lipidome of endometrial fluid aspirate (EFA) in women undergoing an IVF cycle in order to establish the most relevant differences in the lipid patterns of implantative and non-implantative cycles. Thus, the aim of this study was to determine a combination of metabolites that could accurately discriminate between women with implantative and non-implantative cycles and, therefore, predict the implantation outcome.
Materials and methods
Ethical approval
This study was approved by the Institutional Review Board (code CEIC 11/45). All the patients included in the study provided informed consent.
Study population
The population under study consisted of 29 women undergoing an IVF/intracytoplasmic sperm injection (ICSI) cycle at the Reproductive Unit of Cruces University Hospital, in which EFA was obtained at the time of embryo transfer. Out of the 29 women, 15 achieved pregnancy (implantative cycles) and 14 did not (non-implantative cycles).
The inclusion criteria were as follows: (i) first or second IVF/ICSI cycle; (ii) woman’s age between 18 and 40 years; (iii) no known uterine or endometrial conditions (myomas, polyps, malformations, adenomyosis, endometritis, thin endometrium); (iv) absence of hydrosalpinx; (v) no infectious risk; (vi) transfer on day 3 of 1–2 fresh embryos, at least one being of good quality; and (vii) fresh own oocyte cycles without preimplantation genetic diagnosis or testicular biopsy. Patients where difficult transfers were expected (previous difficult mock transfer) were excluded.
The characteristics of the study population are detailed in Table 1. The most common indications for IVF were male factor (37.9%), insemination failure (20.7%), tubal factor (10.3%), and endometriosis (10.3%).
Table 1.
Demographic and clinical characteristics in implantative and non-implantative cycles
| Implantative cycles (n = 15) |
Non-implantative cycles (n = 14) |
|
|---|---|---|
| Age, years | 36.2 ± 2.6 | 36.6 ± 2.8 |
| Body mass index, kg/m2 | 25.4 ± 3.0 | 25.9 ± 3.2 |
| % Smokers | 26.7 | 28.6 |
| % Male factor | 40 | 35.7 |
| % Previous insemination failure | 20 | 21.4 |
| % Tubal factor | 6.7 | 14.3 |
| % Endometriosis | 13.3 | 7.1 |
| % First cycle | 60 | 50 |
| Days of stimulation | 11.9 ± 1.9 | 11.6 ± 1.7 |
| Estradiol the day of hCG (pg/mL) | 2104.6 ± 928.1 | 2031.2 ± 1048.5 |
| P4 the day of hCG (ng/mL) | 0.84 ± 0.22 | 0. 95 ± 0.23 |
| Oocytes obtained | 10.3 ± 6.7 | 10.8 ± 7.3 |
| MII oocytes | 7.9 ± 6.2 | 8.2 ± 5.4 |
| Embryos obtained | 5.3 ± 4.8 | 4.8 ± 4.7 |
| Embryos transferred | 1.4 ± 0.4 | 1.6 ± 0.3 |
| % Twins | 13.3 |
Non-significant differences
Sample collection
Our standard IVF protocol has been described previously [2, 8]. Briefly, it consist of a conventional antagonist protocol, ovarian stimulation being performed with only recombinant follicle-stimulating hormone (rFSH) in women ≤ 35 years, and with combinations of rFSH and human menopausal gonadotropin (hMG) or luteinizing hormone (LH) in women aged 36–39. Ovulation was triggered with recombinant human chorionic gonadotropin (rec hCG) (Ovitrelle, Merck, Madrid, Spain) when ≥ 3 follicles ≥ 18.5 mm were observed. Oocyte retrieval was scheduled 36 h after triggering ovulation. If on the day of administering hCG, the progesterone level was > 1.6 ng/mL, embryos were frozen and transfer was performed in a later cycle (and the case was not included in our study). The luteal phase was supported by progesterone 200 mg every 12 h, starting the day of oocyte pick-up. The hormone was administered orally until embryo transfer, and thereafter vaginally.
Inseminated/microinjected oocytes were cultured in micro drop plates with single culture medium in premixed gas incubator and CO2 incubator. Embryo quality was assessed by means of ASEBIR morphological criteria [19]. Embryos were evaluated on days 2 and 3 after IVF/ICSI. Embryo quality was determined based on the number of blastomeres, the percentage of fragmentation, blastomere symmetry, the characteristics of the vacuoles and of the zona pellucida, and the presence of multinucleated cells [19].
Pregnancy was defined as visualization of a gestational sac 4 weeks after embryo transfer. Biochemical pregnancies were not included in the study.
Women underwent a standard IVF cycle, and a sample of endometrial fluid was taken immediately before embryo transfer, as previously reported [2, 10]. Transfers were performed on day 3 in all cases. The sample was obtained using an embryo transfer catheter connected to a 10-mL syringe, under ultrasound guidance. The catheter was inserted gently, avoiding traumatizing the cervix or touching the uterine fundus. The catheter was inserted directly through the external cervical orifice, without any contact with the vaginal wall or the ectocervix. Aspiration was initiated once the distal end of the catheter was in the uterine cavity (near the fundus) and was interrupted before removal. Endometrial fluid aspiration was poured into the Eppendorf tube without touching its walls. We excluded women in whom we observed abnormal cervical mucus or leucorrhea (n = 2). Aspiration was performed manually. Only samples showing no presence of blood were considered for lipidomic analysis. Four samples were excluded (blood tainted or insufficient material). Samples collected were stored at − 80 °C until analysis.
Lipid extraction and UHPLC-MS analysis
The extraction method was set up using a combination of organic solvents that allows a wide coverage of the endometrial fluid lipidome. Extracts were analyzed on an ultra-high performance liquid chromatography coupled to time-of-flight mass spectrometry (UHPLC-ToF-MS)-based analytical platform, optimized for lipid profiling. Briefly, the endometrial fluid samples were defrosted on ice and mixed with 450 μL of a water/methanol/chloroform mixture (1:2.5:1, v/v/v). After centrifugation at 16,000×g for 20 min at room temperature, 200 μL of the supernatants was collected, dried, and reconstituted in 60 μL of methanol for the UHPLC-ToF-MS profiling of fatty acids (FAs), oxidized fatty acids (oxFAs), N-acylethanolamines, bile acids, and lysoglycerophospholipids (lysophosphatidylcholines, lysophosphatidylethanolamines, and lysophosphatidylinositols, either acyl- or ether-linked species). In addition, another 200 μL of the supernatants was collected and mixed with sodium chloride (50 mM). These extracts were incubated for 30 min at − 20 °C. After centrifugation at 16,000×g for 15 min at 4 °C, 35 μL of the organic phase was collected and the solvent removed. The dried extracts were then reconstituted in 60 μL of acetonitrile/isopropanol (1:1, v/v) for the analysis of glycerolipids (triglycerides and diglycerides), cholesteryl esters, sphingolipids (ceramides, hexosylceramides, and sphingomyelins), and glycerophospholipids (phosphatidylcholines, phosphatidylethanolamines, and phosphatidylinositols, either diacyl or 1-ether, 2-acyl-linked species).
Lipidomic profiles of the endometrial fluid aspirates were semi-quantified as described previously [20, 21] with two separate UHPLC-ToF-MS-based platforms. Chromatographic separation and mass spectrometric detection conditions are detailed in Supplementary Material. For both analytical platforms, randomized sample injections were performed. The study samples were accompanied by repeat extracts of a pooled sample, used for assessing data quality. This pooled sample was analyzed at the same time as the individual samples, evenly distributed over the batch.
Data analysis
The steps followed for data analysis are summarized in Fig. 1. First, data pre-processing was carried out using the TargetLynx application manager for MassLynx 4.1 software (Waters Corp., Milford, USA). The LC-MS features included in this study were identified prior to the analysis, as described in Supplementary Material. Data normalization was performed following the procedure described by Manni et al. [20]. The peak intensities for each ion feature included in the analysis were normalized to the sum of the peak intensities within each sample. There was no significant difference (p value > 0.05) between the sums of the peak intensities for the groups being compared in this study (implantative vs. non-implantative endometrium). Missing values were evaluated in the data matrix. One tenth of the minimum value was assigned to these observations [22–24]. Then, univariate data analysis was performed. Data for each of the groups are represented as means ± SDMs. Metabolites were evaluated by calculating group percentage changes and p values from unpaired Student’s t test or Welch test where the variances were not equal. All calculations were performed using the statistical software package R v.3.4.1 (R Development Core Team, 2017; http://cran.r-project.org).
Fig. 1.
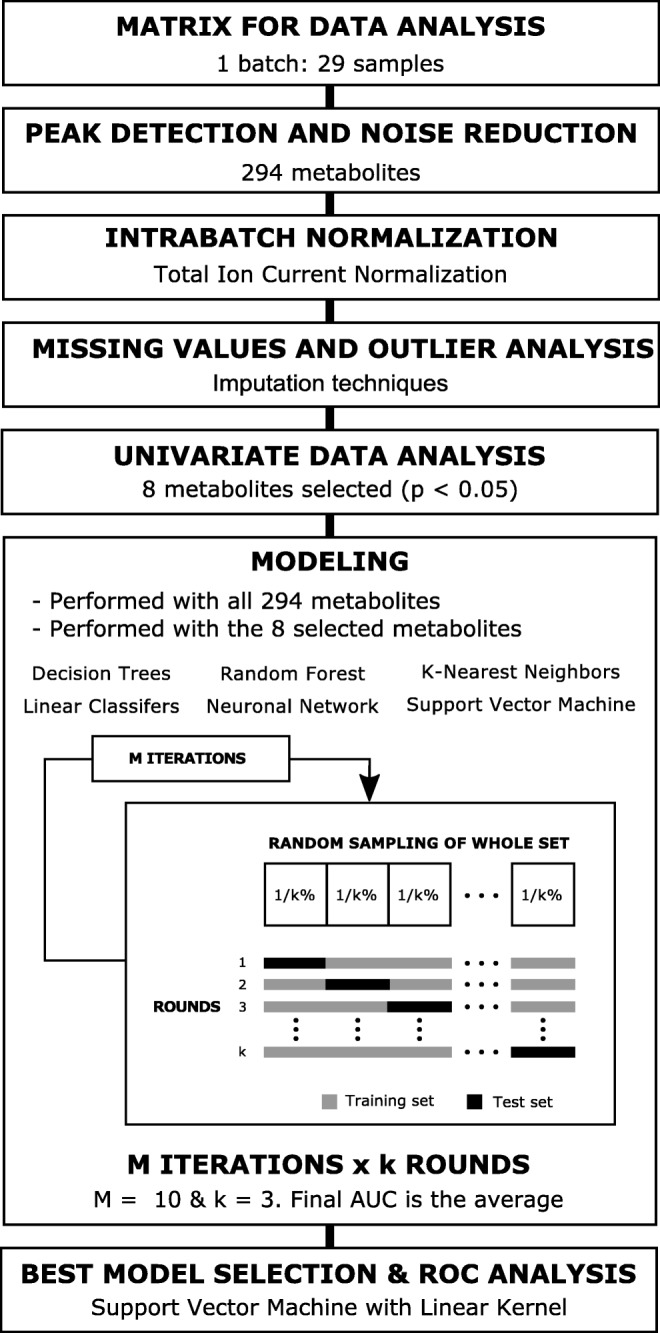
Data analysis workflow. Detailed flowchart of data analysis procedure followed. Data matrix includes 29 samples and 294 metabolites (lipid species). Peak intensity for each metabolite was normalized to the sum of the peak intensities within each sample. Once normalized, outlier analysis was performed and imputation techniques were used for the missing values. Univariate analysis (Student’s t test) was then carried out. Eight metabolites were found to be significantly altered (p < 0.05) in the non-implantative endometrium group, compared to levels in the implantative endometrium. Different modeling approaches were tested taking into account all metabolites and only the metabolites selected after univariate analysis: decision trees, random forests, k-nearest neighbors, linear classifiers, a neural network, and support vector machines. A three-fold cross-validation was performed with a 10 resampling for each modeling. After ROC analysis, the support vector machine model with a linear kernel was selected
Exploratory data modeling was carried out following two approaches: (1) including the significant metabolites (p < 0.05), and (2) all the LC-MS features detected. Several model methodologies were tested: decision trees, linear classifiers, a neural network, a random forest, k-nearest neighbors, and support vector machines (SVMs) (Fig. 1). A detailed description is included in Supplementary Material. Furthermore, pre-processing of the data was also evaluated, comparing non-pre-processed, centered and scaled, Box-Cox transformed [21], and Box-Cox transformed, centered and scaled data [25–27]. The model providing the best results in the exploratory data analysis was subsequently optimized and evaluated applying a three-fold cross-validation with a 10 resampling for each parameter (or tuple of parameters). The caret R-package was used to evaluate this methodology [28].
Receiver operating characteristic (ROC) curve analysis was used to assess the discriminatory power of the model. Overall diagnostic accuracy of the model was evaluated by the area under the ROC curve (AUC) with its associated standard error. Sensitivity, specificity, positive predictive value, and negative predictive value were also calculated as described in Supplementary Material.
Results
We have analyzed the lipidome of EFA samples collected from 15 women that achieved pregnancy and 14 that did not. We have used an UHPLC-ToF-MS-based analytical platform able to detect a total of 294 different lipid species (detailed in Supplementary Table 1), including FAs (24), oxFAs (5), N-acylethanolamines (2), bile acids (5), steroids (1), cholesterol, cholesteryl esters (8), diglycerides (7), triglycerides (48), phosphatidylcholines (106), phosphatidylethanolamines (47), phosphatidylinositols (4), ceramides (11), monohexosylceramides (2), and sphingomyelins (23). Evaluation of the data revealed 174 missing values (< 2%) due to levels being below the limits of detection. Missing values were randomly distributed in implantative and non-implantative groups, and the percentage of missing values remained lower than 7% for all the metabolites.
Differential lipid levels between implantative and non-implantative endometrial fluid
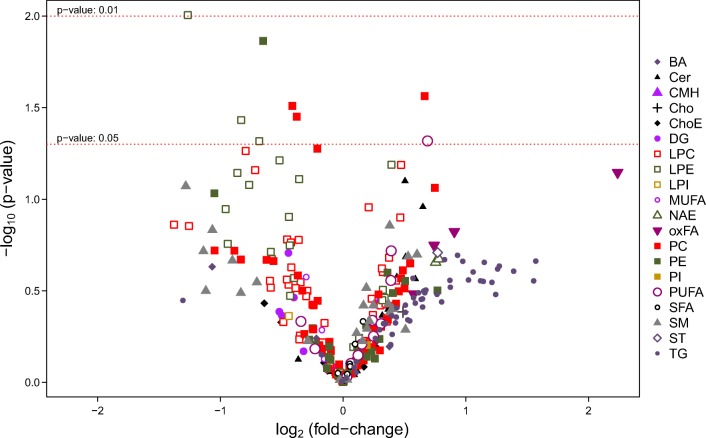
Univariate assessment of the 294 lipids for discriminating between implantative and non-implantative EFA was performed (Fig. 2). Eight lipids were significantly altered in the non-implantative cycles under the t test criterion (p < 0.05) (Table 2). Except the polyunsaturated fatty acid (PUFA) docosapentaenoic acid (22:5n-6), all of them were glycerophospholipids (phosphatidylcholines and phosphatidylethanolamines) with unsaturated acyl chains. Only two of these metabolites appeared at higher levels in the non-implantative groups: polyunsaturated phosphatidylcholine PC(40:8) and omega-6 docosapentaenoic acid (Table 2). The remaining six were significantly lower in the non-implantative cycles.
Fig. 2.
Differential metabolite levels in implantative compared to non-implantative endometrial fluid aspirate (EFA). Volcano plot representation indicating the -log10 (p value) vs. log2 (fold-change) of each individual metabolic ion features for the comparison implantative vs. non-implantative endometrium. BA, bile acid; Cer, ceramide; CMH, monohexosylceramide; Cho, cholesterol; ChoE, cholesteryl ester; DG, diglyceride; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; MUFA, monounsaturated fatty acid; NAE, N-acyl ethanolamine; oxFA, oxidized fatty acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; SM, sphingomyelin; ST, steroid; TG, triglyceride
Table 2.
Significantly (p < 0.05) altered metabolites in non-implantative cycles comparing to implantative cycles. Fold-changes and p values from unpaired Student’s t test (or Welch test where the variances were not equal) are indicated
| Metabolite | Metabolic class | Implantative | Non-implantative | Fold-change | p value |
|---|---|---|---|---|---|
| LPE(20:5) | LPE | 0.105 ± 0.078 | 0.044 ± 0.023 | 0.416 | 0.010 |
| PE(16:1e/20:5) | 1-ether, 2-acyl-PE | 0.014 ± 0.006 | 0.009 ± 0.005 | 0.636 | 0.014 |
| PC(40:8) | Diacyl-PC | 0.008 ± 0.004 | 0.012 ± 0.007 | 1.585 | 0.027 |
| PC(18:0/18:1) | Diacyl-PC | 5.576 ± 1.836 | 4.186 ± 1.405 | 0.751 | 0.031 |
| PC(18:0/18:2) | Diacyl-PC | 8.138 ± 2.602 | 6.260 ± 1.878 | 0.769 | 0.035 |
| LPE(22:5/0:0) | LPE | 0.414 ± 0.287 | 0.233 ± 0.119 | 0.562 | 0.037 |
| Docosapentaenoic acid (22:5n-6) | Omega 6-PUFA | 0.118 ± 0.089 | 0.192 ± 0.102 | 1.611 | 0.048 |
| LPE(18:2/0:0) | LPE | 0.634 ± 0.358 | 0.395 ± 0.250 | 0.623 | 0.048 |
Abbreviations: LPE, lysoglycerophosphatidylethanolamine; 1-ether, 2-acyl-PE, 1-ether, 2-acylglycerophosphatidylethanolamine; Diacyl-PC, diacylglycerophosphatidylcholine; PUFA, polyunsaturated fatty acid
Predictive model for implantative endometrial status
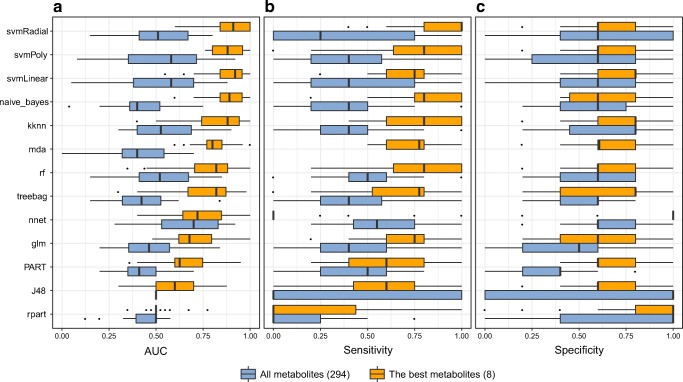
Several different methodologies (decision trees, linear classifiers, a neural network, random forests, k-nearest neighbors, and SVMs) and pre-processing approaches (no pre-processing, centering and scaling, Box-Cox transformation, and Box-Cox transformation plus centering and scaling) were evaluated in order to generate a predictive model of the endometrial implantative status. These methodologies were tested including all the lipids detected and only the eight metabolites selected based on the univariate analysis (Table 2). The discriminatory power of the models was assessed using ROC curve analysis (Supplementary Figure 1). Despite the range of different pre-processing approaches tested, similar results were obtained in all cases. Therefore, we selected the approach without pre-processing for simplicity. A summary of the performance of all the models without a pre-processing step is shown in Fig. 3, showing the distributions of AUC, sensitivity, and specificity values from the cross-validation analysis.
Fig. 3.
Summary of models run without data pre-processing for the prediction of the endometrial implantative status. Boxplots show the distribution of a AUC, b sensitivity, and c specificity in the cross-validation analysis for the following methodologies: decision trees (treebag, rpart, PART, and J48), linear classifiers (Naïve-Bayes, naïve_bayes; generalized linear models, glm; and flexible discriminant analysis, mda), k-nearest neighbors, kknn; a neural network, nnet; random forests, rf; and a support vector machine with several kernels: linear, svmLinear; radial, svmradial; and polynomial, svmPoly. All these methodologies were tested including all variables detected (a total of 294 lipids) and only the eight significant metabolites selected under the t test criterion (p < 0.05). Models are ordered from the best to worst performance based on AUC. Missing boxplots indicate a failure in the model applied
Regardless of the methodology, models considering all the lipids detected performed less well than those considering only the metabolites selected based on the t test criterion (Fig. 3), and therefore, models including all the metabolites were not analyzed further. The discriminatory power of models with the best eight metabolites was then assessed, selecting SVM algorithms as the best choice for classifying endometrial implantative status. The most common kernels to construct SVM models were also considered (linear, radial, and polynomial; each with their specific tuning parameters optimized), all obtaining similar results in the ROC curve analysis (Table 3 and Fig. 4). As the final model, we propose the SVM with a linear kernel and without pre-processing of the data, as it allows for a more parsimonious design and hence efficient parameterization. The cost, or the tuning parameter C, was optimized to C = 0.28. The diagnostic performance of this model showed an AUC, sensitivity, specificity, positive predictive value, and negative predictive value of 0.893 ± 0.072, 0.857, 0.800, 0.800, and 0.857, respectively.
Table 3.
ROC analysis summary of support vector machine models depending on the kernel applied without pre-processing of the data. Analysis performed for the optimal tuning parameters for each kernel (linear kernel: C = 0.28; radial kernel: C = 2.74 and sigma = 0.017; polynomial kernel: C = 1.09, scale = 0.024, and degree = 1)
| Linear | Radial | Polynomial | |
|---|---|---|---|
| Accuracy | 0.828 | 0.862 | 0.828 |
| Sensitivity | 0.857 | 0.857 | 0.857 |
| Specificity | 0.800 | 0.867 | 0.800 |
| Positive predictive value | 0.800 | 0.857 | 0.800 |
| Negative predictive value | 0.857 | 0.867 | 0.857 |
| Positive likelihood ratio | 4.286 | 6.429 | 4.286 |
| Negative likelihood ratio | 0.179 | 0.165 | 0.179 |
Fig. 4.
ROC analysis of the support vector machine models. a ROC curve by kernel. Analysis performed for the optimal tuning parameters: linear kernel: C = 0.28; radial kernel: C = 2.74 and sigma = 0.017; polynomial kernel: C = 1.09, scale = 0.024, and degree = 1. b Accuracy by kernel. A cutoff at 0.5 is considered. Models were calculated with data without pre-processing
Due to the specific condition of patients with endometriosis, performance of the model was assessed excluding these patients. The eight metabolites significantly altered in non-implantative cycles remained significant once patients were excluded (Supplementary Table 2) and the classification model yielded an AUC of 0.893 ± 0.073 (sensitivity, specificity, positive predictive value, and negative predictive value of 0.769, 0.846, 0.833, and 0.786, respectively).
Lipid levels and clinical parameters
None of the eight lipids differentially expressed in endometrial fluid were significantly associated with any of the clinical parameters investigated (maternal age, body mass index, estradiol levels, numbers of oocytes retrieved, mature oocytes, numbers of fertilized, top-quality, and transferred embryos and number of gestational sacs) (data not shown).
Discussion
The endometrium exhibits a number of changes over the course of the ovarian cycle. Many of them are related to the achievement of an adequate secretory phase where implantation can occur. One of the crucial features of secretory endometrium is the development of endometrial glands. In human implantation, the embryo plays the most important role. However, even in the absence of known maternal conditions, a remarkable proportion of chromosomally normal embryos fail to implant. On the other hand, the microenvironment provided by uterine fluid, particularly glandular secretions, is essential for implantation [29–31]. That is why we have directed our attention to the composition of the endometrial fluid.
Lipids play a vital role in modifying the physical properties and biological functions of membranes as well as providing the cell a source of nutrients [32]. In humans, the oocyte fatty acid composition is very different that fatty acid composition of other cells and tissues [33]. Lipid and lipophilic micronutrient concentrations in blood serum the day of oocyte retrieval have been associated with better outcomes in IVF [34]. In endometrial biopsies, it has been shown that premature progesterone elevation disrupts the lipid homeostasis of the endometrium during the peri-implantation period [35]. The altered lipid levels may impair endometrial receptivity and early embryo implantation [35]. Fatty acids oxidation plays an important role in embryo development in mice [36] and in implantation and endometrial stromal cell decidualization in both mice and humans [37].
The assessment of the composition of endometrial fluid is of great interest for two reasons: (i) it could be an indirect marker of implantation, reflecting endometrial status; and (ii) it could itself be a determinant of implantation. Previously, more than 800 proteins have been found by 2-D electrophoresis and mass spectrometry of endometrial fluid [38], and even more than 2200 when trapped ion mobility spectrometry, time-of-flight (TIMS-TOF) was employed [39]. We have also reported a very different proteomic pattern in implantative and non-implantative cycles [2, 10].
In this new study, we have performed a comparative analysis of the lipidomic patterns of endometrial fluid in implantative and non-implantative IVF cycles. The use of a metabolomics approach in the study of endometrial fluid samples aspirated at the very same time as embryo transfer is especially interesting, due to the strong link between the metabolites and the phenotype. Metabolomic profiling aims to provide a close real-time assessment of a specific metabolic state.
Only eight metabolites were significantly altered in endometrial fluid in non-implantative cycles, but remarkably, all of them were glycerophospholipids except one omega-6 PUFA. The levels of the lysophosphatidylethanolamines LPE(22:5/0:0), LPE(18:2/0:0), and LPE(20:5), the ether-linked phosphatidylethanolamine PE(16:1e/20:5), and the diacylphosphatidylcholines PC(18:0/18:1) and PC(18:0/18:2) were lower in the endometrial fluid of women in whom implantation did not occur. In contrast, levels of the docosapentaenoic acid (22:5n-6) and the diacylphosphatidylcholine PC(40:8) were higher.
Lysophospholipids, and particularly lysophosphatidylcholines, have received notable attention as potential biomarkers of infertility and pregnancy-related diseases [40, 41] due to their roles as lipid mediators of intracellular signaling and their dysregulated levels under inflammatory conditions. In addition, conversion to lysophosphatidic acid by lysophospholipase D has been reported to be important in the progress of human pregnancy [42], and several studies support the view that lysophosphatidic acid plays a role in pregnancy in mammals [43, 44]. Although most of the studies reported pro-inflammatory effects of lysophosphatidylcholines, there is also evidence of anti-inflammatory effects [40]. In a previous study, we showed that in non-implantative IVF cycles, proteins related to inflammation were over-expressed in the endometrial fluid [10]. Nonetheless, our present study did not reveal significant changes in the levels of lysophosphatidylcholines, while three lysophosphatidylethanolamines were diminished in non-implantative endometrial fluid. It is also interesting to point out that antiphospholipid antibodies, particularly antiphosphatidylethanolamine antibodies, have previously been linked to recurrent pregnancy loss and IVF failure in non-male factor infertility patients [45]. Altered levels of lysophosphatidylethanolamines have been also related to minimal/mild endometriosis (patients with laparoscopically confirmed endometriosis at stages I-II) [13].
Furthermore, roles of omega-3 and omega-6 PUFAs have also been studied in pregnancy and reproductive diseases [46], as the fetus requires a source of long chain PUFAs as a source of energy and lipid mediators. The serum levels of linoleic acid (LA), an omega-6 PUFA, and of alpha-linolenic acid (ALA), an omega-3 PUFA, have been shown to influence embryo implantation and pregnancy rates in women undergoing IVF [47]. A negative association has been found between serum ALA levels and pregnancy rates after IVF [48], while increased serum LA/ALA ratios have been reported to promote embryo implantation and increase pregnancy rates in these women [47]. This highlights the importance of maintaining an appropriate balance of LA and ALA during the different stages of the pregnancy. In our study, the non-implantative endometrium group had higher levels of docosapentaenoic acid (22:5n-6), which can be synthetized from LA [49]. We have recently shown how omega-3 and omega-6 fatty acid composition of human oocytes changes during oocyte maturation [50] and how fatty acid pattern is different in normal weight and in obese women [50].
Even though few significant differences were obtained when the lipid species were evaluated individually, a predictive lipidomic signature linked to the implantative status of the endometrial fluid was found. Then, the levels of the lipids identified were associated with the presence of alterations in the endometrial fluid linked to non-implantative cycles, and although our findings do not demonstrate a causal role of these metabolites, they likely reflect alterations in key pathways. Indeed, women with a non-implantative cycle are accurately classified with a SVM algorithm including the eight lipid metabolites selected in the univariate analysis. This classification model yielded an AUC of 0.893 ± 0.072, 85.7% sensitivity, 80.0% specificity, and 82.8% accuracy.
Our study has the limitation of underestimating endometrial implantation potential. That is, while in all cases of implantative cycles the endometrium and the embryo are adequate, in non-implantative cycles it cannot be ascertained whether the problem lies in the embryo, the endometrium, or both. Therefore, despite having adequate endometrium and endometrial fluid, pregnancy will not occur in some cases due to embryo conditions and these will be considered non-implantative cycles. This issue will make it more difficult to detect statistically significant differences but in no cases will differences appear to be significant if they are not. One could speculate that in PGT-A cycles, where the confounding of embryo quality is at some extent controlled, the influence of endometrial fluid lipidomics should be more evident. However, more studies are needed to determine whether our findings on day 3 in the stimulated cycles are consistent with what occurs on day 5 in the freeze-thawed cycles (with or without estrogen therapy).
Lipidome is one aspect of the complex process of endometrium receptivity and implantation, where other factors (genomic, proteiomic, metabolomic, cytokines, hormones, and embryo itself) are involved. Our findings, if confirmed, and if a quick test were to be developed, could led to the individualization of embryo transfer strategies, canceling the transfer in presence of an unfavorable lipidomic pattern in the endometrial fluid, or considering the possibility of double embryo transfer. On the other hand, one could speculate regarding a number of future options: endometrial fluid modification through diet or the instillation of certain compounds or modification of embryo culture medium.
Electronic supplementary material
(XLSX 20 kb)
(DOCX 16 kb)
(PDF 62 kb)
(DOCX 38.9 kb)
Funding information
This study was partially funded by a Grant for Fertility Innovation (GFI, 2011) from Merck (Darmstadt, Germany).
Compliance with ethical standards
Conflict of interest
IM-A, EA, MI-L, and CA are OWL Metabolomics’ employees.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matorras R, Urquijo E, Mendoza R, Corcóstegui B, Expósito A, Rodríguez-Escudero FJ. Ultrasound-guided embryo transfer improves pregnancy rates and increases the frequency of easy transfers. Hum Reprod. 2002;17:1762–1766. doi: 10.1093/humrep/17.7.1762. [DOI] [PubMed] [Google Scholar]
- 2.Matorras R, Quevedo S, Corral B, Prieto B, Exposito A, Mendoza R, et al. Proteomic pattern of implantative human endometrial fluid in in vitro fertilization cycles. Arch Gynecol Obstet. 2018;297:1577–1586. doi: 10.1007/s00404-018-4753-1. [DOI] [PubMed] [Google Scholar]
- 3.Vilella F, Ramirez LB, Simón C. Lipidomics as an emerging tool to predict endometrial receptivity. Fertil Steril. 2013;99:1100–1106. doi: 10.1016/j.fertnstert.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez F, Galan A, Martin JJL, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 5.Edgell TA, Rombauts LJF, Salamonsen LA. Assessing receptivity in the endometrium: the need for a rapid, non-invasive test. Reprod BioMed Online. 2013;27:486–496. doi: 10.1016/j.rbmo.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod BioMed Online. 2012;24:23–34. doi: 10.1016/j.rbmo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–1029. doi: 10.1016/s0015-0282(02)03323-x. [DOI] [PubMed] [Google Scholar]
- 8.Zapantis G, Szmyga MJ, Rybak EA, Meier UT. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod. 2013;28:3292–3300. doi: 10.1093/humrep/det358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Gaast MH, Beier-Hellwig K, Fauser B, Beier HM, Macklon NS. Endometrial secretion aspiration prior to embryo transfer does not reduce implantation rates. Reprod BioMed Online. 2003;7:105–109. doi: 10.1016/s1472-6483(10)61737-3. [DOI] [PubMed] [Google Scholar]
- 10.Azkargorta M, Escobes I, Iloro I, Elortza F, Osinalde N, Exposito A, et al. Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum Reprod. 2018;33:1898–1906. doi: 10.1093/humrep/dey274. [DOI] [PubMed] [Google Scholar]
- 11.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. MHR Basic Sci Reprod Med. 2008;14:679–690. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domínguez F, Ferrando M, Díaz-Gimeno P, Quintana F, Fernández G, Castells I, et al. Lipidomic profiling of endometrial fluid in women with ovarian endometriosis. Biol Reprod. 2017;96:772–779. doi: 10.1093/biolre/iox014. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Guan L, Zhang H, Gao Y, Sun J, Gong X, et al. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod Biol Endocrinol. 2018;16:42. doi: 10.1186/s12958-018-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Tan CW, Venkatratnam A, Tannenbaum SR, Cui L, Griffith L, et al. Dysregulated sphingolipid metabolism in endometriosis. J Clin Endocrinol Metab. 2014;99:E1913–E1921. doi: 10.1210/jc.2014-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trousil S, Lee P, Pinato DJ, Ellis JK, Dina R, Aboagye EO, et al. Alterations of choline phospholipid metabolism in endometrial cancer are caused by choline kinase alpha overexpression and a hyperactivated deacylation pathway. Cancer Res. 2014;74:6867–6877. doi: 10.1158/0008-5472.CAN-13-2409. [DOI] [PubMed] [Google Scholar]
- 16.Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril. 2012;97:1078–1084. doi: 10.1016/j.fertnstert.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 17.Vilella F, Ramirez L, Berlanga O, Martínez S, Alamá P, Meseguer M, Simón C. PGE2 and PGF2alpha concentrations in human endometrial fluid as biomarkers for embryonic implantation. J Clin Endocrinol Metab. 2013;98:4123–4132. doi: 10.1210/jc.2013-2205. [DOI] [PubMed] [Google Scholar]
- 18.Braga DPAF, Borges E, Jr, Godoy AT, Montani DA, Setti AS, Zanetti BF, Figueira RCS, Eberlin MN, Lo Turco EG. Lipidomic profile as a noninvasive tool to predict endometrial receptivity. Mol Reprod Dev. 2019;86:145–155. doi: 10.1002/mrd.23088. [DOI] [PubMed] [Google Scholar]
- 19.ASEBIR Special Interest Group of Embryology . Cuadernos de Embriología línica. Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos, 2nd ed. Madrid: ASEBIR; 2008. [Google Scholar]
- 20.Manni MM, Valero JG, Pérez-Cormenzana M, Cano A, Alonso C, Goñi FM. Lipidomic profile of GM95 cell death induced by Clostridium perfringens alpha-toxin. Chem Phys Lipids. 2017;203:54–70. doi: 10.1016/j.chemphyslip.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Barr J, Caballería J, Martínez-Arranz I, Domínguez-Díez A, Alonso C, Muntané J, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11:2521–2532. doi: 10.1021/pr201223p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrydziuszko O, Viant M. Missing values in mass spectrometry based metabolomics: an undervalued step in the data processing pipeline. Metabolomics. 2012;8:161–174. [Google Scholar]
- 23.Hair JF. Multivariate data analysis. New Jersey: Prentice Hall; 2009. [Google Scholar]
- 24.Baraldi AN, Enders CK. An introduction to modern missing data analyses. J Sch Psychol. 2010;48:5–37. doi: 10.1016/j.jsp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B. 1964;26:211–252. [Google Scholar]
- 26.van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Zhao X, Lu X, Lin X, Xu G. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front Mol Biosci. 2015;2:4. doi: 10.3389/fmolb.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn M, Johnson K. Applied predictive modeling. Berlin: Springer Science & Business Media; 2013. [Google Scholar]
- 29.Gray CA, Bazer FW, Taylor KM, Ramsey WS, Spencer TE, Hill JR, et al. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001;64:1608–1613. doi: 10.1095/biolreprod64.6.1608. [DOI] [PubMed] [Google Scholar]
- 30.Dunlap KA, Filant J, Hayashi K, Rucker EB, 3rd, Song G, Deng JM, et al. Postnatal deletion of wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85:386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamonsen LA, Evans J, Nguyen HP, Edgell TA. The microenvironment of human implantation: determinant of reproductive success. Am J Reprod Immunol. 2016;75:218–225. doi: 10.1111/aji.12450. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779:89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 33.Matorras R, Ruiz JI, Mendoza R, Ruiz N, Sanjurjo P, Rodriguez-Escudero FJ. Fatty acid composition of fertilization-failed human oocytes. Hum Reprod. 1998;13:2227–2230. doi: 10.1093/humrep/13.8.2227. [DOI] [PubMed] [Google Scholar]
- 34.Jamro EL, Bloom MS, Browne RW, Kim K, Greenwood EA, Fujimoto VY. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod BioMed Online. 2019;39:665–673. doi: 10.1016/j.rbmo.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Gao Y, Guan L, Zhang H, Chen P, Gong X, Li D, Liang X, Huang M, Bi H. Lipid profiling of peri-implantation endometrium in patients with premature progesterone rise in the late follicular phase. J Clin Endocrinol Metab. 2019;104:5555–5565. doi: 10.1210/jc.2019-00793. [DOI] [PubMed] [Google Scholar]
- 36.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 37.Tsai JH, Chi MM, Schulte MB, Moley KH. The fatty acid beta-oxidation pathway is important for decidualization of endometrial stromal cells in both humans and mice. Biol Reprod. 2014;90:34. doi: 10.1095/biolreprod.113.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, et al. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009;8:4622–4632. doi: 10.1021/pr9004426. [DOI] [PubMed] [Google Scholar]
- 39.Azkargorta M, Bregón-Villahoz M, Escobes I, Iloro I, Iglesias M, Diez Zapirain M, et al. In-depth proteomics and natural peptidomics analyses reveal the presence of antibacterial peptides in human endometrial fluid. (under editorial revision). [DOI] [PubMed]
- 40.Fuchs B, Muller K, Paasch U, Schiller J. Lysophospholipids: potential markers of diseases and infertility? Mini Rev Med Chem. 2012;12:74–86. doi: 10.2174/138955712798868931. [DOI] [PubMed] [Google Scholar]
- 41.Nagamatsu T, Iwasawa-Kawai Y, Ichikawa M, Kawana K, Yamashita T, Osuga Y, et al. Emerging roles for lysophospholipid mediators in pregnancy. Am J Reprod Immunol. 2014;72:182–191. doi: 10.1111/aji.12239. [DOI] [PubMed] [Google Scholar]
- 42.Tokumura A, Kanaya Y, Miyake M, Yamano S, Irahara M, Fukuzawa K. Increased production of bioactive lysophosphatidic acid by serum lysophospholipase D in human pregnancy. Biol Reprod. 2002;67:1386–1392. doi: 10.1095/biolreprod.102.004051. [DOI] [PubMed] [Google Scholar]
- 43.Sordelli MS, Beltrame JS, Cella M, Gervasi MG, Perez Martinez S, Burdet J, et al. Interaction between lysophosphatidic acid, prostaglandins and the endocannabinoid system during the window of implantation in the rat uterus. PLoS One. 2012;7:e46059. doi: 10.1371/journal.pone.0046059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boruszewska D, Kowalczyk-Zieba I, Sinderewicz E, Grycmacher K, Staszkiewicz J, Woclawek-Potocka I. The effect of lysophosphatidic acid together with interferon tau on the global transcriptomic profile in bovine endometrial cells. Theriogenology. 2017;92:111–120. doi: 10.1016/j.theriogenology.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Sher G, Maassarani G, Fisch JD, Chong P, Ching W, Matzner W. Antibodies to phosphatidylethanolamine and phosphatidylserine are associated with increased natural killer cell activity in non-male factor infertility patients. Hum Reprod. 2000;15:1932–1936. doi: 10.1093/humrep/15.9.1932. [DOI] [PubMed] [Google Scholar]
- 46.Akerele OA, Cheema SK. A balance of omega-3 and omega-6 polyunsaturated fatty acids is important in pregnancy. J Nutr Intermed Metab. 2016;5:23–33. [Google Scholar]
- 47.Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab. 2013;98:E1364–E1368. doi: 10.1210/jc.2012-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril. 2011;96:880–883. doi: 10.1016/j.fertnstert.2011.07.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2010;93:211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 50.Matorras R, Exposito A, Ferrando M, Mendoza R, Larreategui Z, Lainz L, et al. Oocytes of obese and overweight women have lower levels of n-3 polyunsaturated fatty acids compared to oocytes of normal weight women. Fertil Steril. (in press). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 20 kb)
(DOCX 16 kb)
(PDF 62 kb)
(DOCX 38.9 kb)