Abstract
Introduction
Kidney transplantation (KT) remains the treatment of choice for end-stage kidney disease (ESKD), but access to transplantation is limited by a disparity between supply and demand for suitable organs. This organ shortfall has resulted in the use of a wider range of donor kidneys and, in parallel, a reexamination of potential alternative renal replacement therapies. Previous studies comparing Canadian intensive home hemodialysis (IHHD) with deceased donor (DD) KT in the United States reported similar survival, suggesting IHHD might be a plausible alternative.
Methods
Using data from the Scientific Registry of Transplant Recipients and an experienced US-based IHHD program in Lynchburg, VA, we retrospectively compared mortality outcomes of a cohort of IHHD patients with transplant recipients within the same geographic region between October 1997 and June 2014.
Results
We identified 3073 transplant recipients and 116 IHHD patients. Living donor KT (n = 1212) had the highest survival and 47% reduction in risk of death compared with IHHD (hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.34–0.83). Survival of IHHD patients did not statistically differ from that of DD transplant recipients (n = 1834) in adjusted analyses (HR: 0.96; 95% CI: 0.62–1.48) or when exclusively compared with marginal (Kidney Donor Profile Index >85%) transplant recipients (HR: 1.35; 95% CI: 0.84–2.16).
Conclusion
Our study showed comparable overall survival between IHHD and DD KT. For appropriate patients, IHHD could serve as bridging therapy to transplant and a tenable long-term renal replacement therapy.
Keywords: intensive home hemodialysis, KDPI, kidney transplant, survival
Graphical abstract
ESKD care is complex and costly. Currently, there are more than 700,000 patients living with ESKD in the United States,1 and this number is expected to rise. Compared with dialysis, KT is known to provide superior quality of life,2 patient survival,3 and cost-effectiveness.3,4 However, not every patient with advanced kidney disease will qualify for transplantation, and those who do, face lengthy waiting times because of critical organ shortage. In 1998, the median waiting time was 2.7 years, rising to 4.2 years by 2008.5 By the end of 2016, 16% of wait-listed patients had been waiting for ≥5 years, and 9.8% (> 9500 individuals) were delisted due to death or becoming too sick to transplant.6 This trend is particularly troublesome for patients with prolonged dialysis exposure, as they face worse allograft and patient survival,4 even when transplanted with living donor (LD) kidney.7,8 Thus, there is a need to use all suitable organs, including those anticipated to have reduced survival, such as high (>85%) Kidney Donor Profile Index (KDPI) kidneys. These marginal organs require specific patient consent, but still provide favorable outcomes compared with remaining on dialysis.9,10 This critical supply-demand mismatch creates a necessity to explore and expand alternative renal replacement therapies that could be comparable to KT.
Generally, survival is known to be worse among dialysis patients than KT recipients. Factors such as blood pressure control,11 volume optimization,12,13 left ventricular mass index improvement,14 and bone mineral metabolism15, 16, 17 directly affect patients’ cardiovascular prognosis. Unsurprisingly, cardiovascular disease is responsible for 48% of deaths in this population, usually due to arrhythmias, cardiac arrest, congestive heart failure, acute myocardial infarction, and atherosclerotic heart disease.1 Conventional in-center hemodialysis, typically delivered over 3- to 5-hour sessions 3 times per week, results in a 2-day interdialytic gap each week, which is associated with increased mortality and morbidity.18,19 IHHD offers a solution to many of these problems, while conferring improved solute clearance15,20 and better survival than in-center hemodialysis.21 There is no standard definition of IHHD,15 but most commonly, it is defined as hemodialysis treatment 4 or more times per week, preferably totaling 20 hours or more, and eliminating the 2-day interdialytic gap.15,22,23 IHHD, in the form of nocturnal home hemodialysis, not only provides survival, cardiovascular, and metabolic advantage, but it is associated with improved sleep apnea and nocturnal oxygen saturation. Moreover, patients receiving nocturnal home hemodialysis usually perceive their dialysis as less cumbersome despite its increased frequency and length. Two previous Canadian studies compared the survival of patients receiving nocturnal IHHD with that of KT recipients. The first, by Pauly et al.,22 showed that survival among a cohort of Canadian nocturnal IHHD patients was not significantly different from among DD KT recipients in the United States. The second study, by Tennankore et al.,24 reported decreased early hospitalization rate but inferior outcome (treatment failure or death) in the IHHD group compared with allograft survival from either living donors or DDs. Most recently, a study by Molnar et al.25 suggested that survival after KT was superior to home hemodialysis in elderly patients. However in this study, home hemodialysis patients received an average of 10 hours of dialysis and fewer than 4 sessions per week, which differs from IHHD.26 Given the conflicting conclusions reported thus far, we sought to compare the overall survival among a large cohort of adult IHHD patients with that of KT recipients stratified by kidney source and quality (defined by KDPI) within the same geographic region.
Methods
IHHD and KT Patients
The IHHD cohort consisted of 116 patients with ESKD who received IHHD from Lynchburg Nephrology Physicians located in Lynchburg, VA, between October 1, 1997, and June 30, 2014. KT recipients’ data were extracted from the Scientific Registry of Transplant Recipients, which includes data on all transplant candidates, donors, and recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight for the activities of the Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients contractors. All KT recipients belong to the same donor service area of Virginia. We obtained Institutional Review Board approval from Central Health, University of Virginia, and Fresenius, as well as written approval from DaVita.
IHHD patients received home hemodialysis >20 hours/week and ≥4 sessions/week during the study period. No patient had >1 day of interdialytic gap. Only patients with ESKD receiving IHHD for the first time were included; 2 patients returning to IHHD after failed KT were excluded. All IHHD patients were ≥18 years old and had a body mass index (BMI) ≥17. Of the 116 patients in this study, 87 patients had been previously described.20 Patients had a mean dialysis duration of 7 hours per session and 40 hours per week, mean blood flow rate and dialysate flow rate were 240 and 287 ml/min, respectively with a mean weekly standardized Kt/V of 5.25. They used the Fresenius (Bad Homburg, Germany) 2008H, 2008K, or 2008K@Home machine. From October 2009 to June 2014, 23 new IHHD patients used the NxStage (Lawrence, MA) System One machine and 12 others changed from the Fresenius machine to NxStage platform. These patients had a mean blood flow rate of 335 ml/min, mean dialysate flow rate of 144 ml/min, mean duration of 6 hours and 57 minutes, received 4 to 5 sessions weekly, and had similar clearances compared with traditional machine users. Just more than 30% of IHHD patients had an arteriovenous fistula for access. Additional characteristics of the IHHD cohort are available in Supplementary Table S1.
The KT cohort consisted of 3073 recipients (1861 DD KT and 1212 LD KT), age ≥18 years old, and transplanted between October 1, 1997, and June 30, 2014, at 3 transplant centers in Virginia. We excluded recipients of en bloc kidneys (2 same-donor pediatric kidneys transplanted as a single unit), recipients of simultaneous organs, repeated KT and those with BMI < 17. DD KT recipients were divided by KDPI categories: low 0% to 20%, standard 21% to 85%, and high >85%. KDRI scores were first calculated for all DD organs according to the Organ Procurement and Transplantation Network instructions and KDPI was then calculated using the most-recent conversion table available on the Organ Procurement and Transplantation Network Web site.9 Because the IHHD cohort consisted only of patients who were black, white, or Asian, we restricted KT recipients to these races. Asian patients (1% or less in both cohorts) were combined with white patients for analysis purposes.
Statistical Analysis
The primary outcome of interest was time to death, which was calculated from start of IHHD or date of first transplant until death or censoring. Patients on IHHD were censored at date of last follow-up, date of stopping IHHD, or study end, whichever was first. KT recipients were censored at the earliest among the following: date of last follow-up, graft failure, study end (December 31, 2014), or date of re-listing on the transplant waiting list (due to failed transplant, if graft failure date was not known). Survival probabilities were estimated using the Kaplan-Meier method, stratified by treatment modality: DD KT, LD KT, and IHHD. The follow-up for Kaplan-Meier survival curves was censored at 8 years because of fewer patients remaining on IHHD. Re-analyses with this cutoff did not change the results. Survival differences among groups were evaluated with log-rank test without adjustment, and then assessed using Cox regression, with adjustment for age, sex, race, BMI, vintage, cause of ESKD, and era. Due to significant advancement in immunosuppression therapies, stratified analysis of KT recipients before or after September 30, 2004 (early or late era), were performed. Data for KDPI calculation was available in 1834 DD KT recipients, leaving out 27 individuals, or 1.5% of the cohort. In addition, for exploratory purposes, survival differences of DD KT versus IHHD or LD KT versus IHHD were evaluated using Cox regression within subgroups of age, sex, race, BMI, vintage, and cause of ESKD, by including their interactions with treatment modalities.
Because treatment modality is not assigned randomly, clinical characteristics could differ considerably between IHHD and KT patients. To address such potential bias, additional analyses with propensity score (PS) methods were performed to evaluate the robustness of the main results. PS of treatment modalities was estimated from logistic regression based on age, sex, race, BMI, vintage, era, and cause of ESKD, separately for IHHD versus DD KT and IHHD versus LD KT. We included PS in the Cox regression along with other covariates and estimated PS-adjusted HR for IHHD versus KT or IHHD versus KDPI groups. We also performed PS matching with 1:2 ratio for IHHD versus KT patients and estimated the HR for IHHD versus KT in the matched subsets, Furthermore, sensitivity analysis adjusting for diabetes, coronary artery disease, cardiovascular disease, peripheral vessel disease, hypertension, and cancer was also performed. A P value < 0.05 was considered statistically significant. Models used complete case analysis. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Raleigh, NC).
Results
The demographic and clinical characteristics for 116 IHHD patients and 3073 KT recipients (1861 DD KT and 1212 LD KT patients) are shown in Table 1. The LD KT group was relatively younger, predominantly white, with shorter dialysis vintage and lower BMI compared with DD KT and IHHD groups. Each cohort had similar proportions of women (41.3% DD KT, 38.6% LD KT, and 41.4% IHHD). LD KT recipients were less likely to have diabetes (33.6%), but the difference was nonsignificant. History of malignancy was more common in IHHD patients (10.3%) than in patients with DD KT (0.9%) or LD KT (0.2%). Compared with other KDPI categories and IHHD patients, high-KDPI recipients (Table 2) were relatively older, more frequently male, and likely to have hypertensive kidney disease. In the IHHD group, >50% identified high school as their highest educational level, and 31% had an arteriovenous fistula for vascular access contrasting with 62.7% prevalence in other US hemodialysis populations1; 44.4% used well water, suggesting a rural place of residence (Supplementary Table S1).
Table 1.
Demographic and clinical characteristics of IHHD and KT patients
| Characteristic | DD KT (n = 1861) | LD KT (n = 1212) | IHHD (n = 116) | P value |
|---|---|---|---|---|
| Age, yr, mean ± SD | 52.1 ± 12 | 47.7 ± 13.6 | 51.0 ± 14.7 | <0.0001a |
| Race, n (%) | ||||
| Black | 1140 (61.3) | 365 (30.1) | 59 (50.9) | <0.0001b |
| White | 721 (38.7) | 847 (69.9) | 57 (49.1) | |
| Sex, F, n (%) | 769 (41.3) | 468 (38.6) | 48 (41.4) | 0.3172 |
| BMI, mean ± SD | 28.5 ± 5.4 | 27.8 ± 5.2 | 30.8 ± 8.9 | <0.0001 |
| ESKD cause, n (%) | ||||
| GN | 278 (14.9) | 233 (19.2) | 38 (32.8) | <0.0001 |
| DM | 519 (27.9) | 313 (25.8) | 30 (25.9) | |
| Hypertension | 613 (32.9) | 290 (23.9) | 28 (24.1) | |
| Polycystic kidney disease | 161 (8.7) | 112 (9.2) | 4 (3.4) | |
| Other | 290 (15.6) | 264 (21.8) | 16 (13.8) | |
| Vintage, mo, mean ± SD | 3.7 ± 2.8 | 1.3 ± 1.8 | 2.7 ± 3.5 | <0.0001c |
| Vintage group, n (%) | ||||
| <3 mo | 137 (7.4) | 382 (31.5) | 21 (18.1) | |
| >3 mo | 1675 (90) | 778 (64.2) | 95 (81.9) | |
| Missing | 49 (2.6) | 52 (4.3) | None | |
| Atherosclerotic disease, n (%) | ||||
| No | 1258 (67.6) | 861 (71) | 82 (70.7) | |
| Yes | 147 (7.9) | 91 (7.5) | 34 (29.3) | |
| Missing | 456 (24.5) | 260 (21.5) | None | |
| Cerebrovascular disease, n (%) | ||||
| No | 1363 (73.2) | 938 (77.4) | 114 (98.3) | |
| Yes | 48 (2.6) | 26 (2.1) | 2 (1.7) | |
| Missing | 450 (24.2) | 248 (20.5) | None | |
| Peripheral vascular disease, n (%) | ||||
| No | 1719 (92.4) | 1138 (93.9) | 100 (86.2) | |
| Yes | 115 (6.2) | 68 (5.6) | 16 (13.8) | |
| Missing | 27 (1.5) | 6 (0.5) | None | |
| Hypertension, n (%) | ||||
| No | 160 (8.6) | 111 (9.2) | 11 (9.5) | |
| Yes | 1260 (67.7) | 858 (70.8) | 105 (90.5) | |
| Missing | 441 (23.7) | 243 (20) | None | |
| Previous cancer,dn (%) | ||||
| No | 1844 (99.1) | 1210 (99.8) | 104 (89.7) | |
| Yes | 17 (0.9) | 2 (0.2) | 12 (10.3) | |
| Diabetes, n (%) | ||||
| No | 1125 (60.5) | 777 (64.1) | 73 (62.9) | |
| Yes | 696 (37.4) | 407 (33.6) | 43 (37.1) | |
| Missing | 40 (2.1) | 28 (2.3) | None | |
| Deaths during study period | 370 (19.9) | 184 (15.2) | 22 (19) | |
BMI, body mass index; DD, deceased donor; DM, diabetes mellitus; ESKD, end-stage kidney disease; GN, glomerulonephritis; IHHD, intensive home hemodialysis; KT, kidney transplantation; LD, living donor.
Mean age compared using 1-way analysis of variance. There is a statistically significant difference when comparing DD and LD groups, IHHD and LD groups, but not when comparing DD and IHHD groups.
Asian race has been combined with white. There was 1 Asian individual in IHHD (0.9%), 26 Asian individuals in DD (1.4%), and 19 Asian individuals in LD (1.6%).
Vintage times compared across groups using Kruskal-Wallis test due to departures from normality.
A small number of patients were missing data on previous cancer. We assumed that missing = no.
Table 2.
Selected demographic and clinical characteristics of deceased donor kidney transplant recipients by KDPI
| Characteristic | KDPI < 20, n = 109 | KDPI 20–85, n = 1352 | KDPI > 85, n = 373 | IHHD, n = 116 |
|---|---|---|---|---|
| Age, yr, mean ± SD | 50.4 ± 13.0 | 51.9 ± 12.0 | 53.8 ± 11.5 | 51.0 ± 14.7 |
| African American, n (%) | 64 (58.7) | 804 (59.5) | 257 (68.9) | 59 (50.9) |
| Females, n (%) | 53 (48.6) | 546 (40.4) | 160 (42.9) | 48 (41.4) |
| BMI, mean ± SD | 28.1 ± 5.5 | 28.5 ± 5.4 | 28.6 ± 5.4 | 30.8 ± 8.9 |
| Cause of ESKD, n (%) | ||||
| GN | 14 (12.8) | 223 (16.5) | 41 (11) | 38 (32.8) |
| DM | 34 (31.2) | 367 (27.1) | 111 (29.8) | 30 (25.9) |
| Hypertension | 31 (28.4) | 416 (30.8) | 155 (41.6) | 28 (24.1) |
| Polycystic kidney disease | 12 (11) | 129 (9.5) | 17 (4.6) | 4 (3.4) |
| Other | 14 (12.8) | 223 (16.5) | 41 (11) | 16 (13.8) |
| Diabetes, n (%) | 43 (39.4) | 502 (37.1) | 140 (37.5) | 43 (37.1) |
| Dialysis vintage, yr, mean ± SD | 3.3 ± 2.6 | 3.7 ± 2.7 | 4.0 ± 3.1 | 2.7 ± 3.5 |
| Dialysis vintage >3 mo, n (%) | 97 (89) | 1211 (89.6) | 343 (92) | 95 (81.9) |
| Deaths, n (%) | 18 (16.5) | 261 (19.3) | 83 (22.3) | 22 (19) |
BMI, body mass index; DM, diabetes mellitus; ESKD, end-stage kidney disease; GN, glomerulonephritis; IHHD, intensive home hemodialysis; KDPI, Kidney Donor Profile Index.
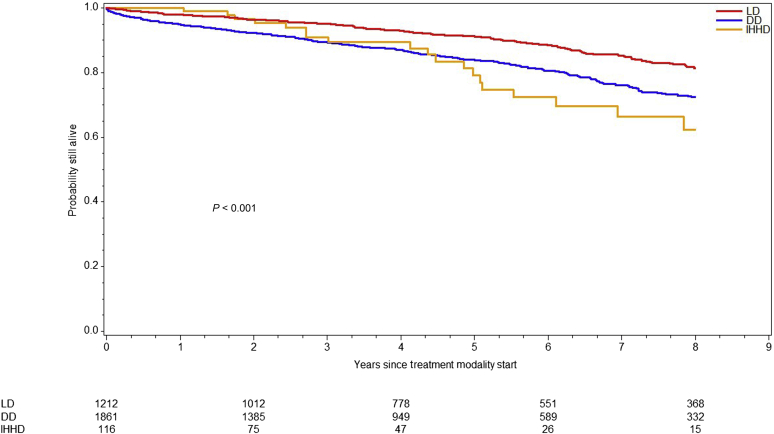
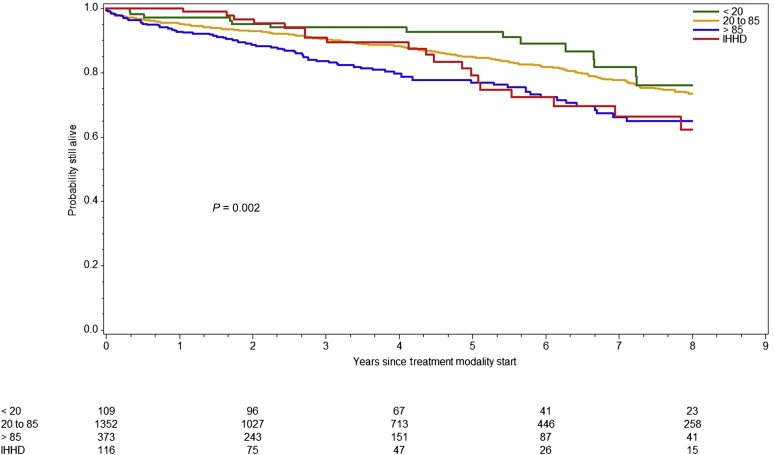
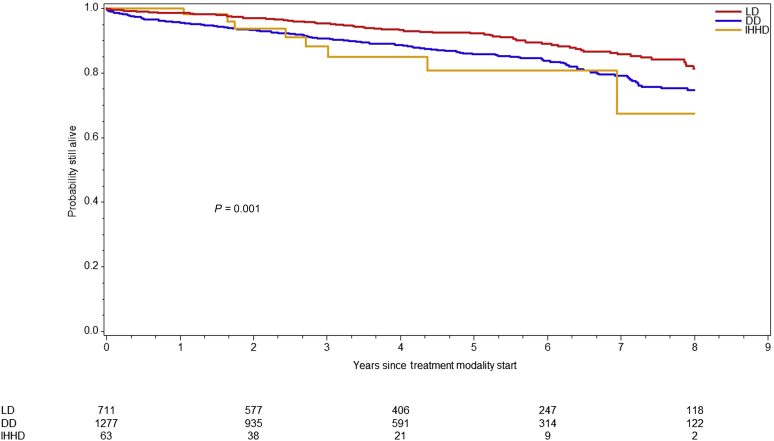
Overall survival differed significantly across modalities (log-rank P < 0.0001) (Figure 1). The LD KT cohort (184 died and 1028 censored) exhibited the best survival among all (184 died and 1028 censored) and did not reach median survival time during follow-up. Conversely, DD KT recipients (370 died and 1491 censored) had a median survival of 14.3 (95% CI: 12.8–15.56) years compared with 10.4 years in IHHD patients (22 died and 94 censored). Because organ quality can affect posttransplant outcomes, we divided the DD KT cohort by KDPI category (low 0%–20%, standard 21%–85% and high KDPI). Median overall recipient survival of high-KDPI KT was 10.6 years. As expected, high-KDPI recipients carried 2.1 times (95% CI: 1.26–3.50, P = 0.0045) higher risk of death than low KDPI recipients. When comparing overall survival between high-KDPI and IHHD cohorts, no significant difference was observed (HR: 1.35; 95% CI: 0.84–2.16; P = 0.2168) (Figure 2).
Figure 1.
Survival by treatment modality. Graph represents the overall survival for living donor (LD) kidney transplant recipients, deceased donor (DD) kidney transplant recipients, and intensive home hemodialysis patients (IHHD). The overall survival differed across modalities (P < 0.001, log-rank test).
Figure 2.
Survival of deceased donor (DD) kidney transplant recipients by organ quality versus intensive home hemodialysis (IHHD). Graph represents the overall survival among those who received DD kidney transplants stratified by Kidney Donor Profile Index (KDPI) category (KDPI <20%, 20%–85%, >85%) compared with IHHD patients. The overall survival differed across modalities (P < 0.002, log-rank test). However, there was no significant difference when comparing high KDPI recipients with IHHD (P = 0.2168).
The HRs associated with demographic and clinical characteristics (age, sex, race, vintage, BMI, cause of ESKD, and era) are shown in Supplementary Table S2. A total of 136 patients (4%) were excluded from multivariable analysis due to missing covariates. There was evidence of association between overall survival and treatment modality, age, race, dialysis vintage, cause of ESKD, and era. Subgroup analysis comparing patients who received DD KT with those who received IHHD noted that dialysis vintage of 2 to 5 years, ESKD due to polycystic kidney disease and hypertension, as well as normal BMI were associated with survival advantage (Supplementary Figure S1). We observed analogous findings between LD KT and IHHD in addition to black race and male gender (Supplementary Figure S2). Table 3 depicts HR for IHHD patients compared with patients who received KT (DD and LD). In the unadjusted model, the risk of death for LD KT recipients was almost half that of IHHD patients (HR: 0.53; 95% CI: 0.34–0.83; P = 0.0053). After covariate adjustment, the adjusted HR was 0.69 (95% CI: 0.44–1.09; P = 0.1075). We did not observe a difference in survival when comparing DD KT with IHHD in unadjusted (HR: 0.92; 95% CI: 0.60–1.41; P = 0.6995) or adjusted (HR: 0.96; 95% CI: 0.62–1.47; P = 0.8378) models. As expected, LD KT was superior to DD KT (HR: 1.39; 95% CI: 1.13–1.70). In addition, sensitivity analysis adjusting for various comorbid conditions revealed similar HR estimates for most covariates despite the fact that >30% of patients in this analysis were excluded due to missing data (data not shown).
Table 3.
Hazard ratios and 95% CIs for treatment modality from Cox regression
| Model type | Comparison |
|||
|---|---|---|---|---|
| DD KT vs. IHHD |
LD KT vs. IHHD |
|||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Unadjusted | 0.92 (0.60–1.41) | 0.6995 | 0.53 (0.34–0.83) | 0.0053 |
| Adjusteda | 0.96 (0.62–1.48) | 0.8395 | 0.69 (0.44–1.08) | 0.1075 |
| PS-adjustedb | 0.96 (0.62–1.49) | 0.8522 | 0.60 (0.37–0.96) | 0.0342 |
| PS-matchedc | 1.067 (0.46–2.49) | 0.8803 | 0.80 (0.47–1.35) | 0.3970 |
CI, confidence interval; DD, deceased donor; IHHD, intensive home hemodialysis; KT, kidney transplantation; LD, living donor; PS, propensity score.
Multivariable Cox regression model adjusted for age, sex, race, vintage, body mass index, cause of end-stage kidney disease, and era (n = 3053).
PS-adjusted model (n = 3053).
PS-matched analysis (2:1 ratio. n = 232 DD KT, n = 232 LD KT, n = 116 IHHD).
The PS-adjusted analysis did not detect a difference in overall survival between DD KT and IHHD (HR: 0.96; 95% CI: 0.62–1.49; P = 0.8522); however, when directly comparing LD KT with IHHD, we observed a survival advantage in the former group (HR: 0.60; 95% CI: 0.37–0.96; P = 0.0342). Likewise, PS-adjusted analysis of KT recipients with marginal organs (KDPI >85%, n = 373) showed a survival comparable with that of IHHD patients (HR: 1.15; 95% CI: 0.7–1.88; P = 0.58). In analyses with a subset of patients matched on PS in a 1:2 ratio, we did not observe a difference between IHHD and DD patients (HR: 1.07; 95% CI: 0.46–2.49; P = 0.88), or between IHHD and LD patients (HR: 0.80; 95% CI: 0.47–1.35; P = 0.40).
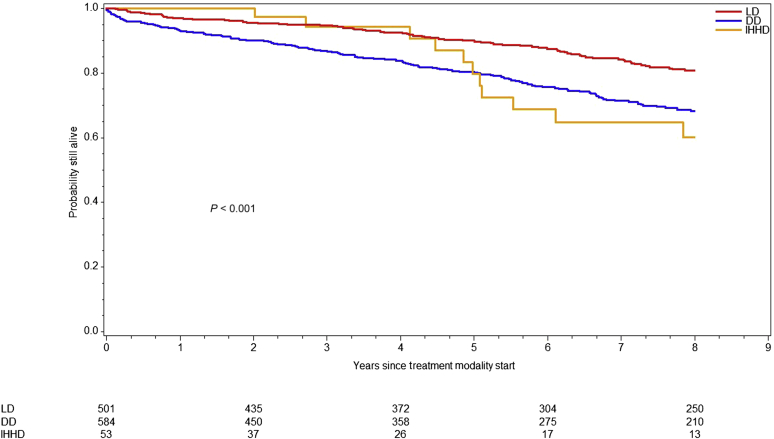
Given changes in practice patterns along with advancement in technology and pharmacotherapy over time, we compared overall survival across treatment modalities within 2 different eras: before and after September 30, 2004 (Figures 3 and 4). In this model, survival was better for all patients during the later era even when adjusting for treatment modality, with an approximately 37% reduction in risk of death (HR: 0.63; 95% CI: 0.53–0.76; P < 0.001). Interestingly, LD KT recipients had superior overall survival compared with IHHD during the earlier era, but this did not carry through in the later cohort (Table 4).
Figure 3.
Survival by treatment modality for patients starting on or before September 30, 2004. Graph represents the overall survival for living donor (LD) kidney transplant recipients, deceased donor (DD) kidney transplant recipients, and intensive home hemodialysis patients (IHHD) starting treatment on or before September 30, 2004. The overall survival differed across modalities (P < 0.001, log-rank test).
Figure 4.
Survival by treatment modality for patients starting after October 1, 2004. Graph represents the overall survival for living donor (LD) kidney transplant recipients, deceased donor (DD) kidney transplant recipients, and intensive home hemodialysis patients (IHHD) starting treatment after October 1, 2004. The overall survival differed across modalities (P < 0.001, log-rank test). Compared with the previous era, survival was better across all treatment modalities.
Table 4.
Hazard ratios and 95% CIs of treatment modality and era from Cox regression
| Variable | Comparison | Hazard ratio (95% CI) | P value |
|---|---|---|---|
| Treatment modality | DD vs. IHHD | 0.956 (0.62–1.47) | 0.8378 |
| LD vs. IHHD | 0.542 (0.35–0.84) | 0.0067 | |
| Era | Late vs. early | 0.761 (0.64–0.91) | 0.0026 |
CI, confidence interval; DD, deceased donor; IHHD, intensive home hemodialysis; LD, living donor.
Multivariable Cox regression model adjusted for treatment modality and era (n = 3189, 576 events).
Discussion
KT has long been considered the treatment of choice for ESKD; however, as incidence of ESKD rises, so is the time patients spend waiting on chronic dialysis. Although LD KT carries superior overall outcomes among all renal replacement therapies, most candidates fail to identify an LD and hence, must wait on the list.25,27 Current data demonstrate conventional hemodialysis is unable to provide survival anywhere near that of KT.28, 29, 30 Many studies have explored risk factors associated with mortality among dialysis patients, and the major players are thought to be suboptimal solute clearance, uncontrolled hypertension, chronic hypervolemia, left ventricular hypertrophy, hyperphosphatemia, and a wide interdialytic gap. Frequent and prolonged hemodialysis treatments, such as what is attained through IHHD, can mitigate those factors and their negative influence on survival.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 In 2019, an executive order pertaining to kidney health and transplantation was issued.31 One of its primary goals is to incentivize home dialysis, through reimbursement restructure, over the next several years. The policy change underscores the timeliness of this study.
Although others have attempted to clarify whether patient survival on IHHD differs from KT, results have been conflicting and questions remain unanswered. Pauly et al.22 compared survival among Canadian IHHD patients and US KT recipients using data from the US Renal Data System database. This study found no significant difference in the adjusted survival of patients receiving DD KT compared with IHHD, but patient follow-up was relatively short. Molnar et al.25 reported patient survival after KT was better than on home hemodialysis in patients ≥65 years old. However, the definition of home hemodialysis of Molnar et al.25 differed from ours: patients received an average of 3.7 sessions of dialysis per week for 165 minutes per session and had short, variable follow-up.26 Our analysis compares IHHD with a broader cohort of KT recipients, including stratification by organ quality in DD KT, which we believe adds to existing literature.
IHHD is known to mitigate and improve several risk factors associated with increased cardiovascular morbidity and mortality.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 This is the most likely explanation for the survival advantage seen with IHHD compared with conventional hemodialysis. Although IHHD has not been conclusively associated with improved quality of life,32,33 studies do show improvement in quality of sleep,34 reduced sleep apnea syndrome,35 restored pituitary-hypothalamic axis resulting in enhanced fertility,36 and improved pregnancy outcomes.37 In addition, IHHD has the potential to provide more flexibility and patient autonomy than conventional in-center hemodialysis.
It is not surprising that our study shows differences among cohorts. LD KT recipients were relatively younger, predominantly white, with shorter dialysis vintage and lower BMI compared with DD KT and IHHD groups. They had lower frequency of diabetes (not statistically significant). Both DD KT and LD KT recipients were less likely to have history of malignancy than the IHHD group (0.9% vs. 0.2% vs. 10.3%, respectively). Although these differences are important and some may be due to incomplete capture in the Scientific Registry of Transplant Recipients dataset, it is unlikely they are the sole reason for the observed survival outcomes. One could argue that KT candidates are on average healthier than IHHD patients, given the thorough pretransplant evaluation that is not required for dialysis patients. Despite this, our adjusted results did not detect a difference in overall survival between DD KT and IHHD groups. Likewise, when exploring the effect of organ quality by dividing DD KT recipients by KDPI categories, we continued to see no difference in overall survival compared with IHHD. We observed a trend toward improved survival during the first 4 years in the IHHD group compared with high KDPI, but further analysis is needed to ascertain this observation. The results comparing LD KT and IHHD were not perfectly consistent (statistically significant in the PS-adjusted model but nonsignificant in the multivariable model and matched model). It may be that the sample size of 116 in the IHHD group did not provide sufficient power.
Between the mid-1990s and mid-2000s, sizeable changes occurred in the transplant landscape. Improvements in induction therapy (transition to predominant use of lymphocyte depleting agents such as antithymocyte globulin and alemtuzumab) and maintenance immunosuppression (moving from azathioprine and cyclosporine regimens to almost universal use of tacrolimus and mycophenolic acid) led to substantial improvement in short-term outcomes with 1-year rejection rate <10% and graft survival rate ≥95%.38 These advances, coupled with changes in pediatric allocation policy during the mid-2000s,39 along with a fall in living donation rates,40 led us to conclude it was important to explore the effect of era on our survival comparisons. All cohorts enjoyed better survival in the later era, but we observed no evidence of a difference in survival between IHHD and DD KT regardless of era. Although newer dialysis machine technology (NxStage) became available during the second era, this was not used in our IHHD cohort until the last 4 years of the study and did not significantly affect our results. The PS analyses mitigate some types of bias, and also demonstrate our main analysis results were not dramatically affected by differences between groups. It is true that PS methods work better with complete and appropriate data, while we had some covariates missing. But we performed additional sensitivity analyses to explore the association between various comorbid conditions and survival. Despite the missing data for some variables, we observed similar effect sizes and no meaningful differences in results across models.
In December 2014, a new kidney allocation system was implemented in the United States, improving access to transplantation for previously disadvantaged groups (i.e., minorities and highly sensitized individuals).41 However, certain candidates (i.e., blood type O recipients and individuals >65 years old) continue to experience extended waiting times and transplant rates that are equal to or less than before the kidney allocation system was implemented.42 In the absence of an LD, candidates such as those mentioned previously or those with perceived increased mortality while waiting, may be offered marginal organs in order to be transplanted in a timely manner, which in turn are linked to inferior outcomes.43,44 Furthermore, most waiting patients receive in-center hemodialysis, which carries worse outcomes than IHHD. Thus, selected candidates could benefit from IHHD as a bridging therapy while waiting for more ideal organs. We believe KT remains the gold-standard treatment for most patients, but our findings clearly indicate more studies are needed to determine circumstances in which IHHD may be a suitable alternative to transplantation.
Worth mentioning is the posttransplant survival disadvantage sometimes experienced in black versus non-black patients. Our study strata were mostly balanced in terms of race, with the exception of the high-KDPI KT recipients (69% black). Compared with the IHHD patients (50% black), this could potentially shift the comparison toward the null, but on the other hand, it is difficult to determine attribution of poor outcome to race versus lower-quality donor organ.
Our study compares a large single-center cohort of adult IHHD patients with KT recipients from the same geographical area. The data cover an extended period, during which there were changes in clinical practice, technology, and pharmacotherapy. We studied patients within the same geographic region. This approach naturally controlled for geographic variation in recipient candidate selection and posttransplant care practices that are known to occur in different regions, but the drawback is that this approach may affect the generalizability of our findings.
ESKD treatment and KT are challenging to study. The subpar number of transplantable organs necessitates the study and implementation of alternatives to transplantation. However, ethical constraints, not unique to kidney failure outcomes research, limit the ability to perform the most scientifically valid study because what is best for each unique patient’s health might not align with an ideal study design. Thus, we have made use of the best-available data and carefully applied statistical methods that allow us to make appropriate and meaningful conclusions.
To thrive as a modality of renal replacement therapy, IHHD requires focused patient training (for approximately 4–6 weeks on average), committed and adherent patients with a dedicated care partner, well-trained and supportive hemodialysis staff nurses who can provide close monitoring, as well as supervision by an experienced physician(s). Building such a program can be time onerous and labor intensive for all stakeholders, but done properly, it results in an effective dialysis modality in which patients are not only highly motivated but engaged with their treatment. Many dialysis programs are unable to offer all these components in a sufficient manner to deliver a successful IHHD program. Thus, it is understandable that the type of IHHD program we are able to deliver may not currently be the norm in the United States. Similarly, it is possible that the outcomes would be less remarkable at smaller, less experienced dialysis centers.
We have discussed several potential limitations that include the relatively small cohort of IHHD patients, its single-center and retrospective observational nature, population restriction to a specific geographic region, and changes in clinical practice and technology. Nonetheless, we have taken steps to overcome these limitations as much as possible. We equally recognize that psychosocial and economic issues, outside the scope of this study, could result in confounding.
In summary, our study demonstrates that in selected patients, IHHD can offer comparable survival benefit to that afforded by successful DD KT. These findings are of particular interest to those who, unfortunately because of a lack of LDs and/or face extended wait times, might be transplanted with marginal organs. Given the recent presidential mandate to appreciably augment home dialysis therapies and transplantation, and in light of major organ shortage, IHHD serves as a suitable treatment option for those who may not be candidates for peritoneal dialysis and bridge therapy while waiting for KT. Increasing awareness in the nephrology community and appropriate patient education are needed to facilitate patients’ access to IHHD as an alternative to standard in-center hemodialysis before KT with the potential to positively impact patient survival.
Disclosure
RSL receives honoraria for educational presentations for DaVita, FMC, and NxStage. All the other authors declared no competing interests.
Acknowledgments
We gratefully acknowledge the staff at the University of Virginia (UVA) Health System Dialysis, the UVA Lynchburg Home Dialysis, DaVita Home Dialysis in Lynchburg, and Fresenius Kidney Care Blue Ridge in Bedford, VA. We thank the support of the Division of Nephrology and the Department of Public Health Sciences at UVA. The interpretation and reporting of kidney recipient data are the responsibilities of the authors and in no way should be seen as an official statement of Scientific Registry of Transplant Recipients.
Footnotes
STROBE Statement.
Table S1. Additional characteristics of patients receiving IHHD.
Table S2. Hazard ratios and 95% CIs for demographic and clinical characteristics from Cox regression. Multivariable Cox regression model adjusted for age, sex, race, vintage, BMI, cause of ESKD, and era.
Figure S1. Association of DD KT recipients and IHHD patients’ characteristics with survival. Graph represents a subgroup analysis showing hazard ratios for selected characteristics. Survival advantage was present for dialysis vintage of 2 to 5 years, ESKD due to polycystic kidney disease and hypertension, as well as normal BMI.
Figure S2. Association of LD KT recipients and IHHD patients’ characteristics with survival. Graph represents a subgroup analysis showing hazard ratios for selected characteristics. Survival advantage was present for dialysis vintage of 2 to 5 years, ESKD due to polycystic kidney disease and hypertension, normal BMI, black race, and male gender.
Supplementary Material
References
- 1.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 2.Tonelli M., Wiebe N., Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche H.U., Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74:1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) Department of Health and Human Services, Health Resources and Services Administration; Rockville, MD: 2014. OPTN/SRTR 2012 Annual Data Report. [Google Scholar]
- 6.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) Department of Health and Human Services, Health Resources and Services Administration; Rockville, MD: 2017. OPTN/SRTR 2016 Annual Data Report. [Google Scholar]
- 7.Haller M.C., Kainz A., Baer H., Oberbauer R. Dialysis vintage and outcomes after kidney transplantation: a retrospective cohort study. Clin J Am Soc Nephrol. 2017;12:122–130. doi: 10.2215/CJN.04120416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramowicz A., Hazzan M., Maggiore U. Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant. 2016;31:691–697. doi: 10.1093/ndt/gfv378. [DOI] [PubMed] [Google Scholar]
- 9.Organ Procurement Transplant Network A Guide to Calculating and Interpreting the Kidney Donor Profle Index. https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf Available at:
- 10.Massie A.B., Luo X., Chow E.K. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 11.Nesrallah G., Suri R., Moist L. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42(Suppl 1):S13–S17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 12.Fagugli R.M., Reboldi G., Quintaliani G. Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis. 2001;38:371–376. doi: 10.1053/ajkd.2001.26103. [DOI] [PubMed] [Google Scholar]
- 13.Susantitaphong P., Koulouridis I., Balk E.M. Effect of frequent or extended hemodialysis on cardiovascular parameters: a meta-analysis. Am J Kidney Dis. 2012;59:689–699. doi: 10.1053/j.ajkd.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culleton B.F., Walsh M., Klarenbach S.W. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 15.Tennankore K.K., Nadeau-Fredette A.C., Chan C.T. Intensified home hemodialysis: clinical benefits, risks and target populations. Nephrol Dial Transplant. 2014;9:1342–1349. doi: 10.1093/ndt/gft383. [DOI] [PubMed] [Google Scholar]
- 16.Pauly R.P., Chan C.T. Reversing the risk factor paradox: is daily nocturnal hemodialysis the solution? Semin Dial. 2007;20:539–543. doi: 10.1111/j.1525-139X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 17.Block G.A., Hulbert-Shearon T.E., Levin N.W. Association of serum phosphorus and calcium X phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 18.Foley R.N., Gilbertson D.T., Murray T. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 19.Bleyer A.J., Russell G.B., Satko S.G. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 20.Lockridge R.S., Kjellstrand C.M. Nightly home hemodialysis: outcome and factors associated with survival. Hemodial Int. 2011;15:211–218. doi: 10.1111/j.1542-4758.2011.00542.x. [DOI] [PubMed] [Google Scholar]
- 21.Nesrallah G.E., Lindsay R.M., Cuerden M.S. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauly R.P., Gill J.S., Rose C.L. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant. 2009;4:2915–2919. doi: 10.1093/ndt/gfp295. [DOI] [PubMed] [Google Scholar]
- 23.Chan C.T., Covic A., Craig J.C. Novel techniques and innovation in blood purification: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 2013;83:359–371. doi: 10.1038/ki.2012.450. [DOI] [PubMed] [Google Scholar]
- 24.Tennankore K.K., Kim S.J., Baer H.J. Survival and hospitalization for intensive home hemodialysis compared with kidney transplantation. J Am Soc Nephrol. 2014;25:2113–2120. doi: 10.1681/ASN.2013111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar M.Z., Ravel V., Streja E. Survival of elderly adults undergoing incident home hemodialysis and kidney transplantation. J Am Geriatr Soc. 2016;64:2003–2010. doi: 10.1111/jgs.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuttykrishnan S., Kalantar-Zadeh K., Arah O.A. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30:1208–1217. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Port F.K., Merion R.M., Roys E.C. Trends in organ donation and transplantation in the United States, 1997–2006. Am J Transplant. 2008;8:911–921. doi: 10.1111/j.1600-6143.2008.02170.x. [DOI] [PubMed] [Google Scholar]
- 28.McDonald S.P., Russ G.R. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant. 2002;17:2212–2219. doi: 10.1093/ndt/17.12.2212. [DOI] [PubMed] [Google Scholar]
- 29.Rao P.S., Schaubel D.E., Wei G. Evaluating the survival benefit of kidney retransplantation. Transplantation. 2006;82:669–674. doi: 10.1097/01.tp.0000235434.13327.11. [DOI] [PubMed] [Google Scholar]
- 30.Port F.K., Wolfe R.A., Mauger E.A. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. [PubMed] [Google Scholar]
- 31.United States, Executive Office of the President Donald Trump Executive Order 13879: On Advancing American Kidney Health. 10 July 2019. Fed Regist. 2019;84:33817–33819. https://www.federalregister.gov/documents/2019/07/15/2019–15159/advancing-american-kidney-health Available at: Accessed July 15, 2019. [Google Scholar]
- 32.Rocco M.V., Lockridge R.S., Jr., Beck G.J. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manns B.J., Walsh M.W., Culleton B.F. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int. 2009;75:542–549. doi: 10.1038/ki.2008.639. [DOI] [PubMed] [Google Scholar]
- 34.Jabber B.L., Schiller B., Burkart J.M. Impact of short daily hemodialysis on restless leg symptoms and sleep disturbances. Clin J Am Soc Nephrol. 2011;6:1049–1056. doi: 10.2215/CJN.10451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanly P.J., Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 36.van Eps C., Hawley C., Jeffries J. Changes in serum prolactin, sex hormones and thyroid function with alternate nightly nocturnal home hemodialysis. Nephrology (Carlton) 2012;17:42–47. doi: 10.1111/j.1440-1797.2011.01520.x. [DOI] [PubMed] [Google Scholar]
- 37.Barua M., Hladunewich M., Keunen J. Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol. 2008;3:392–396. doi: 10.2215/CJN.04110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanriover B., Jaikaransingh V., MacConmara M.P. Acute rejection rates and graft outcomes according to induction regimen among recipients of kidneys from deceased donors treated with tacrolimus and mycophenolate. Clin J Am Soc Nephrol. 2016;11:1650–1660. doi: 10.2215/CJN.13171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham E.C., Wilson A.C., Goebel J. Current kidney allocation rules and their impact on a pediatric transplant center. Am J Transplant. 2009;9:404–408. doi: 10.1111/j.1600-6143.2008.02504.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigue J.R., Schold J.D., Mandelbrot D.A. The decline in living kidney donation in the United States: random variation or cause for concern? Transplantation. 2013;96:767–773. doi: 10.1097/TP.0b013e318298fa61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart D.E., Klassen D.K. Early experience with the new kidney allocation system. Clin J Am Soc Nephrol. 2017;12:2063–2065. doi: 10.2215/CJN.06380617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart D.E., Wilk A.R., Toll A.E. Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant. 2018;18:1924–1935. doi: 10.1111/ajt.14922. [DOI] [PubMed] [Google Scholar]
- 43.Bae S., Massie A.B., Thomas A.G. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am J Transplant. 2019;19:425–433. doi: 10.1111/ajt.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelletier R.P., Pesavento T.E., Rajab A. High mortality in diabetic recipients of high KDPI deceased donor kidneys. Clin Transplant. 2016;30:940–945. doi: 10.1111/ctr.12768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.