Abstract
Chronic kidney disease (CKD) is an important public health concern in developed countries because of both the number of people affected and the high cost of care when prevention strategies are not effectively implemented. Prevention should start at the governance level with the institution of multisectoral polices supporting sustainable development goals and ensuring safe and healthy environments. Primordial prevention of CKD can be achieved through implementation of measures to ensure healthy fetal (kidney) development. Public health strategies to prevent diabetes, hypertension, and obesity as risk factors for CKD are important. These approaches are cost-effective and reduce the overall noncommunicable disease burden. Strategies to prevent nontraditional CKD risk factors, including nephrotoxin exposure, kidney stones, infections, environmental exposures, and acute kidney injury (AKI), need to be tailored to local needs and epidemiology. Early diagnosis and treatment of CKD risk factors such as diabetes, obesity, and hypertension are key for primary prevention of CKD. CKD tends to occur more frequently and to progress more rapidly among indigenous, minority, and socioeconomically disadvantaged populations. Special attention is required to meet the CKD prevention needs of these populations. Effective secondary prevention of CKD relies on screening of individuals at risk to detect and treat CKD early, using established and emerging strategies. Within high-income countries, barriers to accessing effective CKD therapies must be recognized, and public health strategies must be developed to overcome these obstacles, including training and support at the primary care level to identify individuals at risk of CKD, and appropriately implement clinical practice guidelines.
Keywords: chronic kidney disease, multisectoral approach, prevention, public health, risk factors
In recent decades, remarkable progress has been made that has deepened our understanding of the burden and consequences of chronic kidney disease (CKD) around the globe.1, 2, 3 Since its formal definition in 2002 and subsequent classification by the US National Kidney Foundation, the term CKD has been widely adopted in clinical practice and policy.3 In 2016, CKD was the ninth-leading cause of death in high-income countries.4 Although death rates due to ischemic heart disease, stroke, and lower respiratory infections are anticipated to decrease, years of life lost (YLLs) due to CKD are forecasted to more than double globally by 2040.5 These facts are alarming when one considers demographic trends of aging and lifestyle patterns in developed nations, which are associated with increased CKD risk.6 Large-scale public health investments must therefore be made across the health system to support CKD prevention and management.
The prevalence of CKD in high-income countries has been reported to be around 8.6% in males and 9.6% in females over age 20 years.7 Those with CKD have a reduced life expectancy compared with that of the general population, beginning at an estimated glomerular filtration rate (eGFR) of <60 ml/min per 1.73 m2, in large part due to the associated increased risk of cardiovascular disease (CVD).8 CKD and its comorbidities are also important drivers of health care costs.9 Traditional and nontraditional risk factors for CKD span a broad range; they include developmental, physical, social, cultural, structural, environmental, and genetic factors.2 It is likely that an substantial proportion of CKD can be prevented at various levels through primordial (early, upstream), primary, and secondary interventions.10 Effective interventions to prevent and delay progression of CKD are well recognized, but many barriers exist that limit their widespread implementation, including cost, gender, age, race, and socioeconomic status, even in high-income settings.11,12
Diabetes, hypertension, and obesity are important contributors to the global burden of disease and are the most common traditional risk factors for CKD.13 Nontraditional CKD risk factors include developmental and gestational factors; kidney stones; exposure to nephrotoxic medications, climate change, and air pollution; infections; and AKI.2,14 The burden of CKD attributable to these risk factors is likely considerable, given that many coexist with the more traditional risk factors.15, 16, 17, 18, 19, 20, 21
A comprehensive approach to CKD prevention begins with understanding the breadth of CKD risk factors, their frequency and distribution, identifying populations at risk, and subsequently implementing mitigation strategies. Screening for kidney disease has been shown to be cost-effective in high-income countries.22 It is imperative that such activities include vulnerable and disenfranchised populations within these settings. This article discusses major strategies to tackle traditional and nontraditional CKD risk factors and implement CKD prevention in high-income countries, where health system barriers should be surmountable.
Primordial Prevention of CKD
Population-based studies from high-income countries have demonstrated an association between low birth weight (birth weight < 2.5 kg), being born small for gestational age (birth weight < 10th percentile for gestational age), and preterm birth (birth before 37 weeks of gestation) and subsequent risk of CKD or end-stage kidney disease (ESKD).18,20,23 These associations are stronger in children and adolescents but continue up to an advanced age.20,24,25 The link between developmental circumstances and risk of CKD appears, at least in part, to be mediated by altered renal development in utero or shortly after birth, which is associated with a reduced nephron number, subsequent hyperfiltration, and predisposition to hypertension.26 Globally, approximately 10% of babies are born preterm, a proportion that is similar across income regions,27 with the risk factors in developed countries including preeclampsia, prior preterm birth, advanced maternal age, chronic maternal illness, assisted reproduction, and multiple gestations.28 The incidence of low birth weight in high-income countries has been stable at 7% for >20 years, and is most commonly associated with preterm birth.29 Both preterm birth and low birth weight occur more frequently among socioeconomically disadvantaged populations and indigenous communities within high-income countries, where structural, environmental, social, and physical factors impact fetal and maternal health throughout gestation and early childhood.30 Additional developmental exposures include preeclampsia,31 which is associated with higher blood pressure in childhood, and maternal overweight/obesity and/or diabetes, which are associated with increased odds of pediatric kidney disease, dysplasia, and later-life diabetic nephropathy.32,33 In high-income countries, gestational hypertension/preeclampsia occurs in around 3% of pregnancies,34 diabetes in around 16% of pregnancies,35 and obesity (body mass index > 30 kg/m2) in 5%–30% of pregnant women.36 These rates are also generally higher among indigenous, minority, and disadvantaged populations in these countries. Mothers who experience preeclampsia or gestational diabetes are themselves at increased risk of future kidney disease and diabetes. Developmental programming of CKD risk is relevant in high-income settings, and especially so among indigenous, African American, and lower socioeconomic–level populations who have an established increased risk of CKD.14
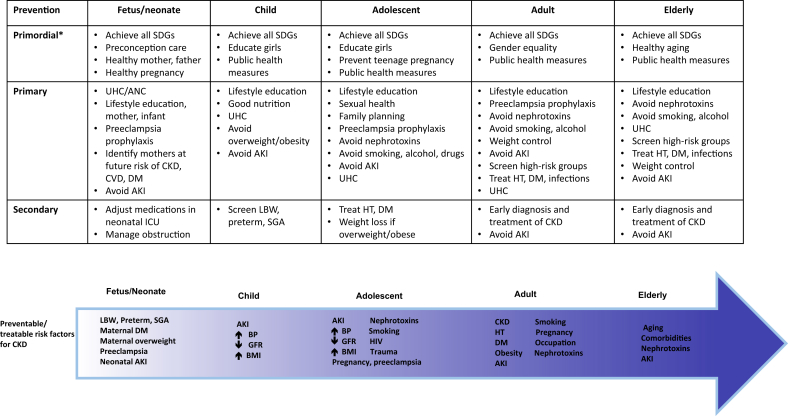
Primordial prevention of CKD therefore includes optimization of maternal health prior to and during pregnancy and ensuring healthy growth and adherence to healthy lifestyles for children born with low birth weight, small for gestational age, or preterm (Figure 137). Healthy mothers begin with healthy girls who receive good nutrition as children, grow up in safe environments with adequate resources permitting healthy and productive lives, are educated, and live in equitable societies. Such circumstances are fostered by the United Nations Sustainable Development Goals, requiring health in all policies, and multisectoral action.38
Figure 1.
Illustration of the spectrum of strategies for chronic kidney disease (CKD) prevention across the life course. *Primordial prevention refers to strategies to optimize upstream factors which may lead to increased risk of CKD at an individual or population level. AKI, acute kidney injury; ANC, ante-natal care; BMI, body mass index; BP, blood pressure;CVD, cardiovascular disease; DM, diabetes mellitus; GFR, glomerular filtration rate; HT, hypertension; ICU, intensive care unit; LBW, low birth weight; SDGs, Sustainable Development Goals37; SGA, small for gestational age at birth; UHC, Universal Health Coverage.
Public Health Approaches to CKD Prevention
For maximal effectiveness and efficiency, strategies to reduce CKD risk should be integrated into a broad approach to noncommunicable disease prevention, especially given the high frequency of comorbidities with CKD (Figure 2).39 Strategies to tackle lifestyle-related noncommunicable disease risk factors are most effective when they are implemented at both the patient and community levels, are supported by regulation and legislation, and incorporate a multi-component approach.40, 41, 42 Successful public health approaches include introduction of economic incentives to reduce prices of healthy food and beverages and increase taxation on unhealthy products, restriction of food advertising, regulation of food composition (salt, trans fats, sugar), support for education and physical activity programs in schools, provision of public recreation facilities, and campaigns to limit advertising and sales of harmful products.43,44 Policies aimed at reducing air pollution are also required.13,44 The World Health Organization, endorsed by member states, has developed multiple packages to guide countries in developing and implementing such strategies.45,46 Implementation of population-level approaches to prevent CKD requires engagement, action, and commitment across multiple sectors of government and society.40
Figure 2.
Highlighting the importance of public health strategies and advocacy for the prevention of chronic kidney disease. AKI, acute kidney injury. Images reproduced with permission from World Kidney Day. Copyright ©World Kidney Day 2006–2020.
Primary Prevention of CKD in Developed Countries
Mitigation of Major Traditional Risk Factors for CKD
The burden of CKD has risen since 1990 in high-income settings, and despite generally good access to primary health care in most developed countries, attributable to ongoing exposure to risk factors, population aging, and population growth.13,47 Within developed countries, the burden of CKD is variable as is the distribution of predisposing risk factors, which tend to be more prevalent in more socioeconomically disadvantaged populations.47,48 Risk factor distribution may also differ between men and women and tends to increase with age; therefore, local epidemiology is relevant in development of strategies to address local risk factor burdens.49,50 In many regions, metabolic risk factors are the major drivers of CKD risk,47,50 and indeed, diabetes remains the most common cause of CKD globally.13 The contribution of hypertension to the burden of CKD in developed countries is more variable but remains significant.13 The contribution of environmental and other nontraditional causes of CKD, including air pollution, smoking, kidney stones, infections, AKI, and other factors are increasingly being recognized.15,51 A holistic approach to disease prevention, as highlighted by the United Nations Sustainable Development Goals, is therefore likely to have a positive impact on the CKD burden from diverse causes (Figure 1).38
In 2019, in high-income countries, 10.4% of people aged 20–79 years are living with diabetes, which translates into 95.2 million individuals, 38.2% whom are undiagnosed.35 A further 11.4% of the population have impaired glucose tolerance and are therefore at risk of diabetes.35 Data from country surveys in 12 high-income countries reported prevalence rates of hypertension (defined as blood pressure >140/90 mm Hg) ranging from 33% to 59% in women and 34% to 59% in men aged 40–79 years.52 Overall, across the 12 countries, awareness of hypertension ranged from 56% to 84%, treatment from 39% to 81%, and control from 17% to 69%.52 In 2014, rates of overweight (body mass index > 25) in adults over 18 years in high-income countries were 61.5% in men and 52.2% in women.53 In 2018, 7.2% of children in high-income countries were overweight, amounting to 5 million children, a 21% increase since 2000.54 Obesity is a major contributor to hypertension and diabetes.53,55 Thus, diabetes, hypertension, and overweight/obesity occur commonly in high-income countries, and despite access to primary care in most settings, a significant number of people will remain undiagnosed and/or undertreated, and therefore at increased risk of developing CKD.
The prevalence of CKD in adults with type 2 diabetes (T2D) ranges from 25% to 40%, depending on the population studied.56, 57, 58 In a United States population-based study, CKD (defined as an eGFR of 15–59 ml/min or microalbuminuria) was present in 40% and 42% of subjects with diagnosed and undiagnosed diabetes, respectively.59 CKD occurred in 17.7% of those with prediabetes and 10.6% without diabetes.59 Similarly, 28% of subjects with diagnosed hypertension were found to have CKD.60 CKD was present in 17%, 22%, and 13% of subjects with prehypertension, undiagnosed hypertension, or normal blood pressure, respectively.60 The prevalence of CKD among obese subjects in this population was 17%.58 A concerning finding is that the majority of individuals with CKD in developed countries are unaware of their diagnosis.61, 62, 63, 64, 65 Systematic screening for diabetes, hypertension, and obesity can identify individuals at risk of CKD and permit early diagnosis and intervention to prevent CKD and other comorbidities related to these conditions (Table 1).61,66, 67, 68
Table 1.
Population-level compliance with strategies for primary and secondary prevention of CKD
| Risk factor | Evidence-based recommendation: guideline-concordant treatment goals and recommended agents | Population-level compliance |
|---|---|---|
| Awareness | Low compliance: 90% of individuals with 2–4 CKD markers and 84% of individuals with ≥5 CKD markers were unaware of their disease61 | |
| Lifestyle | ||
| Overweight | BMI < 25 kg/m2 | |
| Diet | Low salt (<2 g/d) | |
| Smoking | Smoking cessation | |
| Exercise | 30–60 min of exercise 4–7 d/wk | |
| Proteinuria/albuminuria | Monitoring and follow-up Treatment with ACEi/ARBs, with proteinuria >30 mg/mmol or 0.5 g/d |
20% detection rate for CKD-related albuminuria at the community level Prevalence of ACEi/ARB use decreased from 45% in 2006–2008 to 36% in 2012–201466 |
| Blood pressure | <130/80 mmHg (diabetes or proteinuric CKD)a <140/80 mm Hg (nondiabetic or nonproteinuric CKD) |
Prevalence of uncontrolled hypertension (>130/80 mm Hg) was 46%–48% over the past decade66 |
| Diabetes | HbA1c <7% and use of newer agents (i.e., SGLT2 may have role in CKD stages 1–4 for significant cardiovascular and kidney outcomes benefits) | Prevalence of uncontrolled diabetes (HbA1c >7%) was ∼40% in 2012–201466 |
| Dyslipidemia | Use of statinsb | Statin use among patients with CKD aged ≥50 years was low and remained basically unchanged, increasing slightly from 29% in 2006–2008 to 31% in 2012–201466 |
| Cardiovascular | Use of aspirin, beta-blockers among patients with established CVD |
BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; SGLT2, sodium-glucose cotransporter-2.
Use of ACEi/ARBs recommended.
Fire and forget strategy (use of statins among all CKD patients aged >50 years, and no need for serial lipid panels monitoring).
A graded risk for CKD exists with diabetes and hypertension, implying some causality and therefore potential for prevention of CKD through reduction in the burdens of hypertension and diabetes. It is clear that reduction of structural and lifestyle-related risks is key to reduce the burdens of diabetes, hypertension, and obesity, and that many population-level interventions are cost-effective over the long term.69,70 Similarly, optimization of upstream factors, including poverty, unhealthy diets, food insecurity, tobacco consumption, and sedentary lifestyle will address other factors impacting CKD risk and contribute to CKD prevention.71, 72, 73, 74 Interventions to manage hypertension and promote weight loss indeed have been associated with reduced risk of CKD as well as improved outcomes in those with CKD.67,75, 76, 77, 78, 79, 80, 81
Mitigation of Nontraditional Risk Factors for CKD
Not all CKD results from diabetes, hypertension, and obesity; therefore, awareness of additional nontraditional risk factors is key to successful prevention of CKD (Figure 1).48 AKI is being recognized increasingly as an important risk factor for CKD in both adults and children.2,82 In high-income countries, the majority of AKI occurs in hospital, with an incidence rate of 10%–20%.83,84 Major risk factors for AKI include pre-existing CKD, extremes of age, exposure to nephrotoxic substances, renal hypoperfusion, and infection.85 These risk factors in hospitalized patients are often superimposed on existing comorbidities that increase the risk of CKD, such as diabetes, hypertension, and obesity. Incomplete recovery of AKI, and thereby progression to acute kidney disease and CKD occurs in approximately 10% of those experiencing AKI in high-income countries.84 There is still no established targeted treatment for AKI beyond minimization of further kidney injury, so prevention is key. Multiple strategies have been suggested for prevention of AKI, including campaigns to increase awareness of AKI, introduction of electronic alert systems,86,87 development of prediction algorithms,88 and clinical pathways across the spectrum of health care, although the long-term impact of such measures requires further study.85 Documentation of AKI episodes in discharge summaries and follow-up after AKI is important for detecting CKD early.86,89
Nephrotoxic agents such as nonsteroidal anti-inflammatory drugs (NSAIDS), antibiotics, iodinated contrast media, and chemotherapeutic drugs are frequent causes of AKI, and the association is often fairly clear given the short lag-times between administration of the dose and the change in kidney function.87,90 The contribution of nephrotoxic agents to CKD, such as proton-pump inhibitors, is more challenging to define as the onset is more insidious.16 The pathophysiology of chronic nephrotoxicity ranges from interstitial inflammation to tubular and glomerular injury.16,91,92 Also important is that the use of alternative remedies is frequent in high-income settings and may play an under-recognized role in the development of both AKI and CKD.93, 94, 95 Both the public and health care practitioners must be aware of the risks of nephrotoxicity from medication and alternative remedies, as well as of potential interactions between the two, so that use can be minimized or optimized as a strategy to prevent CKD. Calls for better regulation of alternative remedies are required to improve standardization of products and develop appropriate understanding and warning regarding potential toxicities.10
Kidney stones are common in high-income countries, with prevalence rates of 7%– 13% in North America, 5%–9% in Europe, and 20% in Saudi Arabia.96,97 People with kidney stones are at increased risk of developing CKD, in part because of shared risk factors, such as diabetes, hypertension, obesity, metabolic syndrome, and CVD, as well as direct kidney injury from obstruction or crystal precipitation.98,99 Certain forms of bariatric surgery are emerging as an important cause of kidney stones in developed countries.100 Occupational risks and climate change also increase stone risk.96,97,101 The risk of a stone event increases after the first event; therefore, prevention of recurrent stones is important to reduce the risk of CKD.98,102 Dietary changes, including increasing fluid intake, avoiding sweetened beverages, reducing dietary sodium and red meat intake, and ensuring sufficient dietary calcium have all been shown to reduce stone risk.96,102 Population-level strategies to reduce the risk of hypertension, diabetes, metabolic syndrome, and obesity are also likely to reduce stone risk and thereby prevent CKD. Advocacy is required to educate the public and to ensure that occupation and climate risks are mitigated as effectively as possible.
Preeclampsia is being recognized increasingly as a risk factor for CKD. In a Danish population cohort, the risk of CKD in women who had experienced preeclampsia was at least double that seen in women without a history of preeclampsia.103 In a Canadian population-based study, a woman having been born preterm herself was associated with an increased risk of preeclampsia.104 CKD itself increases the risk of preeclampsia, and therefore a subtle programmed decrease in kidney function prior to pregnancy in mothers born preterm may confound this relationship. Women who had been born preterm also had a higher risk of delivering preterm.104 Where the cycle begins is therefore unclear, but preterm birth is both a result of and a risk factor for future CKD, in both the mother and the offspring, and is a risk factor for preeclampsia itself, which is in turn a risk factor for preterm birth and CKD in the mother and the offspring. Both preterm birth and preeclampsia are common, so screening and follow-up of kidney function in individuals who experience these events is important to reduce the CKD burden.18,103 Awareness must be raised among health care professionals such that women and infants at risk can be identified in the delivery room. Education and encouragement regarding adherence to healthy lifestyles, as well as lifelong access to primary care are key to mitigating long-term risk for both. Many structural risk factors likely contribute to both preterm birth and CKD, including poverty, lack of access to antenatal care, poor maternal nutrition, extremes of maternal age, maternal illness, and gender inequality.30 Hypertension, diabetes, CVD, and obesity are all also risks factors for and consequences of preeclampsia and preterm birth that highlight the importance of a life-course approach to CKD prevention (Figure 1).30
The use of illicit substances such as cocaine, heroin, or methamphetamine has also been linked with an increased risk for CKD progression and increased mortality risk among adults with established CKD.105,106 Infections such as endocarditis, hepatitis B and C, and HIV also increase the risk factors for CKD.10 Furthermore, heavy metal pollutants (e.g., arsenic, cadmium, lead, and mercury) and other environmental toxins have been shown to be harmful to the kidneys. Exposure to particulate matter (small particles including dust and dirt ∼2.5–10 mm or smaller) also is associated with the risk of developing de novo CKD at the general population level.15,107 Public health strategies that target these nontraditional factors while promoting better control of traditional factors, such as hypertension, CVD, and diabetes, may significantly impact the future population-based burden of CKD in industrialized nations.10
Secondary Prevention of CKD in the Developed World
Considering the public health burden of CKD and the availability of evidence-based treatment for its management, health systems in developed countries must implement prevention strategies to reduce population impacts of the disease. Secondary prevention in the form of early detection of CKD could promote the use of effective interventions to reduce the risks of adverse outcomes associated with the disease, such as CVD events, ESKD, and increased mortality risk.108
CKD Early Detection and Prevention Programs
Early detection, appropriate risk stratification, and subsequent treatment of CKD may delay or prevent many associated complications and decrease overall health care costs.109,110 Screening the general population for early CKD is not cost-effective, and therefore is unwarranted.6 Selective screening should be directed toward high-risk groups, specifically older persons and those with hypertension and diabetes, as well as some ethnic groups such as African Americans, and indigenous populations of Canada, the United States, and Australia.109,111,112 Targeted screening of high-risk individuals, such as those with diabetes or hypertension or both, is cost-effective.113,114 For instance, the cost-effectiveness of a screening program for proteinuria among US adults with hypertension and diabetes has been clearly demonstrated; angiotensin-converting enzyme inhibitor/angiotensin-receptor blockers have been shown to reduce mortality and slow the progression of kidney disease among at-risk individuals identified through this screening program.114 The guidelines therefore recommend screening only at-risk individuals for CKD (Table 2).115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127
Table 2.
Screening recommendations by the professional medical and nephrology societies
| Organization | Nature of organization | Date | Population | Recommendation statement | Strength and evidence of recommendation |
|---|---|---|---|---|---|
| US Preventive Services Task Force117 | Government/generalist | 2012 | Asymptomatic adults without diagnosed CKD | No recommendation | Grade: I (insufficient Evidence): The evidence is insufficient to assess the balance of benefits and harms of routine screening for CKD in asymptomatic adults. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| ACP118 | Generalist | 2013 | Asymptomatic adults without risk factors for CKD | ACP recommends against screening for CKD in asymptomatic adults without risk factors for CKD. | Grade: weak recommendation, low-quality evidence |
| American Academy of Family Physicians119 | Generalist | 2014 | Endorsed recommendation made by the ACP | ||
| NICE, UK120 | Generalist | 2014; updated 2015 | People prescribed nephrotoxic drugs | 1.1.27 Monitor eGFR at least annually for people prescribed drugs known to be nephrotoxic, such as calcineurin inhibitors (for example, cyclosporin or tacrolimus), lithium, and nonsteroidal anti-inflammatory drugs (NSAIDs). [2008, amended 2014] | Strong |
| People with risk factors for CKD | 1.1.28 Offer testing for CKD using eGFR creatinine and ACR to people with any of the following risk factors: diabetes, hypertension, acute kidney injury, cardiovascular disease (ischemic heart disease, chronic heart failure, peripheral vascular disease, or cerebral vascular disease), structural renal tract disease, recurrent renal calculi or prostatic hypertrophy, and multisystem diseases with potential kidney involvement, e.g., systemic lupus erythematosus, family history of end-stage kidney disease (GFR category G5) or hereditary kidney disease, opportunistic detection of hematuria. | Strong | |||
| Asymptomatic adults | 1.1.29 Do not use age, gender, or ethnicity as risk markers to test people for CKD. In the absence of metabolic syndrome, diabetes or hypertension, do not use obesity alone as a risk marker to test people for CKD. [2008, amended 2014] | Strong | |||
| American Society of Nephrology121 | Renal organization | 2013 | Asymptomatic adults | Strongly recommends regular screening for kidney disease, regardless of an individual’s risk factors | |
| National Kidney Foundation/Kidney Disease Outcomes Quality Initiative122,123 | Renal organization | 2002 | Individuals at risk for CKD | Guideline 3: individuals at increased risk of CKD: Some individuals without kidney damage and with normal or elevated GFR are at increased risk for development of CKD. All individuals should be assessed, as part of routine health encounters, to determine whether they are at increased risk of developing CKD, based on clinical and sociodemographic factors. Individuals at increased risk of developing CKD should undergo testing for markers of kidney damage and to estimate the level of GFR. Individuals found to have CKD should be evaluated and treated as specified in Guideline 2. Individuals at increased risk but found not to have CKD should be advised to follow a program of risk factor reduction, if appropriate, and undergo repeat periodic evaluation. |
Opinion |
| 2013 | Individuals at risk for CKD | The commentary work group endorses the recommendations from the original guideline for screening among individuals at high risk for CKD despite the absence of this specific recommendation in the KDIGO guideline, and as such is in agreement with the recommendation of the ACP to screen asymptomatic adults only if they are at high risk for CKD. | Opinion | ||
| KDIGO124 | Renal organization | 2013 | Screening of CKD was beyond the scope of the CKD work group and no specific recommendations were made. | ||
| Canadian Society of Nephrology125 | Renal organization | 2015 | Asymptomatic individuals | No screening | Consensus |
| At risk of CKD | Practitioners should continue to use case findings in keeping with usual clinical practice: in people with new-onset or long-standing hypertension or diabetes, people with vascular disease, people who are to undergo major surgery or be exposed to other potential causes of acute kidney injury, people with multisystem or generalized symptoms, people who are being considered for nephrotoxic medications or medications that require dose adjustment for renal function, people with a family history of polycystic kidney disease or hereditary nephritis, and people from First Nations populations or other ethnic groups known to be at increased risk. | Consensus | |||
| KHA-CARI Guidelines126 | Renal organization | 2013 | Diabetes, hypertension, or established CVD | We recommend that screening for CKD be targeted and performed in individuals at increased risk of developing CKD, including those with diabetes mellitus, hypertension, and established CVD. | 1B |
| Additional risk factors such as obesity, cigarette smoking; Aboriginal and Torres Strait Islander peoples; family history of stage 5 CKD or hereditary kidney disease in a first- or second-degree relative; and severe socioeconomic disadvantage | We recommend screening in those with additional CKD risk factors identified in guideline 2a (obesity, cigarette smoking, Aboriginal and Torres Strait Islander peoples, family history of stage 5 CKD or hereditary kidney disease in a first- or second-degree relative and severe socioeconomic disadvantage) (1D) | 1D | |||
| UK Renal Association127 | Renal organization | 2011 | At risk for CKD | Guideline 1.4—CKD: detection and monitoring of CKD: We recommend that patients who are at increased risk for developing CKD should be offered screening tests to detect CKD. | 1B |
ACP, American College of Physicians; ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; KDIGO: Kidney Disease: Improving Global Outcomes; KHA-CARI: Kidney Health Australia—Caring for Australasians with Renal Impairment; NICE: National Institute of Health and Care Excellence; UK, United Kingdom; US, United States.
An international survey of kidney care evaluated the practice patterns for routine CKD testing among several high-risk populations.128 Across countries in all income groups, CKD testing was found to be almost universally available for patients with hypertension and diabetes (94%–100%), but minimal for high-risk ethnic populations (<30%).129 More than 50% of countries tested patients with CVD who were taking chronic nephrotoxic medications and had a family history of CKD. Elderly populations (age ≥65 years) also were tested for CKD in more than half of the countries in the high-income and upper-middle–income groups, but in less than half of the countries in the lower-middle–income (47%) and low-income (41%) groups. Overall, CKD testing rates followed the economic development classification pattern, with the highest rates in higher-income countries and the lowest rates in low-income countries. Individual studies report variable screening for CKD, although interventions to improve adherence to guidelines have proved beneficial.130 Follow-up of kidney function after an initial abnormal value and screening for albuminuria is suboptimal even in countries with universal health coverage, and tends to be lower among populations with lower socioeconomic status.131,132 Education of first-line health care workers and primary care physicians is important to improve appropriate screening for early detection and treatment of CKD.130,133 Strengthening of primary care capacity is required, including support from concise guidelines and management protocols, electronic medical records with in-built decision support systems, multidisciplinary teams, and novel knowledge translation tools.
Current State of Secondary Prevention
Effective strategies are available to slow the progression of CKD to ESKD and reduce the risk of cardiovascular events and mortality (Table 1). Lifestyle behavior changes are necessary and have been associated variably with improvements in body mass index, blood pressure, diet, exercise, and renal function in patients with CKD.134 Clinical strategies to manage CKD include dietary manipulations of protein intake, inhibition of the renin–angiotensin–aldosterone system, and strategies to control glycemia and dyslipidemia.135 Among these interventions aimed at slowing CKD progression and mitigating CVD outcomes, inhibition of the renin–angiotensin–aldosterone system has yielded the most impact, is cost-effective in high-income settings, and has been adopted as an integral part of CKD management practices worldwide.6,136
Given that T2D is the major driver of CKD, prevention of CKD in people with diabetes could have a major impact on the global CKD burden. Despite good evidence of their impact, uptake of renin–angiotensin–aldosterone system inhibitors early on was surprisingly slow.115,137 Medications that inhibit the sodium-glucose cotransporter-2 (SGLT2) and glucagon-like peptide-1 receptor agonists (GLP1RAs) are emerging as important additional therapies for secondary prevention of diabetic nephropathy; more awareness is required to optimize uptake. SGLT2 inhibitors were originally developed as glucose-lowering therapies, but they also have multiple other physiological effects, including natriuresis, suppression of pro-inflammatory and pro-fibrotic factors, and reduction in tissue ischemia in the heart and kidney.138, 139, 140 In recent published cardiovascular outcome trials, the SGLT2 inhibitors significantly reduced the risk of cardiorenal endpoints in patients with T2D. For example, in patients with established atherosclerotic CVD, empagliflozin significantly reduced 3-point major adverse cardiac events, cardiovascular and overall mortality, hospitalization for heart failure, and the composite renal secondary endpoint (EMPA-REG OUTCOME).141,142 Subsequently, in a cohort with lower overall cardiovascular risk, canagliflozin also reduced 3-point major adverse cardiac events, hospitalization for heart failure, and renal endpoints, but it did not impact mortality (CANVAS Program).143 In a third trial,144 dapagliflozin reduce the co-primary endpoint (cardiovascular death or hospitalization for heart failure), and also reduced renal risk, even though only 40% of participants had established atherosclerotic CVD at baseline, with a mean eGFR of 85 ml/min per 1.73 m2. In a trial with dedicated renal endpoints, canagliflozin treatment in type 2 diabetic nephropathy (CREDENCE), in addition to renin-angiotensin-aldosterone system blockade, was associated with a 30% decline in the primary composite endpoint, consisting of doubled serum creatinine level, ESKD, renal or cardiovascular death; also reported were significant cardiovascular benefits.145 In this study, canagliflozin was continued in patients with GFR < 30 ml/min, suggesting potential safety beyond this threshold, although more data are required.
Given the profound protection against hospitalization for heart failure in the early trials, several dedicated trials in patients with heart failure are either underway or have been completed. In the first of these studies, dapagliflozin treatment in patients with heart failure and reduced ejection fraction, with or without T2D at baseline (DAPA HF), reduced the risk of cardiovascular death or heart failure endpoints by 24%, regardless of diabetes status.146 The positive cardiovascular impact of SGLT2 inhibitors therefore extends to those without diabetes; however, results of 2 dedicated ongoing trials are awaited to assess their potential role in secondary prevention of nondiabetic kidney disease.147
The GLP1RAs are also used primarily as glucose-lowering therapies and to induce weight loss. In several cardiovascular outcome trials, GLP1RAs have been shown to reduce 3-point major adverse cardiac events and specific atherosclerotic CVD endpoints.148,149 From a renal perspective, GLP1RA, liraglutide (LEADER), and semaglutide (SUSTAIN-6) reduced composite renal endpoints, consisting of progression of albuminuria, doubling of serum creatinine level, renal replacement therapy, or renal death.150 In contrast to SGLT2 inhibitors, with which the risk of hard renal endpoints was reduced, renal benefits of GLP1RA were primarily due to an attenuation of progression of albuminuria.150 Nevertheless, eGFR decline over 52 weeks was significantly lower with dulaglutide versus insulin in patients with CKD stages 3–4 (AWARD-7); eGFR was better preserved in subjects with CKD stage 3 with liraglutide versus placebo (LEADER); and the risk of losing 40% or 50% of kidney function was significantly lower with dulaglutide versus placebo (REWIND), suggesting some benefit for secondary prevention of CKD.150, 151, 152, 153, 154 Semaglutide is currently being studied as a renal protective therapy in patients with renal impairment and albuminuria in the FLOW trial.
Current evidence is encouraging that these new medications may have a significant impact on reducing CKD progression and CVD morbidity, but ongoing vigilance is required. SGLT2 inhibition increases the risk of genital tract infections, and it has been linked with a small but significant increase in the risk of diabetic ketoacidosis, even in patients with T2D.144 In addition, in the CANVAS Program, major and minor amputation and the risk of fracture were higher in canagliflozin-treated patients versus placebo-treated patients—risks that have not been reported in any other trials.145 As a result, clinical concern around these issues has diminished, especially since patients in CREDENCE were at high risk for amputation at baseline given their background of significant CKD. However, good genital/perineal hygiene, awareness of symptoms of ketoacidosis, and regular foot examination are recommended during therapy.155 As confidence builds around the place of these medications in secondary prevention of CKD, advocacy will be required to ensure equitable and affordable access globally.
Awareness, Disparities, Capacity, and Barriers to Effective Prevention
Despite progress, significant gaps in care remain at the policy, health system, and individual levels across the spectrum of CKD prevention.61,66,132 The International Society of Nephrology collected data on country-level capacity for kidney care delivery using a survey aligned with the World Health Organization’s health system building blocks, and published the findings in the first and second edition of the Global Kidney Health Atlas.128 This document highlighted limited awareness and prioritization of CKD, as well as persistent inequities in resources required to tackle the burden of kidney disease globally. Overall, CKD was recognized as a health care priority by just 36% of governments. Surprisingly, the priority accorded to CKD care was inversely related to country income level, but less so in upper-middle– and high-income countries (30%) compared with low- and lower-middle–income countries (50%). This pattern is also reflected in the funding and delivery of CKD care across high-income nations. Government-provided universal health coverage for kidney disease (CKD and ESKD) is common in developed nations, but with substantial variability in how care is funded and delivered. For instance, in the United States, ESKD care is publicly financed for residents; however, optimal treatment of CKD and its risk factors is generally not accessible for persons lacking health insurance.156 Barriers to providing optimal CKD care, including patient factors (e.g., awareness, treatment compliance), care provider factors (e.g., limited awareness and/or inertia in the application of optimal CKD management practices, time and resource constraints), and system factors (e.g., prioritization, funding, availability of functional infrastructures) are frequently reported. Disparities in the burden of disease and quality of care are clearly demonstrated for African Americans in the US, indigenous populations in Australia and Canada, Indo-Asian communities in the UK, and multiple other settings in the developed world.12,74,157,158 Given that the CKD burden tends to be highest in these communities, implementation of appropriate and acceptable prevention strategies is an urgent need.
Despite guidelines regarding management of cardiovascular and renal risk, surprising gaps still exist in guideline-concordant care delivery. In a national study of over 40,000 Canadians with early CKD managed in primary care, significant gaps were found in the use of urine protein excretion as a screening tool and around the rate of use of proven medications to reduce cardiovascular and renal risk associated with proteinuria.132 Many patients received high-quality care for other domains (blood pressure and glycemic control), but awareness about the implications of elevated urine protein and its impact on health outcomes was identified as an area for improvement. This is a familiar picture demonstrated in similar high-income settings.66,159 The proliferation of guideline recommendations targeting primary care, however, constitutes a barrier in itself to sustainable implementation of optimal management of chronic diseases including CKD.66,159, 160, 161 For example, an average primary care physician would require about 7–10 hours per working day to follow preventive recommendations for the common chronic diseases.162,163
Conclusion
Prevention of CKD is possible but requires a broad and holistic approach—ranging from good governance and achievement of the sustainable development goals, to ensuring healthy pregnancies for a good start in life—and access to appropriate screening for early detection and treatment of risk factors for CKD as well as of early CKD (Figure 2). The management of traditional risk factors has improved in certain countries, but significant gaps in care remain even in developed countries, particularly with regard to identifying early risk (e.g., detection and monitoring of albuminuria) and addressing these risks, especially in disadvantaged sectors of the population. Nontraditional risk factors remain underappreciated in many settings. Closing these gaps will require multifaceted stakeholder engagement, including the development and implementation of strong public health measures to prevent CKD risk factors, especially diabetes, hypertension, and obesity; patient advocacy and awareness tools; and promotion of self-management programs. Strong primary care capacity must also be developed to implement target screening, early diagnosis, and early treatment strategies, followed by long-term access to appropriate secondary prevention and care.
Disclosure
DZIC has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck, and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, and Merck. All the other authors declared no competing interests.
Author Contributions
VAL, DZIC, and AKB were responsible for drafting, editing, and finalizing this article for submission.
References
- 1.Thomas B., Matsushita K., Abate K.H. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin A., Tonelli M., Bonventre J. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 3.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization The top 10 causes of death. 2018. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death Available at:
- 5.Foreman K.J., Marquez N., Dolgert A. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016—40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanholder R., Annemans L., Brown E. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 7.Mills K.T., Xu Y., Zhang W. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neild G.H. Life expectancy with chronic kidney disease: an educational review. Pediatr Nephrol. 2017;32:243–248. doi: 10.1007/s00467-016-3383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oosten M.J.M., Logtenberg S.J.J., Leegte M.J.H. Age-related difference in health care use and costs of patients with chronic kidney disease and matched controls: analysis of Dutch health care claims data. Nephrol Dial Transplant. 2019 doi: 10.1093/ndt/gfz146. pii: gfz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luyckx V.A., Tuttle K.R., Garcia-Garcia G. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl (2011) 2017;7:71–87. doi: 10.1016/j.kisu.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton J.M., Moxey-Mims M.M., Eggers P.W. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27:2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caskey F., Dreyer G., Kidney Research UK Kidney health inequalities in the UK. An agenda for change. https://kidneyresearchuk.org/wp-content/uploads/2019/09/Health_Inequalities_lay_report_FINAL_WEB_20190311.pdf Available at:
- 13.Xie Y., Bowe B., Mokdad A.H. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Garcia G., Jha V., World Kidney Day Steering Committee CKD in disadvantaged populations. Kidney Int. 2015;87:251–253. doi: 10.1038/ki.2014.369. [DOI] [PubMed] [Google Scholar]
- 15.Bowe B., Xie Y., Li T. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-022450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarus B., Chen Y., Wilson F.P. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang W., Li L., Ren Y. History of kidney stones and risk of chronic kidney disease: a meta-analysis. PeerJ. 2017;5 doi: 10.7717/peerj.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crump C., Sundquist J., Winkleby M.A. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346. doi: 10.1136/bmj.l1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vikse B.E. Pre-eclampsia and the risk of kidney disease. Lancet. 2013;382:104–106. doi: 10.1016/S0140-6736(13)60741-2. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson J.G., Salonen M.K., Kajantie E. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki Birth Cohort Study, 1924-1944. Am J Kidney Dis. 2018;71:20–26. doi: 10.1053/j.ajkd.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Sykes L., Asar O., Ritchie J. The influence of multiple episodes of acute kidney injury on survival and progression to end stage kidney disease in patients with chronic kidney disease. PloS One. 2019;14 doi: 10.1371/journal.pone.0219828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komenda P., Ferguson T.W., Macdonald K. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis. 2014;63:789–797. doi: 10.1053/j.ajkd.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Ruggajo P., Skrunes R., Svarstad E. Familial factors, low birth weight, and development of ESRD: A nationwide registry study. Am J Kidney Dis. 2016;67:601–608. doi: 10.1053/j.ajkd.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Khalsa D.D., Beydoun H.A., Carmody J.B. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol. 2016;31:1509–1516. doi: 10.1007/s00467-016-3384-7. [DOI] [PubMed] [Google Scholar]
- 25.Hirano D., Ishikura K., Uemura O. Association between low birth weight and childhood-onset chronic kidney disease in Japan: a combined analysis of a nationwide survey for paediatric chronic kidney disease and the National Vital Statistics Report. Nephrol Dial Transplant. 2016;31:1895–1900. doi: 10.1093/ndt/gfv425. [DOI] [PubMed] [Google Scholar]
- 26.Brenner B.M., Lawler E.V., Mackenzie H.S. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 27.Chawanpaiboon S., Vogel J.P., Moller A.B. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrero D.M., Larson J., Jacobsson B. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PloS One. 2016;11 doi: 10.1371/journal.pone.0162506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blencowe H., Krasevec J., de Onis M. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7:e849–e860. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low Birth Weight and Nephron Number Working Group The impact of kidney development on the life course: a consensus document for action. Nephron. 2017;136:3–49. doi: 10.1159/000457967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geelhoed J.J., Fraser A., Tilling K. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu C.W., Yamamoto K.T., Henry R.K. Prenatal risk factors for childhood CKD. J Am Soc Nephrol. 2014;25:2105–2111. doi: 10.1681/ASN.2013060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavkov M.E., Hanson R.L., Knowler W.C. Effect of intrauterine diabetes exposure on the incidence of end-stage renal disease in young adults with type 2 diabetes. Diabetes Care. 2010;33:2396–2398. doi: 10.2337/dc10-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abalos E., Cuesta C., Carroli G. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 35.International Diabetes Federation IDF Diabetes Atlas. https://diabetesatlas.org/en/ Available at:
- 36.Poston L., Caleyachetty R., Cnattingius S. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 37.United Nations Sustainable development goals. http://www.un.org/sustainabledevelopment/news/communications-material/ Available at:
- 38.Luyckx V.A., Tonelli M., Stanifer J.W. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414–422D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonelli M., Wiebe N., Manns B.J. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization Health in all policies. http://apps.who.int/iris/bitstream/10665/112636/1/9789241506908_eng.pdf?ua=1 Available at:
- 41.Hyseni L., Elliot-Green A., Lloyd-Williams F. Systematic review of dietary salt reduction policies: evidence for an effectiveness hierarchy? PloS One. 2017;12 doi: 10.1371/journal.pone.0177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGill R., Anwar E., Orton L. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health. 2015;15:457. doi: 10.1186/s12889-015-1781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mozaffarian D., Afshin A., Benowitz N.L. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta R., Wood D.A. Primary prevention of ischaemic heart disease: populations, individuals, and health professionals. Lancet. 2019;394:685–696. doi: 10.1016/S0140-6736(19)31893-8. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization Tackling NCDs: "Best buys" and other recommended interventions for the prevention and control of noncommunicable diseases. http://www.who.int/ncds/management/best-buys/en/ Available at:
- 46.World Health Organization WHO PEN Protocol 1. Prevention of heart attacks, strokes and kidney disease through integrated management of diabetes and hypertension. http://www.who.int/ncds/management/Protocol1_HeartAttack_strokes_kidneyDisease.pdf?ua=1 Available at:
- 47.Bowe B., Xie Y., Li T. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the Global Burden of Disease Study. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruck K., Stel V.S., Gambaro G. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv J.C., Zhang L.X. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 50.Chen H.Y., Lu F.H., Chang C.J. Metabolic abnormalities, but not obesity per se, associated with chronic kidney disease in a Taiwanese population. Nutr Metab Cardiovasc Dis. 2019 doi: 10.1016/j.numecd.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Noborisaka Y., Ishizaki M., Yamada Y. The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environ Health Prev Med. 2013;18:24–32. doi: 10.1007/s12199-012-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.NCD Risk Factor Collaboration Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394:639–651. doi: 10.1016/S0140-6736(19)31145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization Global report on diabetes. https://apps.who.int/iris/bitstream/handle/10665/204874/WHO_NMH_NVI_16.3_eng.pdf;jsessionid=4FF2FE97F5DC497A6168D6F8605E3709?sequence=1 Available at:
- 54.World Health Organization Joint child malnutrition estimates—levels and trends (2019 edition) https://www.who.int/nutgrowthdb/estimates2018/en/ Available at:
- 55.Hall J.E., do Carmo J.M., da Silva A.A. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–385. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adler A.I., Stevens R.J., Manley S.E. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 57.White S., Chadban S. Diabetic kidney disease in Australia: current burden and future projections. Nephrology (Carlton) 2014;19:450–458. doi: 10.1111/nep.12281. [DOI] [PubMed] [Google Scholar]
- 58.US Renal Data System Chapter 1: CKD in the General Population. https://www.usrds.org/2018/download/v1_c01_GenPop_18_usrds.pdf Available at:
- 59.Plantinga L.C., Crews D.C., Coresh J. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crews D.C., Plantinga L.C., Miller E.R., 3rd Prevalence of chronic kidney disease in persons with undiagnosed or prehypertension in the United States. Hypertension. 2010;55:1102–1109. doi: 10.1161/HYPERTENSIONAHA.110.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuot D.S., Plantinga L.C., Hsu C.Y. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy K.A., Greer R.C., Roter D.L. Awareness and discussions about chronic kidney disease among African-Americans with chronic kidney disease and hypertension: a mixed methods study. J Gen Intern Med. 2019 doi: 10.1007/s11606-019-05540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasparini A., Evans M., Coresh J. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant. 2016;31:2086–2094. doi: 10.1093/ndt/gfw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner M., Wanner C., Schich M. Patient’s and physician’s awareness of kidney disease in coronary heart disease patients—a cross-sectional analysis of the German subset of the EUROASPIRE IV survey. BMC Nephrol. 2017;18:321. doi: 10.1186/s12882-017-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verhave J.C., Troyanov S., Mongeau F. Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol. 2014;9:713–719. doi: 10.2215/CJN.06550613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tummalapalli S.L., Powe N.R., Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol. 2019;14:1142–1150. doi: 10.2215/CJN.00060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jafar T.H., Allen J.C., Jehan I. Health education and general practitioner training in hypertension management: long-term effects on kidney function. Clin J Am Soc Nephrol. 2016;11:1044–1053. doi: 10.2215/CJN.05300515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rios P., Schwedt E., Sola L. Importance of preventive medical examination for early diagnosis of renaldisease in Uruguay—The National Renal Health Program. Arch Med Interna. 2015;37:114–121. [Google Scholar]
- 69.World Health Organization Global action plan for the prevention and control of NCDs 2013–2020. http://www.who.int/nmh/events/ncd_action_plan/en/ Available at: [DOI] [PMC free article] [PubMed]
- 70.World Health Organization Saving lives, spending less: a strategic response to noncommunicable diseases. https://www.who.int/ncds/management/ncds-strategic-response/en/ Available at:
- 71.Ghosh-Dastidar B., Cohen D., Hunter G. Distance to store, food prices, and obesity in urban food deserts. Am J Prev Med. 2014;47:587–595. doi: 10.1016/j.amepre.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutierrez O.M. Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:31–38. doi: 10.1053/j.ackd.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rebholz C.M., Anderson C.A., Grams M.E. Relationship of the American Heart Association’s impact goals (Life’s Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) cohort study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crews D.C., Liu Y., Boulware L.E. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23:298–305. doi: 10.1097/01.mnh.0000444822.25991.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuttle K.R., Bakris G.L., Bilous R.W. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Stenvinkel P., Zoccali C., Ikizler T.A. Obesity in CKD—what should nephrologists know? J Am Soc Nephrol. 2013;24:1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jun M., Hemmelgarn B.R. Strategies for BP control in developing countries and effects on kidney function. Clin J Am Soc Nephrol. 2016;11:932–934. doi: 10.2215/CJN.03690316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couser W.G., Remuzzi G., Mendis S. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 79.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 80.Accord Study Group Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39:701–708. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zoungas S., Chalmers J., Neal B. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 82.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewington A.J., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 85.Kashani K., Rosner M.H., Haase M. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14:941–953. doi: 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selby N.M., Casula A., Lamming L. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. 2019;30:505–515. doi: 10.1681/ASN.2018090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein S.L., Kirkendall E., Nguyen H. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132:e756–e767. doi: 10.1542/peds.2013-0794. [DOI] [PubMed] [Google Scholar]
- 88.Tomasev N., Glorot X., Rae J.W. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572:116–119. doi: 10.1038/s41586-019-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carmody J.B., Swanson J.R., Rhone E.T. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–2043. doi: 10.2215/CJN.05190514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perazella M.A., Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int. 2015;87:909–917. doi: 10.1038/ki.2015.30. [DOI] [PubMed] [Google Scholar]
- 91.Moledina D.G., Perazella M.A. Proton pump inhibitors and CKD. J Am Soc Nephrol. 2016;27:2926–2928. doi: 10.1681/ASN.2016020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radhakrishnan J., Perazella M.A. Drug-induced glomerular disease: attention required! Clin J Am Soc Nephrol. 2015;10:1287–1290. doi: 10.2215/CJN.01010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luyckx V.A. Nephrotoxicity of alternative medicine practice. Adv Chronic Kidney Dis. 2012;19:129–141. doi: 10.1053/j.ackd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Frass M., Strassl R.P., Friehs H. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12:45–56. [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh C.F., Huang S.L., Chen C.L. Increased risk of chronic kidney disease among users of non-prescribed Chinese herbal medicine in Taiwan. Prev Med. 2012;55:155–159. doi: 10.1016/j.ypmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Sorokin I., Mamoulakis C., Miyazawa K. Epidemiology of stone disease across the world. World J Urol. 2017;35:1301–1320. doi: 10.1007/s00345-017-2008-6. [DOI] [PubMed] [Google Scholar]
- 97.Lopez M., Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan S.R., Pearle M.S., Robertson W.G. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. doi: 10.1038/nrdp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keddis M.T., Rule A.D. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens. 2013;22:390–396. doi: 10.1097/MNH.0b013e32836214b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thongprayoon C., Cheungpasitporn W., Vijayvargiya P. The risk of kidney stones following bariatric surgery: a systematic review and meta-analysis. Ren Fail. 2016;38:424–430. doi: 10.3109/0886022X.2015.1137186. [DOI] [PubMed] [Google Scholar]
- 101.Geraghty R.M., Proietti S., Traxer O. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease: evidence from a systematic review of literature. J Endourol. 2017;31:729–735. doi: 10.1089/end.2017.0123. [DOI] [PubMed] [Google Scholar]
- 102.Scales C.D., Jr., Tasian G.E., Schwaderer A.L. Urinary stone disease: advancing knowledge, patient care, and population health. Clin J Am Soc Nephrol. 2016;11:1305–1312. doi: 10.2215/CJN.13251215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kristensen J.H., Basit S., Wohlfahrt J. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:l1516. doi: 10.1136/bmj.l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boivin A., Luo Z.C., Audibert F. Pregnancy complications among women born preterm. CMAJ. 2012;184:1777–1784. doi: 10.1503/cmaj.120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novick T., Liu Y., Alvanzo A. Lifetime cocaine and opiate use and chronic kidney disease. Am J Nephrol. 2016;44:447–453. doi: 10.1159/000452348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mansoor K., Kheetan M., Shahnawaz S. Systematic review of nephrotoxicity of drugs of abuse, 2005-2016. BMC Nephrol. 2017;18:379. doi: 10.1186/s12882-017-0794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jhee J.H., Joo Y.S., Kee Y.K. Secondhand smoke and CKD. Clin J Am Soc Nephrol. 2019;14:515–522. doi: 10.2215/CJN.09540818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vanholder R., Lameire N., Annemans L. Cost of renal replacement: how to help as many as possible while keeping expenses reasonable? Nephrol Dial Transplant. 2016;31:1251–1261. doi: 10.1093/ndt/gfv233. [DOI] [PubMed] [Google Scholar]
- 109.Ferguson T.W., Tangri N., Tan Z. Screening for chronic kidney disease in Canadian indigenous peoples is cost-effective. Kidney Int. 2017;92:192–200. doi: 10.1016/j.kint.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 110.Black C., Sharma P., Scotland G. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14:1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 111.Hoy W.E., Wang Z., Baker P.R. Reduction in natural death and renal failure from a systematic screening and treatment program in an Australian Aboriginal community. Kidney Int Suppl. 2003;83:S66–S73. doi: 10.1046/j.1523-1755.63.s83.14.x. [DOI] [PubMed] [Google Scholar]
- 112.Yarnoff B.O., Hoerger T.J., Simpson S.K. The cost-effectiveness of using chronic kidney disease risk scores to screen for early-stage chronic kidney disease. BMC Nephrol. 2017;18:85. doi: 10.1186/s12882-017-0497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boulware L.E., Jaar B.G., Tarver-Carr M.E. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 114.Qaseem A., Hopkins R.H., Jr., Sweet D.E. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:835–847. doi: 10.7326/0003-4819-159-12-201312170-00726. [DOI] [PubMed] [Google Scholar]
- 115.Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 116.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 117.Moyer V.A. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:567–570. doi: 10.7326/0003-4819-157-8-201210160-00533. [DOI] [PubMed] [Google Scholar]
- 118.Fink H.A., Ishani A., Taylor B.C. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2012;156:570–581. doi: 10.7326/0003-4819-156-8-201204170-00004. [DOI] [PubMed] [Google Scholar]
- 119.Lambert M. ACP releases guideline on screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease. Am Fam Physician. 2014;90:121–122. [Google Scholar]
- 120.National Institute for Health and Care Excellence Chronic kidney disease in adults: assessment and management. Clinical guideline [CG182] https://www.nice.org.uk/guidance/cg182/chapter/Introduction Available at: [PubMed]
- 121.The American Society of Nephrology ASN emphasizes need for early detection of kidney disease, a silent killer. https://www.asn-online.org/news/2013/ASN_COMM_ACP_Screening_Response_102213_R12.pdf Available at:
- 122.Inker L.A., Astor B.C., Fox C.H. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 123.Levey A.S., Coresh J., Bolton K. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 124.Stevens P.E., Levin A., Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 125.Akbari A., Clase C.M., Acott P. Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am J Kidney Dis. 2015;65:177–205. doi: 10.1053/j.ajkd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 126.Johnson D.W., Atai E., Chan M. KHA-CARI guideline: Early chronic kidney disease: detection, prevention and management. Nephrology (Carlton) 2013;18:340–350. doi: 10.1111/nep.12052. [DOI] [PubMed] [Google Scholar]
- 127.MacGregor M.S., Taal M.W. Clinical practice guidelines for the detection, monitoring and care of patients with chronic kidney disease. 5th ed, 2009–2011. https://renal.org/wp-content/uploads/2017/06/detection-monitoring-care-of-patients-with-ckd-5th-edition-1.pdf Available at:
- 128.Bello A.K., Alrukhaimi M., Ashuntantang G.E. Global overview of health systems oversight and financing for kidney care. Kidney Int Suppl. 2017;8:41–51. doi: 10.1016/j.kisu.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bello A.K., Levin A., Tonelli M. Assessment of global kidney health care status. JAMA. 2017;317:1864–1881. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feakins B., Oke J., McFadden E. Trends in kidney function testing in UK primary care since the introduction of the quality and outcomes framework: a retrospective cohort study using CPRD. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee J., Chu C., Guzman D. Albuminuria testing by race and ethnicity among patients with hypertension with and without diabetes. Am J Nephrol. 2019;50:48–54. doi: 10.1159/000500706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bello A.K., Ronksley P.E., Tangri N. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Dipten C., van Berkel S., de Grauw W.J.C. General practitioners’ perspectives on management of early-stage chronic kidney disease: a focus group study. BMC Fam Pract. 2018;19:81. doi: 10.1186/s12875-018-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evangelidis N., Craig J., Bauman A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taal M.W. Staging and management of chronic kidney disease. In: Yu A.S.L., editor. 11th ed. Vol. 2. Elsevier; Philadelphia: 2019. (Brenner and Rector’s The Kidney). [Google Scholar]
- 136.Ettehad D., Emdin C.A., Kiran A. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 137.Kliger A.S., Brosius F.C. Preserving kidney function instead of replacing it. Clin J Am Soc Nephrol. 2019 doi: 10.2215/CJN.07820719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heerspink H.J., Perkins B.A., Fitchett D.H. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 139.Lytvyn Y., Bjornstad P., Udell J.A. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heerspink H.J.L., Kosiborod M., Inzucchi S.E. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39. doi: 10.1016/j.kint.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 141.Wanner C., Inzucchi S.E., Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–1802. doi: 10.1056/NEJMc1611290. [DOI] [PubMed] [Google Scholar]
- 142.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 143.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 144.Wiviott S.D., Raz I., Bonaca M.P. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 145.Perkovic V., Jardine M.J., Neal B. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 146.McMurray J.J.V., Solomon S.D., Inzucchi S.E. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 147.Lytvyn Y., Bjornstad P., van Raalte D.H. The new biology of diabetic kidney disease—mechanisms and therapeutic implications. Endocr Rev. 2019 doi: 10.1210/endrev/bnz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marso S.P., Bain S.C., Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 149.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mann J.F.E., Orsted D.D., Brown-Frandsen K. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 151.Tuttle K.R., Lakshmanan M.C., Rayner B. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 152.Tuttle K.R., Lakshmanan M.C., Rayner B. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7) Diabetes Obes Metab. 2019;21:1493–1497. doi: 10.1111/dom.13668. [DOI] [PubMed] [Google Scholar]
- 153.Gerstein H.C., Colhoun H.M., Dagenais G.R. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 154.Gerstein H.C., Colhoun H.M., Dagenais G.R. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 155.Vardeny O., Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7:169–172. doi: 10.1016/j.jchf.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 156.Hall Y.N., Rodriguez R.A., Boyko E.J. Characteristics of uninsured Americans with chronic kidney disease. J Gen Intern Med. 2009;24:917–922. doi: 10.1007/s11606-009-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Samuel S.M., Palacios-Derflingher L., Tonelli M. Association between First Nations ethnicity and progression to kidney failure by presence and severity of albuminuria. CMAJ. 2014;186:E86–E94. doi: 10.1503/cmaj.130776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van den Beukel T.O., de Goeij M.C., Dekker F.W. Differences in progression to ESRD between black and white patients receiving predialysis care in a universal health care system. Clin J Am Soc Nephrol. 2013;8:1540–1547. doi: 10.2215/CJN.10761012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khanam M.A., Kitsos A., Stankovich J. Chronic kidney disease monitoring in Australian general practice. Austral J Gen Pract. 2019;48:132–137. doi: 10.31128/AJGP-07-18-4630. [DOI] [PubMed] [Google Scholar]
- 160.Harvey G., Oliver K., Humphreys J. Improving the identification and management of chronic kidney disease in primary care: lessons from a staged improvement collaborative. Int J Qual Health Care. 2015;27:10–16. doi: 10.1093/intqhc/mzu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stevens P.E., O’Donoghue D.J., de Lusignan S. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 162.Ostbye T., Yarnall K.S., Krause K.M. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yarnall K.S., Pollak K.I., Ostbye T. Primary care: Is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]