Abstract
Purpose
Chemical fixation is a critical step to retaining cellular targets as naturally as possible. Recent developments in microscopy allow sophisticated detection and measuring techniques with which spatio-temporal molecular alterations are conceivable. In this study, we compare two members of aldehyde fixatives [i.e., glyoxal (Gly) and paraformaldehyde (PFA)] to determine whether Gly, a less toxic dialdehyde fixative that is considered to retain immunoreactivity could provide a successful and consistent cell fixation in favor of PFA in various cell preparations and types.
Methods
We document the fixation competence of Gly and PFA side-by-side (with or without Triton X-100 permeabilization) in live- and fixed-cell preparations in mouse oocytes, embryos, and human somatic cells (human umbilical cord-derived mesenchymal stromal cells) using protein quantification by Western blot assay and super-resolution microscopy.
Results
Although Gly seemed to act faster than PFA, catastrophic consequences were found not acceptable, especially in oocytes and embryos. Due to cell lysate and immunocytochemistry surveys, it was obvious that PFA is superior to Gly in retaining cellular proteins in situ with little/no background staining. In many samples, PFA revealed more reliable and consistent results regarding the protein quantity and cellular localization corresponding to previously defined patterns in the literature.
Conclusion
Although the use of Gly is beneficial as indicated by previous reports, we concluded that it does not meet the requirement for proper fixation, at least for the tested cell types and proteins. However, PFA alone with no addition of TX displayed a significant cytoplasmic loss by generating membrane blebs during fixation.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01666-9) contains supplementary material, which is available to authorized users.
Keywords: Aldehyde fixative, Embryo, Fixation, Glyoxal, Oocyte, Paraformaldehyde
Introduction
Fixation is a fundamental and initial step in histochemical and cytochemical investigations, mainly aiming at preserving tissues, cells, and cellular components from autolytic deterioration. Microscopic structures can subsequently be observed in situ after suitable cell/tissue preparations and labeling procedures. For this purpose, a number of chemical fixatives have been used since the nineteenth century [1]. First introduced in 1893, formaldehyde (FA), as a member of aldehyde fixatives, in the form of a water-based, diluted solution of 1:10 has been the most commonly used chemical fixative, and it is found in many fixative cocktails [2]. Paraformaldehyde (PFA), the polymerized form of FA, is generally favored over FA because PFA cross-links amino groups without altering the tertiary structure of proteins; thereby, cellular epitopes remain relatively well-preserved in a successful labeling protocol with specific antibodies [3–5]. PFA fixation has been widely adopted to preserve cell morphology for immunolabeling where the final concentration of PFA in the fixative solution is around 3–4% [6]. Nonetheless, PFA has also been associated with various problems, ranging from loss of antigenicity to changes in morphology during fixation.
Glyoxal (Gly), the smallest dialdehyde reagent with a structural formula that resembles two formaldehyde molecules bonded back-to-back, has also been tested as a fixative since the early 1960s [7], albeit in fewer studies. Due to a better safety profile, faster reaction rate, and selective control over cross-linking, Gly is considered to retain immunoreactivity and decreases the need for antigen retrieval [8]. With suitable catalysts or other reaction accelerators, Gly forms two-carbon adducts with nearly all end groups in proteins and carbohydrates, leaving most of them unimpaired for subsequent immunohistochemical (IHC) demonstration [8]. Due to the previously reported disadvantages of PFA, Gly has recently been suggested superior to PFA as being less toxic and preserves cells better as investigated using super-resolution microscopy [9]. There are also a number of reports comparing the staining and IHC labeling results between Gly- and FA-fixed tissues. PreFer™, a commercial form of Gly, was found to be unsuitable because of poor preservation of tissue morphology [10]; compared with FA, Gly was found better at preserving the cell membranes and nuclear chromatin in paraffin-embedded tissue sections [11]; estrogen receptor staining with antigen retrieval was found significantly weaker in the Gly-fixed specimens than in FA-fixed specimens [12]. No significant differences were reported between FA and Gly fixation in histomorphometry [13], IHC, Western blot (WB) [14], and fluorescence in situ hybridization [15] assays.
Besides fixation, many samples require a detergent extraction step for exposing antigenic sites to antibodies. As such, using a non-ionic detergent such as Triton X-100 (TX) may be an essential step for improving the penetration of the antibody. TX effectively solubilizes complexes such as biologic membranes, and it apparently does not inhibit the antigen-antibody reaction [16]. However, it is not appropriate for membrane-associated antigens because it destroys lipids among membranous proteins. TX may be added to fixation solutions, thus simultaneously performing fixation and extraction actions. Alternatively, in many settings, TX extraction is applied following cell fixation in daily use.
In this study, we aimed to assess whether Gly could provide a successful and consistent cell fixation in favor of PFA in various cell types and preparations such as isolated mouse granulosa-enclosed or naked oocytes and embryos as well as cultured human umbilical cord mesenchymal stromal cells (hUC-MSCs), all of which were analyzed during and after fixation using a series of antibodies raised against diverse cellular proteins. The study groups were designed to test the PFA (3.5%) and Gly (3%) alone, as well as with the addition of TX (0.1%). Additionally, TX (0.1%) application was also performed after the samples were fixed.
In short, PFA was found to be more consistent and potent as a fixative compared with Gly in many circumstances. Here, we present the relatively poor fixation capacity of Gly, proven by a series of quantitative and qualitative data, some of which were obtained using super-resolution microscopy. The overall results seemed quite different from previously published data. Interestingly, however, PFA alone with no addition of TX displayed a significant cytoplasmic loss by generating membrane blebs during fixation.
Materials and methods
Preparation of PFA stock (10%) and working (3.5%) solutions
Stock PFA (10%, w/v) solution was prepared by adding 100 mg PFA (95% powder) (Merck, Germany) to 650 mL double distilled water (ddH2O), then heated to 60 °C using a magnetic heater/stirrer. Fifty microliters of 1 N NaOH was added to dissolve the PFA completely. After removing from the heater, the suspension was finalized to 1000 mL using ddH2O; the pH was brought to 7.2 using 1 N HCl. Stock PFA solution was filtered and cooled down to 4 °C for storage. Immediately before use, to prepare 3.5% PFA working solution, stock PFA solution was mixed with ddH2O in 1:1.85 ratio (pH = 7.2–7.3).
Preparation of Gly working (3%) solution
For preparing a 1000-mL 3% Gly working solution, 197.25 mL absolute ethanol (Merck, Germany), 79.50 mL 40% Gly (v/v) (Merck, Germany), and 7.5 mL glacial acetic acid (Merck, Germany) were added to 709.75 mL ddH2O. The solution was vortexed and 1 N NaOH (approximately 62.0 mL 1 N NaOH) was added until pH = 5 has been reached [9]. The clear solution was stored at 4 °C and used within few days with no precipitation.
Experimental groups
The fixation potency of 3.5% PFA and 3% Gly solutions were tested in mouse oocytes (mOocytes), mouse embryos (mEmbryos), and cultured (P4) hUC-MSCs, and with or without 0.1% TX detergent. The experiment groups and fixation protocol are summarized in Table 1.
Table 1.
Fixation/extraction protocol and experiment groups
| Fixation/extraction agent | PFA | PFA/TX | PFA + TX | Gly | Gly/TX | Gly + TX |
|---|---|---|---|---|---|---|
| Formula | 3.5% PFA | 3.5% PFA with TX (0.1%) | TX (0.1%) after 3.5% PFA | 3% glyoxal | 3% glyoxal with TX (0.1%) | TX (0.1%) after 3% glyoxal |
| Fixation time and temp (°C) | 15 min @ RT* | 15 min @ RT* | 15 min + 5 min @ RT* | 15 min @ RT* | 15 min @ RT* | 15 min + 5 min @ RT* |
*Twenty minutes at room temperature (RT) for mOocytes and mEmbryos
Collection and preparation of mouse oocytes/embryos and human cells
Oocytes and embryos were obtained from BALB/c (Bagg’s albino, c strain) female mice (n = 8) at 4–6 weeks. The experimental protocol was approved by the IRB (approval number 792/20-18.10.27). All mice were hosted with free access to food and water and kept in a 12-h light/dark cycle. All experiments were carried out in accordance with relevant regulations and guidelines of Ankara University Animal Care and Use Committee. The germinal vesicle (GV)-stage, meiosis-I (MI)-stage oocytes, and zygote/blastocyst-stage embryos (n = 275) were obtained from mice primed with 5 IU (0.1 mL/animal) pregnant mare’s serum gonadotropin (PMSG) (Intervet, UK). The details of the collection of mOocytes can be found elsewhere [17]. For embryo collection, mice were further injected with 5 IU (0.1 mL/animal) human chorionic gonadotropin (hCG) (Sigma-Aldrich, USA). Twenty hours after PMSG injection, the mice were killed and then the ovaries were punctured with a 23-gauge needle in G-MOPS medium (Vitrolife, Sweden). GV-stage oocytes were collected using a mouth-controlled pipette under a dissecting microscope (Nikon, Japan). hCG-induced female mice were mated overnight with mature male mice at a rate of 1 female:1 male. Upon confirming a vaginal plug, zygote-blastocyst stage embryos were collected in the following days from the oviducts/uterus as previously described elsewhere [18].
Fresh umbilical cords (n = 3) were obtained from full-term female infants after cesarean section succeeding the acquisition of informed consent from the mother (Local Ethics Committee, IRB approval number 18-578-12, 2012) that the experiments conformed to the principles set out in the World Medical Association Declaration of Helsinki. The details of obtaining and culture of hUC-MSCs can be found elsewhere [19]. Briefly, umbilical cord pieces were chopped into approximately 0.5-mm3 tissue explants. Following the lag phase, sprouting hUC-MSC cells were expanded in 75-cm2 tissue culture flasks (Corning; 430641, USA) in DMEM-Ham’s F12 media (Biochrom T481-01, Germany) at 37 °C 5% CO2 until passage 4 (P4) cells reached 90–95% confluence. At the final stage, confluent cells were removed from flasks using trypsin-EDTA (0.05/0.02%) (Biochrom, Germany) and then transferred onto poly-L-lysine–coated (0.01%) (Sigma-Aldrich, USA) glass coverslips (5000 cells/cm2) and left for at least 48 h before fixation. Prior to fixation, the procedure cells were washed twice with Dulbecco’s PBS (D-PBS).
Live-cell imaging
Live mEmbryos were cumulated in a 50-μL drops containing GMOPS covered with Ovoil™ (Vitrolife, Sweden) in glass-bottomed 35-mm Petri dishes. hUC-MSCs (1 × 105 cells/cm2) were plated onto glass-bottomed 35-mm Petri dishes (World Precision Instruments, USA) in complete medium (DMEM/HAM’s F12 + 10% FBS) (Merck, Germany). All cells were viewed using live-cell imaging microscopy (controlled temp, CO2, and humidity) before and during the fixation period. Scoping time (min), temperature (°C), and image acquisition interval (s) were aligned according to the cell type and fixation groups (Table 2).
Table 2.
Live-cell imaging groups; fixation and imaging protocols
| mEmbryos (n = 49) | hUC-MSCs (n = 14) | |||||
|---|---|---|---|---|---|---|
| Time (min) | Temp (°C) | Interval* (s) | Time (min) | Temp (°C) | Interval (s) | |
| Before fixation | 20 | 37 | 8 | 15 | 37 | 5 |
| PFA | 20 | RT | 8 | 15 | RT | 5 |
| PFA/TX | 20 | RT | 8 | 15 | RT | 5 |
| PFA + TX | 20 + 5 | RT | 8 | 15 + 5 | RT | 5 |
| Gly | 20 | RT | 8 | 15 | RT | 5 |
| Gly/TX | 20 | RT | 8 | 15 | RT | 5 |
| Gly + TX | 20 + 5 | RT | 8 | 15 + 5 | RT | 5 |
*Image acquisition interval
All movies were recorded in a time-lapse manner for 15/20 min with 5- or 8-s intervals using differential interference microscopy (DIC) configured on a Zeiss LSM-880 confocal system (Zeiss, Germany) equipped with a Zeiss Axio Observer inverted microscope. A 633-nm red laser line (5 mW) was used as a light source. At the objective plane (Zeiss × 20 plan apo/NA 0.8), the laser power was measured 1.97 mW. The laser power was set to 1.2% during image acquisition to keep the excitation energy as low as possible to avoid any photo damage/toxicity; therefore, the cells and embryos were scanned with 0.023 mW (23 μW). Scanning parameters were set in order to achieve pixel dwell time between 0.40 and 0.50 μs.
Western blotting
Total protein, β-actin, vimentin, and α/β-tubulin quantities in unfixed and fixed mOocytes/granulosa cells and hUC-MSCs were determined using WB. mOocytes/granulosa cells were fixed for 20 min; hUC-MSCs were fixed for 15 min, at RT. Fixed and non-fixed cells were dissolved in lysis buffer [1% sodium dodecyl sulphate (SDS), 1.0 mM sodium ortho-vanadate, 10 mM Tris pH 7.4] supplemented with 1x protease inhibitor cocktail (Amresco, USA). The protein concentration was measured using the BCA (bicinchoninic acid) method. Fifty micrograms of protein from each group was separated on 10% Tris-HCl gel using protein electrophoresis (BioRad, USA). The gels were stained in Coomassie brilliant blue overnight (o/n). In the following day, they were de-stained in a 50% methanol, 40% ddH2O, and 10% acetic acid mixture for 3–4 h. The stained gels were scanned and analyzed as described below.
To determine the levels of the aforementioned proteins, 50 μg of cell extract from each group was separated on 10% Tris-HCl gel and then electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Roche, UK) o/n at + 4 °C. Then, the membrane was blocked with 5% (w/v) bovine serum albumin (BSA) prepared in TBS-T (20 mM Tris/HCl and 150 mM NaCl plus 0.05% Tween-20 at pH 7.4) at RT for 1 h. Membranes were incubated with primary antibodies specific to β-actin (Abcam; ab8226), vimentin (Sigma; V6630), or α/β-tubulin (Sigma; T9026, T4026) [1:1000 in 5% (w/v) BSA containing TBS-T] for 2 h at RT. Following a triple-wash in TBS-T for 15 min each, membranes were incubated with goat anti-mouse or anti-rabbit secondary antibody (800 nm) (1:2000 in TBS-T) (Licor Biosciences, USA) at RT for 1 h on a shaker. The SDS-PAGE gels in Figs. 2 and 3 were analyzed by measuring the overall lane intensity that was left after fixation and compared with the non-fixed sample. Protein band intensities were measured using a Li-Cor Odyssey CLx infrared detection system (LICOR Biosciences, USA) following the manufacturer’s guidelines. Relative band intensities were measured and analyzed using ImageJ v.3.91 software.
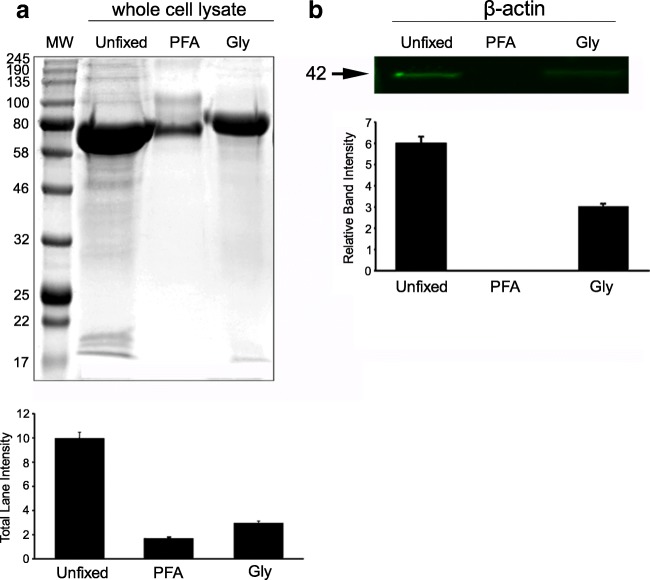
Fig. 2.
Protein fixation capacities of 3.5% PFA and 3% Gly in mOocytes/granulosa cells. a Western blot assays were used to show the percentage of unfixed proteins by measuring the total signal intensity in Coomassie blue-stained whole gel lanes in mOocytes/granulosa cell lysates (n = 5 for each group from independent experiment). b Fluorescent-labeled 42 kD bands specific to β-actin in mOocytes/granulosa cells. All intensity measurement results were found statistically different (p < 0.001) (see Table 4 for the ratio of fixed vs. unfixed proteins). MW molecular weight markers
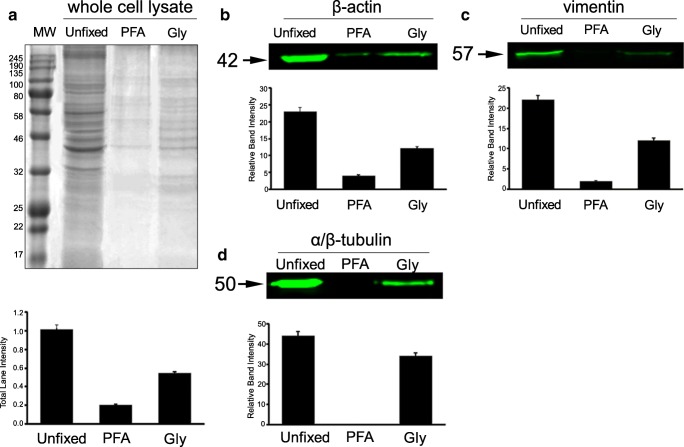
Fig. 3.
Protein fixation capacities of 3.5% PFA and 3% Gly in hUC-MSCs. a Western blot assays were used to show the percentage of unfixed proteins by measuring the total signal intensity in Coomassie blue-stained whole gel lanes in hUC-MSC lysates (n = 6 for each group from independent experiment). b Fluorescent-labeled 42 kD bands specific to β-actin. c Fluorescent-labeled 57 kD bands specific to vimentin. d Fluorescent-labeled 50 kD bands specific to α/β-tubulin. All intensity measurement results were found statistically different (p < 0.001) (see Table 4 for the ratio of fixed vs. unfixed proteins). MW molecular weight markers
Live-cell mitochondrial staining
Live hUC-MSC monolayers on glass-bottom Petri dishes were incubated with 1 mM MitoTracker™ (Molecular Probes; M7512, USA) for 45 min at 37 °C. Cultures were then washed twice with PBS and fixed with six different fixative cocktails for 15 min at RT. In PFA + TX and Gly + TX groups, 0.1% TX was applied for 5 min at RT following fixation and washing. After the final incubation step, monolayers were covered with a glass coverslip and scoped immediately.
Nuclear markers
Fixed hUC-MSCs were incubated with antibodies against nuclear proteins such as CENP-A (1:100 in PBS; o/n at 4 °C + 2 h at 37 °C) (Abcam; ab45694, USA), lamin A/C (1:100 in PBS; following blocking with NGS, 2 h at 37 °C) (Abcam; ab108595, USA), or nucleostemin (1:250 in PBS; 2 h at 37 °C) (Chemicon International; MAB4311, USA). Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Abcam; ab6717, USA) was used as a secondary antibody. For nuclear DNA labeling, Hoechst 33342 (1:1000 in glycerol/PBS) (Invitrogen; LSH3570) in mounting medium (1:1 v/v PBS/glycerol) was used.
Cell-specific markers
Fixed hUC-MSCs with six different fixative cocktails were immunostained with cell-specific markers such as N-cadherin or CD73 as follows: N-cadherin [1:100 in PBS; o/n at 4 °C + 2 h at 37 °C] (Sigma; C3865, USA), CD73 (1:100 in PBS; 2 h at 37 °C) (Abcam; ab54217, USA). FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch; 115-095-166, USA) was used as a secondary antibody.
Cytoskeletal markers
Fixed hUC-MSCs were incubated with antibodies against some cytoskeletal proteins such as vimentin (1:50 in PBS; o/n at 4 °C + 2 h at 37 °C) (Sigma; V6630, USA), pericentrin (1:1000 in PBS; 2 h at 37 °C) (Abcam; ab4448, USA), or α/β-tubulin (1:1 mixture) (1:100 in PBS; 2 h at 37 °C) (Sigma; T9026, T4026, USA). FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch; 115-095-166, USA) was used as a secondary antibody for anti-vimentin and anti-α/β-tubulin antibodies and FITC-conjugated anti-rabbit IgG (Abcam; ab6717, USA) for anti-pericentrin. The slides were covered with mounting medium. Fixed mOocytes and hUC-MSCs were incubated with fluorescein phalloidin (ThermoFisher Scientific; F432, USA) (35 mg/mL in PBS for 60 min) for F-actin staining. All staining steps for mOocytes were performed using micro-well trays (Thermo Scientific, USA). Then, they were gently transferred onto glass-bottomed 35-mm Petri dishes in a 100-μL drop of PBS-based mounting medium containing 1 μg/mL Hoechst 33342 (Invitrogen; LSH3570) for DNA labeling. The top was covered with paraffin oil. Mouse oocytes have a diameter of around 100 μm (including zona pellucida (ZP)); therefore, this type of preparation enabled us to visualize the oocyte surface and the inner structures as naturally as possible.
Fixed-cell imaging with super-resolution confocal microscopy
All fluorescently tagged specimens were examined and imaged using a Zeiss LSM-880 Airyscan system (Zeiss, Germany). Multiple laser lines (405, 488, 543, and 633 nm) were used according to the fluorescent probes. All laser and scanning parameters were set identical for each protein using a reference histogram; × 20 (dry), × 40 (water), and × 63 (oil) immersion objectives, and gallium-arsenide phosphide or airyscan detectors were used to detect signals. Pixel resolution for 2D and 3D images were set automatically and aligned identically for all image acquisitions. For 3D surface topology assessment of GV-MI stage oocytes, 3D module of Zen Desk software (v2.3) was used to analyze the app. 100-μm-thick image stacks.
Quantification and analysis of fluorescent signals
Ten sample images were obtained from each label (protein). The varying signal intensities were analyzed using Zen Blue v2.3 histogram tool to calculate the corrected total cell fluorescence [CTCF = Integrated density − (area of selected cell) × (mean fluorescence of background readings)] [20]. The total image area was used to calculate the CTCF value for N-cadherin, CD73, CENP-A, lamin A/C, nucleostemin, pericentrin, vimentin, and α/β-tubulin.
Statistical analysis
All statistical analyses were performed using the IBM SPSS for Windows v15.0 software package (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the assumption of normality. For non-normally distributed continuous variables, differences between groups were tested using the Kruskal-Wallis test. Continuous variables that did not have normal distribution are expressed as median (minimum-maximum). A two-sided p value < 0.05 was considered as statistically significant. Data are reported as mean ± standard deviation (SD). Sum, average, and SD calculations were performed using MS Excel (Microsoft Corp, Seattle, USA). Significance tests were performed using SPSS 10.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed using one-way analysis of variance (ANOVA) and Student’s t test when values were normally distributed; otherwise, the Mann-Whitney U test was applied. Differences between the experimental and control groups were regarded as statistically significant when p < 0.05.
Results
Live-cell imaging of mEmbryos and hUC-MSCs
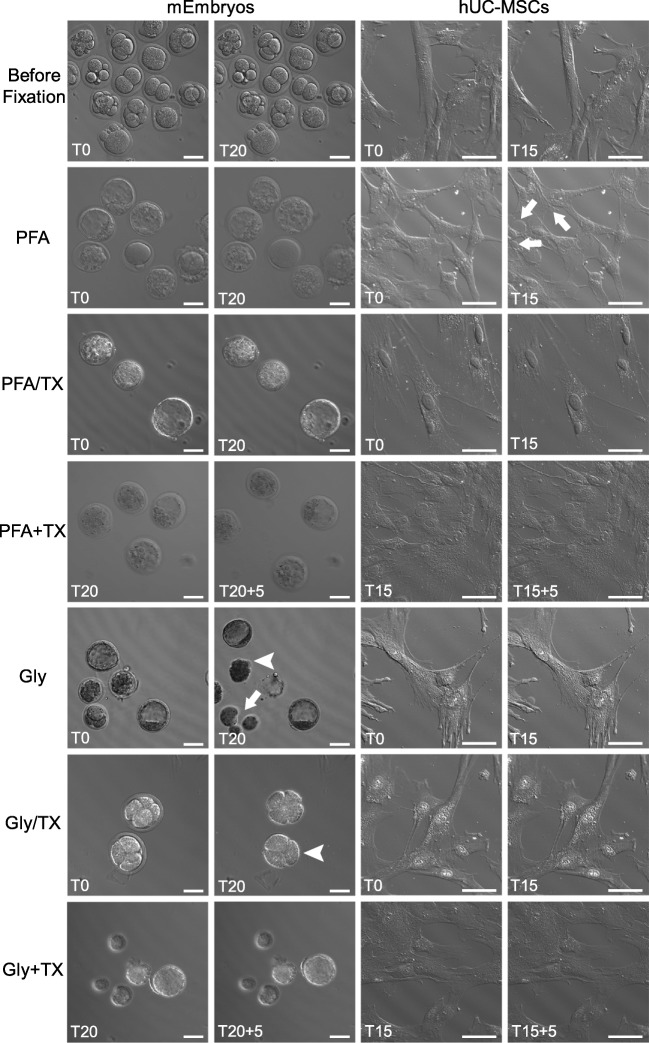
Freshly isolated mouse embryos (mEmbryos) and cultured hUC-MSC monolayers in glass-bottomed Petri dishes were microscopically examined using a laser-illuminated differential interference contrast (DIC) imaging to observe the cell dynamics during fixation. Prior to fixation, live samples in culture media were recorded in a time-lapse manner for 15–25 min (Fig. 1, Electronic supplementary movies). Subsequently, the same samples were directly taken to fixation simply by replacement of the culture media with the fixative solution. As seen in Fig. 1, Electronic supplementary movies, and Table 3, a series of significant changes were noted during the fixation interval (20 min for mEmbryos or 15 min for hUC-MSCs) followed by a 5-min TX incubation.
Fig. 1.
Still images before and during PFA- and Gly-containing fixative cocktails. Live mEmbryos (n = 49) and hUC-MSCs (n = 14) before fixation (first row), and during PFA, PFA/TX, PFA + TX, Gly, Gly/TX, and Gly + TX fixation procedures (second–seventh rows). Note the ZP dissolution (arrowheads) and blastomere dissociation (arrow) in Gly-fixed mEmbryos, and cytoplasmic blebs (arrows) in PFA-fixed hUC-MSCs (see also Table 3, Electronic Supplementary movie files). T refers to time of fixation (20 min for mEmbryos; 15 min for hUC-MSC); additional 5-min TX incubation was applied in +TX groups. Scale bars: 50 μm
Table 3.
Summary of live-cell imaging results before and during fixation
| mEmbryos | hUC-MSCs | |
|---|---|---|
| Before fixation | Embryos from one-cell to morula stage surrounded by an intact ZP | Normal saltatory movement of organelles and vesicles in extremely flat and widened cell bodies. Occasional retraction of cell cytoplasm (see upper part of the Supplementary movie) |
| PFA | Minimal thinning of ZP | Formation of large cytoplasmic blebs proceeded by plasma membrane rupture and thus loss of cytosol |
| PFA/TX | Swelling of blastomeres in morula and blastocysts | A minimal or no change of cell membrane integrity, extracellular flow of tiny cytoplasmic vesicles |
| PFA + TX | No further significant change | No further significant change |
| Gly | Enlargement of perivitelline space, thinning of ZP, dissolution of ZP, and dissociation of blastomeres | Minimal number of tiny blebs with no sign of cytosolic loss |
| Gly/TX | Same effects as described in Gly fixation | No change of cell membrane integrity, no cytosolic loss |
| Gly + TX | No further significant change | No further significant change |
Effectiveness of PFA vs. Gly in whole cell lysates
The protein cross-linking capacities of the two different fixative (PFA and Gly) were tested by evaluating the intensity of unfixed total protein bands per lane (Figs. 2a and 3a). To compare the efficiency of fixation, the bands that survived fixation were summed and expressed as the percentage (%) of an unfixed control [9]. The fixation efficiency of PFA and Gly was assessed in mOocyte/granulosa cell lysates through the evaluation of total WB Coomassie blue-stained and β-actin-labeled bands (Fig. 2a). Seventeen percent of total protein remained unfixed in PFA, whereas 30% of proteins were found unfixed with Gly. We then tested the degree of fixation of PFA and Gly for three major cytoskeletal proteins (i.e., β-actin, vimentin, and α/β-tubulin), all of which have low molecular weights (42, 57, and 50 kDa, respectively), after they were labeled with fluorescent markers. Zero percent of β-actin was unfixed with PFA, whereas 50% was found unfixed with Gly in mOocyte/granulosa cell lysates (Fig. 2b). Fixation of hUC-MSC lysates revealed that PFA was not able to fix only 20% of proteins, while Gly was not able to fix 55% of proteins (Fig. 3a). In hUC-MSC cell lysates, 17% of β-actin was unfixed with PFA, and 52% was unfixed with Gly (Fig. 3b); 9% of vimentin was unfixed with PFA, and 54% was unfixed with Gly (Fig. 3c); and 0.4% of α/β-tubulin was unfixed with PFA, and 77% was unfixed with Gly (Fig. 3d). The fixed protein ratios are summarized in Table 4.
Table 4.
The protein fixation capacity of (% of fixed proteins) in cell lysates after PFA and Gly fixation. The ratio of fixed vs. unfixed proteins was detected significantly higher in the PFA group (p < 0.001)
| mOocytes | hUC-MSCs | |||||
|---|---|---|---|---|---|---|
| Total protein | β-Actin | Total protein | β-Actin | Vimentin | α/β-Tubulin | |
| PFA (3.5%) | 83 | 100 | 80 | 83 | 91 | 99.6 |
| Gly (3%) | 70 | 50 | 45 | 48 | 46 | 23 |
Qualitative and quantitative evaluation of protein labeling
Live-cell mitochondrial staining
Mitotracker™ labeling of living hUC-MSCs revealed fine identification of numerous mitochondria as punctate or rod-like patterns distributed throughout the entire cell cytoplasm (Fig. 4).
Fig. 4.
MitoTracker™ labeling of hUC-MSCs before and after fixed with six fixative cocktails. Live MitoTracker™ labeling of hUC-MSCs (n = 30 experiment) before fixation. The efficiency of fixatives on MitoTracker retaining revealed that the best signal-to-noise ratio was noticed in PFA + TX group. No labeling was retained in the PFA/TX and Gly/TX groups. The Gly + TX group did not display any label, but a strong background. Scale bars: 20 μm
Following live-cell Mitotracker™ labeling, cells were taken into fixative cocktails after a brief wash. PFA fixation alone provided smaller numbers and lesser mitochondrial staining showing a dull appearance within the cytoplasm (Fig. 4). In the PFA/TX group, almost no staining was observed. In contrast, significantly brighter and clearer signals were detected in the PFA + TX group. Gly fixation alone revealed higher intensity and clear signals coming from mitochondria but higher background staining as well. Like in the PFA/TX groups, Gly/TX fixation provided no mitochondrial staining. In the Gly + TX group, no mitochondrial staining was observed but there was a relatively stronger background.
Nuclear proteins and DNA markers
The fluorescent signal intensity and patterns of selected nuclear proteins (CENP-A, nucleostemin, and lamin A/C), some of which are specific to stem/progenitor cells (i.e., nucleostemin), were tested after six different fixation cocktails in hUC-MSCs. Results are summarized in Fig. 5. PFA alone or with TX groups revealed fine nucleoplasmic dots, which seemed to correspond to centromeric nucleosome protein in interphase cells labeled by CENP-A antibody (Fig. 5). PFA alone exhibited slightly higher non-specific background staining, whereas the PFA/TX and PFA + TX groups showed more specific, similar patterns and signal intensity levels. The Gly alone group also displayed punctate staining confined to the nucleus; however, a stronger cytoplasmic and nucleoplasmic background was also noted. In Gly/TX, and more commonly in the Gly + TX groups, nuclear/nucleolar non-specific staining of irregular foci were noted (Fig. 5, arrowheads). In the Gly + TX group, a significantly smaller number of CENP-A loci were labeled with an intense cytoplasmic background where false-positive results in signal intensity measurements were also noted.
Fig. 5.
Nuclear proteins in hUC-MSCs after fixed with six fixative solutions. CENP-A were characterized by nucleoplasmic spots when fixed with PFA alone, PFA/TX, or PFA + TX (first column). The signal intensity levels between the three groups were non-significant (p = 0.999) as quantified using CTCF measurements given in the graphs. Gly alone or Gly with TX groups generally displayed intense cytoplasmic and/or nucleoplasmic background staining as well as irregular nuclear/nucleolar foci (arrowheads) (n = 5 for labeling; n = 50 for signal quantification). Nucleostemin in hUC-MSCs was specific but with varying intensities with no background (second column). The highest intensity was detected in the PFA alone group; the Gly alone Gly/TX groups showed the lowest intensities (p = 0.019) (n = 6 for labeling; n = 60 for signal quantification). Lamin A/C appeared predominantly on the nuclear membrane except in the Gly alone group, which displayed a disrupted arrangement (third column). Scale bars: 10 μm
Nucleostemin, a multilocular nucleolar protein, which was previously illustrated in hUC-MSCs [21], was clearly and strongly labeled in PFA alone or PFA + TX groups in hUC-MSCs (Fig. 5). A significantly weaker staining was noted in the PFA/TX group. Gly alone or Gly/TX groups revealed very weak signals compared with the PFA groups, whereas the Gly + TX group displayed stronger and more specific nucleostemin signals compared with the other two Gly groups.
Lamin A/C, a complex inner nuclear membrane intermediate filament protein, exhibited a fairly homogenous pattern that was restricted to nuclei after hUC-MSCs were fixed with six fixatives (Fig. 5). TX addition either to PFA or Gly showed stronger signals that were finely confined to nuclear membrane, whereas Gly alone group exhibited an extremely devastated lamin A/C pattern.
Cell-specific markers
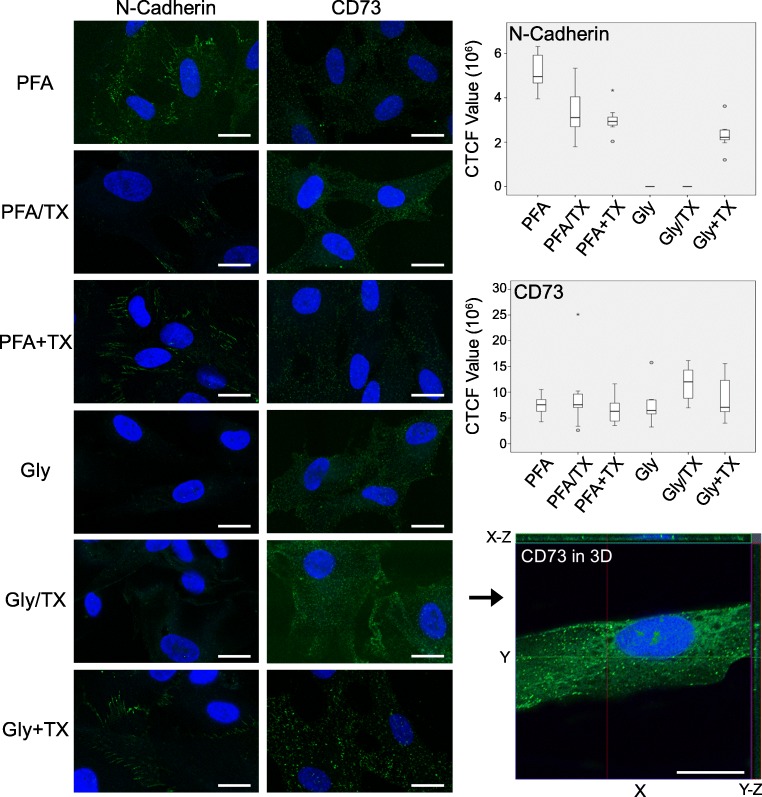
In this section, we first demonstrated the qualitative and quantitative protein fixation by two different fixatives in different cocktails on various cell-specific markers tested in hUC-MSCs. N-Cadherin, a cell adhesion molecule found primarily on the cell surface of mesenchymal cells, was selected to test the fixation capability of the fixatives, and CD73, a plasma membrane glycoprotein specific to various cell types including mesenchymal stem cells. All PFA formulations regardless of TX addition, the Gly alone and Gly + TX groups, exhibited fine, granular staining on the cell membrane without any preferential localization (Fig. 6). N-Cadherin signals were detected as fine string-like patterns along the plasma membranes (Fig. 6) and were clearly visualized in the PFA alone, PFA + TX, and Gly + TX groups. The remaining fixatives were unable to retain the N-cadherin proteins. Interestingly, CD73 signals only in Gly/TX group were consistently detected both on the plasma membrane and in the cytoplasm, which may be due to the translocation of the CD73 epitopes due to the TX incubation (Fig. 6).
Fig. 6.
N-Cadherin and CD73 in hUC-MSCs after they were fixed with six fixative solutions. N-Cadherin was well preserved along the plasma membrane in the PFA alone (highest compared with others; p = 0.042), PFA + TX, and Gly + TX groups (n = 8 for labeling; n = 80 for signal quantification) (first column). CD73 was well preserved in all fixative groups; however, in the Gly/TX group, the signal intensity was detected significantly higher than in the other groups (p = 0.021) (second column). CD73 signals in the Gly/TX group were further analyzed in 3D reconstructed image stacks obtained using super-resolution microscopy in the Z-axis (2.44 μm in thickness). X-Y-axis lateral and X-Z, Y-Z-axis orthogonal sections showed that most of the CD73 epitopes translocated to the cytoplasm and nucleoplasm (n = 6 for labeling; n = 60 for signal quantification). Scale bars: 20 μm
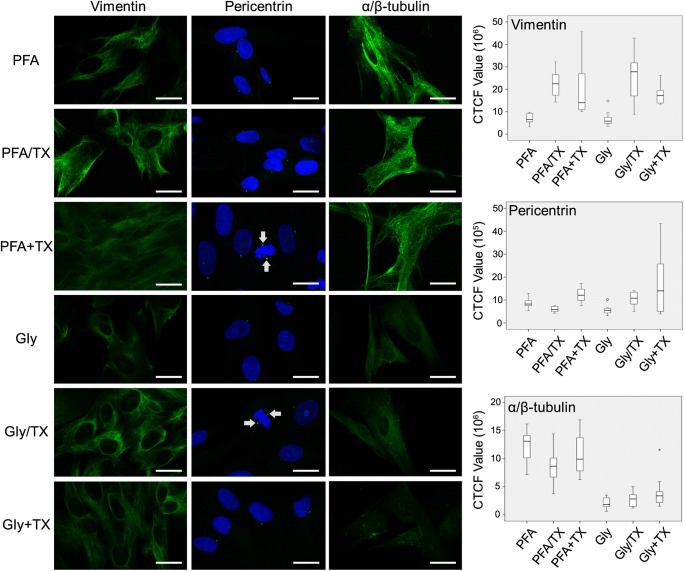
Secondly, we tested the fixation potential of six fixatives on some cytoskeletal proteins (vimentin, pericentrin, and α/β-tubulin). Vimentin, an abundant intermediate filament protein, exclusively expressed in mesenchyme-originated cells, was best retained when cells were fixed with either PFA or Gly with the addition of TX (Fig. 7). When TX was applied after fixation (PFA + TX and Gly + TX groups), the signal intensity became weak. The lowest signal was detected in the PFA alone and Gly alone groups. None of the fixatives caused any background noise or non-specific staining.
Fig. 7.
Preservation of vimentin, pericentrin, and α/β-tubulin in hUC-MSCs after fixing with six fixative solutions. Vimentin was well retained with all fixatives (first column); however, the best and most consistent signals were found in the PFA/TX and Gly/TX groups (p = 0.001); the PFA + TX and Gly groups displayed weak vimentin signals (p < 0.001) (n = 5 for labeling; n = 50 for signal quantification). Pericentrin was best retained in the PFA + TX and Gly + TX groups (p = 0.005); the Gly alone group revealed the lowest signal intensity (p = 0.002) (second column). Pericentrin proteins were also well-preserved in occasionally encountered mitotic cells (arrows) (n = 5 for labeling; n = 50 for signal quantification). α/β-Tubulin staining was best-preserved in all PFA formulations (p < 0.001) (third column), whereas Gly groups in general exhibited vary faint staining. Gly + TX did not preserve any α/β-tubulin (n = 6 for labeling; n = 60 for signal quantification). Scale bars: 20 μm
The staining of pericentrin, which is a conserved protein of the pericentriolar material, an integral component of the centrosome, was exclusively confined to two adjacent juxtanuclear foci (Fig. 7) and best preserved in the PFA + TX and Gly + TX groups; the lowest signal intensity was measured in the Gly alone group.
As the final cytoskeletal element, microtubules composed of α- and β-tubulin were fixed with six fixatives. The results dramatically favored PFA-containing fixatives (Fig. 7). No significant difference was noted among the PFA-containing fixative groups. Gly-containing fixatives revealed very faint microtubule arrays (Gly alone and Gly/TX groups), and no filamentous array was noted in Gly + TX group.
Surface topology
mOocytes
During fixation procedures with different fixatives, we noted some dramatic differences between PFA and Gly fixatives regarding the morphology of naked mOocytes and cumulus enclosed oocytes. After fixing them with either PFA or Gly and then labeling with FITC-conjugated phalloidin toxins, consecutive optical sections obtained using confocal microscope allowed us to visualize those cells as three-dimensional (3D) image stacks. DIC images simply presented the size difference between two fixatives (Fig. 8); Gly-fixed cells were significantly smaller than PFA-fixed ones. As clearly presented in Fig. 1 and the Electronic Supplementary movie (Gly-mEmbryos.wmv), ZP was heavily affected by Gly fixation, whereas it was intact in the PFA group. Surface rendering of image stacks showed a dramatic difference because the plasma membranes of mOocytes (oolemma) were found damaged (Fig. 8).
Fig. 8.
Preservation of cellular processes and plasma membranes in mOocytes and hUC-MSCs. DIC images of mOocytes and cumulus-enclosed (asterisks) oocytes showed the size difference, dissolution (arrowheads), and disappearance of ZP in the Gly group compared with the intact ones in PFA group (arrows) (n = 16 from each independent experiment). DIC images showed the extremely flat, huge cell bodies of hUC-MSCs. Surface topologic renderings confirmed the size difference, ZP dissolution, and oolemma destruction in mOocytes. F-Actin-labeled microspikes (arrows) were detected by surface topological renderings in hUC-MSCs. As indicated by thin arrows, microspikes were well preserved by PFA, whereas Gly was not able to retain those cellular processes (n = 3 for labeling). Scale bars: 20 μm
hUC-MSCs
F-Actin filaments, known as stress fibers in cultured cells, are commonly labeled with F-actin-specific mushroom toxins such as phallotoxins. Thus, we wanted to examine whether any signaling intensity and localization difference existed due to the different fixatives in extremely flat hUC-MSC monolayers. Fine fixation is essential for the preservation of those tiny cellular processes because cells possess wide lamellipodia and microspikes. PFA exhibited finer fixation as evidenced by the preservation of F-actin-labeled microspikes (Fig. 8) compared with Gly, with which no microspikes were preserved.
Discussion
The use of Gly as a fixative in the literature is rather old and rare compared with the other aldehyde fixatives. First presented in 1943 by Wicks and Suntzeff, Gly was proposed to be more efficient than 10% formalin (3.7% FA solution) and was reported to be less harmful by inhalation than FA [7, 22]. Thus, replacing Gly with FA in the chemical fixation of cells and tissues may be reasonable. However, studies comparing the fixation efficiency of FA with Gly in the preservation of different cellular targets (membrane receptors, cytoplasmic, and nuclear targets) as well as the general morphology in tissue sections presented that FA was superior to Gly because Gly frequently resulted in significantly weaker and/or non-specific staining [10, 12, 23].
From the time when the fixation efficacy of PFA was recognized as better for electron microscopy in the 1970s [24] and then for immunocytochemistry (ICC) in the 1980s, it has become the most commonly used aldehyde fixative, especially in precise microscopic observations in cell and molecular biology. In this study, we wanted to compare those two aldehyde fixatives side-by-side using WB assays and super-resolution confocal microscopy to determine whether Gly, as a historic and lesser-known compound, has any superior capacity for fixation over cellular proteins. Very recently, Richter et al. published a comprehensive and well-designed data series pertaining to Gly vs. PFA fixation and concluded that 51 cellular targets were better preserved after Gly fixation [9]. This led us to test Gly for our samples, most of which are related to male and female reproductive organs, early embryonic and adult stem cells. Results emerged from human spermatozoa specimens are in writing process. Our overall comparison of PFA and Gly results are summarized in Table 5, where positive and negative outcomes are designated as green and red colors, respectively.
Table 5.
The overall performance of tested fixatives on various cell markers based on the intensity measurements and qualitative analyses using ICC in hUC-MSCs. Green designates positive outcomes and red refers to negative outcomes
NS not stained, NB no background staining, ND not determined as there is no literature information
aStaining intensity out of three grades
bNonspecific background staining out of three grades
cConcordance with known cellular pattern as defined by poor (★), moderate (★★), and well (★★★)
In the study by Richter et al., the final pH of Gly was determined as 5 after they obtained similar results at pH 4 and 5 for most of their experiments. Therefore, we adopted Gly at pH 5 for all sets of our experiments. They calculated the proportion of the proteins that remained unfixed in brain lysates and found that 40% and 20% of proteins were unfixed with PFA and Gly, respectively. In contrast, as summarized in Table 4, we found that significantly more proteins remained unfixed after Gly fixation. Interestingly, Gly very consistently resulted in higher band intensities than those in PFA ones in hUC-MSCs and mOocyte lysates where fixed proteins either no longer ran into the gel or formed smears only. More specifically, the fixation efficiency of PFA favored Gly because β-actin, vimentin, and α/β-tubulin proteins were retained with greater proportions after PFA. In addition to the degree of β-actin preservation, we also demonstrated the lack of F-actin-decorated microspikes in Gly-fixed somatic cells and surface disruptions in F-actin-labeled oocyte cortex as rendered using high-resolution consecutive fluorescent images. In contrast to our findings, different laboratories that contributed to Richter et al.’s study reported that a relatively higher ratio of β-actin, α- and β-tubulin, and F-actin was preserved with Gly. Nevertheless, they reported that a higher vimentin ratio was retained with PFA based on their ICC staining.
Live-cell imaging of embryos during fixation revealed that Gly caused thinning and then rupture of ZP, and dissociation of blastomeres, whereas tiny infrequent membrane blebs were noted in somatic cells. PFA, on the other hand, maintained the blastocyst morphology as intact as possible while generating the formation of large cytoplasmic blebs in somatic cells. Gly with TX showed similar findings in embryos while maintaining the cytoplasmic and membrane structures in somatic cells. TX application after fixation revealed no further change either in embryos or somatic cells. Based on our live-hUC-MSC videos during fixation, we agree with the statement proposed by Richter et al. [9] that a higher speed of membrane penetration and the sudden interruption of vesicle trafficking was seen with Gly. In contrast to PFA, which resulted in the formation of large membrane blebs and continued almost during the entire fixation period, Gly displayed a very fast and relatively intact vesicle preservation with very few tiny blebs during fixation. However, Gly caused a significant decrease in the size of embryos and oocytes, as demonstrated in Figs. 1 and 8. Although some of the above features may seem advantageous to Gly, the catastrophic changes in the embryo caused by Gly were not acceptable. We assume that Gly-originated perturbations may be due to its acidic nature, which arises from its fast oxidation, and therefore lead to the formation of strong acids, mainly glyoxylic acid [25].
In situ labeling of diverse proteins using ICC after PFA or Gly fixation did not exhibit results superior to Gly. Briefly, Gly caused a higher number of unstained epitopes, a stronger background, and non-specific staining. Gly was occasionally found better than PFA in terms of fluorescence signal intensity.
All cross-linking reagents (all aldehyde group fixatives) form intermolecular bridges, normally through free amino groups, thus creating a network of linked antigens. Cross-linkers preserve cell structure better than organic solvents, but may reduce the antigenicity of some cell components, and require the addition of a permeabilization step to allow access of the antibody to the specimen [26]. Non-ionic detergents such as TX are widely used to permeabilize and solubilize membrane proteins in a gentler manner, allowing the solubilized proteins to retain a native subunit structure and enzymatic activity [27]. Non-ionic detergents are considered non-denaturating because they break lipid-lipid and lipid-protein, but not protein-protein interactions [28]; as such, they are considered for use in the isolation of membrane proteins in cell and molecular biologic applications [27]. Due to our TX-containing fixative results, some protein labels were partially/completely lost (i.e., Mitotracker, CD73, and N-cadherin), whereas the intensity of some labels was enhanced (i.e., CENP-A, lamin A/C, nucleostemin, and vimentin).
Conclusively, the superiority of PFA over Gly was clear, at least for certain cell types and proteins as we have presented here. However, no single type of fixative cocktail and protocol seems to fit to all types of samples and proteins. Thus, it is still noteworthy to suggest that the proper fixative (with or without TX addition) formulae and fixation procedures should be carefully determined through a series of well-designed comparative experiments to optimize protein preservation as naturally as possible.
Electronic supplementary material
mEmbryos. Seven movie files are presented showing 20-min long time-lapse movies (40x) before and during the fixation with PFA and PFA/TX groups. Additional 5 min-TX incubation is shown in the PFA+TX and Gly+TX groups; (n=12 independent experiment). Scale bars: 50 μm. (ZIP 28682 kb).
hUC-MSCs. Seven movie files are presented showing 15-min long time-lapse movies (40x) before and during the fixation with PFA and PFA/TX groups. Additional 5 min-TX incubation is shown in PFA+TX and Gly+TX groups; (n=13 independent experiment). Scale bars: 50 μm. (ZIP 37534 kb).
Abbreviations
- 3D
Three-dimensional
- ACRPB
Acrosin-binding protein
- BCA
Bicinchoninic acid
- BSA
Bovine serum albumin
- CTCF
Corrected total cell fluorescence
- ddH2O
Double distilled water
- DIC
Differential interference contrast
- DMEM
Dulbecco’s modified Eagle’s medium
- F-actin
Filamentous actin
- FA
Formaldehyde
- FITC
Fluorescein isothiocyanate
- Gly
Glyoxal
- GV
Germinal vesicle
- hCG
Human chorionic gonadotropin
- hUC-MSC
Human umbilical cord-derived mesenchymal stem cell
- ICC
Immunocytochemistry
- IHC
Immunohistochemical
- mEmbryos
Mouse embryos
- MI
Meiosis-I
- mOocyte
Mouse oocyte
- P4
Passage 4
- PFA
Paraformaldehyde
- PMSG
Pregnant mare’s serum gonadotropin
- PVDF
Polyvinylidene difluoride
- RT
Room temperature
- SDS
Sodium dodecyl sulphate
- TX
Triton X-100
- WB
Western blot
- ZP
Zona pellucida
Funding information
This study was financially supported by Ankara University Research Fund (17A0230001).
Compliance with ethical standards
The experimental protocol regarding the mouse oocytes and embryos was approved by the IRB (approval number 792/20-18.10.27). All experiments were carried out in accordance with relevant regulations and guidelines of Ankara University Animal Care and Use Committee. Fresh umbilical cords (n = 3) were obtained from full-term female infants after cesarean section succeeding the acquisition of informed consent from the mother (Local Ethics Committee, IRB approval number 18-578-12, 2012) that the experiments conformed to the principles set out in the World Medical Association Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howat WJ, Wilson BA. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods. 2014;70(1):12–19. doi: 10.1016/j.ymeth.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcon N, Bressenot A, Montagne K, Bastien C, Champigneulle J, Monhoven N, Albuisson E, Plénat F. Glyoxal: a possible polyvalent substitute for formaldehyde in pathology? Ann Pathol. 2009;29(6):460–467. doi: 10.1016/j.annpat.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara K. Techniques for localizing contractile proteins with fluorescent antibodies. Curr Top Dev Biol. 1980;14(Pt 2):271–296. doi: 10.1016/S0070-2153(08)60198-2. [DOI] [PubMed] [Google Scholar]
- 4.Robinson RW, Snyder JA. An innovative fixative for cytoskeletal components allows high resolution in colocalization studies using immunofluorescence techniques. Histochem Cell Biol. 2004;122(1):1–5. doi: 10.1007/s00418-004-0656-2. [DOI] [PubMed] [Google Scholar]
- 5.Leyton-Puig D, Kedziora KM, Isogai T, van den Broek B, Jalink K, Innocenti M. PFA fixation enables artifact-free super-resolution imaging of the actin cytoskeleton and associated proteins. Biol Open. 2016;5(7):1001–1009. doi: 10.1242/bio.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SO, Kim J, Okajima T, Cho NJ. Mechanical properties of paraformaldehyde-treated individual cells investigated by atomic force microscopy and scanning ion conductance microscopy. Nano Converg. 2017;4(1):5. doi: 10.1186/s40580-017-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatini DD, Bensch K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dapson RW. Glyoxal fixation: how it works and why it only occasionally needs antigen retrieval. Biotech Histochem. 2007;82(3):161–166. doi: 10.1080/10520290701488113. [DOI] [PubMed] [Google Scholar]
- 9.Richter KN, Revelo NH, Seitz KJ, Helm MS, Sarkar D, Saleeb RS, et al. Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy. EMBO J. 2018;37(1):139–159. doi: 10.15252/embj.201695709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Storkel S. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem. 2004;52(7):893–901. doi: 10.1369/jhc.3A6195.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dapson RW, Feldman AT, Wolfe D. Glyoxal fixation and its relationship to immunohistochemistry. J Histotechnol. 2006;29(2):66–76. doi: 10.1080/01478885.2006.11800879. [DOI] [Google Scholar]
- 12.Umlas J, Tulecke M. The effects of glyoxal fixation on the histological evaluation of breast specimens. Hum Pathol. 2004;35(9):1058–1062. doi: 10.1016/j.humpath.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang YN, Lee K, Pai S, Ledoux WR. Histomorphometric comparison after fixation with formaldehyde or glyoxal. Biotech Histochem. 2011;86(5):359–365. doi: 10.3109/10520295.2010.520275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paavilainen L, Edvinsson A, Asplund A, Hober S, Kampf C, Ponten F, et al. The impact of tissue fixatives on morphology and antibody-based protein profiling in tissues and cells. J Histochem Cytochem. 2010;58(3):237–246. doi: 10.1369/jhc.2009.954321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussolati G, Annaratone L, Berrino E, Miglio U, Panero M, Cupo M, Gugliotta P, Venesio T, Sapino A, Marchiò C. Acid-free glyoxal as a substitute of formalin for structural and molecular preservation in tissue samples. PLoS One. 2017;12(8):e0182965. doi: 10.1371/journal.pone.0182965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitriadis GJ. Effect of detergents on antibody-antigen interaction. Anal Biochem. 1979;98(2):445–451. doi: 10.1016/0003-2697(79)90165-9. [DOI] [PubMed] [Google Scholar]
- 17.Can A, Semiz O, Cinar O. Centrosome and microtubule dynamics during early stages of meiosis in mouse oocytes. Mol Hum Reprod. 2003;9(12):749–756. doi: 10.1093/molehr/gag093. [DOI] [PubMed] [Google Scholar]
- 18.Uysal F, Ozturk S, Akkoyunlu G. DNMT1, DNMT3A and DNMT3B proteins are differently expressed in mouse oocytes and early embryos. J Mol Histol. 2017;48(5–6):417–426. doi: 10.1007/s10735-017-9739-y. [DOI] [PubMed] [Google Scholar]
- 19.Can A, Balci D. Isolation, culture, and characterization of human umbilical cord stroma-derived mesenchymal stem cells. Methods Mol Biol. 2011;698:51–62. doi: 10.1007/978-1-60761-999-4_5. [DOI] [PubMed] [Google Scholar]
- 20.Coskun H, Can A. The assessment of the in vivo to in vitro cellular transition of human umbilical cord multipotent stromal cells. Placenta. 2015;36(2):232–239. doi: 10.1016/j.placenta.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Oktar PA, Yildirim S, Balci D, Can A. Continual expression throughout the cell cycle and downregulation upon adipogenic differentiation makes nucleostemin a vital human MSC proliferation marker. Stem Cell Rev. 2011;7(2):413–424. doi: 10.1007/s12015-010-9201-y. [DOI] [PubMed] [Google Scholar]
- 22.Wicks LF, Suntzeff V. Glyoxal, a non-irritating aldehyde suggested as substitute for formalin in histological fixations. Science. 1943;98(2539):204. doi: 10.1126/science.98.2539.204. [DOI] [PubMed] [Google Scholar]
- 23.Titford ME, Horenstein MG. Histomorphologic assessment of formalin substitute fixatives for diagnostic surgical pathology. Arch Pathol Lab Med. 2005;129(4):502–506. doi: 10.1043/1543-2165(2005)129<502:HAOFSF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Smit JW, Meijer CJ, Decary F, Feltkamp-Vroom TM. Paraformaldehyde fixation in immunofluorescence and immunoelectron microscopy. Preservation of tissue and cell surface membrane antigens. J Immunol Methods. 1974;6(1–2):93–98. doi: 10.1016/0022-1759(74)90093-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhao D, Xu B. Analysis of glyoxal and related substances by means of high-performance liquid chromatography with refractive index detection. J Chromatogr Sci. 2013;51(10):893–898. doi: 10.1093/chromsci/bms186. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry—the red, brown, and blue technique. Vet Pathol. 2014;51(1):42–87. doi: 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- 27.Bhairi SM, Mohan C, Ibryamova S, La Favor T. Detergents: a guide to the properties and uses of detergents in biological systems. San Diego: Calbiochem-Novabiochem Corporation; 2001. [Google Scholar]
- 28.Lórenz-Fonfría Víctor, Perálvarez-Marín Alex, Padrós Esteve, Lazarova Tzvetana. Production of Membrane Proteins. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. Solubilization, Purification, and Characterization of Integral Membrane Proteins; pp. 317–360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mEmbryos. Seven movie files are presented showing 20-min long time-lapse movies (40x) before and during the fixation with PFA and PFA/TX groups. Additional 5 min-TX incubation is shown in the PFA+TX and Gly+TX groups; (n=12 independent experiment). Scale bars: 50 μm. (ZIP 28682 kb).
hUC-MSCs. Seven movie files are presented showing 15-min long time-lapse movies (40x) before and during the fixation with PFA and PFA/TX groups. Additional 5 min-TX incubation is shown in PFA+TX and Gly+TX groups; (n=13 independent experiment). Scale bars: 50 μm. (ZIP 37534 kb).