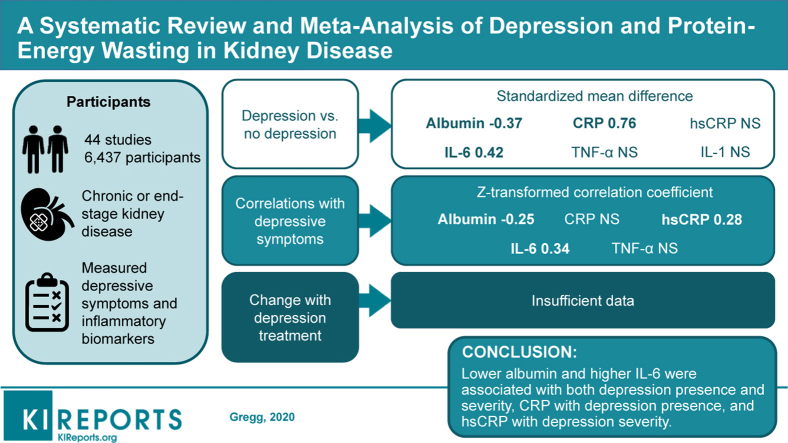
Abstract
Introduction
Depression comorbid with chronic disease may be mediated by inflammation. We sought to characterize relationships between inflammatory biomarkers and depressive symptoms in patients with chronic kidney disease and end-stage kidney disease.
Methods
A systematic literature search was conducted by 2 authors up to March 19, 2019, for studies of patients with chronic kidney disease or end-stage kidney disease evaluating circulating inflammatory biomarkers associated with depression of chronic disease: albumin, C-reactive protein (CRP), high-sensitivity CRP, interleukin-6 (IL-6), tumor necrosis factor-α, and interleukin-1. Standardized mean differences in biomarkers between individuals with and without depression were computed and analyzed using mixed effects models. Correlations between biomarkers and the severity of depressive symptoms were computed.
Results
Thirty-four studies (5652 participants) compared biomarkers between depressed and nondepressed individuals. Individuals with depression had lower albumin levels (standardized mean difference, −0.37; 95% confidence interval [CI], −0.61 to −0.13), higher CRP levels (standardized mean difference, 0.76; 95% CI, 0.16–1.37), and higher IL-6 levels (standardized mean difference, 0.42; 95% CI, 0.21–0.63). Studies were heterogeneous for albumin, CRP, high-sensitivity CRP, and tumor necrosis factor-α. Twenty-three studies (3047 participants) investigated correlations between biomarkers and depressive symptoms. The severity of depressive symptoms correlated with albumin (Z = −0.25; 95% CI, −0.36 to −0.14), high-sensitivity CRP (Z = 0.28; 95% CI, 0.13–0.43), and IL-6 (Z = 0.34; 95% CI, 0.18–0.49). There was heterogeneity across studies of IL-6. Only 6 studies (321 participants) investigated the effect of antidepressant treatment on inflammatory biomarkers, which was insufficient to combine in meta-analysis.
Conclusion
Lower albumin and higher IL-6 were associated with both the presence and severity of depression, CRP with the presence of depression, and high-sensitivity CRP with the severity of depressive symptoms. The effect of interventions to lower inflammation in patients with kidney disease and depression deserves investigation.
Keywords: albumin, chronic kidney disease, C-reactive protein, depression, hemodialysis, inflammation
Graphical abstract
Major depressive disorder is prevalent in up to 25% of individuals with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) compared with ∼7% in the general population and is associated with death, hospitalization, and dialysis initiation.1, 2, 3, 4 In the general population, circulating inflammatory biomarkers, such as interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), and tumor necrosis factor-α (TNF-α), are associated with the presence of depressive symptoms,5, 6, 7 and biomarkers of chronic inflammation predict incident depression.8 Associations between depression, elevated inflammatory cytokine levels, protein catabolism, and cardiovascular disease have led to the proposal of protein–energy wasting syndrome as a potential mechanism underlying the high comorbidity of depression with chronic medical illnesses, poor response to antidepressant medications in patients with chronic disease, and associations of depression with long-term morbidity and mortality.9, 10, 11, 12, 13 Protein–energy wasting refers to the complex constellation of factors including inflammation, malnutrition, comorbid medical illnesses, protein catabolism, metabolic acidosis, hormonal abnormalities, and loss of kidney function that contribute to cardiovascular disease and muscle wasting in patients with CKD and ESKD.14,15 Given that CKD and ESKD are disease states associated with increased underlying systemic inflammation and protein–energy wasting13,16,17 and that depression is prevalent and associated with adverse outcomes in these patient populations, the association of inflammation with depressive symptoms in such patients deserves exploration.
Several observational studies have sought to investigate these associations in patients with dialysis-dependent ESKD, and data are scarcer in those with nondialysis CKD. These studies reported heterogeneous results, likely because many were limited by small sample sizes, which do not allow the derivation of definitive conclusions. The objective was to, therefore, conduct a systematic review and meta-analysis of observational studies that investigated (i) associations of circulating inflammatory biomarkers and common measures of malnutrition with depression prevalence in patients with CKD and ESKD; (ii) associations of these circulating protein–energy wasting biomarkers with depressive symptom severity, as captured by self-report measures of depressive affect; and (iii) the effect of treatment with antidepressant medications on change from baseline in the levels of these biomarkers. The rationale was to summarize relationships between inflammatory biomarkers and depression to provide a resource for future investigation to identify novel depression prediction models and treatment strategies in patients with CKD and ESKD.
Methods
Search Strategy and Study Selection Criteria
We performed a systemic review and meta-analysis in accordance with published guidelines outlined by the Preferred Reporting Items for Systemic Reviews and Meta-Analyses.18 A systemic search was conducted in PubMed for published studies up to March 19, 2019, for the keyword search terms “depression,” “hemodialysis,” “peritoneal dialysis,” “chronic kidney disease,” “end-stage renal disease,” “cytokine,” “inflammation,” “syndrome,” “complex,” and “interleukin.” The search was limited to human studies. Only studies in the English language were included. Studies involving recipients of kidney transplantation were excluded. Two authors (GM and DL) independently evaluated all resulting search citations by title and abstract and the full text of any reference that seemed pertinent to the questions of interest. Discrepancies were resolved by a third author (LPG). We included studies of prespecified circulating inflammatory biomarkers previously reported to be associated with depression of chronic disease: albumin, C-reactive protein (CRP) measured by the regular or high-sensitivity assay, IL-6, TNF-α, and interleukin-1 (IL-1).19,20 Studies included in the analysis involved patients with CKD and/or ESKD and addressed at least 1 of the 3 following areas: (i) comparison of circulating inflammatory biomarker levels between individuals with and without depression, (ii) correlation of circulating inflammatory biomarker levels with severity of depressive symptoms, or (iii) change in inflammatory biomarkers from baseline after treatment with an antidepressant medication. We included all study designs, including retrospective studies, cross-sectional studies, prospective cohort studies, and clinical trials.
Data Extraction and Classification
Two authors (GM and LPG) independently extracted data from all selected studies and individually recorded the data into the meta-analysis electronic database sequentially. Specific data extracted were number of participants, presence of nondialysis CKD or ESKD, type of dialysis therapy (hemodialysis [HD] vs. peritoneal dialysis [PD]), measurement tool and definition or cutoff used for depression diagnosis, and type and level of circulating inflammatory biomarker. Two authors (GM and LPG) independently evaluated factors such as the study design and the risk of selection or publication bias. Any differences between the 2 reviews were resolved through consensus.
Outcome Measures
The prespecified primary outcome was the association of inflammatory biomarkers with the presence of depression, as defined in each study included in the meta-analysis. Secondary outcomes included correlations of inflammatory biomarkers with the severity of depressive symptoms as ascertained by self-report measures and the impact of treatment with antidepressant medications on change from baseline in biomarkers levels.
Statistical Analysis
Meta-analysis was performed using a mixed effects model with study as the random effect and implemented in the “metaphor” package in R.21 Thus, we assumed that the studies included in this analysis were a random sample from a larger population of studies. The Knapp and Hartung adjustment (appropriate for the meta-analysis involving a small number of studies) accounted for the fact that the true effect heterogeneity parameter (τ2) was estimated rather than known.22 Heterogeneity was measured by the I2 statistic, where values between 75% and 100% indicated considerable heterogeneity, and the Q statistic was tested for significant heterogeneity across studies.23 Heterogeneity was investigated with regard to 2 factors with sufficient numbers of studies for analysis: sample composition (HD vs. other) and depression outcome measure (Beck Depression Inventory [BDI] vs. other). No systematic bias was observed because of stratification or adjustment for these factors. Funnel plot asymmetry was tested with a rank correlation test to ascertain potential publication bias.24 The quality of each study was measured using Grading of Recommendations Assessment, Development, and Evaluation criteria.25 Meta-analysis was performed for standardized mean differences between depressed and nondepressed participants and for Fisher r-to-Z transformed correlations between biomarkers and the severity of depressive symptoms for each inflammatory biomarker for each study that provided the appropriate outcome measure. Fisher r-to-Z transformation was used because it provides stable variance estimates and a more normal distribution. Studies providing a linear regression β coefficient rather than a correlation coefficient were not included in the meta-analysis of correlations. In a sensitivity analysis, a meta-analysis of correlations was performed without application of Fisher r-to-Z transformation. In a prespecified analysis plan, we required that only biomarkers with a minimum of ≥4 studies reporting the necessary statistics would be included in the meta-analysis. Although meta-analysis can be performed with only 2 studies, we believe that 2 studies would not be adequate to obtain useful estimates. In contrast, requiring a large number of studies would limit the number of biomarkers that could be analyzed. Therefore, we chose a minimum of 4 studies in an attempt to balance statistical considerations with analysis of as many biomarkers as possible.
Results
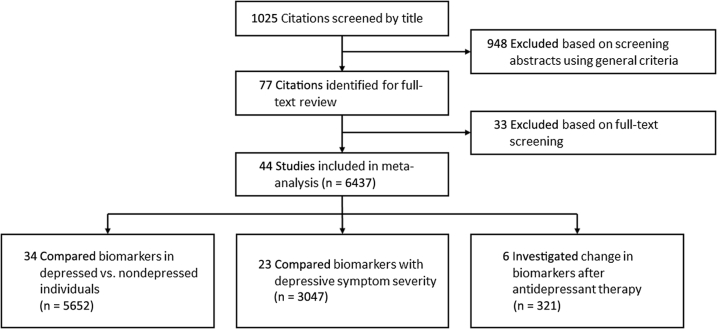
Study Flow and Characteristics
Our search criteria revealed 1025 citations. A total of 948 were excluded by screening or detailed review criteria. Forty-four studies, including 6437 unique participants, met criteria for inclusion (Figure 1). The majority of included studies were conducted in patients with ESKD. Of these, 26 sampled only patients on HD, 5 sampled only patients on PD, and 11 sampled both patients on HD and those on PD. Only 5 studies included participants with nondialysis CKD, and 2 were conducted exclusively in patients with nondialysis CKD. Studies were heterogeneous; several different scales were used to measure depressive symptoms; and inconsistent cutoffs of these scales were used to identify the presence of depression. Because of the observational nature of the majority of the data, most studies were classified as moderate or low according to Grading of Recommendations Assessment, Development, and Evaluation criteria (Tables 1 and 2).25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64
Figure 1.
Identification of eligible studies.
Table 1.
Comparisons of inflammatory biomarkers in CKD or ESKD samples with vs. without depression
| Study | Study design | GRADE score | Sample | No. of patients with depression | Depression definition (categorically) | Biomarkers measured |
|---|---|---|---|---|---|---|
| Dogan et al.26 | Cross-sectional | Low | 43 HD | 21 | HAMD >7 | Albumin, CRP |
| Kalender et al.27 | Cross-sectional | Low | 68 HD, 47 PD, 26 CKD | 34 | DSM IV criteria | Albumin, CRP |
| Micozkadioglu et al.28 | Cross-sectional | Low | 110 HD | 71 | CDI >10 | Albumin, CRP |
| Boulware et al.29 | Prospective cohort | Moderate | 688 HD, 229 PD | 221 | MHI-5 ≤52 | Albumin, CRP, IL-6 |
| Kalender et al.30 | Cross-sectional | Low | 42 PD | 11 | SCID-I | hsCRP, IL-6, TNF-α, IL-1 |
| Simic Ogrizovic et al.9 | Prospective cohort | Low | 77 HD, 51 PD | 58 | BDI ≥14 | Albumin |
| Hsu et al.31 | Cross-sectional | Low | 51 HD | 18 | HADS ≥8 | Albumin, CRP |
| Montinaro et al.32 | Cross-sectional | Very low | 30 HD | 19 | HADS ≥8 | Albumin |
| Bossola et al.33 | Cross-sectional | Low | 80 HD | 42 | BDI ≥15 | Albumin, hsCRP, IL-6 |
| Ko et al.34 | Cross-sectional | Low | 81 PD | 43 | BDI >15 | Albumin, hsCRP, TNF-α |
| Gyamlani et al.35 | Cross-sectional | Low | 71 CKD | 18 | CES-D ≥16 | Albumin, CRP |
| Li et al.36 | Cross-sectional | Moderate | 142 PD | 37 | HAMD ≥10 | Albumin, CRP |
| Hung et al.37 | Cross-sectional | Moderate | 146 HD | 68 | BDI ≥14 | Albumin, hsCRP, IL-6 |
| Chilcot et al.38 | Prospective cohort | Low | 132 HD, 28 PD | 41 | BDI ≥16 | Albumin |
| Bornivelli et al.39 | Cross-sectional | Low | 45 HD | 16 | HAMD >7 | Albumin, CRP |
| Armaly et al.40 | Cross-sectional | Low | 71 HD | 31 | BDI >11 or HADS >18 | Albumin, CRP |
| Kim et al.41 | Cross-sectional | Low | 78 HD | 35 | BDI ≥20 | Albumin |
| Cilan et al.42 | Prospective cohort | Low | 40 HD | 9 | DSM IV criteria | IL-6, TNF-α, IL-1 |
| Wang et al.43 | Cross-sectional | Moderate | 195 HD | 47 | MINI | Albumin, CRP, IL-6, TNF-α, IL-1 |
| Taraz et al.44 | Cross-sectional | Moderate | 83 HD | 51 | BDI ≥16 | Albumin, hsCRP, IL-6, TNF-α, IL-1 |
| Su et al.45 | Cross-sectional | Low | 274 HD | 118 | BDI ≥14 | Albumin, hsCRP |
| Cilan et al.46 | Prospective cohort | Low | 40 PD | 10 | DSM IV criteria | Albumin |
| Preljevic et al.47 | Cross-sectional | Low | 84 HD, 25 PD | 24 | SCID-I | Albumin |
| Nowak et al.48 | Cross-sectional | Low | 694 HD | 268 | BDI >16 | CRP |
| Knuth et al.49 | Cross-sectional | Low | 75 HD | 36 | BDI ≥14 | Albumin, IL-6 |
| Fan et al.50 | Prospective cohort | Moderate | 323 HD | 83 | CES-D ≥16 | Albumin |
| Loosman et al.51 | Prospective cohort | Low | 100 CKD | 34 | BDI ≥11 | Albumin |
| Bossola et al.52 | Cross-sectional | Moderate | 100 HD | 74 | BDI ≥10 | Albumin, hsCRP, IL-6 |
| Ekramzadeh et al.53 | Cross-sectional | Low | 110 HD | 80 | BDI ≥10 | Albumin |
| Gok Oguz et al.54 | Cross-sectional | Low | 40 PD | 16 | BDI ≥10 | Albumin |
| Barros et al.55 | Prospective cohort | Low | 104 HD | 32 | BDI ≥15 | Albumin |
| Haverkamp et al.56 | Prospective cohort | Moderate | 436 HD, 54 PD | 211 | BDI ≥13 | TNF-α |
| Jong et al.57 | Cross-sectional | Low | 129 HD | 43 | BDI ≥14 | Albumin, hsCRP, IL-6, TNF-α |
| Chilcot et al.58 | Cross-sectional | Low | 396 HD | 121 | BDI ≥16 | Albumin |
BDI, Beck Depression Index; CDI, Cognitive Depression Index; CES-D, Center for Epidemiologic Studies-Depression; CKD, chronic kidney disease; CRP, C-reactive protein; DSM IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ESKD, end-stage kidney disease; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Scale; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; MHI-5, Medical Outcomes Study Short Form-36; MINI, Mini International Neuropsychiatric Interview; PD, peritoneal dialysis; SCID-I, Structured Clinical Interview for DSM-IV Axis I Disorders; TNF-α, tumor necrosis factor-α.
Table 2.
Correlations of the severity of depressive symptoms with inflammatory biomarkers in patients with CKD and ESKD
| Study | Study design | GRADE score | Sample | Depressive symptom measure (continuous score) | Biomarkers measured |
|---|---|---|---|---|---|
| Dogan et al.26 | Cross-sectional | Low | 43 HD | HAMD | Albumin, CRP |
| Kalender et al.27 | Cross-sectional | Low | 68 HD, 47 PD, 26 CKD | BDI | Albumin, CRP |
| Kalender et al.59 | Cross-sectional | Low | 68 HD, 47 PD, 26 CKD, 66 controls | SF-36 mental composite | Albumin, CRP |
| Dervisoglu et al.60 | Cross-sectional | Low | 31 HD, 31 PD, 31 CKD | BDI | IL-6, TNF-α |
| Simic Ogrizovic et al.9 | Prospective cohort | Low | 77 HD, 51 PD | BDI | Albumin, hsCRP, IL-6 |
| Bossola et al.33 | Cross-sectional | Low | 80 HD | BDI | Albumin, hsCRP, IL-6 |
| Ko et al.34 | Cross-sectional | Low | 81 PD | BDI | Albumin, hsCRP, TNF-α |
| Sonikian et al.61 | Cross-sectional | Low | 27 HD, 17 PD | Zung Self-Rating Depressoin Scale | IL-6 |
| Chilcot et al.38 | Prospective cohort | Low | 132 HD, 28 PD | BDI | Albumin, CRP |
| Hung et al.37 | Cross-sectional | Moderate | 146 HD | BDI | Albumin, hsCRP, IL-6 |
| Li et al.36 | Cross-sectional | Moderate | 142 PD | HAMD | Albumin, CRP |
| Choi et al.13 | Prospective cohort | Low | 81 HD | BDI | Albumin, hsCRP |
| Kim et al.41 | Cross-sectional | Low | 78 HD | BDI | Albumin, hsCRP |
| Taraz et al.44 | Cross-sectional | Moderate | 83 HD | BDI | Albumin, hsCRP, IL-6, TNF-α, IL-1 |
| Bornivelli et al.39 | Cross-sectional | Low | 45 HD | HAMD | CRP |
| Wang et al.43 | Cross-sectional | Moderate | 195 HD | HADS + SF-36 depression | Albumin, CRP, IL-6, TNF-α, IL-1 |
| Nowak et al.48 | Cross-sectional | Low | 694 HD | BDI | CRP |
| Knuth et al.49 | Cross-sectional | Low | 75 HD | BDI | IL-6 |
| Ekramzadeh et al.53 | Cross-sectional | Low | 110 HD | BDI | Albumin |
| Uglesic et al.62 | Cross-sectional | Low | 52 HD, 36 PD | BDI | Albumin, CRP, IL-6 |
| Jong et al.57 | Cross-sectional | Low | 129 HD | BDI | Albumin, hsCRP, IL-6, TNF-α |
| Zhao et al.63 | Randomized trial | High | 189 HD | BDI | IL-6 |
| Schricker et al.64 | Cross-sectional | Low | 26 HD, 13 PD | Allgemeine Depressionsskala | CRP, IL-6 |
BDI, Beck Depression Index; CKD, chronic kidney disease; CRP, C-reactive protein; ESKD, end-stage kidney disease; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Scale; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; PD, peritoneal dialysis; SF-36, Short-Form Health-Related Quality of Life 36-item questionnaire; TNF-α, tumor necrosis factor-α.
Inflammation and the Presence of Depression
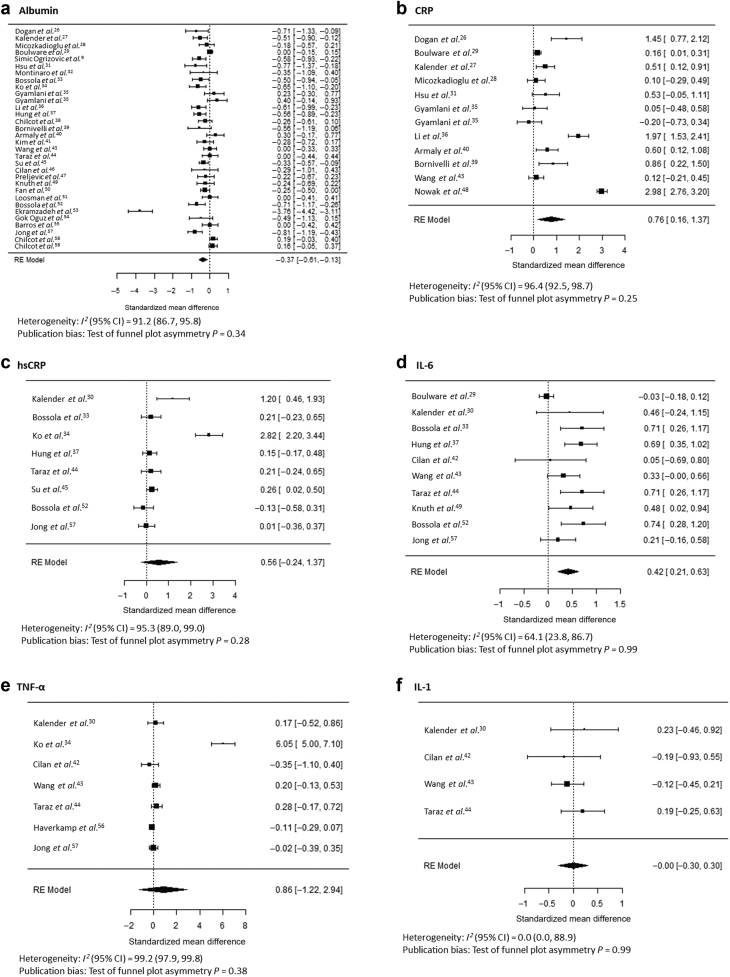
In CKD and ESKD samples, of the 44 studies included, 34 tested whether levels of inflammatory biomarkers differed between individuals with and without depression (Table 1). Serum albumin was the most frequently studied biomarker, tested in 30 studies. Overall, serum albumin level was found to be lower in depressed than in nondepressed individuals (standardized mean difference, −0.37; 95% confidence interval [CI], −0.61 to −0.13) (Figure 2a9,26, 27, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58). CRP reached statistical significance in the meta-analysis (standardized mean difference, 0.76; 95% CI, 0.16–1.37), but hsCRP did not (Figure 2b and c). IL-6 level was higher in depressed than in nondepressed individuals (standardized mean difference, 0.42; 95% CI, 0.21–0.63) (Figure 2d). The few studies of TNF-α (n = 7) and IL-1 (n = 4) showed no association of levels of these biomarkers with the presence of depression (Figure 2e and f). Studies of each biomarker except IL-1 were significantly heterogeneous (P < 0.001), with I2 statistic >75% for all biomarkers except IL-1 (I2 = 0%) and IL-6 (I2 = 64%), but all tests of funnel plot asymmetry were nonsignificant (Figure 2a–f; Supplementary Figure S1). The underlying data used to compare biomarkers in those with versus without depression are presented in Supplementary Table S1.
Figure 2.
Standardized mean differences between individuals with and without depression, as defined in each study, in albumin (a), C-reactive protein (CRP) (b), high-sensitivity CRP (hsCRP) (c), interleukin-6 (IL-6) (d), tumor necrosis factor-α (TNF-α) (e), and interleukin-1 (IL-1) (f) levels.9,26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 RE, random effects.
Inflammation and the Severity of Depressive Symptoms
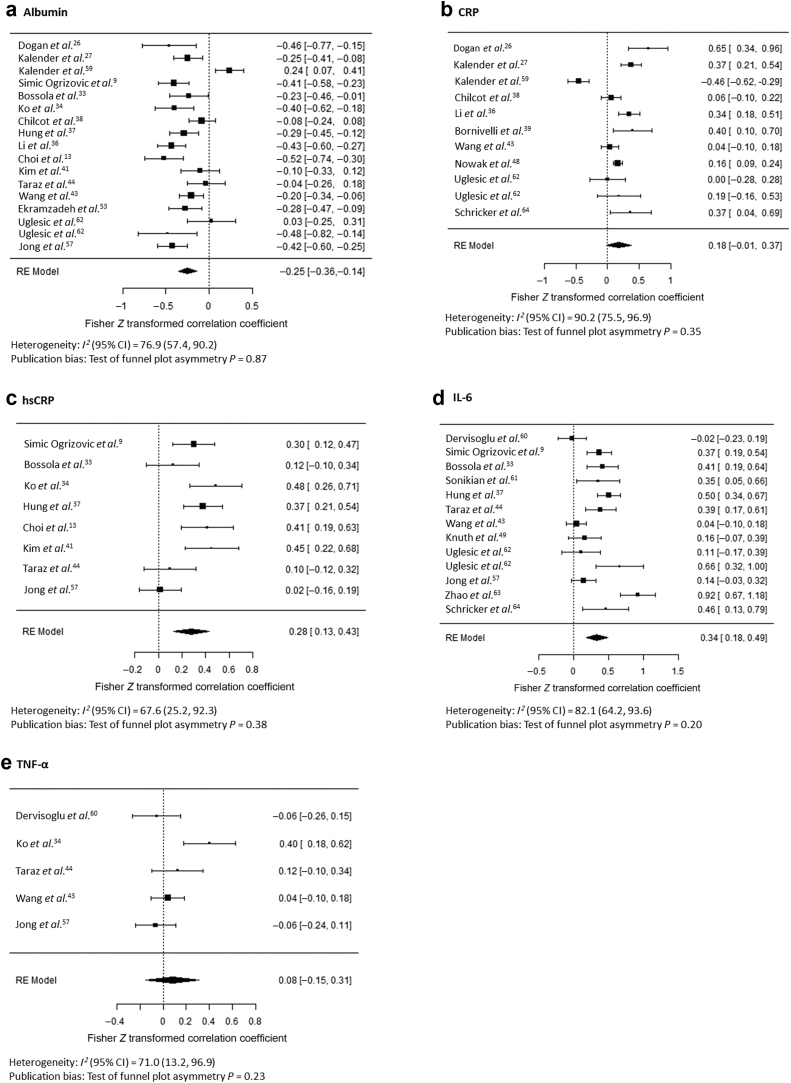
A total of 23 studies reported cross-sectional correlations of inflammatory biomarkers with the severity of depressive symptoms in patients with CKD and ESKD (Table 2). By Fisher r-to-Z transformation, the serum albumin level negatively correlated with the severity of depressive symptoms (Z = −0.25; 95% CI, −0.36 to −0.14) (Figure 3a9,13,26,27,33,34,36, 37, 38, 39,41,43,44,48,49,53,57,59, 60, 61, 62, 63, 64). CRP and hsCRP levels correlated positively with the severity of depression in several individual studies. In meta-analysis, CRP did not reach statistical significance (Figure 3b) but hsCRP levels correlated significantly with the severity of depressive symptoms (Z = 0.28; 95% CI, 0.13–0.43) (Figure 3c). IL-6 levels also associated with the severity of depressive symptoms in the majority of studies and in pooled analysis (Z = 0.34; 95% CI, 0.18–0.49) (Figure 3d). TNF-α level, investigated in 5 studies, was not associated with the severity of depressive symptoms (Figure 3e). Only 2 studies investigated correlations of IL-1 levels with the severity of depressive symptoms, so these were not combined in meta-analysis. Studies were significantly heterogeneous for each biomarker, with studies showing considerable heterogeneity except for TNF-α (I2 = 74%) and hsCRP (I2 = 70%), but tests of funnel plot asymmetry were nonsignificant (Figure 3a–e; Supplementary Figure S2). The results were similar using nontransformed correlation coefficients (Supplementary Figure S3A–E). The underlying data used to evaluate correlations of depressive symptoms with inflammatory biomarkers are presented in Supplementary Table S2.
Figure 3.
Correlations of depressive symptoms, taken continuously, with albumin (a), C-reactive protein (CRP) (b), high-sensitivity CRP (hsCRP) (c), interleukin-6 (IL-6) (d), and tumor necrosis factor-α (TNF-α) (e) levels.9,13,26,27,33,34,36, 37, 38, 39,41,43,44,48,49,53,57,59, 60, 61, 62, 63, 64 RE, random effects.
Effect of Antidepressant Treatment on Inflammatory Biomarkers
Only 6 studies, all in ESKD samples, investigated whether treatment with antidepressant medications affected systemic levels of inflammatory biomarkers (Table 3).65, 66, 67 Selective serotonin reuptake inhibitors (SSRIs) were used for depression treatment in all the studies. Serum albumin level, measured in 3 studies, was not shown to change with antidepressant therapy.46,66,67 CRP level was found to decrease with sertraline treatment in 1 study,67 but 3 others, 2 of which used the high-sensitivity assay, found no difference.46,65,66 The most commonly studied biomarker was IL-6, which was included in 5 studies. Of these, 2 showed no difference in IL-6 levels,42,63 2 showed that IL-6 level increased in those whose depressive symptoms responded to treatment,46,65 and 1 showed that IL-6 level significantly decreased with sertraline treatment.66 Three of the 4 studies of TNF-α showed no difference after treatment with sertraline or fluoxetine,42,46,65 but the largest study showed that TNF-α level decreased after 12 weeks of sertraline treatment.66 Two of the 3 studies that reported IL-1 showed a significant change, but in one the level decreased and in the other it increased.46,65
Table 3.
Clinical trials of change in inflammatory biomarker levels with treatment of depression in patients with ESKD
| Study | Sample | Intervention | Depression definition | Timing | Albumin levela (g/dl) | CRP levela (mg/dl) | hsCRP levela (mg/dl) | IL-6 levela (pg/ml) | TNF-α levela (pg/ml) | IL-1 levela (pg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al.65 | 28 HD | Fluoxetine 20 mg/d (8 wk) | BDI ≥11 and HAMD ≥7 | Baseline | 219 ± 50b | 7.26 ± 2.51 | 80.67 ± 11.4 | 18.87 ± 2.1 | ||
| Study exit | 234 ± 60b | 10.58 ± 2.37c | 65.37 ± 5.97 | 10.52 ± 1.18c | ||||||
| Cilan et al.42 | 9 HD | Sertraline 50 mg/d (8 wk) | DSM IV criteria | Baseline | 143.78 ± 85.84 | 109.22 ± 260.15 | 135.89 ± 171.46 | |||
| Study exit | 54.63 ± 83.71 | 56.56 ± 162.61 | 127.11 ± 216.27 | |||||||
| Cilan et al.46 | 10 PD | Sertraline 50 mg/d (8 wk) | DSM IV criteria | Baseline | 3.40 ± 0.60 | 9.01 (3.17–57.9) | 46.5 (5–200) | 25.5 (1–800) | 25 (3–340) | |
| Study exit | 3.21 ± 0.44 | 6.48 (3.17–41.4) | 200 (160–200)c | 220 (1–800) | 375 (3–500)c | |||||
| Taraz et al.66 | 50 HD (n = 21 in the sertraline group) | Sertraline 100 mg/d vs. placebo (12 wk) | BDI ≥16 | Baseline | 4.3 ± 0.3 | 4.0 (IQR, 2.8) | 7.8 (IQR, 6.6) | 13 (IQR, 8.9) | ||
| Study exit | 4.4 ± 0.3 | 3.9 (IQR, 3.0) | 4.7 (IQR, 4.9)c | 8.5 (IQR, 6)c | ||||||
| Zahed et al.67 | 35 HD with CRP level >5 mg/dl | Sertraline 50–200 mg/d (12 wk) | BDI >14 | Baseline | 3.09 ± 0.86 | 33.5 ± 24.2 | ||||
| Study exit | 4.17 ± 0.57 | 15.4 ± 12.6c | ||||||||
| Zhao et al.63 | 189 HD (n = 63 in the escitalopram group) | Escitalopram 20 mg/d and/or exercise (18 wk) | BDI ≥14 | Values not reported; P > 0.05 for comparison of baseline to study exit |
BDI, Beck Depression Index; CRP, C-reactive protein; DSM IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ESKD, end-stage kidney disease; HAMD, Hamilton Depression Scale; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; IQR, interquartile range; PD, peritoneal dialysis; TNF-α, tumor necrosis factor-α.
Presented as mean ± SD or median (IQR) at baseline (before antidepressant treatment) and at study exit.
Reported as 2.19 ± 0.5 mg/ml at baseline and 2.34 ± 0.6 mg/ml at study exit. Values have been converted to mg/dl for consistency in this table.
P < 0.05 compared to the baseline value.
Discussion
We report the following findings in patients with CKD and ESKD: (i) the presence of depression, albeit ascertained by variable scales and definitions, was associated with lower serum albumin and higher plasma CRP and IL-6 levels; (ii) more severe depressive symptoms correlated with lower albumin and higher IL-6 and hsCRP levels; and (iii) there were insufficient data to determine whether treatment with SSRI antidepressant medications was associated with changes in inflammatory biomarkers from baseline.
Serum albumin is a major biomarker of protein–energy wasting in patients with CKD and ESKD. The levels decrease in states of acute inflammation owing to increased protein catabolism or altered hepatic protein synthesis, and in CKD, it can also be lost in the urine in the setting of nephrotic range proteinuria.68 In addition, hypoalbuminemia is considered a highly sensitive but nonspecific biomarker of malnutrition68 and the contribution of malnutrition to protein–energy wasting may explain the association of low circulating albumin level with death in patients with ESKD.69, 70, 71, 72 We showed that serum albumin level was lower in depressed than in nondepressed patients with CKD and ESKD and negatively correlated with the severity of depressive symptoms. The heterogeneous results of these studies likely in part reflect small sample sizes, as larger studies more often detected a difference that smaller studies may have been underpowered to demonstrate. Thus, hypoalbuminemia and protein–energy wasting may in part explain the connection between depression and poor outcomes in patients with CKD and ESKD.3,13 It is unclear from these data whether the association of decreased albumin with worse depressive symptoms is due to effects of depression on appetite and weight, whether protein–energy wasting augments depressive symptoms, or whether hypoalbuminemia, associated with worse overall poor health in patients with kidney disease, may be reflected in physical and emotional symptoms.
The observed associations of plasma CRP and hsCRP with depression and depressive symptoms proved to be less parsimonious. Both the traditional and high-sensitivity assays for this biomarker had point estimates supporting higher levels in those with depression. This was particularly true of the traditional assay, with a standardized mean difference of 0.76 (95% CI, 0.16–1.37) and a Z-transformed correlation coefficient of 0.18 (95% CI, −0.01 to 0.37). Given the association of CRP with the presence of depression and that of hsCRP with the severity of depressive symptoms, overall these data suggest that there likely is an association between depression and systemic inflammation, but this analysis may have been limited by power to detect this association using only the high-sensitivity or traditional assay or by the heterogeneous definitions of depression in the included studies.
The mixed associations of CRP and hsCRP with depression did not clearly vary by study sample size, such that some small studies showed a highly significant difference30,34 and some large studies showed none.37,43 The differences in results were most likely driven by the heterogeneity of depression scales used to ascertain the presence of depression diagnosis or depressive affect or the way depression was defined in the study. Although no studies using cutoffs of the BDI found a difference in CRP levels, 2 found a difference in hsCRP levels.34,45 In correlation analyses of CRP, almost all the nonsignificant results were seen in comparison with the BDI score, whereas correlations with other measures such as the Hamilton Depression Scale score were found to be significant, even in smaller studies.26,36,39,64 Consequently, in addition to the previously discussed limitations of these studies, depressive symptoms measured by the BDI may reflect different qualities than did other depression scales, which may have influenced these results.
Higher IL-6 level was strongly associated with both the presence of depression and the severity of depressive symptoms. The smallest studies did not show a difference in IL-6 levels between depressed and nondepressed participants, which may have been limited by sample size.30,42 Larger studies tended to show higher IL-6 level associated with depression,33,37,43 but the largest study showed no association.29 Mean IL-6 levels were similar in both the negative and positive studies, suggesting the possibility of a moderating effect. Protein–energy wasting may explain the variability of these results; studies that showed no relationship between the BDI and IL-6 level reported lower hemoglobin level, lower albumin level, and generally lower body mass index, which could indicate that the severity of protein–energy wasting obfuscates the relationship between IL-6 and the BDI. Most of the studies using the BDI to define depression detected a difference in IL-6 level, whereas studies using other metrics failed to detect a difference, suggesting that the questions on the BDI may more accurately elicit symptom burden exemplified by IL-6–mediated pathways. Many depression scales incorporate questions about both mental and physical symptoms, and it remains unclear whether IL-6 level associates more strongly with physical symptoms such as fatigue than with emotional qualities such as anhedonia. Another possibility is that dialysis modality affects this association; the studies that showed a difference were all conducted exclusively in patients on HD, whereas 2 that showed no difference were conducted in patients on PD or a combination of patients on PD and those on HD.29,30
A few studies measured TNF-α or IL-1 levels, and most of these were small, with few participants with depression. Only 1 study that measured TNF-α levels identified a difference associated with depression and a correlation with severity of depressive symptoms,34 and none of the 4 studies that measured IL-1 levels detected a difference between depressed and nondepressed individuals.30,42, 43, 44 Ultimately there were too few data to determine whether associations between these inflammatory biomarkers and depressive symptoms exist in patients with CKD and ESKD.
Similarly, too few studies have investigated the effect of antidepressant medication therapy on inflammatory biomarkers in CKD or ESKD samples to draw definitive conclusions. Most studies were small, with only 1 including >50 participants.63 The 2 smallest studies, with 9 and 10 participants, were the only to diagnose depression by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria,42,46 while the rest used variable cutoffs of the BDI or the Hamilton Depression Scale. Four studies administered low doses of SSRIs (fluoxetine 20 mg/d or sertraline 50–100 mg/d), despite that no dose adjustments are recommended in patients with CKD or dialysis-dependent patients,73 leaving questions about whether an adequate dose was trialed. Most of the studies had no control arm to compare against SSRI therapy to determine whether SSRIs accounted for the changes in inflammatory biomarkers or whether some change occurred as part of the natural history of the major depressive episode. Some studies treated participants for only 8 weeks,42,46,65 so may have missed a time-dependent effect of treatment on inflammation. Interestingly however, the largest study, which also offered the longest treatment course of 18 weeks, showed no difference in IL-6 levels, but unfortunately did not report levels of albumin, CRP, hsCRP, or TNF-α.63 The limitations of these studies may account for the variability seen in the results, and it remains promising yet unclear whether treatment of depression affects inflammatory biomarkers and whether this correlates with improvement in depressive symptoms in response to treatment. More studies are needed to determine whether existing traditional therapies to treat depressive symptoms may improve systemic inflammation and its association with poor long-term outcomes in patients with CKD and ESKD.
Our analysis has several important limitations. Studies of depression and inflammatory biomarkers were limited by small sample size, variable instruments and thresholds to define depression, and inadequate dosing or treatment duration of antidepressant medications. The heterogeneity, low to moderate quality according to Grading of Recommendations Assessment, Development, and Evaluation criteria, and limitations of the included studies must be considered when interpreting the meta-analysis results. Variability in inclusion and exclusion criteria, sampling methods, or depression measurement scales for individual studies may influence the results. It is also unclear whether some of the symptoms measured by the BDI and other depression scales such as insomnia, fatigue, and changes in appetite may be a result of kidney disease itself, which is known to be associated with inflammation, rather than similar symptoms caused by depression. The included studies used variable scales and cutoffs to define depression, which may have diminished the differences in inflammatory biomarkers seen between depressed and nondepressed individuals. Despite this, we identified associations between the presence of depression and lower albumin and higher CRP and IL-6 levels, suggesting that these findings are likely robust. Most of the included studies lacked adjustment for covariates, so it is unclear whether the associations that were seen could be accounted for by age, dialysis vintage, body mass index, comorbid medical conditions, protein–energy wasting, or other possible confounding factors. Larger studies controlling for these variables will be important to further detail the relationships between these factors and determine whether confounding variables might account for the variability in the results seen in prior studies.
Conclusion
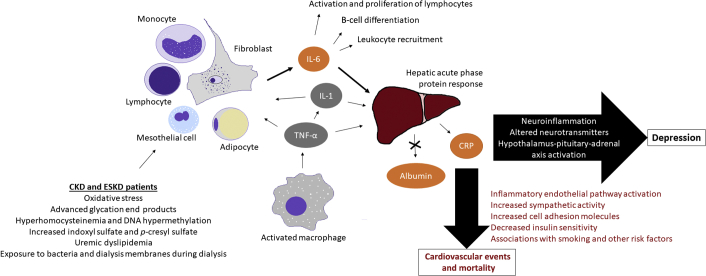
These data strongly support that there may be a relationship between protein–energy wasting, particularly pathways mediated by IL-6 and CRP, and depressive symptoms in patients with kidney disease. Future studies investigating the relationships between depression and protein–energy wasting in patients with CKD and ESKD should be adequately powered, include measurements of multiple biomarkers at baseline and after any intervention, and use criterion standard definitions of depression as well as continuous measures of depressive symptoms. Ideally any clinical trials would include clinically appropriate doses and treatment durations of any therapy for depression, as well as a control group to determine whether antidepressant therapy affects biomarkers or whether any changes could be accounted for by the natural course of the disease. A proposed conceptual model describing the relationship between inflammation, protein–energy wasting, and depression in patients with CKD and ESKD is shown in Figure 4.19,74, 75, 76
Figure 4.
Summary of proposed roles of inflammatory mediators of depression in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in protein–energy wasting. Orange inflammatory biomarkers are those shown to associate with depression and depressive symptoms, while gray indicates factors that were not shown to associate significantly with depression or depressive symptoms. Clearance of inflammatory cytokines may be decreased in patients with CKD and ESKD, contributing to elevated circulating levels. Furthermore, in patients with CKD and ESKD, oxidative stress, bacterial endotoxins, glycation end products, hyperhomocysteinemia, DNA hypermethylation, uremic toxins, and uremic dyslipidemia can stimulate various pro-inflammatory pathways, particularly leading to the generation of interleukin-6 (IL-6). In addition, primarily activated macrophages produce tumor necrosis factor-α (TNF-α), which has multiple downstream effects, including stimulation of interleukin-1 (IL-1) production and regulating much of the cytokine cascade. TNF-α and IL-1 both stimulate the production of IL-6 by monocytes, lymphocytes, mesothelial cells, fibroblasts, and adipocytes. IL-6, IL-1, and TNF-α in turn each activate the acute phase protein response in the liver, which induces hepatic production of C-reactive protein (CRP) and downregulates albumin production. Enhanced inflammation may contribute to depression via neuroinflammation, alterations in various neurotransmitter pathways, and activation of the hypothalamus-pituitary-adrenal axis. Inflammation-mediated cardiovascular disease may also explain associations between depression and cardiovascular events and death. Elevated high-sensitivity CRP, which is used for cardiovascular risk stratification, may be associated with these outcomes due to confounding factors such as smoking and diabetes mellitus in patients with CKD and ESKD, but some studies suggest that CRP and other inflammatory cytokines may have a pathophysiological role in vascular damage via the activation of inflammatory endothelial pathways, increased sympathetic activity, increased cell adhesion molecules, and decreased insulin sensitivity. Inflammatory cytokine networks are complex and interrelated, so consideration of these elevated biomarkers without the context of their soluble receptors or other cytokines in the inflammatory pathways likely oversimplifies these relationships. Nonetheless, studies have consistently shown relationships between elevated IL-6, CRP by the traditional or high-sensitivity assay, and albumin with depression, cardiovascular outcomes, and death in patients with CKD and ESKD, suggesting that these cytokines may be promising therapeutic targets to improve outcomes in these populations.
Many important knowledge gaps remain regarding the association of depression with protein–energy wasting and inflammation in CKD and ESKD, their relationship to outcomes, and the impact of various treatment regimens on depressive symptoms, inflammatory biomarkers, and long-term morbidity and mortality. As more research is done, it will be important to gather the molecular data to better identify patients who may benefit from traditional antidepressant therapy or anti-inflammatory–based treatment regimens. Understanding the relationship between depression and inflammation may be a key next step to identify patients with CKD and ESKD who are at risk of depression or who may variably respond to existing therapies.
Disclosure
MT has served as an advisor or consultant to the following organizations: Allergan Sales LLC, Alkermes, Arcadia Pharmaceuticals Inc., AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Eli Lilly and Company, Evotec, Johnson & Johnson, Lundbeck, MedAvante, Merck, MSI Methylation Sciences Inc., Nestle Health Science-Pamlab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceutical Co., Ltd., Roche Products Ltd., SHIRE Development LLC, Takeda Pharmaceutical Co., Ltd., and Tal Medical/PureTech Ventures. All the other authors declared no competing interests.
Acknowledgments
This work was supported by a grant (R01DK085512) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) awarded to SSH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, the National Institutes of Health, or the Department of Veterans Affairs. Parts of this work were presented in abstract form at the University of Texas Southwestern 2019 Donald W. Seldin Research Symposium on May 9, 2019.
Footnotes
Figure S1. Funnel plots for underlying data used for comparisons of inflammatory biomarkers in CKD or ESKD samples with vs. without depression.
Figure S2. Funnel plots for underlying data used for correlations of the severity of depression with inflammatory biomarkers in CKD and ESKD patients.
Figure S3. Correlations of depressive symptoms, taken continuously, with (A) albumin, (B) CRP, (C) hsCRP, (D) IL-6, and (E) TNF-α.
Table S1. Underlying data used for comparisons of inflammatory biomarkers in CKD or ESKD samples with vs. without depression, presented as mean (SD).
Table S2. Underlying data used for correlations of the severity of depression with inflammatory biomarkers in CKD and ESKD patients, presented as Spearman or Pearson rho.
PRISMA Checklist.
Supplementary Material
References
- 1.Palmer S., Vecchio M., Craig J.C. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179–191. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 2.Waraich P., Goldner E.M., Somers J.M. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry. 2004;49:124–138. doi: 10.1177/070674370404900208. [DOI] [PubMed] [Google Scholar]
- 3.Hedayati S.S., Minhajuddin A.T., Afshar M. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303:1946–1953. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilcot J., Guirguis A., Friedli K. Depression symptoms in haemodialysis patients predict all-cause mortality but not kidney transplantation: a cause-specific outcome analysis. Ann Behav Med. 2018;52:1–8. doi: 10.1007/s12160-017-9918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wium-Andersen M.K., Orsted D.D., Nielsen S.F. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry. 2013;70:176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- 6.Fan N., Luo Y., Ou Y. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum Psychopharmacol. 2017;32(4) doi: 10.1002/hup.2588. [DOI] [PubMed] [Google Scholar]
- 7.Haapakoski R., Mathieu J., Ebmeier K.P. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasco J.A., Nicholson G.C., Williams L.J. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 9.Simic Ogrizovic S., Jovanovic D., Dopsaj V. Could depression be a new branch of MIA syndrome? Clin Nephrol. 2009;71:164–172. doi: 10.5414/cnp71164. [DOI] [PubMed] [Google Scholar]
- 10.Hedayati S.S., Gregg L.P., Carmody T. Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: the CAST randomized clinical trial. JAMA. 2017;318:1876–1890. doi: 10.1001/jama.2017.17131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glassman A.H., O’Connor C.M., Califf R.M. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 12.Angermann C.E., Gelbrich G., Stork S. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315:2683–2693. doi: 10.1001/jama.2016.7635. [DOI] [PubMed] [Google Scholar]
- 13.Choi M.J., Seo J.W., Yoon J.W. The malnutrition-inflammation-depression-arteriosclerosis complex is associated with an increased risk of cardiovascular disease and all-cause death in chronic hemodialysis patients. Nephron Clin Pract. 2012;122:44–52. doi: 10.1159/000348509. [DOI] [PubMed] [Google Scholar]
- 14.Fouque D., Kalantar-Zadeh K., Kopple J. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 15.Carrero J.J., Stenvinkel P., Cuppari L. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Gupta J., Mitra N., Kanetsky P.A. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B.T., Ahmed F.A., Hamm L.L. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015;16:77. doi: 10.1186/s12882-015-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taraz M., Taraz S., Dashti-Khavidaki S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: a review of literature. Hemodial Int. 2015;19:11–22. doi: 10.1111/hdi.12200. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y., Feng L., Feng L. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53. doi: 10.1186/1465-9921-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metaphor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 22.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22 doi: 10.1002/sim.1482. 2963–2710. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Balshem H., Helfand M., Schunemann H.J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Dogan E., Erkoc R., Eryonucu B. Relation between depression, some laboratory parameters, and quality of life in hemodialysis patients. Renal Fail. 2005;27:695–699. doi: 10.1080/08860220500242728. [DOI] [PubMed] [Google Scholar]
- 27.Kalender B., Ozdemir A.C., Koroglu G. Association of depression with markers of nutrition and inflammation in chronic kidney disease and end-stage renal disease. Nephron Clin Pract. 2006;102:c115–c121. doi: 10.1159/000089669. [DOI] [PubMed] [Google Scholar]
- 28.Micozkadioglu H., Micozkadioglu I., Zumrutdal A. Relationship between depressive affect and malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrology (Carlton) 2006;11:502–505. doi: 10.1111/j.1440-1797.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 29.Boulware L.E., Liu Y., Fink N.E. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1:496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 30.Kalender B., Dervisoglu E., Sengul E. Depression, nutritional status, and serum cytokines in peritoneal dialysis patients: is there a relationship? Perit Dial Int. 2007;27:593–595. [PubMed] [Google Scholar]
- 31.Hsu H.J., Chen C.K., Wu M.S. Lower prevalence of depression in hemodialysis patients who use polysulfone dialyzers. Am J Nephrol. 2009;29:592–597. doi: 10.1159/000193144. [DOI] [PubMed] [Google Scholar]
- 32.Montinaro V., Iaffaldano G.P., Granata S. Emotional symptoms, quality of life and cytokine profile in hemodialysis patients. Clin Nephrol. 2010;73:36–43. doi: 10.5414/cnp73036. [DOI] [PubMed] [Google Scholar]
- 33.Bossola M., Ciciarelli C., Di Stasio E. Correlates of symptoms of depression and anxiety in chronic hemodialysis patients. Gen Hosp Psychiatry. 2010;32:125–131. doi: 10.1016/j.genhosppsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Ko G.J., Kim M.G., Yu Y.M. Association between depression symptoms with inflammation and cardiovascular risk factors in patients undergoing peritoneal dialysis. Nephron Clin Pract. 2010;116:c29–c35. doi: 10.1159/000314548. [DOI] [PubMed] [Google Scholar]
- 35.Gyamlani G., Basu A., Geraci S. Depression, screening and quality of life in chronic kidney disease. Am J Med Sci. 2011;342:186–191. doi: 10.1097/MAJ.0b013e3182113d9e. [DOI] [PubMed] [Google Scholar]
- 36.Li Z.J., An X., Mao H.P. Association between depression and malnutrition-inflammation complex syndrome in patients with continuous ambulatory peritoneal dialysis. Int Urol Nephrol. 2011;43:875–882. doi: 10.1007/s11255-011-9917-x. [DOI] [PubMed] [Google Scholar]
- 37.Hung K.C., Wu C.C., Chen H.S. Serum IL-6, albumin and co-morbidities are closely correlated with symptoms of depression in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2011;26:658–664. doi: 10.1093/ndt/gfq411. [DOI] [PubMed] [Google Scholar]
- 38.Chilcot J., Davenport A., Wellsted D. An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dialy Transplant. 2011;26:1628–1634. doi: 10.1093/ndt/gfq611. [DOI] [PubMed] [Google Scholar]
- 39.Bornivelli C., Aperis G., Giannikouris I. Relationship between depression, clinical and biochemical parameters in patients undergoing haemodialysis. J Ren Care. 2012;38:93–97. doi: 10.1111/j.1755-6686.2012.00259.x. [DOI] [PubMed] [Google Scholar]
- 40.Armaly Z., Farah J., Jabbour A. Major depressive disorders in chronic hemodialysis patients in Nazareth: identification and assessment. Neuropsychiatr Dis Treat. 2012;8:329–338. doi: 10.2147/NDT.S31903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.K., Kim S.G., Kim H.J. Serum S100B protein is associated with depressive symptoms in patients with end-stage renal disease. Clin Biochem. 2012;45:1573–1577. doi: 10.1016/j.clinbiochem.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Cilan H., Oguzhan N., Unal A. Relationship between depression and proinflammatory cytokine levels in hemodialysis patients. Renal Fail. 2012;34:275–278. doi: 10.3109/0886022X.2011.647292. [DOI] [PubMed] [Google Scholar]
- 43.Wang L.J., Wu M.S., Hsu H.J. The relationship between psychological factors, inflammation, and nutrition in patients with chronic renal failure undergoing hemodialysis. Int J Psychiatry Med. 2012;44:105–118. doi: 10.2190/PM.44.2.b. [DOI] [PubMed] [Google Scholar]
- 44.Taraz M., Khatami M.R., Gharekhani A. Relationship between a pro- and anti-inflammatory cytokine imbalance and depression in haemodialysis patients. Eur Cytokine Netw. 2012;23:179–186. doi: 10.1684/ecn.2013.0326. [DOI] [PubMed] [Google Scholar]
- 45.Su S.F., Ng H.Y., Huang T.L. Survey of depression by Beck Depression Inventory in uremic patients undergoing hemodialysis and hemodiafiltration. Ther Apher Dial. 2012;16:573–579. doi: 10.1111/j.1744-9987.2012.01094.x. [DOI] [PubMed] [Google Scholar]
- 46.Cilan H., Sipahioglu M.H., Oguzhan N. Association between depression, nutritional status, and inflammatory markers in peritoneal dialysis patients. Renal Fail. 2013;35:17–22. doi: 10.3109/0886022X.2012.741643. [DOI] [PubMed] [Google Scholar]
- 47.Preljevic V.T., Osthus T.B., Os I. Anxiety and depressive disorders in dialysis patients: association to health-related quality of life and mortality. Gen Hosp Psychiatry. 2013;35:619–624. doi: 10.1016/j.genhosppsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Nowak L., Adamczak M., Wiecek A. Is inflammation a new risk factor of depression in haemodialysis patients? Int Urol Nephrol. 2013;45:1121–1128. doi: 10.1007/s11255-012-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knuth B., Radtke V., Rocha P. Prevalence of depression symptoms and serum levels of interleukin-6 in hemodialysis patients. Psychiatry Clin Neurosci. 2014;68:275–282. doi: 10.1111/pcn.12130. [DOI] [PubMed] [Google Scholar]
- 50.Fan L., Sarnak M.J., Tighiouart H. Depression and all-cause mortality in hemodialysis patients. Am J Nephrol. 2014;40:12–18. doi: 10.1159/000363539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loosman W.L., Rottier M.A., Honig A. Association of depressive and anxiety symptoms with adverse events in Dutch chronic kidney disease patients: a prospective cohort study. BMC Nephrol. 2015;16:155. doi: 10.1186/s12882-015-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bossola M., Di Stasio E., Giungi S. Fatigue is associated with serum interleukin-6 levels and symptoms of depression in patients on chronic hemodialysis. J Pain Symptom Manage. 2015;49:578–585. doi: 10.1016/j.jpainsymman.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Ekramzadeh M., Mazloom Z., Sagheb M. Association of depression with selenium deficiency and nutritional markers in the patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2015;25:381–387. doi: 10.1053/j.jrn.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Gok Oguz E., Akoglu H., Ulusal Okyay G. Serum apelin is associated with affective disorders in peritoneal dialysis patients. Renal Fail. 2016;38:1059–1066. doi: 10.1080/0886022X.2016.1193873. [DOI] [PubMed] [Google Scholar]
- 55.Barros A., Costa B.E., Mottin C.C., d’Avila D.O. Depression, quality of life, and body composition in patients with end-stage renal disease: a cohort study. Braz J Psychiatry. 2016;38:301–306. doi: 10.1590/1516-4446-2015-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haverkamp G.L., Loosman W.L., Franssen C.F. The role of tryptophan degradation in the association between inflammatory markers and depressive symptoms in chronic dialysis patients. Nephrol Dial Transplant. 2017;32:1040–1047. doi: 10.1093/ndt/gfw212. [DOI] [PubMed] [Google Scholar]
- 57.Jong I.C., Tsai H.B., Lin C.H. Close correlation between the ankle-brachial index and symptoms of depression in hemodialysis patients. Int Urol Nephrol. 2017;49:1463–1470. doi: 10.1007/s11255-017-1598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chilcot J., Friedli K., Guirguis A. C reactive protein and depressive symptoms in hemodialysis patients: a questionable association. Hemodial Int. 2017;21:542–548. doi: 10.1111/hdi.12500. [DOI] [PubMed] [Google Scholar]
- 59.Kalender B., Ozdemir A.C., Dervisoglu E. Quality of life in chronic kidney disease: effects of treatment modality, depression, malnutrition and inflammation. Int J Clin Pract. 2007;61:569–576. doi: 10.1111/j.1742-1241.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 60.Dervisoglu E., Kir H.M., Kalender B. Depressive symptoms and proinflammatory cytokine levels in chronic renal failure patients. Nephron Clin Pract. 2008;108:c272–c277. doi: 10.1159/000126907. [DOI] [PubMed] [Google Scholar]
- 61.Sonikian M., Metaxaki P., Papavasileiou D. Effects of interleukin-6 on depression risk in dialysis patients. Am J Nephrol. 2010;31:303–308. doi: 10.1159/000285110. [DOI] [PubMed] [Google Scholar]
- 62.Uglesic B., Ljutic D., Lasic D. Depression and serum interleukin-6 levels in patients on dialysis. Psychiatr Danub. 2015;27:168–173. [PubMed] [Google Scholar]
- 63.Zhao C., Ma H., Yang L. Long-term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin-6 and interleukin-18. Neuropsychiatr Dis Treat. 2017;13:91–100. doi: 10.2147/NDT.S124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schricker S., Heider T., Schanz M. Strong associations between inflammation, pruritus and mental health in dialysis patients. Acta Derm Venereol. 2019;99:524–529. doi: 10.2340/00015555-3128. [DOI] [PubMed] [Google Scholar]
- 65.Lee S.K., Lee H.S., Lee T.B. The effects of antidepressant treatment on serum cytokines and nutritional status in hemodialysis patients. J Korean Med Sci. 2004;19:384–389. doi: 10.3346/jkms.2004.19.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taraz M., Khatami M.R., Dashti-Khavidaki S. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: results of a randomized double-blind, placebo-controlled clinical trial. Int Immunopharmacol. 2013;17:917–923. doi: 10.1016/j.intimp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Zahed N.S., Sharifi M., Karimi M. Impact of sertraline on serum concentration of CRP in hemodialysis patients with depression. J Renal Inj Prev. 2017;6:65–69. doi: 10.15171/jrip.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cabrerizo S., Cuadras D., Gomez-Busto F. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015;81:17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Amaral S., Hwang W., Fivush B. Serum albumin level and risk for mortality and hospitalization in adolescents on hemodialysis. Clin J Am Soc Nephrol. 2008;3:759–767. doi: 10.2215/CJN.02720707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Combe C., McCullough K.P., Asano Y. Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Dialysis Outcomes and Practice Patterns Study (DOPPS): nutrition guidelines, indicators, and practices. Am J Kidney Dis. 2004;44:39–46. doi: 10.1053/j.ajkd.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 71.de Mutsert R., Grootendorst D.C., Indemans F. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19:127–135. doi: 10.1053/j.jrn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Cooper B.A., Penne E.L., Bartlett L.H. Protein malnutrition and hypoalbuminemia as predictors of vascular events and mortality in ESRD. Am J Kidney Dis. 2004;43:61–66. doi: 10.1053/j.ajkd.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 73.Hedayati S.S., Yalamanchili V., Finkelstein F.O. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012;81:247–255. doi: 10.1038/ki.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baragetti I., El Essawy B., Fiorina P. Targeting immunity in end-stage renal disease. Am J Nephrol. 2017;45:310–319. doi: 10.1159/000458768. [DOI] [PubMed] [Google Scholar]
- 75.Stenvinkel P., Ketteler M., Johnson R.J. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 76.Han Q.Q., Yu J. Inflammation: a mechanism of depression? Neurosci Bull. 2014;30:515–523. doi: 10.1007/s12264-013-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.