Abstract
In the visual pathway, optic nerve (ON) injury may cause secondary degeneration of neurons in distal regions, such as the visual cortex. However, the role of the neuroinflammatory response in regulating secondary impairment in the visual cortex after ON injury remains unclear. The NOD-like receptor family pyrin domain containing 3 (NLRP3) is an important regulator of neuroinflammation. In this study, we established a mouse model of unilateral ON crush (ONC) and showed that the expression of NLRP3 was significantly increased in the primary visual cortex (V1) as a response to ONC and that the NLRP3 inflammasome was activated in the contralateral V1 1 days–14 days after ONC. Ablation of the NLRP3 gene significantly decreased the trans-neuronal degeneration within 14 days. Visual electrophysiological function was improved in NLRP3−/− mice. Taken together, these findings suggest that NLRP3 is a potential therapeutic target for protecting visual cortical neurons against degeneration after ON injury.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00445-x) contains supplementary material, which is available to authorized users.
Keywords: NLRP3, Visual cortex, Optic nerve injury, Visual cortical degeneration
Introduction
In the visual system, in addition to the direct damage to the axons and somata in the primary site under pathological conditions such as ocular trauma and high intraocular pressure, neurons within the visual pathway also suffer from anterograde (retina to visual cortex) or retrograde (visual cortex to retina) injuries, which are characterized by distal neural degeneration, programmed cell death, and increased inflammatory cytokines, accompanied by the recruitment and activation of glial cells [1–4]. However, little is known about the initial factors and pathological mechanisms responsible for these secondary changes; relevant therapies to prevent dysfunction are still unavailable.
Inflammation performs the dual roles of damage and repair in the central nervous system (CNS) and has also been reported to be a key factor in secondary injury [5]. An important component of human innate immunity, cell pattern-recognition receptors, such as toll-like receptors [6] and NOD-like receptors [7], are activated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), fused into a multimeric complex by binding to the adaptor molecule apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC) and procaspase-1, named the inflammasome. The inflammasome is capable of converting immature pro-interleukin (IL)-1β and pro-IL-18 to mature forms by procaspase-1 self-cleavage, thereby triggering the innate inflammatory response [8–10]. In addition, activated caspase-1 promotes the maturation of gasdermin D (GSDMD), which ultimately mediates the pro-inflammatory form of programmed cell death termed pyroptosis [11]. In the inflammasome family, the nucleotide-binding domain leucine-rich repeat (LRR)–pyrin domain containing 3 or NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome has been well characterized, for the abnormal activation of the NLRP3 inflammasome has been shown to be implicated in many CNS disorders [12, 13], diabetes mellitus [14, 15], and autoimmune diseases [16, 17]. Accumulating evidence has shown that inflammasomes participate in the onset and progression of secondary brain injury [18–20] and glaucoma [21]. Furthermore, activation of the NLRP3 inflammasome in microglia has been implicated in retinal ganglion cell loss after optic nerve (ON) injury [22].
Studies on secondary lesions have shown that the breach of the blood-brain barrier following ON injury is associated with the recruitment of peripheral mononuclear macrophages and neutrophils [3] and the inflammatory response upon NLRP3 activation of glia [22, 23]. Furthermore, the expression of NLRP3 in neurons has been documented [24–27]. Concerning its possible involvement in secondary/distal damage in the CNS, it is imperative to establish whether the NLRP3 inflammasome is activated trans-synaptically after ON injury and whether its effect on visual cortical neurons is responsible for secondary neuronal degeneration. To address this issue, we established a mouse model of unilateral ON crush (ONC). Activation of the NLRP3 inflammasome in the primary visual cortex (V1) was found to be associated with the progression of neuronal injury. The inflammatory cytokines in V1 of the NLRP3−/− brain were decreased within 14 days, the number of apoptotic and degenerative neurons was decreased, and the indexes of visual electrophysiology were also improved in NLRP3-knockout mice.
Materials and Methods
Animals
Adult C57BL/6J male mice (WT) 8–10 weeks old and weighing 20 g–24 g were purchased from the Animal Center of The Army Medical University (AMU). Age- and weight-matched NLRP3−/− mice (JAX Cat. No. 021302) were purchased from The Jackson Laboratory (Bar Harbor, ME). The NLRP3−/− mice were confirmed by polymerase chain reaction (PCR) (Figs. S1 and S2). The primer sequences used were as follows: 5′-TGCCTGCTCTTTACTGAAGG-3′ (mutant forward); 5′-TCAGTTTCCTTGGCTACCAGA-3′ (WT forward); 5′-TTCCATTACAGTCACTCCAGATGT-3′ (common reverse). The animals were housed in a 12/12 h light/dark schedule in a specific pathogen-free facility, with free access to irradiated food and sterile water.
Experimental Design
The sample size per experiment was determined based on previous publications with similar methodologies. All experiments were performed 3 times. All the data were included in analysis.
Surgical Procedure for Optic Nerve Crush
The control group consisted of mice that received no operation (naïve) and mice that received sham surgery on the left eye (sham). In the experimental group, the left eye was subjected to ONC. Briefly, animals were anesthetized by intraperitoneal injection of 1% pentobarbital. Using aseptic technique, a minimal incision was performed in the temporal area of the conjunctiva of the left eye, and the extraocular muscles were gently separated to expose the ON, which was crushed with fine, self-closing forceps 1.5 mm behind the eyeball for 10 s without damaging the blood vessels [28, 29]. In the sham operation, the ON was exposed by using the same protocol but without crushing. The eyes were treated with antibiotic ointment after surgery. The mice were then kept on a warming pad until fully awake. There were no postoperative infections. All animal procedures were conducted in accordance with the Guidelines on the Use of Animals from the NIH and the Institutional Animal Care and Use Committee of AMU and were approved by the Animal Ethics Committee of AMU.
RNA Isolation and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Animals were euthanized and V1 was dissected out according to the mouse brain atlas (The Mouse Brain in Stereotaxic Coordinates, Academic Press, New York, 2001). Total RNA of the contralateral and ipsilateral V1 was extracted with an RNeasy RNA isolation kit (Qiagen, Hilden, Germany), and reverse transcribed with a Prime Script™ RT reagent Kit (Takara, Dalian, China) according to the manufacturer’s instructions. qRT-PCR was performed on a CFX96 real-time instrument/C1000 Thermal Cycler (Bio-Rad, Hercules, CA) using the SYBR method (Quanta BioSciences, Gaithersburg, MA).
The primer sequences used for qRT-PCR were as follows:
NLRP3: F-AAAGGAAGTGGACTGCGAGA, R-TTCAAACGACTCCCTGGAAC;
NLRP1: F-TTAGATGAGCATGCCATTGC, R-ACTCCTGAAGACACAAGTGG;
NLRC4[NLR family CARD (caspase recruitment domain)-containing 4]: F-GGAAAGTGCAAGGCTCTGAC, R-TGTCTGCTTCCTGATTGTGC;
NLRP6: F-CTGTTCTGAGCTACTGCGTGAG, R-AGGCTCTTCTTCTTCTTCTCCTG;
AIM2(Absent in Melanoma 2): F-AGCCTGAACAGAAACAGATGG, R-CTTCTTGGGTCTCAAACGTGA.
Western Blot
Freshly-dissected V1 tissue was combined with RIPA (89900, Thermo Fisher Scientific, Waltham, MA) and protease inhibitor cocktail (78438, Thermo Fisher Scientific, Waltham, MA) to obtain the lysate. After centrifugation at 12,000 × g at 4°C for 15 min, the supernatant was collected and the protein concentration was measured with an Enhanced BCA Protein Assay Kit (P0009, Beyotime, Shanghai, China). Equal amounts of lysate (50 mg) were separated on SDS polyacrylamide gel and transferred to polyvinylidene difluoride membranes (IPVH00010, Millipore, Burlington, MA) with continuous current (250 mA) for 0.5 h–2 h. The membranes were washed in Tris-buffered saline (TBS, pH 7.4) containing 0.1% Tween-20 (TBS-T) and blocked in 5% non-fat milk in TBS-T for 2 h at room temperature (RT). Then, the membranes were incubated with primary antibodies recognizing mouse NLRP3 (AG-20B-0014-C100, 1:500, AdipoGen Life Science, Liestal, Switzerland), ASC (Santa Cruz, Dallas, TX), caspase-1 (AB 1871, 1:1000, Chemicon, Temecula, CA), IL-1β (AF-401-NA, 1:1000, R&D Systems, Minneapolis, MN), AIM2 (ab93015, 1:500, Abcam, Cambridge, MA), and β-actin (AP0731, 1:5000, Bioworld, Bloomington, MN) overnight at 4°C. After three washes with TBS-T for 10 min each, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, R&D Systems, Minneapolis, MN) for 2 h at RT. The signals were detected with a chemiluminescence reagent (ECL, GE Healthcare, Shanghai, China), and images were acquired by a chemiluminescent detection system (Thermo Fisher Scientific, Waltham, MA). Densitometric quantification of the specific band intensities was normalized to β-actin in the same blot.
Immunostaining
Mouse brains were fixed in freshly prepared 4% paraformaldehyde at 4°C for 24 h and then embedded in OCT compound (Sakura Finetek, San Diego, CA). Cryosections (20 μm) were permeabilized with PBS containing 0.2% Triton X-100 at RT for 30 min. Sections were blocked with the appropriate sera and then incubated overnight at 4°C with primary antibodies for NLRP3 (1:400, AdipoGen Life Science, Liestal, Switzerland) and NeuN (#24307, 1:200, Cell Signaling Technology, Danvers, MA). After three washes, the sections were incubated with the appropriate fluorescent secondary antibodies (Alexa Fluor 488 and 594, 1:400, Life Technologies, Carlsbad, CA) for 1 h at RT. The slides were then stained with DAPI and mounted. Images were captured using an SP-8 confocal microscope (Leica, Wetzlar, Germany).
TUNEL
Apoptotic neurons in contralateral V1 sections were stained using an In Situ Cell Death Detection Kit according to the manufacturer’s protocol (TMR red, 12156792910, Roche, Mannheim, Germany). Briefly, the cryosections were washed with PBS, permeabilized in citrate-Triton buffer (0.1%), and then incubated with TUNEL working solution for 1 h at 37°C in a humid cassette avoiding light. The samples were then processed for NeuN double-labeling (see above) after a PBS wash. The images were captured with the SP-8 confocal microscope. TUNEL+NeuN+ cells were randomly counted via FIJI software (https://imagej.nih.gov/ij/), and the numbers of positive cells from the same optical fields (low-power) in each animal were averaged.
Fluoro-Jade C (FJC) Staining
Staining was carried out according to the manufacturer’s protocol, with modifications. Briefly, slides were first immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min, followed by 70% ethanol and a distilled water rinse for 2 min. Then, the slides were incubated in 0.06% potassium permanganate for 9 min. Following a 2-min water rinse, a 0.0001% solution of Fluor-Jade® C (AG325-30MG, Chemicon, Temecula, CA) dissolved in a 0.1% acetic acid vehicle was added to the slides and left for 15 min. The slides were then rinsed through three changes of distilled water (1 min per change), dried on a slide warmer at 50°C for 5 min, cleared in xylene for 1 min, and then coverslipped with DPX (Sigma). The stained slides were photographed with the SP-8 confocal microscope. The number of FJC-positive cells from the same optical fields (low-power) of each animal was averaged.
Flash Visual Evoked Potentials (F-VEPs)
To evaluate the visual electrophysiological function of mice with ONC or sham surgery, F-VEPs were measured on days 7 and 14 after ONC. Electrodes located at the primary visual cortexes were the active (positive) electrodes, and an electrode placed at the frontal cortex was the reference (negative) electrode. The ground electrode was inserted into the tail. While an eye was being tested, the contralateral eye was covered by a lightproof patch; the apparatus reduced interference from electrical noise (60 Hz) and the heartbeat. In each F-VEP experiment, 50 successive flash stimuli of 10 μs duration and 1 Hz frequency were delivered (intensity 120 mJ–200 mJ). In each animal, the amplitudes of three main waveforms from stimulus onset were recorded, and the P2 amplitude with respect to baseline was analyzed.
Statistical Analysis
Data are presented as the mean ± SEM. The unpaired Student’s t-test was applied to assess the differences between two groups. One-way analysis of variance (ANOVA) followed by the Tukey test was used for multiple comparisons. A P-value < 0.05 was considered statistically significant. All results were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
NLRP3 Expression is Elevated in Contralateral Primary Visual Cortex After Optic Nerve Crush
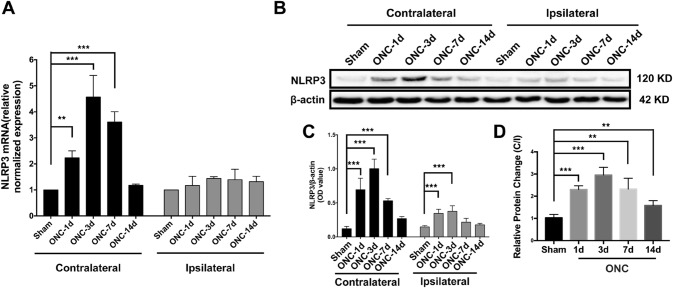
In previous studies, increased expression of NLRP3 has been reported in the local cerebral cortex after traumatic brain injury and cerebral ischemia [30, 31]. However, no study has described its expression pattern in V1 after anterior visual pathway injury. First, using qRT-PCR, we demonstrated elevated NLRP3 levels in the contralateral V1 after ONC. The RNA expression of NLRP3 was upregulated on day 1 and was further increased to its highest level on day 3. Then, the relative mRNA level decreased to the levels in the naïve (Fig. S3A) and sham groups on day 14 (Fig. 1A). However, no significant changes in the mRNA levels of NLRP3 in the ipsilateral V1 were found throughout the 14 days after ONC. Consistent with the qRT-PCR results, increased protein levels of NLRP3 in the contralateral V1 in response to ONC were shown by Western blotting (Fig. 1B). The densitometric ratio of NLRP3 to β-actin in ONC mice was nearly 22-fold higher than that of the naïve (Fig. S3 B, C) and sham groups on day 1 and progressively increased until day 3; subsequently, the ratio gradually decreased. On the ipsilateral side, however, the protein level of NLRP3 in the ONC mice was significantly higher on days 1 and 3 than in their sham-treated littermates, and then gradually declined to levels similar to those of the sham controls (Fig. 1B, C).
Fig. 1.
NLRP3 expression is significantly upregulated in primary visual cortex after optic nerve crush (ONC). A, B The mRNA and protein levels of NLRP3 in contralateral and ipsilateral V1 as determined by qRT-PCR and Western blot, respectively. C Quantification of NLRP3 protein levels in V1. D Relative protein change is presented as the ratio of contralateral to ipsilateral protein levels (C/I ratio). **P < 0.01, ***P < 0.001 versus sham group, n = 4.
The ratio of NLRP3 protein levels in the contralateral V1 to those in the ipsilateral V1 (C/I) was significantly higher 1 day after ONC than in the sham group and further increased on day 3. Then, a substantial decrease in the ratio occurred on day 7 but was still significantly higher than that in the sham mice (Fig. 1D). Taken together, these results suggest that the pronounced activation of NLRP3 in the contralateral V1 acts as a response to ONC.
NLRP3 is Upregulated in Contralateral V1 Neurons Following Optic Nerve Crush
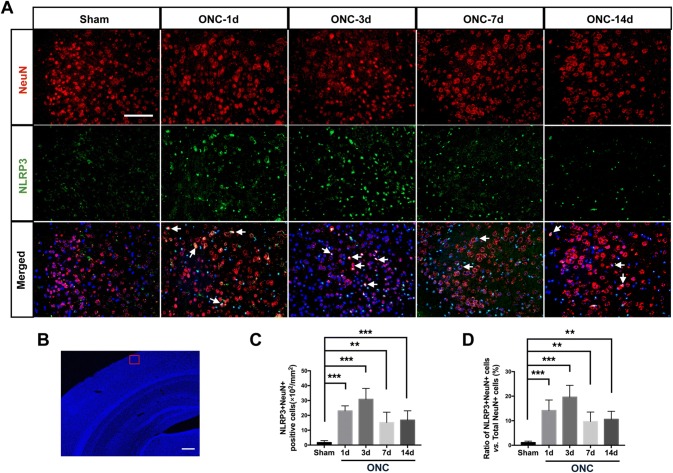
In rodent species, > 95% of the axons of retinal ganglion cells decussate at the chiasma and project to the contralateral recipient nuclei [32]. qRT-PCR and Western blot demonstrated that the increased expression of NLRP3 induced by ONC was prominent in contralateral V1. To determine whether the upregulated NLRP3 was located within the neurons of V1, we performed double immunolabeling using NLRP3 and neuronal nuclei (NeuN) antibodies. NLRP3 expression was hardly detectable in neurons in both the naïve (data not shown) and sham groups, while a significant increase in NLRP3-positive staining was observed in the neurons 1 day after ONC (Fig. 2A). A schematic overview of the regions of interest used for quantification in contralateral V1 is shown in Fig. 2B. The number of NeuN+NLRP3+ double stained cells was significantly higher and was approximately 13.4-fold greater than that of the sham group on day 3. A notable decrease in double-positive cells occurred 7 days after ONC (Fig. 2A, C).
Fig. 2.
NLRP3 is activated in neurons of contralateral V1 1 day after ONC. A Double immunostaining for NeuN (red) and NLRP3 (green) in contralateral V1. Nuclear counterstaining by DAPI in merged images is pseudocolored in dark blue. Arrows indicate NeuN+NLRP3+ cells. Scale bar, 100 μm. B Region of interest for quantification in contralateral V1. Scale bar, 500 μm. C Quantification of NeuN+NLRP3+ cells. D Ratio of NeuN+NLRP3+ to total NeuN+ neurons. Data are presented as the mean ± SEM. **P < 0.01, ***P < 0.001 vs sham group, n = 6.
We next calculated the ratios of NeuN+NLRP3+ versus total NeuN+ neurons in the contralateral V1 of the ONC and sham-treated mice. The ratio was significantly higher on day 1 in the ONC than in the sham groups and reached a peak on day 3, when the maximum number of NeuN+NLRP3+ double-positive cells occurred. The ratio was significantly lower on day 7 due to the decreased number of double-positive neurons (Fig. 2D), and remained significantly higher than the sham group on day 14 (Fig. 2D). These results demonstrate that NLRP3 in the contralateral V1 neurons is upregulated in response to ONC within 14 days.
NLRP3 Inflammasome Components ASC, Caspase-1 (p20), and GSDMD are Activated After ONC
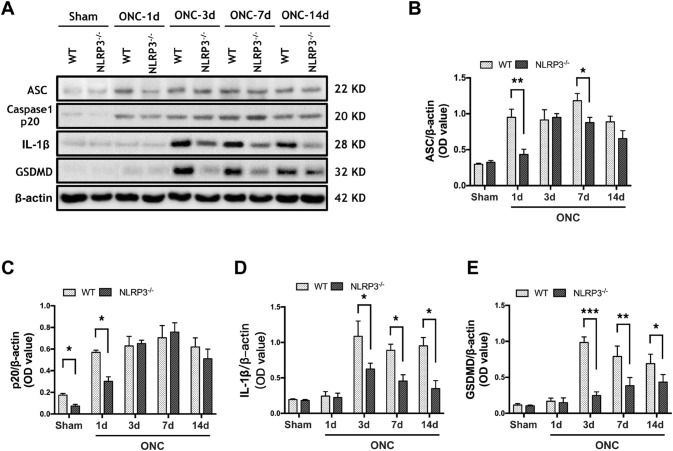
Since NLRP3 was upregulated mainly in the contralateral V1, we next asked whether the increased NLRP3 mediated the formation of inflammasomes in the same region. We examined the inflammasome components ASC and caspase-1 and downstream cytokines. In WT mice, the protein level of ASC was significantly upregulated in the contralateral V1 on day 1 and continued to increase in the period between 3 and 14 days, indicating the active assembly of NLRP3 inflammasomes in response to ONC (Fig. 3A, B). The increase of cleaved caspase-1 (p20) was evident 1 day after ONC, had progressively increased by days 3 and 7 (Fig. 3A, C), and was maintained up to day 14. No significant difference in the protein level of IL-1β in ONC mice and sham controls was found on day 1, then it significantly increased on day 3 and persisted on days 7 and 14 after ONC (Fig. 3A, D). However, in NLRP3−/− mice with ONC, the protein level of ASC was significantly lower than that in the WT group on days 1 and 7. No difference was found on day 3 due to the significantly increased protein level of ASC in the V1 of NLRP3−/− brains (Fig. 3A, B). The protein level of p20 in V1 of NLRP3−/− brains was significantly lower than that of WT 1 day after ONC; however, there was no significant difference between the two genotypes over the rest of the experimental period (Fig. 3A, C). Although no significant difference occurred 1 day after ONC, the protein level of IL-1β in NLRP3−/− V1 was significantly lower than in WT littermates on days 3, 7, and 14 (Fig. 3A, D). We also found changes in the protein level of GSDMD, the dominant factor mediating pyroptosis, which is processed by cleaved caspase-1. In the V1 of WT brains, the expression of GSDMD was significantly upregulated on days 3, 7, and 14. As expected, the protein levels of GSDMD in V1 of NLRP3−/− brains were significantly lower than those of the WT group at each time point. Of note, a significant increase occurred in NLRP3−/− V1 14 days after ONC (Fig. 3A, E). These results suggest that the ONC-induced NLRP3 inflammasome activation partially contributes to the increases of caspase-1, IL-1β, and GSDMD in the contralateral V1. In addition, GSDMD-mediated pyroptosis may occur in the contralateral V1 after ONC.
Fig. 3.
The expression levels of ASC, caspase-1 (p20), IL-1β, and cleaved GSDMD in the contralateral V1 of wild type (WT) mice and NLRP3-/- mice change following ONC. A Western blots showing the protein levels in contralateral V1. β-actin served as loading control. B–E Quantification of the changes in protein levels of ASC, caspase-1 (p20), IL-1β, and cleaved GSDMD. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, n = 4.
Ablation of NLRP3 Alleviates Apoptosis of Neurons in V1 Following ONC
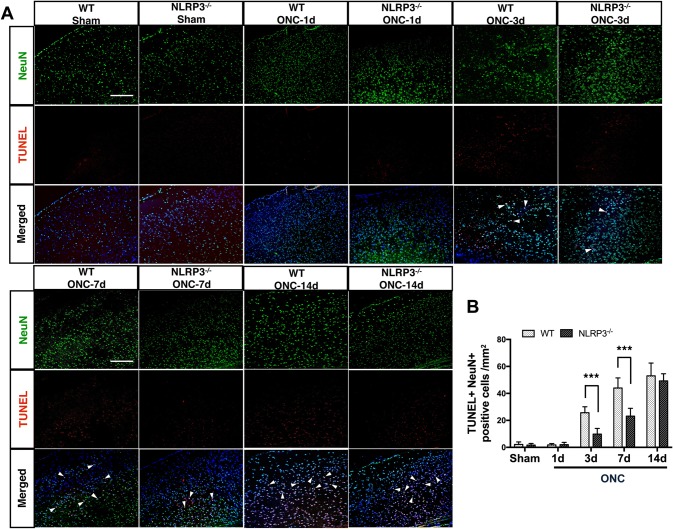
Programmed cell death is known to play a major role in the spread of neurodegeneration [33]. As neuronal apoptosis in V1 has been reported after ON axotomy [3], we next determined the effect of NLRP3 deletion on neuronal apoptosis using TUNEL staining. The numbers of TUNEL+NeuN+ double-positive cells showed little difference in the contralateral V1 of both WT and NLRP3−/− brains 1 day after ONC compared to sham controls. In WT V1, the number of double-positive cells was significantly higher on day 3 than in NLRP3−/− mice and sham controls, and was further increased in the 7- to 14- day period. However, the differences in the number of double-positive cells between WT and NLRP3−/− were not significant on day 14 due to the substantial increase of TUNEL+NeuN+ cells in NLRP3−/− V1 (Fig. 4A, B). In other words, the results suggest that knocking out the NLRP3 gene delays ONC-induced neuronal apoptosis within 7 days.
Fig. 4.
Apoptotic neurons in the contralateral V1 of WT and NLRP3−/− brains after ONC. A Double staining for TUNEL (red) and NeuN (green) in the contralateral V1. Nuclear counterstaining by DAPI in merged images is pseudocolored in dark blue. Arrowheads indicate NeuN+TUNEL+ cells. Scale bars, 200 μm. B Quantification of TUNEL+NeuN+ cells. Data are presented as the mean ± SEM. ***P < 0.001, n = 6.
Ablation of NLRP3 Delays Neuronal Degeneration in Contralateral V1 after ONC
Given that the ONC-induced trans-neuronal injury resulted not only in apoptosis but also in degeneration, to further determine the protective effect of NLRP3 depletion on the secondary degeneration in V1, we used FJC, an anionic fluorochrome, to selectively identify degenerating neurons [34]. The numbers of FJC-positive cells in the contralateral V1 of both WT and NLRP3−/− brains were not significantly higher than that in sham controls on day 1 (Fig. 5A). The number of FJC-positive cells in WT mice was significantly higher on day 3 than in sham-treated littermates and continued to increase through 7 and 14 days. As expected, the numbers of FJC-positive cells were significantly lower in V1 of NLRP3−/− brains than in WT littermates for the remainder of the observation period (Fig. 5A, C). A schematic overview of the regions of interest used for quantification in contralateral V1 is shown in Fig. 5B. The results suggest that a portion of neurons in the contralateral V1 undergo degeneration due to ONC, and ablation of NLRP3 delays the degeneration for at least 14 days.
Fig. 5.
FJC staining of contralateral V1 in WT and NLRP3-/- mice after ONC. A Confocal images showing the differences in FJC staining between WT and NLRP3-/- mice. Scale bar, 200 μm. B Region of interest used for quantification. Scale bar, 500 μm. C Quantification of FJC-positive cells. Data are presented as the mean ± SEM. **P < 0.01, ***P < 0.001, n = 6.
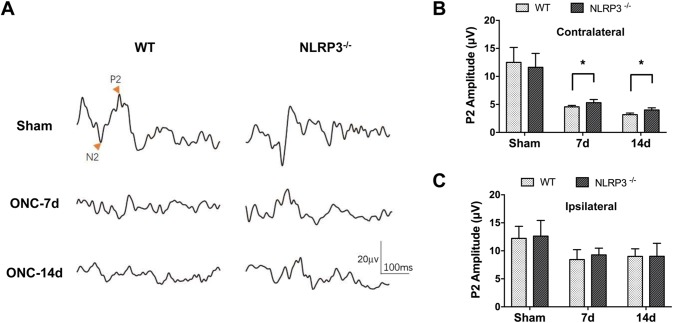
Visual Function is Improved in NLRP3-Knockout Mice Following ONC
The results of TUNEL and FJC staining demonstrated that knocking out the NLRP3 gene plays a protective role in the apoptosis and degeneration of V1 neurons after crush injury. Next, we asked whether visual cortical function was also improved by ablation of NLRP3. F-VEPs were recorded to determine the differences in the visual electrophysiological function between WT and NLRP3−/− mice after ONC. The VEP P2 amplitudes were significantly lower in both WT and NLRP3−/− mice after ONC than in sham controls. Notably, the VEP P2 waveform and amplitude from the contralateral hemisphere were significantly improved in the NLRP3−/− mice compared to the WT mice on 7 and 14 days (Fig. 6A, B), while the differences were barely detectable in the ipsilateral hemisphere in these mice (Fig. 6C), indicating that the visual cortical activity in contralateral hemisphere suffered more severely from ONC due to the decussation of axonal projection, and the mitigation effect of NLRP3 gene knockout within 14 days after ONC.
Fig. 6.
Ablation of NLRP3 mitigates the ONC-induced decrease in the VEP P2 amplitude. A Representative waveforms in the contralateral hemisphere (with respect to ONC) of WT and NLRP3-/- mice. B, C Quantification of the VEP P2 amplitude in the contralateral (B) and ipsilateral hemispheres (C). Data are presented as the mean ± SEM. *P < 0.05, n = 6 for sham groups and n = 8 for ONC groups.
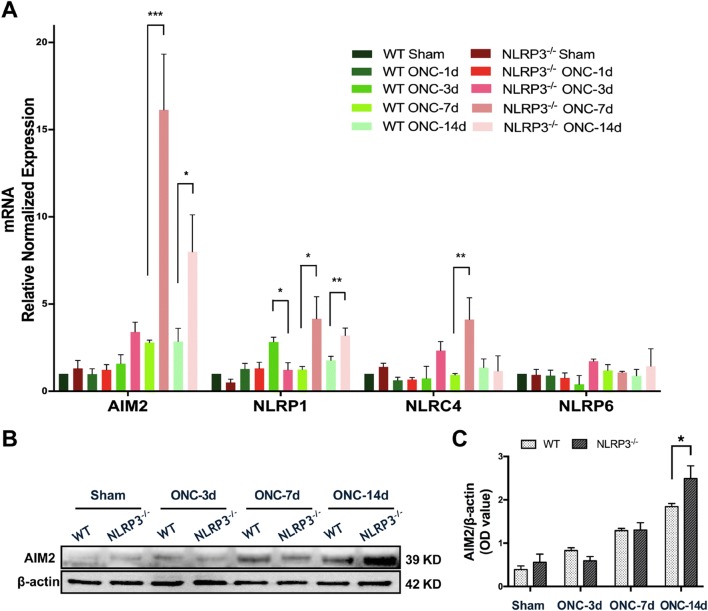
AIM2 Expression is Altered in Contralateral V1 in a Time-Dependent Manner Following ONC
Considering that the NLRP3 gene was globally knocked out in the animals used in this study, and the expression of ASC, caspase-1 (p20), and IL-1β was not substantially suppressed in V1 of the NLRP3−/− brain after ONC (Fig. 3C, D), we speculated that there is a compensatory effect of other inflammasomes that might be responsible for cytokine maturation. Therefore, we next determined whether other inflammasome analogues were activated in the contralateral V1 after crush injury, especially in the absence of NLRP3. The mRNA levels of AIM2, NLRP1, NLRP6, and NLRC4 were assessed by qRT-PCR. The results showed that the mRNA expression of AIM2 in the V1 of NLRP3−/− brains was significantly upregulated by ~ 5.9-fold (16.14 ± 3.19 vs 2.79 ± 0.13) compared with WT littermates on day 7, and the difference remained significant through day 14. The mRNA level of NLRP1 was also significantly increased in NLRP3−/− V1 on days 7 and 14, as was that of NLRC4 on day 7, all compared to WT littermates. However, the changes in NLRP6 expression were hardly detectable over the observational period in both WT and NLRP3−/− mice (Fig. 7A).
Fig. 7.
The expression levels of AIM2, NLRP1, NLRC4, and NLRP6 are altered in contralateral V1 after ONC. A qRT-PCR results of the mRNA levels of AIM2, NLRP1, NLRC4, and NLRP6 in the contralateral V1 of WT and NLRP3-/- mice. B Western blots showing the expression of AIM2 in the contralateral V1 of WT and NLRP3-/- mice. C Quantification of the protein levels of AIM2. Data are presented as the mean ± SEM. *P < 0.05, n = 5.
Since the mRNA level of AIM2 increased dramatically in V1 of the NLRP3−/− brain after ONC, we speculated that AIM2 was a candidate for further study. We next determined whether the protein level of AIM2 was also prominent in the V1 of the NLRP3−/− brain. Western blots revealed that the protein level of AIM2 was significantly upregulated 3 days after crush injury in both WT and NLRP3−/− mice, while the level was significantly higher in the V1 of NLRP3−/− brains than in WT littermates on day 14 (Fig. 7B, C). These findings suggest that AIM2 is activated in V1 after ONC and that its significant upregulation in NLRP3−/−V1 may account for the compensatory effect on inflammatory cytokine maturation.
Discussion
The spread of neuronal degeneration, such as delayed secondary damage, occurs in distal brain regions that are anatomically connected to the proximal site of the initial infarction by axonal projections [35]. In the field of neuro-ophthalmology, prior to vision loss at the end stage, dysfunctionality of the distal regions in the visual pathway has been reported in the early or progressive stage of glaucoma and ocular trauma [36–38]. For instance, ocular hypertension undermines synaptic connections and induces progressive thinning of the visual cortex [39, 40]. So more comprehensive neurological examinations and visual functional analyses of the visual cortex have been recommended [41–44], and treatment should not be limited to rescue of the optic nerve and the alleviation of ocular hypertension but should also include protection of the distal related brain regions in the early stage. Therefore, it is urgent to seek effective therapies to meet this clinical need.
In the current study, ONC triggered upregulation of NLRP3 in contralateral V1, together with activated NLRP3 inflammasome components, indicating the immediate response of this canonic inflammasome in visual cortex spatially separated from the site of the initial lesion. Meanwhile, co-localization of NLRP3 and NeuN suggests that NLRP3 can be activated in visual cortical neurons upon ONC. Depletion of NLRP3 attenuated the apoptosis and degeneration of V1 neurons susceptible to ONC within 7 days. The index of visual electrophysiology was also improved in NLRP3 gene-knockout mice. To our knowledge, this is the first evidence of NLRP3 activation in the visual cortex and its implications in anterograde neurodegeneration.
The receptor molecules that sense exogenous threats or endogenous stress, such as pathogen-associated molecular patterns and DAMPs, recruit scaffold partners (ASCs) and lead to the assembly of inflammasomes. The inflammasome family has > 20 members [45, 46], among which the NLRP3 inflammasome is the best characterized because of its activation by diverse stimuli and its extensive participation in a variety of immunoresponsive processes, especially sterile inflammation [47]. Involvement of the NLRP3 inflammasome in the secondary insults of traumatic brain injury [48], intracerebral hemorrhage [49], and cerebral ischemia [50] has been well documented, suggesting that NLRP3 can be activated trans-neuronally due to its high sensitivity. On the other hand, cortical neurons are ‘blinded’ by the interruption of input after ON injury. Taken together, we speculated that the direct and immediate response possibly occurs in the visual cortical neurons.
Along with NLRP3 activation, neurons in the primary visual cortex were compromised in our findings. There is a possibility that some form of ‘damage initiators’ are delivered trans-synaptically via the visual pathway. Whether NLRP3 itself is a form of ‘transmitter’ or is activated by other stimuli, such as high-mobility group box 1 (HMGB1) [51], reactive oxygen species (ROS) [52], and DAMPs [53], remains unclear. In addition, the current study did not exclude the activation of NLRP3 in microglia and astrocytes, which may also contribute to anterograde degeneration. Future work is needed on the NLRP3 triggering mechanism and the combined effect of NLRP3 activation in neurons and glia in this type of trans-neuronal injury. Moreover, in primate species, ~ 40% of retinal ganglion cell axons decussate to the contralateral side at the chiasma and terminate in layers I, IV, and VI of the lateral geniculate nucleus before projecting to the visual cortex [32]. This poses a more complicated scenario in terms of anterograde degeneration that we cannot thoroughly investigate in rodent models.
Interestingly, the activated NLRP3 inflammasome was not fully responsible for the maturation of caspase-1 (p20) and IL-1β after ONC because paradoxically increased expression of ASC and p20 occurred in NLRP3−/−mice at specific time points. Therefore, we hypothesize that a compensatory mechanism of other inflammatory mediators exists. Like NLRP3, AIM2, NLRP1, NLRP6, and NLRC4 are also mediators of the inflammatory response through inflammasome formation. Previous studies have elucidated their effects in CNS disorders, especially in CNS injury [54–57]. Strikingly, AIM2, a member of the hemopoietic IFN-inducible nuclear 200 family [58], which is activated by viral, bacterial, and host ectopic dsDNA, was significantly upregulated in the contralateral V1 of the NLRP3−/− brain after ONC. Our studies are now focusing on the interaction and synergistic effect of NLRP3 and AIM2 in secondary neuronal degeneration.
In conclusion, our findings demonstrate that the activation of NLRP3 is responsible for the anterograde degeneration of visual cortical neurons after ONC. Inhibition of NLRP3, especially in the early stage, might be a potential method of neuroprotection.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Prof. Yuan-Guo Zhou from the Army Medical Center of the People’s Liberation Army and Prof. Feng Mei from the Army Medical University (AMU) for project consultation and data evaluation, and thank Prof. Bo Peng from Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, for technical support. This work was supported by the National Natural Science Foundation of China (81570840 and 81200926), the Academician-Led Science and Technological Innovation of Chongqing (cstc2017jcyj-yszxX0006), and the Research Foundation of the Department of Ophthalmology in Daping Hospital, AMU (9-2543).
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Sen Lin, Email: sam.lin@tmmu.edu.cn.
Jian Ye, Email: yejian1979@163.com.
References
- 1.Shou TD. Visual functional changes during acute elevation of intraocular pressure. Neurosci Bull. 2006;22:235–238. [PubMed] [Google Scholar]
- 2.You Y, Gupta VK, Graham SL, Klistorner A. Anterograde degeneration along the visual pathway after optic nerve injury. PLoS One. 2012;7:e52061. doi: 10.1371/journal.pone.0052061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith NM, Giacci MK, Gough A, Bailey C, McGonigle T, Black AMB, et al. Inflammation and blood-brain barrier breach remote from the primary injury following neurotrauma. J Neuroinflammation. 2018;15:201. doi: 10.1186/s12974-018-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dengler-Crish CM, Smith MA, Inman DM, Wilson GN, Young JW, Crish SD. Anterograde transport blockade precedes deficits in retrograde transport in the visual projection of the DBA/2J mouse model of glaucoma. Front Neurosci. 2014;8:290. doi: 10.3389/fnins.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Front Cell Infect Microbiol. 2012;2:142. doi: 10.3389/fcimb.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol. 2013;16:23–31. doi: 10.1016/j.mib.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 12.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu SB, Mi WL, Wang YQ. Research progress on the NLRP3 inflammasome and its role in the central nervous system. Neurosci Bull. 2013;29:779–787. doi: 10.1007/s12264-013-1328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlos D, Costa FR, Pereira CA, Rocha FA, Yaochite JN, Oliveira GG, et al. Mitochondrial DNA activates the NLRP3 inflammasome and predisposes to type 1 diabetes in murine model. Front Immunol. 2017;8:164. doi: 10.3389/fimmu.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Wan J, Lee L, Peng C, Xie H, Lee C. Study of the NLRP3 inflammasome component genes and downstream cytokines in patients with type 2 diabetes mellitus with carotid atherosclerosis. Lipids Health Dis. 2017;16:217. doi: 10.1186/s12944-017-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu A, Li H, Niu J, Wu S, Xue G, Yao X, et al. Hyperactivation of the NLRP3 inflammasome in myeloid cells leads to severe organ damage in experimental lupus. J Immunol. 2017;198:1119–1129. doi: 10.4049/jimmunol.1600659. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Chen PH, Siecinski S, Li QJ, Liu C, Steinman L, et al. An interferon-beta-resistant and NLRP3 inflammasome-independent subtype of EAE with neuronal damage. Nat Neurosci. 2016;19:1599–1609. doi: 10.1038/nn.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma MW, Wang J, Dhandapani KM, Brann DW. NADPH oxidase 2 regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid Med Cell Longev. 2017;2017:6057609. doi: 10.1155/2017/6057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SW, de Rivero Vaccari JP, Truettner JS, Dietrich WD, Keane RW. The role of microglial inflammasome activation in pyroptotic cell death following penetrating traumatic brain injury. J Neuroinflammation. 2019;16:27. doi: 10.1186/s12974-019-1423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH. The P2X7 receptor primes IL-1beta and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front Cell Neurosci. 2017;11:227. doi: 10.3389/fncel.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puyang Z, Feng L, Chen H, Liang P, Troy JB, Liu X. Retinal ganglion cell loss is delayed following optic nerve crush in NLRP3 knockout mice. Sci Rep. 2016;6:20998. doi: 10.1038/srep20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Fouda AY, Lemtalsi T, Shosha E, Rojas M, Liu F, et al. Retinal neuroprotection from optic nerve trauma by deletion of arginase 2. Front Neurosci. 2018;12:970. doi: 10.3389/fnins.2018.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou J, Crews FT. Inflammasome-IL-1beta signaling mediates ethanol inhibition of hippocampal neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P, He L, Pang X, Wang X, Yang T, Wu H. NLRP3 is expressed in the spiral ganglion neurons and associated with both syndromic and nonsyndromic sensorineural deafness. Neural Plast. 2016;2016:3018132. doi: 10.1155/2016/3018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, et al. Evidence that NF-kappaB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55:1082–1096. doi: 10.1007/s12035-017-0394-9. [DOI] [PubMed] [Google Scholar]
- 27.von Herrmann KM, Salas LA, Martinez EM, Young AL, Howard JM, Feldman MS, et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:24. doi: 10.1038/s41531-018-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Liang Y, Zhang J, Bian C, Zhou H, Guo Q, et al. Microglial TIR-domain-containing adapter-inducing interferon-beta (TRIF) deficiency promotes retinal ganglion cell survival and axon regeneration via nuclear factor-kappaB. J Neuroinflammation. 2012;9:39. doi: 10.1186/1742-2094-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou JX, Liu YJ, Chen X, Zhang X, Xu J, Yang K, et al. Low-intensity pulsed ultrasound protects retinal ganglion cell from optic nerve injury induced apoptosis via Yes associated protein. Front Cell Neurosci. 2018;12:160. doi: 10.3389/fncel.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismael S, Nasoohi S, Ishrat T. MCC950, the selective inhibitor of nucleotide oligomerization domain-like receptor protein-3 inflammasome, protects mice against traumatic brain injury. J Neurotrauma. 2018;35:1294–1303. doi: 10.1089/neu.2017.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye X, Shen T, Hu J, Zhang L, Zhang Y, Bao L, et al. Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp Neurol. 2017;292:46–55. doi: 10.1016/j.expneurol.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Leamey CA, Protti D, Dreher B. Comparative survey of the mammalian visual system with reference to the mouse. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge: MIT Press; 2008. pp. 35–60. [Google Scholar]
- 33.Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun. 1876;2013:4. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmued L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75:342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT. Glaucoma and the brain: Trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol. 2018;63:296–306. doi: 10.1016/j.survophthal.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Lam DY, Kaufman PL, Gabelt BT, To EC, Matsubara JA. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci. 2003;44:2573–2581. doi: 10.1167/iovs.02-0779. [DOI] [PubMed] [Google Scholar]
- 38.Vasalauskaite A, Morgan JE, Sengpiel F. Plasticity in adult mouse visual cortex following optic nerve injury. Cereb Cortex. 2019;29:1767–1777. doi: 10.1093/cercor/bhy347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekeyster E, Aerts J, Valiente-Soriano FJ, De Groef L, Vreysen S, Salinas-Navarro M, et al. Ocular hypertension results in retinotopic alterations in the visual cortex of adult mice. Curr Eye Res. 2015;40:1269–1283. doi: 10.3109/02713683.2014.990983. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Xie L, Dai C, Xie B, Liang M, Zhao L, et al. Progressive thinning of visual cortex in primary open-angle glaucoma of varying severity. PLoS One. 2015;10:e0121960. doi: 10.1371/journal.pone.0121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsey JD, Scadeng M, Dubowitz DJ, Crowston JG, Weinreb RN. Magnetic resonance imaging of the visual system in vivo: transsynaptic illumination of V1 and V2 visual cortex. Neuroimage. 2007;34:1619–1626. doi: 10.1016/j.neuroimage.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Chan KC, So KF, Wu EX. Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp Eye Res. 2009;88:65–70. doi: 10.1016/j.exer.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Li X. The Antidepressant Effect of Light Therapy from Retinal Projections. Neurosci Bull. 2018;34:359–368. doi: 10.1007/s12264-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan J, Fu H, Zhang J. Activation of parvalbumin-positive neurons in both retina and primary visual cortex improves the feature-selectivity of primary visual cortex neurons. Neurosci Bull. 2017;33:255–263. doi: 10.1007/s12264-016-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6:a016287. doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janowski AM, Sutterwala FS. Atypical inflammasomes. Methods Mol Biol. 2016;1417:45–62. doi: 10.1007/978-1-4939-3566-6_2. [DOI] [PubMed] [Google Scholar]
- 47.Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214:1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irrera N, Pizzino G, Calo M, Pallio G, Mannino F, Fama F, et al. Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol. 2017;8:459. doi: 10.3389/fphar.2017.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong Y, Ding ZH, Zhan FX, Cai L, Yin X, Ling JL, et al. The NLRP3 inflammasome and stroke. Int J Clin Exp Med. 2015;8:4787–4794. [PMC free article] [PubMed] [Google Scholar]
- 51.Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun. 2016;55:215–224. doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev. 2016;2016:2183026. doi: 10.1155/2016/2183026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubartelli A. DAMP-mediated activation of NLRP3-inflammasome in brain sterile inflammation: the fine line between healing and neurodegeneration. Front Immunol. 2014;5:99. doi: 10.3389/fimmu.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22:1676–1686. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang PF, Li ZG, Zhang Y, Ju XH, Liu XW, Zhou AM, et al. NLRP6 inflammasome ameliorates brain injury after intracerebral hemorrhage. Front Cell Neurosci. 2017;11:206. doi: 10.3389/fncel.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, et al. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A. 2015;112:4050–4055. doi: 10.1073/pnas.1419090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.