Abstract
Optoacoustic imaging (OAI), or photoacoustic imaging (PAI), has fundamentally influenced basic science by providing high-resolution visualization of biological mechanisms. With the introduction of multispectral optoacoustic tomography (MSOT), these technologies have now moved closer to clinical applications. MSOT utilizes short-pulsed near-infrared laser light to induce thermoelastic expansion in targeted tissues. This results in acoustic pressure waves, which are used to resolve specific endo- and exogenous chromophores. Especially in the pediatric population, this non-invasive imaging approach might hold fundamental advantages compared to conventional cross-sectional imaging modalities. As this technology allows the visualization of quantitative molecular tissue composition at high spatial resolution non-invasively in sufficient penetration depth, it paves the way to personalized medicine in pediatric diseases.
Technologic background
Up to now, ultrasound has played a central role in pediatric diagnostics. Due to its neglectable risk profile and easy availability, it is the diagnostic tool of choice for a variety of different indications [1–4]. As current state of the art ultrasound technologies, such as contrast-enhanced ultrasound (CEUS) do, on the one hand, allow functional flow-based tissue analysis [5, 6], but they essentially require the application of “acoustic” labels on the other. In addition, when compared to new cross-sectional imaging techniques (e.g., PET-CT), ultrasound is limited in visualizing subcellular composition of tissues and therefore diagnostic possibilities. All of this led to the development of new functional and molecular, non-invasive imaging approaches in recent years.
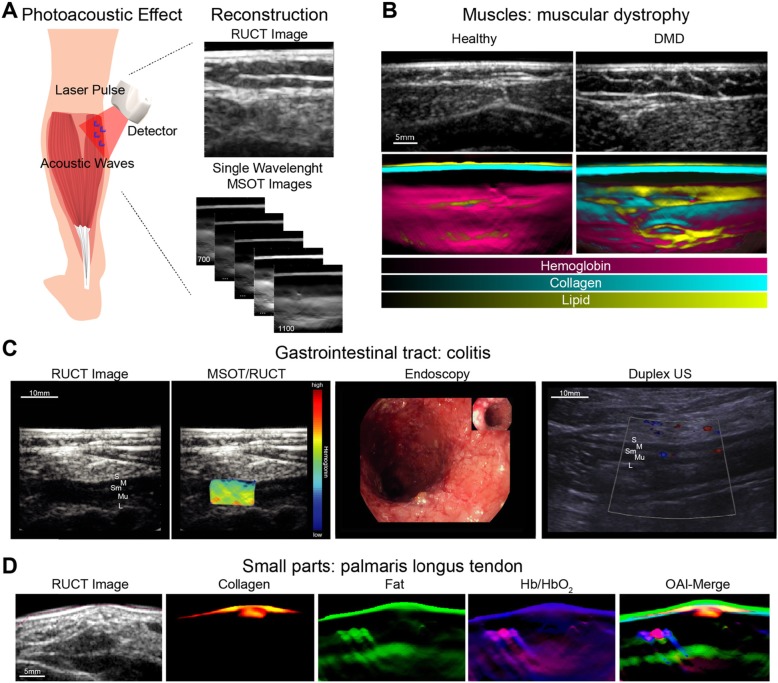
In this regard, multispectral optoacoustic tomography (MSOT) is rapidly evolving as one promising key technology. MSOT utilizes short-pulsed laser light in the near- and extended near-infrared range (NIR/exNIR) for tissue illumination. As light in these wavelengths has fewer tissue absorption, it enables deeper tissue penetration. The NIR is typically represented in the wavelength between 700 and 900 m followed by extend NIR (exNIR)/NIR II window around 900–1600 nm. In OAI, this light is absorbed by tissue molecules and converted into acoustic pressure waves, which can be recorded and formed into optoacoustic images. In the NIR, endogenous absorbers such as deoxyhemoglobin, oxyhemoglobin, and melanin possess distinct absorption spectra, which gives them a unique signature in MSOT [7, 8]. In the exNIR, further absorption peaks for lipids, water, and collagens can be found [9]. Multispectral imaging, e.g., the utilization of different wavelengths, enables the quantification and spatial resolution of these molecules in MSOT images [10]. A translatable system (MSOT Acuity, iThera Medical GmbH, Munich, Germany) comprises a tunable optical parametric oscillator (OPO) that is pumped by an Nd:YAG laser to provide exact excitation pulses. The pulse energy (30 mJ peak at 730 nm) is as low as to adhere with ANSI limits of maximum permissible exposure (MPE) of the skin. Using different center frequencies, the resolution ranges between 290 μm (4 MHz) and 345 μm (3 MHz), respectively. The same imaging probe can then detect the delivered light and resulting sound waves. The probe is comparable to standard ultrasound systems (Fig. 1). In order to translate the use in clinical scenarios, the technique has been fused with conventional ultrasound imaging, called reflectance ultrasound computed tomography (RUCT) [11]. For RUCT generation, an ultrasound imaging platform is used, which synchronizes ultrasound and optoacoustic image streams [12].
Fig. 1.
Principle of optoacoustic imaging. a The terms “photoacoustic” or “optoacoustic” describe the use of “light” and “sound.” The illumination of tissue by pulsed laser light and the subsequent recording of thermoelastic pressure waves combines the advantages optical (high contrast) and acoustic (high resolution) imaging. Subsequent image reconstruction and spectral unmixing enables non-invasive real-time visualization and quantification of specific endogenous chromophores such as hemoglobin, lipids, and collagens. RUCT reflectance ultrasound computed tomography, MSOT multispectral optoacoustic tomography. b Imaging example from muscular imaging resolving hemoglobin, collagen, and fat signals. DMD Duchenne muscular dystrophy. c MSOT imaging of the colon unmixed total hemoglobin signal. S serosa, M muscularis mucosa, Sm submucosa, L lumen, US ultrasound. d Example for imaging of palmaris longus tendon at the wrist with unmixing for different tissue components. Note the clear delineation of yellow/red containing tendon in comparison of hemoglobin signals from vessels
Current clinical applications
OAI has so far been used in a number of various clinical applications; selected studies are summarized in Table 1. The strength of MSOT is the ability to quantify endogenous chromophores, of which hemoglobin is well known and best absorbing in human tissues. Traditionally, a wavelength of 700 nm is used to detect the peak for deoxygenated hemoglobin (HbR), 800 nm as measure for total hemoglobin (isosbestic point, HbT), and 850 nm for oxygenated hemoglobin (HbO2).
Table 1.
Selected clinical OAI studies
| Anatomical region/organ | Chromophores | Wavelength (nm) | Clinical output | References |
|---|---|---|---|---|
| Non-melanoma skin cancer | HbR, HbO2, melanin | 700, 715, 730, 760, 780, 790, 800, 825, 850, 900 | Mapping and visualization of skin cancer | [13, 14] |
| Skin (hair follicles) | HbR, HbO2, melanin, lipids | 660–1300 | Measurement of the structural and physiological features of intact hair follicles | [15] |
| Vasculature | HbR, HbO2 | 700–850 | Noninvasive diagnosis and monitoring of vascular malformations | [16] |
| HbR, HbO2 | 700–960 | Clinical flow-mediated dilatation measurements | [17] | |
| HbO2. HbR, SO2 | 730, 750, 800, 830 | Visualization of blood vessels, microvasculature and the respective oxygen saturation | [18] | |
| HbO2, HbR, melanin | 730, 760, 790, 820, 850 | Real-time visualization of blood vessels in 3D | [19, 20] | |
| HbO2, HbR, SO2 | 760, 850 | Movement correction and artifact-free imaging | [21] | |
| HbO2, HbR, melanin | 700, 720, 740, 760, 780, 800, 820, 840, 860 | Human angiography | [22, 23] | |
| HbO2, HbR | 680–950, 1064 | Visualization of human carotid artery | [24] | |
| Sentinel lymph node (SLN) | Melanin, ICG | 700, 730, 760, 800, 850, 900 | Identification of SLNs | [25] |
| Melanin, ICG | 700, 730, 760, 800, 850 | Metastatic status of lymph nodes | [26] | |
| Hb, methylene blue | 650, 1064 | Identification of SLNs | [27] | |
| Inflammatory bowel disease | HbT, HbO2, HbR, SO2 | 700, 730, 760, 800, 850, 900 | Inflammatory disease activity in Crohn’s disease | [28, 29] |
| Breast (cancer) | HbT, HbO2, HbR, SO2 | 700, 730, 760, 800, 850 | Characterization of healthy tissue and malignant lesions | [30] |
| HbO2, HbR, water, lipid | 700–970 (10 nm steps) | [31] | ||
| HbO2, HbR | 730, 760, 850 | [32] | ||
| Thyroid | HbT, HbO2, HbR, SO2, water, lipids | 700, 730, 760, 800, 850, 900, 920, 950 | Non-invasive diagnostics by tissue characterization | [33] |
| – | – | Optical features of thyroid anatomy | [34] | |
| HbO2, HbR, water, lipid | 760, 850, 930, 970 | Differentiation between malignant and benign thyroidal nodules | [35] | |
| HbO2 | 1064 | Photoacoustic/ultrasound dual imaging of human thyroid cancer | [36] | |
| Systemic sclerosis | HbT, HbO2, HbR | 700, 730, 750, 800, 850 | Disease activity | [37] |
| Muscle | HbT, HbO2, HbR, lipids, collagens | 680, 700, 730, 760, 800, 850, 920, 1000, 1030, 1064, 1100 | Disease extent in DMD patients | [38] |
HbR deoxygenated hemoglobin, HbO2 oxygenated hemoglobin, HbT total hemoglobin, SO2 MSOT-derived oxygen saturation
When examining vasculature, MSOT is capable of visualizing major blood vessels and microvasculature [18]. As MSOT resolves hemoglobin in its oxygenated and deoxygenated states through optic absorption properties (whereas conventional Doppler ultrasound measures flow), it can also provide images of hemoglobin oxygen saturation and pulsation [18]. Successful studies were performed for mapping human feet blood vessels [18] as well as carotid arteries [24]. The ability to resolve vessels as small as 100 μm in diameter and within 1-cm depth could also aid to diagnose vascular malformations [16].
In a consecutive study, the subcutaneous finger tissue of patients with systemic sclerosis (SSc) provided significantly lower MSOT values for oxygenated hemoglobin as well as total hemoglobin in comparison to healthy controls, reflecting microvascular dysfuntion in SSc as a possible marker of disease activity [37].
Further investigations explored the quantification of hemoglobin in the intestinal wall of patients with Crohn’s disease (CD) with MSOT. These measures have been shown to be increased in patients with active CD, demonstrating robust correlation with endoscopic and microscopic intestinal inflammation [28]. MSOT could therefore be used to differentiate between active disease and remission in affected patients in a non-invasive fashion [29]. Its application is currently further explored in order to develop a clinically (CE) certified technology (www.euphoria2020.eu).
One of the main tissues examined with MSOT is breast tissue. In healthy breast tissue, hormone-related physiological changes of breast parenchyma were visualized with MSOT using only a three wavelengths illumination (700, 800, and 850 nm wavelengths) [39]. The authors found that intensity values were significantly higher at all excitation wavelengths in the secretory compared to the proliferative/follicular phase [39]. In malignant breast tissue, MSOT allowed the visualization of peripheral tumor vascularization, disruption of fat, and water tissue layers [31]. In addition, increased signals for hemoglobin were found in invasive breast cancer when compared to healthy breast tissue [30].
The high resolution in OAI/PAI makes it possible to reveal more vessels than with conventional Doppler as shown in a photoacoustic/ultrasound dual imaging study of human thyroid cancers [36]. While Doppler has sensitivity limits in terms of detecting movements/flow in small vessels, the precise visualization of hemoglobin containing tumor-related bloods vessels could improve cancer diagnosis [40], raising the hope to develop radiation-free screening technologies or treatment monitoring of other (solid) tumors for application in children.
In near-filed applications such as non-melanoma skin cancers MSOT already successfully distinguished skin cancer from normal skin, being helpful for the visualization of lesion margins [13, 14]. Even the detection of metastatic spread of melanoma to sentinel lymph nodes appears to be improved with MSOT when compared to current sentinel lymph node excision protocols [25, 26]. Further, it was demonstrated that the injection of indocyanine green (ICG) and the detection via MSOT could be a proper replacement strategy for scintigraphy approaches [26].
Besides these promising first clinical studies, OAI has similar limitations like ultrasound. Namely, penetration depth of up to several centimeters as well as air, thick bones, body fat, and body hair can significantly influence image quality in adults. Therefore, children and adolescents might be excellent candidates for MSOT imaging as their organs and/or muscles are closer to the body surface.
Consequently, the first pediatric study showed that MSOT imaging is able to visualize the molecular composition of muscles in children. Muscular collagen content in children with Duchenne muscular dystrophy was increased as compared to healthy controls, suggesting non-invasive measured collagen content as a novel non-invasive biomarker for disease severity and potential monitoring tool for novel therapies [38]. Given the rise of genetic therapies, these findings underline the potential of MSOT for further personalized molecular imaging applications in children. A great advantage is that MSOT imaging can be performed without sedation or general anesthesia, which is especially important in patients with muscular disorders with respiratory depression. By using MSOT imaging, a substantial number of invasive procedures could hopefully be saved in the future.
Limitations
For translation into clinical practice, there are still several challenges to overcome: firstly, standardization of OAI imaging is required. It was already reported that preclinical OAI imaging systems consistently perform equal or even better (with variations smaller than 10%) compared to other preclinical imaging modalities, which underlines their clinical potential [41]. In agreement with these findings, a small clinical study suggests that clinical MSOT provides consistent and reproducible results in humans as well [42]. For further validation, besides comparisons with other cross-sectional imaging modalities (US/MRI), prospective clinical trials with higher case number and multi-centric approaches with clinically meaningful outcomes are required. With the International Photoacoustic Standardization Consortium (IPASC), first efforts are currently made to facilitate an international debate and consensus to address this need [43]. Currently, there are two CE-marked OAI systems for clinical use: Imagio by Seno Medical Instruments and MSOT Acuity by iThera Medical GmbH. The high costs for OAI systems, especially when compared to current high-end ultrasound systems, are a main challenge for clinical implementation. Various approaches for reducing these costs are already in discussion, including alternate illumination sources and signal detection methods [44]. A higher number of commercially available systems might also help to reduce costs and allow a widespread adoption of MSOT in clinical practice.
Future perspective
MSOT is capable of visualizing endogenous chromophores such as hemoglobin, melanin, lipids, and recently collagens. Hemoglobin and collagens in particular display specific properties that are promising for monitoring the course of various diseases. Furthermore, the use of exogenous chromophores (e.g., optoacoustic targeted therapies) as contrast agents for OAI imaging in pediatric patients is still unexplored. Therefore, the combination of endogenous and exogenous molecular information could pave the way for novel tailored theranostic approaches in pediatrics in the future.
Acknowledgements
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” for ALW and in (partial) fulfillment of the requirements for obtaining the degree “Dr. rer. biol. hum.” for FK.
Abbreviations
- CD
Crohn’s disease
- DMD
Duchenne muscular dystrophy
- exNIR
Extended near-infrared range
- HBO2
Oxygenated hemoglobin
- HbR
Deoxygenated hemoglobin
- HbT
Total hemoglobin
- MPE
Maximum permissible exposure
- MSOT
Multispectral optoacoustic tomography
- NIR
Near-infrared range
- OAI
Optoacoustic imaging
- RUCT
Reflectance ultrasound computed tomography
- SaO2
MSOT-derived oxygen saturation
Authors’ contributions
APR, ALW, JC, MJW, and FK carried out the literature research. APR, ALW, and FK wrote the first draft of the manuscript. JC wrote the technological background section. The manuscript was critically reviewed by all authors. The author(s) read and approved the final manuscript.
Authors’ information
Ferdinand Knieling has been working for 9 years in non-invasive preclinical and clinical imaging (ultrasound, CEUS, ultra-high frequency US, micro-CT, MSOT, RSOM). He has a dedicated scientific education in the field of ultrasound imaging (Prof. D. Strobel, Erlangen and Prof. J.-K. Willmann, Stanford, USA) and is the author of numerous leading publications in the field of optoacoustic imaging (New England Journal of Medicine, Nature Medicine, Gastroenterology). In 2019, he was awarded the Adalbert Czerny Prize by The German Society of Pediatrics and Adolescent Medicine (Deutsche Gesellschaft für Kinder- und Jugendmedizin - DGKJ) for “new diagnostics in pediatric medicine.”
Funding
Else Kröner–Fresenius–Stiftung, Interdisciplinary Center for Clinical Research (IZKF) of the Friedrich-Alexander-University Erlangen-Nuremberg, and ELAN fund by the IZKF.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Displayed patient data (Fig. 1) were acquired within approved study protocols by the local Ethical Review Board of the FAU Erlangen-Nuremberg and registered at clinicaltrials.gov (ID: NCT03490214, NCT02622139).
Consent for publication
Consent for publication was obtained.
Competing interests
APR and FK patent pending (EP 19 163 304.9). APR and FK received travel support by iThera Medical GmbH, Germany. JC is an employee from iThera Medical GmbH, Germany.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fenster ME, Hokanson JS. Heart murmurs and echocardiography findings in the normal newborn nursery. Congenit Heart Dis. 2018;13(5):771–775. doi: 10.1111/chd.12651. [DOI] [PubMed] [Google Scholar]
- 2.Orman G, Benson JE, Kweldam CF, Bosemani T, Tekes A, de Jong MR, et al. Neonatal head ultrasonography today: a powerful imaging tool! J Neuroimaging. 2015;25(1):31–55. doi: 10.1111/jon.12108. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40(1):27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliev MM, Dekhqonboev AA, Yuldashev RZ. Advantages of abdominal ultrasound in the management of infants with necrotizing enterocolitis. Pediatr Surg Int. 2017;33(2):213–216. doi: 10.1007/s00383-016-4017-8. [DOI] [PubMed] [Google Scholar]
- 5.Knieling F., Waldner M., Goertz R., Zopf S., Wildner D., Neurath M., Bernatik T., Strobel D. Early Response to Anti-Tumoral Treatment in Hepatocellular Carcinoma - Can Quantitative Contrast-Enhanced Ultrasound Predict Outcome? Ultraschall in der Medizin - European Journal of Ultrasound. 2012;34(01):38–46. doi: 10.1055/s-0032-1330387. [DOI] [PubMed] [Google Scholar]
- 6.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34(1):11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 7.Tzoumas S, Deliolanis N, Morscher S, Ntziachristos V. Unmixing molecular agents from absorbing tissue in multispectral optoacoustic tomography. IEEE Trans Med Imaging. 2014;33(1):48–60. doi: 10.1109/TMI.2013.2279994. [DOI] [PubMed] [Google Scholar]
- 8.Taruttis A, Ntziachristos V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat Photonics. 2015;9(4):219–227. doi: 10.1038/nphoton.2015.29. [DOI] [Google Scholar]
- 9.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nature Methods. 2016;13(8):639–650. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 10.Ntziachristos V, Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT) Chem Rev. 2010;110(5):2783–2794. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 11.Mercep E, Dean-Ben XL, Razansky D. Combined pulse-echo ultrasound and multispectral optoacoustic tomography with a multi-segment detector array. IEEE Trans Med Imaging. 2017;36(10):2129–2137. doi: 10.1109/TMI.2017.2706200. [DOI] [PubMed] [Google Scholar]
- 12.Mercep E, Jeng G, Morscher S, Li PC, Razansky D. Hybrid optoacoustic tomography and pulse-echo ultrasonography using concave arrays. IEEE Trans Ultrason Ferroelectr Freq Control. 2015;62(9):1651–1661. doi: 10.1109/TUFFC.2015.007058. [DOI] [PubMed] [Google Scholar]
- 13.Chuah SY, Attia ABE, Ho CJH, Li X, Lee JS, Tan MWP, et al. Volumetric multispectral optoacoustic tomography for 3-dimensional reconstruction of skin tumors: a further evaluation with histopathologic correlation. J Invest Dermatol. 2019;139(2):481–485. doi: 10.1016/j.jid.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Attia ABE, Chuah SY, Razansky D, Ho CJH, Malempati P, Dinish US, et al. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics. 2017;7:20–26. doi: 10.1016/j.pacs.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford SJ, Bigliardi PL, Sardella TCP, Urich A, Burton NC, Kacprowicz M, et al. Structural and functional analysis of intact hair follicles and pilosebaceous units by volumetric multispectral optoacoustic tomography. J Invest Dermatol. 2016;136(4):753–761. doi: 10.1016/j.jid.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Masthoff M, Helfen A, Claussen J, Karlas A, Markwardt NA, Ntziachristos V, et al. Use of multispectral optoacoustic tomography to diagnose vascular malformations. JAMA Dermatol. 2018;154(12):1457–1462. doi: 10.1001/jamadermatol.2018.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlas A, Reber J, Diot G, Bozhko D, Anastasopoulou M, Ibrahim T, et al. Flow-mediated dilatation test using optoacoustic imaging: a proof-of-concept. Biomed Opt Express. 2017;8(7):3395–3403. doi: 10.1364/BOE.8.003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taruttis A, Timmermans AC, Wouters PC, Kacprowicz M, van Dam GM, Ntziachristos V. Optoacoustic imaging of human vasculature: feasibility by using a handheld probe. Radiology. 2016;281(1):256–263. doi: 10.1148/radiol.2016152160. [DOI] [PubMed] [Google Scholar]
- 19.Dean-Ben X, Fehm TF, Razansky D. Universal hand-held three-dimensional optoacoustic imaging probe for deep tissue human angiography and functional preclinical studies in real time. J Vis Exp. 2014;93:e51864. doi: 10.3791/51864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean-Ben XL, Ozbek A, Razansky D. Volumetric real-time tracking of peripheral human vasculature with GPU-accelerated three-dimensional optoacoustic tomography. IEEE Trans Med Imaging. 2013;32(11):2050–2055. doi: 10.1109/TMI.2013.2272079. [DOI] [PubMed] [Google Scholar]
- 21.Dean-Ben XL, Bay E, Razansky D. Functional optoacoustic imaging of moving objects using microsecond-delay acquisition of multispectral three-dimensional tomographic data. Sci Rep. 2014;4:5878. doi: 10.1038/srep05878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean-Ben XL, Razansky D. Functional optoacoustic human angiography with handheld video rate three dimensional scanner. Photoacoustics. 2013;1(3-4):68–73. doi: 10.1016/j.pacs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean-Ben XL, Razansky D. Portable spherical array probe for volumetric real-time optoacoustic imaging at centimeter-scale depths. Opt Express. 2013;21(23):28062–28071. doi: 10.1364/OE.21.028062. [DOI] [PubMed] [Google Scholar]
- 24.Ivankovic I, Mercep E, Schmedt CG, Dean-Ben XL, Razansky D (2019) Real-time volumetric assessment of the human carotid artery: handheld multispectral optoacoustic tomography. Radiology. 181325 [DOI] [PubMed]
- 25.Stoffels I, Jansen P, Petri M, Goerdt L, Brinker TJ, Griewank KG, et al. Assessment of nonradioactive multispectral optoacoustic tomographic imaging with conventional lymphoscintigraphic imaging for sentinel lymph node biopsy in melanoma. JAMA Netw Open. 2019;2(8):e199020. doi: 10.1001/jamanetworkopen.2019.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoffels I, Morscher S, Helfrich I, Hillen U, Leyh J, Burton NC, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci Transl Med. 2015;7(317):317ra199. doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Uribe A, Erpelding TN, Krumholz A, Ke H, Maslov K, Appleton C, et al. Dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection in patients with breast cancer. Sci Rep. 2015;5:15748. doi: 10.1038/srep15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldner MJ, Knieling F, Egger C, Morscher S, Claussen J, Vetter M, et al. Multispectral optoacoustic tomography in Crohn’s disease: noninvasive imaging of disease activity. Gastroenterology. 2016;151(2):238–240. doi: 10.1053/j.gastro.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 29.Knieling F, Neufert C, Hartmann A, Claussen J, Urich A, Egger C, et al. Multispectral optoacoustic tomography for assessment of Crohn’s disease activity. N Engl J Med. 2017;376(13):1292–1294. doi: 10.1056/NEJMc1612455. [DOI] [PubMed] [Google Scholar]
- 30.Becker A, Masthoff M, Claussen J, Ford SJ, Roll W, Burg M, et al. Multispectral optoacoustic tomography of the human breast: characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur Radiol. 2018;28(2):602–609. doi: 10.1007/s00330-017-5002-x. [DOI] [PubMed] [Google Scholar]
- 31.Diot G, Metz S, Noske A, Liapis E, Schroeder B, Ovsepian SV et al (2017) Multispectral optoacoustic tomography (MSOT) of human breast cancer. Clin Cancer Res. [DOI] [PubMed]
- 32.Dean-Ben XL, Fehm TF, Gostic M, Razansky D. Volumetric hand-held optoacoustic angiography as a tool for real-time screening of dense breast. J Biophotonics. 2016;9(3):253–259. doi: 10.1002/jbio.201500008. [DOI] [PubMed] [Google Scholar]
- 33.Roll W, Markwardt NA, Masthoff M, Helfen A, Claussen J, Eisenblatter M et al (2019) Multispectral optoacoustic tomography of benign and malignant thyroid disorders - a pilot study. J Nucl Med. [DOI] [PMC free article] [PubMed]
- 34.Dima A, Ntziachristos V. In-vivo handheld optoacoustic tomography of the human thyroid. Photoacoustics. 2016;4(2):65–69. doi: 10.1016/j.pacs.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dogra VS, Chinni BK, Valluru KS, Moalem J, Giampoli EJ, Evans K, et al. Preliminary results of ex vivo multispectral photoacoustic imaging in the management of thyroid cancer. AJR Am J Roentgenol. 2014;202(6):W552–W558. doi: 10.2214/AJR.13.11433. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Zhao L, He X, Su N, Zhao C, Tang H, et al. Photoacoustic/ultrasound dual imaging of human thyroid cancers: an initial clinical study. Biomed Opt Express. 2017;8(7):3449–3457. doi: 10.1364/BOE.8.003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masthoff M, Helfen A, Claussen J, Roll W, Karlas A, Becker H, et al. Multispectral optoacoustic tomography of systemic sclerosis. J Biophotonics. 2018;11(11):e201800155. doi: 10.1002/jbio.201800155. [DOI] [PubMed] [Google Scholar]
- 38.Regensburger AP, Fonteyne LM, Jungert J, Wagner AL, Gerhalter T, Nagel AM, et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat Med. 2019;25(12):1905–1915. doi: 10.1038/s41591-019-0669-y. [DOI] [PubMed] [Google Scholar]
- 39.Abeyakoon O, Morscher S, Dalhaus N, Ford SJ, Mendichovszky IA, Manavaki R et al (2018) Optoacoustic imaging detects hormone-related physiological changes of breast parenchyma. Ultraschall Med [DOI] [PubMed]
- 40.Toi M, Asao Y, Matsumoto Y, Sekiguchi H, Yoshikawa A, Takada M, et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci Rep. 2017;7:41970. doi: 10.1038/srep41970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph J, Tomaszewski MR, Quiros-Gonzalez I, Weber J, Brunker J, Bohndiek SE. Evaluation of precision in optoacoustic tomography for preclinical imaging in living subjects. J Nucl Med. 2017;58(5):807–814. doi: 10.2967/jnumed.116.182311. [DOI] [PubMed] [Google Scholar]
- 42.Helfen Anne, Masthoff Max, Claussen Jing, Gerwing Mirjam, Heindel Walter, Ntziachristos Vasilis, Eisenblätter Michel, Köhler Michael, Wildgruber Moritz. Multispectral Optoacoustic Tomography: Intra- and Interobserver Variability Using a Clinical Hybrid Approach. Journal of Clinical Medicine. 2019;8(1):63. doi: 10.3390/jcm8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohndiek S. Addressing photoacoustics standards. Nature Photonics. 2019;13(5):298. doi: 10.1038/s41566-019-0417-3. [DOI] [Google Scholar]
- 44.Fatima A, Kratkiewicz K, Manwar R, Zafar M, Zhang R, Huang B, et al. Review of cost reduction methods in photoacoustic computed tomography. Photoacoustics. 2019;15:100137. doi: 10.1016/j.pacs.2019.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.