Abstract
Introduction
Hypertensive nephrosclerosis is considered the second most common cause of end-stage renal disease (ESRD), but it is still an insufficiently studied and controversial disease entity. More information on the phenotype and prognosis is needed to improve clinical diagnostics and treatment.
Methods
We included all Norwegian patients with chronic kidney disease (CKD) referred for kidney biopsy between 1988 and 2012 whose clinical presentation was consistent with, but not primarily suspicious for, hypertensive nephrosclerosis (n = 4920); follow-up continued until 2013.
Results
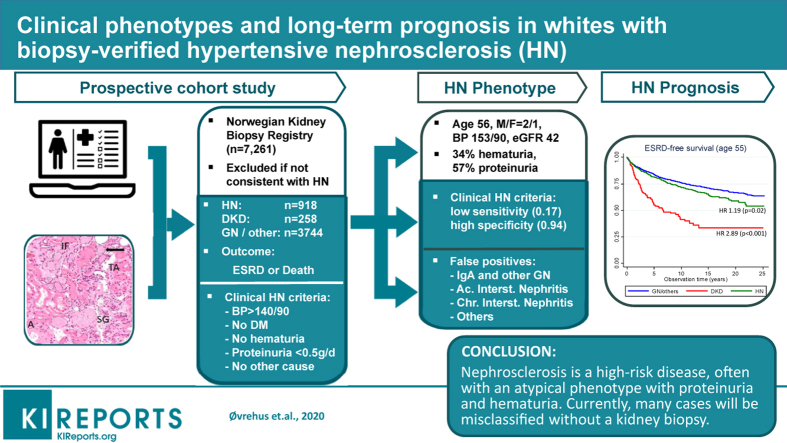
A total of 918 patients (19%) had biopsy-verified hypertensive nephrosclerosis (i.e., arterionephrosclerosis). Their most common biopsy indications were proteinuria (57%), low estimated glomerular filtration rate (eGFR) (44%), hematuria (34%), or combinations of these indications. Multivariable logistic regression analysis revealed that arterionephrosclerosis was significantly associated with higher age, male sex, not having diabetes, higher blood pressure, lower proteinuria, and not having hematuria (P < 0.01 for all). Body mass index, cholesterol, high-density lipoprotein cholesterol, and eGFR were not significantly associated with arterionephrosclerosis (P > 0.05 for all). The most common biopsy-verified diagnoses in patients fulfilling the clinical criteria for hypertensive nephrosclerosis were arterionephrosclerosis (40%), glomerulonephritis (22%), and interstitial nephritis (14%), reflecting that the criteria had low sensitivity (0.17) and high specificity (0.94). ESRD and mortality risks did not differ in patients with arterionephrosclerosis compared to patients with glomerulonephritis, interstitial nephritis, or other relevant diagnoses (P > 0.1 for both), whereas patients with diabetic kidney disease had a 2-fold higher risk (P < 0.001 for both).
Conclusion
Arterionephrosclerosis is a high-risk disease, often with an atypical phenotype with proteinuria and hematuria contributing to low accuracy for current clinical criteria for hypertensive nephrosclerosis.
Keywords: arterionephrosclerosis, diagnostic accuracy, hypertensive nephrosclerosis, renal biopsy
Graphical abstract
The incidence of ESRD has increased strongly in the United States and most Western countries over the past 20 years due to lifestyle-related chronic kidney disease (CKD).1 Diabetic kidney disease and hypertensive nephrosclerosis are now assumed to constitute 45% and 28% of new ESRD cases, respectively.2 However, the US Renal Data System makes a cautionary note on the reliability of these 2 diagnosis as primary causes of ESRD, reflecting that our underlying knowledge base, especially for hypertensive nephrosclerosis, is quite weak and often not evidence-based. Nephrosclerosis is diagnosed in the majority of patients without a kidney biopsy, even though the accuracy of a clinical nephrosclerosis diagnosis has been highly debated over the past 2 decades.3, 4, 5 Histologic findings typically include hyalinosis and narrowing of the afferent arterioles, global glomerulosclerosis, and interstitial fibrosis, and these are often named arterionephrosclerosis.
Only a few diagnostic studies with relatively small patient populations and suboptimal design have been published,6, 7, 8, 9, 10, 11 and prevalence, prognosis, and other important aspects of the nephrosclerosis phenotype are not well studied. Clinical criteria for nephrosclerosis—long-standing hypertension, no diabetes, no hematuria, and no overt proteinuria—had a positive predictive value of 97% in African American patients (n = 39), but it was only 48% in Italian patients (n = 56).9,10 African Americans with 2 risk allele variants of the apolipoprotein L1 (APOL1) gene have, after receiving some second environmental hit, a high risk of focal segmental glomerulosclerosis, arterionephrosclerosis, HIV nephropathy, and kidney failure.12,13 Recent findings indicate that pathologic ion-channel creation leads to disrupted endocytosis, autophagy, and mitochondrial dysfunction in the kidneys, which especially harms terminally differentiated cells such as the podocytes.13,14 Conversely, in whites arterionephrosclerosis is postulated to more often be due to a process initiated by hypertension and cardiovascular risk factors.5 These functional and structural changes indicate that kidney ischemia could be an important mechanism, but they could also resemble changes observed in normal aging. This has led some to question whether arterionephrosclerosis is simply an accelerated normal aging process.15
Therefore, to improve our understanding of hypertensive nephrosclerosis, we describe the clinical phenotype, prognosis, and diagnostics in white Norwegians with biopsy-verified arterionephrosclerosis.
Methods
The Norwegian Kidney Biopsy Registry has collected extensive clinical and histopathologic data for all patients undergoing kidney biopsy in Norway since 1988 (5.0 million inhabitants, >90% whites, and a biopsy frequency of 150 per million inhabitants per year in 2013).16 The registry classifies the biopsy as arterionephrosclerosis if typical findings occur in the absence of other primary renal diagnosis. In patients with other findings (such as diabetes, glomerulonephritis, amyloid, and so on), combined with arterionephrosclerosis, the latter is registered as an additional diagnosis only and not considered in the current study.
Standard evaluation of kidney biopsies in Norway is based on light microscopy, immunohistochemistry (staining for immunoglobulins IgG, IgA, IgM, κ/λ light chains, complements C3 and C1q), and electron microscopy combined with other types of staining as needed. Each biopsy is first evaluated by an experienced regional nephropathologist and later by the national registry nephropathologist using the criteria given in the World Health Organization monographs of renal disease for consistent diagnoses.17 Typical findings in arterionephrosclerosis are arterial medial thickening and hyaline arteriolosclerosis in afferent arterioles, leading to narrowing of the lumen. Furthermore, arterial medial hypertrophy, intimal sclerosis, and duplication of elastic laminae may be seen. Varying degrees of focal glomerular ischemic changes with thickening and wrinkling of the glomerular basement membrane, mesangial matrix increase, capillary collapse, and global glomerulosclerosis along with tubular atrophy and interstitial fibrosis are also seen.17
In addition, representative clinical data obtained before the biopsy are provided by the local nephrologist: the indication(s) for kidney biopsy (nephrotic syndrome, proteinuria, hematuria, progressive decline in kidney function, acute decline in kidney function, nephritic syndrome), age, sex, height, weight, systolic and diastolic blood pressure, antihypertensive medication, diabetes mellitus, and selected blood and urine laboratory values (eGFR, total cholesterol, high-density lipoprotein cholesterol, proteinuria, hematuria).
To generate a clinically relevant group of patients with biopsy-verified diagnoses, we excluded patients whose clinical presentation made arterionephrosclerosis very unlikely or when a specific diagnosis could be made with high probability based on clinical criteria and noninvasive blood testing alone; patients with nephrotic syndrome, antineutrophilic cytoplasmic nuclear antibody–associated vasculitis, anti–glomerular basement membrane glomerulonephritis, thrombotic microangiopathy, or light-chain cast nephropathy were excluded. The included patients thus represented a relevant mix of cases, consisting of both arterionephrosclerosis and other kidney diseases when arterionephrosclerosis could not be accurately ruled out by clinical presentation or noninvasive tests.
All patients were observed until December 2012 by linkage to the Norwegian Cause of Death Registry and the Norwegian Nephrology Registry. Mandatory reporting makes both registries more than 99% complete regarding death and ESRD status, respectively. We used Stata 13 software for statistical analysis (StataCorp LP, College Station, Texas). Baseline clinical variables were described as means or percentages, and we compared the group with arterionephrosclerosis to those with diabetic kidney disease or glomerulonephritis/other diseases using t test or χ2 test. We also used logistic regression to study the association between arterionephrosclerosis (yes/no) and various baseline characteristics. Prognosis was described with Kaplan-Meier plots, and the associations of kidney diagnosis with death and ESRD after adjusting for covariates were assessed with Cox regression analysis. Diagnostic accuracy was evaluated as sensitivity/specificity and positive/negative likelihood ratios, because these measures are less dependent on prevalence and enable proper adjustment of pretest probability in individual patients.
All participants gave informed consent when included in the Norwegian Kidney Biopsy Registry. Our study was approved by the Regional Committee for Medical and Health Research Ethics of Central Norway.
Results
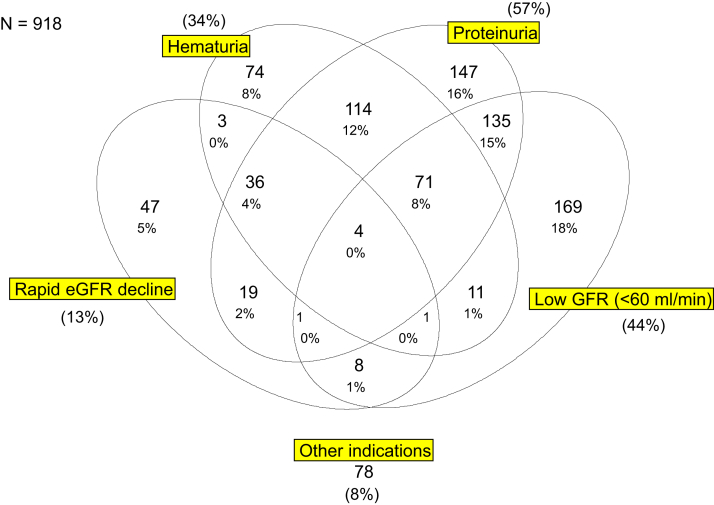
We included 4920 patients with biopsy-verified kidney diagnoses in whom arterionephrosclerosis could not be reasonably ruled out with noninvasive methods. Even though kidney biopsy is rarely performed when hypertensive nephrosclerosis is suspected, 918 (18.6%) of these relevant patients had arterionephrosclerosis as the main diagnosis. The majority of these patients with arterionephrosclerosis underwent biopsy owing to combinations of proteinuria (57%), low GFR (44%), and/or hematuria (34%) (Figure 1).
Figure 1.
Venn diagram showing clinical indications for kidney biopsy in all patients with histopathological arterionephrosclerosis included in the current study. GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate.
Baseline characteristics are shown in Table 1 by groups of arterionephrosclerosis, diabetic kidney disease, and glomerulonephritis/other diseases. The mean age of patients in the arterionephrosclerosis group was 57 years, 69% were men, and 10% had diabetes mellitus. Their mean systolic blood pressure was 153 mm Hg, eGFR was 42 ml/min per 1.73 m2, and urine protein excretion was 1.7 g/day. Compared with the group with glomerulonephritis/other diseases, this represented substantially higher age, more males, higher blood pressure, lower proteinuria, and less often hematuria (P < 0.001 for all). Correspondingly, patients with arterionephrosclerosis had higher diastolic blood pressure, lower body mass index, and lower proteinuria than those with diabetic kidney disease (P < 0.001 for all).
Table 1.
Baseline characteristics in diabetic kidney disease, arterionephrosclerosis, and glomerulonephritis/other relevant diseases
| Characteristics | DKD (n = 258) | Significance | ANS (n = 918) | Significance | GN/Other (n = 3744) |
|---|---|---|---|---|---|
| Age (yr) | 55.5 (14.4) | a | 56.8 (13.9) | b | 47.9 (17.2) |
| Male sex | 171 (66.2) | a | 632 (68.8%) | b | 2253 (60.2) |
| Body mass index (kg/m2) | 27.6 (4.1) | b | 25.8 (3.4) | c | 25.4 (3.9) |
| Diabetes mellitus | 228 (88.4) | b | 87 (9.5) | b | 201 (5.4) |
| Systolic blood pressure (mm Hg) | 153.9 (24.1) | a | 152.5 (27.8) | b | 139.8 (22.5) |
| Diastolic blood pressure (mm Hg) | 84.5 (11.8) | b | 90.0 (16.0) | b | 83.6 (13.2) |
| Cholesterol (mmol/l) | 5.2 (1.2) | c | 5.4 (0.8) | a | 5.5 (1.3) |
| HDL cholesterol (mmol/l) | 1.25 (0.26) | a | 1.26 (0.23) | c | 1.29 (0.26) |
| Proteinuria (g/24 h) | 3.2 (2.8) | b | 1.7 (2.0) | b | 2.0 (2.33) |
| Hematuria | 65 (25.2%) | c | 314 (34.2%) | b | 2087 (55.7) |
| eGFR (ml/min per 1.73 m2) | 41.1 (29.3) | a | 42.4 (27.9) | b | 53.1 (34.3) |
ANS, arterionephrosclerosis; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Data are shown as the mean (1 SD) or numbers (percentages).
None of the tests had P 0.01–0.05.
Nonsignificant tests with P > 0.05.
P < 0.001 based on 2-sided t test and χ2 tests.
P < 0.01.
The multivariate logistic regression analyses presented in Table 2 showed that arterionephrosclerosis was significantly associated with higher age, male sex, not having diabetes, higher blood pressure, not having hematuria, and lower proteinuria (P < 0.01 for all). Body mass index, cholesterol, high-density lipoprotein cholesterol, and eGFR were not associated with a histologic diagnosis of arterionephrosclerosis (P > 0.05 for all). Higher age and diastolic blood pressure were most strongly associated with arterionephrosclerosis. For example, if the diastolic blood pressure increased by 1 SD (i.e., 15 mm Hg), the odds of arterionephrosclerosis increased by 53% (odds ratio 1.53, P < 0.001). Corresponding standardized odds ratio for age was 1.64 (SD, 17 years, P < 0.001).
Table 2.
Association between clinical variables and histologically confirmed nephrosclerosis
| Unadjusted |
Age- + sex-adjusted |
Multivariable |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR std | 1 SD | ||||
| Age (per 1 yr) | 1.03 | 1.03 | 1.04 | 0.000 | 1.03 | 1.03 | 1.04 | 0.000 | 1.03 | 1.03 | 1.04 | 0.000 | 1.64 | 16.9 |
| Male sex (no/yes) | 1.44 | 1.23 | 1.68 | 0.000 | 1.43 | 1.23 | 1.68 | 0.000 | 1.30 | 1.101 | 1.53 | 0.004 | — | — |
| BMI (per 1 kg/m2) | 1.02 | 1.00 | 1.04 | 0.06 | 1.01 | 0.99 | 1.03 | 0.319 | 1.01 | 0.99 | 1.03 | 0.773 | 1.01 | 3.9 |
| Diabetes mellitus (no/yes) | 0.87 | 0.68 | 1.11 | 0.268 | 0.66 | 0.52 | 0.85 | 0.001 | 0.62 | 0.46 | 0.79 | 0.001 | — | — |
| Systolic BP (per 1 mm Hg) | 1.02 | 1.02 | 1.02 | 0.000 | 1.01 | 1.01 | 1.02 | 0.000 | 1.01 | 1.00 | 1.01 | 0.021 | 1.15 | 25.1 |
| Diastolic BP (per 1 mm Hg) | 1.03 | 1.03 | 1.04 | 0.000 | 1.03 | 1.03 | 1.04 | 0.000 | 1.03 | 1.02 | 1.04 | 0.000 | 1.53 | 14.5 |
| Hematuria (no/yes) | 0.45 | 0.38 | 0.52 | 0.000 | 0.53 | 0.45 | 0.62 | 0.000 | 0.48 | 0.42 | 0.58 | 0.000 | — | — |
| Proteinuria (per 1g/24 h) | 0.91 | 0.88 | 0.94 | 0.000 | 0.89 | 0.86 | 0.93 | 0.000 | 0.87 | 0.84 | 0.91 | 0.000 | 0.71 | 2.5 |
| eGFR (per 1ml/min per 1.73 m2) | 0.99 | 0.99 | 0.99 | 0.000 | 0.99 | 0.99 | 1.00 | 0.038 | 1.00 | 1.00 | 1.00 | 0.887 | 0.99 | 33.8 |
| Cholesterol (per 1 mmol/l) | 0.94 | 0.88 | 1.01 | 0.060 | 0.94 | 0.89 | 1.01 | 0.079 | 0.92 | 0.84 | 1.00 | 0.085 | 0.90 | 1.2 |
| HDL cholesterol (per 1mmol/l) | 0.69 | 0.51 | 0.93 | 0.020 | 0.87 | 0.66 | 1.20 | 0.368 | 1.10 | 0.80 | 1.57 | 0.967 | 1.00 | 0.3 |
BMI, body mass index; BP, blood pressure; CI, confidence interval; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; OR, odds ratio; OR std, odds ratio for outcome associated with 1-SD increase in x-variable.
Multivariable logistic regression analysis data. Age matching to population-based (older) cases did not change results.
Table 3 shows the distributions of histologic diagnoses among patients fulfilling the current clinical diagnostic criteria for hypertensive nephrosclerosis (hypertension with blood pressure >140/90 mm Hg, proteinuria <0.5 g/24 hours, no hematuria, and no diabetes mellitus) as well as all patients included in the study. Among the 380 patients with positive clinical criteria, there were 153 with biopsy-verified arterionephrosclerosis. The most common histologic diagnoses among those with a false-positive clinical diagnosis (227 of 380 patients) were primary glomerulonephritis (82), acute (33), and chronic (17) tubulointerstitial nephropathy, amyloidosis (15), lupus nephritis (5), and others (60). There were also 15 cases with normal or unclassifiable findings. This indicates that the diagnostic accuracy of current clinical arterionephrosclerosis criteria was low (Table 4). The clinical criteria detected only 153 of 918 cases with histopathologic arterionephrosclerosis (sensitivity 0.17), whereas 3775 of 4002 patients without arterionephrosclerosis had a negative test result (specificity 0.94). Corresponding likelihood ratios were 2.9 for a positive test result and 0.9 for a negative test result. This means that a positive test result would increase the post-test probability of disease moderately, whereas a negative test result would not give a clinical meaningful decrease of the disease probability (see the example in Table 4).
Table 3.
Distribution of histopathologic diagnoses in patients fulfilling current clinical criteria for arterionephrosclerosisa
| Histopathologic diagnoses | Positive clinical ANS criteria |
All relevant included |
Total Norwegian Kidney Biopsy Registry |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Amyloidosis | 15 (4.0) | 139 (2.8) | 299 (4.1) |
| Anti-GBM glomerulonephritis | — | — | 43 (0.6) |
| Arterionephrosclerosis | 153 (40.3) | 918 (18.7) | 947 (13.0) |
| Diabetic nephropathy | 0 (0.0) | 258 (5.2) | 359 (4.9) |
| Focal segmental glomerulosclerosis | 5 (1.4) | 218 (4.4) | 354 (4.9) |
| Glomerulonephritis, ANCA/crescentic | — | — | 561 (7.7) |
| Glomerulonephritis, mesangioproliferative, including IgA | 47 (12.4) | 1522 (30.9) | 1662 (22.9) |
| Glomerulonephritis, membranoproliferative | 6 (1.7) | 56 (1.1) | 133 (1.8) |
| Glomerulonephritis, membranous | 2 (0.4) | 118 (2.4) | 404 (5.6) |
| Glomerulonephritis, minimal change | 1 (0.4) | 68 (1.4) | 226 (3.1) |
| Glomerulonephritis, other/unclassified | 21 (5.6) | 337 (6.8) | 399 (5.5) |
| Interstitial nephritis, chronic | 17 (4.4) | 183 (3.7) | 186 (2.6) |
| Interstitial nephritis, acute | 33 (8.6) | 311 (6.3) | 316 (4.4) |
| Light-chain cast nephropathy/Ig deposit | — | — | 110 (1.5) |
| Lupus nephritis | 5 (1.3) | 199 (4.0) | 280 (3.9) |
| Thrombotic microangiopathy | — | — | 34 (0.5) |
| Unclassifiable | 40 (10.5) | 177 (3.6) | 186 (2.6) |
| Normal/very slight and unspecific changes | 15 (4.0) | 202 (4.1) | 211 (2.9) |
| Inadequate biopsy material | — | — | 309 (4.3) |
| Other/rare diagnosis | 20 (5.3) | 214 (4.3) | 242 (3.3) |
| Sum | 380 (100.0) | 4920 (100.0) | 7261 (100.0) |
ANCA, anti-neutrophil cytoplasmic nuclear antibodies; ANS, arterionephrosclerosis; GBM, glomerular basement membrane; NKBR, Norwegian Kidney Biopsy Register.
Positive clinical criteria for arterionephrosclerosis were hypertension (>140/90 mm Hg), proteinuria <0.5 g/24 h, no hematuria, and no diabetes mellitus.
The table includes all relevant patients in the current study and all biopsies in the Norwegian Kidney Biopsy Register.
Table 4.
Diagnostic performance of current clinical criteria of arterionephrosclerosis in relevant biopsy-verified cases
| Nephrosclerosis | Biopsy findings |
||
|---|---|---|---|
| Positive | Negative | ||
| Clinical diagnosis | |||
| Positive | 153 (TP) | 227 (FP) | 380 |
| Negative | 765 (FN) | 3775 (TN) | 4540 |
| 918 | 4002 | 4920 | |
| Sensitivity = TP/(TP + FN) = 0.167 (95% CI, 0.143–0.192) Specificity = TN/(TN + FP) = 0.943 (95% CI, 0.936–0.950) |
Positive LR = Sens./(1 – Spec.) = 2.94 (95% CI, 2.42–3.56) Negative LR = (1 – Sens.)/Spec. = 0.88 (95% CI, 0.86–0.91) |
||
Illustrative example (post-test odds = pretest odds × LR):
| |||
CI, confidence interval; FN, false negative, FP, false positive; LR, likelihood ratio; Sens., sensitivity; Spec., specificity; TN, true negative; TP, true positive.
The lower boxe shows how the probability of disease changes with a positive vs. a negative test result with the given test performance measures.
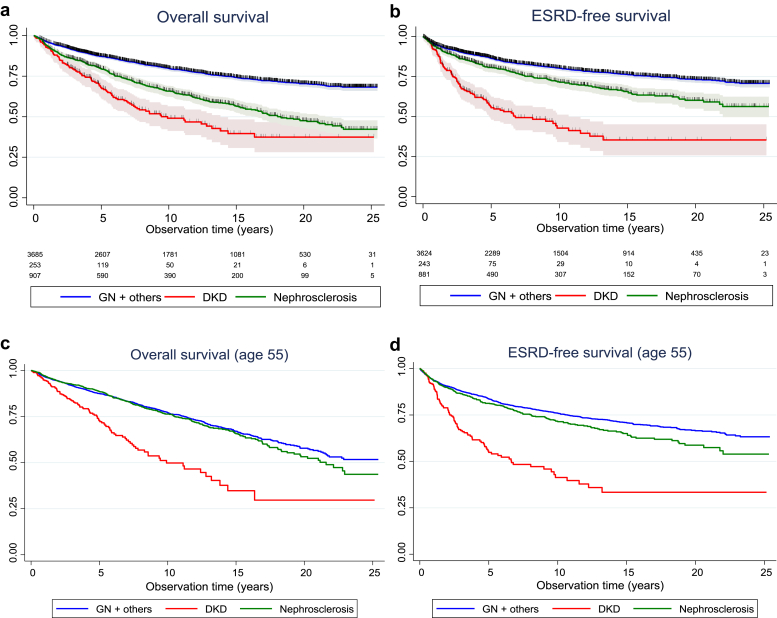
The long-term (25-year) prognosis for patients with biopsy-verified arterionephrosclerosis, diabetic kidney disease, and glomerulonephritis/other diagnoses is shown in Figure 2. The Kaplan-Meier curves represent the estimated probability of survival (with 95% confidence intervals) for hypothetical cohorts of interest, not the actual percentage surviving. Figure 2a presents overall survival curves and numbers at risk and shows that our cohort of patients with biopsy-verified arterionephrosclerosis has a better survival curve compared with those with diabetic kidney disease (log rank P < 0.001) but worse survival compared with those with glomerulonephritis or other diagnoses at biopsy (log-rank P < 0.001). After 10 years, approximately 80% of those with glomerulonephritis, 65% of arterionephrosclerosis, and only 50% of patients with diabetic kidney disease would be alive. Figure 2b presents the ESRD-free survival curves and shows that this cohort of patients with arterionephrosclerosis patients has a slightly higher ESRD risk than those with glomerulonephritis. Those with diabetic kidney disease had a substantially worse prognosis, with a 45% cumulative probability of having started renal replacement therapy after 5 years. Corresponding numbers for arterionephrosclerosis and glomerulonephritis were approximately 10% and 20%, respectively. However, patients with arterionephrosclerosis or diabetic kidney disease were on average 8 years older than those with glomerulonephritis or other diseases. Figures 2c and 2d therefore present overall mortality and ESRD-free survival curves adjusted to age 55 years. The age-adjusted morality curves were similar for arterionephrosclerosis and glomerulonephritis, whereas diabetic patients with kidney disease still had a much worse prognosis. Age-adjusted ESRD curves showed a similar picture, but with a slightly higher risk in arterionephrosclerosis compared with glomerulonephritis.
Figure 2.
Overall (a,d) and end-stage renal disease (ESRD)–free survival (b,d) by kidney diagnosis in biopsy-verified cases. The numbers of patients at risk during follow-up and censored cases are marked. Kaplan-Meier plots also show data risk-adjusted to age 55 years owing to the younger age of patients with glomerulonephritis (GN) or other disease (c,d). DKD, diabetic kidney disease.
Cox regression analysis (Table 5) revealed age- and sex-adjusted hazard ratios for death of 2.0 (P < 0.001) for diabetic kidney disease and 1.1 (P = 0.46) for patients with arterionephrosclerosis compared to those with glomerulonephritis (the reference group). Corresponding hazard ratios for ESRD were 2.2 (P < 0.001) and 1.1 (P = 0.05), respectively. Additional adjustment for other risks, such as blood pressure and obesity, as well as eGFR and proteinuria did not change these findings. Therefore, patients with arterionephrosclerosis experience risks similar to those with glomerulonephritis but lower than those of patients with diabetic kidney disease.
Table 5.
Association between biopsy-verified kidney diagnoses and clinical outcomes after adjusting for various risk factors
| Adjusted for age + sex |
Adjusted for age + sex + BP + BMI |
Adjusted for age + sex + BP + BMI + eGFR + proteinuria |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Death | ||||||||||||
| Arterionephrosclerosis | 1.09 | 0.96 | 1.23 | 0.18 | 1.00 | 0.84 | 1.20 | 0.98 | 0.99 | 0.82 | 1.20 | 0.94 |
| Diabetic kidney disease | 2.04 | 1.67 | 2.50 | 0.000 | 1.93 | 1.42 | 2.60 | 0.000 | 1.81 | 1.32 | 2.48 | 0.000 |
| Glomerulonephritis/other | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||||
| ESRD | ||||||||||||
| Arterionephrosclerosis | 1.19 | 1.03 | 1.39 | 0.023 | 0.92 | 0.75 | 1.13 | 0.43 | 0.95 | 0.77 | 1.17 | 0.64 |
| Diabetic kidney disease | 2.89 | 2.34 | 3.56 | 0.000 | 2.84 | 2.16 | 3.75 | 0.000 | 2.68 | 2.00 | 3.58 | 0.000 |
| Glomerulonephritis/other | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||||
BMI, body mass index; BP, blood pressure; CI, confidence interval; ESRD, end-stage renal disease; HR, hazard ratio.
Data are based on Cox proportional hazard regression analysis.
Discussion
Current clinical criteria for hypertensive nephrosclerosis had low sensitivity but high specificity, and the associated false-positive cases included a substantial proportion with histopathologic glomerulonephritis and interstitial nephritis. Many patients with arterionephrosclerosis had substantial proteinuria and other unconventional characteristics, and the diagnosis carried a substantial risk for ESRD and death.
The diagnostic process of arterionephrosclerosis, the second most common cause of ESRD,2,16,18 is hampered by the lack of generally accepted objective criteria. Schlesinger et al.6 reviewed 43 patients with ESRD who had presumed arterionephrosclerosis and found that few had undergone biopsy and only one of the patients had biopsy-verified arterionephrosclerosis. Likewise, Zarif et al.8 evaluated 607 patients with ESRD and found that less than 30% of 225 patients with hypertensive nephrosclerosis actually fulfilled the clinical criteria, kidney biopsy had only been performed in 4 patients; in only one of these were the biopsy findings consistent with a diagnosis of arterionephrosclerosis. Furthermore, few previous studies have evaluated the diagnostic accuracy of the current clinical criteria for nephrosclerosis.7,9, 10, 11 We previously found that disease was confirmed at biopsy in 38 of 39 patients in the African American Study of Kidney and Hypertension study who fulfilled the classic criteria of nephrosclerosis (i.e., positive predictive value 0.97).10 However, patients with negative test results did not have a kidney biopsy, so we could not calculate sensitivity and specificity. A similar limitation was present in all other studies.7,9,11 Our results from this study show that the clinical criteria used today have suboptimal diagnostic accuracy; a positive test result can increase post-test probability moderately, but a negative test result will not decrease the post-test probability at all.
Hypertension has always been assumed to be an important element of the nephrosclerosis phenotype, but the level of blood pressure does not directly predict degree of end-organ damage.5,19,20 Additional risk factors, such as age, obesity, hyperlipidemia, smoking, chronic inflammation, and oxidative stress, are necessary to trigger clinical kidney disease by inducing arterial stiffening, loss of preglomerular autoregulation, and ischemia. The biopsy-verified phenotype of white Northern Europeans in our study displayed some of these features (age), but we did not find an association with body mass index or lipids. Systolic blood pressure has long been recognized as a stronger risk factor for cardiovascular events compared with diastolic values, especially for coronary heart disease and in the elderly.21,22 Less information is available regarding kidney disease risk, and systolic and diastolic blood pressure have been found to predict ESRD rather equally in the general population.23 Many patients had substantial proteinuria, and although this has been described in previous case reports and small series,24, 25, 26 our data confirm that overt proteinuria is highly prevalent. Podocyte damage leading to proteinuria and thereby contributing to tubulointerstitial fibrosis has recently been identified as an important process in arterionephrosclerosis as well as other forms of glomerulosclerosis.27,28 Hematuria was also common, so although it decreases the probability of arterionephrosclerosis, it far from excludes the diagnosis.
In line with our findings, a severe prognosis has been demonstrated for patients with biopsy-verified nephrosclerosis in the rather few other studies available. A Japanese study found that 29% of the 401 patients included had severe progression defined as ESRD, doubling of the serum creatinine level, or a 50% reduction of eGFR after 5 years of follow-up.29 Other studies have described that 60% to 65% of patients with arterionephrosclerosis progressed to ESRD within 10 years, but these patients typically had much more severe hypertension compared with patients in our study.30,31 Large population-based studies of hypertensive patients without CKD at baseline have also demonstrated a strong graded risk with higher blood pressures starting in the high-normal range and increasing to a relative risk of 4 in those with severe hypertension (grade III).32 However, the absolute risk was very low with, for example, only 199 ESRD events among 21,340 patients with systolic blood pressure in the 160- to 180-mm Hg range with follow-up over 25 years—that is, 1% compared with more than 25% in our study of patients with established biopsy-verified nephrosclerosis. Furthermore, randomized intervention studies of hypertensive patients with nephrosclerosis fail to demonstrate a benefit of antihypertensive treatment,33 and it has been suggested that many cases of nephrosclerosis could represent normal aging.34 Therefore, the absolute risk of developing severe CKD among hypertensive patients is low, but for those with established hypertensive nephrosclerosis, the risk of ESRD or death equals the risk among those with glomerulonephritis or other classic nondiabetic kidney diseases.
Our findings could have important clinical consequences. A significant number of false-positive arterionephrosclerosis cases with undiagnosed IgA nephropathy and other primary glomerulopathies, amyloidosis, and acute as well as chronic tubulointerstitial nephritis could potentially have been offered different and more specific treatment if they had undergone biopsy. This is in line with a more active use of kidney biopsy in groups traditionally not offered this diagnostic test (i.e., the elderly, patients with assumed diabetic kidney disease, and patients with assumed arterionephrosclerosis).35,36 However, although kidney biopsy in general is a safe procedure when performed lege artis,37 recommendations highlight the need to ensure that the risk of the procedure and treatment is acceptable for the patient.38 However, whether and how much the indications for biopsy should be widened warrants further discussions and studies.
Some important study limitations merit discussion. The optimal study design would be to systematically obtain biopsies and prospectively follow up all incident patients with a clinical phenotype of hypertensive nephropathy. However, it is unlikely that patients, physicians, and ethics boards would accept such a liberal biopsy policy. Thus, we probably need to accept, but actively recognize, the inherited selection bias in biopsy-verified studies. Our study probably included more atypical cases than found in clinical practice, so it could be that we underestimate the diagnostic accuracy of the current clinical criteria. However, we base our findings on a large number of biopsy-verified cases combined with relevant clinical data, which seldom have been available for studies on hypertensive nephrosclerosis. A very long follow-up, as in our study, is also necessary in ESRD studies because progression is often slow and it may take many years before differences between various phenotypes and treatments become clear. Finally, we included only white Northern European subjects, and generalization to other regions should be done with caution (and for African Americans not at all).
In conclusion, the current clinical criteria for arterionephrosclerosis have suboptimal diagnostic accuracy in whites because many patients have an atypical phenotype with proteinuria and hematuria. Arterionephrosclerosis carries an increased ESRD and mortality risk comparable with other causes of nondiabetic CKD. Further studies should focus on optimizing the clinical criteria for hypertensive nephrosclerosis and developing new diagnostic tests, including evidence-based indications for kidney biopsy in this large group of patients with CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre at Norwegian University of Science and Technology, Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
Author Contributions
MAØ and SIH designed the study; TSO, AD, RB, KIA, and SIH collected the data; ABF, JHI, and SIH analyzed the data; and MAØ and SIH drafted and revised the article. All authors revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
References
- 1.Saran R., Li Y., Robinson B. US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 suppl 1):Svii. doi: 10.1053/j.ajkd.2015.12.014. S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health. U.S Renal Data System, annual data report 2018. https://www.usrds.org/2018/view/Default.aspx Available at:
- 3.Weisstuch J.M., Dworkin L.D. Does essential hypertension cause end-stage renal disease? Kidney Int Suppl. 1992;36:S33–S37. [PubMed] [Google Scholar]
- 4.Luft F.C. Hypertensive nephrosclerosis—a cause of end-stage renal disease? Nephrol Dial Transplant. 2000;15:1515–1517. doi: 10.1093/ndt/15.10.1515. [DOI] [PubMed] [Google Scholar]
- 5.Meyrier A. Nephrosclerosis: a term in quest of a disease. Nephron. 2015;129:276–282. doi: 10.1159/000381195. [DOI] [PubMed] [Google Scholar]
- 6.Schlessinger S.D., Tankersley M.R., Curtis J.J. Clinical documentation of end-stage renal disease due to hypertension. Am J Kidney Dis. 1994;23:655–660. doi: 10.1016/s0272-6386(12)70275-5. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y.S., Yuan W.J., Yu J.P. [Clinical and pathological characteristics in patients with clinically presumed hypertensive nephrosclerosis] [in Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:391–395. [PubMed] [Google Scholar]
- 8.Zarif L., Covic A., Iyengar S. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15:1801–1807. doi: 10.1093/ndt/15.11.1801. [DOI] [PubMed] [Google Scholar]
- 9.Zucchelli P.Z., Auccalà A. Recent data on hypertension and progressive renal disease. J Hum Hypertens. 1996;10:679–682. [PubMed] [Google Scholar]
- 10.Fogo A., Breyer J.A., Smith M.C. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 11.Caetano E.R., Zatz R., Saldanha L.B., Praxedes J.N. Hypertensive nephrosclerosis as a relevant cause of chronic renal failure. Hypertension. 2001;38:171–176. doi: 10.1161/01.hyp.38.2.171. [DOI] [PubMed] [Google Scholar]
- 12.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckerman P., Susztak K. APOL1: the balance imposed by infection, selection, and kidney disease. Trends Mol Med. 2018;24:682–695. doi: 10.1016/j.molmed.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman B.I., Cohen A.H. Hypertension-attributed nephropathy: what's in a name? Nat Rev Nephrol. 2016;12:27–36. doi: 10.1038/nrneph.2015.172. [DOI] [PubMed] [Google Scholar]
- 15.Rule A.D., Amer H., Cornell L.D. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwegian Kidney Biopsy Register Annual reports. 2018. https://www.nephro.no/nnr/AARSRAPPORT_NNR_2017.pdf Available at:
- 17.Churg JB J, Glassock R.J. 2nd ed. Igaku-Shoin; New York: 1995. Renal disease: classification and atlas of glomerular diseases. [Google Scholar]
- 18.ERA-EDTA Registry. Annual report 2014. https://www.era-edta-reg.org/index.jsp?p=14 Available at:
- 19.Kopp J.B. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens. 2013;22:266–272. doi: 10.1097/MNH.0b013e3283600f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill G. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- 21.Black H.R. The paradigm has shifted to systolic blood pressure. J Hum Hypertens. 2004;18(suppl 2):S3–S7. doi: 10.1038/sj.jhh.1001795. [DOI] [PubMed] [Google Scholar]
- 22.Lewington S., Clarke R., Qizilbash N. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 23.Klag M.J., Whelton P.K., Randall B.L. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 24.Obialo C.I., Hewan-Lowe K., Fulong B. Nephrotic proteinuria as a result of essential hypertension. Kidney Blood Press Res. 2002;25:250–254. doi: 10.1159/000066345. [DOI] [PubMed] [Google Scholar]
- 25.Kimura N., Yonemoto S., Machiguchi T. Synthetic/secreting and apoptotic phenotypes in renal biopsy tissues from hypertensive nephrosclerosis patients. Hypertens Res. 2006;29:573–580. doi: 10.1291/hypres.29.573. [DOI] [PubMed] [Google Scholar]
- 26.Innes A., Johnston P.A., Morgan A.G. Clinical features of benign hypertensive nephrosclerosis at time of renal biopsy. Q J Med. 1993;86:271–275. [PubMed] [Google Scholar]
- 27.Fogo A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11:76–87. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seccia T.M., Caroccia B., Calo L.A. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J Hypertens. 2017;35:205–212. doi: 10.1097/HJH.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 29.Yamanouchi M., Hoshino J., Ubara Y. Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: a biopsy-based cohort study. Nephrol Dial Transplant. 2019;34:1182–1188. doi: 10.1093/ndt/gfy121. [DOI] [PubMed] [Google Scholar]
- 30.Dasgupta I., Porter C., Innes A., Burden R. “Benign” hypertensive nephrosclerosis. QJM. 2007;100:113–119. doi: 10.1093/qjmed/hcl139. [DOI] [PubMed] [Google Scholar]
- 31.Liang S., Le W., Liang D. Clinico-pathological characteristics and outcomes of patients with biopsy-proven hypertensive nephrosclerosis: a retrospective cohort study. BMC Nephrol. 2016;17:42. doi: 10.1186/s12882-016-0254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu C.Y., McCulloch C.E., Darbinian J. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 33.Ku E., Gassman J., Appel L.J. BP Control and long-term risk of ESRD and mortality. J Am Soc Nephrol. 2017;28:671–677. doi: 10.1681/ASN.2016030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hommos M.S., Glassock R.J., Rule A.D. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28:2838–2844. doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S.G., Bomback A.S., Radhakrishnan J. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–1724. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhaun N., Bellamy C.O., Cattran D.C., Kluth D.C. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85:1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- 37.Tondel C., Vikse B.E., Bostad L., Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591–1597. doi: 10.2215/CJN.02150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]