Abstract
Background
Tumors are highly plastic metabolic entities composed of cancer and host cells that can adopt different metabolic phenotypes. For energy production, cancer cells may use 4 main fuels that are shuttled in 5 different metabolic pathways. Glucose fuels glycolysis that can be coupled to the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) in oxidative cancer cells or to lactic fermentation in proliferating and in hypoxic cancer cells. Lipids fuel lipolysis, glutamine fuels glutaminolysis, and lactate fuels the oxidative pathway of lactate, all of which are coupled to the TCA cycle and OXPHOS for energy production. This review focuses on the latter metabolic pathway.
Scope of review
Lactate, which is prominently produced by glycolytic cells in tumors, was only recently recognized as a major fuel for oxidative cancer cells and as a signaling agent. Its exchanges across membranes are gated by monocarboxylate transporters MCT1-4. This review summarizes the current knowledge about MCT structure, regulation and functions in cancer, with a specific focus on lactate metabolism, lactate-induced angiogenesis and MCT-dependent cancer metastasis. It also describes lactate signaling via cell surface lactate receptor GPR81.
Major conclusions
Lactate and MCTs, especially MCT1 and MCT4, are important contributors to tumor aggressiveness. Analyses of MCT-deficient (MCT+/- and MCT−/-) animals and (MCT-mutated) humans indicate that they are druggable, with MCT1 inhibitors being in advanced development phase and MCT4 inhibitors still in the discovery phase. Imaging lactate fluxes non-invasively using a lactate tracer for positron emission tomography would further help to identify responders to the treatments.
Keywords: Cancer metabolism, Monocarboxylate transporters (MCTs), GPR81, Metabolic symbiosis, Angiogenesis, Metastasis
Highlights
-
•
In cancer, hypoxia and cell proliferation are associated to lactic acid production.
-
•
Lactate exchanges are at the core of tumor metabolism.
-
•
Transmembrane lactate trafficking depends on monocarboxylate transporters (MCTs).
-
•
MCTs are implicated in tumor development and aggressiveness.
-
•
Targeting MCTs is a therapeutic option for cancer treatment.
1. Introduction
Tumors are metabolic entities that comprise cancer and host cells. Their metabolic activities depend on their access to nutrients, biological activities, and spatiotemporal localization. While most cells in the body are oxidative and fully oxidize glucose to CO2, cells exposed to hypoxia (i.e., a local concentration of O2 below physiological cell needs) and proliferating cells preferentially convert glucose to lactate in processes known as anaerobic and aerobic glycolysis, respectively. These metabolic phenotypes are at the core of tumor biology. In solid tumors, the glycolytic switches associated with adaptation to hypoxia and cell proliferation operate via different mechanisms. Indeed, hypoxic adaptation is a survival mechanism that involves hypoxia-inducible transcription factors (HIFs), whereas metabolic adaptation to cell proliferation involves growth factors and their effectors, such as c-Myc and Ras, with reported overlaps [1]. Other cancer cells are oxidative. However, at the whole tumor level, increased conversion of glucose to lactate associated with a high glycolytic rate generates millimolar concentrations of lactic acid that is released to the extracellular compartment [2]. Because lactic acid is hydrophilic and a weak acid, its transport across membranes necessitates transporters that belong to the monocarboxylate transporter (MCT) family. Their contribution to tumor progression deserves attention.
MCTs are encoded by the solute carrier 16 (SLC16) family of genes. Among the 14 members of the family, MCT1/SLC16A1, MCT2/SLC16A7, MCT3/SLC16A8, and MCT4/SLC16A3 (hereafter referred to as MCTs) convey monocarboxylate ions together with protons (Figure 1). These passive transporters are primarily localized at the plasma membrane where they can operate bidirectionally depending on the concentration gradient of their substrates [3], [4], [5]. They comprise 12 transmembrane (TM) helices, intracellular N- and C-termini and a large cytosolic loop between TM6 and TM7 [4], [5], [6]. Their structure has not been resolved by X-ray crystallography yet but has been modeled on the basis of the structure of E. coli glycerol-3-phosphate transporter (GlpT) and site-directed mutagenesis experiments [4], [7], [8]. Human MCT1 modeling determined that lysine 38, aspartate 302, and arginine 306 are of particular importance for substrate binding and transporter activity [4].
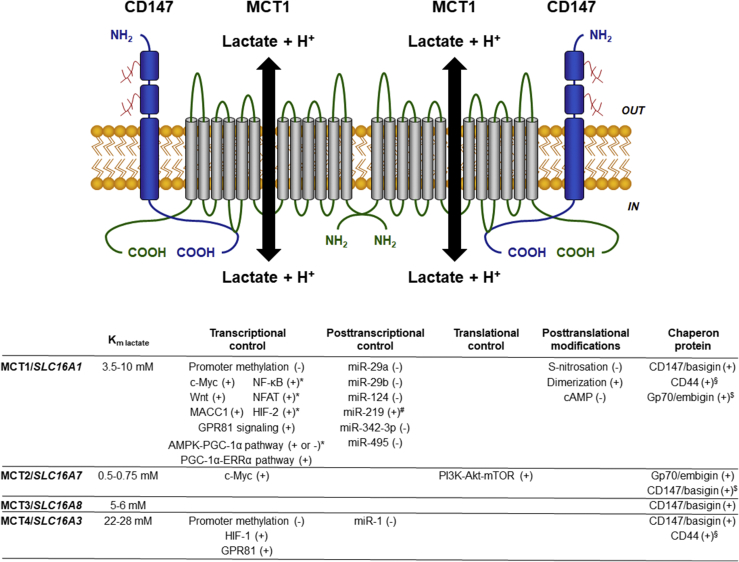
Figure 1.
Main characteristics of lactate transporters MCT1-4. The cartoon depicts the predicted structure of functional MCT1 that, as a dimer, interacts with 2 CD147/basigin ancillary proteins at the cell membrane. Like MCT2-4, MCT1 is a passive symporter that shuttles lactate together with a proton along their concentration gradients across membranes. On the bottom is a summary of know regulators of MCT expression and stability, together with MCT affinities for lactate. + indicates induction/stabilization; - indicates repression/destabilization; * refers to pathways that are not yet fully characterized; # refers to indirect influence; $ refers to an unlikely still existing possibility; § refers to a situation reported only in cancer cells. For abbreviations, see list.
While MCTs share common substrates, including pyruvate, l-lactate, ketone bodies aceto-acetate and d-β-hydroxybutyrate, and short chain fatty acid propionate and butyrate, they differ by their relative affinities. MCT2/SLC16A7 is the transporter with the highest affinity for monocarboxylates (Km range = 0.1–1.2 mM according to the substrate; 0.5–0.75 mM for lactate), followed by MCT1/SLC16A1 (Km range = 1–12.5 mM according to the substrate; 3.5–10 mM for lactate), MCT3/SLC16A8 that has an affinity for lactate comparable to MCT1 (Km = 5–6 mM), and MCT4/SLC16A3 that has a low affinity for lactate (Km = 22–28 mM) and pyruvate (Km = 153 mM) [9], [10] (Figure 1). Although lactate is not the only substrate of MCTs, it is the most characterized in the literature and the most abundant in vivo, particularly in tumors where it reaches concentrations up to 40 mM [11]. Hence, this review mainly addresses MCT-mediated lactate transport in cancer.
2. MCT expression
2.1. MCT expression in normal tissues
MCTs are most often described to be functionally active at the cell membrane, but expression in organelle membranes (mitochondria and peroxisomes) has been reported as well. For example, MCTs have been proposed to mediate lactate uptake by mitochondria for in situ oxidation by lactate-dehydrogenases (LDHs) and putative mitochondrial lactate oxidases [12], [13], [14], [15], [16], [17], and lactate release by peroxisomes following in situ production by LDHs [18]. However, the validity of these observations has been questioned [3], and neither a peptidic sequence nor chaperones sending MCTs to organelles have been identified to date.
MCTs have different patterns of expression related to different functions in normal tissues. Notably, none of them are expressed by β-cells of the islets of Langerhans in the pancreas, which prevents lactic acid-induced ATP generation and insulin secretion during exercise, as it would result in hypoglycemia [3].
MCT1 is ubiquitously expressed. In the gut epithelium, its localization at both apical and basolateral membranes has been suggested to promote the absorption of short chain fatty acids produced by the gut microbiota [19]. MCT1 is also expressed in the heart and in red skeletal muscle fibers that use lactate as an oxidative fuel for mitochondrial respiration [20], [21], [22]. It mediates lactic acid influx in the proximal convoluted tubule of the kidney and in the liver parenchyma, where lactate fuels gluconeogenesis after exercise [23]. However, rather than influx, MCT1 facilitates lactic acid efflux in glycolytic cells, including white skeletal muscle fibers, erythrocytes, astrocytes, oligodendrocytes, hypoxic cells, and immune cells, such as activated T-lymphocytes [4].
Compared to MCT1, MCT2 expression is restricted to specific tissues that differ when considering different species [4], [5], [6]. In humans, MCT2 is, e.g., expressed in the liver parenchyma where it participates in lactate-fueled gluconeogenesis (the Cori cycle) and in the proximal convoluted tubule of the kidney for the clearance of lactic acid [24]. In neurons, MCT2 fuels mitochondrial respiration with lactic acid produced by astrocytes, a metabolic pathway associated with memory [25].
MCT3 is only expressed in the retinal pigment and choroid plexus epithelia of the eye [26]. By facilitating lactic acid exchanges between the retinal pigment epithelium and the choroid, it regulates the pH of the subretinal space.
MCT4, which has the lowest affinity for lactate among MCTs, primarily facilitates lactic acid efflux from glycolytic cells, including exercising white skeletal muscle fibers, astrocytes, immune cells, chondrocytes, and hypoxic cells [3], [6]. It has a very low affinity for pyruvate and a higher affinity for lactate, which ensures that pyruvate is converted to lactate before export [10], thus promoting NAD+ regeneration by the LDH-5 reaction, which is necessary for maintaining a high glycolytic flux.
In some tissues, MCTs facilitate lactate exchanges between lactic acid-producing cells and lactic acid-consuming cells. It is the case for skeletal muscles, where MCT4-expressing glycolytic white muscle fibers provide lactate to MCT1-expressing, oxidative red muscle fibers [27], [28] and for the central nervous system, where MCT1-and MCT4-expressing glycolytic astrocytes and oligodendrocytes provide lactate to MCT2-expressing oxidative neurons [28], [29], [30].
2.2. MCT expression in cancers
MCT1, MCT2, and MCT4 expression has been extensively characterized in cancer cell lines and in multiple tumor types from patients. Their presence and cell type localization in human cancers are displayed in Table 1. Upregulation of MCT1, MCT2, and MCT4 during tumor progression from normal to tumor epithelium has also been repeatedly observed in human samples (Table 2). In one study, decreased MCT2 expression was reported in hepatocellular carcinomas compared to healthy parenchyma [31] (Table 2). Surprisingly, MCT2 expression has often been observed in the cytosol rather than at the plasma membrane of cancer cells in breast [32], cervix [33], colorectal [32], [34], lung [32], ovary [32], prostate [35], and soft tissue [36] cancers. Whether such atypical localization results from impaired trafficking or from targeted expression at the membrane of organelles is not yet known.
Table 1.
Presence of MCTs in human cancers.
| Cancer type | Cell type | MCT1 | MCT2 | MCT4 | Ref. |
|---|---|---|---|---|---|
| Adrenocortical carcinoma | Bulk tissue | ± | ± | ± | [152] |
| Bladder cancer | Cancer cells | ± | ± | [220], [221] | |
| Brain cancer | Cancer cells in all tumor types | + | [89], [160], [188], [190], [222] | ||
| Cancer cells in glioblastoma | + | ||||
| Cancer cells in diffuse astrocytoma | ± | ||||
| Breast cancer | Bulk tissue | ++ | + | + | [32], [223], [224], [225] |
| Cancer cells in basal-like breast cancer | + | ||||
| Cancer cells in TNBC | + | ||||
| Cancer cells in invasive ductal carcinoma | + | ||||
| Cervix cancer | Cervix cancer cells | ++ | ± | [33], [150] | |
| Epithelial cells | ± | ||||
| Colorectal cancer | Cancer cells in colon adenocarcinoma | ± | ± | ± | [32], [34], [156], [157], [226] |
| Gastric cancer | Bulk tissue | + | [153], [155] | ||
| Cancer cells in well-differentiated cancers | + | − | |||
| Head and neck cancer | Cancer cells in esophageal carcinoma | ± | + | [151], [162] | |
| Cancer cells in oral squamous cell carcinoma | + | ||||
| Kidney cancer | Cancer cells in clear renal cell carcinoma | ± | ± | [227] | |
| Liver cancer | Cancer cells in hepatocellular carcinoma | − | ± | [31] | |
| Lung cancer | Cancer cell in NSCLC | ++ | + | + | [32] |
| Lymphoma | Cancer cells in B-cell lymphoma, | ++ | − | [198] | |
| Cancer cells in Burkitt lymphoma | ++ | − | |||
| Ovary cancer | Bulk tissue | ± | ± | [182] | |
| Prostate cancer | Bulk tissue (primary tumors) | − | + | + | [35], [93] |
| Bulk tissue (metastasis) | ± | ± | |||
| Cancer cells in prostatic intraepithelial neoplasia | ± | ± | ± | ||
| Skin cancers | Melanoma cells | ± | ± | [96], [159], [228] | |
| Cancer cells in squamous cell skin cancer | + | ++ | |||
| Soft tissue cancers | Cancer cells in soft tissue sarcoma | + | + | + | [36], [80] |
−, no expression; ±, mild expression; +, high expression; ++ very high expression.
Table 2.
Changes of MCT expression during progression from normal to tumor epithelium in humans.
| Cancer type | MCT1 | MCT2 | MCT4 | Ref. |
|---|---|---|---|---|
| Adrenocortical cancer | + | [152] | ||
| Brain cancer | + | + | [89], [160], [188], [190], [222] | |
| Breast cancer | (+) | + | [32], [54], [223] | |
| Cervix cancer | + | + | [33] | |
| Colorectal cancer | (+) | + | [34], [157], [229] | |
| Esophageal adenocarcinoma | + | + | [151] | |
| Gastric cancer | (+) | [153], [155] | ||
| Hepatocellular carcinoma | − | + | [31] | |
| Melanoma | + | + | [96] | |
| Oral squamous cell carcinoma | + | [162] | ||
| Prostate cancer | (+) | [35], [93] |
+, upregulation in all reported cases; (+), non-systematic upregulation; -, downregulation.
2.3. Regulation of MCT expression
2.3.1. Physiological regulation of MCT expression
Physiologically, MCTs are subject to transcriptional, posttranscriptional, translational, and posttranslational regulations (summarized in Figure 1).
At the transcriptional level, exercise upregulates MCT1/SLC16A1 and MCT4/SLC16A3 gene expression in muscles through a putative mechanism implicating activation of the AMP-activated protein kinase (MAPK)-peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) pathway [37], [38]. Conversely, AMPK activation downregulates MCT1/SLC16A1 and MCT4/SLC16A3 transcription in Sertoli cells [39]. Further investigations are thus required to understand the dual role of AMPK in different tissues. The observation that PGC-1α promotes MCT1/SLC16A1 transcription in skeletal muscles [40] is interesting, knowing that lactate induces both PGC-1α and MCT1 expression after binding to lactate receptor G protein-coupled receptor 81 (GPR81) in L6 cells, a rat myoblast cell line [41]. Whether or not MCT1 induction is downstream of PGC-1α and/or AMPK has not been characterized in these independent studies. However, it is tempting to propose the existence of a lactate/GPR81/PGC-1α/MCT1 or of a lactate/AMPK/PGC-1α/MCT1 signaling axis through which lactate would induce its own metabolism in target cells, in a way similar to what butyrate does in the colon epithelium [42].
Hypoxia has been reported to stimulate MCT4/SLC16A3 transcription owing to the presence of hypoxia response elements (HREs) in the promoter region of the gene, making it a direct HIF-1-target gene [43]. The hypoxic induction of MCT4 could be particularly important for wound healing, where cells downstream of damaged blood vessels must switch to a glycolytic metabolism, hence export lactic acid, to survive [44]. However, experimental evidence of the contribution of MCT4 to wound healing is currently lacking. Interestingly, although their respective promoters, genes, and 3′-UTR sequences do not contain HREs, several reports clearly indicate that hypoxia is also capable of inducing MCT1/SLC16A1 and MCT2/SLC16A7 transcription [45], [46], [47], [48], [49], [50]. Expression studies performed in cancer cells suggested the involvement of other hypoxia-activated transcription factors, such as HIF-2 (which enhances Myc transcriptional activity) [51], [52], [53] and nuclear factor-κB (NF-κB) [46].
Interestingly, the promoter sequence of MCT1/SLC16A1 also contains consensus sequences for the binding of nuclear factor of activated T-cells (NFAT) [3], but, to our knowledge, the significance of this potential regulatory pathway has never been investigated. Furthermore, a single study in cancer cells reported that the MCT1/SLC16A1 promoter can be methylated, reducing gene expression [54].
Several studies evidenced that MCTs are under the control of miRNAs. Accordingly, miR-29a, miR-29b, miR-124, and miR-495 target the 3′-untranslated sites in MCT1/SLC16A1 mRNA, accounting for the repression of its expression in pancreatic β-cells [55], [56]. This effect has been confirmed for miR-29a and miR-29b in mature mouse islets [55]. Conversely, by suppressing the expression of oligodendrocyte differentiation inhibitors, miR-219 can enhance MCT1 expression in oligodendrocyte precursors, which participates in their differentiation and in the generation of the myelin sheath in mice [57]. While additional miRNAs have been found to regulate MCT1 and MCT4 expression in cancer cells, to our knowledge, none have been reported to date to influence MCT2 and MCT3 expression.
Little is known concerning translational control of MCTs, except for MCT2/SLC16A7 mRNA translation that is increased in the brain in response to noradrenaline, insulin, insulin-like growth factor 1 (IGF1), and brain-derived neurotrophic factor (BDNF) in a PI3K/Akt/mTOR-dependent manner [58], [59], [60].
To date, no posttranslational modifications of MCTs have been described, except for an inhibitory S-nitrosation of cysteine residues in MCT1 [61]. However, this modification was induced pharmacologically and did not result from an endogenous production of reactive nitrogen species. Its relevance in physiology and physiopathology thus remains to be investigated.
The stability of MCTs, their subcellular localization at the plasma membrane and their functions require their physical interaction with chaperone glycoproteins of the multifunctional immunoglobulin family. CD147/basigin is the main chaperone of MCT1, MCT3 and MCT4 [62], [63], and gp70/embigin the main chaperone of MCT2 [64] (Figure 1). Interactions of MCT1 with gp70 and of MCT2 with CD147 have also been observed and could be species-dependent [65]. CD147 and gp70 transmembrane and intracellular C-terminus domains interact with MCTs TM3-6 and C-terminus domains, respectively [7], [62], [65], [66]. An elegant study using fluorescence resonance energy transfer (FRET) further revealed that functionally active MCT1 forms dimers that interact with two CD147 proteins at the cell membrane [67]. These functional units can recruit additional proteins to form supercomplexes. Hence, MCT1 was reported to interact with cytochrome oxidase (COX) in L6 cell mitochondria [14]; MCT1 and MCT4 with carbonic anhydrases CA2 [68] and CA6 [69] that facilitate lactic acid transport across the cell membrane independently of CA catalytic activities; and MCT4 with β1-integrin in epithelial cells, where the complex polarized at the basolateral membrane and in the leading edge lamellipodia of migrating cells [70]. Additional interactions have been described in cancer cells (see Section 2.3.2).
The stability of MCTs and their associated chaperone proteins is interdependent, as silencing one often reduces the expression of the other [45], [71], [72], [73]. Loss of the chaperone leads to inappropriate expression of MCTs in intracellular vesicles, indicating that CD147 and gp70 target the transporters to the plasma membrane [62], [74]. In epithelial cell monolayers, CD147 was shown to ensure MCT1 polarization, whereas MCT3 and MCT4 reciprocally influenced CD147 polarity [75], [76], [77]. Of note, CD147 gene expression is induced by hypoxia [78], which could account for hypoxia-inducible MCT1 expression. There is no report addressing the sensitivity of gp70 to hypoxia.
Finally, the abundance of MCTs at the cell plasma membrane depends on their turnover and recycling rates. c-AMP signaling has been shown to target MCT1 to autophagosomes and lysosomes in endothelial cells, thus decreasing the pool of functional MCT1 at the plasma membrane [79].
2.3.2. Regulation of MCT expression in cancer
Human cancers often express MCTs at high level (see Table 1, Table 2). From a transcriptional standpoint, at least nine different mechanisms have been evidenced to date to explain this observation (summarized in Figure 1). Hypoxia can induce MCT1/SLC16A1, MCT2/SLC16A7, and MCT4/SLC16A3 gene expression directly via HIF-1 activation for MCT4/SLC16A3 [43], or indirectly for the two other isoforms [45], [46], [48], [50], [80]. Oncogenic Myc signaling can trigger MCT1/SLC16A1 and MCT2/SLC16A7 either directly of via the loss of translation repressors miR-29a and miR-29c [52], [81]. MCT1/SLC16A1 is also a direct Wnt-target gene, coupling Wnt activation to increased lactate export in glycolytic colon cancer cells [82]. NF-κB signaling and loss of function of p53 can further trigger MCT1/SLC16A1 transcription [46]. Transcription factor metastasis-associated in colon cancer 1 (MACC1) signaling was reported to induce MCT1/SLC16A1 transcription in gastric cancer cell lines [83]. PGC-1α-estrogen-related receptor α (ERRα) signaling supports MCT1/SLC16A1 transcription, and this axis was repressed in exercising tumor-bearing mice [84]. Extracellular acidosis can activate HIF-2 and Myc, which then stimulate MCT1/SLC16A1 transcription [53], [85]. Glutamine availability supports HIF-1 activity and MCT4/SLC16A3 expression [86]. Finally, GPR81 stimulation by extracellular lactate can trigger a yet unknown signaling cascade increasing MCT1/SLC16A1 and MCT4/SLC16A3 gene expression in pancreatic ductal adenocarcinoma (PDAC) cell lines [87]. Conversely, decreased MCT expression can be due to hypermethylation of gene promoters, leading to the silencing of MCT1/SLC16A1 in breast cancer [54] and of MCT4/SLC16A3 in colorectal carcinoma [88].
Increased MCT1/SLC16A1 mRNA stability can be due to a loss of MCT1 translation repressor miR-124, as observed in medulloblastoma [89] and in PDAC [82]. Loss of miR-342-3p, which normally acts downstream of the estrogen receptor, was further found to increase MCT1 expression in triple negative breast cancer (TNBC) cells [90]. This study revealed that MCT1/SLC16A1 mRNA is a direct target of the miRNA. An indirect regulation of MCT4 expression was reported for miR-1, which acted by decreasing Smad3-HIF-1 signaling, ultimately resulting in decreased MCT4 expression in glycolytic colorectal cancer cells [91].
Posttranslational MCT1 stabilization has been observed in nutrient stress conditions. It involves a poorly characterized mechanism dependent on mitochondrial reactive oxygen species (mtROS) [48]. Interestingly, glucose deprivation induces autophagy and activates Wnt-β-catenin signaling, whereas β-catenin downregulation was recently found to reduce MCT1 expression in hepatocarcinoma cells, thus positively coupling autophagy to high MCT1 expression [92]. Furthermore, interactions of MCT1, MCT4, and CD147 with hyaluronan receptor CD44 were reported in breast and prostate cancer cell lines [93], [94]. In the complex, CD44 would act as an additional chaperone for MCT1 and MCT4, and impairment of CD44 signaling decreased the plasma membrane expression and impaired the activity of both transporters in breast cancer cell lines [94].
3. MCT functions in cancer
As reported in Table 1, Table 2, MCTs are widely expressed in different tumor types, not only in cancer cells but also in stromal cells. It is therefore not surprising that they exert multiple activities in cancer, including in metabolic exchanges, metabolic signaling, and cancer metastasis.
3.1. MCTs facilitate lactate exchanges in tumors
In solid tumors, lactate accumulating in the extracellular matrix has for a long time been considered as a mere metabolic waste. However, there is now strong evidence that this energy-rich metabolite is a substrate for a subpopulation of oxygenated cancer cells (Figure 2). That oxidative cancer cells expressing MCT1 are capable of taking up lactate secreted by glycolytic cancer cells expressing MCT4 has initially be reported in 2008 [95] and has since been confirmed in several studies [49], [96], [97], [98], [99], [100], [101], [102]. Because oxidative cancer cells preferentially use lactate as an oxidative fuel compared to glucose, they spare glucose that becomes more available for glycolytic cancer cells [95]. This cooperative relationship has been coined ‘metabolic symbiosis’ [95], [103]. In addition to MCTs, lactate can be transferred efficiently from cell to cell through connexin 43 that forms intercellular channels allowing lactate diffusion from its production to its consumption site in a tumor syncitium [104], and stromal cells can be hijacked by oxidative cancer cells to produce lactate [105], [106], [107]. Of note, this stromal contribution has been found to be dispensable in a model of tongue cancer in MCT4/SLC16A3-knockout mice [108]. Once present in the cytosol of oxidative cancer cells, lactate is oxidized to pyruvate by LDH-1, which implies the simultaneous reduction of NAD+ in NADH + H+. Pyruvate and NADH (through the malate-aspartate shuttle) can then fuel the TCA cycle [95], [97], [109]. In non-small cell lung carcinoma (NSCLC) models, especially upon orthotopic transplantation in mice, the contribution of lactate to the TCA cycle exceeds that of glucose [101]. An opposite situation can be encountered in metastatic breast cancer, where glycolytic cancer cells in the bone fuel oxidative osteoclast metabolism with lactate, promoting bone resorption [110].
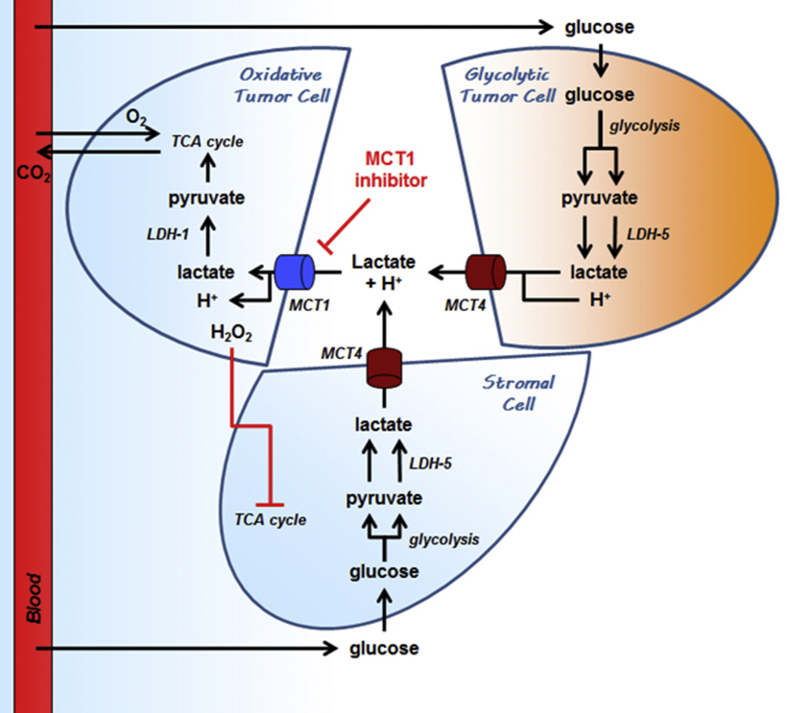
Figure 2.
Metabolic symbiosis and commensalism based on the exchange of lactate in cancer. The cartoon depicts a tumor-feeding blood vessel delivering glucose and oxygen to cancer cells. An oxidative cancer cell is represented close to the blood vessel, a hypoxic cancer cells remotely, and a host cell on the bottom. From a metabolic standpoint, the hypoxic cancer cell has no choice but to perform anaerobic glycolysis to survive, which implies having access to high amounts of glucose. Comparatively, the oxidative cancer cell can use several different metabolic fuels. When nearby glycolytic cells provide lactate (usually a MCT4-dependent process), it uses lactate as an oxidative fuel preferentially to glucose (usually a MCT1-dependent process), which increases glucose availability for the glycolytic cancer cell. The oxidative cancer cell can obtain additional lactate by forcing the host cell to adopt a glycolytic metabolism. When lactate is not available or when MCTs are inhibited, the oxidative cancer cell switches to a glucose-based metabolism, thus depriving other cells from this important resource, which ultimately kills the hypoxic cancer cell. MCT1 and MCT4 inhibitors can, thus, destroy both the metabolic symbiosis and the commensalism based on the exchange of lactate in cancer. For abbreviations, see list. Adapted from reference [219].
Compared to glucose-fueled respiration, oxidative lactate metabolism could offer at least four advantages to oxygenated cancer cells. First, the yield of ATP produced per molecule of lactate consumed is up to 7.5 times higher as compared to aerobic glycolysis. Then, cancer cells preferentially utilizing lactate spare the energy required for glycolytic enzyme synthesis and maintenance and for the phosphorylation of glucose and fructose-6-phosphate during glycolysis. Lactate oxidation by LDH-1 also provides the cell with energy-rich NADH, which can fuel the mitochondrial electron transport chain (ETC) through the malate-aspartate shuttle [109]. Finally, the LDH-1-catalyzed reaction promotes lysosome acidification and autophagic vesicle maturation [111]. In the process, LDH-1 physically interacts with vacuolar-type proton-ATPase (V-ATPase) at the lysosome surface, which suggests that the LDH-1 reaction aliments a pool of H+ that feed V-ATPase in order to acidify lysosomes. Autophagy would be of particular importance for oxidative cancer cells, because this process is necessary for the recycling of oxidized proteins and organelles, including mitochondria that can be damaged by their own production of mtROS [112].
To track oxidative lactate metabolism in tumors, (±)-[18F]-3-fluoro-2-hydroxypropionate (18-Flac) was developed as a tracer of lactate for positron emission tomography (PET) imaging [113]. Imaging lactate uptake could be of particular interest in the context of anti-angiogenic therapy evasion that can occur, notably, when oxidative cancer cells establish a metabolic symbiosis with glycolytic ones, as observed in pancreatic neuroendocrine tumors and in renal cell carcinomas [98], [99], [100].
Importantly, several cancer cell lines, mostly highly glycolytic cancer cells, are not able to import and/or to oxidize lactate in normoxia [95], [97], [98]. This could result from low oxidative and high glycolytic activities and/or from inadequate MCT and LDH expression patterns, resulting in a net outward lactate flux that opposes lactate uptake. Moreover, because MCTs are passive transporters, a high concentration of exogenous lactate can inhibit the efflux of lactate from highly glycolytic cells. This constitutes a serious problem for anticancer immunity, where extracellular lactate impairs the glycolytic activity of cytotoxic T-lymphocytes and activated monocytes, their proliferation and their function, thus promoting immune resistance [114], [115].
Of note, MCTs transport other monocarboxylates than lactate. In particular, β-hydroxybutyrate released by adipocytes is consumed by MCT2-expressing breast cancer cells [116]. In addition to its role as a metabolic fuel, β-hydroxybutyrate is a HDAC inhibitor that induces the expression of tumor promoter genes in breast cancer cells, such as cytokine IL-1β and growth factor lipocalin 2. MCT inhibition at large could thus interfere with the transfer of monocarboxylates different than lactate between distinct cancer cell subpopulations and between stromal and cancer cells.
3.2. MCTs and lactate receptor GPR81 control lactate signaling
In addition to its role as a metabolic substrate, lactate is also a signaling molecule regulating gene expression and protein activation. Lactate can indeed be considered a tumor-promoting metabolite that influences angiogenesis, amino acid metabolism, histone deacetylases (HDACs), GPR81 signaling, and immunity in processes associated with tumor progression (Figure 3).
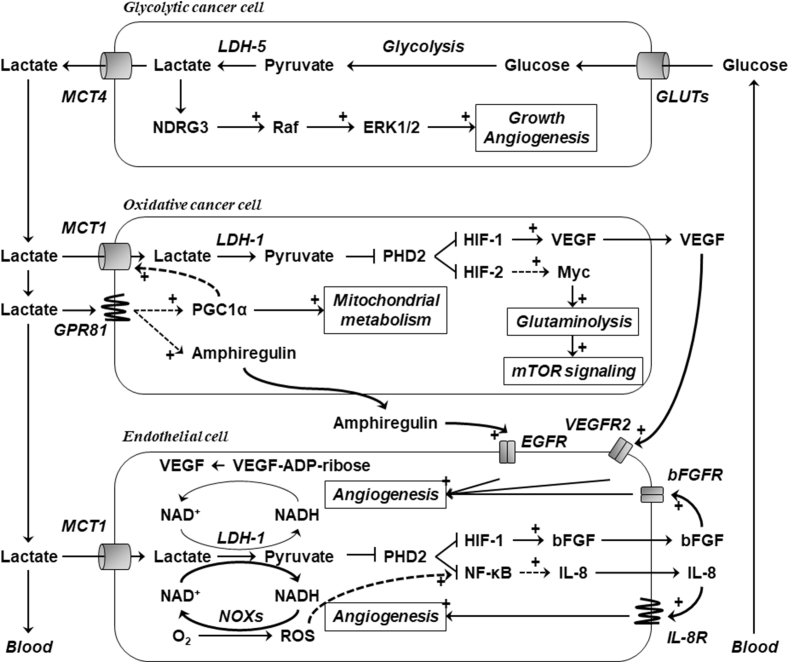
Figure 3.
Lactate is a signaling molecule in cancer and endothelial cells. The cartoon depicts on endothelial cell in close proximity of an oxidative cancer cell, and a hypoxic/glycolytic cancer cell is further away. Lactate is produced from glucose in the hypoxic/glycolytic cancer cell, and intracellular lactate activates a NDRG3-Raf-ERK1/2 tumor growth-promoting pathway. Once exported (usually a MCT4-dependent process), lactate diffuses along its concentration gradient and influences the oxidative cancer cell and the endothelial cell. Extracellular lactate can bind to lactate receptor GPR81 in the oxidative cancer cell, supporting mitochondrial biogenesis, lactate transport and signaling. It can also enter into the cell (usually a MCT1-dependent process), where it promotes pro-angiogenic signaling and glutaminolysis. Similarly, the endothelial cell expresses MCT1 and can therefore take up lactate. There, lactate triggers additional pro-angiogenic pathways. Plain lines and dotted lines represent well-established and presumptive cascades, respectively. + refers to stimulation. For abbreviations, see list.
3.2.1. Lactate induces tumor angiogenesis
Lactate is a pro-angiogenic agent. In oxidative cancer cells and in endothelial cells, lactate influx through MCT1 and its oxidation by LDH-1 generates pyruvate, which acts as a pro-angiogenic cytosolic signal. Comparatively, owing to an unfavorable transmembrane gradient, highly glycolytic cancer cells are resistant to lactate influx [117]. Pyruvate, but not lactate itself, has been described as a pseudo-hypoxic signal that acts on HIF prolylhydoxylases (PHDs), especially PHD2 [118], [119]. In the presence of oxygen and cofactors α-ketoglutarate and vitamin C, this family of enzymes catalyzes the hydroxylation of HIF-1α on two proline residues, targeting this HIF-1 subunit to proteasome-mediated degradation (see reference [44] for a review). Thus, even in the presence of enough oxygen, PHDs can be inhibited either by oxidants or by competitors of α-ketoglutarate. In this molecular context, pyruvate competes with α-ketoglutarate, resulting in PHD inhibition in normoxic cancer cells [117], [120], [121]. Yet, some doubts persist on the exact nature of the competition [122].
PHD inhibition by lactate-derived pyruvate exerts different effects according to the cell type: it stabilizes HIF-1α in both oxidative cancer cells and in endothelial cells, and subsequently activate HIF-1 and triggers the transcription of vascular endothelial growth factor-A (VEGF-A) in cancer cells [117] and of VEGF receptor 2 (VEGFR2) and basic fibroblast growth factor (bFGF) in endothelial cells [121], [123]. Both VEGF-A and bFGF are well characterized pro-angiogenic factors, and activation of their receptors expressed by endothelial cells stimulates endothelial cell proliferation and migration, leading to neovessel formation from pre-existing blood vessels [124]. In endothelial cells, PHDs not only tag HIF-1α for proteasomal degradation but also negatively regulate inhibitor of κB-kinase β (IκKβ) expression and activity [125]. It follows that lactate activates IκKβ, resulting in the phosphorylation of inhibitor of κBα (IκBα) and its subsequent proteasomal degradation, the nuclear translocation of NF-κB and the transcription of pro-angiogenic factor interleukin-8 (IL-8) [120]. Of note, together with pyruvate, NADH produced by the LDH-1 reaction is required for NF-κB activation: in the cytosol, NADH fuels NAD(P)H oxidases (NOXs) that start to generate ROS, contributing to activation of IκK and/or inhibition of PHDs [120]. This signaling pathway is not activated in oxidative cancer cells because NADH generated by the LDH-1 reaction preferentially fuels OXPHOS rather than NOXs in these cells [109]. Altogether, lactate produced e.g. by hypoxic cancer cells elicits a coordinated pro-angiogenic transcriptional program that induces neovessel formation in tumors [11].
N-myc downstream-regulated gene 3 (NDRG3), another PHD2 target, has also been described as an angiogenesis regulator in tumors [126]. In normoxic conditions, NDRG3 is targeted by PHD2 to the proteasome for degradation. However, under prolonged hypoxia, intracellular lactate accumulation is responsible for NDRG3 activation and the consecutive stimulation of the Raf-extracellular signal-regulated kinase (ERK) pathway, supporting tumor growth and angiogenesis [126]. Interestingly, although NDRG3 is negatively regulated by PHD2, intracellular lactate does not stimulate NDRG3 via PHD2 inhibition, but primarily via direct binding to NDRG3. The relevance of NDRG3 in the pseudo-hypoxic response to extracellular lactate is questionable, because exposure of normoxic cancer cells to high levels of exogenous lactate led only to limited NDRG3 stabilization and signaling when compared to hypoxia. Consequently, whether MCTs control this pathway is unknown.
In endothelial cells and macrophages, additional pro-angiogenic effects of lactate have been described and are linked to a decrease in VEGF (poly)ADP-ribosylation, i.e., a NAD+-dependent posttranslational modification that limits VEGF activity. LDH-1 and (poly)ADP-ribosyltransferases indeed compete for NAD+: lactate oxidation to pyruvate decreases NAD+ levels, and, thereby, VEGF (poly)ADP-ribosylation [127], [128], [129], [130]. If the pathway is well described, the involvement of MCTs would be logical but has not been demonstrated to date.
Overall, by various mechanisms, cancer cell, endothelial cell, and/or macrophage exposure to lactate was phenotypically shown to induce endothelial cell proliferation [123], [131], migration [120], [121], [123], [131], [132], tube formation [120], [121], [123], [133], vessel sprouting from aortic explants [121], angiogenesis in the egg chorioallantoic membrane [130], [131] and rabbit cornea [127] models, and tumor angiogenesis in mice [117], [120], [121], [134].
3.2.2. Lactate stimulates amino acid metabolism
In cancer cells expressing MCT1, a second signaling activity of lactate is linked to its positive regulation of amino acid metabolism. In these cells, PHD inhibition by lactate-derived pyruvate not only leads to HIF-1α but also to HIF-2α stabilization and HIF-2 activation [135]. HIF-2 was reported to enhance Myc signaling, thereby promoting glutamine uptake and metabolism through enhanced expression of the inward glutamine transporter ASCT2 and of glutamine-metabolizing enzyme glutaminase 1 (GLS1) [135]. Moreover, lactate-induced glutamine metabolism was shown to activate mTOR [98], [136], a key nutrient sensor and master regulator of cell growth, which e.g. stimulates protein synthesis. Whether lactate can simultaneously stimulate autophagy via LDH-1 and protein synthesis via mTOR in cancer cells is an open question.
3.2.3. Lactate inhibits histone deacetylases
Extracellular lactate inhibits HDACs, resulting in histone hyperacetylation, reduced chromatin compactness, and changes in gene expression [137], [138]. In particular, lactate-induced hyperacetylation was reported to facilitate DNA repair and to promote cancer cell resistance to chemotherapy. GPR81 silencing and MCT1 inhibition were both shown to interfere with this process [138]. Lactate-induced histone hyperacetylation thus links extracellular lactate to intracellular epigenome regulation, genome stability and therapy evasion.
3.2.4. Lactate activates lactate receptor GPR81
Besides intracellular activities depending on its uptake, extracellular lactate can bind to GPR81, a lactate-activated G-protein-coupled receptor. In a physiological context, GPR81 activation inhibits lipolysis in adipose cells in vitro and decreases the concentration of circulating free fatty acids in vivo [139], [140]. However, to our knowledge, neither an impact of GPR81 on cancer cell lipolysis nor a potential involvement of GPR81 on a systemic release of fatty acid to fuel cancer cells have been investigated to date.
GPR81 is expressed in colon, lung, hepatocellular, salivary gland, PDAC, and breast cancer cell lines, and in PDAC and breast tumors in patients [87], [141]. In PDAC cells, GPR81 stimulation was found to transcriptionally induce the expression of MCT1, MCT4, CD147 and PGC-1α, thereby promoting lactate uptake and oxidative (lactate) metabolism [87]. Accordingly, GRP81 expression promoted, whereas its downregulation decreased, PDAC xenograft growth in mice. In breast cancer cells, GPR81 silencing reduced ATP production, stimulated apoptosis and decreased c-AMP response element binding protein (CREB)-dependent transcription of pro-angiogenic factor amphiregulin [141]. In agreement with the latter observation, conditioned medium of GPR81-silenced breast cancer cells decreased endothelial cell tube formation, and GPR81-silenced breast tumor xenografts showed reduced angiogenesis and growth [142]. These observations suggest that GPR81 stimulation by lactate supports both lactate uptake by cancer cells and lactate-induced angiogenesis, thus collaborating with intracellular lactate processing to sustain tumor growth and progression through non-redundant molecular mechanisms.
3.2.5. Lactate induces immune tolerance
In tumors, lactic acid impairs anticancer immunity by repressing T-cell proliferation, dendritic cell (DC) maturation, and natural killer (NK) cell activity.
Lactic acid inhibits the proliferation of cytotoxic T lymphocytes (CTLs) in a dose-dependent manner [114]. When proliferating, activated CTLs indeed depend on glycolysis and export lactate via MCT1 [143]. Because the activity of the transporter is driven by the concentration of lactate and protons across the cell membrane, lactic acid accumulating in the extracellular compartment of tumors opposes lactate efflux from these cells, hence their proliferation [114], [144], [145]. Molecularly, lactic acid was further shown to repress NFAT expression in T and NK cells [146]. These observations could have applications in other fields than cancer: given the importance of MCT1 for CTL proliferation in immune responses, MCT1 inhibitors were initially developed as immunosuppressors to inhibit tissue graft rejection [143], [145], [147].
In antigen-presenting DCs, a single study reported that lactic acid can impair DC maturation by decreasing the binding efficiency of NF-κB to DNA [148]. Immature DCs displayed an anti-inflammatory phenotype. Conversely, lactate promoted the differentiation of peripheral blood mononuclear cells in myeloid-derived suppressor cells (MDSCs), which contributed to immunosuppression [149]. It also decreased NK cell activity [146], [149], and the authors showed that knocking down LDHA expression in cancer cells resulted in a smaller tumor size, an increased number of NK cells and a decreased number of MDSCs. A ketogenic diet, which, to some extent, prevents intratumoral lactate accumulation, partially recapitulated this effect [149].
3.3. MCTs promote cancer metastasis
The clinical significance of MCT expression patterns has been evaluated in patient tumor samples. In humans, high MCT1 and high MCT4 expression is usually associated with poor prognosis, whereas MCT2 expression correlates with a favorable outcome (prognostic values of MCT expression are reported in Table 3). In the same line, clinical data showed that high MCT1 expression is associated with invasion in colorectal carcinoma [34] and metastasis in cervix [150] and esophageal adenocarcinomas [151]; in adrenocortical [152] and gastric [153] carcinomas, and in bladder cancer [154]; and high MCT4 expression correlated with invasion in gastric cancer [155] and metastasis in esophageal adenocarcinoma [151], and in colorectal carcinoma [156], [157]. In metastatic lesions compared to the primary tumor, overexpression of MCT1 was reported in NSCLC [158] and overexpression of MCT4 in melanoma [159], although an independent study showed no statistically significant upregulation of the expression of the transporter [96]. These observations suggest a contribution of MCT1 and MCT4 to the metastatic process.
Table 3.
Prognostic value of MCT expression in human cancers.
| Cancer type | High MCT1 | High MCT2 | High MCT4 | Ref. |
|---|---|---|---|---|
| Adrenocortical carcinoma | good | [152] | ||
| Bladder cancer | poor | Uncertain | [154], [220], [221] | |
| Breast cancer | poor | poor | [196], [223], [224], [225] | |
| Clear renal cell cancer | poor | poor | [227], [230] | |
| Colorectal cancer | poor | [231] | ||
| Glioblastoma | poor | [203] | ||
| Head and neck cancer | poor | [162], [232] | ||
| Hepatocellular carcinoma | good | poor | [31] | |
| Lung cancer | poor | [193], [196] | ||
| Melanoma | poor | poor | [96], [159] | |
| Neuroblastoma | poor | [188] | ||
| Osteosarcoma | poor | [80] | ||
| Ovarian Cancer | poor | poor | [182] | |
| Prostate cancer | poor | [35], [93] | ||
| Soft tissue sarcoma | poor | poor | [36] | |
| Testicular germ cell cancer | poor | poor | [164] |
That MCT1 promotes cancer cell migration and invasion has been evidenced in vitro. Indeed, glucose deprivation, a situation occurring in tumors [2], posttranslationally induced MCT1 and CD147 expression at the plasma membrane of human cervix carcinoma cells in a mtROS-dependent manner, which stimulated cancer cell migration towards glucose [48]. Other studies confirmed MCT1-induced migration and invasion in breast and lung carcinoma, glioblastoma, and osteosarcoma cell lines [49], [50], [71], [73], [80], [160], [161]. The amplitude of the effect varied with the expression pattern of MCTs and the relative reliance of cancer cells on glycolysis.
Mechanistically, Zhao et al. [80] proposed decreased NF-κB signaling as a molecular mechanism coupling MCT1 repression to decreased osteosarcoma cell migration. This has been recently confirmed in another study in cervix and breast cancer cells [161]. Interestingly, the promigratory activity of MCT1 was shown to be independent of its transporter activity, as MCT1 silencing, but not its pharmacological inhibition, repressed cancer cell migration. Moreover, knock-in experiments with a transporter-deficient version of human MCT1 in MCT1-deficient mouse cancer cells was sufficient to restore migration. While MCT1 silencing inhibited NF-κB signaling as well as cancer cell migration and metastatic dissemination from primary breast cancer in vivo, restoring its expression simultaneously restored NF-κB-dependent cancer cell migration. MCT1 could thus directly or indirectly interact with upstream components of the NF-κB signaling pathway, supporting its activity. Of note, MCT1 activity in stromal cells might also contribute to metastasis: blocking lactate influx in endothelial cells [120], [121], [123] and in osteoclasts [110] impaired tumor-induced angiogenesis and bone resorption, respectively.
Similar to MCT1, MCT4 knockdown impaired the migration and invasion of various cell lines [70], [71], [74], [162], [163], with the exception of JEG-3 testicular germ cell cancer cells on which, surprisingly, it had the opposite effect [164]. MCT4 silencing led to abnormal CD147 trafficking and accumulation in lysosomes [74], increased focal adhesion size [70], upregulated epithelial markers, and downregulated mesenchymal markers [162]. MCT4, but not MCT1, directly interacts with β1-integrin at the lamellipodium of migrating cells [70]. Because integrin conformation is pH-sensitive [165], loss of MCT4 activity could locally modify the transmembrane pH gradient and modify integrin signaling and cell adhesion. This hypothesis does not rule out that the physical interaction between MCT4 and β1-integrin could per se regulate β1-integrin-mediated adhesion and cancer cell migration. A selective MCT4 inhibitor would allow to discriminate between both hypotheses by evaluating whether the activity of the transporter is dispensable or not.
Importantly, CD147, the chaperone protein shared by MCT1 and MCT4, is well known to trigger cancer cell migration, invasion, and metastasis, notably through activation of matrix metalloproteinases (MMPs) (see reference [166] for a recent review). Because MCT1 and CD147 on the one hand and MCT4 and CD147 on the other hand mutually stabilize their expression at the cell plasma membrane [45], [71], [72], [73], silencing MCT1 or MCT4 might impair CD147 expression and function. This could explain, at least in part, how MCT1 and MCT4 can promote cancer cell migration and invasion independently of their transport activities. Because CD44 signaling stimulates breast cancer cell invasion [167], the hypothesis of a similar relationship between CD44 and MCTs is attractive, but has, to our knowledge, never been tested.
4. Therapeutic MCT inhibition
4.1. MCT inhibitors
Several compounds non-specifically inhibit MCTs (Table 4). Among known modes of actions, 4′-diisothiocyano-2,2′-stilbenedisulphonate (DIDS) irreversibly binds to a lysine residue on MCT1 and MCT2, thus inactivating the transporters, but not on MCT4 [8]; and organomercurial compounds such as p-chloromercuribenzenesulphonate (p-CMBS) disrupt MCT-CD147 interactions, thus interfering with MCT1, MCT3, and MCT4 expression and activity, but not with MCT2 [64]. These compounds do not present a high clinical hope for disrupting MCT functions in cancer.
Table 4.
MCT inhibitors.
| Inhibitor | MCT1 Ki (μM) |
MCT2 Ki (μM) |
MCT4 Ki (μM) |
Other targets |
|---|---|---|---|---|
| Non-selective MCT inhibitors | ||||
| Phloretin | 5 | 14 | 41 | Glucose transporters |
| Quercetin | 10 | 5 | 40 | ERβ |
| DIDS | 434 | ND | NI | Bicarbonate transporters |
| Simvastatin | >200 | ND | >200 | HMG-CoA reductase |
| p-CMBS | 25 | NI | 25 | Anion transporters |
| Lonidamine | 36 | 36 | 40 | Mitochondrial hexokinase |
| CHC | 166 | 24 | 991 | Mitochondrial pyruvate carrier |
| Selective MCT inhibitors | ||||
| AR-C155858 | 0.002 | <0.01a | NI | - |
| AZD3965 | 0.002 | 0.02 | NI | - |
| BAY-8002 | Nanomolar range | Nanomolar range | NI | - |
Pharmacological interest for MCT1 inhibitors rose in 2007 with the identification of MCT1 as the target of a new class of immunomodulatory drugs [145]. These compounds and optimized compounds AR-C155858 and AZD3965 were shown to inhibit lactic acid efflux from activated T-lymphocytes [145], [168]. They reduce the glycolytic rate of these cells and acidify their cytosol, ultimately blocking their proliferation and preventing acute rejection following organ transplantation in mice. Lactic acid accumulation in the tumor microenvironment has been proposed to mediate immune evasion through a similar mechanism of inhibition of lactate secretion [114], [146]. AR-C155858 and AZD3965 inhibit both MCT1 and MCT2, although AZD3965 is 6 times more selective for MCT1 than for MCT2 (see Table 4). AR-C155858 directly binds to TM7-10 of MCT1, but not to MCT2 unless when MCT2 undergoes a conformational change induced by its interaction with CD147 [65], [169], [170], [171]. Because MCT2 preferentially interacts with gp70, both inhibitors could thus reasonably be considered as selective MCT1 inhibitors, and no off-target effects except those involving MCT2 have been reported to date. AR-C155858 and AZD3965 do not inhibit MCT4, even at high doses [169], [170], [171], [172]. Similarly, MCT3-mediated lactate transport was not inhibited by a 1 h treatment of rat pancreatic cells with 10 μM of AZD3965 [172]. Recently, BAY-8002 was reported as a novel selective MCT1 inhibitor, with a 6-fold selectivity for MCT1 compared to MCT2, no activity on MCT4 and no off-target effects [173]. Competition studies using radiolabeled compounds indicated that BAY-8002 and AZD3965 acted in a similar way, as they displaced each other. An alternative strategy to specifically target a given MCT isoform consists in the delivery of small interfering RNAs loaded in PEGylated chitosan nanoparticles, as documented for MCT1 [174].
4.2. Targeting MCT-mediated lactic acid influx by cancer cells
The metabolic symbiosis based on the exchange of lactate is of particular importance for cancer cell adaptation to glucose depletion [95] and for tumor resistance to anti-angiogenic therapies [98], [99], [100]. When MCT1 gates lactate uptake by oxidative cancer cells, MCT1 targeting can result in lactate influx impairment, a metabolic switch from lactate-fueled OXPHOS to aerobic glycolysis in these cells, and the indirect death of hypoxic cancer cells consecutive to glucose deprivation [95]. In particular, the use of small interfering RNAs validated the key role exerted by MCT1 in such symbiont. In murine models of cancer, daily administration of MCT inhibitor α-cyano-4-hydroxycinnamate (CHC) resulted in decreased tumor growth, an increased tumor necrotic core associated with the eradication of hypoxic tumor areas, and a higher sensitivity of the remaining oxygenated tumor rim to radiotherapy [95]. The antitumor selectivity of the approach relies on the metabolic interdependency of oxidative and glycolytic cancer cells in a same tumor: while oxidative cancer cells adapt to MCT1 inhibition by switching to alternative substrates (as would also do other oxidative cells in the body), glycolytic cancer cells depending on the symbiosis for survival cannot.
MCT1 inhibition can also interfere with oxidative cancer cells hijacking stromal cells to get additional lactate (see Section 3.1). While in normal conditions the co-culture of cancer-associated fibroblasts (CAFs) would fuel cancer cell proliferation, the administration of CHC or a MCT1 knockdown was sufficient to disrupt this relationship; thus impairing cancer cell proliferation [107], [175]. Similarly, MCT1 inhibition or knockdown delayed the growth of mixed cancer cell-CAF xenografts in mice. Interestingly, other studies reported MCT1 expression and the presence of markers of an oxidative metabolism in CAFs, and the expression of markers of a glycolytic metabolism in cancer cells [176], [177], [178]. These features may reflect metabolic heterogeneity in cancer. Acid clearance by CAFs was reported in an independent study [179] and might be beneficial for cancer cells.
MCT1-mediated lactate uptake is also a characteristic of angiogenic endothelial cells (see Section 3.2.1). Consequently, MCT1 targeting in endothelial and cancer cells, using a silencing approach or CHC administration, impaired lactate-induced angiogenesis in vitro and in murine models of cancer in vivo [117], [120], [121]. Intriguingly, in the only study to our knowledge where angiogenesis was assessed upon AZD3965 treatment, the drug had no effect on small cell lung carcinoma xenograft vascularization [180].
In the context of combination therapy, MCT1 was identified as a main transporter facilitating the uptake of anticancer agent 3-bromopyruvate by cancer cells [181]. A potential interference between MCT1 inhibitors and 3-bromopyruvate should thus be addressed in future studies. The evaluation of combination treatments should further take into account the potential involvement of MCT1 in multidrug resistance [93], [182] and the observation that MCTs can transport additional exogenous molecules [183].
4.3. Targeting MCT-mediated lactic acid efflux by cancer cells
MCTs facilitate lactic acid efflux from glycolytic cancer cells and are, therefore, important pH regulators [184], [185]. It is tempting to inhibit this function in order to acidify the cytosol of glycolytic cancer cells, inducing their death. To achieve that aim, dozens of studies used non-specific MCT inhibitors, selective MCT1 inhibitors and genetic approaches targeting MCT1 and/or MCT4 [45], [49], [50], [52], [72], [73], [101], [160], [162], [172], [180], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198]. Impairment of MCT-mediated efflux generally resulted in decreased pyruvic and/or lactic acid release from glycolytic cancer cells, increased intracellular pyruvic and/or lactic acid content resulting in cytosol acidification, inhibition of glycolysis and an increased dependence of the cells on mitochondrial oxidative metabolism sometimes associated with increased oxidative stress and a drop in ATP levels [6], [11]. These effects were found to be exacerbated by hypoxia, when cancer cells mainly relied on glycolysis, produced important amounts of lactic acid, and expressed high MCT levels [45], [49], [50], [180]. In several studies, targeting CD147 recapitulated the metabolic effects of MCT impairment [45], [72], [199], [200], [201].
Interestingly, in cases when MCT1 inhibition increased oxidative mitochondrial metabolism, cancer cells generally became more sensitive to ETC Complex I inhibitors metformin, phenformin and BAY87-2243 [52], [72], [196], [197], [198], [202] and to GLS1 inhibitor bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) [172]. Synthetic lethality is possible, as exemplified by the combination of a dual MCT1 and MCT4 inhibitor with metformin, which depleted cancer cells of NAD+ [202]. Moreover, additive or synergetic effects were observed upon MCT1, MCT2, or MCT4 inhibition in combination with chemotherapy [80], [83], [155], [172], [192], [194] and radiotherapy [155], [180], [189]. They could be attributed to decreased extracellular acidity, decreased intracellular pH, and/or increased oxidative stress. Importantly, inhibition of lactic acid secretion is the main incentive for the current evaluation of AZD3965 in phase I/II clinical trial for Burkitt and large B cell lymphoma, and gastric and prostate cancers (ClinicalTrials.gov NCT01791595).
Using AR-C155858 and AZD3965 or knockdown/knockout of selected MCT isoforms, compensatory mechanisms were identified, including upregulation of other MCT isoforms. In particular, upregulation of MCT4 expression decreased the sensitivity of cancer cells to AR-C155858, AZD3965 and to a short hairpin RNA targeting MCT1 [45], [71], [72], [180], [193]. This resistance mechanism may result from alleviation of a competition between both transporters for CD147, and could be reciprocal [45], [72].
MCT4 expression has been associated with the tumorigenic potential of glioblastoma cells: glioblastoma cells expressing stemness marker CD133 expressed MCT4 at high levels, and a knockdown of MCT4 decreased CD133 expression, the ability of the cells to form neurospheres and xenograft growth in mice [203]. Interestingly, lactate transport was not influenced by MCT4 knockdown in this model. A thorough characterization of MCT expression in tumor-initiating cells could thus potentially lead to the identification of new therapeutic approaches.
4.4. Targeting MCT-dependent metastasis
Quercetin, lonidamine, DIDS, and simvastatin can reduce cancer cell migration and invasion [71]. However, due to their multiple molecular targets, linking these results and those obtained with CHC [48], [80] to MCTs is not straightforward. To our knowledge, only three studies were performed with AR-C155858 and AZD3965 in this context, and showed a very limited reduction [163] or a total absence [161], [204] of effects on cancer cell migration and invasion, despite evidence of inhibition of lactic acid transport. Because MCT1 knockdown, but not its pharmacological inhibition, impaired cancer cell migration and invasion, it was concluded that the activity of the transporter is not necessary for its pro-metastatic functions [161], [204]. In breast and prostate cancer cells, MCT1 silencing, but not its pharmacological inhibition, decreased the phosphorylation of hepatic growth factor (HGF) receptor c-Met [204] and NF-κB activity [161], thereby decreasing cancer cell migration. Hence, it is probable that selective MCT1 inhibitors would have limited direct antimetastatic effects.
4.5. Potential toxicities of MCT inhibitors: learning from MCT-deficient mice and patients
The important roles exerted by MCTs in physiology call for attention on possible toxicities associated with MCT inhibitors. Mouse models have been engineered for MCT1, MCT2, and MCT3 deficiency, and patients with heterozygous missense mutations of the MCT1/SLC16A1 gene have been reported.
In genetically engineered mouse models, a full knockout of MCT1/SLC16A1 was found to be embryonically lethal due to neuronal defects [205]. Comparatively, a systemic MCT1/SLC16A1+/− genotype and an oligodendrocyte-selective MCT1/SLC16A1 knockdown produced living mice, but these animals had impaired axon myelination, leading to axon damage and decreased neuron survival in the central nervous system [206]. The regeneration of motor and sensory peripheral nerves after a lesion was also delayed in MCT1/SLC16A1+/− mice. These results are consistent with the decreased expression of MCT1 observed in neurodegenerative human diseases, such as amyotrophic lateral sclerosis (ALS) [205], [207] and Alzheimer's disease [208], suggesting an important role of this transporter in the maintenance of axon integrity, putatively because it facilitates lactate shuttles between oligodendrocytes and neurons [30], [205]. In another study, MCT1/SLC16A1+/− mice showed increased resistance to high fat diet-induced obesity, higher insulin and leptin secretion, as well as decreased food intake, fat absorption, fat mass, and liver steatosis [209]. For what concerns pharmacological treatments, chronic administration of CHC (4.7 mg daily) impaired hind limb reperfusion following femoral artery and vein ligation in mice [134]. This effect was attributed to an interference with lactate-induced angiogenesis, but a specific MCT1 inhibitor would be required for further validation.
In the brain, MCT2 is preferentially expressed in neurons where it conveys lactate uptake [6]. Adult rats injected with antisense oligonucleotides in the hippocampus showed memory defects. MCT2-deficiency did not alter short-term memory but significantly disrupted long-term memory [25]. Neither glucose nor lactate rescued amnesia, indicating that yet unknown processes dependent on MCT2 are essential for long-term memory. Accordingly, MCT2 expression was found to be decreased in animal models of Alzheimer's disease [208], [210].
In eyes, MCT3 facilitates lactate export by the retina. It is therefore not surprising that MCT3/SLC16A8-knockout mice developed visual defects [211]. They were attributed to a decrease in photoreceptor currents in response to light and associated to a 4-fold increase in lactate levels in the retina and, possibly, acidification of the subretinal space. However, histological features of the eyes were preserved.
MCT4/SLC16A3-knockout mice have been generated in 2018 and are viable [108]. However, a detailed analysis of phenotypic changes and possible compensation of the deficiency by other MCT isoforms has not yet been performed.
In humans, genetic polymorphisms of MCT1/SLC16A1 impact the oxidative clearance of lactate by slow-twitching muscle fibers, with carriers of 1470T>A, 2917(1414)C>T, and IVS3-17A>C variants showing poorer lactate clearance during high intensity exercise [212], [213], [214]. In the same line, a single case report concluded that lactate transport deficiency caused lactate accumulation in the exercising muscle, which was potentially responsible for episodes of rhabdomyolysis and myoglobinuria [215]. However, the patient was not genotyped. In 3 additional patients with “cryptic exercise intolerance,” heterozygous missense mutations of the MCT1/SLC16A1 gene have been reported to cause muscle and chest pain after prolonged exercise [216], but, surprisingly, one of these mutations did not impair MCT1 function in Xenopus oocytes [3]. Assuming that cryptic exercise intolerance symptoms result from an impairment of MCT1 functions, they could be caused by impaired lactic acid efflux in skeletal muscles and/or impaired lactic acid clearance by various tissues. More recently, novel MCT1 mutations (either homozygous or heterozygous) have been identified in 9 patients [217]. They resulted in recurrent and severe episodes of keto-acidosis, i.e., accumulation of ketone bodies in the blood due to an imbalance between their production in the liver and their use in peripheral tissues, possibly resulting from a decreased uptake capacity of ketone bodies by MCT1-deficient cells. Thus, keto-acidosis is important to consider upon therapeutic MCT1 inhibition as well. To our knowledge, no polymorphisms and no missense mutations have been found for MCT2, MCT3 and MCT4 in patients. MCT4 polymorphism has been reported in horses, but it was silent [218].
5. Conclusive remarks
Whereas glycolysis coupled to lactic fermentation has been reported as the preferential metabolic mode adopted by hypoxic and proliferating cancer and host cells, tumors also contain cells relying on an oxidative metabolism. Like other oxidative cells in the body, oxidative cancer cells may use several metabolic substrates, of which the main ones are glucose, lipids, glutamine, and lactate. What distinguishes tumors from normal tissues is that they accumulate lactate owing to a high glycolytic activity in some tumor areas and inefficient clearance by the abnormal tumor vasculature in general. Lactate levels in cancer may reach 40 mM with an average concentration in human tumors of about 10 mM [2], [11]. Therefore, this metabolic resource is of particular importance for tumor growth and development. Importantly, the export of lactate by glycolytic cells and its import by oxidative cells are gated by MCTs.
In this review, we summarized the current knowledge about the regulation of MCT expression, their functions in cancer, and their druggability. We emphasized that they are expressed not only in cancer cells but also in host cells, where they exert multiple pro-tumoral activities. Via MCTs, lactate sustains metabolic cooperation and commensalism (Figure 2), as well as metabolic and pro-angiogenic signaling (Figure 3). MCTs, especially MCT1, can further promote cancer metastasis independently of their transport activities. As such, high MCT expression is generally correlated with poor prognosis for cancer patients (Table 3). However, lactate may also exert part of its pro-tumoral activity independently of MCTs, notably by binding to the GPR81 receptor at the cell surface [87]. This information is included in our review, because it has not been covered previously. For therapy, it is therefore important to consider MCTs as well as other potential therapeutic targets.
Based on their functions in cancer and analyzes of the phenotypes of MCT-deficient mouse models and patients, we believe that MCTs, especially MCT1 and MCT4 and also probably MCT2, have good chances to be confirmed as potent anticancer targets for patient treatment. A first MCT1 inhibitor, AZD3965, is currently undergoing clinical trials for several types of cancer (ClinicalTrials.gov NCT01791595). While we sincerely hope that this compound will exert appreciable anticancer effects, one has to realize that anti-MCT drug development is still in its infancy, calling for refined drug development. In our opinion, a key parameter to solve and about which the literature is largely silent is to reach full selectivity for a given MCT isoform. As can be noticed in Table 4, MCT3 has been poorly studied, and the selectivity of existing compounds preferentially targeting MCT1 versus MCT2 is based on an unlikely, yet possible, change of MCT2 conformation when binding to either gp70 of CD147. Selective MCT4 inhibitors have not been disclosed in the scientific literature yet, and little is known about the role of other MCT substrates than lactate, including pyruvate, ketone bodies and exogenous drugs, on tumor progression.
Conclusively, the last 10 years have witnessed an impressive increase in the scientific knowledge about lactate and MCTs in cancer. MCTs are promising anticancer targets. However, several grey zones and black boxes are still present, that range from the (epi)genetic control of cancer and host cells by lactate in tumors to the potential contribution of MCTs to cancer growth at the systemic level (for example, the Cori cycle of lactate-fueled gluconeogenesis in the liver [23]) and the contribution of other substrates than lactate.
Author's contribitions
VLP structured and drafted the manuscript and produced tables and figures. EM contributed to review the literature and produced tables. VFVH wrote a section of the manuscript. PEP and PS supervised the work. All authors revised and edited the manuscript, figures and tables.
Funding
Works at authors' labs are supported by European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreements No 642623 RADIATE and No 722605 TRANSMIT, the Belgian Fonds National de la Recherche Scientifique (F.R.S.-FNRS), the Belgian Télévie, the Belgian Fondation contre le Cancer (Fundamental Research grant FAF-F/2018/1282) and the Fondation Louvain (all to PS), and the Italian Ministry for University and Research (MIUR, Rita Levi-Montalcini program for young researchers 2014) to PEP. PS is a F.R.S.-FNRS Senior Research Associate. Sponsors were not involved in the writing of the report and in the decision to submit the article for publication.
Conflict of interest
PS is inventor of PCT international application number PCT/EP2017/072582 “[18F]-labelled lactate as a PET radiotracer”. Authors declare no other conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.07.006.
Abbreviations
- 18-FLAC
(±)-[18F]-3-fluoro-2-hydroxypropionate
- ALS
Amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- BDNF
Brain-derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- BPTES
Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- CAF
Cancer-associated fibroblast
- CHC
α-cyano-4-hydroxycinnamate
- COX
Cytochrome oxidase
- CREB
c-AMP response element binding protein
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- DIDS
4′-diisothiocyano-2,2′-stilbenedisulphonate
- EGFR
Epithelial growth factor
- ERK
Extracellular signal-regulated kinase
- ERRα
Estrogen-related receptor α
- ETC
Electron transport chain
- FRET
Fluorescence resonance energy transfer
- GlpT
Glycerol-3-phosphate transporter
- GLS1
Glutaminase 1
- GLUT
Glucose transporter
- GPR82
G protein-coupled receptor 81
- HDAC
Histone deacetylase
- HGF
Hepatic growth factor
- HIF
Hypoxia-inducible transcription factor
- HRE
Hypoxia response element
- IGF1
Insulin-like growth factor 1
- IκBα
Inhibitor of κBα
- IκKβ
Inhibitor of κB-kinase β
- IL
Interleukin
- LDH
Lactate dehydrogenase
- MACC1
Metastasis-associated in colon cancer 1
- MCT
Monocarboxylate transporter
- MDSC
Myeloid-derived suppressor cell
- MMP
Matrix metalloproteinase
- mTOR
Mammalian target of rapamycin
- mtROS
Mitochondrial reactive oxygen species
- miR
MicroRNA
- NDRG3
N-myc downstream-regulated gene 3
- NF-κB
Nuclear factor-κB
- NFAT
nuclear factor of activated T-cells
- NK
Natural killer
- NOX
NAD(P)H oxidase
- NSCLC
Non-small cell lung carcinoma
- OXPHOS
Oxidative phosphorylation
- p-CMBS
p-chloromercuribenzenesulphonate
- PDAC
Pancreatic ductal adenocarcinoma
- PET
Positron-emission tomography
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator 1-α
- PHD
(HIF) prolylhydoxylase
- ROS
Reactive oxygen species
- SLC16A
Solute carrier 16A
- SMCT
Sodium-dependent monocarboxylate transporter
- TCA
Tricarboxylic acid (cycle)
- TM
Transmembrane
- TNBC
Triple negative breast cancer
- V-ATPase
Vacuolar-type proton-ATPase
- VEGF
Vascular endothelial growth factor
- VEGFR2
Vascular endothelial growth factor receptor 2
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marchiq I., Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. Journal of Molecular Medicine (Berlin) 2016;94:155–171. doi: 10.1007/s00109-015-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walenta S., Snyder S., Haroon Z.A., Braun R.D., Amin K., Brizel D. Tissue gradients of energy metabolites mirror oxygen tension gradients in a rat mammary carcinoma model. International Journal of Radiation Oncology, Biology, Physics. 2001;51:840–848. doi: 10.1016/s0360-3016(01)01700-x. [DOI] [PubMed] [Google Scholar]
- 3.Halestrap A.P., Wilson M.C. The monocarboxylate transporter family--role and regulation. IUBMB Life. 2012;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 4.Halestrap A.P. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 5.Pinheiro C., Longatto-Filho A., Azevedo-Silva J., Casal M., Schmitt F.C., Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. Journal of Bioenergetics and Biomembranes. 2012;44:127–139. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Escuredo J., Van Hee V.F., Sboarina M., Falces J., Payen V.L., Pellerin L. Monocarboxylate transporters in the brain and in cancer. Biochimica et Biophysica Acta. 2016;1863:2481–2497. doi: 10.1016/j.bbamcr.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manoharan C., Wilson M.C., Sessions R.B., Halestrap A.P. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Molecular Membrane Biology. 2006;23:486–498. doi: 10.1080/09687860600841967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson M.C., Meredith D., Bunnun C., Sessions R.B., Halestrap A.P. Studies on the DIDS-binding site of monocarboxylate transporter 1 suggest a homology model of the open conformation and a plausible translocation cycle. Journal of Biological Chemistry. 2009;284:20011–20021. doi: 10.1074/jbc.M109.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimmer K.S., Friedrich B., Lang F., Deitmer J.W., Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochemical Journal. 2000;350 Pt 1:219–227. [PMC free article] [PubMed] [Google Scholar]
- 10.Manning Fox J.E., Meredith D., Halestrap A.P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. Journal of Physiology. 2000;529 Pt 2:285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhup S., Dadhich R.K., Porporato P.E., Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Current Pharmaceutical Design. 2012;18:1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 12.Brooks G.A., Brown M.A., Butz C.E., Sicurello J.P., Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. Journal of Applied Physiology. 1999;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. 1985. [DOI] [PubMed] [Google Scholar]
- 13.Butz C.E., McClelland G.B., Brooks G.A. MCT1 confirmed in rat striated muscle mitochondria. Journal of Applied Physiology. 2004;97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto T., Hussien R., Brooks G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. American Journal of Physiology. Endocrinology and Metabolism. 2006;290:E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T., Hussien R., Cho H.S., Kaufer D., Brooks G.A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One. 2008;3:e2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bari L., Valenti D., Atlante A., Passarella S. L-lactate generates hydrogen peroxide in purified rat liver mitochondria due to the putative L-lactate oxidase localized in the intermembrane space. FEBS Letters. 2010;584:2285–2290. doi: 10.1016/j.febslet.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Brooks G.A., Dubouchaud H., Brown M., Sicurello J.P., Butz C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proceedings of the National Academy of Sciences of the U S A. 1999;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland G.B., Khanna S., Gonzalez G.F., Butz C.E., Brooks G.A. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochemical and Biophysical Research Communications. 2003;304:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Rymenant E., Abranko L., Tumova S., Grootaert C., Van Camp J., Williamson G. Chronic exposure to short-chain fatty acids modulates transport and metabolism of microbiome-derived phenolics in human intestinal cells. The Journal of Nutritional Biochemistry. 2017;39:156–168. doi: 10.1016/j.jnutbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley W.C., Gertz E.W., Wisneski J.A., Neese R.A., Morris D.L., Brooks G.A. Lactate extraction during net lactate release in legs of humans during exercise. Journal of Applied Physiology. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. 1985. [DOI] [PubMed] [Google Scholar]
- 21.McCullagh K.J., Poole R.C., Halestrap A.P., O'Brien M., Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. American Journal of Physiology. 1996;271:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- 22.Brooks G.A. Intra- and extra-cellular lactate shuttles. Medicine & Science in Sports & Exercise. 2000;32:790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Garcia C.K., Goldstein J.L., Pathak R.K., Anderson R.G., Brown M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 24.Becker H.M., Mohebbi N., Perna A., Ganapathy V., Capasso G., Wagner C.A. Localization of members of MCT monocarboxylate transporter family Slc16 in the kidney and regulation during metabolic acidosis. American Journal of Physiology - Renal Physiology. 2010;299:F141–F154. doi: 10.1152/ajprenal.00488.2009. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philp N.J., Yoon H., Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. American Journal of Physiology - Cell Physiology. 2001;280:C1319–C1326. doi: 10.1152/ajpcell.2001.280.5.C1319. [DOI] [PubMed] [Google Scholar]
- 27.Juel C. Lactate-proton cotransport in skeletal muscle. Physiological Reviews. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- 28.Brooks G.A. Cell-cell and intracellular lactate shuttles. Journal of Physiology. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellerin L., Pellegri G., Bittar P.G., Charnay Y., Bouras C., Martin J.L. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Developmental Neuroscience. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 30.Funfschilling U., Supplie L.M., Mahad D., Boretius S., Saab A.S., Edgar J. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves V.A., Pinheiro C., Morais-Santos F., Felipe-Silva A., Longatto-Filho A., Baltazar F. Characterization of monocarboxylate transporter activity in hepatocellular carcinoma. World Journal of Gastroenterology. 2014;20:11780–11787. doi: 10.3748/wjg.v20.i33.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinheiro C., Reis R.M., Ricardo S., Longatto-Filho A., Schmitt F., Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. Journal of Biomedicine and Biotechnology. 2010:427694. doi: 10.1155/2010/427694. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinheiro C., Longatto-Filho A., Ferreira L., Pereira S.M., Etlinger D., Moreira M.A. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. International Journal of Gynecological Pathology. 2008;27:568–574. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro C., Longatto-Filho A., Scapulatempo C., Ferreira L., Martins S., Pellerin L. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Archiv. 2008;452:139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 35.Pertega-Gomes N., Vizcaino J.R., Miranda-Goncalves V., Pinheiro C., Silva J., Pereira H. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer. 2011;11:312. doi: 10.1186/1471-2407-11-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro C., Penna V., Morais-Santos F., Abrahao-Machado L.F., Ribeiro G., Curcelli E.C. Characterization of monocarboxylate transporters (MCTs) expression in soft tissue sarcomas: distinct prognostic impact of MCT1 sub-cellular localization. Journal of Translational Medicine. 2014;12:118. doi: 10.1186/1479-5876-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takimoto M., Takeyama M., Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism. 2013;62:1633–1640. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Kitaoka Y., Takahashi Y., Machida M., Takeda K., Takemasa T., Hatta H. Effect of AMPK activation on monocarboxylate transporter (MCT)1 and MCT4 in denervated muscle. The Journal of Physiological Sciences. 2014;64:59–64. doi: 10.1007/s12576-013-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galardo M.N., Riera M.F., Pellizzari E.H., Cigorraga S.B., Meroni S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. Journal of Molecular Endocrinology. 2007;39:279–288. doi: 10.1677/JME-07-0054. [DOI] [PubMed] [Google Scholar]
- 40.Benton C.R., Yoshida Y., Lally J., Han X.X., Hatta H., Bonen A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiological Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T., Hussien R., Oommen S., Gohil K., Brooks G.A. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. The FASEB Journal. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 42.Cuff M.A., Lambert D.W., Shirazi-Beechey S.P. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. Journal of Physiology. 2002;539:361–371. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullah M.S., Davies A.J., Halestrap A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. Journal of Biological Chemistry. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 44.Payen V.L., Brisson L., Dewhirst M.W., Sonveaux P. Common responses of tumors and wounds to hypoxia. Cancer Journal. 2015;21:75–87. doi: 10.1097/PPO.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 45.Le Floch R., Chiche J., Marchiq I., Naiken T., Ilk K., Murray C.M. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proceedings of the National Academy of Sciences of the U S A. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boidot R., Vegran F., Meulle A., Le Breton A., Dessy C., Sonveaux P. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Research. 2012;72:939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 47.Cheng C., Edin N.F., Lauritzen K.H., Aspmodal I., Christoffersen S., Jian L. Alterations of monocarboxylate transporter densities during hypoxia in brain and breast tumour cells. Cellular Oncology (Dordrecht) 2012;35:217–227. doi: 10.1007/s13402-012-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Saedeleer C.J., Porporato P.E., Copetti T., Perez-Escuredo J., Payen V.L., Brisson L. Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene. 2014;33:4060–4068. doi: 10.1038/onc.2013.454. [DOI] [PubMed] [Google Scholar]
- 49.Morais-Santos F., Granja S., Miranda-Goncalves V., Moreira A.H., Queiros S., Vilaca J.L. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget. 2015;6:19177–19189. doi: 10.18632/oncotarget.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miranda-Goncalves V., Granja S., Martinho O., Honavar M., Pojo M., Costa B.M. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget. 2016;7:46335–46353. doi: 10.18632/oncotarget.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keith B., Johnson R.S., Simon M.C. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature Reviews Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doherty J.R., Yang C., Scott K.E., Cameron M.D., Fallahi M., Li W. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Research. 2014;74:908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corbet C., Draoui N., Polet F., Pinto A., Drozak X., Riant O. The SIRT1/HIF2alpha axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Research. 2014;74:5507–5519. doi: 10.1158/0008-5472.CAN-14-0705. [DOI] [PubMed] [Google Scholar]
- 54.Asada K., Miyamoto K., Fukutomi T., Tsuda H., Yagi Y., Wakazono K. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology. 2003;64:380–388. doi: 10.1159/000070297. [DOI] [PubMed] [Google Scholar]
- 55.Pullen T.J., da Silva X.G., Kelsey G., Rutter G.A. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1) Molecular and Cellular Biology. 2011;31:3182–3194. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang D., Zhang Y., Han J., Wang W., Liu Y., Li J. Embryonic stem cell-derived pancreatic endoderm transplant with MCT1-suppressing miR-495 attenuates type II diabetes in mice. Endocrine Journal. 2015;62:907–920. doi: 10.1507/endocrj.EJ15-0186. [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Ren C., Qu X., Wu X., Dong F., Chand Y.K. miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1. European Journal of Neuroscience. 2017;45:249–259. doi: 10.1111/ejn.13485. [DOI] [PubMed] [Google Scholar]
- 58.Chenal J., Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. Journal of Neurochemistry. 2007;102:389–397. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- 59.Chenal J., Pierre K., Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. European Journal of Neuroscience. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- 60.Robinet C., Pellerin L. Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience. 2011;192:155–163. doi: 10.1016/j.neuroscience.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 61.Diers A.R., Broniowska K.A., Chang C.F., Hill R.B., Hogg N. S-nitrosation of monocarboxylate transporter 1: inhibition of pyruvate-fueled respiration and proliferation of breast cancer cells. Free Radical Biology and Medicine. 2014;69:229–238. doi: 10.1016/j.freeradbiomed.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirk P., Wilson M.C., Heddle C., Brown M.H., Barclay A.N., Halestrap A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. The EMBO Journal. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philp N.J., Ochrietor J.D., Rudoy C., Muramatsu T., Linser P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Investigative Ophthalmology & Visual Science. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 64.Wilson M.C., Meredith D., Fox J.E., Manoharan C., Davies A.J., Halestrap A.P. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) Journal of Biological Chemistry. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 65.Ovens M.J., Manoharan C., Wilson M.C., Murray C.M., Halestrap A.P. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochemical Journal. 2010;431:217–225. doi: 10.1042/BJ20100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finch N.A., Linser P.J., Ochrietor J.D. Hydrophobic interactions stabilize the basigin-MCT1 complex. The Protein Journal. 2009;28:362–368. doi: 10.1007/s10930-009-9202-3. [DOI] [PubMed] [Google Scholar]
- 67.Wilson M.C., Meredith D., Halestrap A.P. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. Journal of Biological Chemistry. 2002;277:3666–3672. doi: 10.1074/jbc.M109658200. [DOI] [PubMed] [Google Scholar]
- 68.Becker H.M., Klier M., Schuler C., McKenna R., Deitmer J.W. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proceedings of the National Academy of Sciences of the U S A. 2011;108:3071–3076. doi: 10.1073/pnas.1014293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forero-Quintero L.S., Ames S., Schneider H.P., Thyssen A., Boone C.D., Andring J.T. Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70. Journal of Biological Chemistry. 2019;294:593–607. doi: 10.1074/jbc.RA118.005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallagher S.M., Castorino J.J., Philp N.J. Interaction of monocarboxylate transporter 4 with beta1-integrin and its role in cell migration. American Journal of Physiology - Cell Physiology. 2009;296:C414–C421. doi: 10.1152/ajpcell.00430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izumi H., Takahashi M., Uramoto H., Nakayama Y., Oyama T., Wang K.Y. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Science. 2011;102:1007–1013. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 72.Marchiq I., Le Floch R., Roux D., Simon M.P., Pouyssegur J. Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Research. 2015;75:171–180. doi: 10.1158/0008-5472.CAN-14-2260. [DOI] [PubMed] [Google Scholar]
- 73.Morais-Santos F., Miranda-Goncalves V., Pinheiro S., Vieira A.F., Paredes J., Schmitt F.C. Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocrine-Related Cancer. 2014;21:27–38. doi: 10.1530/ERC-13-0132. [DOI] [PubMed] [Google Scholar]
- 74.Gallagher S.M., Castorino J.J., Wang D., Philp N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Research. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 75.Philp N.J., Wang D., Yoon H., Hjelmeland L.M. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Investigative Ophthalmology & Visual Science. 2003;44:1716–1721. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- 76.Settle P., Mynett K., Speake P., Champion E., Doughty I.M., Sibley C.P. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta. 2004;25:496–504. doi: 10.1016/j.placenta.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Deora A.A., Philp N., Hu J., Bok D., Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proceedings of the National Academy of Sciences of the U S A. 2005;102:16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ke X., Fei F., Chen Y., Xu L., Zhang Z., Huang Q. Hypoxia upregulates CD147 through a combined effect of HIF-1alpha and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis. 2012;33:1598–1607. doi: 10.1093/carcin/bgs196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uhernik A.L., Li L., LaVoy N., Velasquez M.J., Smith J.P. Regulation of monocarboxylic acid transporter-1 by cAMP dependent vesicular trafficking in brain microvascular endothelial cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Z., Wu M.S., Zou C., Tang Q., Lu J., Liu D. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer Letters. 2014;342:150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 81.Gan L., Xiu R., Ren P., Yue M., Su H., Guo G. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene. 2016;35:3037–3048. doi: 10.1038/onc.2015.360. [DOI] [PubMed] [Google Scholar]
- 82.Sprowl-Tanio S., Habowski A.N., Pate K.T., McQuade M.M., Wang K., Edwards R.A. Lactate/pyruvate transporter MCT-1 is a direct Wnt target that confers sensitivity to 3-bromopyruvate in colon cancer. Cancer & Metabolism. 2016;4:20. doi: 10.1186/s40170-016-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C., Wen Z., Xie J., Zhao Y., Zhao L., Zhang S. MACC1 mediates chemotherapy sensitivity of 5-FU and cisplatin via regulating MCT1 expression in gastric cancer. Biochemical and Biophysical Research Communications. 2017;485:665–671. doi: 10.1016/j.bbrc.2017.02.096. [DOI] [PubMed] [Google Scholar]
- 84.Aveseh M., Nikooie R., Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. Journal of Physiology. 2015;593:2635–2648. doi: 10.1113/JP270463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corbet C., Pinto A., Martherus R., Santiago de Jesus J.P., Polet F., Feron O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metabolism. 2016;24:311–323. doi: 10.1016/j.cmet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Cacace A., Sboarina M., Vazeille T., Sonveaux P. Glutamine activates STAT3 to control cancer cell proliferation independently of glutamine metabolism. Oncogene. 2016;36:2074–2084. doi: 10.1038/onc.2016.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roland C.L., Arumugam T., Deng D., Liu S.H., Philip B., Gomez S. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Research. 2014;74:5301–5310. doi: 10.1158/0008-5472.CAN-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Viswanath P., Najac C., Izquierdo-Garcia J.L., Pankov A., Hong C., Eriksson P. Mutant IDH1 expression is associated with down-regulation of monocarboxylate transporters. Oncotarget. 2016;7:34942–34955. doi: 10.18632/oncotarget.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li K.K., Pang J.C., Ching A.K., Wong C.K., Kong X., Wang Y. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Human Pathology. 2009;40:1234–1243. doi: 10.1016/j.humpath.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Romero-Cordoba S.L., Rodriguez-Cuevas S., Bautista-Pina V., Maffuz-Aziz A., D'Ippolito E., Cosentino G. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Scientific Reports. 2018;8:12252. doi: 10.1038/s41598-018-29708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu W., Zhang Z., Zou K., Cheng Y., Yang M., Chen H. MiR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death & Disease. 2017;8:e2761. doi: 10.1038/cddis.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan Q., Yang L., Zhang X., Ma Y., Li Y., Dong L. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/beta-catenin signaling pathway activation in hepatocellular carcinoma cells. Journal of Experimental & Clinical Cancer Research. 2018;37:9. doi: 10.1186/s13046-018-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hao J., Chen H., Madigan M.C., Cozzi P.J., Beretov J., Xiao W. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. British Journal of Cancer. 2010;103:1008–1018. doi: 10.1038/sj.bjc.6605839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slomiany M.G., Grass G.D., Robertson A.D., Yang X.Y., Maria B.L., Beeson C. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Research. 2009;69:1293–1301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonveaux P., Vegran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. Journal of Clinical Investigation. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ho J., de Moura M.B., Lin Y., Vincent G., Thorne S., Duncan L.M. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Molecular Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kennedy K.M., Scarbrough P.M., Ribeiro A., Richardson R., Yuan H., Sonveaux P. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allen E., Mieville P., Warren C.M., Saghafinia S., Li L., Peng M.W. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Reports. 2016;15:1144–1160. doi: 10.1016/j.celrep.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimenez-Valerio G., Martinez-Lozano M., Bassani N., Vidal A., Ochoa-de-Olza M., Suarez C. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Reports. 2016;15:1134–1143. doi: 10.1016/j.celrep.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pisarsky L., Bill R., Fagiani E., Dimeloe S., Goosen R.W., Hagmann J. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Reports. 2016;15:1161–1174. doi: 10.1016/j.celrep.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faubert B., Li K.Y., Cai L., Hensley C.T., Kim J., Zacharias L.G. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Curry J.M., Tuluc M., Whitaker-Menezes D., Ames J.A., Anantharaman A., Butera A. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–1384. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Semenza G.L. Tumor metabolism: cancer cells give and take lactate. Journal of Clinical Investigation. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dovmark T.H., Saccomano M., Hulikova A., Alves F., Swietach P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene. 2017;36:4538–4550. doi: 10.1038/onc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A.K., Frank P.G. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 106.Whitaker-Menezes D., Martinez-Outschoorn U.E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A.K. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fiaschi T., Marini A., Giannoni E., Taddei M.L., Gandellini P., De Donatis A. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Research. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 108.Bisetto S., Whitaker-Menezes D., Wilski N.A., Tuluc M., Curry J., Zhan T. Monocarboxylate transporter 4 (MCT4) knockout mice have attenuated 4NQO induced carcinogenesis; a role for MCT4 in driving oral squamous cell cancer. Frontiers in Oncology. 2018;8:324. doi: 10.3389/fonc.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Hee V.F., Perez-Escuredo J., Cacace A., Copetti T., Sonveaux P. Lactate does not activate NF-kappaB in oxidative tumor cells. Frontiers in Pharmacology. 2015;6:228. doi: 10.3389/fphar.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lemma S., Di Pompo G., Porporato P.E., Sboarina M., Russell S., Gillies R.J. MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: a new rationale for the pathogenesis of osteolytic bone metastases. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2017;1863:3254–3264. doi: 10.1016/j.bbadis.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 111.Brisson L., Banski P., Sboarina M., Dethier C., Danhier P., Fontenille M.J. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell. 2016;30:418–431. doi: 10.1016/j.ccell.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 112.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nature Reviews Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Hee V.F., Labar D., Dehon G., Grasso D., Gregoire V., Muccioli G.G. Radiosynthesis and validation of (+/-)-[18F]-3-fluoro-2-hydroxypropionate ([18F]-FLac) as a PET tracer of lactate to monitor MCT1-dependent lactate uptake in tumors. Oncotarget. 2017;8:24415–24428. doi: 10.18632/oncotarget.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 115.Dietl K., Renner K., Dettmer K., Timischl B., Eberhart K., Dorn C. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. The Journal of Immunology. 2010;184:1200–1209. doi: 10.4049/jimmunol.0902584. [DOI] [PubMed] [Google Scholar]
- 116.Huang C.K., Chang P.H., Kuo W.H., Chen C.L., Jeng Y.M., Chang K.J. Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via beta-hydroxybutyrate. Nature Communications. 2017;8:14706. doi: 10.1038/ncomms14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Saedeleer C.J., Copetti T., Porporato P.E., Verrax J., Feron O., Sonveaux P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu H., Forbes R.A., Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. Journal of Biological Chemistry. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 119.Lu H., Dalgard C.L., Mohyeldin A., McFate T., Tait A.S., Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. Journal of Biological Chemistry. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 120.Vegran F., Boidot R., Michiels C., Sonveaux P., Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Research. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 121.Sonveaux P., Copetti T., De Saedeleer C.J., Vegran F., Verrax J., Kennedy K.M. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hewitson K.S., Lienard B.M., McDonough M.A., Clifton I.J., Butler D., Soares A.S. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. Journal of Biological Chemistry. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 123.Miranda-Goncalves V., Bezerra F., Costa-Almeida R., Freitas-Cunha M., Soares R., Martinho O. Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Molecular Carcinogenesis. 2017;56:2630–2642. doi: 10.1002/mc.22707. [DOI] [PubMed] [Google Scholar]
- 124.Cross M.J., Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends in Pharmacological Sciences. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 125.Cummins E.P., Berra E., Comerford K.M., Ginouves A., Fitzgerald K.T., Seeballuck F. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proceedings of the National Academy of Sciences of the U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee D.C., Sohn H.A., Park Z.Y., Oh S., Kang Y.K., Lee K.M. A lactate-induced response to hypoxia. Cell. 2015;161:595–609. doi: 10.1016/j.cell.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 127.Constant J.S., Feng J.J., Zabel D.D., Yuan H., Suh D.Y., Scheuenstuhl H. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair and Regeneration. 2000;8:353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 128.Zabel D.D., Feng J.J., Scheuenstuhl H., Hunt T.K., Hussain M.Z. Lactate stimulation of macrophage-derived angiogenic activity is associated with inhibition of poly(ADP-ribose) synthesis. Laboratory Investigation. 1996;74:644–649. [PubMed] [Google Scholar]
- 129.Xiong M., Elson G., Legarda D., Leibovich S.J. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. American Journal Of Pathology. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar V.B., Viji R.I., Kiran M.S., Sudhakaran P.R. Endothelial cell response to lactate: implication of PAR modification of VEGF. Journal of Cellular Physiology. 2007;211:477–485. doi: 10.1002/jcp.20955. [DOI] [PubMed] [Google Scholar]
- 131.Murray B., Wilson D.J. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis. 2001;4:71–77. doi: 10.1023/a:1016792319207. [DOI] [PubMed] [Google Scholar]
- 132.Beckert S., Farrahi F., Aslam R.S., Scheuenstuhl H., Konigsrainer A., Hussain M.Z. Lactate stimulates endothelial cell migration. Wound Repair and Regeneration. 2006;14:321–324. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 133.Burns P.A., Wilson D.J. Angiogenesis mediated by metabolites is dependent on vascular endothelial growth factor (VEGF) Angiogenesis. 2003;6:73–77. doi: 10.1023/a:1025862731653. [DOI] [PubMed] [Google Scholar]
- 134.Porporato P.E., Payen V.L., De Saedeleer C.J., Preat V., Thissen J.P., Feron O. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15:581–592. doi: 10.1007/s10456-012-9282-0. [DOI] [PubMed] [Google Scholar]
- 135.Perez-Escuredo J., Dadhich R.K., Dhup S., Cacace A., Van Hee V.F., De Saedeleer C.J. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle. 2016;15:72–83. doi: 10.1080/15384101.2015.1120930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duran R.V., Oppliger W., Robitaille A.M., Heiserich L., Skendaj R., Gottlieb E. Glutaminolysis activates Rag-mTORC1 signaling. Molecular Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 137.Latham T., Mackay L., Sproul D., Karim M., Culley J., Harrison D.J. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Research. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wagner W., Ciszewski W.M., Kania K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Communication and Signaling. 2015;13:36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. Journal of Biological Chemistry. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 140.Cai T.Q., Ren N., Jin L., Cheng K., Kash S., Chen R. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochemical and Biophysical Research Communications. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 141.Staubert C., Broom O.J., Nordstrom A. Hydroxycarboxylic acid receptors are essential for breast cancer cells to control their lipid/fatty acid metabolism. Oncotarget. 2015;6:19706–19720. doi: 10.18632/oncotarget.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee Y.J., Shin K.J., Park S.A., Park K.S., Park S., Heo K. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget. 2016;7:70898–70911. doi: 10.18632/oncotarget.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murray C.M., Hutchinson R., Bantick J.R., Belfield G.P., Benjamin A.D., Brazma D. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nature Chemical Biology. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 144.Schroeder T., Yuan H., Viglianti B.L., Peltz C., Asopa S., Vujaskovic Z. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Research. 2005;65:5163–5171. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- 145.Bueno V., Binet I., Steger U., Bundick R., Ferguson D., Murray C. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 146.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metabolism. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 147.Ekberg H., Qi Z., Pahlman C., Veress B., Bundick R.V., Craggs R.I. The specific monocarboxylate transporter-1 (MCT-1) inhibitor, AR-C117977, induces donor-specific suppression, reducing acute and chronic allograft rejection in the rat. Transplantation. 2007;84:1191–1199. doi: 10.1097/01.tp.0000287541.53389.be. [DOI] [PubMed] [Google Scholar]
- 148.Puig-Kroger A., Pello O.M., Selgas R., Criado G., Bajo M.A., Sanchez-Tomero J.A. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. Journal of Leukocyte Biology. 2003;73:482–492. doi: 10.1189/jlb.0902451. [DOI] [PubMed] [Google Scholar]
- 149.Husain Z., Huang Y., Seth P., Sukhatme V.P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. The Journal of Immunology. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 150.Pinheiro C., Longatto-Filho A., Pereira S.M., Etlinger D., Moreira M.A., Jube L.F. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Disease Markers. 2009;26:97–103. doi: 10.3233/DMA-2009-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huhta H., Helminen O., Palomaki S., Kauppila J.H., Saarnio J., Lehenkari P.P. Intratumoral lactate metabolism in Barrett's esophagus and adenocarcinoma. Oncotarget. 2017;8:22894–22902. doi: 10.18632/oncotarget.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pinheiro C., Granja S., Longatto-Filho A., Faria A.M., Fragoso M.C., Lovisolo S.M. Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget. 2015;6:44403–44421. doi: 10.18632/oncotarget.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pinheiro C., Longatto-Filho A., Simoes K., Jacob C.E., Bresciani C.J., Zilberstein B. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. European Journal of Cancer. 2009;45:2418–2424. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 154.Zhang G., Zhang Y., Dong D., Wang F., Ma X., Guan F. MCT1 regulates aggressive and metabolic phenotypes in bladder cancer. Journal of Cancer. 2018;9:2492–2501. doi: 10.7150/jca.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee J.Y., Lee I., Chang W.J., Ahn S.M., Lim S.H., Kim H.S. MCT4 as a potential therapeutic target for metastatic gastric cancer with peritoneal carcinomatosis. Oncotarget. 2016;7:43492–43503. doi: 10.18632/oncotarget.9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakayama Y., Torigoe T., Inoue Y., Minagawa N., Izumi H., Kohno K. Prognostic significance of monocarboxylate transporter 4 expression in patients with colorectal cancer. Experimental and Therapeutic Medicine. 2012;3:25–30. doi: 10.3892/etm.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martins S.F., Amorim R., Viana-Pereira M., Pinheiro C., Costa R.F., Silva P. Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer. 2016;16:535. doi: 10.1186/s12885-016-2566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee G.H., Kim D.S., Chung M.J., Chae S.W., Kim H.R., Chae H.J. Lysyl oxidase-like-1 enhances lung metastasis when lactate accumulation and monocarboxylate transporter expression are involved. Oncology Letters. 2011;2:831–838. doi: 10.3892/ol.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pinheiro C., Miranda-Goncalves V., Longatto-Filho A., Vicente A.L., Berardinelli G.N., Scapulatempo-Neto C. The metabolic microenvironment of melanomas: prognostic value of MCT1 and MCT4. Cell Cycle. 2016;15:1462–1470. doi: 10.1080/15384101.2016.1175258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miranda-Goncalves V., Honavar M., Pinheiro C., Martinho O., Pires M.M., Pinheiro C. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro-Oncology. 2013;15:172–188. doi: 10.1093/neuonc/nos298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Payen V.L., Hsu M.Y., Radecke K.S., Wyart E., Vazeille T., Bouzin C. Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Research. 2017;77:5591–5601. doi: 10.1158/0008-5472.CAN-17-0764. [DOI] [PubMed] [Google Scholar]
- 162.Zhu J., Wu Y.N., Zhang W., Zhang X.M., Ding X., Li H.Q. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kong S.C., Nohr-Nielsen A., Zeeberg K., Reshkin S.J., Hoffmann E.K., Novak I. Monocarboxylate transporters MCT1 and MCT4 regulate migration and invasion of pancreatic ductal adenocarcinoma cells. Pancreas. 2016;45:1036–1047. doi: 10.1097/MPA.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 164.Silva E.C.A., Carcano F.M., Bonatelli M., Zaia M.G., Morais-Santos F., Baltazar F. The clinicopathological significance of monocarboxylate transporters in testicular germ cell tumors. Oncotarget. 2018;9:20386–20398. doi: 10.18632/oncotarget.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwab A., Fabian A., Hanley P.J., Stock C. Role of ion channels and transporters in cell migration. Physiological Reviews. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 166.Kumar D., Vetrivel U., Parameswaran S., Subramanian K.K. Structural insights on druggable hotspots in CD147: a bull's eye view. Life Sciences. 2019;224:76–87. doi: 10.1016/j.lfs.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 167.Bourguignon L.Y., Singleton P.A., Diedrich F., Stern R., Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. Journal of Biological Chemistry. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 168.Guile S.D., Bantick J.R., Cooper M.E., Donald D.K., Eyssade C., Ingall A.H. Optimization of monocarboxylate transporter 1 blockers through analysis and modulation of atropisomer interconversion properties. Journal of Medicinal Chemistry. 2007;50:254–263. doi: 10.1021/jm060995h. [DOI] [PubMed] [Google Scholar]
- 169.Ovens M.J., Davies A.J., Wilson M.C., Murray C.M., Halestrap A.P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochemical Journal. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Critchlow S.E., Hopcroft L., Mooney L., Curtis N., Whalley N., Zhong H. Abstract 3224: pre-clinical targeting of the metabolic phenotype of lymphoma by AZD3965, a selective inhibitor of monocarboxylate transporter 1 (MCT1) Cancer Research. 2012;72:3224. [Google Scholar]
- 171.Nancolas B., Sessions R.B., Halestrap A.P. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochemical Journal. 2015;466:177–188. doi: 10.1042/BJ20141223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Curtis N.J., Mooney L., Hopcroft L., Michopoulos F., Whalley N., Zhong H. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt's lymphoma anti-tumor activity. Oncotarget. 2017;8:69219–69236. doi: 10.18632/oncotarget.18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quanz M., Bender E., Kopitz C., Grunewald S., Schlicker A., Schwede W. Preclinical efficacy of the novel monocarboxylate transporter 1 inhibitor BAY-8002 and associated markers of resistance. Molecular Cancer Therapeutics. 2018;17:2285–2296. doi: 10.1158/1535-7163.MCT-17-1253. [DOI] [PubMed] [Google Scholar]
- 174.Corbet C., Ragelle H., Pourcelle V., Vanvarenberg K., Marchand-Brynaert J., Preat V. Delivery of siRNA targeting tumor metabolism using non-covalent PEGylated chitosan nanoparticles: identification of an optimal combination of ligand structure, linker and grafting method. Journal of Controlled Release. 2016;223:53–63. doi: 10.1016/j.jconrel.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 175.Sanita P., Capulli M., Teti A., Galatioto G.P., Vicentini C., Chiarugi P. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer. 2014;14:154. doi: 10.1186/1471-2407-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Koukourakis M.I., Giatromanolaki A., Harris A.L., Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Research. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 177.Koukourakis M.I., Giatromanolaki A., Bougioukas G., Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biology & Therapy. 2007;6:1476–1479. doi: 10.4161/cbt.6.9.4635. [DOI] [PubMed] [Google Scholar]
- 178.Koukourakis M.I., Kalamida D., Mitrakas A.G., Liousia M., Pouliliou S., Sivridis E. Metabolic cooperation between co-cultured lung cancer cells and lung fibroblasts. Laboratory Investigation. 2017;97:1321–1331. doi: 10.1038/labinvest.2017.79. [DOI] [PubMed] [Google Scholar]
- 179.Hulikova A., Black N., Hsia L.T., Wilding J., Bodmer W.F., Swietach P. Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proceedings of the National Academy of Sciences of the U S A. 2016;113:E5344–E5353. doi: 10.1073/pnas.1610954113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bola B.M., Chadwick A.L., Michopoulos F., Blount K.G., Telfer B.A., Williams K.J. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Molecular Cancer Therapeutics. 2014;13:2805–2816. doi: 10.1158/1535-7163.MCT-13-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Birsoy K., Wang T., Possemato R., Yilmaz O.H., Koch C.E., Chen W.W. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nature Genetics. 2013;45:104–108. doi: 10.1038/ng.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen H., Wang L., Beretov J., Hao J., Xiao W., Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clinical & Experimental Metastasis. 2010;27:557–569. doi: 10.1007/s10585-010-9345-9. [DOI] [PubMed] [Google Scholar]
- 183.Follman K.E., Morris M.E. Treatment of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) overdose with two potent monocarboxylate transporter 1 (MCT1) inhibitors, AZD3965 and AR-C155858. Journal of Pharmacology and Experimental Therapeutics. 2019 doi: 10.1124/jpet.119.256503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wahl M.L., Owen J.A., Burd R., Herlands R.A., Nogami S.S., Rodeck U. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Molecular Cancer Therapeutics. 2002;1:617–628. [PubMed] [Google Scholar]
- 185.Chiche J., Le Fur Y., Vilmen C., Frassineti F., Daniel L., Halestrap A.P. In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. International Journal of Cancer. 2012;130:1511–1520. doi: 10.1002/ijc.26125. [DOI] [PubMed] [Google Scholar]
- 186.Ben-Horin H., Tassini M., Vivi A., Navon G., Kaplan O. Mechanism of action of the antineoplastic drug lonidamine: 31P and 13C nuclear magnetic resonance studies. Cancer Research. 1995;55:2814–2821. [PubMed] [Google Scholar]
- 187.Ben-Yoseph O., Lyons J.C., Song C.W., Ross B.D. Mechanism of action of lonidamine in the 9L brain tumor model involves inhibition of lactate efflux and intracellular acidification. Journal of Neuro-Oncology. 1998;36:149–157. doi: 10.1023/a:1005819604858. [DOI] [PubMed] [Google Scholar]
- 188.Mathupala S.P., Parajuli P., Sloan A.E. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. [DOI] [PubMed] [Google Scholar]
- 189.Colen C.B., Seraji-Bozorgzad N., Marples B., Galloway M.P., Sloan A.E., Mathupala S.P. Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study. Neurosurgery. 2006;59:1313–1323. doi: 10.1227/01.NEU.0000249218.65332.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fang J., Quinones Q.J., Holman T.L., Morowitz M.J., Wang Q., Zhao H. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Molecular Pharmacology. 2006;70:2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 191.Colen C.B., Shen Y., Ghoddoussi F., Yu P., Francis T.B., Koch B.J. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13:620–632. doi: 10.1593/neo.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee I., Lee S.J., Kang W.K., Park C. Inhibition of monocarboxylate transporter 2 induces senescence-associated mitochondrial dysfunction and suppresses progression of colorectal malignancies in vivo. Molecular Cancer Therapeutics. 2012;11:2342–2351. doi: 10.1158/1535-7163.MCT-12-0488. [DOI] [PubMed] [Google Scholar]
- 193.Polanski R., Hodgkinson C.L., Fusi A., Nonaka D., Priest L., Kelly P. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clinical Cancer Research. 2014;20:926–937. doi: 10.1158/1078-0432.CCR-13-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Amorim R., Pinheiro C., Miranda-Goncalves V., Pereira H., Moyer M.P., Preto A. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Letters. 2015;365:68–78. doi: 10.1016/j.canlet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 195.Hanson D.J., Nakamura S., Amachi R., Hiasa M., Oda A., Tsuji D. Effective impairment of myeloma cells and their progenitors by blockade of monocarboxylate transportation. Oncotarget. 2015;6:33568–33586. doi: 10.18632/oncotarget.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hong C.S., Graham N.A., Gu W., Espindola C.C., Mah V., Maresh E.L. MCT1 modulates cancer cell pyruvate export and growth of tumors that co-express MCT1 and MCT4. Cell Reports. 2016;14:1590–1601. doi: 10.1016/j.celrep.2016.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Beloueche-Babari M., Wantuch S., Casals G.T., Koniordou M., Parkes H.G., Arunan V. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Research. 2017;77:5913–5924. doi: 10.1158/0008-5472.CAN-16-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Noble R.A., Bell N., Blair H., Sikka A., Thomas H., Phillips N. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2017;102:1247–1257. doi: 10.3324/haematol.2016.163030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baba M., Inoue M., Itoh K., Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochemical and Biophysical Research Communications. 2008;374:111–116. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 200.Schneiderhan W., Scheler M., Holzmann K.H., Marx M., Gschwend J.E., Bucholz M. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58:1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 201.Su J., Chen X., Kanekura T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Letters. 2009;273:140–147. doi: 10.1016/j.canlet.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 202.Benjamin D., Robay D., Hindupur S.K., Pohlmann J., Colombi M., El-Shemerly M.Y. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Reports. 2018;25:3047–3058. doi: 10.1016/j.celrep.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lim K.S., Lim K.J., Price A.C., Orr B.A., Eberhart C.G., Bar E.E. Inhibition of monocarboxylate transporter-4 depletes stem-like glioblastoma cells and inhibits HIF transcriptional response in a lactate-independent manner. Oncogene. 2014;33:4433–4441. doi: 10.1038/onc.2013.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gray A.L., Coleman D.T., Shi R., Cardelli J.A. Monocarboxylate transporter 1 contributes to growth factor-induced tumor cell migration independent of transporter activity. Oncotarget. 2016;7:32695–32706. doi: 10.18632/oncotarget.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Morrison B.M., Tsingalia A., Vidensky S., Lee Y., Jin L., Farah M.H. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Experimental Neurology. 2015;263:325–338. doi: 10.1016/j.expneurol.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Golubczyk D., Malysz-Cymborska I., Kalkowski L., Janowski M., Coates J.R., Wojtkiewicz J. The role of glia in canine degenerative myelopathy: relevance to human amyotrophic lateral sclerosis. Molecular Neurobiology. 2019;56:5740–5748. doi: 10.1007/s12035-019-1488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang M., Cheng X., Dang R., Zhang W., Zhang J., Yao Z. Lactate deficit in an Alzheimer disease mouse model: the relationship with neuronal damage. Journal of Neuropathology & Experimental Neurology. 2018;77:1163–1176. doi: 10.1093/jnen/nly102. [DOI] [PubMed] [Google Scholar]
- 209.Lengacher S., Nehiri-Sitayeb T., Steiner N., Carneiro L., Favrod C., Preitner F. Resistance to diet-induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS One. 2013;8:e82505. doi: 10.1371/journal.pone.0082505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lu W., Huang J., Sun S., Huang S., Gan S., Xu J. Changes in lactate content and monocarboxylate transporter 2 expression in abeta(2)(5)(-)(3)(5)-treated rat model of Alzheimer's disease. Neurological Sciences. 2015;36:871–876. doi: 10.1007/s10072-015-2087-3. [DOI] [PubMed] [Google Scholar]
- 211.Daniele L.L., Sauer B., Gallagher S.M., Pugh E.N., Jr., Philp N.J. Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. American Journal of Physiology - Cell Physiology. 2008;295:C451–C457. doi: 10.1152/ajpcell.00124.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cupeiro R., Benito P.J., Maffulli N., Calderon F.J., Gonzalez-Lamuno D. MCT1 genetic polymorphism influence in high intensity circuit training: a pilot study. Journal of Science and Medicine in Sport. 2010;13:526–530. doi: 10.1016/j.jsams.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 213.Gonzalez-Haro C., Soria M., Vicente J., Fanlo A.J., Sinues B., Escanero J.F. Variants of the solute carrier SLC16A1 gene (MCT1) associated with metabolic responses during a long-graded test in road cyclists. The Journal of Strength & Conditioning Research. 2015;29:3494–3505. doi: 10.1519/JSC.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 214.Onali F., Calo C.M., Massidda M., Alvarez-Alvarez M.M., Esteban M.E. An unexpected world population variation of MCT1 polymorphism 1470T > A involved in lactate transport. European Journal of Sport Science. 2018;18:1376–1382. doi: 10.1080/17461391.2018.1491629. [DOI] [PubMed] [Google Scholar]
- 215.Fishbein W.N. Lactate transporter defect: a new disease of muscle. Science. 1986;234:1254–1256. doi: 10.1126/science.3775384. [DOI] [PubMed] [Google Scholar]
- 216.Merezhinskaya N., Fishbein W.N., Davis J.I., Foellmer J.W. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle & Nerve. 2000;23:90–97. doi: 10.1002/(sici)1097-4598(200001)23:1<90::aid-mus12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 217.van Hasselt P.M., Ferdinandusse S., Monroe G.R., Ruiter J.P., Turkenburg M., Geerlings M.J. Monocarboxylate transporter 1 deficiency and ketone utilization. New England Journal of Medicine. 2014;371:1900–1907. doi: 10.1056/NEJMoa1407778. [DOI] [PubMed] [Google Scholar]
- 218.Mykkanen A.K., Koho N.M., Reeben M., McGowan C.M., Poso A.R. MCT1, MCT4 and CD147 gene polymorphisms in healthy horses and horses with myopathy. Research in Veterinary Science. 2011;91:473–477. doi: 10.1016/j.rvsc.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 219.Spugnini E.P., Sonveaux P., Stock C., Perez-Sayans M., De Milito A., Avnet S. Proton channels and exchangers in cancer. Biochimica et Biophysica Acta. 2015;1818:2715–2726. doi: 10.1016/j.bbamem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 220.Choi J.W., Kim Y., Lee J.H., Kim Y.S. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. 2014;84 doi: 10.1016/j.urology.2014.03.031. 245.e9-15. [DOI] [PubMed] [Google Scholar]
- 221.Afonso J., Santos L.L., Miranda-Goncalves V., Morais A., Amaro T., Longatto-Filho A. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Molecular Carcinogenesis. 2015;54:1451–1466. doi: 10.1002/mc.22222. [DOI] [PubMed] [Google Scholar]
- 222.Froberg M.K., Gerhart D.Z., Enerson B.E., Manivel C., Guzman-Paz M., Seacotte N. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. NeuroReport. 2001;12:761–765. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- 223.Pinheiro C., Albergaria A., Paredes J., Sousa B., Dufloth R., Vieira D. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 224.Doyen J., Trastour C., Ettore F., Peyrottes I., Toussant N., Gal J. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochemical and Biophysical Research Communications. 2014;451:54–61. doi: 10.1016/j.bbrc.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 225.Johnson J.M., Cotzia P., Fratamico R., Mikkilineni L., Chen J., Colombo D. MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Frontiers in Cell and Developmental Biology. 2017;5:27. doi: 10.3389/fcell.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim H.K., Lee I., Bang H., Kim H.C., Lee W.Y., Yun S.H. MCT4 expression is a potential therapeutic target in colorectal Cancer with peritoneal carcinomatosis. Molecular Cancer Therapeutics. 2018;17:838–848. doi: 10.1158/1535-7163.MCT-17-0535. [DOI] [PubMed] [Google Scholar]
- 227.Kim Y., Choi J.W., Lee J.H., Kim Y.S. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and the Cancer Genome Atlas data analyses. Human Pathology. 2015;46:104–112. doi: 10.1016/j.humpath.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 228.Sweeny L., Dean N.R., Frederick J.W., Magnuson J.S., Carroll W.R., Desmond R.A. CD147 expression in advanced cutaneous squamous cell carcinoma. Journal of Cutaneous Pathology. 2012;39:603–609. doi: 10.1111/j.1600-0560.2012.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lambert D.W., Wood I.S., Ellis A., Shirazi-Beechey S.P. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. British Journal of Cancer. 2002;86:1262–1269. doi: 10.1038/sj.bjc.6600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cao Y.W., Liu Y., Dong Z., Guo L., Kang E.H., Wang Y.H. Monocarboxylate transporters MCT1 and MCT4 are independent prognostic biomarkers for the survival of patients with clear cell renal cell carcinoma and those receiving therapy targeting angiogenesis. Urologic Oncology. 2018;36:311. doi: 10.1016/j.urolonc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 231.Abe Y., Nakayama Y., Katsuki T., Inoue Y., Minagawa N., Torigoe T. The prognostic significance of the expression of monocarboxylate transporter 4 in patients with right- or left-sided colorectal cancer. Asia-Pacific Journal of Clinical Oncology. 2019;15:e49–e55. doi: 10.1111/ajco.13077. [DOI] [PubMed] [Google Scholar]
- 232.Curry J., Tassone P., Gill K., Tuluc M., BarAd V., Mollaee M. Tumor metabolism in the microenvironment of nodal metastasis in oral squamous cell carcinoma. Otolaryngology - Head and Neck Surgery. 2017;157:798–807. doi: 10.1177/0194599817709224. [DOI] [PubMed] [Google Scholar]
- 233.Broer S., Broer A., Schneider H.P., Stegen C., Halestrap A.P., Deitmer J.W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochemical Journal. 1999;341(Pt 3):529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Halestrap A.P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 235.Kobayashi M., Otsuka Y., Itagaki S., Hirano T., Iseki K. Inhibitory effects of statins on human monocarboxylate transporter 4. International Journal of Pharmaceutics. 2006;317:19–25. doi: 10.1016/j.ijpharm.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 236.Nancolas B., Guo L., Zhou R., Nath K., Nelson D.S., Leeper D.B. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochemical Journal. 2016;473:929–936. doi: 10.1042/BJ20151120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.