Introduction
The serum creatinine level is one of the widely used indices for estimating renal function and is used in some equations to calculate the estimated glomerular filtration rate (eGFR).1,2 Two methods, namely the Jaffe method and the enzymatic method, are widely used for measuring creatinine levels. Creatinine measurement with the enzymatic method is less susceptible to interference by glucose or bilirubin and has a smaller coefficient of variance, resulting in more accurate staging of chronic kidney disease than the Jaffe method.3, 4, 5 Thus, enzymatic assays are recommended for creatinine measurement in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines,2 and almost all laboratories in Japan perform enzymatic assays on a number of platforms. The enzymatic method is sometimes affected by interference due to monoclonal gammopathy or catecholamines.6, 7, 8, 9,S1 The mechanism of interference is assumed to be precipitation of globulin or chemical reactions between globulin and substances included in reagents, but it has not been fully elucidated. Here, we describe a case of pseudohypercreatininemia associated with precipitation of IgM caused by low ionic strength of the reagent of the creatinine assay.
Case Presentation
A 78-year-old man was referred to the department of thoracic surgery in our hospital for evaluation of lung tumor. He was being treated with nifedipine for hypertension. At the initial visit to our hospital, his blood creatinine level was 4.68 mg/dl, with an eGFR of 10.3 ml/min per 1.73 m2, which was much higher than his previous report (1.03 mg/dl; eGFR 53.8 ml/min per 1.73 m2), obtained in another clinic just 4 days before the visit. He was generally well and did not complain of anorexia, fever, or oliguria. Urinalysis and abdominal ultrasonography were unremarkable. Biochemical test, serum protein electrophoresis, and immunofixation electrophoresis showed a serum IgM of 1366 mg/dl and monoclonal proliferation of IgM-kappa (Table 1). Results of immunoblotting prompted us to perform bone marrow aspiration immunophenotyping and biopsy, yielding a diagnosis of lymphoplasmacytic lymphoma with macroglobulinemia. Kidney biopsy showed mild mesangial proliferation but failed to reveal the cause of acute elevation of blood creatinine level. Despite the high creatinine levels measured in our hospital, those measured in outside laboratories soon after the biopsy were nearly normal. To elucidate the cause of the discrepancy in the creatinine concentrations, the measurement methods were analyzed.
Table 1.
Laboratory findings of this case
| Parameters (units) | Values | Reference ranges |
|---|---|---|
| Leukocytes (109/l) | 7.8 | 4.0–8.0 |
| Hemoglobin (g/dl) | 10.8 | 13.5–17.5 |
| Platelets (109/l) | 248 | 140–400 |
| Albumin (g/dl) | 3.9 | 3.9–4.8 |
| Total bilirubin (mg/dl) | 0.5 | 0.2–1.1 |
| Blood urea nitrogen (mg/dl) | 19 | 8–20 |
| Creatinine (mg/dl) | 4.68 | 0.5–1.1 |
| eGFR (ml/min per 1.73 m2) | 10 | |
| Sodium (mmol/l) | 142 | 136–145 |
| Potassium (mmol/l) | 4.7 | 3.5–4.8 |
| IgG (mg/dl) | 850 | 870–1700 |
| IgA (mg/dl) | 91 | 110–350 |
| IgM (mg/dl) | 1366 | 30–180 |
| FLC, kappa (mg/l) | 29.7 | 3.3–19.4 |
| FLC, lambda (mg/l) | 11.4 | 5.7–26.3 |
| Kappa/Lambda ratio | 2.61 | 0.26–1.65 |
| β2MG (mg/l) | 2.66 | 0.9–2.66 |
eGFR, estimated glomerular filtration rate; FLC, free light chain; β2MG, β2-microglobulin.
Only a kit used in our hospital (Sekisui Medical Co., Ltd., Tokyo, Japan) showed abnormally high creatinine values, whereas creatinine levels obtained using kits from other manufacturers (Shino-Test Corporation, Tokyo, Japan; Kyowa Medex Co., Ltd., Tokyo, Japan; Kainos, Tokyo, Japan; and Showa Medical Science, Tokyo, Japan) ranged from 1.1 to 1.5 mg/dl (Table 2). Measurement with the Jaffe method (Cayman Chemical, Ann Arbor, MI) showed a creatinine level of 1.77 mg/dl. Blood levels of urea nitrogen and β2-microglobulin that are generally elevated in patients with renal failure were lower than those of creatinine. Moreover, cystatin C–based eGFR was 33.3 ml/min per 1.73 m2. Taken together, the eGFR in this case was 30 ml/min per 1.73 m2 or higher.
Table 2.
Estimated glomerular filtration rate (eGFR) estimated from creatinine and cystatin C levels
| Parameters | Manufacturers | Methods | Values | eGFR |
|---|---|---|---|---|
| Creatinine | Sekisui Medical | Enzymatic | 4.68 | 10.3 |
| Kyowa Medex | Enzymatic | 1.30 | 41.7 | |
| Kainos | Enzymatic | 1.50 | 35.7 | |
| Showa Medical Science | Enzymatic | 1.03 | 41.7 | |
| Cayman Chemical | Jaffe | 1.77 | 29.8 | |
| Cystatin C | 1.82 | 33.3 |
The modified Modification of Diet in Renal Disease equation for the Japanese population was used for calculating creatinine-based eGFR. Regarding cystatin C, the Chronic Kidney Disease Epidemiology Collaboration equation was used.
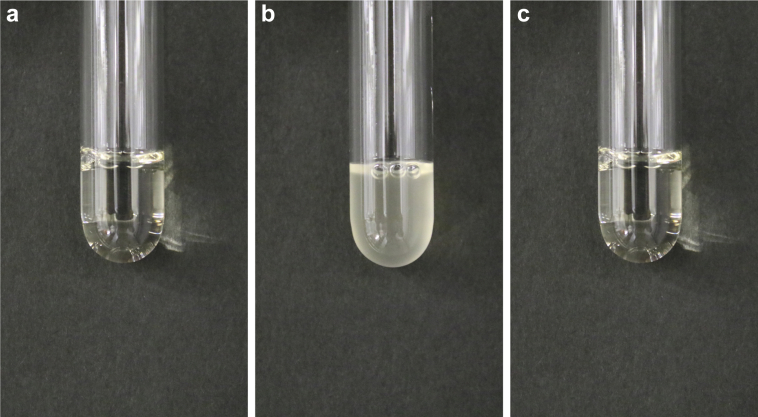
In the enzymatic method, the first reagent, including catalase, eliminates interference by endogenous creatine. With the second reagent, creatinine is sequentially catalyzed by creatininase, creatinase, and sarcosine oxidase, resulting in the production of hydrogen peroxide. Creatinine levels are calculated from the amount of quinone pigments generated by the reaction between hydrogen peroxide and chromogens. With the kit used in our hospital, the patient’s serum became turbid when the second reagent was added (Figure 1). In the reaction curve, that is, a plot of serial absorbance in the process of the chemical reaction, a rapid increase was observed immediately after mixing the patient’s serum and the second reagent (Figure 2). Turbidity due to addition of the second reagent and the distorted reaction curve were not seen when using a control serum of another patient with creatinine levels of 5.5 mg/dl and without paraprotein; this suggested that the white precipitate caused positive interference with the kit used in our hospital.
Figure 1.
Emergence of turbidity, due to mixture of the patient’s serum and the reagents in the kit of our laboratory, and its disappearance following the addition of salt solution. (a) Serum and first reagent; (b) panel (a) and second reagent; (c) panel (b) and 5 mol/l sodium chloride solution.
Figure 2.
Reaction curves in creatinine measurement. The curve is distorted in this case (black line), but not in control serum without paraproteinemia with creatinine levels of 5.5 mg/dl (blue line).
Next, the Sia euglobulin precipitation test, which involves mixing distilled water and the patient’s serum, was performed on the basis that Igs do not dissolve in water but do dissolve in salt solution. A white precipitate emerged soon after mixing, and this precipitate vanished after the addition of 5 mol/l sodium chloride solution (Figure 1), indicating that the low ionic strength of the second reagent was the trigger for paraprotein aggregation. As expected, the concentrations of sodium and chloride in the second reagent of the kits used in our laboratory were 33 mmol/l and 9 mmol/l, respectively, lower than those of the kits from other manufacturers.
Lastly, the substance of the white precipitate was examined. Turbidity resulting from the mixture of serum and distilled water did not emerge after total protein removal with acetonitrile, perchloric acid, or centrifugal ultrafiltration (Nanosep, Nihon Pall Ltd., Tokyo, Japan) from the patient’s serum. Creatinine values measured with the kit used in our hospital after protein removal were comparable to those determined by other kits, thus again indicating that the components of the white precipitate were proteins. In addition, the reaction curve was examined after elimination of non-IgM proteins with protein G–coupling agarose, which binds IgG, and Jacalin-coupling agarose, which binds non-IgG and non-IgM globulin (Thermo Fisher Scientific K.K., Tokyo, Japan). This pretreatment attenuated the rapid increase in the curves but did not normalize the distortion (Figure 3), which confirmed that the aggregated protein was IgM.
Figure 3.
Differences in reaction curves depending on sample pretreatment. A sharp elevation due to addition of the second reagent is observed in the serum of this case without pretreatment (black line), which is ablated by elimination of total protein (red line). This distortion is partially attenuated but present after removal of non-IgM protein with 2 agarose-embedded proteins (blue line).
Discussion
Some researchers reported that high pH, low pH, and low ionic strength of the reagents were associated with coagulation of IgM and the resultant interference with the enzymatic assay (Figure 4).S2 In the present study, it was demonstrated that low ionic strength of the second reagent in the creatinine kit was linked to aggregation of paraproteins in the patient’s serum, and that the white precipitate included IgM when using agarose-embedded proteins that can specifically bind non-IgM proteins. The Sia euglobulin precipitation test (Sia test) is a classical method for detecting clonal gammopathy by mixing the patient’s sera and water, because euglobulin and macroglobulin are insoluble in water.S3 In addition to the positivity of the Sia test, the fact that 5% sodium chloride solution eradicated the white precipitate suggests that the ionic strength of the reagents plays an important role in the aggregation of globulins, and that the importance of components other than salt in the reagents is limited. This assumption is supported by the present findings that the ionic strength of the reagents in the kit used in our laboratory was lower than that of the kits from the other manufacturers, which indicated the “true” creatinine levels of this patient. Interference with the assay was not observed when measuring serum creatinine levels of other patients with IgM-paraproteinemia (data not shown). Although it can be assumed that the solubility of IgM in water varies depending on the differences in the structures of IgM, the detailed cause remains unknown. Given that the reagents of the Jaffe method include a solution with high pH, the pH of the reagents does not seem to have been related to the white precipitate in the present case.
Figure 4.
Summary of creatinine measuring methods and mechanisms of interference. Mechanisms involved in this case are highlighted in red. *Adequate pretreatments eliminate interfering substances, including proteins. ID-LC/MS, isotope dilution liquid chromatography/tandem mass spectrometry.
Several issues need to be considered in clinical practice (Table 3). First, clinicians should take pseudohypercreatininemia into account when an unrealistic increase in creatinine levels alone is observed. This report highlights the limitation of the enzymatic method, even though this method is less affected by interference by non-creatinine substances than the Jaffe method. Notably, the kit on a specific platform including reagents with low ionic strength may lead to falsely elevated or decreased creatinine levels. In a case in which pseudohypercreatininemia is suspected, it is necessary to consult laboratory staff about errors in the reaction process, such as changes in the appearance of sera and distorted reaction curves.
Table 3.
Teaching points in the case
| 1. Paraproteins are associated with pseudohypercreatininemia even when using the enzymatic method. |
| 2. Low ionic strength of the reagents in the enzymatic assays may lead to precipitation of paraproteins and resultant false elevations of serum creatinine levels. |
| 3. Determination of white precipitate and of distorted reaction curve is necessary to demonstrate spurious elevations of creatinine levels. |
| 4. Combination of measurement of serum creatinine levels after deproteinizing pretreatment and tests that do not involve serum creatinine levels, such as cystatin C or scintigraphy, can provide true estimated glomerular filtration rate in patients with paraproteinemia. |
Second, the determination of correct serum creatinine concentrations is needed. There are some options for measuring accurate serum creatinine levels of patients with paraproteinemia. The present results showed that deproteinization with acetonitrile, perchloric acid, or centrifugal ultrafiltration successfully eliminated the interference caused by paraproteinemia and normalized the distorted reaction curves. From the clinical perspective, creatinine measurement following protein removal from sera seems to be one of the best ways to obtain serum creatinine concentrations, because the protein extraction process enables the use of the same kit, even if it leads to the diagnosis of pseudohypercreatininemia secondary to IgM paraproteinemia. The kits from other manufacturers may lead to correct creatinine level measurements unless a white precipitate forms. It should be kept in mind that measured values include small but non-negligible differences from kit to kit. The Jaffe method measures creatinine levels through a chemical reaction different from the enzymatic method, but this method is susceptible to interference by non-creatinine chromogens such as glucose, ascorbic acid, pyruvate, and cephalosporin antibiotics (Figure 4). Paraprotein precipitation owing to the high pH of the reagents may disrupt the normal process of creatinine measurement with the Jaffe method. Creatinine quantification with liquid chromatography-isotope dilution mass spectrometry, a reference procedure for the calibration of creatinine levels measured by the other methods, can provide correct serum creatinine levels regardless of co-existing paraproteins (Figure 4).6,S4 However, this method requires time- and cost-consuming sample preparation and special equipment, thereby limiting the facilities that can perform the measurement.
Last, the true renal function of patients with paraproteinemia should be determined using tests that do not involve serum creatinine levels. Blood urea nitrogen testing kits are widely available, but it is affected by gastrointestinal bleeding or volume depletion. Interference by paraprotein with the kit for blood urea nitrogen has also been reported.S5 Serum β2-microglobulin concentrations are often elevated in patients with multiple myeloma or lymphoplasmacytic lymphoma with IgM-type monoclonal gammopathy reflecting the tumor burden, and they are associated with the prognosis even without renal impairment,S6,S7 thus limiting the usefulness of this marker. There are no reports, to date, that paraproteins interfere with the cystatin C assay. Some researchers suggest that tumor burden in patients with multiple myeloma is not associated with serum cystatin C levels,S8 but this report cannot rule out the possibility of interference by paraproteins with this assay. Renal scans using the radioisotope technetium Tc-99m mertiatide or Tc-99m succimer provide better renal excretory function, but they have the disadvantages of high cost and limited availability. Combinations of the tests described above are helpful for measuring the renal function of patients with pseudohypercreatininemia accurately.
Conclusion
A case presenting with pseudohypercreatininemia caused by precipitation of IgM paraprotein resulting from mixture of the sera and the reagent solution with low ionic strength was reported. Measurement of serum globulin levels, especially IgM and IgG, and examination of the reaction process are required when an elevation of creatinine levels alone is observed. To rule out pseudohypercreatininemia, comprehensive assessment with measurement of serum creatinine level after deproteinization and other methods providing GFR without creatinine is required.
Disclosure
TW has received honoraria from Chugai Pharmaceutical, Daichi Sankyo, Kyowa Kirin, and Otsuka Pharmaceutical; and grant from Daichi Sankyo and Otsuka Pharmaceutical. HM has received consulting fees from BML Inc., SRL Inc., and Eiken Chemical Co. Ltd.; and research support from Tosoh Co. Ltd. and Kanto Chemical Industry Co. Ltd. MF declared having received speaker honoraria from Bayer HealthCare, Kissei Pharmaceutical, Kyowa Kirin, and Torii Pharmaceutical; consultancy fees from Kyowa Kirin, Ono Pharmaceutical, and Torii Pharmaceutical; and grants from Kyowa Kirin and Ono Pharmaceutical. All the other authors declared no competing interests.
Acknowledgments
The results of this report were presented at the American Society of Nephrology Kidney Week 2018 in San Diego, CA, on October 27, 2018, and the 48th Eastern Regional Meeting of the Japanese Society of Nephrology in Tokyo, Japan, on October 20, 2018.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 3.Lee E., Collier C.P., White C.A. Interlaboratory variability in plasma creatinine measurement and the relation with estimated glomerular filtration rate and chronic kidney disease diagnosis. Clin J Am Soc Nephrol. 2017;12:29–37. doi: 10.2215/CJN.05400516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killeen A.A., Ashwood E.R., Ventura C.B., Styer P. Recent trends in performance and current state of creatinine assays. Arch Pathol Lab Med. 2013;137:496–502. doi: 10.5858/arpa.2012-0134-CP. [DOI] [PubMed] [Google Scholar]
- 5.Drion I., Cobbaert C., Groenier K.H. Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol. 2012;13:133. doi: 10.1186/1471-2369-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hummel K.M., von Ahsen N., Kühn R.B. Pseudohypercreatininemia due to positive interference in enzymatic creatinine measurements caused by monoclonal IgM in patients with Waldenström’s macroglobulinemia. Nephron. 2000;86:188–189. doi: 10.1159/000045741. [DOI] [PubMed] [Google Scholar]
- 7.Rudofsky G., Villalobos M., Waldherr R. The Case ∣ Renal failure in a male with Waldenström's macroglobulinemia. Kidney Int. 2010;77:371–372. doi: 10.1038/ki.2009.465. [DOI] [PubMed] [Google Scholar]
- 8.McGill M.R., Vijayan A., Trulock E.P. Falsely elevated plasma creatinine due to an immunoglobulin M paraprotein. Am J Kidney Dis. 2016;68:789–792. doi: 10.1053/j.ajkd.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Kadatz M.J., Lee E.S., Duncan J. A peculiar case of paraproteinemia and elevated creatinine. Am J Kidney Dis. 2018;71:A15. doi: 10.1053/j.ajkd.2017.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.