Pediatric nephrotic syndrome (PNS) is a major medical problem without a known cure nor clear treatment regimen and has been the focus of intense investigation. Its most severe manifestion is steroid-resistant nephrotic syndrome (SRNS), the second most frequent cause of pediatric end-stage kidney disease (ESKD).1
New concepts about PNS have been built on fundamental genetic discoveries. Aided considerably by the advent of next-generation sequencing and its application to familial cases in the research setting, more than 60 single gene (“Mendelian”) causes of SRNS have been discovered. Non-Mendelian genetic forms of nephrotic syndrome (NS) also have been identified, including high-risk APOL1 genotypes with focal and segmental glomeruloesclerosis2,3 and HLA-DQ alleles with pediatric steroid-sensitive NS.4
The translation of scientific knowledge into clinical outcomes requires robust clinical trials and epidemiological studies with appropriate size, design, and comprehensiveness. Studies in the United States, the United Kingdom, and the European Union, such as NEPTUNE, PodoNet, and PredNos, are driving forward rigorous and novel scientific inquiries in PNS. However, there is a noted lack of similar studies being performed outside of developed countries. It is already clear that the prevalence and natural history of NS differs by geographic location and genetic ancestry, with prevalence of Mendelian SRNS differing across countries, and APOL1-associated NS being a condition specific to those of recent African ancestry. This creates an opportunity for studies of NS in developing countries to add significant information to the field.
Brazil is the fifth largest country in the world, with a highly admixed population, derived mainly from European colonizers and immigrants, African slaves, and indigenous Amerindians. The diversity of people and climates provides an opportunity to study the genetic and environmental influence on NS, in a way that has not been matched elsewhere around the world. It is possible, moreover, that the prevalent tropical climate present in Brazil may influence the prevalence and diversity of infections, potentially affecting PNS epidemiology.
In the past 6 years, research on the genetics of PNS in Brazil has gained momentum in main universities of São Paulo. This interest has been translated into some reports (Watanabe A, Neves PD, Watanabe EH, et al. APOL1 risk alleles are critical for the development of collapsing glomerulopathy in Brazilian children [abstract]. J Am Soc Nephrol. 2018;29:716).5, 6, 7, 8
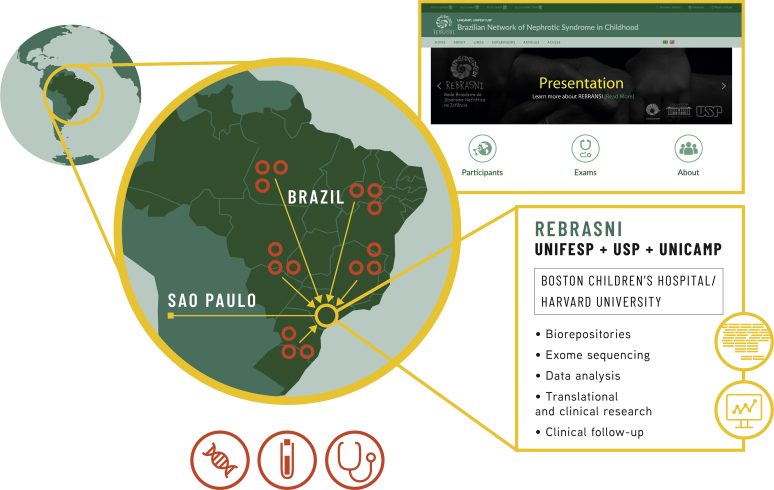
The REBRASNI
In light of the need to better understand the etiology and natural history of NS, and the unique opportunity to do this in Brazil, physicians and physician scientists from the Divisions of Pediatric Nephrology and Nephrology of 3 renowned Brazilian medical schools, University of São Paulo, Federal University of São Paulo, and State University of Campinas, created the Brazilian Network of Pediatric Nephrotic Syndrome (REBRASNI, Rede Brasileira de Sindrome Nefrótica na Infância, in Portuguese) early in 2018 (Figure 1). The mission of REBRASNI is to generate knowledge to improve diagnosis, evaluate prognosis, and contribute to personalized treatment and potential cure of PNS.
Figure 1.
Structure and initiatives of Brazilian Network of Pediatric Nephrotic Syndrome (REBRASNI). UNICAMP; State University of Campinas; UNIFESP, Federal University of São Paulo; USP, University of São Paulo.
REBRASNI uses an electronic platform (www.rebrasni.sites.unifesp.br) to register cases of PNS within the Brazilian territory. Epidemiologic, clinical, and laboratory data will be prospectively obtained from all patients with NS, whereas biosamples will be initially collected from individuals with SRNS. Patient samples (whole blood and urine) and a fragment of kidney biopsy (when clinically indicated) will be stored in biorepositories, according to Brazilian regulations (see the section Research Plans later in the article, Supplementary Methods, and Figure 2).
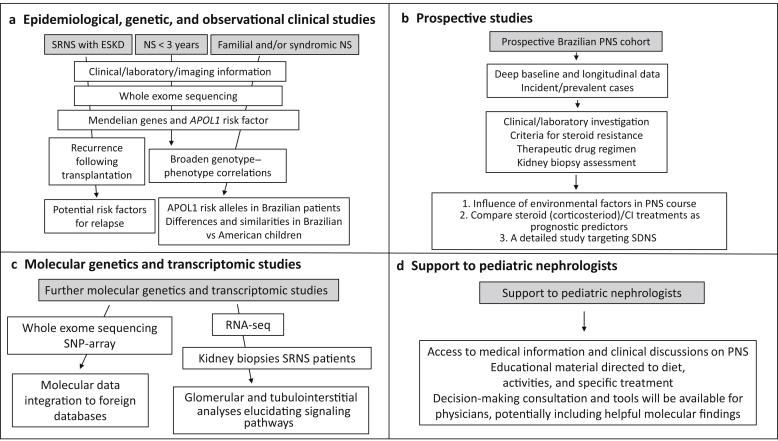
Figure 2.
Research plans. CI, calcineurin inhibitor; ESKD, end-stage kidney disease; NS, nephrotic syndrome; PNS, pediatric nephrotic syndrome; RNA-seq, RNA sequencing; SDNS, steroid-dependent nephrotic syndrome; SNP, single nucleotide polymorphism; SRNS, steroid-resistant nephrotic syndrome.
Results
Preliminary data have been collected in the state of Sao Paulo. There were 454 patients with NS within the age range of 0.1 to 18 years followed between 2017 and 2018 at State University of Campinas and University of São Paulo, and 1 major affiliated center: University of São Paulo at Ribeirão Preto. The median age of NS onset was 3.1 (0.3–14.9) years; 268 patients (57.3%) were male, and 349 (76.9%) were Caucasian and 95 (20.9%) were mixed race/black (Table 1). Eighty-seven of them (19.2%) harbored the clinical diagnosis of SRNS/congenital nephrotic syndrome. Of these 87 patients, 30 (34.5%; or 6.6% of all patients with NS) developed ESKD.
Table 1.
Demographics and age of onset of patients with pediatric nephrotic syndrome followed at State University of Campinas, University of São Paulo-São Paulo, and University of São Paulo-Ribeirão Preto
| Variable | Result |
|---|---|
| Total number of patients | 454 |
| Sex, % | Male: 57.3 Female: 42.7 |
| Median age at onset (range) of nephrotic syndrome, yr | 3.1 (0.1–14.9) |
| Ethnicity, % | Caucasian: 76.9 Mixed/Black: 20.9 Other: 2.2 |
Seventy of the 87 patients with SRNS (80.4%) had undergone a kidney biopsy. Forty-four (62.9%) had the diagnosis of focal and segmental glomeruloesclerosis and 8 (11.4%) collapsing glomerulopathy. Seven of the patients with collapsing glomerulopathy (87.5%) developed ESKD, a condition not reached by any patient with minimal change disease during the 2-year follow-up. In biopsied patients with steroid-dependent NS, the most common diagnosis was minimal change disease (Table 2).
Table 2.
Histological diagnoses of biopsied pediatric patients with nephrotic syndrome followed at State University of Campinas, University of São Paulo-São Paulo, and University of São Paulo-Ribeirão Preto
| Variable | Steroid-resistant nephrotic syndrome | Steroid-dependent nephrotic syndrome |
|---|---|---|
| Number of biopsied patients, n/N (%) | 70/87 (80.4) | 67/147 (45.6) |
| Minimal change disease, % | 18.6 | 56.7 |
| Focal and segmental glomerulosclerosis, % | 62.9 | 34.3 |
| Collapsing glomerulopathy, % | 11.4 | 0.0 |
| Diffuse mesangial sclerosis, % | 4.3 | 0.0 |
| Acute tubular necrosis, % | 0.0 | 3.0 |
| Tubulointerstitial nephropathy, % | 0.0 | 1.5 |
| Proliferative mesangial glomerulopathy, % | 2.9 | 1.5 |
| Focal interstitial fibrosis, % | 0.0 | 1.5 |
| Membranous nephropathy, % | 0.0 | 1.5 |
Among the 1606 children with ESKD submitted to kidney transplantation at Federal University of São Paulo, University of São Paulo-São Paulo, and State University of Campinas (1982–2018), 154 (9.6%) had the primary diagnosis of NS. A total of 135 patients have available medical records for appropriate studies. Eighty-three of them (61.5%) were male, the median age of NS onset was 4.0 (0.2–15.0) years, the median time to ESKD was 4.0 (1.0–15.2) years, and the median age of first kidney transplantation was 12.0 (2.5–18.7) years. Thirty-one patients (23.0%) had NS recurrence after transplantation (Table 3).
Table 3.
Demographics and clinical features of kidney transplantation in patients with pediatric nephrotic syndrome followed at Federal University of São Paulo, University of São Paulo-São Paulo, and State University of Campinas
| Demographic and clinical features | Result |
|---|---|
| Time period | 1982–2018 |
| Number of transplanted patients with nephrotic syndrome with available data | 135 |
| Sex, % | Male: 61.5 Female: 38.5 |
| Median age at onset (range) of nephrotic syndrome, yr | 4.0 (0.2–15.0) |
| Median time to end-stage of kidney disease (range), yr | 4.0 (1.0–15.2) |
| Median age at first kidney transplantation (range), yr | 12.0 (2.5–18.7) |
| Number of patients with recurrence of nephrotic syndrome in the graft (first kidney transplantation), n/N (%) | 31/135 (23.0) |
The high proportion of SRNS, ESKD, and steroid dependency observed in our cohort is likely explained by the fact that State University of Campinas, University of São Paulo, and Federal University of São Paulo are tertiary pediatric nephrology units to which a significant number of patients with difficult-to-treat NS and ESKD cases are referred.
Research Plans
One of the most important aims of REBRASNI is to discover the genetic architecture and describe the epidemiologic characteristics of PNS in Brazil. To accomplish these goals, our network will prioritize performance of a number studies in the settings itemized as follows, summarized in Figure 2, and more comprehensively described in the Supplementary Methods.
Epidemiologic, Genetic, and Observational Clinical Studies
-
(i)Genotype–phenotype correlations based on whole exome sequencing (including APOL1 genotyping) and a broad spectrum of clinical, biopsy, imagenologic, and laboratory characterization, will address the following outcomes:
-
•ESKD;
-
•onset when younger than 3 years old; and
-
•familial and/or syndromic NS.
-
•
-
(ii)
Clinical analyses will aim to identify and characterize potential risk factors for occurrence of NS relapse following kidney transplantation, treatment efficacy, and safety.
Prospective Studies
-
(i)Establish a prospective Brazilian PNS cohort:
-
•to evaluate the influence of environmental factors associated with the onset and/or clinical course of PNS;
-
•to compare failure of steroid and calcineurin inhibitor treatments as prognostic predictors of progression of chronic kidney disease in PNS;
-
•to study the potential roles of persistent microscopic hematuria, selective proteinuria index, level of hypoalbuminemia, and/or new biomarkers in the PNS clinical course; and
-
•to study steroid-dependent patients with a particular focus on steroid pharmacokinetics.
-
•
Molecular Genetics and Transcriptomic Studies
-
(i)
Children without a known Mendelian cause of their disease will undergo expanded, exome-wide genetic analysis. Molecular genetics data will be integrated into worldwide, NS databases and are also expected to provide independent families to strengthen Mendelian claims of new SRNS-associated genes.
-
(ii)
Analyze the clinical impact of APOL1 risk alleles in Brazilian patients with SRNS and factors that modify its penetrance.
-
(iii)
Study glomerular and tubulointerstitial transcriptomic analyses in kidney biopsies of SRNS REBRASNI patients using RNA sequencing.
Support to Pediatric Nephrologists and Families
-
(i)
Create access to medical information in PNS through scientific papers and clinical discussions via online site and provide educational material to patients focused on diet, activities, and specific treatments.
Financial Support and Sustainability
Funding from grant mechanisms, including binational ones, are expected to be the main source of financial support along the next years, followed by private donations. Applications to government funds and private health institutions are under way.
Conclusion
Brazilian pediatric nephrology is hoping and expecting that the success of REBRASNI may bring a major step forward in PNS knowledge and actions in our country and worldwide.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank Regiane Suemy Higa and Feppile Augusto Machado da Conceição for the informatics work and support required to build the REBRASNI registry. Our work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo: grant 2013/02162–8 to LFO as 1 of the 4 principal investigators, and grant 2014/27198–8 to JBP and PCKN as 2 of the 4 principal investigators.
Footnotes
Contributor Information
Vera M.S. Belangero, Email: vmsbelangero@gmail.com.
Paulo C. Koch Nogueira, Email: pckoch@uol.com.br.
Luiz F. Onuchic, Email: lonuchic@usp.br.
Supplementary Material
References
- 1.Stokman M.F., Renkema K.Y., Giles R.H. The expanding phenotypic spectra of kidney diseases: insights from genetic studies. Nat Rev Nephrol. 2016;12:472–483. doi: 10.1038/nrneph.2016.87. [DOI] [PubMed] [Google Scholar]
- 2.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzur S., Rosset S., Shemer R. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gbadegesin R.A., Adeyemo A., Webb N.J. HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. 2015;26:1701–1710. doi: 10.1681/ASN.2014030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guaragna M.S., Lutaif A.C., Piveta C.S. Two distinct WT1 mutations identified in patients and relatives with isolated nephrotic proteinuria. Biochem Biophys Res Commun. 2013;441:371–376. doi: 10.1016/j.bbrc.2013.10.064. [DOI] [PubMed] [Google Scholar]
- 6.Guaragna M.S., Lutaif A.C., Piveta C.S. NPHS2 mutations account for only 15% of nephrotic syndrome cases. BMC Med Genet. 2015;16:88. doi: 10.1186/s12881-015-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guaragna M.S., Cleto T.L., Souza M.L. NPHS1 gene mutations confirm congenital nephrotic syndrome in four Brazilian cases: a novel mutation is described. Nephrology (Carlton) 2016;21:753–757. doi: 10.1111/nep.12667. [DOI] [PubMed] [Google Scholar]
- 8.Feltran L.S., Varela P., Silva E.D. Targeted next-generation sequencing in brazilian children with nephrotic syndrome submitted to renal transplant. Transplantation. 2017;101:2905–2912. doi: 10.1097/TP.0000000000001846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.