Abstract
Purpose
To determine age-adjusted overall success rates for patients undergoing clomiphene citrate only minimal stimulation cycle (mini) in vitro fertilization (IVF) without any gonadotropin administration.
Methods
Eight hundred thirty-nine women (mean age: 38.4 ± 0.1 years; 2488 cycles) underwent clomiphene citrate only mini-IVF. Their first oocyte retrieval was between January 2009 and December 2009, with follow-up until December 2014. The cumulative live birth rate (CLBR) per oocyte retrieval cycle started and live birth rate per oocyte was retrospectively analyzed. The basic CLBR was calculated as the number of women who achieved a live birth divided by the total number of women who started oocyte retrieval.
Results
The mean number of oocytes retrieved was 1.5. The basic CLBRs for all ages after the first and third cycles were 22.6% and 39.2%, respectively. For ≤ 34 years, 35–37 years, 38–40 years, 41–42 years, and ≥ 43 years, CLBRs after the first and third cycles were 42.5% and 70.1%, 32.9% and 49.1%, 20.0% and 38.6%, 12.6% and 25.2%, and 4.4% and 8.8%, respectively. These rates had a significant relationship with age (P < 0.01). The LBR per oocyte for all ages was 9.6%.
Conclusion
Acceptable overall IVF success rates can be achieved in clomiphene citrate only mini-IVF, as well as acceptable LBR. The CLBRs and LBRs per oocyte are evidently influenced by women’s age.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01662-z) contains supplementary material, which is available to authorized users.
Keywords: Minimal stimulation cycle IVF, Cumulative live birth rates, Woman’s age, The live birth rate per oocyte
Introduction
Minimal ovarian stimulation and natural-cycle in vitro fertilization (IVF) were designed to reduce patients’ physical and mental burdens, as well as reduce treatment cost [1], and improve oocyte quality [2–9]. There are, however, a wide range of ovarian stimulation protocols, advocated as minimal stimulation generally aimed at retrieving 3–5 oocytes per cycle [10]. Minimal stimulation often is defined as a fixed low dose (up to 150 IU daily) of follicle-stimulating hormone (FSH) or human menopausal gonadotropin (hMG), concurrently administrated with oral compounds, such as an estrogen agonist (e.g., clomiphene citrate) or aromatase inhibitors (e.g., letrozole), according to the proposal suggested by the International Society for Mild Approaches in Assisted Reproduction [10]. Even more “minimal” is non gonadotropin IVF, using clomiphene citrate tablets (50 mg/day) from days 3 of menstruation until before oocyte maturation trigger but without gonadotropin administration, and a gonadotropin-releasing hormone (GnRH) agonist in the form of nasal spray for triggering maturation [11, 12]. However, the success rates of these regimens are controversial, because the number of oocytes retrieved per cycle is less than with other minimal stimulation protocols which may affect overall success rate. Silber et al. [7] demonstrated that the intrinsic fertility of the human oocyte is higher in natural cycle IVF than what has been reported in IVF with COH [13–15]. In fact, however, natural cycle IVF is complicated and problematic to administer. The question that this paper wishes to address is whether clomiphene citrate only minimal stimulation cycle-IVF can give similarly acceptable live birth rate per oocyte, but be more reliable than “natural” cycle. Furthermore of course, the overall success rates per patient in clomiphene citrate only minimal stimulation cycle IVF should be addressed.
Cumulative live birth rate (CLBR) is generally defined as the delivery of at least one live born infant in relation to the scheduled oocyte retrieval cycle. CLBR is the ultimate measure of success rate in the mind of most patients because it summarizes the chance of a live birth over the IVF treatment period [16–19]. Several studies have evaluated the live birth rate in minimal stimulation and natural cycle [4, 5, 20–23]. Among them, two randomized controlled trials [4, 24] demonstrated that CLBR with mini IVF was comparable to controlled ovarian hyperstimulation (COH) cycles. However, these studies were mostly based on gonadotropin-administrated minimal stimulation IVF or with the natural cycle. Therefore, it is still unclear how simple clomiphene citrate only stimulation for IVF would compare to gonadotropin cycles or to natural cycle IVF.
In the present study, we investigated the CLBRs of patients who underwent clomiphene citrate only minimal stimulation cycle IVF followed by single embryo transfer exclusively and evaluated the probability of live births with this protocol according to female age, even over 43 years. CLBR was calculated by dividing the number of women who achieved a live birth by the total number of women who started oocyte retrieval cycles. Started oocyte retrieval cycles included canceled cycles and cycles obtaining no oocytes after oocyte retrievals. A secondary goal was to see whether the high live birth rate per oocyte demonstrated in natural cycles could be duplicated in clomiphene citrate only minimal stimulation cycles.
Materials and methods
Study patients
During this retrospective study period between January 2009 and December 2009, 3281 patients were scheduled their first oocyte retrieval cycle in our center. The main strategy for ovarian stimulation in our center is minimal stimulation and natural cycle IVF. COH cycle IVF is rarely performed in our center. Patients consulted with gynecologists to determine their treatment plan based on patients’ preference or their menstrual cycle: minimal stimulation cycle or natural cycle IVF. The aim of this study was to evaluate the cumulative live birth rate when patients exclusively undergo minimal stimulation cycle IVF using clomiphene citrate without any gonadotropin administration. Therefore, patients who had been treated with controlled ovarian stimulation cycle (n = 132), natural cycle (n = 1792), or clomiphene citrate-based minimal stimulation cycle with gonadotropin administration (n = 518) for at least one cycle of their IVF treatments during the study period were excluded. A total of 839 women (mean age: 38.4 ± 0.1 years old) whose entire ovarian stimulation regimen were solely minimal stimulation cycle IVF using clomiphene citrate were included in the present study. We examined the pregnancy outcomes following an elective single fresh or frozen embryo transfer and followed up with the women for 5 years, until discontinuation of the treatment, or until the first live birth, whichever occurred first. The surplus embryos were used for transfer before moving forward to the subsequent oocyte retrieval cycle. For the evaluation of live birth rate per oocyte, to exclude bias of surplus embryos, 2186 cycles in 713 women, including women who retrieved only one oocyte and women with more than one oocytes at retrieval but they completed the transfers of all embryos resulting from that retrievals, were extracted from the study group afore-mentioned and retrospectively analyzed. Informed consent was obtained from all individual participants included in the study. The institutional review board of our center approved the present study (approval number: 13–08).
Clomiphene citrate only minimal stimulation cycle protocol
Clomiphene citrate (CC)-based minimal stimulation cycle IVF was performed as described previously [11, 25]. Briefly, CC (50–100 mg/day, Fuji Pharma Co., Ltd.) was orally administered with an extended regimen from day 3 of the menstrual cycle until the day before inducing final oocyte maturation (day 11 of the menstrual cycle). Neither FSH nor hMG was administered. Ovulation triggering was performed with a gonadotropin-releasing hormone (GnRH) agonist, buserelin (600 μg, Mochida Pharmaceutical Co., Ltd., Fuji Pharma Co., Ltd.) administered in the form of a nasal spray. The criteria for triggering is by confirming the dominant follicle diameter of ≥ 18 mm and an estradiol (E2) level of ≥ 250 pg/mL Oocyte retrieval was performed 34–35 h after triggering. No anesthesia was administrated for oocyte retrieval. The absence of follicles on ultrasound monitoring before starting oocyte retrieval was considered premature ovulation.
Conventional insemination, intracytoplasmic sperm injection, and embryo culture
Conventional insemination or intracytoplasmic sperm injection (ICSI) was performed as described previously [25]. Normally fertilized zygotes with two pronuclei were cultured individually in Quinn Advantage Protein Plus cleavage medium (CooperSurgical, Inc. USA) from days 1 to 3. Then, the embryos were transferred to a Quinn Advantage Protein Plus blastocyst medium (CooperSurgical, Inc. USA) from days 4 to 6. All embryos were cultured at 37.0 °C under a gas phase of 5% O2, 5% CO2, and 90% N2 in a water jacket or non-humidified incubators (Astec Co. Ltd., Japan).
Cryopreservation and embryo transfer
During the study period, single embryo transfers were performed exclusively; among 1539 of embryo transfer cycles, 50.1% (n = 771) were fresh cleavage stage embryos, 0.5% (n = 8) were fresh blastocyst transfer cycles, 5.1% (n = 79) were vitrified-warmed cleavage stage embryos, and 44.3% (n = 681) were vitrified-warmed blastocyst transfer (SVBT) cycles. In other words, usually fresh transfers were cleavage stage, and frozen transfers were blastocyst. In our center, a single cleavage stage embryo transfer is the primary embryo transfer regimen. If tubal factor infertility (tubal obstruction, hydrosalpinx, or a history of ectopic pregnancy) was observed in women, SVBT was indicated. In addition, SVBT was indicated following previous failed cycles with single cleavage stage embryo transfer and when surplus embryos were available following cleavage-stage embryo transfer. The Cryotop® vitrification method (Kitazato Biopharma, Japan) was used as described previously [25] for embryo vitrification. Fresh or vitrified-warmed embryo transfers were performed in spontaneous natural (92.4%, n = 702) or hormonal replacement (7.6%, n = 58) cycles [11, 25, 26]. In hormonal replacement cycles, transdermal estradiol patches (1.44 mg/day) were started from day 2 and dydrogesterone (30 mg/day) was added from day 11 of the cycle; cleavage-stage embryos or blastocysts were transferred 1 or 7 days later, respectively. Dydrogesterone (30 mg/day) was routinely administered orally during the early luteal phase after both fresh and vitrified-warmed embryo transfer procedures. Intramuscular or intravaginal progesterone was also administered until the 9th week of pregnancy in cases where the luteal function was deemed as insufficient.
Data analysis
All analyses were conducted using JMP (SAS, NC, USA). The successful oocyte retrieval rate, number of retrieved oocytes and matured oocytes, clinical pregnancy rate (confirmed gestational sac at 6–7 weeks of pregnancy), live birth rate (over 22 weeks of pregnancy), and miscarriage rate stratified by women’s age groups according to the Society for Assisted Reproductive Technology (SART) classification (age: ≤ 34, 35–37, 38–40, 41–42, and ≥ 43 years) were calculated for the cohort.
The effect of the women’s age on the CLBR was assessed using a Cox proportional hazard model with a 95% confidence interval (CI). They were classified into five subgroups (age: ≤ 34, 35–37, 38–40, 41–42, and ≥ 43 years), and the pregnancy outcomes were compared among the subgroups by using the Chi-square test, Kruskal-Wallis test, or Cochran-Armitage test for trend as appropriate. P < 0.05 was considered statistically significant. Statistical P values were further adjusted using the Bonferroni method for multiple comparisons.
Subsequently, the five subgroups of CLBRs were calculated. The CLBR was calculated as the outcome by dividing the cumulative number of women achieving a live birth (according to the number of treatment cycles started) by the total number of women who started oocyte retrieval. A Cox proportional hazard model with 95% CI was used to analyze the correlation between age subgroups and CLBRs. Finally of interest was to compare the live birth rate per oocyte in clomiphene citrate only cycles. We calculated the live birth rate per retrieved oocyte and compared between age subgroups.
Results
Patient characteristics and success rates
During the study period, 834 patients had 2488 oocyte retrieval cycles via clomiphene citrate only minimal stimulation cycle IVF. The patients’ characteristics are shown in Supplementary Table 1. Patients with the polycystic ovarian syndrome and hypothalamus and hypothalamus pituitary gland-related amenorrhea were excluded. In addition, women in which donor oocyte and donor sperm were used were not included in the study cohort; therefore, there was no need to exclude such patients. Women with surgically retrieved sperm were not excluded. The overall successful oocyte retrieval (OR) rate was 86.2% (2145/2488); the average number of mature oocytes retrieved was 1.5 ± 0.0 which were used for conventional insemination or ICSI. Overall, 90.4% of women (754/834) underwent embryo transfer (1539 cycles). The clinical pregnancy rate per single embryo transfer cycle for all ages averaged 35.3% (544/1539). The live birth rate for all ages averaged 27.1% (417/1539). The number of oocytes retrieved, fertilized, and cleaved, as well as clinical pregnancy and live birth rates decreased (and miscarriage rates increased) as the women’s age increased (P < 0.01). When clinical pregnancy and live birth rates were stratified by types of embryo transfer, clinical pregnancy rates were 24.0% (185/771), 50.0% (4/8), 30.4% (24/79), and 48.6% (331/681) and live birth rates were 18.7% (144/771), 50.0% (4/8), 24.1%(19/79), and 36.7% (250/681) in fresh cleavage-stage embryo, fresh blastocyst-stage embryo, frozen cleavage-stage embryo, and frozen blastocyst-stage embryo transfers, respectively (Tables 1 and 2). Clinical pregnancy and live birth rates stratified by types of transfer also decreased as women’s age increased (Supplementary Table 2).
Table 1.
Patient cycle-based success rates stratified by women’s age subgroups
| Total | Group A (age: ≤ 34 years) | Group B (age: 35–37 years) | Group C (age: 38–40 years) | Group D (age: 41–42 years) | Group E (age: ≥ 43 years) | P value | |
|---|---|---|---|---|---|---|---|
| No. of patients who started the OPU cycle | 839 | 134 | 161 | 280 | 151 | 113 | NA |
| No. of patients who received oocyte retrieval | 832 | 133 | 159 | 278 | 149 | 113 | NA |
| No. of oocyte retrieval cycles started | 2541 | 258 | 372 | 864 | 599 | 448 | |
| No. of oocyte retrieval cycles achieved | 2488 | 256 | 366 | 840 | 583 | 443 | |
| (%, oocyte retrieval cycles started) | (97.9) | (99.2) | (98.4) | (97.2) | (97.3) | (98.9) | |
| No. of successful oocyte retrieval cycles, n | 2145 | 235 | 320 | 747 | 487 | 356 | < 0.01 |
| (%, per oocyte retrieval cycles) | (86.2) | (91.8)ab | (87.4)ab | (88.9)a | (83.5)bc | (80.4)c | |
| No. of retrieved oocytes, mean ± SEM | 1.6 ± 0.0 | 1.9 ± 0.1a | 1.7 ± 0.1a | 1.7 ± 0.0a | 1.5 ± 0.0b | 1.4 ± 0.0b | < 0.01 |
| No. of inseminated oocytes, mean ± SEM | 1.5 ± 0.0 | 1.8 ± 0.1a | 1.6 ± 0.1a | 1.6 ± 0.0a | 1.4 ± 0.0b | 1.3 ± 0.0b | < 0.01 |
| No. of fertilized oocytes, mean ± SEM | 1.3 ± 0.0 | 1.5 ± 0.1a | 1.4 ± 0.1ab | 1.3 ± 0.0bc | 1.2 ± 0.1cd | 1.1 ± 0.0d | < 0.01 |
| No. of cleaved embryos, mean ± SEM | 1.2 ± 0.0 | 1.4 ± 0.1a | 1.2 ± 0.1ab | 1.2 ± 0.0bc | 1.0 ± 0.0cd | 1.0 ± 0.0d | < 0.01 |
Different letters represent significant differences between the groups (P < 0.01)
NA not applicable, OPU oocyte pick-up, SEM standard error of the mean, no. number
Table 2.
Patient cycle-based success rates stratified by women’s age subgroups
| Total | Group A (age: ≤ 34 years) | Group B (age: 35–37 years) | Group C (age: 38–40 years) | Group D (age: 41–42 years) | Group E (age: ≥ 43 years) | P value | |
|---|---|---|---|---|---|---|---|
| No. of patients received embryo transfer | 754 | 123 | 143 | 258 | 134 | 96 | NA |
| No. of embryo transfer cycles | 1539 | 225 | 268 | 561 | 304 | 181 | |
| Types of transferred embryos, n (%) | |||||||
| Fresh-cleavage stage embryo | 771 (50.1) | 104 (46.2)ab | 113 (42.2)a | 292 (52.1)b | 146 (48.0)ab | 116 (64.1)c | < 0.01 |
| Fresh-blastocyst stage embryo | 8 (0.5) | 3 (1.3) | 3 (1.1) | 2 (0.4) | 0 (0) | 0 (0) | 0.1088 |
| Frozen-cleavage stage embryo | 79 (5.1) | 8 (3.6) | 13 (4.9) | 25 (4.5) | 19 (6.3) | 14 (7.7) | 0.2857 |
| Frozen-blastocyst stage embryo | 681 (44.3) | 110 (48.9)ab | 139 (51.9)a | 242 (43.1)b | 139 (45.7)a | 51 (28.2)c | < 0.01 |
| Endometrial preparation in frozen embryo transfer cycles, n (%) | |||||||
| Spontaneous ovulation cycle | 702 (92.4) | 105 (86.8)a | 154 (96.9)b | 255 (94.8)bc | 144 (89.4)a | 60 (89.6)ac | 0.0048 |
| Hormone replacement cycle | 58 (7.6) | 16 (13.2) | 5 (3.1) | 14 (5.2) | 17 (10.6) | 7 (10.5) | 0.0048 |
| Clinical pregnancy, n (%) | 544 (35.4) | 122 (54.2)a | 120 (44.8)b | 204 (36.4)c | 73 (24.0)d | 25 (13.8)e | < 0.01 |
| Live birth, n (%) | 417 (27.1) | 106 (47.1)a | 95 (35.5)b | 156 (27.8)c | 47 (15.5)d | 13 (7.2)e | < 0.01 |
| Miscarriage, n (%) | 127 (23.4) | 16 (13.1)a | 25 (20.8)ab | 48 (23.5)b | 26 (35.6)c | 12 (48.0)c | < 0.01 |
Different letters represent significant differences between groups (P < 0.01)
NA not applicable, no. number
Effect of women’s age on the cumulative live birth rate and live birth rate per oocyte
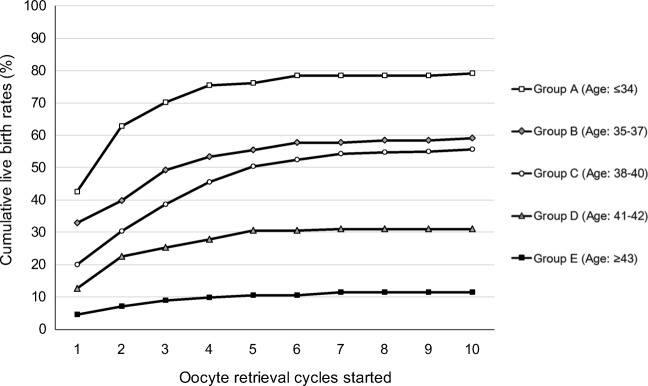
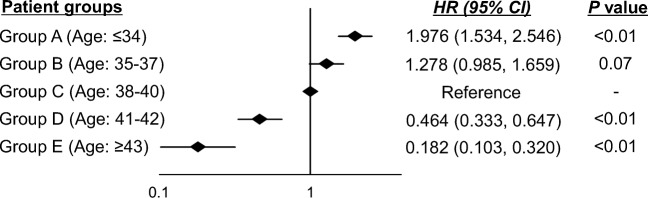
Figure 1 shows basic CLBRs according to the women’s age subgroups. The detailed data, including the number of deliveries and dropout rates, are shown in Supplementary Table 3. The CLBRs on average for all age groups after the first, third, and fifth started oocyte retrieval cycles were 22.6%, 39.2%, and 46.5%, respectively. The CLBRs plateaued after approximately 6 cycles (data not shown in Fig. 1). After the first, third, and fifth oocyte retrieval cycles started, the CLBRs for women aged ≤ 34 years (group A), 35–37 years (group B), 38–40 years (group C), 41–42 years (group D), and ≥ 43 years (group E) were 42.5%, 70.1%, and 76.1%; 32.9%, 49.1%, and 55.3%; 20.0%, 38.6%, and 50.4%; 12.6%, 25.2%, and 30.5%; and 4.4%, 8.8%, and 10.6%, respectively. When group C (age 38–40 years) was set as a referent category, the adjusted hazard ratios of basic CLBRs were 1.976 (95% CI: 1.534–2.546, p < 0.01) in group A, 1.278 (95% CI: 0.985–1.659, p = 0.07) in group B, 0.464 (95% CI: 0.333–0.647, p < 0.01) in group D, and 0.182 (95% CI: 0.103–0.320, p < 0.01). The adjusted hazard ratio significantly decreased with increasing age groups, although there were no significant differences between groups B and C (P < 0.01; Fig. 2).
Fig. 1.
The basic cumulative live birth rates (CLBRs) according to the women’s age subgroups
Fig. 2.
The adjusted hazard ratio between women’s age subgroups and basic cumulative live birth rates. HR: hazard ratio, CI: confidence interval, adjusted factor: women’s body mass index (BMI) and cause of infertility
The live birth rates based on retrieved oocytes are shown in Table 3. The overall live birth rate per oocyte for all ages was 9.6%. For women in group A, there was a 24.0% live birth rate per oocyte; for women in groups B, C, D, and E, live birth rates per oocyte were 15.3%, 10.1%, 4.9%, and 2.0%, respectively. There was a significant trend between live birth rates and woman’s age (P < 0.0001).
Table 3.
Live birth rate per matured oocytes in clomiphene citrate only minimal stimulation cycle IVF
| Total | Group A (age: ≤ 34 years) | Group B (age: 35–37 years) | Group C (age: 38–40 years) | Group D (age: 41–42 years) | Group E (age: ≥ 43 years) | P value | |
|---|---|---|---|---|---|---|---|
| Number of patients | 713 | 99 | 134 | 238 | 135 | 107 | – |
| Number of oocytes retrieved | 3264 | 304 | 476 | 1167 | 758 | 559 | – |
| Number of live births | 312 | 73 | 73 | 118 | 37 | 11 | – |
| Live birth rate per oocyte (%) | 9.6 | 24.0a | 15.3b | 10.1c | 4.9d | 2.0e | P < 0.01 |
Different letters represent significant differences between groups (P < 0.01)
Discussion
The effect of patient age on the success rate of fertility treatment has been well documented. Ovarian reserve decreases with advanced age, and consequently, the number of oocytes obtained per OPU cycle decreases [27–29]. Additionally, older age leads to an increased incidence of aneuploidy and poor embryo quality [30–35]. In the present study, the live birth rates per embryo transfer cycle were stratified into five age subgroups classified by SART and correlations between the subgroups were examined. The successful OPU rate and the number of matured, fertilized, and cleaved oocytes decreased with advancing age. Moreover, clinical pregnancy and live birth rates significantly decreased with advancing age. The Cox proportion hazard model confirmed that cumulative live birth rates (CLBRs) decreased as women’s ages increased which verify the previous findings reported by others [13, 14, 19, 20, 28].
In clomiphene citrate only mini-IVF, the number of oocytes retrieved per oocyte retrieval cycle is lower than the number likely to be retrieved in standard IVF with controlled gonadotropin ovarian hyperstimulation. A higher number of oocyte pick-up (OPU) cycles with clomiphene citrate only mini-IVF only, therefore, might possibly be required in order to reach the same CLBR as controlled ovarian hyperstimulation. However as can be seen in Fig. 1, the live birth rate for the first oocyte retrieval cycle started in women aged ≤ 34 was 42.5%, certainly comparable to single embryo transfer in COH cycles. In fact, at every age group, the live birth rate per cycle was comparable to that which is achieved with COH [19] [36].
Malizia et al. reported the CLBRs among patients (mean age: 35.8 ± 4.7 years) who underwent controlled ovarian hyperstimulation cycles in the USA, yielding similar basic CLBRs to the present study. Additionally, a similar result was observed in a large cohort study from the United Kingdom. Although the different characteristics in the cohort used for these comparisons would confuse the conclusions, these results encourage that even in clomiphene citrate only minimal stimulation IVF, one could achieve satisfactory CLBRs in comparison to standard IVF with controlled gonadotropin ovarian hyperstimulation (COH).
We previously have demonstrated that with natural cycle IVF, the live birth rate per oocyte is higher than with standard controlled ovarian stimulation cycle IVF [7]. Therefore, it needed to be seen whether a higher live birth rate per oocyte makes up for the lower number of oocytes in clomiphene citrate only or natural cycles. The present study indicated that live birth rates per oocyte in women aged ≤ 34 years, 35–37 years, 38–40 years, 41–42 years, and ≥ 43 years were 24.0%, 15.3%, 10.1%, 4.9%, and 2.0%, respectively, which was similar to what we have previously reported in natural cycle [7]. These results suggested that even in clomiphene citrate only mini-IVF, it is possible to obtain a similar benefit of higher live birth per oocyte as in natural cycle IVF. As a matter of choice clomiphene citrate, only mini-IVF seems superior to natural cycle because of higher successful oocyte retrieval rates, easier control, and obviously more oocytes.
The strength of the present study was the use of a large cohort of women who underwent clomiphene citrate only mini-IVF followed exclusively by a single embryo transfer. This enabled us to determine a realistic cumulative live birth rate, and also to evaluate the live birth rate per retrieved oocyte. The large sample size allowed us to maximize the generalizability of prognostic factors. However, this study also has limitations. For example, in each cycle rank, there was heterogeneity due to different insemination methods, the status of embryos for transfer, and embryo transfer protocols. In addition, in order to evaluate the live birth rate per oocyte, we excluded the data of patients with more than one oocytes at retrieval and had surplus embryos that has not completed the transfer in the present study. Since our database could not trace each individual oocyte, we could not accurately calculate the rate in cases in which more than two oocytes were retrieved. Therefore, this was the only calculation of the live birth rate per oocyte we could arrive at.
In conclusion, the present study indicates that cumulative live birth rates (CLBRs) are acceptable in clomiphene citrate only minimal stimulation cycle IVF, even though the number of retrieved oocytes is minimal because of a higher live birth rate per oocyte. Clearly, CLBRs is significantly influenced by the woman’s age. The benefits in terms of cost and patient acceptability of clomiphene citrate only minimal stimulation cycle IVF are considerable.
Electronic supplementary material
(DOCX 43 kb)
Authors’ contributions
T.A., A.Y., K.K., and S.S were responsible for the supervision, study design, data collection and interpretation, and manuscript writing. K.E. was responsible for study design and data collection. K.E. and H.S. performed statistical data analysis. J.F., S.U., Y.F., S.G., and T.K. were responsible for the study design and literature review.
Funding information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takashi Abe, Email: t-abe@shinjukuart.com.
Akiko Yabuuchi, Email: a-yabuuchi@towako-kato.com.
Kenji Ezoe, Email: k-ezoe@towako-kato.com.
Helen Skaletsky, Email: skaletsky@wi.mit.edu.
Junichiro Fukuda, Email: j-fukuda@towako-kato.com.
Satoshi Ueno, Email: s-ueno@towako-kato.com.
Yuting Fan, Email: sysufanyut@gmail.com.
Sierra Goldsmith, Email: sierra@infertile.com.
Tamotsu Kobayashi, Email: t-kobayashi@towako-kato.com.
Sherman Silber, Email: silber@infertile.com.
Keiichi Kato, Email: k-kato@towako.net.
References
- 1.Sophonsritsuk A, Choktanasiri W, Weerakiet S, Rojanasakul A. Comparison of outcomes and direct cost between minimal stimulation and conventional protocols on ovarian stimulation in in vitro fertilization. J Obstet Gynaecol Res. 2005;31(5):459–463. doi: 10.1111/j.1447-0756.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 2.Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22(4):980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 3.Fauser BC, Devroey P, Yen SS, Gosden R, Crowley WF, Jr, Baird DT, et al. Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks. Hum Reprod. 1999;14(11):2681–2686. doi: 10.1093/humrep/14.11.2681. [DOI] [PubMed] [Google Scholar]
- 4.Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet. 2007;369(9563):743–749. doi: 10.1016/S0140-6736(07)60360-2. [DOI] [PubMed] [Google Scholar]
- 5.Pelinck MJ, Vogel NE, Arts EG, Simons AH, Heineman MJ, Hoek A. Cumulative pregnancy rates after a maximum of nine cycles of modified natural cycle IVF and analysis of patient drop-out: a cohort study. Hum Reprod. 2007;22(9):2463–2470. doi: 10.1093/humrep/dem164. [DOI] [PubMed] [Google Scholar]
- 6.Polinder S, Heijnen EM, Macklon NS, Habbema JD, Fauser BJ, Eijkemans MJ. Cost-effectiveness of a mild compared with a standard strategy for IVF: a randomized comparison using cumulative term live birth as the primary endpoint. Hum Reprod. 2008;23(2):316–323. doi: 10.1093/humrep/dem372. [DOI] [PubMed] [Google Scholar]
- 7.Silber SJ, Kato K, Aoyama N, Yabuuchi A, Skaletsky H, Fan Y, Shinohara K, Yatabe N, Kobayashi T. Intrinsic fertility of human oocytes. Fertil Steril. 2017;107(5):1232–1237. doi: 10.1016/j.fertnstert.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Verberg MF, Eijkemans MJ, Macklon NS, Heijnen EM, Baart EB, Hohmann FP, Fauser BC, Broekmans FJ. The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis. Hum Reprod Update. 2009;15(1):5–12. doi: 10.1093/humupd/dmn053. [DOI] [PubMed] [Google Scholar]
- 9.Verberg MF, Macklon NS, Nargund G, Frydman R, Devroey P, Broekmans FJ, Fauser BC. Mild ovarian stimulation for IVF. Hum Reprod Update. 2009;15(1):13–29. doi: 10.1093/humupd/dmn056. [DOI] [PubMed] [Google Scholar]
- 10.Nargund G, Fauser BC, Macklon NS, Ombelet W, Nygren K, Frydman R. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum Reprod. 2007;22(11):2801–2804. doi: 10.1093/humrep/dem285. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, et al. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-Centre, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35. doi: 10.1186/1477-7827-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teramoto S, Kato O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod BioMed Online. 2007;15(2):134–148. doi: 10.1016/S1472-6483(10)60701-8. [DOI] [PubMed] [Google Scholar]
- 13.Patrizio P, Sakkas D. From oocyte to baby: a clinical evaluation of the biological efficiency of in vitro fertilization. Fertil Steril. 2009;91(4):1061–1066. doi: 10.1016/j.fertnstert.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Cobo A, Garrido N, Pellicer A, Remohi J. Six years' experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril. 2015;104(6):1426–1434. doi: 10.1016/j.fertnstert.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 15.McLernon DJ, Maheshwari A, Lee AJ, Bhattacharya S. Cumulative live birth rates after one or more complete cycles of IVF: a population-based study of linked cycle data from 178,898 women. Hum Reprod. 2016;31(3):572–581. doi: 10.1093/humrep/dev336. [DOI] [PubMed] [Google Scholar]
- 16.De Brucker M, Haentjens P, Evenepoel J, Devroey P, Collins J, Tournaye H. Cumulative delivery rates in different age groups after artificial insemination with donor sperm. Hum Reprod. 2009;24(8):1891–1899. doi: 10.1093/humrep/dep085. [DOI] [PubMed] [Google Scholar]
- 17.Garrido N, Bellver J, Remohi J, Simon C, Pellicer A. Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil Steril. 2011;96(1):40–46. doi: 10.1016/j.fertnstert.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, Ng EH. Role of baseline antral follicle count and anti-Mullerian hormone in prediction of cumulative live birth in the first in vitro fertilisation cycle: a retrospective cohort analysis. PLoS One. 2013;8(4):e61095. doi: 10.1371/journal.pone.0061095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 20.Bodri D, Kawachiya S, De Brucker M, Tournaye H, Kondo M, Kato R, et al. Cumulative success rates following mild IVF in unselected infertile patients: a 3-year, single-Centre cohort study. Reprod BioMed Online. 2014;28(5):572–581. doi: 10.1016/j.rbmo.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Ferraretti AP, Gianaroli L, Magli MC, Devroey P. Mild ovarian stimulation with clomiphene citrate launch is a realistic option for in vitro fertilization. Fertil Steril. 2015;104(2):333–338. doi: 10.1016/j.fertnstert.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Nargund G, Waterstone J, Bland J, Philips Z, Parsons J, Campbell S. Cumulative conception and live birth rates in natural (unstimulated) IVF cycles. Hum Reprod. 2001;16(2):259–262. doi: 10.1093/humrep/16.2.259. [DOI] [PubMed] [Google Scholar]
- 23.Pelinck MJ, Knol HM, Vogel NE, Arts EG, Simons AH, Heineman MJ, et al. Cumulative pregnancy rates after sequential treatment with modified natural cycle IVF followed by IVF with controlled ovarian stimulation. Hum Reprod. 2008;23(8):1808–1814. doi: 10.1093/humrep/den155. [DOI] [PubMed] [Google Scholar]
- 24.Casano S, Guidetti D, Patriarca A, Pittatore G, Gennarelli G, Revelli A. MILD ovarian stimulation with GnRH-antagonist vs. long protocol with low dose FSH for non-PCO high responders undergoing IVF: a prospective, randomized study including thawing cycles. J Assist Reprod Genet. 2012;29(12):1343–1351. doi: 10.1007/s10815-012-9863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato K, Ueno S, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, Segawa T, Teramoto S. Women's age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod BioMed Online. 2014;29(4):411–416. doi: 10.1016/j.rbmo.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda J, Abe T, Okuno T, Kobayashi T, Kato K. Administering human chorionic gonadotropin injections for triggering follicle maturation could impact fertility during the subsequent menstrual cycle. Int J Gynaecol Obstet. 2016;132(3):309–313. doi: 10.1016/j.ijgo.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Knowlton NS, Craig LB, Zavy MT, Hansen KR. Validation of the power model of ovarian nongrowing follicle depletion associated with aging in women. Fertil Steril. 2014;101(3):851–856. doi: 10.1016/j.fertnstert.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET) Sci China Life Sci. 2012;55(8):694–698. doi: 10.1007/s11427-012-4357-0. [DOI] [PubMed] [Google Scholar]
- 29.Yarali H, Bozdag G, Polat M, Esinler I, Tiras B. Intracytoplasmic sperm injection outcome of women over 39: an analysis of 668 cycles. Arch Gynecol Obstet. 2010;281(2):349–354. doi: 10.1007/s00404-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 30.Feichtinger M, Stopp T, Gobl C, Feichtinger E, Vaccari E, Madel U, et al. Correction: increasing live birth rate by Preimplantation genetic screening of pooled polar bodies using Array comparative genomic hybridization. PLoS One. 2015;10(7):e0133334. doi: 10.1371/journal.pone.0133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragouli E, Alfarawati S, Goodall NN, Sanchez-Garcia JF, Colls P, Wells D. The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod. 2011;17(5):286–295. doi: 10.1093/molehr/gar024. [DOI] [PubMed] [Google Scholar]
- 32.Gabriel AS, Thornhill AR, Ottolini CS, Gordon A, Brown AP, Taylor J, Bennett K, Handyside A, Griffin DK. Array comparative genomic hybridisation on first polar bodies suggests that non-disjunction is not the predominant mechanism leading to aneuploidy in humans. J Med Genet. 2011;48(7):433–437. doi: 10.1136/jmg.2010.088070. [DOI] [PubMed] [Google Scholar]
- 33.Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, Harper J, Schmutzler A, Collins J, Goossens V, van der Ven H, Vesela K, Gianaroli L. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011;26(11):3173–3180. doi: 10.1093/humrep/der294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handyside AH, Montag M, Magli MC, Repping S, Harper J, Schmutzler A, Vesela K, Gianaroli L, Geraedts J. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet. 2012;20(7):742–747. doi: 10.1038/ejhg.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak JJ. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod BioMed Online. 2011;22(1):2–8. doi: 10.1016/j.rbmo.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Smith A, Tilling K, Nelson SM, Lawlor DA. Live-birth rate associated with repeat in vitro fertilization treatment cycles. Jama. 2015;314(24):2654–2662. doi: 10.1001/jama.2015.17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 43 kb)