Abstract
Introduction
Patients with phospholipase A2 receptor (PLA2R)–associated membranous nephropathy and stage 4 or 5 chronic kidney disease are at high risk of end-stage kidney disease. In recent years, rituximab (RTX) emerged as a safe and efficient treatment for patients with PLA2R-associated membranous nephropathy. Whether its use is also appropriate in patients with an estimated glomerular filtration rate <30 ml/min per 1.73 m2 has not been investigated.
Methods
We retrospectively reviewed characteristics and outcome of 13 patients with PLA2R-associated membranous nephropathy and stage 4 or 5 chronic kidney disease who received a total of 14 consecutive RTX treatments from January 2012 to March 2018. The treatment regimen consisted of either 2 weekly infusions of 375 mg/m2 or 2 RTX infusions of 1 g/d two weeks apart. When needed, the regimen was repeated to achieve immunological remission.
Results
The mean estimated glomerular filtration rate, serum albumin level, and urinary protein level at the first RTX infusion were 18 ± 7 ml/min per 1.73 m2, 25.2 ± 5.4 g/l, and 13.2 ± 7.5 g/d, respectively, with all patients being tested positive for serum PLA2R antibodies. Ten treatment courses led to an increase in estimated glomerular filtration rate and remission of nephrotic syndrome after a median follow-up of 40.8 months (interquartile range, 14.8–46.8). Conversely, 4 RTX treatments were unsuccessful, with patients requiring chronic hemodialysis within 1 year. The urinary albumin-to-protein ratio before treatment was predictive of renal response. Immunological remission occurred after 11 treatment courses and was associated with clinical response in 10 of 11 patients. Three patients experienced severe adverse events.
Conclusion
RTX seems effective and reasonably safe in PLA2R-associated membranous nephropathy with stage 4 or 5 chronic kidney disease. Immunological remission is associated with a good clinical outcome.
Keywords: CKD, ESKD, immunosuppressive treatment, membranous nephropathy, PLA2R, rituximab
Graphical abstract
Membranous nephropathy (MN) is one of the leading causes of nephrotic syndrome (NS) in adults. Primary MN is associated with a significant risk of developing end-stage kidney disease.1 Until recently, the urinary protein level was the main biomarker to assess severity and guide therapeutic indications to prevent the development of kidney failure, while it remains to date the primary criterion of remission definitions.2,3
A giant leap in the understanding of the pathophysiological mechanisms of primary MN was made in 2009 with the discovery of autoantibodies directed against a podocyte surface antigen, the M-type phospholipase A2 receptor (PLA2R), found in 70% of adult patients with primary MN. After binding to a conformational epitope of PLA2R, those antibodies (Abs) induce characteristic in situ immune complex deposits.4 Accumulating evidence suggests that high titers of anti-PLA2R antibodies (PLA2R Abs) are correlated with clinical evolution, response to treatment, and renal survival.5, 6, 7, 8, 9 Therefore, agents that specifically interfere with B-cell Ab production are the first step toward selective therapy for primary MN. Several retrospective studies and the 2 randomized controlled trials showed that rituximab (RTX) efficiently and safely induced PLA2R Ab depletion and that the decrease in PLA2R Ab titer preceded remission of proteinuria by several months,10,11 suggesting Ab depletion as the first therapeutic target.12
The use of immunosuppressive therapies, including alkylating agent-corticosteroid combination, calcineurin inhibitors, or RTX, is widely recognized as beneficial in selected patients, that is, high-risk patients with NS and either no improvement over a 6-month period of antiproteinuric therapy, life-threatening symptoms, or progressive kidney failure.3 Nonetheless, latest treatment algorithms, in line with 2012 Kidney Disease: Improving Global Outcomes guidelines, do not suggest using such treatments in patients with an estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73 m2, because of a potential reversal of the risk-benefit balance resulting from both poor efficiency and higher toxicity.12
Moreover, despite the lack of demonstrated impact of the eGFR level on RTX pharmacokinetics and tolerance, patients with an eGFR <30 ml/min per 1.73 m2 were excluded from the 2 RTX-based randomized controlled trials (eGFR ≥ 45 ml/min per 1.73 m2 in the GEMRITUX trial and ≥ 40 ml/min per 1.73 m2 in the MEmbranous Nephropathy Trial Of Rituximab [MENTOR]), and conflicting data exist on whether efficacy could be preserved in altered kidney function.13, 14, 15
In the present study, we analyzed the efficacy and tolerance of RTX in a cohort of 13 consecutive patients presenting with PLA2R MN and receiving therapy at stage 4 or 5 chronic kidney disease (CKD).
Methods
Patients and Study Design
We retrospectively identified 13 consecutive patients treated with RTX for PLA2R MN and an eGFR <30 ml/min per 1.73 m2 from January 2012 to February 2019. Diagnosis of PLA2R MN was based on histopathological criteria, or positive PLA2R Ab testing when kidney biopsy was contraindicated. Twelve patients were screened at the Nephrology and Dialysis Department of Tenon Hospital, Paris, France, and 1 patient at the Nephrology Department of Saint-Luc Academic Hospital, Brussels, Belgium. Eight patients received RTX for the initial flare, 4 patients were treated for relapse, and 1 for both the initial flare and relapse for a total of 14 treatment courses. Previous treatments, for example, renin-angiotensin system blockade or immunosuppressive therapies, were not regarded as study criteria. The treatment regimen consisted of either 2 weekly RTX doses of 375 mg/m2 or 2 RTX infusions of 1 g/d two weeks apart. Treatment was repeated if needed to achieve PLA2R Ab complete depletion.
Patients’ clinical and biological data at diagnosis, at RTX initiation, and at last follow-up (i.e., last evaluation, last day before hemodialysis, or last follow-up before relapse, as appropriate) were retrospectively recorded. The glomerular filtration rate was estimated using the Modification of Diet in Renal Disease equation as the standardized serum creatinine method was not available for all patients. Serum PLA2R Abs were measured by enzyme-linked immunosorbent assay, using a 14 relative units (RU)/ml positivity threshold, and by indirect immunofluorescence assay (both tests developed by EUROIMMUN AG, Lübeck, Germany). Features suggestive of chronic kidney injury were reviewed from the patient’s records and included histological data (i.e., glomerular sclerosis and tubulointerstitial fibrosis), kidney size, and urinary protein composition (i.e., urinary albumin-to-protein ratio, IgG-to-creatinine ratio, alpha-1 microglobulin-to-creatinine ratio, retinol binding protein-to-creatinine ratio).
Outcomes after RTX treatment were defined as follows: responders referred to patients with partial remission or complete remission according to Kidney Disease: Improving Global Outcomes guidelines (i.e., urinary protein level between 0.3 and 3.5 g/d with a decrease by at least 50% from the initial value and <0.3 g/d, respectively) and stabilization or improvement in eGFR3; nonresponders referred to patients with no complete remission or partial remission and a decline in eGFR. Relapse was defined by the reappearance of proteinuria and circulating PLA2R Abs after partial remission/complete remission and PLA2R Abs negativation. Immunological remission was defined by negative tests for circulating PLA2R Ab detection.
Each patient was fully informed of risks and benefits of the use of RTX at low eGFR.
Statistical Analysis
Normality was confirmed using the D’Agostino-Pearson and Shapiro-Wilk normality tests, allowing the comparison of means using 2-tailed paired t tests and the comparison of odds ratios using the 2-sided Fisher exact test. All statistics were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, La Jolla, CA). Normally distributed variables are expressed as means ± SD, and nonnormally distributed variables are expressed as median (interquartile range [IQR]).
Results
Patients’ Characteristics at Diagnosis and at RTX Infusion
Baseline characteristics at diagnosis are reported in Table 1. The mean age was 60.7 ± 9.7 years, and the male/female ratio was 2.2. The mean serum creatinine level was 165 ± 90 μmol/l, and the mean eGFR was 48 ± 24 ml/min per 1.73 m2, with 4 patients having an eGFR between 15 and 30 ml/min per 1.73 m2. The mean urinary protein level was 7.9 ± 5.1 g/d and serum albumin level 21.9 ± 6.7 g/l, and the 10 patients tested at diagnosis had circulating PLA2R Abs detected by either enzyme-linked immunosorbent assay, indirect immunofluorescence assay, or both. Three patients were not tested for circulating PLA2R Abs at diagnosis but were serologically positive during follow-up.
Table 1.
Patients’ characteristics at diagnosis, RTX initiation, and last follow-up
| S. no. | Sex | Age (yr) | Histology |
Morphology |
sCreat level (μmol/l) | eGFR (ml/min per 1.73 m2) | uProt level (g/d) | sAlb level (g/l) | PLA2R Abs level |
Time (mo) |
Previous IS treatmenta | RTX retreatment | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S Glom (%) | IFTA (%) | Time (mo) : Bx to RTX | Kidney size (left/right; cm) | Time (mo) : imaging to RTX | ELISA (RU/ml) | IIFT | Diagn. to RTX | RTX to last FU | ||||||||||||||||||||||
| 1b,c | M | 63 | NA | 40 | 104 | 11.2/9.4 | 2 | 103 | 996 | 1112 | 67 | 5 | 4 | 13 | 17 | 6.8 | 16 | 24 | 34 | 718 | 85 | Neg | 1/1000 | 1/100 | Neg | 104.4 (1.5d) | 5.1 | 1 | ||
| 2e | F | 58 | – | – | – | NA | – | 160 | 340 | 218 | 30 | 13 | 20 | 14 | 11 | 0.9 | 25 | 30 | 39 | NA | 84 | Neg | NA | 1/200 | Neg | 35.4 | 13.9 | 1 | ||
| 3c | F | 66 | 0 | 33 | 18 | 11.2/11.2 | 1 | 128 | 202 | 603 | 47 | 27 | 7 | 7 | 12 | 11 | 20 | 26 | 30 | NA | 602 | Neg | 1/100 | 1/1000 | 1/50 | 18.4 | 11.9 | RTX | 4 | |
| 4e | M | 65 | – | – | – | x/13.2 | 26 | 180 | 258 | 205 | 35 | 23 | 28 | 12 | 12 | 0.6 | 16 | 29 | 41 | NA | 97 | Neg | 1/1000 | 1/500 | Neg | 36.6 | 20.6 | 1 | ||
| 5e | F | 62 | – | – | – | 9.0/x | 1 | 370 | 408 | 201 | 11 | 10 | 21 | 6 | 25 | 0.7 | 17 | 19 | 40 | NA | NA | Neg | 1/200 | 1/500 | Neg | 2.0 | 52.2 | 0 | ||
| 6 | M | 73 | 18 | 20 | 4 | 9.3/9.6 | 4 | 290 | 250 | 338 | 19 | 21 | 15 | 19 | 9 | 0.9 | 23 | 29 | 39 | 36 | 34 | Neg | 1/100 | 1/100 | Neg | 3.6 | 41.2 | 0 | ||
| 6b,f | 18 | 20 | 51 | NA | – | 310 | 300 | 18 | 18 | 2 | 1.7 | 34 | 39 | 34 | Neg | 1/50 | Neg | 47.0 (3.0d) | 15.1 | RTX | 0 | |||||||||
| 7b,g | M | 59 | 0 | 10 | 46 | NA | – | 105 | 460 | 185 | 67 | 11 | 34 | 5 | 16 | 0.5 | 26 | 19 | 38 | NA | 130 | Neg | NA | 1/500 | Neg | 45.9 (12.0d ) | 42.8 | Cy-Cs | 0 | |
| 8b | M | 34 | 0 | 10 | 128 | 10.0/10.0 | 1 | 130 | 321 | 140 | 56 | 18 | 47 | 2 | 7 | 0.3 | 14 | 29 | 38 | 270 | NA | Neg | 1/1000 | 1/100 | Neg | 128.0 (7.0d ) | 40.4 | RTX; C | 0 | |
| 9b | M | 58 | 9 | 10 | 45 | 10.4/10.8 | 30 | 100 | 230 | 146 | 71 | 27 | 43 | 4 | 14 | 0.3 | 15 | 22 | 37 | NA | 81 | Neg | 1/1000 | 1/1000 | Neg | 45.0 (8.45d) | 83.1 | RTX; C | 0 | |
| 10c | M | 61 | 0 | 0 | 17 | 10.9/11.3 | 3 | 85 | 415 | 459 | 84 | 14 | 11 | 4 | 28 | 31 | 33 | 17 | 12 | NA | NA | NA | 1/1000 | 1/1000 | 1/500 | 17.1 | 4.8 | Cy-Cs; C | 1 | |
| 11c | F | 72 | 11 | 5 | 9 | 10.3/11.0 | 1 | 120 | 366 | 453 | 41 | 11 | 8 | 3 | 20 | 6 | 21 | 19 | 9 | NA | NA | NA | NA | 1/500 | 1/500 | 9.0 | 8.3 | 1 | ||
| 12 | M | 64 | 23 | 20 | 6 | 10.3/11.0 | 6 | 273 | 270 | 231 | 20 | 22 | 26 | 8 | 8 | 0.2 | 24 | 25 | 41 | NA | NA | Neg | 1/200 | 1/200 | Neg | 6.2 | 45.0 | 0 | ||
| 13b | M | 54 | 23 | 10 | 348 | 10.0/10.3 | 2 | 97 | 186 | 155 | 74 | 29 | 35 | 6 | 4 | 0.5 | 35 | 31 | 43 | NA | 52 | Neg | NA | NA | NA | 348.0 (13.0d) | 11.0 | 0 | ||
Abs, antibodies; Bx, biopsy; C, cyclosporine; Cy-Cs, cyclophosphamide-corticosteroid combination therapy; Diagn., diagnosis; eGFR, estimated glomerular filtration rate (according to the Modification of Diet in Renal Disease formula); ELISA, enzyme-linked immunosorbent assay; F, female; FU, follow-up; IFTA, interstitial fibrosis and tubular atrophy; IIFT, indirect immunofluorescence testing; IS, immunosuppressive; M, male; NA, not available; Neg, negative; PLA2R, phospholipase A2 receptor; RTX, rituximab; sAlb, serum albumin; sCreat, serum creatinine; S Glom, sclerotic glomeruli; uProt, urinary protein; x, absent or severely atrophic kidney.
Age is reported at diagnosis; sCreat, eGFR, uProt, sAlb, and PLA2R Ab levels are reported at diagnosis, rituximab, and last follow-up in each parameter column.
A detailed description of previous treatments is provided in Supplementary Figure S1.
Patients treated for relapse.
Definitive hemodialysis.
Delay from relapse.
Insufficient or no histological data (small sample size in patient 2 and serology-based diagnosis in patients 4 and 5 owing to solitary kidney).
Patient treated twice with rituximab (see Supplementary Figure S1).
Transient hemodialysis (started 5 mo after rituximab treatment and discontinued after 4 mo).
Histological features indicative of chronicity, that is, glomerular sclerosis and tubulointerstitial fibrosis, respectively, involved mean rates of 10% ± 10% and 16% ± 12% of the kidney biopsy sample at diagnosis. The median delay between kidney biopsy and the first RTX infusion was 17.1 months (IQR, 8.45–45.9 months). Renal imaging, performed in a median time of 2.0 months (IQR, 1.0–5.0 months) before RTX therapy, showed a mean kidney size of 10.5 ± 0.9 cm.
As depicted in Supplementary Figure S1, 6 patients previously received immunosuppressive treatments. In those with previous RTX treatment experience, the time interval since the last dose was at least 16 months.
Patients’ characteristics at RTX initiation are detailed in Table 1. The estimated GFR was below 30 ml/min per 1.73 m2 in all patients, with a mean eGFR of 18 ± 7 ml/min per 1.73 m2. Six RTX courses (43%) were administered in patients who had reached stage 5 CKD. The median serum albumin and urinary protein levels were 25.2 ± 5.4 g/l and 13.2 ± 7.5 g/d, respectively. Serum PLA2R Abs were tested positive in all patients. RTX treatment was administered in a median delay of 12.0 months (IQR, 6.2–18.4 months) after diagnosis and of 7.0 months (IQR, 3.0–8.5 months) after relapse.
One patient (patient 6) received RTX twice, the first course at diagnosis and the second course at relapse 4 years later. Both episodes occurred with eGFR < 30 ml/min per 1.73 m2 and were separated by a period of complete clinical and immunological remission with eGFR stabilization (up to 22 ml/min per 1.73 m2). Altogether, 14 treatment courses for a total of 13 patients were recorded.
Clinical and Immunological Outcomes
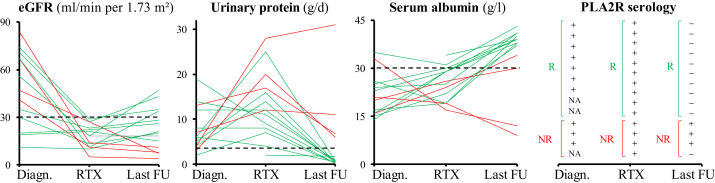
Clinical and immunological outcome after RTX infusion is detailed in Table 1 and Figures 1 and 2.
Figure 1.
Evolution of estimated glomerular filtration rate (eGFR), urinary protein level, serum albumin level, and phospholipase A2 receptor (PLA2R) antibodies at diagnosis (Diagn.), at the first rituximab (RTX) infusion, and at last follow-up (FU). Green lines represent responders (R); and red lines represent nonresponders (NR). +, patient with positive serology; −, patient with negative serology; NA, patient not tested.
Figure 2.
Differential evolution of estimated glomerular filtration rate (eGFR), urinary protein level, and serum albumin level at diagnosis (Diagn.), at the first rituximab (RTX) infusion, and at last follow-up (FU) among responders (a) and nonresponders (b). Data presented as mean ± SD. ns, P > 0.03.
Overall, over a median follow-up period of 17.8 months (IQR, 10.3–43.3 months), the mean eGFR, urinary protein level, and serum albumin level evolved from 18 ± 7 to 23 ± 13 ml/min, from 13 ± 7 to 0.8 ± 8 g/d, and from 25 ± 5 to 34 ± 11 g/l, respectively.
Ten treatment courses performed in 9 patients resulted in a renal response (responder group), while 4 (29%) patients had a progression to end-stage kidney disease (nonresponder group) within 1 year.
In the responder group, the mean eGFR increased from 19 ± 6 to 29 ± 11 ml/min per 1.73 m2 (P = 0.01), the serum albumin level increased from 26.7 ± 5.2 to 39.5 ± 1.8 g/l (P < 0.001), and the urinary protein level decreased from 10.8 ± 6.6 to 0.7 ± 0.4 g/d (P < 0.001) from RTX initiation to last follow-up (median duration, 40.8 months [IQR, 14.8–46.8 months]). Importantly, all the 10 successful treatment courses were associated with complete immunological remission. Two patients received a second course of RTX (administered at 7 and 10 months after the first dose) to reach complete immunological remission.
In the 4 nonresponders patients, the median time between RTX initiation and the start of hemodialysis was 6.7 months (IQR, 4.9–11.0 months). Three patients never reached immunological remission despite repeated RTX infusions (2 courses in 2 patients and 4 in 1 patient). The fourth patient, treated at an eGFR of 5 ml/min per 1.73 m2, reached immunological remission after RTX treatment but required chronic hemodialysis. All the nonresponders were treated with a high-dose regimen (i.e., 2 RTX infusions of 1 g/d two weeks apart).16
As shown in Table 2, at RTX initiation, the urinary protein composition was found to be predictive of renal response. Indeed, the mean urinary albumin-to-protein ratio differed significantly between nonresponders and responders (55.2% ± 3.9% and 74.3% ± 8.2%, respectively; P < 0.001) and was predictive of outcome using a 60% threshold (odds ratio, 189.0; 95% confidence interval, 3.2–11110; P = 0.001). Moreover, the urinary IgG level and urinary IgG-to-protein ratio were both significantly higher in nonresponders versus responders (157.6 ± 68.0 mg/mmol vs. 63.3 ± 40.4 mg/mmol; P = 0.01 and 11.3% ± 2.9% vs. 6.7% ± 2.8%; P = 0.02, respectively). By contrast, no significant difference was observed between the 2 groups regarding age, eGFR, serum albumin level, urinary protein level, urinary albumin level, urinary alpha-1 microglobulin level, and urinary retinol binding protein level at RTX initiation.
Table 2.
Comparison between responders and nonresponders at rituximab initiation
| Characteristic | Responders | Nonresponders | P |
|---|---|---|---|
| Age (yr) | 65.6 ± 10.2 | 68.6 ± 4.1 | 0.58 |
| eGFR (ml/min per 1.73 m2) | 19.2 ± 6.5 | 14.2 ± 9.3 | 0.27 |
| Serum albumin level (g/l) | 26.7 ± 5,2 | 21.5 ± 4.2 | 0.10 |
| Urinary protein level (g/d) | 10.8 ± 6.6 | 19.2 ± 6.7 | 0.05 |
| Urinary albumin level (g/d) | 7.1 ± 2.4 | 6.9 ± 2.3 | 0.88 |
| Urinary albumin-to-protein ratio (%) | 74.3 ± 8.2 | 55.2 ± 3.9 | <0.001 |
| Urinary IgG level (mg/mmol) | 63.3 ± 40.4 | 157.6 ± 68.0 | 0.01 |
| Urinary IgG-to-protein ratio (%) | 6.7 ± 2.8 | 11.3 ± 2.9 | 0.02 |
| Urinary α1M level (mg/mmol) | 23.0 ± 14.3 | 30.6 ± 20.3 | 0.46 |
| Urinary α1M-to-protein ratio (%) | 3.1 ± 1.8 | 2.3 ± 1.3 | 0.48 |
| Urinary RBP level (mg/mmol) | 11.1 ± 7.2 | 14.0 ± 5.6 | 0.50 |
| Urinary RBP-to-protein ratio (%) | 1.5 ± 1.5 | 1.0 ± 0.1 | 0.54 |
α1M, alpha-1 microglobulin; eGFR, estimated glomerular filtration rate (according to the Modification of Diet in Renal Disease formula); RBP, retinol binding protein.
Data presented as mean ± SD.
Immunological remission after RTX treatment was the second parameter associated with renal response (odds ratio, 49.0; 95% confidence interval, 1.6–1502; P = 0.01).
Of the 6 patients with stage 5 CKD before treatment, 3 (50%) had a progression to end-stage kidney disease, including 1 with complete immunological response. The remaining 3 patients reached complete immunological response, had improved kidney function, and were dialysis-free at last follow-up (median, 60 months [IQR, 36–65 months]). One of them required only transient hemodialysis but recovered 5 months after immunological remission.
Safety
Four severe adverse events requiring hospitalization were reported in 3 patients for a total of 52 RTX infusions. Patient 1 underwent infectious chronic obstructive pulmonary disease exacerbation 1 month after the first RTX infusion. Patient 6 presented with prostatitis 1 month after the first course of RTX and underwent severe laryngospasm and bronchospasm after the first RTX injection of the second course, warranting treatment withdrawal, but achieved immunological remission. Patient 10 presented with Clostridium difficile colitis and giardiasis 1 week after the first RTX administration. No death related to treatment was reported; 1 patient died of status epilepticus of unknown origin 1 year after the last RTX injection.
Discussion
Management of MN has been a source of controversy for decades. The course of the disease is highly heterogeneous and poorly predictable with, on the one side, spontaneous remission occurring in at least one-third of the patients and, on the other side, progression to end-stage kidney disease within 10 years in 35% of the cases.17 Therefore, except when MN is associated with life-threatening complications or rapid decline in kidney function, current international guidelines still recommend the use of conventional antiproteinuric treatments during the first 6 months of the disease to avoid potentially toxic immunosuppressive treatments in patients who may reach spontaneous remission. In the case of persistent NS after this period, guidelines recommend starting immunosuppressive therapy such as corticosteroid-cytotoxic agent combination or calcineurin inhibitors.3,18 For more than a decade, RTX emerged as an effective alternative option to those agents, as suggested in several retrospective studies19, 20, 21, 22 and further confirmed by 2 randomized controlled trials: the French trial GEMRITUX and the North-American trial MENTOR.10,11
Following Kidney Disease: Improving Global Outcomes Controversies Conference on glomerular diseases, an international consortium recently proposed that only small kidneys, and not eGFR level, should restrain the use of immunosuppressive agents.23 Indeed, as suggested by previous studies, immunosuppressive therapy, such as alkylating agent-corticosteroid regimen or cyclosporine, could still be efficient in patients with severely deteriorating kidney function, although encumbered by substantial relapse rate and serious treatment-related adverse events in half of cases.24,25
To date, there is no study evaluating the efficacy of RTX in patients with MN and an eGFR <30 ml/min per 1.73 m2, apart from 2 reported cases with stage 4 CKD.14,15
In our study, 13 consecutive patients with PLA2R MN and stage 4 or 5 CKD were treated with RTX for a total of 14 treatment courses. The characteristics of patients at diagnosis in this cohort were in accordance with the previously described classical PLA2R MN, that is, predominantly middle-aged men with initial NS and positive PLA2R serology, except that 4 patients had stage 4 CKD at diagnosis.
After RTX treatment, renal outcome was favorable in 10 cases. In all these responders, treatment induced remission of the NS, stabilization or improvement in eGFR, and disappearance of circulating PLA2R Abs. By contrast, none of the 4 nonresponders achieved NS remission, with only 1 patient reaching immunological remission. The follow-up duration was markedly shorter in nonresponders (6.7 months) owing to rapid progression to dialysis as compared with the follow-up duration of 40.8 months in responders.
High levels of low- and/or high-molecular-weight urinary proteins other than albumin were demonstrated to be predictive of interstitial fibrosis in CKD and renal prognosis in MN.26,27 In our study, the urinary albumin-to-protein ratio and urinary IgG level before RTX initiation were identified as predictive factors of renal response. Although low-molecular-weight protein excretion was found to be markedly increased in both groups, indicating chronic tubulointerstitial damage, higher levels of urinary IgG suggest more severe alterations in the glomerular filtration barrier in nonresponders, presumably indicative of irreversible glomerular scarring in those patients.
PLA2R Abs disappearance after RTX treatment was associated with clinical remission, suggesting that immunological remission (i.e., complete depletion of PLA2R Abs by both enzyme-linked immunosorbent assay and indirect immunofluorescence assay) may be a surrogate for renal survival after RTX treatment in stage 4 or 5 CKD and PLA2R MN.
The therapeutic protocol consisted of courses of RTX (either two weekly 375 mg/m2 infusion or 2 RTX infusions of 1 g/d 2 weeks apart), repeated as needed to achieve complete immunological remission. In 6 patients, RTX treatment was repeated after 3 to 24 months. We previously showed that RTX was less efficacious at 2 weekly doses of 375 mg/m2 than was cyclophosphamide/steroid combination therapy in inducing immunological remission in patients with high levels of PLA2R Abs.28 In addition, we previously reported that a second course of RTX had a dramatic effect on PLA2R Abs titers, with all but 1 patient reaching complete immunological remission and all retreated patients showing clinical remission.29 The retreatment regimen was stimulated by the GEMRITUX trial where PLA2R Abs levels were markedly decreased as early as 3 months after RTX but not followed by further decrease at 6 months, suggesting an early loss of efficacy of the monoclonal Ab. A previous study showed that RTX blood levels were lower and the B-cell depletion significantly shorter in patients with MN than in patients with rheumatoid arthritis or antineutrophil cytoplasmic antibody vasculitis.21 Urinary loss of the monoclonal Ab in the context of NS likely accounts for those changes in RTX pharmacokinetics and potentially underpowers RTX regimen commonly used in MN.
After a total of 52 RTX infusions, severe adverse events (grade 3+) included 3 infections requiring hospitalization (6% of all treatments) and 1 life-threatening infusion reaction (2%), but no death. RTX toxicity does not seem dramatically enhanced by the severity of renal dysfunction as compared to patients with preserved kidney function.10,11 In the GEMRITUX trial, which included younger patients (53 years old vs. 66 years old), 1 severe infection was reported for a total of 74 infusions (1.3%) without an infusion reaction. In the MENTOR trial, a trend toward the lower incidence of severe and serious all-cause adverse events was observed with RTX compared with cyclosporine.11 Even if the rate of severe adverse events appears higher in the population of patients with advanced CKD, RTX remains safer than cyclophosphamide-corticosteroid combination therapy with a 3- to 4-fold lower hazard rate of any kind of adverse events.30
We acknowledge some limitations due to the retrospective uncontrolled design of our study, the small number of patients, and the heterogeneity of previous therapeutic lines. Clinicopathological correlations could not be analyzed because of the large time intervals between kidney biopsy performed at diagnosis and at RTX therapy.
In conclusion, RTX therapy in PLA2R MN seems to achieve effective clinical and immunological remission in patients with severely altered kidney function, with acceptable safety. The urinary albumin-to-protein ratio appears to be predictive of renal response to treatment and may represent a valuable and readily available tool to guide therapeutic decision. Given the association of complete immunological remission and clinical remission in our study and the already known association between PLA2R serology, disease progression,5, 6, 7, 8 and posttransplant recurrence,9 we suggest that PLA2R Abs complete depletion should be a therapeutic target in MN. To allow the translation of these findings into routine clinical practice, our observations will need to be confirmed in larger prospective cohorts of patients.
Disclosure
NH reports travel grants from Fresenius Kabi and Fresenius Medical Care (outside the submitted work). KD reports consulting fees from Alexion, Amicus Therapeutics, and Boehringer Ingelheim (outside the submitted work). J-JB reports research grants from Roche (outside the submitted work). JM reports speaker honoraria from Baxter Healthcare and Fresenius Medical Care, travel grants from Sanofi Genzyme, and research grants from Baxter Healthcare and Alexion (outside the submitted work). All the other authors declared no competing interests.
Acknowledgments
An abstract of the present work has been presented as a poster at the 56th ERA-EDTA Congress, June 14, 2019, Budapest, Hungary.
Author Contributions
NH and KD contributed to study design and data collection. EE was responsible for statistical analysis. CJ provided phospholipase A2 receptor antibody measurements. NH, KD, and JM drafted the manuscript under the supervision of J-JB, EP, and PR. All authors contributed to the interpretation of the results and approved the final version of the paper.
Footnotes
Figure S1. Detailed description of previous immunosuppressive treatments.
Supplementary Material
References
- 1.Ponticelli C., Glassock R.J. Glomerular diseases: membranous nephropathy—a modern view. Clin J Am Soc Nephrol. 2014;9:609–616. doi: 10.2215/CJN.04160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieppati A., Mosconi L., Perna A. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;8:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;(suppl 2):186–197. [Google Scholar]
- 4.Beck L.H., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic MN. N Engl Med J. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggenenti P., Debiec H., Ruggiero B. Antiphospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofstra J.M., Beck L.H., Beck D.M. Anti-phospholipase A2 receptor antibodies correlate with clinical status in primary membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha E., Thiele I., Zahner G. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bech A.P., Hofstra J.M., Brenchley P.E. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;7:1386–1392. doi: 10.2215/CJN.10471013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta G., Fattah H., Ayalon R. Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin Transplant. 2016;30:461–469. doi: 10.1111/ctr.12711. [DOI] [PubMed] [Google Scholar]
- 10.Dahan K., Debiec H., Plaisier E. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fervenza F.C., Appel G.B., Barbour S.J. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 12.De Vriese A.S., Glassock R.J., Nath K.A. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggenenti P., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–748. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]
- 14.Dahan K., Gillion V., Johanet C. The role of PLA2R antibody in treatment of membranous nephropathy. Kidney Int Rep. 2018;3:498–501. doi: 10.1016/j.ekir.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X.J., Zhou F.D., Wang S.X. A case report of remission of refractory membranous nephropathy progressing to stage 4 chronic kidney disease using low-dose rituximab: a long-term follow-up. Medicine (Baltimore) 2018;97:e11184. doi: 10.1097/MD.0000000000011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seitz-Polski B., Dahan K., Debiec H. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1173–1182. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofstra J.M., Fervenza F.C., Wetzels J.F. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2013;9:443–458. doi: 10.1038/nrneph.2013.125. [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi G., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 20.Cravedi P., Ruggenenti P., Sghirlanzoni M.C. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 21.Ruggenenti P., Cravedi P., Chianca A. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fervenza F.C., Cosio F.G., Erickson S.B. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 23.Floege J., Barbour S.J., Cattran D.C. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 24.du Buf-Vereijken P.W., Branten A.J., Wetzels J.F., Membranous Nephropathy Study Group Cytotoxic therapy for membranous nephropathy and renal insufficiency: improved renal survival but high relapse rate. Nephrol Dial Transplant. 2004;19:1142–1148. doi: 10.1093/ndt/gfh036. [DOI] [PubMed] [Google Scholar]
- 25.Cattran D.C., Greenwood C., Ritchie S., et al, Canadian Glomerulonephritis Study Group A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 26.Pallet N., Chauvet S., Chassé J.F. Urinary retinol binding protein is a marker of extent of interstitial kidney fibrosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Brand J., Hofstra J.M., Wetzetls J.F. Low-molecular-weight proteins as prognostic markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:2846–2853. doi: 10.2215/CJN.04020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Logt A.E., Dahan K., Rousseau A. Immunological remission in PLA2R-antibody–associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 2018;93:1016–1017. doi: 10.1016/j.kint.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Dahan K., Johannet C., Esteve E. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 2019;95:233–234. doi: 10.1016/j.kint.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Van den Brand J., Ruggenenti P., Chianca A. Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol. 2017;28:2729–2737. doi: 10.1681/ASN.2016091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.