Abstract
BACKGROUND
Three patients enrolled in a clinical trial of 5-aminolevulinic-acid (5-ALA)-induced fluorescence-guidance, which has been demonstrated to facilitate intracranial tumor resection, were found on neuropathological examination to have focal cortical dysplasia (FCD).
OBJECTIVE
To evaluate in this case series visible fluorescence and quantitative levels of protoporphyrin IX (PpIX) during surgery and correlate these findings with preoperative magnetic resonance imaging (MRI) and histopathology.
METHODS
Patients were administered 5-ALA (20 mg/kg) approximately 3 h prior to surgery and underwent image-guided, microsurgical resection of their MRI- and electrophysiologically identified lesions. Intraoperative visible fluorescence was evaluated using an operating microscope adapted with a commercially available blue light module. Quantitative PpIX levels were assessed using a handheld fiber-optic probe and a wide-field imaging spectrometer. Sites of fluorescence measurements were co-registered with both preoperative MRI and histopathological analysis.
RESULTS
Three patients with a pathologically confirmed diagnosis of FCD (Types 1b, 2a, and 2b) underwent surgery. All patients demonstrated some degree of visible fluorescence (faint or moderate), and all patients had quantitatively elevated concentrations of PpIX. No evidence of neoplasia was identified on histopathology, and in 1 patient, the highest concentrations of PpIX were found at a tissue site with marked gliosis but no typical histological features of FCD.
CONCLUSION
FCD has been found to be associated with intraoperative 5-ALA-induced visible fluorescence and quantitatively confirmed elevated concentrations of the fluorophore PpIX in 3 patients. This finding suggests that there may be a role for fluorescence-guidance during surgical intervention for epilepsy-associated FCD.
Keywords: 5-Aminolevulinic acid, Epilepsy, Fluorescence guided surgery, Protoporphyrin IX, Focal cortical dysplasia, Optical spectroscopy
ABBREVIATIONS
- 5-ALA
5-aminolevulinic acid
- BBB
blood–brain-barrier
- ECoG
electrocorticography
- EEG
electroencephalography
- FCD
focal cortical dysplasia
- FDG
18-fluoro-2-deoxyglucose
- FLAIR
fluid attenuation inversion recovery
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PpIX
protoporphyrin IX
Fluorescence-guidance in the resection of malignant glioma and other intracranial tumors has been increasingly utilized over the past decade. The majority of published studies report experiences using 5-aminolevulinic acid (5-ALA), which when orally administered to the patient preoperatively leads to accumulation of the fluorophore protoporphyrin IX (PpIX) preferentially in tumor tissue. Excitation of the fluorophore by blue light illumination of the surgical field results in visible pink/red fluorescence, which in turn can assist in surgical resection. For malignant glioma, Class I evidence demonstrates improved extent of tumor resection and increased progression-free survival using fluorescence-guidance,1 and other tumor types, including meningioma,2-6 metastatic tumor,7-10 and low-grade glioma,11-14 have exhibited potentially useful fluorescence.
As part of a prospective clinical trial investigating 5-ALA-iduced fluorescence in intracranial tumor surgery, a 58-yr-old man was operated upon for the resection of a presumed low-grade tumor in the region of the amygdala. The lesion revealed moderate levels of visible red fluorescence during surgery, but the final histopathological diagnosis was focal cortical dysplasia (FCD). Subsequently, 2 additional patients with FCD were operated upon under the same protocol. This report presents the findings from these 3 patients, which suggest possible utility of intraoperative fluorescence-guidance in this nonneoplastic condition.
METHODS
Patients enrolled in the Institutional Review Board (IRB)-approved, Investigational Device/Drug Exception (IND)-supported, prospective, Informed Consent study satisfy inclusion criteria of magnetic resonance imaging (MRI)-demonstrated intracranial tumor (high- or low-grade glioma, meningioma, metastatic tumor, or pituitary adenoma) amenable to surgical resection, and absence of liver, dermatologic, or psychiatric disease. Patients with nonstudy final pathology diagnoses are excluded from the primary analysis of the principal investigation and are the basis of separate, independent evaluation. The first case was preoperatively diagnosed with a presumed low-grade glioma; the second and third cases were preoperatively thought to have focal cortical dysplasia (FCD) and were enrolled in the study under special waiver from our institutional Committee for the Protection of Human Subjects (IRB).
Informed consent was obtained for all patients. Preoperative laboratory studies confirmed normal liver function, electrolyte, creatinine, and when applicable negative pregnancy testing. 5-ALA (DUSA Pharmaceuticals Inc, Wilmington, Massachusetts) was administered orally (20 mg/kg) approximately 3 h before surgery. MRI-based image-guidance was active in all cases, using the Stealth S7 system (Medtronic Navigation Inc, Dublin, Ireland) and scalp fiducials. Intermittently during resection as well as at the completion of resection, fluorescence was assessed both visually, using a fluorescence-adapted operating microscope (Pentero operating microscope with Blue 400 module, Carl Zeiss Meditec Inc, Jena, Germany), and quantitatively using a handheld fiber optic probe system and a wide-field, multispectral imaging system, as described previously.13,15,16 Placed in contact with tissue, the probe interrogates in situ an area less than 1 mm across; the noncontact wide-field system, incorporated into the operating microscope, assesses in situ the whole surgical field. The MRI-co registered location of each assessed location was recorded, and a biopsy was obtained from that site for correlation of fluorescence with histology. All patients adhered to standard 5-ALA fluorescence precautions and monitoring postoperatively.
RESULTS
Case 1
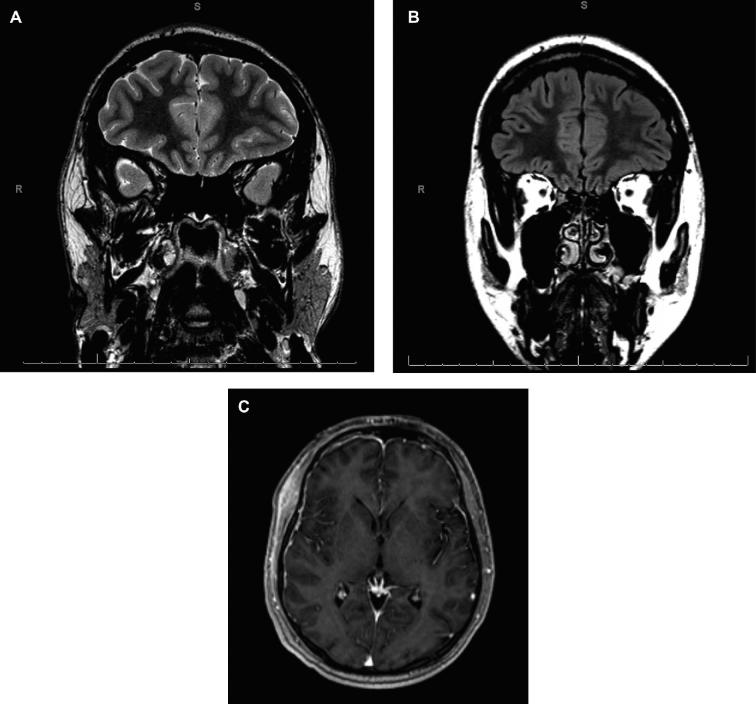
This 58-yr-old right-handed man presented with the new onset of brief episodes characterized by visual disturbance that he described as “distortion” and “pixilation”. He had a history of severe headaches as a youth and had been diagnosed as having ocular migraine but never experienced a generalized or motor seizure. Scalp electroencephalography (EEG) showed focal slowing and sharp waves with phase reversals in the left temporal region. As part of his evaluation he underwent MRI of his head, which revealed a lesion involving the left amygdala that was associated with mild volume increase, several small cyst-like structures, and no enhancement (Figure 1); the remainder of the study was normal. The differential diagnosis for this lesion was low-grade glial neoplasm, dysembryoplastic neuroepithelial tumor, and ganglioglioma; cortical dysplasia could not be excluded. He elected to have this lesion resected and signed consent for enrollment in the 5-ALA fluorescence-guided resection investigational study.
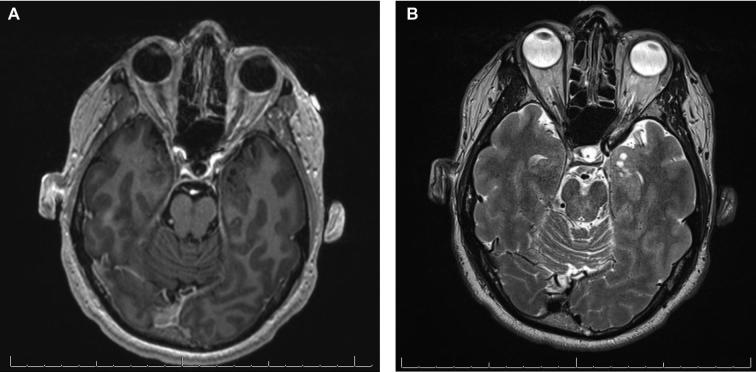
FIGURE 1.
Case 1. The preoperative magnetic resonance imaging (MRI) shows a nonenhancing, multicystic, T2-hyperintense mass in the region of the left anterior hippocampus and amygdala. A, Fast inversion-recovery-prepared 3-dimensional gradient echo with gadolinium contrast-enhancement, axial plane. B, Axial T2W.
On the morning of surgery, the patient was administered 5-ALA orally (20 mg/kg) approximately 3 h prior to incision. A preoperative MRI was obtained with scalp fiducials for intraoperative image-guidance. He was positioned supine with his head secured in 3-point pin fixation. A 5-cm linear incision in the left temporal region, small craniotomy, and corticotomy through the middle temporal gyrus were performed. Upon exposure of the lesion, the tissue was observed to be abnormal in texture and gray in color. With blue light illumination, visible red fluorescence was readily observed from most but not all of the lesion (Figure 2). Intraoperative probe measurements over the lesion showed levels of PpIX from 0.02 to 0.20 μg/ml. A complete resection was performed, with no evidence of residual fluorescence, and this was confirmed with intraoperative MRI. He was discharged to home on postoperative day 3. At 6-mo follow-up he has had no recurrent episodes of visual disturbance or other symptoms suggestive of seizure activity.
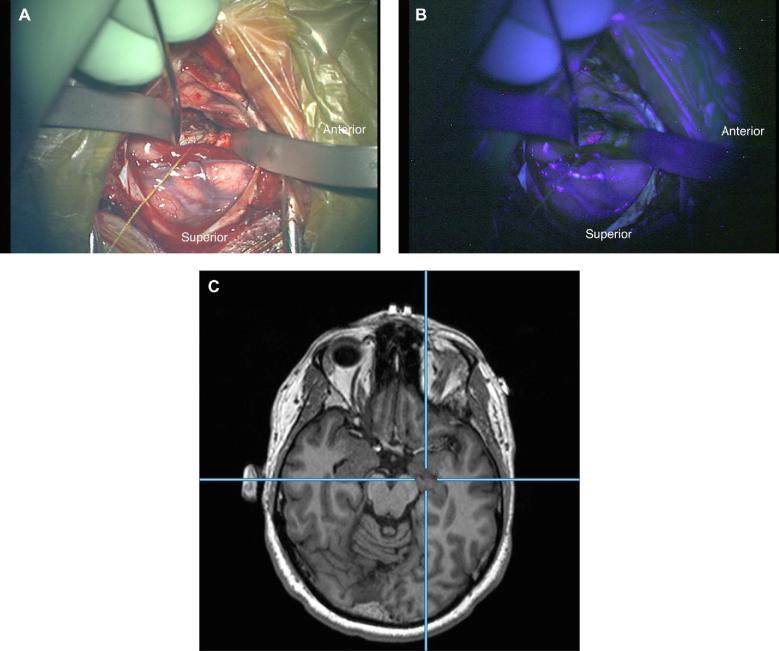
FIGURE 2.
Case 1. A, White light image through the operating microscope of the surgical field during resection. B, Blue light image of the same surgical field demonstrating visible fluorescence of the lesion. C, Axial reformatted MRI corresponding to the intraoperative location of operating microscope.
Pathological review of the surgical specimen showed no evidence of a neoplastic process. Abnormal tangential cortical lamination was noted but no balloon cells or huge neurons as seen in cortical dysplasia Type 2 (Figure 3). Corpora amylacea and mild gliosis were present. The pathological diagnosis was cortical dysplasia Type 1b.
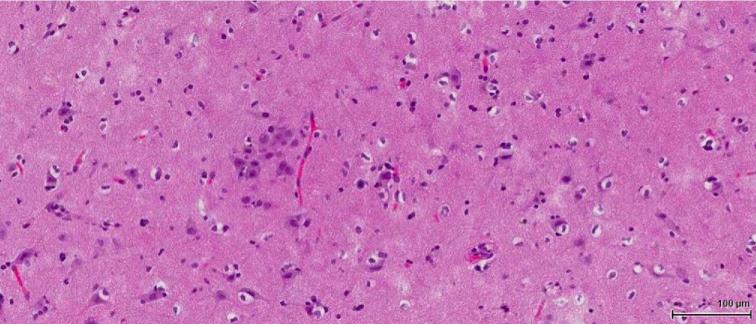
FIGURE 3.
Case 1. Micrograph of representative H & E—stained slide of surgical specimen with abnormal tangential cortical lamination, mild gliosis, and corpora amylacea. No balloon cells or huge neurons were present. The pathological diagnosis was focal cortical dysplasia (FCD) Type 1b.
Case 2
This 23-yr-old woman presented with a medically intractable seizure disorder characterized by multiple seizure types, including nightly nocturnal seizures consisting of arousal, screaming and flailing of all extremities, drop attacks occurring 3 to 4 times a month, frequent staring spells, and rare generalized tonic-clonic seizures. She had a history of delayed language development as a child, but her neurological examination at presentation was normal. Her MRI revealed a nonenhancing left frontal lesion most consistent with FCD (Figure 4); there was no evidence of mesial temporal sclerosis. Functional MRI confirmed left hemisphere language dominance. 18-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) demonstrated hypometabolism in the left frontal lobe. Her interictal electroencephalogram showed left frontal spikes and sharp waves. Multiple seizure onsets arising from the left frontopolar region were recorded.
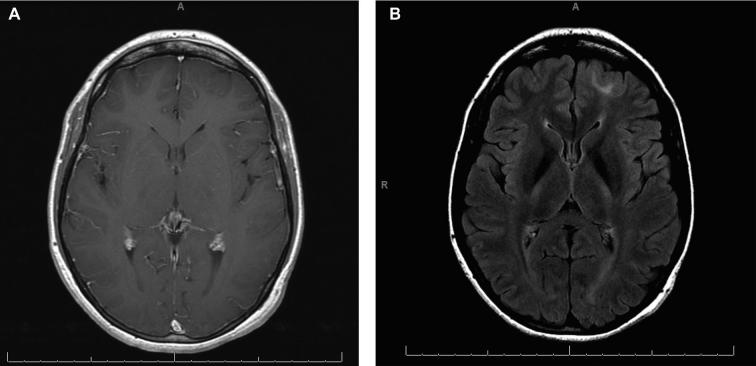
FIGURE 4.
Case 2. Preoperative MRI. A, Axial T1W spin echo postgadolinium MRI showing thickening of the left frontal cortex and blurring of the gray–white junction. There is no contrast enhancement. B, Axial T2W Fluid Attenuation Inversion Recovery (FLAIR) with increased signal intensity demonstrating the left frontal lesion.
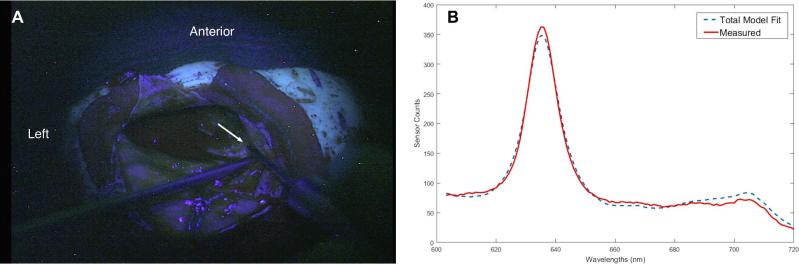
A recommendation was made for surgical resection of her left frontal lesion and epileptogenic seizure focus. Based on the experience of Case 1, a waiver was requested and granted for enrollment in the 5-ALA-induced-fluorescence surgery protocol, and the patient chose to participate in the study. Approximately 3 h prior to surgery, she was administered 5-ALA (20 mg/kg) orally, and surgery was then performed under general anesthesia, using image-guidance and electrocorticography (ECoG). A left frontal 4 × 5 contact subdural grid revealed sharp and slow wave rhythmic discharges corresponding to the location of the lesion. A volumetric resection of the T2-weighted MRI abnormality, comprising a grossly expanded gyrus and underlying abnormally firm, heterogeneous in texture tissue. Faint visible fluorescence was seen at a single site, corresponding to a region of Fluid Attenuation Inversion Recovery (FLAIR) hyperintensity on MRI; no other area of fluorescence was observed during resection, including other areas of MRI FLAIR hyperintensity. Quantitative levels of PpIX were measured at multiple sites, with the highest concentration at the location of faint visible fluorescence being 0.24 μg/ml (Figure 5). At the completion of resection, no epileptiform discharges were seen on ECoG. Postoperatively she experienced no neurological deficit or other complication, and she was discharged from hospital on postoperative day 3. Four months following surgery, she has experienced no seizure activity and continues to do well.
FIGURE 5.
Case 2. Intraoperative probe spectroscopy. A, A blue-light image of the surgical field through the operating microscope during lesion resection shows the hand-held probe at the point of highest measured protoporphyrin IX (PpIX) concentration in this case. The white arrow points to the tissue being interrogated by the tip of the probe (a hand-held suction coming in from the bottom left is seen below). B, Spectra from the same site. The continuous, red line spectrum represents the actual measured data; the dashed line is the fit calculated by a light transport model.
Pathological examination of the surgical specimen showed dyslamination of neuronal layers, disorientation of neurons, dysmorphic forms, and balloon cells; reactive gliosis was noted. Histologic examination of tissue from the site with faint visible fluorescence and the highest level of PpIX concentration described above revealed expected enlarged neuronal cells that were mis-spaced and mis-oriented on a background of mild gliosis (Figure 6). No evidence of neoplasia was found. The neuropathologic diagnosis was FCD, Type 2b.
FIGURE 6.
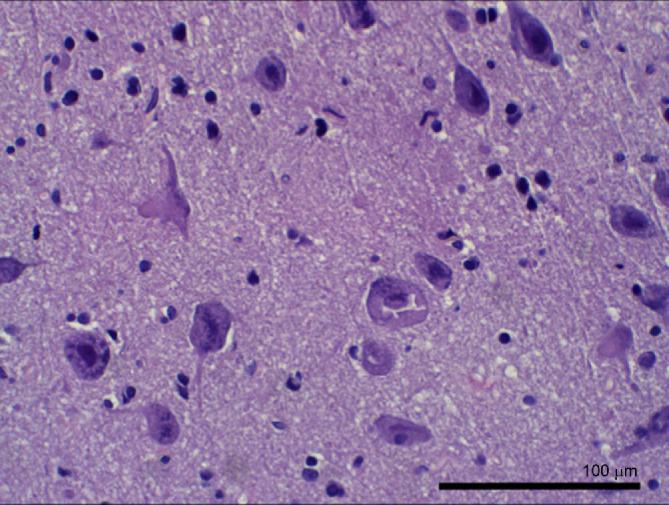
Case 2. H & E histology of the specimen taken at the site of faint visible fluorescence and the highest level of PpIX concentration revealed expected atypical enlarged neuronal cells that were mis-spaced and mis-oriented on a background of mild gliosis. Scale bar, 100 μm.
Case 3
This 20-yr-old man presented with medically intractable epilepsy. His seizures, which began at the age of 11 yr and now occurred several times daily, typically involved extension of the left arm and flexion of the right arm, lasting 15 to 20 s, occasionally associated with loss of awareness. His last generalized seizure was more than a year earlier. His neurological exam was normal. MRI revealed indistinct cortex in the left frontal lobe with T2 prolongation extending to the ventricle, consistent with FCD (Figure 7). FDG PET was normal. Interictal EEG showed spike-wave complexes and polyphasic complex discharges over the right frontal lobe and less often bilaterally. Ictal EEGs demonstrated bilateral frontal complex discharges. An intracranial electrode study was performed with left frontal depth and subdural strip electrodes, right frontal subdural grid and strip electrodes, and an interhemispheric double-sided subdural grid electrode. Multiple seizures, all behaviorally similar, were recorded with ictal onset variably localized to either the left or right frontal lobe, leading to a decision to proceed to an anterior corpus callosotomy. Because of the unusual preoperative EEG findings, subdural strip and depth electrodes were left for 4 d postoperatively, and further seizure activity now localized to the left side. A decision was then made to proceed with left frontal resection, and a waiver to enroll the patient in the 5-ALA-induced fluorescence study obtained.
FIGURE 7.
Case 3. Preoperative MRI. A, Oblique coronal T2W image shows indistinct cortex in the left frontal region with T2 prolongation extending deep into the white matter. B, Coronal T2W FLAIR demonstrates the same left frontal abnormality. C, Three-dimension magnetization-prepared rapid gradient echo T1W axial image with gadolinium.
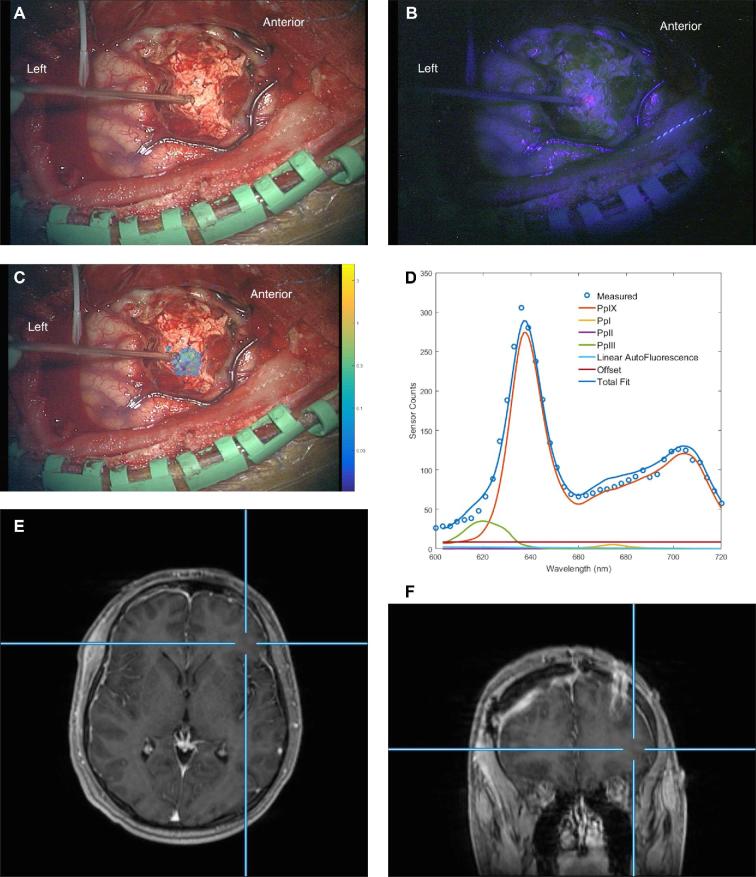
The patient was administered 5-ALA orally (20 mg/kg), approximately 3 h before surgery. The surgery was performed with image-guidance and the patient awake for language mapping and monitoring. ECoG prior to resection demonstrated multiple sharp waves over the left frontal cortex. Volumetric resection was begun away from language areas, and moderate visible fluorescence corresponding to the MRI-demonstrated abnormality and continuing down to the ventricle was observed (Figure 8). The highest PpIX concentration recorded, by wide-field imaging spectrometry, was 0.22 μg/ml. Resection was performed until subcortical language-eloquent sites precluded further tissue removal. Post-resection ECoG showed significantly reduced but not eliminated sharp waves. Postoperatively he experienced temporary slowness of speech. He has had residual although reduced seizure activity.
FIGURE 8.
Case 3. A, Intraoperative white-light image through the operating microscope during lesion resection. B, Blue-light image of the same surgical field. C, White-light image with superimposed color wash showing quantitative levels of PpIX. The white circle region of interest has a PpIX concentration of 0.22 μg/ml. The color bar is a log scale in μg/ml. D, Spectrum for region of interest shown in 8C. The blue circles are measured values (by the wide-field imaging system) of emission at wavelengths represented on the abscissa. The total model fit (highest continuous line spectrum) and the modeled components for the fluorescence are shown; the modeled spectrum for PpIX is the second highest line spectrum. E, Co-registered, contrast-enhanced axial T1W MR image of this location. F, Corresponding contrast-enhanced coronal T1W MR image.
Histopathology showed blurring of the gray–white junction with scattered heterotopic neuronal cells within the white matter. Cortical dyslamination, dysmorphic neurons, and reactive gliosis were present; no balloon cells were seen. The final diagnosis was FCD Type 2a. Interestingly, histology from the location shown in Figure 8 revealed no dysmorphic neuronal forms of FCD but marked astro- and microgliosis (Figure 9).
FIGURE 9.
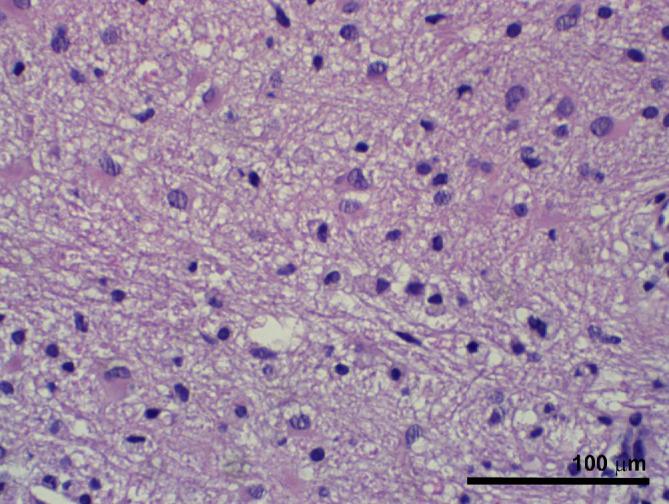
Case 3. H & E histology of specimen from the site depicted in Figure 8. No atypical neuronal forms of FCD are seen, but marked astro- and microgliosis is present. Scale bar, 100 μm.
DISCUSSION
5-ALA-induced fluorescence has been increasingly utilized in the surgical resection of malignant glioma as well as other intracranial tumors. Its association with nonneoplastic intracranial pathological entities is not well understood, and although its application in nonneoplastic conditions in other fields is well recognized,17-19 it is currently not being used in intracranial surgery for nontumor indications. This small case series in which the final diagnosis proved to be FCD afforded the opportunity to assess visible and quantitative PpIX fluorescence in resection of this entity.
While high-grade malignant gliomas exhibit vivid fluorescence, intracranial tumors of lesser grade have also been shown to have elevated levels of the PpIX. Visible fluorescence has been reported in 16% of low-grade gliomas, and in many of these tumors without visible fluorescence intraoperative quantitative measurements of PpIX have revealed elevated levels.13,14 In addition, considerable experience now exists with 5-ALA-induced fluorescence in surgery for meningioma2-6 and metastatic tumor.7-10 The number of quantitative measurements in this small case series is low, but PpIX concentrations in pathologically abnormal specimens from these 3 cases had a median value of 0.056 μg/ml; measurements over presumed normal cortex in these cases had a median value of 0.009 μg/ml (Table and Figure 10). To place these values in context, PpIX concentrations in low-grade glioma specimens in a recently reported study had a median value of 0.015 μg/ml;14 measurements in high-grade gliomas, meningiomas, and metastases are generally higher.13
TABLE.
Patient Summary, Fluorescence Measurements, and Histopathology of Sampled Lesional Tissue
| Case number | Age, gender | Cortical dysplasia type | Probe measurement | Visible fluorescence (y/n) | PpIX concentration (μg/ml) | Specimen histopathology |
|---|---|---|---|---|---|---|
| 1 | 58, M | 1b | ||||
| 1 | No | 0.003 | Nonspecifica | |||
| 2 | Yes | 0.157 | (no specimen) | |||
| 3 | No | 0.022 | FCD + Gliosis | |||
| 2 | 23, F | 2b | ||||
| 1 | No | 0.008 | FCD + Gliosis | |||
| 2 | No | 0.170 | FCD + Gliosis | |||
| 3 | No | 0.012 | FCD + Gliosis | |||
| 4 | No | 0.004 | FCD + Gliosis | |||
| 5 | Yes | 0.611 | FCD + Gliosis | |||
| 6 | No | 0.018 | FCD + Gliosis | |||
| 3 | 20, M | 2a | ||||
| 1 | No | 0.017 | (no specimen) | |||
| 2 | No | 0.006 | (no specimen) | |||
| 3 | No | 0.017 | (no specimen) | |||
| 4 | No | 0.017 | (no specimen) | |||
| 5 | No | 0.002 | FCD | |||
| 6 | No | 0.032 | FCD | |||
| 7 | No | 0.168 | Gliosis | |||
| 8 | No | 0.013 | (no specimen) | |||
| 9 | Yes | 0.601 | Gliosis | |||
| 10 | Yes | 0.681 | Gliosis | |||
| 11 | No | 0.079 | Gliosis | |||
| 12 | No | 0.012 | (no specimen) | |||
| 13 | No | 0.041 | Gliosis | |||
| 14 | No | 0.532 | Gliosis | |||
| 15 | No | 0.071 | Gliosis | |||
| 16 | Yes | 0.307 | (no specimen) | |||
| 17 | Yes | 0.409 | (no specimen) | |||
| 18 | Yes | 3.644 | Gliosis |
aCalcified vessels focally.
FIGURE 10.
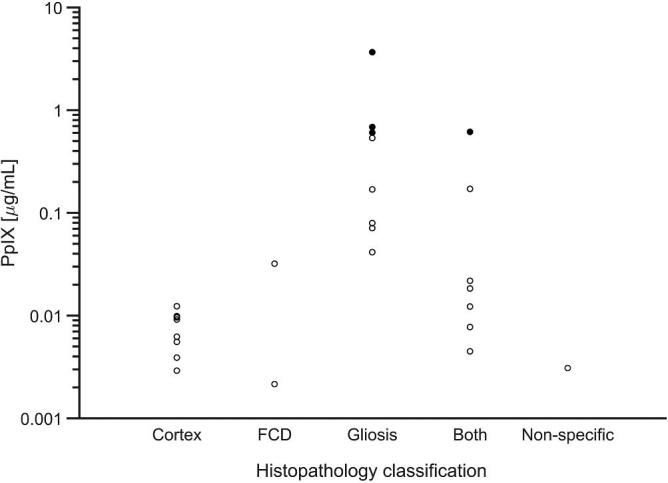
Concentration of ALA-induced PpIX for presumed normal cortex and for specimens histologically demonstrating FCD, gliosis, or both. Open circles represent measurement sites without associated visible fluorescence; filled circles were measurement sites that demonstrated visible fluorescence. No normal cortex sites were visibly fluorescent. The Y-axis is logarithmic. The nonspecific histology findings in one specimen were calcified vessels focally.
Factors known to be associated with elevated protoporphyrin levels in tumor include blood–brain-barrier (BBB) breakdown related to tumor vasculature,20,21 increased capillary density,20 proliferative index,22,23 histologic neoplastic grade,21,23 cell density,24,25 mitochondrial content,26 age of patient,23 and possible aquaporin channel up-regulation.27 The role of prior radiation therapy,28 inflammation,24 and gliosis24,29,30 has been discussed. The lesions in our patients did not have evidence of gadolinium contrast-enhancement on MRI imaging, evidence against absence of the BBB, and increased capillary density, cell density or proliferation was not appreciated on histology. The patients were 58, 23, and 20 yr of age, and none had a history of prior radiation therapy or inflammatory disease.
Reactive astrocytes have been associated with 5-ALA-induced fluorescence, and underlie at least some of the false positive observations during tumor surgery.25,29 Utsuki and colleagues29 in their study of ALA-induced fluorescence false positives found associations with infiltration of reactive astrocytes and macrophages in recurrent glioma and with peritumoral edema and infiltration of reactive astrocytes in surgeries for recurrent malignant glioma and metastasis, respectively. In another study of surgery for recurrent malignant glioma after prior surgical resection and adjuvant therapy, by Kamp et al,30 visible solid or faint fluorescence was seen in 12 of 13 patients (12 of whom had received radiation therapy and temozolomide and 1 only chemotherapy) found to have only reactive changes but no tumor on histology. Notably, reactive gliosis was present in all 3 of our FCD patients, and in our third patient, the tissue specimen associated with the highest concentration of PpIX demonstrated only marked astro- and micro-gliosis. Further, the 2 sites evidencing FCD without associated gliosis on histopathology demonstrated low concentrations of PpIX. Edema was not present, and in the absence of lower concentrations of PpIX in the histological areas of FCD, PpIX leakage—a phenomenon previously described,31 particularly with respect to metastatic tumor—is difficult to implicate.
Observations in an animal model show that seizure activity or histologic changes of gliosis can be associated with 5-ALA-induced fluorescence. Reactive gliosis as well as temporary disturbance of the BBB in association with seizure activity itself may play a role in such fluorescence. Using an established rat model of chronic seizure activity induced with an acute dose of pilocarpine, Kleen and colleagues32 provided preliminary evidence of fluorescence in the CA1 subfield of the posterior hippocampus and Layer II of the piriform cortex, areas classically associated with epileptogenesis, as well as, to a lesser degree, in adjacent parahippocampal cortex.
All 3 of our FCD patients had experienced seizures, and 2 of them had long-standing intractable epilepsy. Although the number of patients may be too small to necessarily expect correlation, the level of PpIX concentration observed did not appear to be related to the frequency or severity of seizures.
Localized malformations of cortical development and their association with epilepsy, first highlighted by Taylor et al33 and classified most notably by Barkovich et al,34 Palmini et al,35 and Blümcke et al,36 represent a group of nonneoplastic conditions characterized by features associated with disordered neuronal proliferation, maturation, and migration. The most recent classification scheme for FCD, developed by a task force of the International League Against Epilepsy, is 3-tiered, with Type 1 featuring abnormal cortical lamination (1a: radial, 1b: tangential, and 1c: radial and tangential), Type 2 having dysmorphic neurons, without (2a) or with (2b) balloon cells, and Type 3 associated with principal other lesions (3a, hippocampal sclerosis; 3b, adjacent glial or glioneuronal tumor; 3c, adjacent vascular malformation, and 3d, adjacent other lesion acquired early in life).36
The role of surgical intervention in the treatment of medically intractable epilepsy associated with FCD has long been recognized. With advances in neuroimaging, that role continues to increase. In one recent study, 7T MRI was able to identify FCD in 29% of patients who previously had MRI-negative epilepsy with conventional imaging.37 In a condition often refractory to anticonvulsant therapy, the success of surgical resection with respect to seizure outcomes (65% Engel Class 1 at 1 yr in a recent large series38) has been widely reported.38-42 In studies investigating the factors associated with unsuccessful surgery, however, incompleteness of resection of a FCD lesion and surrounding abnormal tissue concordant with electrophysiological epileptogenesis has repeatedly been strongly implicated.43-46
Reported rates of complete resection in patients with FCD range from recently published single-institution retrospective studies range from 54.5% to 85.9%.39,41,47-49 The high degree of variability represents a lack of consensus regarding the definition of complete resection. Some studies use radiographic criteria (absence of residual imaging abnormalities), others use normalization of intraoperative ECoG, and still others advocate for histologically negative resection margins.41 Further complicating the analysis is inclusion of FCD located in eloquent cortex that was treated by multiple subpial transection or large diffuse lesions that were deliberately treated with subtotal resection. As a result, the rate of gross total resection in FCD that is preoperatively deemed completely resectable is not reported. An alternate, potentially more useful metric is the rate of re-operation, which likely represents the percentage of cases in which there was residual epileptogenic tissue that was missed during the primary surgery. Re-operation in patients with FCD occurs in 6.9% to 22.7% of large series.39,41,50 In a review of 115 epilepsy resections, Englot and colleagues50 found that patients with FCD were more likely to suffer recurrent seizures than those with mesial temporal sclerosis (MTS) or tumors. Taken together, these data suggest that a significant percentage of patients with FCD are at risk for incomplete resections, and consequently, poor seizure outcomes.
Methods that may improve the likelihood of complete surgical resection warrant investigation and, if utility is shown, adoption. Evaluative and intraoperative electrophysiology,41,45 image-guidance,41,45 intraoperative histopathology,41 and intraoperative MRI51 have all been utilized to improve the likelihood of complete surgical resection in FCD, and the early experience reported here suggests that fluorescence-guidance may have a useful role as well. A larger clinical experience correlating PpIX fluorescence with histopathological findings of FCD and gliosis is required to better understand the diagnostic performance of fluorescence-guidance in surgery for this condition.
CONCLUSION
Three patients with medically intractable epilepsy, MRI-demonstrated lesions concordant with electrophysiologic epileptogenesis, and subsequent histologic confirmation of FCD (Types 1b, 2a, and 2b) underwent surgical resection under a protocol evaluating 5-ALA-induced fluorescence for intraoperative guidance. Visible fluorescence (faint or moderate) was observed in each case, and quantitative assessment of PpIX concentration during surgery with a fiber optic probe system and by wide-field imaging spectroscopy confirmed elevated levels of the fluorophore. In 1 case the highest PpIX concentration was found in tissue that histologically demonstrated no typical features of FCD but marked gliosis. This experience suggests that fluorescence-guidance in surgical resection of this epilepsy-associated, nonneoplastic lesion may offer some utility.
Disclosures
This work was supported in part by NIH grant R01NS052274 (D.W.R.) awarded by the National Institute of Neurological Disorders and Stroke. Carl Zeiss Surgical GmbH and Medtronic Navigation provided the fluorescence-enabled OPMI Pentero operating microscope and StealthStation navigation system, respectively. DUSA Pharmaceuticals supplied the 5-ALA. Drs Paulsen (with Dartmouth College), Roberts (with Dartmouth College), and Wilson (with University Health Network, Toronto) are patent holders and have patent applications on several aspects of the fluorescence technologies discussed in this study. Drs Roberts, Paulsen, and Wilson are co-founders and equity holders in InSight Surgical Technologies LLC.
COMMENT
The authors have extended the utility of ALA-induced PpIX fluorescence beyond tumor pathologies to epilepsy, specifically focal cortical dysplasia (FCD). The novelty of this work rests on the discovery that ALA-induced PpIX might be a useful biomarker for guiding epilepsy surgeries. Following the successful template of their prior work, the authors analyzed FCD tissue using a commercially available microscope adapted for fluorescence imaging, and their quantitative technologies using both a handheld spectroscopy probe and a wide field imaging spectrometer. They have previously demonstrated the improved detection capabilities of these quantitative systems, specifically because of their higher sensitivity for PpIX fluorescence. They have exploited these tools to provide more sensitive detection of pathologic tissue in FCD specimens, demonstrating that in tissue without visible PpIX fluorescence (ie, fluorescence detected via the commercially available microscope), significant levels of PpIX can be detected. Specifically, they note greater than one order of magnitude higher PpIX concentrations in non-visibly fluorescent tissues (eg, case#2, specimen 2, 0.170 ug/ml) compared to their previously published background levels found in normal tissues (<0.10 ug/ml). Furthermore, the authors also provided an interesting finding associating a biological factor, ie, gliosis, with areas of higher PpIX levels that merits further investigation.
At least 2 key lessons can be learned from this work. First, that PpIX can be a useful biomarker in pathologies other than tumors such as FCD and epilepsy. This is a preliminary study on only 3 patients that will now require further work to determine the ultimate clinical utility of this technology as an adjunct for FCD. The authors are thus commended for this work, which should encourage the community to investigate the utility of PpIX on not just tumors and FCD but on additional pathologies. Second, that quantitative technologies are paramount for improved guidance and accuracy using fluorescence technologies. As more fluorescent markers become available and more clinicians use fluorescence, quantitative technologies should be developed to help standardize fluorescence assessments across surgeons as well as to help improve overall accuracies. This work further substantiates the need for making such technologies more widely available to the neurosurgical community.
Pablo A. Valdes Quevedo
Alexandra J. Golby
Boston, Massachusetts
REFERENCES
- 1. Stummer W, Pichlmeier U, Meinel Tet al.. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 2. Bekelis K, Valdes PA, Erkmen Ket al.. Quantitative and qualitative 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in skull base meningiomas. Neurosurg Focus. 2011;30(5):E8–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster N, Eljamel S. ALA-induced fluorescence image guided surgery of meningiomas: a meta-analyses. Photodiagnosis Photodyn Ther. 2016;15:73–78. [DOI] [PubMed] [Google Scholar]
- 4. Potapov AA, Goryaynov SA, Okhlopkov VAet al.. Laser biospectroscopy and 5-ALA fluorescence navigation as a helpful tool in the meningioma resection. Neurosurg Rev. 2016;39(3):437–447. [DOI] [PubMed] [Google Scholar]
- 5. Valdes PA, Bekelis K, Harris BTet al.. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurgery. 2014;10(suppl 1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilbers E, Hargus G, Wolfer J, Stummer W. Usefulness of 5-ALA (Gliolan®)-derived PPX fluorescence for demonstrating the extent of infiltration in atypical meningiomas. Acta Neurochir. 2014;156(10):1853–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamp MA, Grosser P, Felsberg Jet al.. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir. 2012;154(2):223–228. [DOI] [PubMed] [Google Scholar]
- 8. Kamp MA, Fischer I, Buhner Jet al.. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget. 2016;7(41):66776–66789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marbacher S, Klinger E, Schwyzer Let al.. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014;36(2):E1–E10. [DOI] [PubMed] [Google Scholar]
- 10. Utsuki S, Miyoshi N, Oka Het al.. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol. 2007;24(2):53–55. [DOI] [PubMed] [Google Scholar]
- 11. Sanai N, Snyder LA, Honea NJet al.. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115(4):740–748. [DOI] [PubMed] [Google Scholar]
- 12. Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Med Chir.(Tokyo). 2007;47(2):53–57. [DOI] [PubMed] [Google Scholar]
- 13. Valdes PA, Leblond F, Kim Aet al.. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. 2011;115(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valdes PA, Jacobs V, Harris BTet al.. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg. 2015;123(3):771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valdes PA, Leblond F, Jacobs VL, Wilson BC, Paulsen KD, Roberts DW. Quantitative, spectrally-resolved intraoperative fluorescence imaging. Sci Rep. 2012;2(1):E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim A, Khurana M, Moriyama Y, Wilson BC. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J. Biomed. Opt. 2010;15(6):067006(1-12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fritsch C, Goerz G, Ruzicka T. Photodynamic therapy in dermatology. Arch Dermatol. 1998;134(2):207–214. [DOI] [PubMed] [Google Scholar]
- 18. Dirschka T, Radny P, Dominicus Ret al.. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166(1):137–146. [DOI] [PubMed] [Google Scholar]
- 19. Brand S, Wang TD, Schomacker KTet al.. Detection of high-grade dysplasia in Barrett's esophagus by spectroscopy measurement of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Gastrointest Endosc. 2002;56(4):479–487. [DOI] [PubMed] [Google Scholar]
- 20. Valdes PA, Moses ZB, Kim Aet al.. Gadolinium- and 5-aminolevulinic acid-induced protoporphyrin IX levels in human gliomas: an ex vivo quantitative study to correlate protoporphyrin IX levels and blood-brain barrier breakdown. J Neuropathol Exp Neurol. 2012;71(9):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts DW, Valdes PA, Harris BTet al.. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg. 2011;114(3):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valdes PA, Kim A, Brantsch Met al.. Delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011;13(8):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaber M, Wolfer J, Ewelt Cet al.. The value of 5-ALA in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, MRI, 18F-FET PET, and tumor molecular factors. Neurosurgery. 2016;78(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lau D, Hervey-Jumper SL, Chang Set al.. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg. 2016;124(5):1300–1309. [DOI] [PubMed] [Google Scholar]
- 25. Stummer W, Tonn JC, Goetz Cet al.. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibbs SL, Chen B, O’Hara JA, Hoopes PJ, Hasan T, Pogue BW. Protoporphyrin IX level correlates with number of mitochondria, but increase in production correlates with tumor cell size. Photochem Photobiol. 2006;82(5):1334–1341. [DOI] [PubMed] [Google Scholar]
- 27. Suero Molina EJ, Ardon H, Schroeteler Jet al.. Aquaporin-4 in glioma and metastatic tissues harboring 5-aminolevulinic acid-induced porphyrin fluorescence. Clin Neurol Neurosurg. 2013;115(10):2075–2081. [DOI] [PubMed] [Google Scholar]
- 28. Miyatake S, Kuroiwa T, Kajimoto Y, Miyashita M, Tanaka H, Tsuji M. Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: case report. Neurosurgery. 2007;61(5):E1101–E1104. [DOI] [PubMed] [Google Scholar]
- 29. Utsuki S, Oka H, Sato Set al.. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol. Med. Chir (Tokyo). 2007;47(5):210–214. [DOI] [PubMed] [Google Scholar]
- 30. Kamp MA, Felsberg J, Sadat Het al.. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2015;157(2):207–214. [DOI] [PubMed] [Google Scholar]
- 31. Stummer W, Stocker S, Novotny Aet al.. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B. 1998;45(2-3):160–169. [DOI] [PubMed] [Google Scholar]
- 32. Kleen JK, Valdes PA, Harris BT, Holmes GL, Paulsen KD, Roberts DW. ALA-induced PpIX fluorescence in epileptogenic tissue. Proc SPIE. 2011;7883:78833S-78831-78833S-78837. [Google Scholar]
- 33. Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34(4):369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barkovich AJ, Kuzniecky RI, Dobyns WB, Jackson GD, Becker LE, Evrard P. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27(02):59–63. [DOI] [PubMed] [Google Scholar]
- 35. Palmini A, Najm I, Avanzini Get al.. Terminology and classification of the cortical dysplasias. Neurology. 2004;62(6 suppl 3):S2–S8. [DOI] [PubMed] [Google Scholar]
- 36. Blumcke I, Thom M, Aronica Eet al.. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission1. Epilepsia. 2011;52(1):158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Ciantis A, Barba C, Tassi Let al.. 7T MRI in focal epilepsy with unrevealing conventional field strength imaging. Epilepsia. 2016;57(3):445–454. [DOI] [PubMed] [Google Scholar]
- 38. Fauser S, Essang C, Altenmuller DMet al.. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015;56(1):66–76. [DOI] [PubMed] [Google Scholar]
- 39. Fauser S, Schulze-Bonhage A, Honegger Jet al.. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127(11):2406–2418. [DOI] [PubMed] [Google Scholar]
- 40. Oluigbo C, Sacino M, Myseros JS, Magge SN, Gaillard W, Keating RF. 131 Resective surgery for focal cortical dysplasia in children: a comparative analysis of the utility of intraoperative magnetic resonance imaging. Neurosurgery. 2016;63(suppl 1):153–154. [DOI] [PubMed] [Google Scholar]
- 41. Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004;101(1):55–65. [DOI] [PubMed] [Google Scholar]
- 42. Park CK, Kim SK, Wang KCet al.. Surgical outcome and prognostic factors of pediatric epilepsy caused by cortical dysplasia. Childs Nerv Syst. 2006;22(6):586–592. [DOI] [PubMed] [Google Scholar]
- 43. Sacino MF, Ho CY, Whitehead MTet al.. Repeat surgery for focal cortical dysplasias in children: indications and outcomes. J Neurosurg Pediatr. 2017;19(2):174–181. [DOI] [PubMed] [Google Scholar]
- 44. Krsek P, Maton B, Jayakar Pet al.. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72(3):217–223. [DOI] [PubMed] [Google Scholar]
- 45. Oluigbo CO, Wang J, Whitehead MTet al.. The influence of lesion volume, perilesion resection volume, and completeness of resection on seizure outcome after resective epilepsy surgery for cortical dysplasia in children. J Neurosurg Pediatr. 2015;15(6):644–650. [DOI] [PubMed] [Google Scholar]
- 46. Kim DW, Lee SK, Chu Ket al.. Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology. 2009;72(3):211–216. [DOI] [PubMed] [Google Scholar]
- 47. Hader WJ, Mackay M, Otsubo Het al.. Cortical dysplastic lesions in children with intractable epilepsy: role of complete resection. J Neurosurg. 2004;100(2 Suppl Pediatrics):110–117. [DOI] [PubMed] [Google Scholar]
- 48. Jin B, Wang J, Zhou J, Wang S, Guan Y, Chen S. A longitudinal study of surgical outcome of pharmacoresistant epilepsy caused by focal cortical dysplasia. J Neurol. 2016;263(12):2403–2410. [DOI] [PubMed] [Google Scholar]
- 49. Wagner J, Urbach H, Niehusmann P, von Lehe M, Elger CE, Wellmer J. Focal cortical dysplasia type IIb: completeness of cortical, not subcortical, resection is necessary for seizure freedom. Epilepsia. 2011;52(8):1418–1424. [DOI] [PubMed] [Google Scholar]
- 50. Englot DJ, Han SJ, Rolston JDet al.. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014;14(4):386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sacino MF, Ho CY, Murnick Jet al.. Intraoperative MRI-guided resection of focal cortical dysplasia in pediatric patients: technique and outcomes. J Neurosurg Pediatr. 2016;17(6):672–678. [DOI] [PubMed] [Google Scholar]