Abstract
The epidemiologic transition occurring in low- and middle-income countries (LMICs) has led to a surge in chronic kidney disease (CKD) prevalence because of a combination of highly prevalent chronic noncommunicable diseases (NCDs) and communicable diseases (CDs). The progressive rise in CKD prevalence in LMICs threatens the existing weak health systems in these countries as care for advanced CKD remains largely unavailable and unaffordable. An interplay of low literacy levels, poor health-seeking behavior, inadequate health care funding, weak health systems, and lack of skilled nephrology workforce has made it difficult for adequate CKD preventive measures to be implemented. Primary, secondary, and tertiary prevention measures need to be instituted in LMICs by a collaboration of governmental and nongovernmental organizations to stem this tide and help prevent deaths from other NCDs that share similar risk factors with CKD. For these to be effective, locally relevant knowledge is needed to contextualize existing prevention and control solutions, or to develop novel and more appropriate solutions for LMICs.
Keywords: CKD, low- and middle-income countries, noncommunicable diseases, prevention
CKD is defined as the presence of functional (estimated glomerular filtration rate [GFR] <60 mL/min per 1.73 m2) or structural abnormalities of the kidney persisting beyond 3 months.1 CKD has long become, and persistently remains, an NCD of public health dimensions and importance that cannot be overstated. Although in 1990, CKD was the 27th leading global cause of death2 accounting for a mean age-standardized death rate of 11.1 per 100,000 population, it rapidly gained prominence as a global cause of death by becoming the 18th cause-specific cause of death 2 decades later. Relentlessly still, between 2007 and 2017, it remained the 16th leading cause of premature death, accounting for a mean of 359.4 years of life lost per 100,000 population globally in 2017.3 According to recently published Global Burden of Disease data, CKD accounted for as high as 369.6 per 100,000 age-standardized years of life lost in 2017. To contextualize the magnitude of this statistic, HIV/AIDS and drug-susceptible tuberculosis coinfection accounted for a maximum of 180.0 per 100,000 age-standardized years of life lost in the same year.3 These dismal figures reflect a global average of the burden of CKD, however, and it must be noted that national and regional epidemiologic differences exist; these differences reveal disproportionate ramifications of the burden of CKD, with LMICs most affected.4 The United States Renal Data System data indicate that the prevalence of CKD has remained stable in the United States in the last 2 decades, with prevalence rates of all stages of CKD being 14.8% between 2013 and 2016.5 Similarly, across Europe,6 prevalence rates of stages 3–5 of CKD have been demonstrated to be between 4.1% and 25.3%. This review discusses the peculiarities of CKD in LMICs and various preventive measures to reduce the prevalence of CKD in these regions.
CKD in LMICs
Though an NCD by its very nature, CKD occurs as a chronic complication of either other NCDs such as systemic hypertension, diabetes mellitus, or a complication of CDs such as HIV, hepatitis B, hepatitis C, and schistosomiasis. Race and ethnicity are also established nonmodifiable determinants of CKD.7 The epidemiologic transition plaguing LMICs presents a precarious position in which NCDs such as diabetes mellitus and systemic hypertension are the most frequent causes of CKD and end-stage renal disease (ESRD) in developed nations, whereas a distinctive mélange of nonmodifiable risk factors, NCDs, and CDs are at play in LMICs. Unlike in high-income countries where prevalence rates are relatively homogeneous, rates are rather heterogeneous in LMICs, varying as widely as between 5.5% in Bolivia and 29.9% in China.8 Although variability in prevalence can be explained by different sample population characteristics as well as a nonuniform definition of CKD, it also possibly reflects the differing contributions of the various CKD risk factors to disease occurrence across different countries and regions. Globally, for example, HIV-related CKD is most prevalent in Africa, with prevalence rates of 7.9%.9 Furthermore, within the African continent, the prevalence of HIV-related CKD is highest within the West African subregion at 14.6% compared with Southern Africa, which has estimated rates of 3.2%.9
CKD in LMICs tends to occur earlier and appears to be more severe. This is not unrelated to genetic and CD factors. The frequent occurrence of APOL1 renal risk variants in West Africa may be a primary reason for the disproportionately high prevalence of CKD in this region.10,11 This genetic risk has been complicated by the high prevalence of HIV in Sub-Saharan Africa, which acts as a “second hit,” initiating and facilitating the progression of CKD.12 It is possible that other CDs also act as factors modulating CKD progression in the milieu of genetic risk. Rural agricultural communities in Sri Lanka,13 India,14 and Central America15,16 have frequent occurrences of CKD of unknown etiology. The renal disadvantage of indigenes of LMICs occurs early in fetal life as maternal protein malnutrition is linked with reduced fetal nephron numbers and subsequent increased risk of CKD.17 Another peculiarity of CKD in LMICs includes the widespread use of herbal medications that cause chronic interstitial nephritis.18 Also, the high prevalence of fake and counterfeit medicines (as much as 10%–60%) in LMICs19,20 may be a significant cause of CKD though not well quantified. The relatively high incidence of hematologic disorders like sickle cell anaemia21,22 and the sickle cell trait23,24 and other hemoglobinopathies24 in West African communities may also contribute to the higher CKD prevalence and increased variability regarding CKD in LMICs. An understanding of these peculiarities of CKD in LMICs underscores the need for appropriate and targeted CKD preventive strategies at all levels (primary, secondary, and tertiary) if the current burden in LMICs is to be mitigated. Stemming the tide of CKD in these regions and its ensuing complications should thus become a global health priority. These figures underscore, in addition, the urgent call to action against CKD, especially in LMICs that bear the disproportionate burden of disease.
Prevention of CKD
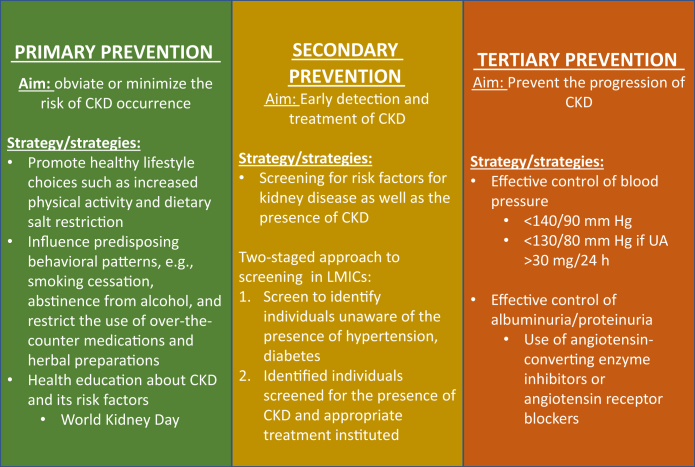
Preventive approaches to CKD and its attendant complications can be primary, secondary, or tertiary—the appropriate level at which to deploy a strategy being determined by CKD severity, that is, CKD staging (Figure 1). CKD is classified into 5 stages, 1 through 5, with the progression from stages 1 to 5 representing the worsening renal function and increasing morbidity (such as cardiovascular disease [CVD], anaemia, and mineral bone disease) and mortality. The epidemiologic spectrum of CKD can be described as an inverted pyramid in which an inordinately larger proportion of patients are represented in the earlier stages of CKD 1–3 relative to the later stages of 4 and 5.25 Additionally, the earlier stages of CKD are usually asymptomatic, with ESRD—CKD stage 5—representing the end of the spectrum. Renal replacement therapies must be instituted in ESRD; otherwise, mortality is inevitable. The disproportionately top-heavy range of disease is accounted for by the fact that patients in the earlier stages of CKD are more likely to die from the disease than progress to ESRD.
Figure 1.
Overview of chronic kidney disease (CKD) prevention strategies in low- and middle-income countries (LMICs). UA, urine albumin.
Nevertheless, both CKD and ESRD are associated with enormous health care costs as previously highlighted. Increasing the awareness of CKD risk factors before the onset of CKD in at-risk cohorts and the general population is the objective of primary preventive efforts whereas early detection and diagnosis is the objective of secondary efforts. In those already known with CKD, tertiary prevention focuses on retarding the progression of CKD; specifically, tertiary prevention modifies the natural history of CKD.
Primary Prevention of CKD
There are shared risk factors between CKD and CVD, with CKD equally being a risk factor for CVD. This makes preventive strategies for CVD quite relevant and applicable to CKD prevention, especially at the level of primary prevention. CKD primary prevention aims at reducing the incidence of CKD risk factors such as hypertension, diabetes, and smoking in the general population26 as well as lowering CKD occurrence as a complication in at-risk groups. Preventive measures to obviate the occurrence of CKD risk factors are directed at influencing predisposing behavioral patterns and include the promotion of healthy lifestyle choices, such as dietary salt restriction for hypertension prevention27; abstinence from smoking and alcohol; and increased physical activity and adoption of low-calorie, low-fat diets to reduce the risk of obesity and diabetes. A 58% reduction in the incidence of type 2 diabetes with intense lifestyle intervention over 3 years was demonstrated in the Diabetes Prevention Program28,29; the US Diabetes Prevention Program Outcomes Study similarly showed a 27% reduction over 15 years. A population strategy influences collective behavior, which in turn improves the health outcomes of a population while also improving the distribution of these outcomes within the population. As seminally highlighted by Rose,30 the determinants of the incidence or prevalence rates of disease are a product of population characteristics rather than that of individual traits. If the disease burden of CKD is to be significantly diminished, population-based strategies cannot be overemphasized. Population-focused, lifestyle-directed plans, however, affect long-term changes that are only prospectively appreciated and not instantaneously. Integration into public health policies together with long-term government support is, therefore, a necessity for this strategy.
Health education to increase awareness about CKD and its risk factors is an equally important strategy in the primary prevention of CKD at the population level. Increased awareness potentially improves health-seeking behavior and utilization of health services among individuals as well as health care providers at the primary care level. A heightened level of awareness is especially applicable in LMICs, where the knowledge of CKD and determinants is low.31 The vehicle of the World Kidney Day has been a most useful tool for raising and spreading awareness about CKD, including screening for CKD and its risk factors in LMICs. In LMICs, the screening programs associated with the World Kidney Day may be the only opportunity to have a free assessment of renal function as many cannot afford the cost of screening or the basic screening tests are not routinely available in these regions. Governments, nongovernmental organizations, and private entities could use the World Kidney Day vehicle for early identification of CKD and institution of adequate treatment modalities to prevent CKD progression even in difficult-to-reach and underserved communities. We suggest a World Kidney Day theme that focuses on the rural populace in LMICs, which would encourage channeling of funds to these disadvantaged populations.
Secondary Prevention of CKD
Early detection and treatment of CKD have been demonstrated to impact positively on the morbidity and mortality outcomes and constitute the cornerstone of secondary prevention in CKD. As stated earlier, the early stages of CKD are relatively asymptomatic, and individuals in the general population or individuals with predisposing high-risk conditions, especially in LMICs, are usually unaware of the presence of CKD. Screening as a preventive strategy endeavors to appropriately identify such individuals to institute early treatment. Early management of the asymptomatic stages of CKD is known to have the potential to prevent the progression of CKD. Albuminuria as an attribute of CKD is an integral component of the definition, staging, and prognostication of CKD and is consequently a standard parameter used in CKD screening. Although there is broad consensus on the utility of albuminuria screening of at-risk groups for CKD, no adequate evidence is available to justify screening of the general population, the primary reason being the low yield of treatable cases with albuminuria-based screening, especially in young adults. The average age of onset of CKD in LMICs, however, is the fourth decade of life,8 and this reasoning may not hold in this region. An argument against population-based screening strategies may hold in high-income countries but not in LMICs, where the level of awareness of CKD and its risk factors is low and thus remains undiagnosed in the general population. In a community screen of semiurban and rural people, the prevalence of CKD was 11.4%,32 whereas the age- and gender-adjusted prevalence rate of hypertension was 26.1%. In a community-based CKD screening program in Dharan, Nepal, the prevalence rate of hypertension was 38.7%33 in the study population with a mean age of 42.9±14.9 years; in approximately 1 in 2 individuals, hypertension was newly diagnosed, and only 51% of patients with known hypertension were receiving treatment. Data by Ulasi et al.34 show even more staggering figures from a community CKD screening in an urban area of Nigeria in which 7 of 10 individuals who received a diagnosis of hypertension were previously unaware of their condition. Population or community screening is needed in these regions and would require customization to increase the yield of positive cases, minimize screening-related costs, and avoid unwarranted treatment of false positives. A 2-staged screening strategy is proposed, one in which there is a first screening to identify individuals with previously unknown CKD risk factors, with those duly identified then getting screened for CKD. Kengne et al.35 had in an earlier review proposed the use of risk scores and questionnaires to identify CKD risk and subsequent biochemical testing such as estimated GFR and albuminuria determination alone or in combination.
Tertiary Prevention of CKD
CKD is a progressive disease, with increasing severity being associated with increased risks of CVD, CVD-related mortality, and all-cause mortality in both the general and at-risk population.36 Matsushita et al.37 demonstrated that a GFR of 15–29 ml/min per 1.73 m2 with clinically significant albuminuria (albumin-to-creatinine ratio 30–299 mg/g) was associated with a 300% increase in the hazard of all-cause mortality relative to a GFR of 90–104 ml/min per 1.73 m2 in a general population cohort.38 This same trend obtains among high-risk cohorts. Relative to an estimated GFR of 75–89 ml/min per 1.73 m2, the hazard for all-cause mortality in CKD stage 4 (estimated GFR 15-29 ml/min per 1.73 m2) was 300% the hazard for CKD stage 2 (estimated GFR 60–89 ml/min per 1.73 m2). Tertiary prevention in CKD, therefore, aims at retarding the progression of CKD along its continuum of worsening renal function, which in turn reduces CVD and mortality. Two important modifiable risk factors for CKD progression are hypertension and proteinuria,39,40 and their effective treatment should be the target of tertiary prevention. Meta-analytic data41 show that intensive control of blood pressure (BP) reduces the composite outcome of renal failure by 17% (hazard ratio 0.82, 95% confidence interval 0.68–0.98) and progression to ESRD by 18% (hazard ratio 0.79, 95% confidence interval 0.67–0.93). The Kidney Disease: Improving Global Outcomes guideline for BP management in CKD recommends targeting systolic BP <140 mm Hg and diastolic BP <90 mm Hg in both diabetic and nondiabetic CKD patients irrespective of CKD stage. Even lower targets of systolic BP <130 mm Hg and diastolic BP <80 mm Hg are recommended in all CKD patients with clinically significant urine albumin levels, that is, >30 mg per 24 hours. Blood pressure reduction with antihypertensives such as angiotensin-converting enzyme inhibitors, angiotensin aldosterone receptor blockers, non-dihydropyridine calcium channel blockers such as verapamil, or diltiazem is pervasively used clinically. There are, however, no firm recommendations for their use as the antihypertensives of choice for BP control over other agents when albuminuria is between 30 and 300 mg per 24 hours,42 as the evidence for their supposedly superior roles in this cohort come from observational data and subgroup and post hoc data analysis of clinical trials.43, 44, 45, 46, 47 Indeed, the Kidney Disease: Improving Global Outcomes guideline only makes a grade level 2D suggestion for their use in this scenario. The evidence is, however, more robust for the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for BP control over other agents when urine albumin is >300 mg per 24 hours in both diabetic and nondiabetic CKD, with Kidney Disease: Improving Global Outcomes recommending their use for BP control in patients when urine albumin >300 mg per 24 hours.48, 49, 50, 51
The reduction of proteinuria as a strategy in retarding CKD progression is closely linked with BP control as there are shared pathophysiologic mechanisms52 and therapeutic agents, that is, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Agents such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have dual pharmacologic capabilities—BP and albuminuria reduction. These unique abilities are possible because of their actions on both systemic and intrarenal renin–angiotensin–aldosterone system, which result in the lowering of not only systemic hypertension but also intraglomerular hypertension and glomerular hyperfiltration—pathophysiologic processes that are central to the occurrence of proteinuria.53 In the Ramipril Efficacy in Nephropathy study,54,55 investigators explored the effect of Ramipril with standard therapy versus placebo with standard treatment in patients with nondiabetic, proteinuric CKD and found Ramipril to significantly slow the decline in GFR (0.53 ml/min [standard error 0.08] vs 0.88 ml/min [standard error 0.13]; P = 0.03). This effect remained statistically significant even after adjusting for BP control. Follow-up data of the subsets with proteinuria between 1–3 g per 24 hours and >3 g per 24 hours showed a continued deceleration in the rate of GFR decline and progression to ESRD.48 Among patients with non-nephrotic proteinuria, lower baseline GFR, and higher baseline urine protein levels, a more significant effect of Ramipril on renal endpoints was evident.48 The GISEN (Gruppo Italiano di Studi Epidemiologici in Nefrologia) investigators further have been able to also prove the independent role of proteinuria reduction, apart from BP control, in slowing CKD progression and improving outcomes.39
The scarce availability of resources, reduced availability and access to dialytic therapies, few to nonexistent kidney transplantation programs, and competing health care–related costs from CDs and other NCDs, emphasize the opinion that promoting tertiary prevention of CKD over primary and secondary level prevention in LMICs may not have the very much desired impact of appreciably changing the epidemiology of CKD in these countries. An understanding of the double-barreled contribution of CDs and NCDs to CKD occurrence underscores the need for appropriate and targeted CKD preventive strategies, as one-size-fits-all preventive approaches may not be of value across the board; prevention programs that have been successfully deployed in high-income countries may not succeed in LMICs. Given the more enormous economic and population health ramifications of CKD and its complications in LMICs, preventive strategies ought to have a more public health and policy thrust.
Hindrances to CKD Prevention in LMICs
Poor Funding of Health Care
Many LMICs have a median health expenditure per capita per annum of between US$100 and US$400 compared with US$2000 in high-income countries.56 This is despite having only 20% of the world population living in high-income countries. The competition for the scarce resources is worsened by a double burden of disease caused by the growing epidemiologic transition in LMICs. Policymakers in LMICs must make difficult decisions about the allocation of resources between infectious diseases killing the young and under-5 population and chronic NCDs like CKD, which affect mostly adults and the elderly. The lack of funds will, for a long time, impede progress in CKD prevention and early detection.
Weak Health Systems
In many LMICs, especially Sub-Saharan Africa, the health systems have been weakened by lack of human resources and skilled workforce, lack of facilities, and a nonsustainable structure. Many LMICs have a dysfunctional primary health care system, which is pivotal for the success of the earlier-discussed screening programs. The nonfunctionality of the primary health systems makes the follow-up of individuals with early CKD extremely difficult. One of the primary pillars of primary health care is the encouragement of an integrated approach to health care instead of running parallel programs for specific disease entities. This approach would seem to work very well in LMICs where resources are scarce and there is a double burden of CDs and NCDs.
An example is the integration of diabetes and hypertension care in existing HIV clinic systems in Cambodia.57 Another vital principle of primary health care is community participation.58 Health promotion activities that ensure primary prevention of CKD may be more easily implemented in LMICs where there is still communal living, especially in the rural communities. One of the guiding principles for screening programs is that case finding should be a continuous process and not a “once and for all” project59 as it is being done in many LMICs for CKD. A functional primary health system will ensure that screening the members of the community is a continuous process. Governments of LMICs need to get their primary health care system to function well if there is any hope of tackling the surge of NCDs including CKD.
Cost of Screening Programs
A significant concern about the success of screening programs is the question of who pays for the screening. The Screening and Early Evaluation of Kidney Disease (SEEK) program in Thailand60 was partly funded by the government and a big pharmaceutical company. A pharmaceutical company funded a similar screening program in India.61 Similar large community-based screening programs for CKD are sparse in Sub-Saharan Africa. Out-of-pocket expenditure for CKD screening services will most likely discourage inhabitants of the rural areas of LMICs.
Moreover, there has been little data in LMICs showing the cost-effectiveness of community-based screening programs,34 which makes it difficult for health policy makers in these regions to take well-informed decisions about paying for CKD screening programs. Health policy makers in LMICs should provide funds for these screening programs, especially for the at-risk population. Multinational corporations operating in LMICs should be encouraged to sponsor screening programs as part of their corporate social responsibility.
Lack of Locally Relevant Data
Many LMICs, especially in sub-Saharan Africa, do not have local data that document the burden of CKD and its traditional risk factors. This limitation makes planning for prevention and treatment programs difficult. The heterogeneity of the different racial groups in LMICs requires local data in various ethnic and language groups. Also, the different GFR estimating equations may have different sensitivities across the various ethnic groups and CKD etiologies, leading to possible CKD classification errors. Local research is therefore vital, not just for the documentation of the prevalence of CKD and its risk factors but also in generating knowledge regarding attitudes and practices that would facilitate the implementation of CKD prevention and treatment programs in LMICs.
Lack of Skilled Workforce
Globally, there is a shortage of skilled nephrology personnel, which is more acute in the developing world.61 This shortage makes secondary and tertiary prevention programs challenging to see through as the number of persons with CKD requiring prevention of progression may overwhelm the available workforce. The various intervention programs of the International Society of Nephrology, including short- and long-term fellowships and educational ambassador programs, have helped train the different cadre of the nephrology workforce in the LMICs, but there is still a considerable gap that could be filled by long-term training programs instituted by health policy makers in various LMICs. As part of the effective use of health care personnel in LMICs for the prevention of CKD, task-shifting may be most useful in bridging the wide gap caused by a paucity of skilled nephrology workforce.62 LMICs can leverage on the various International Society of Nephrology educational programs to train non-nephrology workforce personnel to acquire basic laboratory skills necessary for the various screening programs. Also, with the improvement in penetration of mobile phone and telemedicine systems in LMICs, remote provision of educational resources and early intervention become increasingly possible.
Conclusion
The scourge of CKD in LMICs is likely to increase in severity and possibly weaken further the already faulty health systems in these countries if governments, nongovernmental organizations, and individuals do not evolve cost-effective and functional programs to enhance early identification and prevention of CKD progression.
Disclosure
All the authors declared no competing interests.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Roth G.A., Abate D., Abate K.H. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanifer J.W., Muiru A., Jafar T.H., Patel U.D. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31:868–874. doi: 10.1093/ndt/gfv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Renal Data System . USRDS Coordinating Center; Minneapolis, MN: 2018. 2018 Annual Data Report. [Google Scholar]
- 6.Brück K., Stel V.S., Gambaro G. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman B.I., Divers J., Palmer N.D. Population ancestry and genetic risk for diabetes and kidney, cardiovascular, and bone disease: modifiable environmental factors may produce the cures. Am J Kidney Dis. 2013;62:1165–1175. doi: 10.1053/j.ajkd.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ene-Iordache B., Perico N., Bikbov B. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Global Health. 2016;4:e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 9.Ekrikpo U.E., Kengne A.P., Bello A.K. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limou S., Nelson G.W., Kopp J.B., Winkler C.A. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulasi I.I., Tzur S., Wasser W.G. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract. 2013;123:123–128. doi: 10.1159/000353223. [DOI] [PubMed] [Google Scholar]
- 12.Kasembeli A.N., Duarte R., Ramsay M. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26:2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmon J.H., Elledge M.F., Womack D.S. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka—lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:125. doi: 10.1186/1471-2369-15-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguli A. Uddanam nephropathy/regional nephropathy in India: preliminary findings and a plea for further research. Am J Kidney Dis. 2016;68:344–348. doi: 10.1053/j.ajkd.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Ramírez-Rubio O., Amador J.J., Kaufman J.S. Urine biomarkers of kidney injury among adolescents in Nicaragua, a region affected by an epidemic of chronic kidney disease of unknown aetiology. Nephrol Dial Transplant. 2015;31:424–432. doi: 10.1093/ndt/gfv292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almaguer M., Herrera R., Orantes C.M. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16:9–15. doi: 10.37757/MR2014.V16.N2.3. [DOI] [PubMed] [Google Scholar]
- 17.Barker D.J., Osmond C., Kajantie E., Eriksson J.G. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–458. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- 18.Stanifer J.W., Lunyera J., Boyd D. Traditional medicine practices among community members with chronic kidney disease in northern Tanzania: an ethnomedical survey. BMC Nephrol. 2015;16:170. doi: 10.1186/s12882-015-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston A., Holt D.W. Substandard drugs: a potential crisis for public health. Br J Clin Pharmacol. 2014;78:218–243. doi: 10.1111/bcp.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackey T.K., Liang B.A., York P., Kubic T. Counterfeit drug penetration into global legitimate medicine supply chains: a global assessment. Am J Trop Med Hyg. 2015;92(6 suppl):59–67. doi: 10.4269/ajtmh.14-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdu A., Emokpae M., Uadia P., Kuliya-Gwarzo A. Proteinuria among adult sickle cell anemia patients in Nigeria. Ann Afr Med. 2011;10:34–37. doi: 10.4103/1596-3519.76578. [DOI] [PubMed] [Google Scholar]
- 22.Arogundade F., Sanusi A., Hassan M. An appraisal of kidney dysfunction and its risk factors in patients with sickle cell disease. Nephron Clin Pract. 2011;118:c225–c231. doi: 10.1159/000321138. [DOI] [PubMed] [Google Scholar]
- 23.Naik R.P., Derebail V.K., Grams M.E. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312:2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derebail V.K., Nachman P.H., Key N.S. High prevalence of sickle cell trait in African Americans with ESRD. J Am Soc Nephrol. 2010;21:413–417. doi: 10.1681/ASN.2009070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 26.Levey A.S., Schoolwerth A.C., Burrows N.R. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53:522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Chobanian A.V., Bakris G.L., Black H.R. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.Knowler W.C., Barrett-Connor E., Fowler S.E., Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427–432. doi: 10.1093/ije/30.3.427. [DOI] [PubMed] [Google Scholar]
- 31.Obrador G.T., García-García G., Villa A.R. Prevalence of chronic kidney disease in the Kidney Early Evaluation Program (KEEP) Mexico and comparison with KEEP US. Kidney Int. 2010;77:S2–S8. doi: 10.1038/ki.2009.540. [DOI] [PubMed] [Google Scholar]
- 32.Ulasi I.I., Ijoma C.K., Onodugo O.D. Towards prevention of chronic kidney disease in Nigeria: a community-based study in Southeast Nigeria. Kidney Int Suppl. 2013;3:195–201. [Google Scholar]
- 33.Sharma S.K., Dhakal S., Thapa L. Community-based screening for chronic kidney disease, hypertension and diabetes in Dharan. JNMA J Nepal Med Assoc. 2013;52:205–212. [PubMed] [Google Scholar]
- 34.Ulasi I.I., Ijoma C.K., Onwubere B.J. High prevalence and low awareness of hypertension in a market population in Enugu, Nigeria. Int J Hypertens. 2011;2011:869676. doi: 10.4061/2011/869675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George C., Mogueo A., Okpechi I. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Global Health. 2017;2 doi: 10.1136/bmjgh-2016-000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 37.Consortium CKDP Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Der Velde M., Matsushita K., Coresh J. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 39.Ruggenenti P., Perna A., Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int. 2003;63:2254–2261. doi: 10.1046/j.1523-1755.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 40.Peterson J.C., Adler S., Burkart J.M. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Lv J., Ehteshami P., Sarnak M.J. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maki D.D., Ma J.Z., Louis T.A., Kasiske B.L. Long-term effects of antihypertensive agents on proteinuria and renal function. Arch Intern Med. 1995;155:1073–1080. [PubMed] [Google Scholar]
- 43.Ruggenenti P., Gaspari F., Perna A., Remuzzi G. Cross sectional longitudinal study of spot morning urine protein: creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ. 1998;316:504–509. doi: 10.1136/bmj.316.7130.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugliese G., Solini A., Fondelli C. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transplant. 2011;26:3950–3954. doi: 10.1093/ndt/gfr140. [DOI] [PubMed] [Google Scholar]
- 45.Newman D.J., Pugia M.J., Lott J.A. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta. 2000;294:139–155. doi: 10.1016/s0009-8981(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 46.Howey J., Browning M., Fraser C. Selecting the optimum specimen for assessing slight albuminuria, and a strategy for clinical investigation: novel uses of data on biological variation. Clin Chem. 1987;33:2034–2038. [PubMed] [Google Scholar]
- 47.Carter J.L., Tomson C.R., Stevens P.E., Lamb E.J. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant. 2006;21:3031–3037. doi: 10.1093/ndt/gfl373. [DOI] [PubMed] [Google Scholar]
- 48.Ruggenenti P., Perna A., Gherardi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 49.Panek R., Lawen T., Kiberd B.A. Screening for proteinuria in kidney transplant recipients. Nephrol Dial Transplant. 2010;26:1385–1387. doi: 10.1093/ndt/gfq503. [DOI] [PubMed] [Google Scholar]
- 50.Thakar C.V., Worley S., Arrigain S. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 51.Kshirsagar A.V., Bang H., Bomback A.S. A simple algorithm to predict incident kidney disease. Arch Intern Med. 2008;168:2466–2473. doi: 10.1001/archinte.168.22.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remuzzi G., Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 53.Remuzzi G., Perico N., Macia M., Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. 2005;68:S57–S65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 54.Ruggenenti P., Perna A., Gherardi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Lancet. 1998;352:1252–1256. doi: 10.1016/s0140-6736(98)04433-x. [DOI] [PubMed] [Google Scholar]
- 55.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 56.World Health Organization . World Health Organization; Geneva: 2018. Public Spending on Health: A Closer Look at Global Trends. [Google Scholar]
- 57.Kheang S, Janssens B, Damme W, Zachariah R, eds. Delivering anti-retroviral treatment within the framework of a chronic diseases clinic, MSF’s experience in Cambodia. Paper presented at: International Conference on AIDS; 2004.
- 58.Demaio A.R., Nielsen K.K., Tersbøl B.P. Primary Health Care: a strategic framework for the prevention and control of chronic non-communicable disease. Global Health Action. 2014;7:24504. doi: 10.3402/gha.v7.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson J.M.G., Jungner G., World Health Organization . World Health Organization; Geneva: 1968. Principles and Practice of Screening for Disease. [Google Scholar]
- 60.Ingsathit A., Thakkinstian A., Chaiprasert A. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant. 2009;25:1567–1575. doi: 10.1093/ndt/gfp669. [DOI] [PubMed] [Google Scholar]
- 61.Osman M.A., Alrukhaimi M., Ashuntantang G.E. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl. 2018;8:52–63. doi: 10.1016/j.kisu.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okpechi IG, Bello AK, Ameh OI, Swanepoel CR, eds. Integration of care in management of CKD in resource-limited settings. Semin Nephrol. 2017;37:260–272. [DOI] [PubMed]