Abstract
Introduction
Much of the higher risk for end-stage kidney disease (ESKD) in African American individuals relates to ancestry-specific variation in the apolipoprotein L1 gene (APOL1). Relative to kidneys from European American deceased-donors, kidneys from African American deceased-donors have shorter allograft survival and African American living-kidney donors more often develop ESKD. The National Institutes of Health (NIH)–sponsored APOL1 Long-term Kidney Transplantation Outcomes Network (APOLLO) is prospectively assessing kidney allograft survival from donors with recent African ancestry based on donor and recipient APOL1 genotypes.
Methods
APOLLO will evaluate outcomes from 2614 deceased kidney donor-recipient pairs, as well as additional living-kidney donor-recipient pairs and unpaired deceased-donor kidneys.
Results
The United Network for Organ Sharing (UNOS), Association of Organ Procurement Organizations, American Society of Transplantation, American Society for Histocompatibility and Immunogenetics, and nearly all U.S. kidney transplant programs, organ procurement organizations (OPOs), and histocompatibility laboratories are participating in this observational study. APOLLO employs a central institutional review board (cIRB) and maintains voluntary partnerships with OPOs and histocompatibility laboratories. A Community Advisory Council composed of African American individuals with a personal or family history of kidney disease has advised the NIH Project Office and Steering Committee since inception. UNOS is providing data for outcome analyses.
Conclusion
This article describes unique aspects of the protocol, design, and performance of APOLLO. Results will guide use of APOL1 genotypic data to improve the assessment of quality in deceased-donor kidneys and could increase numbers of transplanted kidneys, reduce rates of discard, and improve the safety of living-kidney donation.
Keywords: African Americans, APOL1, chronic kidney disease, graft failure, kidney transplantation, outcomes
Graphical abstract
See Commentary on Page 252
Implementation of a new Kidney Allocation System in 2014 was an attempt to remedy the shortage of kidneys for transplantation and premature loss of functioning allografts caused by recipient death from nonrenal causes.1,2 The new Kidney Allocation System attempts to increase renal allograft survival by better matching recipients with quality of donor kidneys. The Kidney Allocation System also improves equity for highly sensitized patients and those with longer dialysis vintage. An essential aspect of the new Kidney Allocation System is the Kidney Donor Risk Index (KDRI), a scoring system based on 10 deceased-donor factors that objectively determines the quality of kidneys and estimates graft life span. African American ethnicity/race is an important characteristic considered in the KDRI because it projects an approximate 20% higher risk for allograft loss.
The KDRI was designed before widespread recognition of the role of apolipoprotein L1 gene (APOL1) G1 and G2 renal-risk variants (RRVs) on susceptibility to ESKD in populations with recent African ancestry.3,4 These populations have similar genetic composition to individuals currently residing in Africa. APOL1 RRVs are present nearly exclusively in individuals with recent African ancestry and cause a range of kidney diseases in an autosomal recessive inheritance pattern.5 A minority of individuals with APOL1 high-risk genotypes (2 RRVs; G1G1, G2G2, or G1G2) develop nephropathy.5 Retrospective studies support that variation in donor APOL1 genotypes, not donor African American ancestry per se, increases the risk for shorter renal allograft survival.6, 7, 8, 9 Donor high-risk APOL1 genotypes are also associated with kidney disease in living-kidney donors.10
To improve safety in living-kidney donation and outcomes for kidney transplant recipients, as well as increase knowledge of APOL1 effects after transplantation, the National Institute of Diabetes and Digestive and Kidney Diseases issued a request for applications entitled the APOLLO in November 2016.11 The National Institute on Minority Health and Health Disparities and the National Institute of Allergy and Infectious Diseases provide cofunding and research expertise. This U01 Consortium supports a Scientific and Data Research Center (SDRC or Coordinating Center) including a cIRB and Clinical Laboratory Improvement Amendments–approved genotyping laboratory. APOLLO supports 13 Clinical Centers (CCs) to recruit and follow participants. APOLLO focuses on key aspects of the 2019 Presidential Executive Order on Kidney Care, addressing improved care of the African American population and emphasizing increasing rates of transplantation, improving efficiency of transplantation, and streamlining organ procurement processes (https://www.whitehouse.gov/briefings-statements/remarks-president-trump-signing-executive-order-advancing-american-kidney-health/).
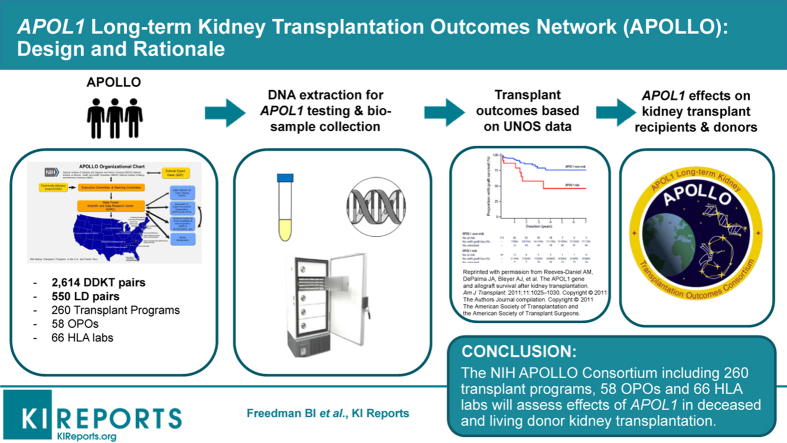
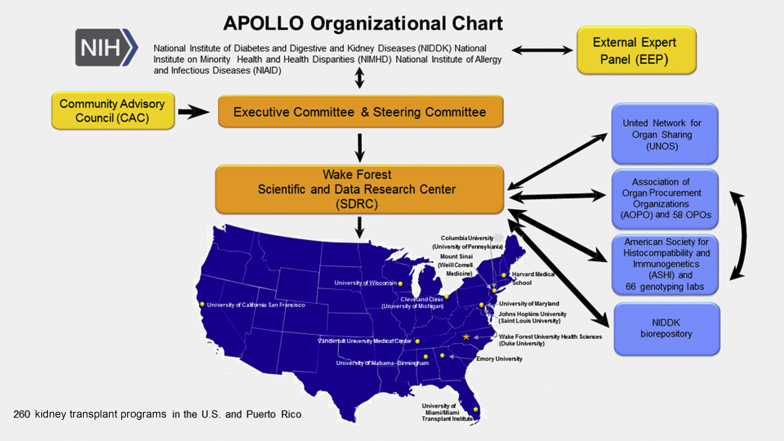
Implementing the APOLLO protocol across 260 kidney transplant programs, 58 OPOs, and 66 histocompatibility laboratories is challenging; OPOs and histocompatibility laboratories participate voluntarily. Figure 1 displays the APOLLO organizational chart and Table 1 lists the 17 APOLLO CCs and co-CCs, with principal investigators. The APOLLO Steering Committee (SC) consists of representatives from NIH, an external study chair, chair of the community advisory council (CAC), the Wake Forest School of Medicine SDRC and principal investigators at 13 APOLLO CCs.12 Leadership of UNOS and Association of Organ Procurement Organizations (AOPO) participate (nonvoting members).
Figure 1.
APOL1 Long-term Kidney Transplantation Outcomes Network (APOLLO) organizational chart (developed using clker.com free clipart; http://www.clker.com/clipart-blank-gray-usa-map-white-lines-1.html). NIH, National Institutes of Health; OPO, organ procurement organization.
Table 1.
APOLLO clinical centers and principal investigators
| Clinical center | Principal investigator(s) |
|---|---|
| University of Wisconsin, Madison, Wisconsin, USA | Brad C. Astor |
| Vanderbilt University Medical Center, Nashville, Tennessee, USA | Kelly A. Birdwell |
| Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; and Saint Louis University School of Medicine, St. Louis, Missouri, USA | Daniel C. Brennan and Krista L. Lentine |
| University of Alabama School of Medicine in Birmingham, Birmingham, Alabama, USA | Bruce A. Julian and Roslyn B. Mannon |
| Columbia University, New York, New York, USA; and University of Pennsylvania, Philadelphia, Pennsylvania, USA | Sumit Mohan and Deirdre Sawinski |
| Mount Sinai School of Medicine, New York, New York, USA; and Weill Cornell Medical Center, New York, New York, USA | Barbara Murphy and Darshana M. Dadhania |
| Cleveland Clinic, Cleveland, Ohio, USA; and University of Michigan, Ann Arbor, Michigan, USA | Emilio D. Poggio and Mona D. Doshi |
| Emory University School of Medicine, Atlanta, Georgia, USA | Stephen O. Pastan and Kenneth A. Newell |
| Joslin Diabetes Center, Boston, Massachusetts, USA | Sylvia E. Rosas |
| University of Maryland, Baltimore, Maryland, USA | Jonathan Bromberg and Matthew R. Weir |
| University of Miami, Miami, Florida, USA | Alessia Fornoni, Giselle Guerra, and Mariella Ortigosa-Goggins |
| University of California, San Francisco, San Francisco, California, USA | Chi-yuan Hsu and Meyeon Park |
| Wake Forest School of Medicine, Winston-Salem, North Carolina, USA; and Duke University School of Medicine, Durham, North Carolina, USA | Amber M. Reeves-Daniel, Barry I. Freedman, and Rasheed A. Gbadegesin |
Background
APOLLO is an observational study prospectively evaluating outcomes after kidney transplantation from deceased- and living-donors with recent African ancestry.11 APOLLO includes donors with self-reported or family-reported African American, Hispanic black, Afro-Caribbean, and African ancestry. Clinical data, DNA, serum, and urine from kidney donors and recipients of their kidneys are collected. To prevent alterations in clinical practice and minimize variability, APOL1 genotypes will be determined after recruitment ends. UNOS will provide outcomes data. Additional information is on the APOLLO Web site: http://TheApolloNetwork.org.
The primary objective of APOLLO is determining whether the presence of APOL1 renal-risk genotypes in deceased-donors is associated with death-censored renal allograft survival. Secondary objectives include the following:
-
•
defining whether the presence of APOL1 high-risk genotypes in kidney donors is associated with poorer kidney function or greater proteinuria after transplantation;
-
•
defining whether the presence of APOL1 high-risk genotypes is associated with poorer kidney outcomes in living-kidney donors after nephrectomy; and
-
•
identifying modifying factors that increase susceptibility to shortened allograft survival, reduced kidney allograft function, or association with greater proteinuria in recipients of kidneys from APOL1 high-risk genotype donors, under the assumption that donor APOL1 high-risk genotypes are associated with poorer kidney function in recipients of renal allografts.
APOLLO results could prompt a revision of the current formula to calculate the KDRI by replacing the ethnicity/race component with APOL1 genotype.13,14 This refinement could reduce discard of APOL1 low-risk genotype deceased-donor kidneys, leading to more transplants, improved quality of life for recipients, and reduced health care costs. Despite consistent retrospective results, data from a well-powered national prospective study would provide stronger justification for implementing universal APOL1 genotyping in deceased-donors.15
Clarification of the role of APOL1 genotyping in potential living-donors is also urgently needed. Although recommending APOL1 genotyping be considered in the living-donor candidate evaluation, the 2017 Kidney Disease: Improving Global Outcomes Guideline for the Evaluation and Care of Living Donors identified the need to define the role of APOL1 genotyping in the evaluation of donor candidates with recent African ancestry as a key research priority.16 APOLLO and its ancillary studies address this call for additional evidence.
Organization of Kidney Transplant Programs in APOLLO
APOLLO aims to enroll all eligible kidney donors and kidney transplant recipients from U.S. transplant programs. This effort includes 260 academic and community-based programs with kidneys recovered from 58 OPOs (Figure 1). Approximately half of these programs perform 90% of kidney transplants eligible for APOLLO. To foster universal participation, the SDRC aligned each of the 13 CCs with multiple transplant programs to include all programs. CC principal investigators are working with medical directors and physicians at their assigned programs. To date, a small number of kidney transplant programs (each anticipated to perform <10 eligible kidney transplants per year) have declined to participate. This mainly involved programs that do not perform clinical research.
Partnerships
The APOLLO SC developed partnerships with UNOS, AOPO, and its 58 OPOs and 66 OPO-contracted histocompatibility laboratories to assess deceased-donor APOL1 genotype effects on kidney transplantation. Close relationships quickly developed among the APOLLO SDRC, APOLLO SC, American Society of Transplantation (represented on the SC), and leadership of AOPO, UNOS, and the American Society for Histocompatibility and Immunogenetics (ASHI). With completion of a data use agreement, UNOS worked at nominal cost with the SDRC to transfer information electronically for optimizing recruitment and determining outcomes. UNOS provides the SDRC with a daily list of recipients of kidneys transplanted from eligible donors. This list is shared with APOLLO CCs to ensure eligible donors are approached for recruitment. AOPO and ASHI proved to be motivated partners and participate voluntarily. APOLLO produces additional work for their members; however, the vast majority agreed to participate to advance the science of transplantation and improve the lives of patients with kidney disease.
APOLLO Community Advisory Council
Shortly after inception, APOLLO developed a CAC composed of individuals with self-reported recent African ancestry and knowledge of kidney disease or transplantation.12 CAC members represent nearly all facets of the study population, including living-kidney donors, kidney transplant recipients, and patients with chronic kidney disease/ESKD (or their relatives). The CAC includes 2 representatives from the 13 CCs and an at-large member who serves on the SC. The CAC advises the NIH and SC on policy and practice matters, bridges the African American community with APOLLO investigators to engage the community, builds trust, and enlists allies to improve communication.
Members of the CAC directly participated in the design of APOLLO by providing guidance on how to conduct the research, along with input on the participant information documents and study videos. Highlighting their critical role, the CAC stimulated many changes in study design: (i) a 1-page bullet summary of the APOLLO study for page 1 of the consent form; (ii) requirement that living-donor candidates be informed about APOLLO (or have APOL1 genotyping) at least 2 weeks before the date of the donor nephrectomy (otherwise, they will not be eligible); (iii) development of color infographics to explain the benefits of participating in the study in lay terms, as well as the pros and cons of requesting APOL1 genotype data at the conclusion of the study; (iv) contributed to the decision allowing the authorized family decision maker for deceased-donors to request donor APOL1 gene test results; and (v) helped craft language in the consent form and return of results letters/Web site information. Every aspect of the protocol was reviewed with the CAC.
Protocol Overview
APOLLO inclusion criteria for kidney donors require family-reported or self-reported recent African ancestry. Exclusion criteria include deceased-donors whose families do not agree that their loved ones’ tissues be used for research and living-donors unwilling to provide informed consent. NIH mandated attempts at universal enrollment of all eligible kidney transplant recipients in the APOLLO Request for Applications. The study provides English and Spanish consent forms. If an APOLLO CC wishes the consent form in another language, they can arrange translation and certification, then share it with the APOLLO cIRB for study-wide approval.
Inclusion criteria for transplant recipients include those receiving kidneys from an eligible living- or deceased-donor with recent African ancestry, regardless of the recipients’ ancestry. Multi-organ transplants, such as a kidney with an additional organ or pediatric en bloc and dual kidney transplants, are eligible. There is no age cutoff for inclusion of kidneys from eligible deceased pediatric donors or for recipients of a kidney transplant. Pediatric recipients will be enrolled with the consent of their parent or legal guardian and with their assent if between the ages of 7 and 17 years. Exclusion criteria for recipients include those unwilling to provide informed consent.
Teams from OPOs assess whether families of eligible deceased-donors agree that the donor’s tissues and bio-samples may be included in research studies. If they do, samples of blood and urine are collected. Bio-samples are sent to regional histocompatibility laboratories with clinical samples; DNA is extracted and serum is processed. APOLLO DNA, serum, and urine samples are shipped to the SDRC quarterly. Samples from deceased-donors will be thawed once at the SDRC, aliquoted, and portions sent to the National Institute of Diabetes and Digestive and Kidney Diseases Repository (see Manual of Procedures Stage 1 in the Supplementary Material for details).
Based on data from September 1, 2015, to August 31, 2016, APOLLO could enroll approximately 2048 recipients of a kidney (1881 kidney-alone; 167 simultaneous kidney-pancreas and other multi-organ transplants) from eligible deceased-donors per year if all deceased-donor families permitted their loved ones’ tissues and organs to be included in research and all kidney transplant recipients consented. For adequate power to assess the primary APOLLO study outcome, recruitment will target 2614 deceased-donor kidney-recipient pairs (Tables 2 and 3).
Table 2.
Power for the primary analysis, assumes 18-month recruitment, 2614 recipients, and 1634 deceased-donors, mean follow-up 3 years with marginal failure rates 15%, 12.5%, and 10%
| Correlation within-donor/effective sample size |
0.3/2162 |
0.5/1960 |
0.7/1807 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure rates/no. failures | 15%/432 | 12.5%/378 | 10%/324 | 15%/392 | 12.5%/343 | 10%/294 | 15%/361 | 12.5%/316 | 10%/271 |
| Hazard ratio 1.7 | 0.895 | 0.835 | 0.747 | 0.864 | 0.798 | 0.705 | 0.836 | 0.765 | 0.670 |
| Hazard ratio 1.5 | 0.690 | 0.611 | 0.518 | 0.647 | 0.569 | 0.480 | 0.612 | 0.536 | 0.449 |
Table 3.
Power for the primary analysis, assumes 18-month recruitment, 2614 recipients and 1634 deceased-donors, mean follow-up 4 years with marginal failure rates 20%, 17.5%, and 15%
| Correlation within-donor/effective sample size |
0.3/2162 |
0.5/1960 |
0.7/1807 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure rates/no. failures | 20%/432 | 17.5%/378 | 15%/324 | 20%/392 | 17.5%/343 | 15%/294 | 20%/361 | 17.5%/316 | 15%/271 |
| Hazard ratio 1.7 | 0.960 | 0.935 | 0.895 | 0.942 | 0.911 | 0.864 | 0.924 | 0.888 | 0.836 |
| Hazard ratio 1.5 | 0.809 | 0.756 | 0.690 | 0.770 | 0.714 | 0.647 | 0.736 | 0.679 | 0.612 |
Demographic and clinical data on donors and recipients extracted from the UNOS and Scientific Registry of Transplant Recipients databases will be sent to the SDRC. To provide additional data missing in the large databases, clinical data from consenting participants arising from routine, standard-of-care, follow-up visits will be extracted from the medical records at transplant programs for upload to the SDRC.
The APOLLO study will attempt to enroll living-donors with recent African ancestry and recipients of their kidneys during the recruitment phase until 2614 recipients of eligible deceased-donor kidneys are recruited. Living-donors younger than 18 years will not be enrolled, as this is not a typical clinical scenario. Transplant programs will discuss APOLLO study participation with all living-donor candidates with recent African ancestry early in the evaluation process and must obtain informed consent no less than 2 weeks before planned nephrectomy. The affiliated CC will perform these functions at their aligned transplant programs. Collection, processing, and shipment of bio-samples from living-kidney donors are the same as those for transplant recipients (outlined in Manual of Procedures Stage 2, Supplementary Material). Clinical data from routine, standard-of-care, follow-up visits will be collected from medical records at transplant programs and extracted from UNOS to the SDRC (see Supplementary Material for APOLLO Data Collection, including timing of data collection, types of data elements collected, and data included in primary analyses).17
Statistical Considerations
The primary APOLLO analysis is the association of donor APOL1 genotypes with time to death-censored renal allograft failure in recipients of a kidney from deceased-donors with recent African ancestry. Return to dialysis/renal replacement therapy or retransplantation qualify as graft failure, but listing for a kidney transplant would not qualify. This will be a proportional hazards model extended to allow for correlation between pairs of recipients of kidneys from the same donor, stratified by transplant program and covariate adjusted using recipient age, sex, recipient’s self-reported ancestry (reported as race in the UNOS database), human leukocyte antigen match, cold ischemia time, panel reactive antibodies, donor age, and the KDRI.8 Supporting analyses will include recipient APOL1 genotype and exploration of other covariate effects, including transplant center characteristics that may be used to pool similar smaller centers into larger strata.
Sample Size
APOLLO is designed to detect an APOL1 high-risk genotype (2 APOL1 RRVs) effect on the hazard for time to graft failure of 1.7 with power between 50% and 94% depending on length of recruitment, recruitment yield, the correlation between 2 outcomes from a single donor, and observed failure rates. It was initially anticipated that follow-up of recipients would be approximately 3 years; however, if follow-up can be extended by 1 year beyond the current study timeline, and the proposed recruitment yield of 2614 is obtained within 18 months, the minimum power to detect a 15% failure rate would be 80%. The analysis estimating the increased hazard attributable to high-risk APOL1 genotypes will include African American deceased-donor kidney transplant (DDKT) recipients, allowing for correlation between outcomes from 2 recipients receiving kidneys from a single deceased-donor.
Data from UNOS on transplants between September 1, 2015, and August 31, 2016, included 2545 recipients of kidneys from African American donors, representing 497 living-donors and 1285 deceased-donors. Among the deceased-donors, 763 donated 2 kidneys and 522 1 kidney, a total of 2048 transplants. To project recruitment yield for APOLLO, the calculations described in this article assume 2050 DDKT occur in 12 months and 3075 in 18 months. To allow for refusals and fluctuations in the number of DDKT over time, the expected yield below is reduced by 15% to 2614 DDKT recruited over 18 months and 1742 over 12 months. The number of deceased-donors donating to 2 recipients is assumed to be 60% of all deceased-donors, so that 2614 DDKT represents 1634 deceased-donors, 980 donating 2 kidneys and 654 2 kidney.
Correlation of Transplant Outcomes Within Donors
Because the primary aim assesses the association of APOL1 genotypes of the deceased kidney donor, and transplant outcomes on 2 kidneys from the same donor may be correlated, the effective sample size for that analysis falls between the number of transplants and the number of donors and depends on the within-donor correlation for donors providing 2 kidneys. The sample size numbers represent an effective number of African American DDKT, meaning that the total number of recipients is reduced by a factor of 1/(1+r), where r represents the correlation in outcomes from 2 recipients receiving kidneys from a single deceased-donor. An analysis of the dataset used for Freedman et al.8 estimated the correlation to be between 0.5 and 0.7. The actual correlation in the APOLLO analysis may differ but will likely be between 0.3 and 0.8. For Tables 2 and 3, there is a calculation for effective sample sizes corresponding to correlations of 0.3, 0.5, and 0.7. Gangnon and Kosorok18 provide a formula for sample size calculation with clustered survival data.
Estimated Graft Failure Rate
The failure rates in Tables 2 and 3 were estimated from published data.8 Based on retrospective data, the 3-year cumulative failure rate approximated 12% for low-risk APOL1 genotypes (<2 RRVs) and 16% for high-risk APOL1 genotypes (2 RRVs). The 4-year cumulative failure rates were approximately 15% and 20% for low-risk and high-risk APOL1 genotypes, respectively. Because there is some uncertainty in these estimates, Tables 2 and 3 include a range expected to cover the actual observed failure rates. Note that the power is driven by the number of observed events regardless of the length of follow-up. The number of failures for estimates in which the assumed correlation is not zero represents an effective number after a (downward) adjustment for the correlation.
Length of Recruitment and Follow-up Periods
The initial study timeline was set to last 60 months. Allowing the initial months for startup and the last 3 months for analysis, the total length of recruitment and follow-up would be approximately 51 months. An 18-month recruitment period produces an average follow-up of 3 years for the cohort, assuming annual updates of outcome data through Month 51. With 18 months of recruitment, unless the within-donor correlation is less than 0.3 (lower than the literature suggests; not presented in Tables 2 or 3), or the cumulative 3-year failure rate is at least 15% (which would be somewhat high), the effective number of observed failures will not provide 80% power for a hazard ratio of 1.7 with 3-year follow-up (Table 2). The total number of observed failures could be increased by lengthening the average follow-up period to 4 years. Table 3 contains power estimates assuming the study timeline increases to 72 months by extending follow-up for 1 year after an average 18-month recruitment period. If recruitment is slower than expected or site initiation delayed, the recruitment period might need to be extended.
Expected Effect Size of APOL1 RRVs
Prior reports provide relevant estimates of effect size from data comparable to what APOLLO will collect; a hazard ratio indicating increased risk of allograft failure for kidneys from deceased-donors with 2 APOL1 RRVs in the range of 1.5 to 3.0.8 Because APOLLO is designed to capture outcomes from a larger, more diverse population, and to be conservative, we estimated the power using hazard ratios 1.5 to 2.0. This range includes 1.7, the hazard ratio cutoff defining risk for a high-KDPI kidney (formerly expanded-criteria-donor kidneys).19 APOLLO was designed not to fail in detecting a smaller effect that would still be clinically important. An effect of 1.7 represents the cutoff for defining risk for a high-KDPI kidney and 1.5 represents an effect that could be of interest but for which power is not likely to exceed 80%.
Power Estimates
Table 2 contains calculated power to detect hazard ratios of 1.7 and 1.5 between subgroups making up 13% and 87% of the total sample, and a range of sample sizes and marginal failure rates using Schoenfeld’s formula.20 They assume 18 months of recruitment and mean follow-up period of 51 months. The bolded estimates in Tables 2 and 3 were calculated assuming the within-donor correlation is 0.7, and the increased risk of failure in the high-risk APOL1 genotype group is 1.7 times that in the low-risk APOL1 genotype group. Estimates in Table 3 are calculated with the same assumptions except for the length of follow-up, which is assumed to average 1 additional year. Power analysis assumes a type 1 error rate of 0.05 for a 2-sided test.
Live Kidney Donor Transplantation and Living-Kidney Donors
APOLLO is underpowered to detect an effect of the APOL1 high-risk genotype in eligible living-donors on recipient graft outcomes. Assuming 497 kidneys from living-donors are transplanted annually, a type 1 error rate of α = 0.05 and a 3-year failure rate of 0.05, the power to detect hazard ratios of 1.5, 1.7, 2.0, and 2.5 is 0.10, 0.14, 0.21, and 0.34, respectively. Thus, extended recruitment and/or longer follow-up are needed to appropriately power the APOL1 RRV comparison for living-donors. As more transplant programs screen living-donor candidates for APOL1 and exclude some with high-risk genotypes, APOLLO could be enriched for kidney transplants from APOL1 low-risk living-donors.
To address these concerns and clarify the safety of living-kidney donation, an NIH-funded APOLLO Ancillary Study entitled Living-donor Extended Time Outcomes (LETO) started in June 2019 (R01 DK120551). LETO will retrospectively assess kidney function and proteinuria in African American individuals who served as living-kidney donors approximately 15 to 20 years ago, and assess graft outcomes in their recipients, based on APOL1 genotype (C-yH, MP, and KLL, principal investigators).
The SC recognizes that many African American donor candidates will be genotyped for APOL1 outside of APOLLO and some may be excluded from donation based on their genotype. This reduces the numbers of living-donors with APOL1 high-risk genotypes that APOLLO will follow. Therefore, the LETO study will perform retrospective analyses of this type. APOLLO also recognizes that some transplant programs did not address APOL1 when the study was planned; however, APOLLO requires transplant programs to discuss current knowledge about APOL1 with living-donor candidates. The vast majority of programs are following study recommendations. APOLLO investigators hope that APOL1 genotyping will reassure most African American donor candidates who have low-risk genotypes; this could increase numbers of living-donors in the future. The APOLLO External Expert Panel and NIH have asked the Coordinating Center to track numbers of living U.S. African American kidney donors in the years before APOLLO and during APOLLO recruitment.
Statistical Analysis
The APOLLO Consortium provides the opportunity to explore an array of questions related to kidney donor and kidney transplant recipient characteristics. The primary analysis is the influence of deceased-donor APOL1 high-risk genotypes on time to renal allograft failure. APOL1 G1 and G2 RRVs will be genotyped by real-time polymerase chain reaction in a Clinical Laboratory Improvement Amendments–certified laboratory at Wake Forest School of Medicine, with subsequent genetic and statistical quality control processes. Time to death-censored allograft failure will be the primary outcome and a failure-time modeling framework will be used.
Regulatory and Ethical Considerations, Informed Consent, and the cIRB
Wake Forest School of Medicine serves as the APOLLO cIRB. The cIRB reviews site-specific consent forms and local context worksheets to ensure that they are compatible with the protocol and cIRB policies and procedures. CCs and participating transplant programs collect written informed consent for the main study from living-donors and recipients, including APOL1 genotyping and other genetic testing, poststudy contact, and recall for participation in ancillary studies. The Wake Forest School of Medicine cIRB approved the APOLLO protocol in January 2019 and requested a Manual of Procedures with further details of the study before the start of recruitment. The first recipient of an APOLLO deceased-donor kidney was enrolled May 16, 2019.
Benefits of the cIRB include the following: (i) version control at the time of submission of amendments and continuing reviews (all approved at one time and all work on the same version of the protocol), and (ii) standardized terms in the consent form promote consistency with information presented to participants at all sites. Challenges using the cIRB include the following: (i) even with 1 IRB, most if not all sites require a duplicate registration of the study locally, (ii) issues with institutional preferences versus policy or local law requirements regarding terms in consent forms, and (iii) addressing differences in state and local issues (e.g., age of majority, return of genetic results, Health Insurance Portability and Accountability Act).
Return of APOL1 Genotype Data
The APOLLO SDRC and SC strongly support the call to return individual research results to participants and the CAC mandated its inclusion in the protocol.11,21, 22, 23, 24 In addition, APOLLO investigators must inform eligible living-kidney donors that clinical APOL1 gene testing is currently available outside the study and potentially at their own cost, before their decision whether to participate. The CAC requested that eligible living-donors be informed about APOLLO and the opportunity to receive Clinical Laboratory Improvement Amendments–certified APOL1 testing outside the study at least 2 weeks before donor nephrectomy. This is intended to allow donors time to ask questions, perform research, and make informed decisions about participation. Test results must not alter medical care in this observational study. Therefore, the SDRC will perform research APOL1 genotyping after recruitment ends.
To permit APOL1 results to be returned to participants, testing had to be performed in a Clinical Laboratory Improvement Amendments laboratory. This requirement altered the original genotyping plan at a standard laboratory and incurred additional cost. Bio-ethicist Dr. Ana Iltis, with input from the CAC, developed a series of APOLLO infographics providing information on kidney disease, genes, and APOL1 directed to the public, kidney transplant recipients, potential living-kidney donors, and families of deceased-donors (see Supplementary Material). These documents have been distributed to APOLLO transplant programs to assist with the informed consent process and help living-kidney donors, transplant recipients, and deceased-donors’ next-of-kin decide whether to request return of APOL1 test results in later years. Infographics show the benefits and risks of requesting APOL1 research results. The SDRC will inform participants, and OPOs will be able to inform deceased-donor next-of-kin when APOL1 results become available.
Operations
The NIH announced APOLLO awards in late September 2017, and an SC was rapidly formed (Table 4). Study startup began with a series of SC conference calls followed by bi-annual meetings at NIH commencing in early 2018. When the protocol was complete, the SDRC, AOPO, and ASHI leadership worked closely with select OPOs and histocompatibility laboratories to optimize approaches for identifying, collecting, and processing bio-samples from eligible deceased-donors. Only donors whose families permitted research use of their loved one’s organs and tissues are included. OPOs enroll all eligible deceased-donors whose families permit research. APOLLO will include donors reported to them as African American, Hispanic black, Afro-Caribbean, or African by family or medical records. To assist OPO staff, AOPO modified their computer-entry system to provide alerts when eligible donors were under consideration.
Table 4.
Anticipated APOLLO study timeline
| September 2017 | May 2019 | January 2021 | April 2022 | May 2022 |
|---|---|---|---|---|
| Initiation of funding | First recipient recruited | Last recipient recruited | Follow-up ends | End of funding |
In spring 2019, the SDRC shipped supplies to 66 histocompatibility laboratories with instructions for sample handling, DNA extraction, serum processing, sample storage, and shipping to the SDRC quarterly, including cryovials for DNA and serum, and storage and shipping containers. ASHI backed the effort with newsletters, emails, and a letter from the SDRC to ensure participation of histocompatibility laboratories. The efforts of AOPO and ASHI leadership cannot be overstated; they tirelessly promoted APOLLO at local, regional, and national meetings. In addition, AOPO worked with the SDRC to call individual OPO and histocompatibility laboratory directors to answer questions and ensure participation. Supplementary Figure S1 displays recruitment targets.
Conclusions
APOLLO is an observational study addressing critical questions in kidney transplantation consistent with newly outlined federal initiatives. It will determine whether replacing deceased-donor race/ethnicity in the current KDRI with APOL1 genotype better describes organ quality. Deceased-donors are tested for viral infections using polymerase chain reaction–based technology and results are available in hours. APOL1 genotyping also can be performed within hours to permit results to be included in decisions on allocation of kidneys. APOLLO results could lead to fewer discarded kidneys, improved donor and recipient selection, additional kidneys transplanted, longer renal allograft survival, and substantial savings. In addition, APOLLO and LETO hold great promise for determining the safety of living-kidney donation from African American individuals with APOL1 high-risk genotypes. Additional ancillary studies will be performed.
There have been, and will continue to be, challenges ensuring broad national participation with limited resources. However, the compelling questions addressed by APOLLO, and the possibility to increase the numbers and improve the outcomes of transplanted kidneys have galvanized the transplant community. Providing equity to the optimal treatment of ESKD that disproportionately affects individuals with recent African ancestry remains critical to the mission of the sponsor, investigators, and CAC. All members of APOLLO, including community-based members, are performing outreach and education of critical importance to the population at risk. Investigators and coordinators have derived tremendous purpose from participating in this landmark study. APOLLO is an important component of the increased efforts in participatory research of the National Institute of Diabetes and Digestive and Kidney Diseases. OPOs are now enrolling deceased-donors, histocompatibility laboratories are processing samples, and recipients of APOLLO donor kidneys and living-kidney donors are being recruited. Real-world community-based studies such as APOLLO could optimize outcomes in kidney transplantation and improve the lives of patients with and at risk for nephropathy.
Disclosure
BIF and Wake Forest University Health Sciences have rights to an issued U.S. patent related to APOL1 genetic testing (www.apol1genetest.com). BIF is a consultant for AstraZeneca and Renalytix AI. JB has current contracts with Novartis, Angion, Medimmune, CareDx, Astellas, Quark, and Sphingotec, and current consulting with Natera. EDP has speaking honorariums from CareDx and Gador Argentina, and a consulting honorarium from Renalytix and Reata. All the other authors declared no competing interests.
Acknowledgments
Support was received from the following National Institutes of Health grants (recipient’s affiliations included): 5U01DK116041 (BIF, DMR, RJS, and DWB; Wake Forest School of Medicine, Winston-Salem, North Carolina, USA), 5U01DK116043 (C-yH and MP; University of California, San Francisco, San Francisco, California, USA), 5U01DK116099 (SOP and KAN; Emory University School of Medicine, Atlanta, Georgia, USA), 5U01DK116040 (AMR-D, BIF, and RAG; Wake Forest School of Medicine, Winston-Salem, North Carolina, USA; Duke University School of Medicine, Durham, North Carolina, USA), 5U01DK116093 (KAB, Vanderbilt University, Nashville, Tennessee, USA), 5U01DK116042 (DCB and KLL; Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Saint Louis University School of Medicine, St. Louis, Missouri, USA), 5U01DK115997 (BAJ and RBM; University of Alabama School of Medicine in Birmingham, Birmingham, Alabama, USA), 5U01DK116095 (JB and MRW; University of Maryland School of Medicine, Baltimore, Maryland, USA), 5U01DK116097 (EDP and MDD; Cleveland Clinic, Cleveland, Ohio, USA; University of Michigan School of Medicine, Ann Arbor, Michigan, USA), 5U01DK116066 (SM and DS; Columbia University, New York, New York, USA; University of Pennsylvania, Philadelphia, Pennsylvania, USA), 5U01DK116092 (BCA, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA), 5U01DK116101 (AF, GG, and MO-G; University of Miami Miller School of Medicine, Miami, Florida, USA), 5U01DK116102 (SER, Joslin Diabetes Center, Boston, Massachusetts, USA), 5U01DK116100 (BM and DMD; Icahn School of Medicine at Mount Sinai, New York, New York, USA; Weill Cornell Medicine, New York, New York, USA), R01 DK120551 (APOLLO ancillary study) (C-yH, MP, and KLL; University of California, San Francisco, San Francisco, California, USA; Saint Louis University School of Medicine, St. Louis, Missouri, USA), and R01 MD014161 (APOLLO ancillary study) (James Dubois, SM; Washington University School of Medicine, St. Louis, Missouri, USA; Columbia University, New York, New York, USA).
Organ Procurement Organizations and American Society for Histocompatibility and Immunogenetics laboratories face daily operational and financial challenges. Nonetheless, APOLLO reminds us of the transplant community’s unwavering devotion to acquire knowledge and improve patient outcomes. The voluntary participation of these organizations is integral to APOLLO’s success. The study team is indebted to the members and leadership of these organizations for their selfless contributions to research.
APOLLO is registered in clinicaltrials.gov as NCT03615235.
Footnotes
Figure S1. APOLLO recruitment target.
APOLLO manual of procedures stage 1 for OPOs and HLA labs.
APOLLO manual of procedures stage 2 for kidney transplant programs.
APOLLO data collection.
APOLLO infographic for participants and the general public.
APOLLO infographic for return of research results.
APOLLO infographic for deceased-donor next-of-kin.
SPIRIT Guidelines.
Supplementary Material
References
- 1.Israni A.K., Salkowski N., Gustafson S. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25:1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao P.S., Schaubel D.E., Guidinger M.K. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 3.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzur S., Rosset S., Shemer R. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman B.I., Limou S., Ma L. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis. 2018;72:S8–S16. doi: 10.1053/j.ajkd.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves-Daniel A.M., Depalma J.A., Bleyer A.J. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman B.I., Julian B.A., Pastan S.O. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant. 2015;15:1615–1622. doi: 10.1111/ajt.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman B.I., Pastan S.O., Israni A.K. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation. 2016;100:194–202. doi: 10.1097/TP.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B.T., Kumar V., Williams T.A. The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant. 2012;12:1924–1928. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi M.D., Ortigosa-Goggins M., Garg A.X. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018;29:1309–1316. doi: 10.1681/ASN.2017060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman B.I., Moxey-Mims M. The APOL1 Long-Term Kidney Transplantation Outcomes Network-APOLLO. Clin J Am Soc Nephrol. 2018;13:940–942. doi: 10.2215/CJN.01510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimmel P.L., Jefferson N., Norton J.M. How community engagement is enhancing NIDDK research. Clin J Am Soc Nephrol. 2019;14:768–770. doi: 10.2215/CJN.14591218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julian B.A., Gaston R.S., Brown W.M. Effect of replacing race with apolipoprotein L1 genotype in calculation of kidney donor risk index. Am J Transplant. 2017;17:1540–1548. doi: 10.1111/ajt.14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadkarni G.N., Wyatt C.M., Murphy B. APOL1: a case in point for replacing race with genetics. Kidney Int. 2017;91:768–770. doi: 10.1016/j.kint.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Newell K.A., Formica R.N., Gill J.S. Integrating APOL1 gene variants into renal transplantation: considerations arising from the American Society of Transplantation Expert Conference. Am J Transplant. 2017;17:901–911. doi: 10.1111/ajt.14173. [DOI] [PubMed] [Google Scholar]
- 16.Lentine K.L., Kasiske B.L., Levey A.S. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101:S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massie A.B., Kucirka L.M., Segev D.L. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangnon R.E., Kosorok M.R. Sample-size formula for clustered survival data using weighted log-rank statistics. Biometrika. 2004;91:263–275. [Google Scholar]
- 19.Metzger R.A., Delmonico F.L., Feng S. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld D.A. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 21.Freedman B.I., Fletcher A.J., Sanghani V.R. Perceptions regarding genetic testing in populations at risk for nephropathy. Am J Nephrol. 2013;38:453–457. doi: 10.1159/000356244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon E.J., Amomicronrtegui D., Blancas I. African American living donors' attitudes about APOL1 genetic testing: a mixed methods study. Am J Kidney Dis. 2018;72:819–833. doi: 10.1053/j.ajkd.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeukeje E.M., Young B.A., Fullerton S.M. You are just now telling us about this? African American perspectives of testing for genetic susceptibility to kidney disease. J Am Soc Nephrol. 2019;30:526–530. doi: 10.1681/ASN.2018111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan S., Iltis A.S., Sawinski D. APOL1 genetic testing in living kidney transplant donors. Am J Kidney Dis. 2019;74:538–543. doi: 10.1053/j.ajkd.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.