Abstract
Background
The TP53 gene is one of the most commonly inactivated tumor suppressors in human cancers. p53 functions during cancer progression have been linked to a variety of transcriptional and non-transcriptional activities that lead to the tight control of cell proliferation, senescence, DNA repair, and cell death. However, converging evidence indicates that p53 also plays a major role in metabolism in both normal and cancer cells.
Scope of review
We provide an overview of the current knowledge on the metabolic activities of wild type (WT) p53 and highlight some of the mechanisms by which p53 contributes to whole body energy homeostasis. We will also pinpoint some evidences suggesting that deregulation of p53-associated metabolic activities leads to human pathologies beyond cancer, including obesity, diabetes, liver, and cardiovascular diseases.
Major conclusions
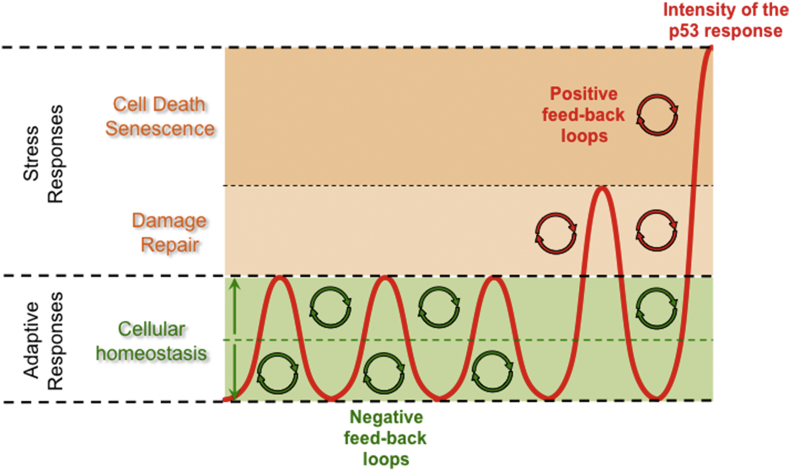
p53 is activated when cells are metabolically challenged but the origin, duration, and intensity of these stresses will dictate the outcome of the p53 response. p53 plays pivotal roles both upstream and downstream of several key metabolic regulators and is involved in multiple feedback-loops that ensure proper cellular homeostasis. The physiological roles of p53 in metabolism involve complex mechanisms of regulation implicating both cell autonomous effects as well as autocrine loops. However, the mechanisms by which p53 coordinates metabolism at the organismal level remain poorly understood. Perturbations of p53-regulated metabolic activities contribute to various metabolic disorders and are pivotal during cancer progression.
Keywords: p53, Metabolism, Normal tissue homeostasis, Cancer
Highlights
-
•
The tumor suppressor p53 is a key metabolic regulator.
-
•
p53-mediated control of metabolism is important in normal and cancer cells.
-
•
Deregulation of p53 metabolic functions contributes to cancer development and to metabolic disorders.
1. Introduction
p53 is a major tumor suppressor as highlighted by the high prevalence of somatic mutations in TP53 in many cancer types, and the strong predisposition to multiple early-onset cancers in Li-Fraumeni (LFS) patients that carry germline mutations of TP53. Approximately 50% of human tumors harbor TP53 mutations while the remaining malignancies expressing WT p53 display functional inactivation of the p53 pathway by alternative mechanisms implicating viral oncoproteins or regulators of p53 such as MDM2 or MDM4. p53 acts mainly as a transcription factor that is activated in response to multiple stressors to regulate the expression of genes controlling proliferation and senescence, DNA repair, and cell death [1]. Several laboratories have also highlighted a major role of p53 in metabolism and showed that p53-associated metabolic functions contribute to its tumor suppressive activities [2,3]. Of note, most tumor-associated p53 mutants are missense, encoding a stable form of the protein devoid of its WT transcriptional activities but endowed with novel gain-of-function activities that are not fully understood. Interestingly, some p53 mutants acquire metabolic functions that contribute to their pro-tumoral activities [[4], [5], [6], [7], [8]]. An additional notion emerging from recent studies relates to the metabolic activities of other key regulators of the p53 pathway but it is currently unclear how the different components of the p53 pathway orchestrate their metabolic activities and by which mechanisms the deregulation of this complex metabolic network contributes to tumorigenesis.
Although the metabolic program that is controlled by p53 at the transcriptional level is becoming clearer, many questions remain unanswered, such as those related to the molecular mechanisms by which metabolic changes signal to p53 and to how p53 controls different subsets of its target genes during metabolic challenges. By integrating multiple input signals that reflect the metabolic status of a cell, p53 behaves as a metabolic sensor and in turn coordinates an adapted metabolic response. The outcome of the p53-driven metabolic response is influenced by the cellular and tissular contexts but the mechanisms underlying the diversity of the metabolic responses regulated by p53 remain poorly understood. Hence, the p53 pathway and metabolism are functionally intertwined, and this has major significance not only to cancer progression but also to normal tissue homeostasis and human diseases beyond cancer.
2. P53 controls multiple metabolic pathways
Experimental evidence of the multiple roles of p53 in metabolism and their importance in normal tissue function and tumor progression is growing at an impressive rate. While some of these metabolic activities contribute to stress responses during which p53 ultimately leads to cell demise, they are also essential for maintaining cellular homeostasis and guaranteeing cell survival under conditions of non-genotoxic stresses. p53 was initially linked to the control of glycolysis and mitochondrial respiration, but more recent data have highlighted the high connectivity of p53 with multiple metabolic pathways. In this section, we summarize the current knowledge of the complex roles of WT-p53 in metabolism both in normal and cancer cells. We refer the reader to other recent reviews for the description of the metabolic roles of various p53 mutants [[4], [5], [6], [7], [8]] (See Figure 1 and Table 1).
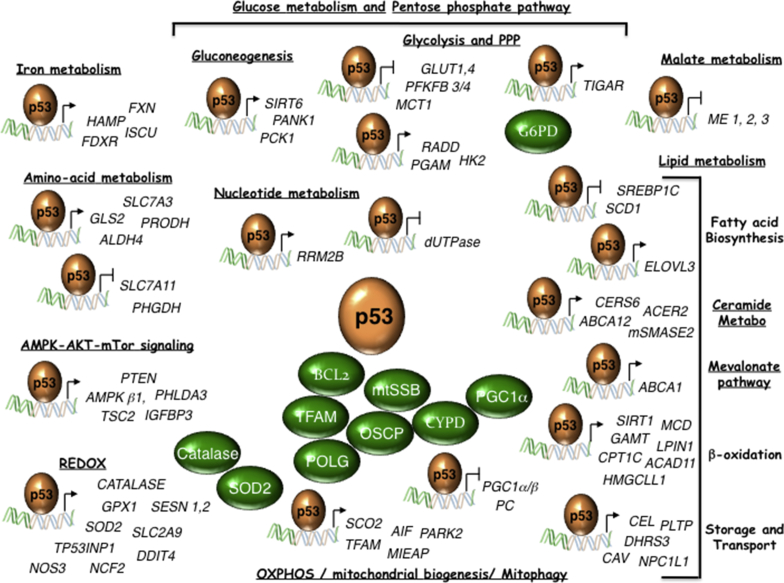
Figure 1.
WT-p53 controls multiple metabolic pathways. p53 direct target genes, and p53-interacting proteins (in green) implicated in metabolism are indicated. ABCA1 (ATP binding cassette subfamily A member 1); ABCA12 (ATP binding cassette subfamily A member 12); ACAD11 (AcylCoA Dehydrogenase Family member 11); ACER2 (Alkaline ceramidase 2); AIF (Apoptosis Inducing Factor); ALDH4 (Aldehyde dehydrogenase 4); AMPKb1 (AMP-activated kinase b1 subunit); BCL2 (B-cell lymphoma 2); Catalase; CAV (Caveolin); CEL (Carboxy Ester Lipase); CERS6 (Ceramide synthetase 6); CPT1C (Carnitine Palmitoyl transferase 1C); CYPD (Cyclophilin D); DDIT4 (DNA Damage inducible transcript 4); DHRS3 (Dehydrogenase reductase 3); dUTPase (deoxyuridine triphosphate nucleotidohydrolase), ELOVL3 (Elongation of very long chain fatty acids-like 3); FDXR (Ferredoxin Reductase); FXN (Frataxin); GAMT (Guanidinoacetate methyltransferase); GLS2 (Glutaminase 2); GLUT1 (Glucose transporter 1); GLUT4 (Glucose transporter 4); GPX1 (Glutathione Peroxidase 1); HAMP (Hepcidin); HK2 (Hexokinase II); HMGCLL1 (3-hydroxymethyl-3-methylglutaryl-CoA lyase like 1); mtSSB (Single Stranded DNA Binding protein 1); IGFBP3 (IGF-Binding protein 3); ISCU (Iron-sulfur cluster assembly enzyme); LPIN1 (Lipin 1); MCD (Malonyl-CoA Decarboxylase); MCT1 (Mono-Carboxylate transporter 1); ME1 (Malic Enzyme 1); ME2 (Malic Enzyme 2); ME3 (Malic Enzyme 3); MIEAP (Mitochondria-eating protein); mSMASE2 (Neutral sphingomyelinase); NCF2 (Neutrophilic cytosolic factor 2); NPC1L1 (Nieman-Pick C1-like 1); NOS3 (Nitric Oxide Synthase 3); OSCP (Oligomycin sensitivity-conferring protein); PANK1 (Panthotenate Kinase 1); PARK2 (Parkin); PC (Pyruvate carboxylase); PCK1 (Phosphoenolpyruvate carboxykinase 1); PFKFB4 (6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4); PFKFB3 (6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3); PGC1α (Peroxisome proliferator-activated receptor Gamma, Coactivator 1 alpha); PGC1β (Peroxisome proliferator-activated receptor Gamma, Coactivator 1 beta); PGAM (Muscle specific phosphoglycerate mutase); PHGDH (Phosphoglycerate Dehydrogenase); PHLDA3 (Pleckstrin homology-like domain, Family A, member 3); PLTP (Phospholipid Transfer Protein); POLG (Polymerase γ); PRODH (Proline Dehydrogenase); PTEN (Phosphatase and tensin homolog); RRAD (Ras-related associated with Diabetes); RRM2B (Ribonucleotide reductase regulatory subunit M2B); SCD1 (Stearoyl-CoA desaturase 1); SCO2 (Synthesis of cytochrome c oxidase 2); SESN1 (Sestrin 1); SESN2 (Sestrin 2); SIRT1 (Sirtuin 1); SIRT6 (Sirtuin 6); SLC2A9 (Solute carrier family 2 member 9); SLC7A3 (Solute carrier family 7 member 3); SLC7A11 (Solute carrier family 7 member 11); SOD2 (Superoxide Dismutase 2); SREBP1C (Sterol regulatory element binding transcription factor 1); TFAM (Mitochondrial Transcription Factor A); TIGAR (TP53-induced glycolysis and apoptosis regulator); TP53INP1 (Tumor Protein 53-Induced Nuclear Protein 1); TSC2 (TSC Complex subunit 2).
Table 1.
P53 direct target genes implicated in metabolism. This table references p53-controlled genes for which experimental evidence supports a direct role of p53 in their transcription.
| Activated/Repressed by p53 | Experimental evidences | Bibliography | ||
|---|---|---|---|---|
| Nucleotide Synthesis | ||||
| dUTPase | Deoxyuridine triphosphate nucleotidohydrolase | repressed | reporter assays, ChIP | [106] |
| RRM2B/p53R2 | Ribonucleotide reductase regulatory/TP53 inducible subunit M2B | activated | ChIP-seq | [107,108,251] |
| Glucose Metabolism | ||||
| Glycolysis and Pentose Phosphate Pathway | ||||
| GLUT1 | Glucose transporter 1 | repressed | reporter assays, EMSA | [9] |
| GLUT4 | Glucose transporter 4 | repressed | reporter assays, EMSA | [9] |
| RRAD | Ras-related associated with Diabetes | repressed | ChIP | [13] |
| MCT1/SLC16A1 | Mono-Carboxylate transporter 1 | repressed | ChIP | [11] |
| M-PGAM | Muscle specific phosphoglycerate mutase | activated | reporter assays, EMSA | [249] |
| HK2 | Hexokinase 2 | activated | reporter assays, EMSA, DNAse I Footprint | [250] |
| TIGAR | TP53-induced glycolysis and apoptosis regulator | activated | ChIP, EMSA, ChIP-seq | [12] |
| PFKFB4 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | repressed | ChIP | [14] |
| PFKFB3 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | repressed | reporter assays, ChIP | [15] |
| Gluconeogenesis | ||||
| PANK1 | Panthotenate Kinase 1 | activated | ChIP, ChIP-on-ChIP, ChIP-seq, EMSA, reporter assays | [210] |
| PCK1 | Phosphoenolpyruvate carboxylase 1 | activated | ChIP | [235] |
| SIRT6 | Sirtuin 6 | activated | ChIP | [209] |
| Lipid metabolism | ||||
| Fatty Acid Oxidation | ||||
| MCD | Malonyl-CoA Decarboxylase | activated | ChIP-qPCR | [22] |
| LPIN1 | Lipin 1 | activated | ChIP-qPCR, ChIP-seq | [23,25,39] |
| CPT1C | Carnitine Palmitoyl transferase 1C | activated | ChIP-qPCR, ChIP-seq | [24,25,39] |
| ACAD11 | AcylCoA Dehydrogenase Family member 11 | activated | ChIP-qPCR, ChIP-seq | [25,39] |
| HMGCLL1 | 3-hydroxymethyl-3-methylglutaryl-CoA lyase like 1 | activated | ChIP-qPCR, ChIP-seq | [25,39] |
| Fatty Acid synthesis and storage, lipid transport and lipoprotein metabolism | ||||
| SREBP1C | Sterol regulatory element binding transcription factor 1 | repressed | reporter assays | [221] |
| ABCA1 | ATP binding cassette subfamily A member 1 | activated | ChIP | [45] |
| NPC1L1 | Nieman-Pick C1-like 1 | activated | ChIP | [54,235] |
| SCD1 | Stearoyl-CoA desaturase 1 | repressed | ChIP-qPCR, ChIP-seq | [49,51] |
| ELOVL3 | Elongation of very long chain fatty acids-like 3 | activated | reporter assays, ChIP, EMSA | [224] |
| ME1 | Malic Enzyme 1 | repressed | reporter assays, ChIP | [46] |
| ME2 | Malic Enzyme 2 | repressed | reporter assays, ChIP | [46] |
| PLTP | Phospholipid Transfer Protein | activated | reporter assays, ChIP | [54] |
| CEL | Carboxy Ester Lipase | activated | reporter assays, ChIP, ChIP-seq | [54,251] |
| CAV | Caveolin | activated | reporter assays, EMSA | [215] |
| DHRS3 | Dehydrogenase reductase 3 | activated | reporter assays, EMSA, ChIP | [47,48] |
| SIRT1 | Sirtuin 1 | activated/repressed | reporter assays, EMSA, ChIP | [191,192] |
| CYP19 | Aromatase | activated | ChIP | [213] |
| Sphingolipid metabolism | ||||
| CERS6 | Ceramide synthetase 6 | activated | reporter assays, EMSA | [59] |
| ACER2 | Alkaline ceramidase 2 | activated | reporter assays, ChIP-qPCR, ChIP-seq | [54,61,251] |
| ABCA12 | ATP binding cassette subfamily A member 12 | activated | ChIP | [60] |
| nSMANE2 | Neutral spingomyelinase | activated | reporter assays | [54] |
| Amino-acid and ammonia metabolism | ||||
| GLS2 | Glutaminase 2 | activated | ChIP | [20,21,84] |
| SLC7A3 | Solute carrier family 7 member 3 | activated | ChIP | [91] |
| PHGDH | Phosphoglycerate Dehydrogenase | repressed | ChIP, EMSA | [92] |
| P5CDH/ALDH4 | P5C dehydrogenase/Aldehyde dehydrogenase 4 | activated | reporter assays, ChIP, EMSA | [95] |
| PRODH/POX | Proline Dehydrogenase/Proline Oxidase | activated | ChIP, ChIP-PET | [97,98] |
| CPS1 | Carbamoyl phosphate synthetase 1 | repressed | reporter assays, ChIP | [102] |
| OTC | Ornithine transcarbamoylase | repressed | reporter assays, ChIP | [102] |
| ARG1 | Arginase 1 | repressed | reporter assays, ChIP | [102] |
| Mitochondrial Biogenesis, integrity and Respiration | ||||
| SCO2 | Synthesis of cytochrome c oxidase 2 | activated | [26] | |
| TFAM | Mitochondrial Transcription Factor A | activated | reporter assays, ChIP, | [27] |
| PGC1α | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha | repressed | ChIP | [195] |
| AIF | Apoptosis inducing factor | activated | ChIP | [28] |
| PC | Pyruvate carobxylase | repressed | reporter assays, ChIP | [230] |
| PARK2 | Parkin | activated | reporter assays, ChIP | [40] |
| MIEAP | Mitochondria-eating protein | activated | reporter assays, ChIP | [41] |
| Iron Metabolism and Ferroptosis | ||||
| Iron Metabolism | ||||
| ISCU | Iron-sulfur cluster assembly enzyme | activated | reporter assays, ChIP | [66] |
| FDXR | Ferredoxin reductase | activated | reporter assays, ChIP, ChIP-seq | [67,68,76,251] |
| HAMP | Hepcidin | activated | reporter assays, EMSA, ChIP | [65] |
| FXN | Frataxin | activated | reporter assays, EMSA, ChIP | [69] |
| Ferroptosis | ||||
| SLC7A11 | Solute carrier family 7 member 11 | repressed | ChIP-qPCR, EMSA | [79,84] |
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 | activated | ChIP-qPCR | [83] |
| Redox | ||||
| SESN1 | Sestrin 1 | activated | reporter assays, EMSA, ChIP, ChIP-seq | [116,251] |
| SESN2 | Sestrin 2 | activated | ChIP-seq | [39,117,251] |
| ALDH4 | Aldehyde dehydrogenase 4 | activated | reporter assays, EMSA, ChIP | [95] |
| GPX1 | Glutathione Peroxidase 1 | activated | reporter assays, EMSA | [113,252] |
| SOD2 | Manganese dependent superoxide dismutase | activated/repressed | reporter assays, ChIP | [113,125] |
| CATALASE | Catalase | activated | ChIP | [115] |
| NCF2/p67phox | Neutrophilic cytosolic factor 2 | activated | reporter assays, ChIP | [124] |
| DDIT4/REDD1 | DNA Damage inducible transcript 4 | activated | reporter assays | [156] |
| NOS3 | Nitric Oxide Synthase 3 | activated | reporter assays, EMSA, ChIP | [203] |
| TP53INP1 | Tumor Protein 53-Induced Nuclear Protein 1 | activated | reporter assays, EMSA, ChIP, ChIP-seq, | [120,121,251] |
| SLC2A9/GLUT9 | Solute carrier family 2 member 9 | activated | reporter assays, ChIP | [123] |
| Creatine Biosynthesis | ||||
| GAMT | Guanidinoacetate methyltransferase | activated | reporter assays, EMSA, ChIP | [55,96] |
| Autophagy | ||||
| ATG2B | Autophagy related 2B | activated | ChIP-seq | [39] |
| ATG4A | Autophagy related 4A | activated | ChIP-seq | [39] |
| ATG4C | Autophagy related 4C | activated | ChIP-seq | [39] |
| ATG7 | Autophagy related 7 | activated | ChIP-seq | [39] |
| ATG10 | Autophagy related 10 | activated | ChIP-seq | [39] |
| ULK1 | Unc-51 like kinase 1 | activated | ChIP-seq, EMSA, reporter assays | [39,139] |
| ULK2 | Unc-51 like kinase 2 | activated | ChIP-seq, EMSA, reporter assays | [39,139] |
| UVRAG | UV radiation resistance associated gene | activated | ChIP-seq | [39] |
| VMP1/TMEM49 | Vacuole membrane protein 1 | activated | ChIP-seq | [39] |
| DAPK1 | Death Associated Protein Kinase 1 | activated | ChIP, EMSA | [141] |
| AEN/ISG20L1 | Apoptosis enhancing nuclease | activated | ChIP | [138] |
| DRAM1 | DNA damage regulated autophagy modulator 1 | activated | reporter assays, ChIP, ChIP-seq | [140,251] |
| EI24 | Etoposide-induced 2.4 kb transcript | activated | ChIP-seq | [251,253] |
| AMPK, AKT and mTOR signaling | ||||
| AMPKβ1 | AMP-activated kinase beta 1 subunit | activated | reporter assays, ChIP | [152] |
| TSC2 | TSC Complex subunit 2 | activated | reporter assays, ChIP | [152] |
| PTEN | Phosphatase and tensin homolog | activated | reporter assays, EMSA | [157] |
| IGFBP3 | IGF-Binding protein 3 | activated | reporter assays, EMSA | [158] |
| PHLDA3 | Pleckstrin homology-like domain, Family A, member 3 | activated | reporter assays, ChIP, ChIP-seq | [159] |
2.1. p53-mediated regulation of glycolysis, the TCA cycle and oxidative phosphorylation
Although p53 promotes glycolysis and inhibits respiration in specialized cells such as pancreatic β-cells or hepatocytes, most data related to p53-associated metabolic activities support the notion that p53 favors oxidative phosphorylation (OXPHOS) over glycolysis in most cell types. Consistent with this notion, p53 deficiency contributes to the metabolic reprogramming of cancer cells towards a more glycolytic profile. p53 functions in glucose uptake involves the transcriptional repression of SLC2A1/4 that encode the glucose transporters GLUT1/4, or by restricting IKK–NF-κB activation that leads to decreased GLUT3 expression [9,10]. p53 also inhibits glycolysis by regulating the transcription of other genes that directly or indirectly modulate glycolysis including RRAD, PFKFB3/4, TIGAR, and the monocarboxylate transporter 1 (MCT1) -encoding gene SLC16A1 [[11], [12], [13], [14], [15]]. The role of p53 in this metabolic pathway also involves post-transcriptional mechanisms such as those implicating the regulation of miR-34a, a microRNA that targets several glycolytic enzymes, and the downregulation of the glycolytic enzyme phosphoglycerate mutase (PGM) [16,17].
At the same time, p53 promotes OXPHOS by several complementary mechanisms. First, p53 favors pyruvate oxidation in mitochondria through the down-regulation of the pyruvate dehydrogenase (PDH) kinase PDK2, a negative regulator of the PDH complex (PDC) that converts pyruvate into AcetylCoenzyme A (AcCoA) to sustain the tri-carboxylic acid (TCA) cycle [18]. The importance of p53 in fueling the TCA cycle with glucose-derived pyruvate has recently been highlighted in pancreatic cancer models in which restoration of p53 activity was shown to increase α-Ketoglutarate (αKG) levels. By changing the αKG/succinate ratio, p53 loss modulates the activity of αKG-dependent chromatin modifying enzymes, thereby influencing the epigenome of cancer cells and engaging them into a more malignant state [19]. To sustain the TCA cycle, p53 can also concomitantly enhance the flux of several anapleurotic pathways, including glutaminolysis and fatty-acid oxidation (FAO) [[20], [21], [22], [23], [24], [25]]. Moreover, p53 favors mitochondrial respiration by promoting mitochondrial biogenesis, maintaining mitochondrial genome integrity, and enhancing the activity of the electron-transport chain through transcriptional regulation of Synthesis of Cytochrome c Oxidase 2 (SCO2), Mitochondrial Transcription Factor A (TFAM), Ferredoxin Reductase (FDXR), and Apoptosis Inducing Factor (AIF). Beside the regulation of these target genes, p53 also sustains mitochondrial activity through its direct interaction with several mitochondrial proteins that play a key role in mitochondrial genome replication and repair, including the mitochondrial polymerase gamma (POLG), the human mitochondrial single-stranded DNA-binding protein (HmtSSB), the F1F0-ATP synthase subunit OSCP and TFAM [[26], [27], [28], [29], [30], [31], [32], [33]]. Another mechanism by which p53 favors mitochondrial function is through the expression of several components of the mitochondrial protein import machinery, including TOM20, TIM23, mtHSP70, and mtHSP60 [34]. In addition, p53 controls mitochondrial architecture and dynamics. More specifically, p53 supports mitochondrial fission by increasing the expression of the GTPase Dynamin-related protein 1 (DRP1), and by interacting with Prohibitin 1 and subsequent processing of the long form of Opa1 (L-Opa1) that promotes mitochondrial fusion [[35], [36], [37]]. Finally, p53 ensures the quality control and turnover of mitochondria through mitophagy by increasing the expression of Parkin and Mitochondria Eating Protein (MIEAP) [[38], [39], [40], [41]].
2.2. p53 and the Pentose Phosphate Pathway (PPP)
The control of glycolysis by p53 is tightly coordinated with its ability to channel glycolytic intermediates into branched anabolic pathways, among which is the Pentose Phosphate Pathway (PPP). The regulation of the PPP by p53 is important for the production of NADPH to contribute to maintain the redox status by reducing oxidized glutathione, and for the generation of precursors of nucleotide synthesis for DNA repair if cells face DNA damage. However, the role of p53 in the PPP remains ambivalent since several reports suggest that different pools of the p53 protein can either stimulate or repress this metabolic pathway depending on the cell type and stress conditions. Thus, on the one hand, nuclear p53 controls the efficiency of the glycolytic flux and the channeling of glucose-6-phosphate into the PPP by regulating the transcription of TIGAR, PFKFB3, and PFKFB4, 3 genes encoding enzymes that directly modulate the levels of Fructose-(2,6) bisphosphate (F2,6BP), an allosteric activator of phosphofructokinase 1 (PFK1) [12,14,15]. On the other hand, cytoplasmic p53 inhibits directly the activity of G6P-dehydrogenase (G6PD), the enzyme that controls the first and rate limiting step of the PPP, by preventing the formation of the active dimer [42]. Altogether, these data suggest that the dynamic control of p53 subcellular localization is an important regulatory mechanism of the PPP.
2.3. p53 controls lipid metabolism
Another important aspect of p53 metabolic activities relates to its multiple roles in lipid homeostasis, including in lipid transport and storage, in fatty acid and cholesterol biosynthesis, in sphingolipid metabolism, as well as in FAO [43,44]. At the systemic level, p53 controls the expression of proteins that contribute to complex lipid breakdown and their absorption in the gut (NPC1L1), their transport in the systemic circulation through lipoproteins (PLTP, CEL), and their intracellular flux (ABCA1, ABCA12, CAV). At the cellular level, p53 limits lipid anabolism through convergent mechanisms that decrease de novo fatty acid synthesis and impact the mevalonate pathway, a key metabolic pathway involved in cholesterol and non-sterol isoprenoids biosynthesis. Thus, p53 transcriptionally inhibits SREBP1c, a gene encoding the sterol regulatory element-binding protein 1 (SREBP1) transcription factor that activates the transcription of many lipogenic genes. An additional mechanism involves the transcriptional regulation of ABCA1, a transporter controlling retrograde cholesterol transport and therefore its abundance in the endoplasmic reticulum (ER), an event regulating SREBP2 maturation and its nuclear translocation [45]. In addition, induction of p53 impinges on lipid synthesis by limiting NADPH production, an essential co-factor for de novo fatty acid synthesis, through the repression of Malic Enzymes 1 and 2 (ME1/2) and, as previously mentioned, by inhibiting the PPP [42,46]. Paradoxically, p53 promotes lipid droplet formation by directly regulating the transcription of Dehydrogenase reductase 3 (Dhrs3), a family member of the short chain alcohol dehydrogenase/reductase superfamily that is important for retinoid metabolism and associates with lipid droplets [47,48]. It is plausible that the regulation of Dhrs3 by p53 is part of a specialized program that regulates the concentration of cytosolic retinol, and its product retinoic acid, in order to control cell proliferation and differentiation.
Another important aspect of p53 functions relates to its function in fatty acid desaturation. This activity is partly mediated through the transcriptional repression of Stearoyl-CoA desaturase 1 (SCD1), which encodes the enzyme converting saturated to mono-unsaturated fatty acids (MUFAs) [49]. p53-mediated repression of SCD1 has been documented in several cellular contexts, including in cancer cells in which it impacts membrane phospholipid composition and AKT-signaling, but also in normal fibroblasts engaged in premature senescence, a process during which the abundance of unsaturated fatty acids could change the structure and function of organelles, in particular mitochondria, that play a central role in senescence [[50], [51], [52], [53]].
During nutrient starvation, p53 inhibits fatty acid synthesis and concomitantly enhances lipid catabolism by increasing FAO, a metabolic process that replenishes the pool of mitochondrial AcCoA and provides reducing power (FADH2 and NADH) to support the activity of the electron transport chain. This involves the transcriptional activation of genes encoding carnitine acyl-transferases that facilitate fatty acids efflux out of the peroxisome (CROT) and their transport into the mitochondria (CPT1A and CPT1C), as well as that of several genes that promote directly or indirectly FAO (LPIN1, ACAD11, HMGCLL1, GAMT, MCD) [[23], [24], [25],54,55].
p53 also plays a key function in the metabolism of sphingolipids, a class of lipids that play multiple roles in signaling pathways involved in proliferation, cell death, and differentiation [56]. Indeed, p53 inhibits the expression of sphingosine kinase-1 (SK1), a central enzyme in that metabolic pathway that modulates Sphingosine-1-Phosphate (S1P) levels, through a post-translational mechanism involving cysteine-proteases and the proteasome [57]. The importance of SK1 regulation by p53 during cancer development was confirmed in mice in which genetic inactivation of Sk1 abrogated lymphomagenesis in Trp53 knock-out (KO) mice [58]. In addition, in cancer cells, p53 increases the expression of Ceramide synthase 5 (CERS5) and 6 (CERS6) and of the neutral Sphingomyelinase-2 (nSMASE2), three ceramide-generating enzymes, upon DNA damage or folate deprivation [59,60]. These data indicate that p53 can concomitantly increase the synthesis of pro-apoptotic ceramides and decrease the synthesis of the anti-apoptotic S1–P. Paradoxically, p53 also induces the transcription of human Alkaline ceramidase 2 (ACER2) which gene product catalyzes the hydrolysis of ceramides into sphingosine, the precursor of S1P [61]. Finally, it is noteworthy that p53 is part of a feedback loop involving ceramides. Thus, the well-described buildup of ceramides following DNA damage was found to occur in a p53-dependent manner. Interestingly, the massive up-regulation of p53 occurring upon transient induction of CerS6 during serum or folate deprivation results from the direct binding of C16-ceramide to the core DNA Binding Domain of p53 and inhibition of MDM2-mediated degradation by the proteasome [62,63]. Altogether, these studies indicate that p53 is both a target and a modulator of sphingolipid metabolism.
2.4. p53, iron metabolism and ferroptosis
Iron plays a critical role in a variety of biological processes including cell proliferation, and cancer cells display a stronger dependence on iron than do normal cells [64]. Cells utilize free iron (also called labile iron) to synthesize cofactors such as heme and iron sulfur (Fe–S) clusters that are essential for the activity of several enzymes involved in DNA synthesis and repair, as well as those implicated in many oxido–reduction reactions. Iron also functions as a cofactor for lipoxygenases (LOXs). Beyond these essential roles, high iron concentration is deleterious due to the Fenton reaction during which a ferrous iron donates an electron in a reaction with hydrogen peroxide (H2O2) to generate a highly reactive hydroxyl radical. Therefore, the uptake, storage, and usage of iron must be tightly controlled and p53 appears to be pivotal in a complex network controlling iron metabolism both at the systemic and the cellular levels by regulating the transcription of several key iron regulators including Hepcidin (HAMP) [65], iron-sulfur cluster assembly enzyme (ISCU) [66], Ferredoxin reductase (FDXR) [67,68], and Frataxin (FXN) [69]. Different teams have reported that iron chelators, as well as iron overload, lead to p53 stabilization through distinct mechanisms implicating either Hypoxia-Inducible Factor 1 alpha (HIF1α) or MDM2, two direct regulators of p53 which activities are modulated by intracellular iron levels [[70], [71], [72], [73]]. Changes in iron metabolism can also directly impact on p53 transcriptional activities. Thus, the iron polyporphyrin heme was shown to interfere with p53's interaction with DNA, thereby promoting its nuclear export and cytosolic degradation [73]. Finally, ferritin, an iron storage protein, was also reported to bind and activate p53 under oxidative stress [74]. Interestingly, the gene network implicating p53 in iron metabolism involves several feedback loops that in turn modulate p53 expression and activity [75]. Thus, FDXR, a bona fide p53 target gene, encodes a protein that influences p53 translation through a mechanism implicating the binding of the RNA-binding protein IRP2 to the 3′ untranslated region (UTR) region of p53 mRNA [76]. In neuronal cells and astrocytes, interfering with FXN, that is also regulated by p53 at the transcriptional level, triggers a p53-dependent apoptotic response [77]. It is currently unknown whether modulation of p53 activity by FXN influences the development of the various types of cancer observed in patients suffering Friedreich's ataxia, a rare early-onset degenerative disease linked to FXN deficiency. However, the p53-dependent cell death occuring upon FXN inactivation likely contributes to their neurological symptoms. Altogether, these data illustrate the high connectivity between p53 and iron metabolism and show that perturbations of this network can lead to mitochondrial iron overload, a process associated with tumor predisposition [76].
The importance of p53 in iron metabolism extends to the control of ferroptosis, a non apoptotic cell death mechanism characterized by iron-dependent lipid peroxidation. Interestingly, p53 can promote opposite effects on ferroptosis, through both transcriptional and non-transcriptional mechanisms that are influenced by the cellular context. Several studies indicate that p53 activation promotes ferroptosis and that this function is important for its tumor suppressive activities. The first evidence linking p53 to ferroptosis was identified in genetically engineered mouse models (GEMMs) harboring combined lysine-to-arginine (K→R) mutations located in p53 DNA binding domain. Unexpectedly, knock-in mice expressing a p53 acetylation-defective mutant on K117, K161, and K162 were not cancer prone despite these mutations completely abrogated p53's ability to induce cell cycle arrest, senescence, and cell death in response to acute DNA damage or oncogenic stress. Interestingly, these mutations did not alter the regulation of a subset of p53 metabolic target genes, suggesting that p53 metabolic activities play a key role in tumor suppression in vivo [78]. Later, Slc7a11, a p53-repressed gene encoding a component of the Xc− cystine/glutamate antiporter, was identified as an important mediator of p53-associated control of ferroptosis and tumor suppression in this mouse model [[79], [80], [81]]. The lipoxygenase ALOX12, that binds directly to and is inhibited by SLC7A11, was found to be indispensable for p53-mediated ferroptosis [82]. Other p53 metabolic target genes also contribute to its role in ferroptosis, such as Glutaminase 2 (GLS2) that will provide precursors for GSH synthesis (see below), or SAT1, which gene product is involved in polyamine catabolism by catalyzing acetylation of spermidine and spermine, a process linked to H2O2 production, activation of the ALOX15 lipoxygenase and lipid peroxidation [83]. The role of p53 in sensitizing cells to ferroptosis was confirmed in another GEMM mimicking the p53-S47 genetic variant that is the second most common Single Nucleotide Polymorphism (SNP) within the TP53 locus found in the human population of African origin. In this animal model, increased cancer pre-disposition was linked to the inability of the S47-p53 variant to properly regulate some p53 metabolic target genes including Gls2 and Slc7a11 [84]. Nevertheless, p53 was also reported to limit or delay ferroptosis in some cancer cells by preventing the relocalisation from the nucleus to the plasma membrane of dipeptidyl-peptidase-4 (DPP4), a positive regulator of ferroptosis, or as a result of p21-mediated cell cycle arrest that contributes to maintain glutathione levels [85,86].
2.5. p53 and amino-acid metabolism
The transcriptional regulation of GLS2 by p53, which gene product converts glutamine into glutamate and ammonia, was the first evidence linking p53 to amino-acid metabolism. p53-mediated control of GLS2 contributes to different metabolic pathways and replenishes TCA intermediates when pyruvate oxidation is impaired. It is also important to maintain the redox status of cells by fueling glutathione synthesis [20,21,84]. Although the protective function of p53 during metabolic challenges was initially observed upon glucose starvation [87], it was later extended to conditions where different amino-acids become limiting [[88], [89], [90], [91]]. Consistent with this notion, p53-deficient cells are more sensitive to serine/glycine or glutamine deprivation. In response to glutamine deprivation, p53 drives an adaptive response by inducing the expression of the arginine transporter SLC7A3 that increases temporarily intracellular arginine levels to sustain mTORC1 activity [91], while the induction of the aspartate transporter SLC1A3 supports mitochondrial respiration and nucleotide synthesis [90]. Different components of the p53 pathway are also involved in the cellular response to serine deprivation. Cells facing a limited supply of exogenous serine induce de novo serine synthesis, an anabolic pathway that converts the glycolytic intermediate 3-phosphoglycerate (3 PG) into serine through a multi-step enzymatic process implicating phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH). The link between p53 and serine metabolism was initiallty described by Vousden and colleagues. In serine/glycine-deprived cells, they identified p21 as an important router that constrains the channeling of the remaining pool of serine towards glutathione synthesis at the expense of nucleotide synthesis in order to maintain a proper redox status and to promote cell survival [89]. At the molecular level, p53 and MDM2, independently of each other, have antagonistic roles on the transcriptional control of genes encoding key enzymes involved in serine synthesis. For instance, p53 was reported to repress the PHGDH promoter in melanoma cells cultured in complete medium [92], whereas our team more recently reported direct and p53-independent MDM2-mediated activation of PHGDH, PSAT1, and PSPH upon serine/glycine deprivation [93]. In the Ou et al. study, the recruitment of p53 on PHGDH promoter was not assessed upon serine–glycine deprivation but only after treatment with the DNA-damaging agent Doxorubicin or Nutlin3A that lead to the full activation of p53. Induction of p53-mediated repression of PHGDH by Nutlin3A potentiates cell death in serine/glycine-deprived melanoma cells, suggesting that this combination of treatments impacts on serine/glycine levels synergistically to reach a lethal threshold. It is noteworthy that the cell death induced upon p53 activation by Nutlin3A in these cells depends on ATF4, a transcription factor that is key to recruit MDM2 on the promoter of its metabolic target genes [93]. Therefore, it is plausible that the activities of MDM2, p53, and ATF4 are finely tuned to control the flux through this key metabolic pathway in a biphasic manner. Decreased serine availability may initially promote cell survival through MDM2/ATF4-mediated regulation of serine synthesis and through a p53-p21 axis that maintain the redox balance of these cells. Later, more prolonged or severe depletion of serine/glycine pools (that may ultimately induce to DNA damage) would initiate a vicious cycle during which the repression of PHGDH by p53 and the activation of NOXA and PUMA by ATF4 lead to cell death.
Another pathway involving amino-acids in which p53 plays an important role is proline metabolism. In recent years, proline metabolism has received considerable attention as a mechanism of NAD/NADP regeneration, an anaplerotic source, a potential producer of reactive oxygen species (ROS), and also as a cell signaling hub [94]. p53 induces the transcription of both Proline dehydrogenase (PRODH)/Proline oxidase (POX), initially named PIG6 for “p53-induced gene 6”, and Pyrroline-5-Carboxylate (P5C) dehydrogenase (P5CDH), also referred to as aldehyde dehydrogenase 4 (ALDH4) [[95], [96], [97], [98]]. These two p53 target genes encode enzymes that catalyze the first and second reactions involved in proline degradation, respectively. PRODH/POX is a mitochondrial enzyme linked to complex II of the electron transport chain (ETC) with a flavine adenine dinucleotide at the active site that transfers electrons to Coenzyme Q. By doing so, proline can serve as an alternative source of energy when glucose and glutamine are limiting, but proline-derived electrons also produce superoxides through complex III, thereby contributing to p53-dependent apoptosis or senescence [96,99,100]. ALDH4 is a NAD+-dependent enzyme localized in the mitochondrial matrix that catalyzes the second step of proline degradation by converting L-Glutamic-γ-semialdehyde into glutamate. The anti-oxidant effect of ALDH4 has been linked to its ability to exhaust the proline pool [95]. Consistent with its anti-oxidant function, ALDH4 depletion in worms induces the expression of the ROS-sensitive nuclear factor erythroid 2-related factor 2 (NRF2) transcription factor that coordinates proline and fatty acid metabolism [101]. It remains to be determined when p53 preferentially induces ALDH4 over PRODH expression, but the finely tuned regulation of these two enzymes by p53 differentially impacts the redox status of the cells and changes the outcome of p53-mediated responses. Given the metabolic interlock between proline metabolism and the PPP, it is plausible that p53-mediated control of proline metabolism influences some of its multiple effects on the PPP [12,14,15,42]. Finally, proline catabolism is also tightly connected to ureagenesis and polyamines biosynthesis through the conversion of P5C into ornithine, a precursor of polyamines and key intermediate of the urea cycle. Interestingly, p53 has also been shown to influence polyamine biosynthesis through the repression of three key enzymes of the urea cycle, Carbamoyl phosphate synthetase 1 (CPS1), Ornithine transcarbamoylase (OTC) and Arginase 1 (ARG1). p53 deficiency increases ureagenesis and putrescine levels, thereby promoting proliferation. Moreover, defects in the urea cycle activates p53 in a MDM2-dependent manner, suggesting a positive regulatory loop between p53 and the urea cycle [102]. Thus, while the model remains to be confirmed, p53-coordinated actions on amino-acid catabolism and on the urea cycle might be necessary to regulate polyamine levels.
2.6. p53 and nucleotide synthesis
By inhibiting nucleotide synthesis, p53 limits cell proliferation in unchallenged conditions, but it can also temporarily stimulate metabolic pathways that contribute to both purine and pyrimidine synthesis in response to DNA damage to facilitate DNA repair. Thus, the p53-inducible microRNA-34a (miR-34a) represses inosine 5′-monophosphate dehydrogenase (IMPDH), a rate-limiting enzyme involved in de novo guanosine triphosphate (GTP) biosynthesis [103]. p53 also inhibits guanosine monophosphate (GMP) synthesis by repressing the expression of guanosine 5′-monophosphate synthase (GMPS), one of three glutamine amidotransferases that converts xanthosine 5′-monophosphate (XMP) into GMP [104]. An interesting feedback loop involves p53 and GMPS; the latter is required for p53 stabilization in response to genotoxic stress or upon nucleotide depletion. Nuclear translocation of GMPS in these conditions facilitates p53 stabilization by promoting its transfer from MDM2 to a multiprotein complex containing GMPS and the USP7 (also called HAUSP) deubiquitylating enzyme [105]. In addition, p53 inhibits DNA synthesis in a p21-independent, but SP1-dependent manner, by repressing the transcription of deoxyuridine triphosphate nucleotidohydrolase (dUTPase), which gene product catalyzes the hydrolysis of dUTP into dUMP, a precursor of dTTP [106]. On the other hand, p53 activates ribonucleotide reductase (RNR) upon DNA damage, an enzyme reducing nucleotide diphosphates (NDPs, including ADP, GDP, CDP, and UDP) at the 2′ position of the ribose sugar to generate deoxyribonucleotides (dNTPs). RNR is a tightly regulated tetrameric enzyme consisting of two catalytic subunits (RRM1) and two regulatory subunits, either RRM2 or p53R2/RRM2B, that supplies cells with dNTPs for DNA replication or for DNA repair and mitochondrial DNA synthesis. p53 activates the transcription of p53R2/RRM2B, thereby contributing to its role in DNA repair [107,108]. Paradoxically, p53 was reported to suppress RRM1 and RRM2 expression at the post-transcriptional level via the inhibition of mammalian target of rapamycin complex 1 (mTORC1) [109]. It is notework that opposing roles of p53 in nucleotide synthesis have been described during amino-acid deprivation. Indeed, whereas p53 inhibits nucleotide synthesis when an exogenous source of serine becomes limiting, it sustains nucleotide synthesis during glutamine deprivation [89,90]. Finally, p53 can also modulate nucleotide synthesis indirectly through the regulation of the PPP and the one carbon cycle [15]. Hence, although several lines of evidence suggest that p53 coordinates the DNA repair machinery with nucleotide synthesis, further studies are needed to provide definitive conclusions about its exact functions in nucleotide metabolism.
2.7. p53 and REDOX balance
The control of ROS levels is certainly not the only mechanism by which p53 limits carcinogenesis but its importance is supported by in vivo data showing that tumor incidence in Trp53 KO mice is significantly attenuated when these animals are supplemented with the ROS-scavenger N-acetyl-cysteine (NAC) [110]. In agreement with these findings, the Super-p53/ARF mice that have extra-copies of the Trp53 and Cdkn2a tumor suppressor loci display a continuous activation of p53-dependent anti-oxidant genes that correlates with extended lifespan and reduced cancer incidence [111]. Under physiological conditions, different metabolic functions of p53 can limit ROS levels, including those that promote mitochondrial integrity, or through the down-regulation of ROS-generating enzymes such as nitric oxide synthase 2 (NOS2) [112]. In addition, p53 prevents ROS accumulation by controlling the transcription of genes encoding several important ROS-detoxifying enzymes. These p53-target genes include Superoxide Dismutase 2 (SOD2 or Mn-SOD), the gene product of which prevents the accumulation of superoxide radicals (O2−) in the mitochondria by converting them into H2O2 [113]. In retinal ganglion cells, p53 stimulates the transcription of Catalase, and it was also reported to regulate catalase activity via a direct protein–protein interaction and by controlling the expression of p53R2/RRM2 [114,115]. By regulating catalase activity, p53 promotes the degradation of H2O2 into water and free oxygen. In addition, p53 is intrinsically linked to glutathione metabolism through the transcriptional regulation of Glutathione Peroxidase 1 (GPX1) and genes involved in several metabolic pathways that contribute to glutathione biosynthesis such as SLC7A11 and GLS2, as well as by channeling serine/glycine towards GSH production at the expense of nucleotide synthesis [20,21,79,89]. p53-target genes that favor NADPH production through the PPP, such as TIGAR and PFKFB3, participate in the maintenance of a reduced GSH pool [12,46]. SESN1 and SESN2 belong to p53's anti-oxidant arsenal through their direct effect on peroxiredoxin proteins, by stimulating the degradation of KEAP1, an inhibitor of NRF2, and through the regulation of the AMPK-mTOR pathway [[116], [117], [118], [119]]. Finally, other p53 direct target genes such as the uric acid transporter SLC2A9/GLUT9, TP53INP1, and ALDH4 also contribute to p53 anti-oxidant activities through different mechanisms [95,[120], [121], [122], [123]].
Nevertheless, there is also clear evidence that p53 can paradoxically increase ROS production. A plausible explanation regarding the divergent roles of p53 in controlling ROS levels is that p53 prevents oxidative damages and promotes their repair in order to favor cell survival in response to moderate ROS levels, but increases the production of ROS to lethal levels to induce several forms of cell death, including ferroptosis, apoptosis, and necrosis. The pro-oxidant effects of p53 that lead to cell death involve the transcriptional activation of genes encoding proteins with ROS-generating capacities including LGALS7 (PIG1), the NADPH-quinone oxidoreductase homolog TP53I3 (PIG3), and neutrophil cytosol factor 2 (NCF2/p67phox), a gene encoding a component of NADPH oxidase, the critical enzyme responsible for cytosolic O2− production [96,124]. p53-mediated control of PRODH/POX also contributes to produce ROS by the ETC during p53-induced apoptosis [99]. Paradoxically, p53 has also been reported to repress SOD2 promoter by binding to its transcriptional activator SP1 [125]. The complex interplay between p53 and SOD2 is also illustrated by p53's ability to translocate to mitochondria, where it binds and blocks the ROS-scavenging activity of SOD2 [126]. Stabilization of the pro-oxidant molecule p66shc by p53 increases mitochondrial ROS levels and p53-dependent cell death [127]. The enhanced recruitment of p53 in mitochondria through its interaction with DRP1 has also been associated with mitochondria fragmentation, increased mitochondrial ROS levels, and decreased viability of neuronal cells in patients with huntington disease [128]. Finally, apoptosis can be initiated by p53 independently of its transcriptional activity through its binding to BCL2 family members at the outer mitochondrial membrane, leading to the subsequent release of cytochrome C from the ETC and to electron leak [129,130].
Strikingly, a strong body of evidence indicates that p53 transcriptional activity is modulated in response to oxidative stress, thereby defining an important feedback-loop. Several cysteine residues (including Cys 124, 141, 176, 238, 242, 277) located in the DNA binding domain of p53 are targets for redox regulation and p53 binding to its responsive elements is clearly dependent on reducing conditions. Moreover, the Apurinic/apyrimidinic endonuclease 1/reduction-oxidation factor 1 (APE1/Ref-1) protein has been shown to influence the DNA binding properties of several transcription factors, including p53, by maintaining specific cysteine residues in the reduced state [[131], [132], [133]]. p53 activation during oxidative stress involves its trafficking through nuclear structures that sense ROS called Promyelocytic Leukemia (PML) nuclear bodies. Increased sumoylation of p53 in PML bodies, as well as that of several of its regulators (ARF, MDM2, HIPK2, CBP), has been associated to the induction of p53-regulated anti-oxidant genes [134,135]. Interestingly, PML was also identified as a p53 direct target gene [136], highlighting a potential feedforward mechanism that contributes to p53-mediated senescence, a process during which ROS play a central role. Interestingly, oxidative stress can also promote the translocation of p53 to the mitochondrial matrix where it interacts with cyclophilin D, a key regulator of the mitochondrial permeability transition pore (mPTP), resulting in dissipation of the mitochondrial membrane potential (ΔΨm) and triggering necrosis in neuronal cells during ischemia-reperfusion injury [137].
2.8. p53-mediated regulation of autophagy
There is a complex interplay between p53, autophagy, and cancer. Basal autophagy is an evolutionary conserved quality control process but this catabolic self-eating program can be induced to provide essential metabolites when other sources of nutrients become limiting. p53 functions in autophagy involves transcriptional and non transcriptional effects that have been mainly studied in the context of cancer cells although it is likely that they also contribute to normal tissue homeostasis. Several components of the autophagy machinery have been identified as direct p53 target genes including genes encoding proteins of the autophagy core machinery, autophagy regulators, and lysosomal proteins [39,[138], [139], [140], [141]]. p53 was also found to control autophagy through the AMPK-mTOR pathway [142]. The transcriptional activation of autophagic genes by nuclear p53 has been linked to its ability to induce cell death during genotoxic stress, but, paradoxically, cytoplasmic p53 was shown to repress autophagy [143]. In agreement with the inhibitory role of p53 on autophagy, depletion of Cep-1, the nematode ortholog of p53, triggers autophagy and increases life span in Caenorhabditis elegans [144]. Altogether, these data suggest that both activation and inhibition of p53 can trigger autophagy. In addition, p53 is involved in a feedback loop in which the ATG7 protein modulates p53 functions, likely to restrain its pro-apoptotic activities and to maintain cellular homeostasis during mild metabolic challenges. In agreement with this notion, in absence of the ATG7 protein, nutrient-starved cells exhibit impaired cell-cycle arrest but are more prone to p53-induced cell death, a process that was linked to ATG7 direct binding to p53 and its co-recruitment to the p21Cdkn1a promoter [145]. It is therefore plausible that p53 somehow senses decreased autophagic flux through several mechanisms and modulates its transcriptional program to compensate for such defect in order to facilitate cell survival. However, p53 can stimulate autophagy to ultimately kill cells, a mechanism aiming at preventing the survival of cells accumulating damages. The complex connections between p53 and autophagy in cancer development have been illustrated in several cancer-prone animal models. In a mouse model of hereditary breast cancer based on Palb2 inactivation, loss of Atg6/Beclin 1 reduced tumorigenesis and extended the lifespan of Palb2-deficient mice, an effect that was mitigated by the concomitant inactivation of Tp53 [146]. In addition, Atg7 or Atg5 deficiency reduced tumor burden by turning on p53 in a KRASG12D-driven lung cancer model [147]. Finally, in a model of activated KRAS-driven pancreatic cancer, the dual role of autophagy in cancer progression was shown to be intrinsically connected to the p53 status. Thus, whereas inhibition of ATG7 favored the developement of pre-malignant intraepithelial neoplasias but blocked their evolution into pancreatic ductal adenocarcinoma (PDAC), it accelerated tumor onset in absence of p53 [148]. Based on these data, it is currently difficult to provide a unifying model of p53 functions in autophagy. A more detailed analysis of p53 functions in the different types of autophagy (macroautophagy versus selective forms of autophagy) is required to unvail its subtle roles in this key cellular process (See Table 1).
3. p53: a metabolic sensor involved in multiple signaling pathways
As described in other parts of this review, p53 activities are modulated in response to many metabolic challenges. Moreover, it plays a central role in metabolism by acting upstream and downstream of key signaling pathways that sense and control the energetic status of cells.
3.1. p53 and LKB1-AMPK-mTOR signaling
Given its pleiotropic roles in metabolism, it is not surprising that p53 plays an important role in the LKB1-AMPK-mTOR pathway, a central energy sensing pathway that coordinates cell metabolism. Multiple evidences indicate that p53 and the LKB1-AMPK-mTOR pathway are intimately intertwined, although the effect of p53 on this pathway depends on the stress situation. Thus, p53 was initially shown to be activated by AMPK during energetic stress through its phosphorylation on serine 15 [87,142,149]. However, depending on the cell type, the increased phosphorylation of p53 on Ser15 (Ser18 in the mouse) that occurs upon glucose deprivation was attributed either to AMPK or to ATM, another kinase activated in response to oxidative stress and DNA damage [23,87,150]. It is interesting to note that knock-in mice expressing a mutant form of p53 that cannot be phosphorylated on Ser 18 (S18A) exhibit metabolic phenotypes and defects in glucose homeostasis, providing further evidence for the involvement of p53 phosphorylation on this key residue in metabolism [151]. At the other end of this metabolic feedback loop, it is well known that p53 modulates LKB1-AMPK-mTOR signaling during genotoxic stress or glucose deprivation through the transcriptional control of Liver Kinase B1 (LKB1/STK11), AMP-kinase β1 (AMPKβ1), DNA Damage inducible transcript 4 (DDIT4/REDD1), SESN1/2, and TSC complex subunit 2 (TSC2), thereby leading to inhibition of mTORC1 and decreased cell growth [118,[152], [153], [154], [155], [156]]. Paradoxically, p53-proficient cancer cells respond to glutamine deprivation by inducing a rapid adaptive response that sustains arginine levels and mTORC1 activity, indicating that the crosstalks between p53 and mTORC1 are complex and stimulus-specific [91].
3.2. p53 and the insulin-PI3K-AKT pathway
There is a well-described reciprocical interplay between AKT and p53. p53 induction leads to potent inhibition of AKT signaling by activating the transcription of Phosphatase and Tensin Homolog (PTEN), a gene encoding a PIP3 phosphatase that opposes the effects of PI3K, IGF-binding protein 3 (IGFBP3) that produces a secreted protein binding to free IGF1, and Phlda3, the gene product of which blocks AKT translocation to the plasma membrane [152,[157], [158], [159]]. The impact of p53 on this signaling cascade also involves the transcriptional repression of the Insulin Receptor and Insulin-like Growth Factor Receptor 1 [160]. In addition, p53-mediated regulation of the abundance of mono-unsaturated phospholipids through the repression of SCD1 also influences indirectly AKT activity [50]. The links between AKT and the p53 pathway are also illustrated by AKT-mediated phosphorylation of MDM2, the key negative regulator of p53 [161,162]. Interestingly, p53-mediated regulation of PTEN (and TSC2) occurs preferentially in tissues that respond to exogenous glucose levels, including heart, muscle, liver, white adipose tissue (WAT), and kidneys, suggesting that the regulation of the AKT and mTOR signaling cascades by p53 is an important component of its nutritional sensing functions in vivo [152].
3.3. p53 and hypoxia
Under low oxygen conditions, cells rapidly adapt their metabolism by stabilizing the HIF-1α transcription factor, but it is now recognized that p53 also contributes to the adaptive response of cells to hypoxia. Modulation of p53 activities during hypoxia occurs through both HIF- dependent and independent mechanisms. Several groups have linked HIF induction to decreased MDM2-mediated ubiquitination of p53, an effect that controls its nuclear export and proteasome-mediated degradation [[163], [164], [165], [166]]. Other HIF-dependent mechanisms have been proposed, such as the control of p53 translation by the HIF-inducible RNA-binding protein HuR [167]. However, other investigators have shown that ATR can phosphorylate and activate p53 during S phase independently of HIF1α in low oxygen conditions [168]. Other mechanisms linked to hypoxia, such as the production of mitochondrial ROS or the associated acidosis, can also stabilize p53 [169,170]. Coordination of HIF and p53 activities during hypoxia involves a complex network of common regulators in which the Von Hippel Lindau (VHL) tumor suppressor and the MDM2 oncoprotein play a central role. The complex relationship between HIF, VHL, MDM2, and p53 allows cells to adapt their response to different oxygen concentrations. As for many adaptive responses in which p53 is involved, the fine tuning of this network is important to either promote its pro-survival effects in transient hypoxic conditions or trigger cell death upon more severe or prolonged hypoxia. From a metabolic standpoint, HIF and p53 display opposite effects on glycolysis and mitochondrial respiration and it is therefore logical that the activities of these two transcription factors are coordinated during hypoxia. Thus, whereas HIF stimulates glycolysis by inducing the expression of most glycolytic enzymes and glucose and lactate transporters, p53 represses glycolysis by various mechanisms described in section 2.1. These two transcription factors also mediate antagonistic activities on mitochondrial respiration. Some of their effects on this OXPHOS to glycolytic switch involve the transcriptional regulation of pyruvate dehydrogenase kinases (PDKs) that inhibit mitochondrial pyruvate oxidation as well as the assembly/activity of the ETC [[171], [172], [173], [174]]. Intuitively, induction of p53 during hypoxia should balance some of the metabolic effects mediated by HIF1α. However, the p53 response to mild hypoxic conditions seems to be initially biased towards a subset of its repressed target genes, a process implicating the mSin3a co-repressor [175]. Competition between HIF1α and p53 for common transcriptional co-activators such as CBP/p300 and specific post-translational modifications of p53 also seem to play a role in p53's ability to regulate a subset of its target genes during hypoxia [176,177]. Nevertheless, during more severe and/or prolonged hypoxia that can lead to the accumulation of DNA damage, p53 turns on a cell death program that is mediated, at least in part, by Bnip3L and NIX [178,179]. It is noteworthy that HIF and p53 are key regulators of intracellular ROS levels, but their activities are also responsive to redox changes, thereby defining an additional feedback loop that contributes to the cellular response to hypoxia.
3.4. Connections between p53, AcCoA and NAD metabolism
There is an important crosstalk between p53, NAD, and AcCoA metabolism. Indeed, an important aspect of p53 functions relates to its acetylation on key residues involved in different important domains of the protein, including its DNA binding domain and the c-terminal regulatory domain. The acetylation status of p53 reflects the activity of different enzymes that acetylate or deacetylate p53 on specific residues. Several acetyltrasferases (P300, CBP, hMOF, TIP60) and deacetylases (HDAC1/8, SIRT1), define a complex regulatory network standing at the interface between metabolism and p53 since their co-factors, AcCoA for acetyltransferases and NAD for sirtuins, are also key metabolites [[180], [181], [182], [183]]. Interestingly, pyruvate oxidation by the PDH and FAO, two important metabolic pathways targeted by p53, regulate AcCoA abundance. Although this has not been proven yet, the control of AcCoA levels by p53 likely contributes to maintain cellular homeostais since many metabolic enzymes are regulated by acetylation [184,185]. NAD metabolism also plays a pivotal role in the p53 network. NAD+ binds to p53 tetramers with a millimolar range affinity constant, inducing a conformational change and modulating p53 DNA binding specificity [186]. Similarly to the AcCoA-p53 interplay, several metabolic pathways regulated by p53 can modulate the NAD+/NADH ratio, and changes in NAD levels directly impact on the activity of SIRT1, a NAD-dependent class III deacetylase that is highly connected to p53 [187]. SIRT1 acts as a signalling hub, linking stress/nutrient sensing pathways such as the p38, mTOR, and AMPK pathways to p53. SIRT1-mediated deacetylation of p53 represses its transactivation activity in both normal and cancer cells. Consistent with this notion, SIRT1 deficiency leads to p53 hyperacetylation and enhances the DNA-damage response in lymphocytes [188]. SIRT1-mediated control of p53 acetylation was also shown to regulate its subcellular localization and its transcription-independent mitochondrial functions [189]. Of note, SIRT1 substrates include acetyltransferases that target p53, including p300 and TIP60, as well as several metabolic enzymes controlling AcCoA availability, such as ATP-citrate lyase (ACLY) and AcCoA synthetase 1 (ACSS1) [190]. Finally, additional levels of regulation of this network involve p53-mediated repression of SIRT1 promoter, and the regulation of several miRNAs by p53 that bind to the 3′-UTR of Sirt1, Sirt2, Sirt6, and Sirt7 mRNAs and inhibit their translation [[191], [192], [193], [194]].
3.5. p53 and PGC1α
Several studies have highlighted important links between p53 and the transcriptional co-activator Peroxisome proliferator-activated receptor Gamma, Coactivator 1 alpha (PGC1α), an important metabolic regulator. Depinho and colleagues showed that p53 inhibits the transcription of PGC1α (and PGC1β) in cells undergoing premature aging in the setting of telomere dysfunction [195]. Moreover, a direct interaction between PGC1α and p53 that promotes p53's transcriptional activities and influences cell fate in response to metabolic challenges was also reported. Thus, in glucose-deprived hepatocytes, PGC1α favors the induction of p53 target genes involved in cell cycle arrest and metabolism at the expense of pro-apoptotic genes, likely to initially protect cells and allow them to adapt to nutrient starvation. When PGC1α was inhibited in this experimental model, p53 then induced a cell death response [196]. Conversely, p53 binding to PGC1α in adipocytes was also shown to repress PGC1α activity on its target genes, including Ucp1, a gene encoding a proton channel that uncouples the electron transport chain with ATP production to favor heat production in brown adipocytes [197]. It is interesting to note that in metabolically challenged cells, p53 also activates the transcription of LPIN1, a gene encoding a bifunctional protein that acts as a nuclear transcriptional co-activator with PGC1α and Peroxisome proliferator-activated receptors (PPARs) to promote FAO or behave as a phosphatidate phosphatase enzyme that catalyzes the conversion of phosphatidate to diacylglycerol, a key step in the biosynthesis of triacylglycerol [23]. Hence, p53, PGC1α and their co-factors define a finely tuned network that allows cells to mount transient adaptive responses upon metabolic challenges, before engaging cells into a more detrimental fate when these stresses increase in intensity.
4. Contribution of P53-Associated metabolic functions to normal tissue homeostasis
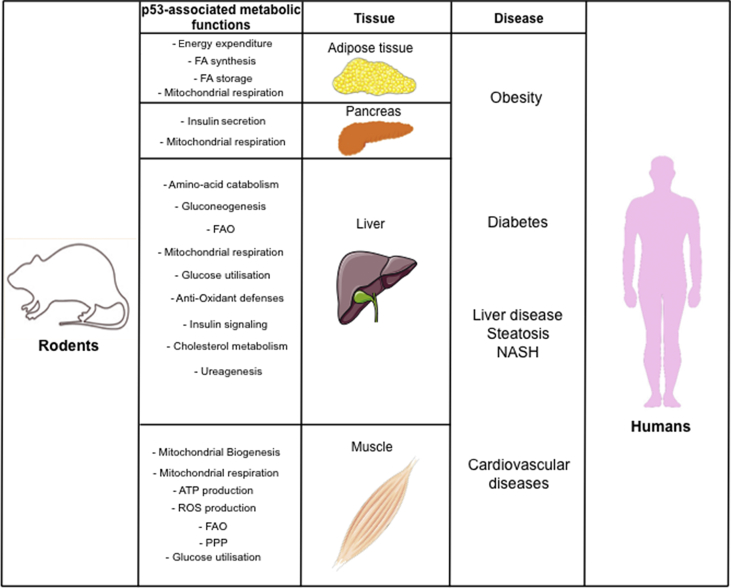
We still lack an integrated vision of p53-associated metabolic functions at the whole organismal level but growing evidence suggests that the deregulation of p53 metabolic activities impacts normal tissue homeostasis, in particular under conditions of nutritional challenge. In line with p53 functions in multiple metabolic pathways, its deregulation results in dysfunction of major metabolic tissues such as skeletal muscles, liver, pancreas, and adipose tissue (See Figure 2).
Figure 2.
Overview of the known in vivo metabolic functions of WT-p53 and the consequences of their deregulation on human diseases (beyond cancer). FA, Fatty acids; FAO, Fatty Acid Oxidation; PPP, Pentose Phosphate Pathway; ROS, Reactive Oxygen Species; ATP, Adenosine Tri Phosphate.
4.1. Role of P53-Associated metabolic functions in muscles
It is well-recognized that Trp53 KO mice have an altered endurance capacity that reflects, at least in part, the importance of p53 in the control of oxidative phosphorylation and proper mitochondrial function in skeletal muscles [26,27,198]. p53 translocates to mitochondria during an acute bout of exercice where it binds to TFAM, a key regulator of mtDNA replication and transcription [27,199]. Both acute and chronic exercice have been shown to diminish p53 mRNA levels, but concommitantly increase its phosphorylation [199]. Beside its role in respiration, mitochondrial p53 maintains the integrity of mitochondria in muscle cells by regulating mtDNA repair [200]. Several metabolic activities of p53, among which are its aforementioned anti-oxidant functions that protect muscle cells from respiration-linked ROS production, and its role in mitochondrial biogenesis, ensure proper myocyte function on the long term [201]. These data suggest that maximal aerobic capacity and mtDNA integrity in muscle cells involve distinct pools of the p53 protein that control the transcription of genes encoded either by the nuclear or the mitochondrial genomes.
The role of the p53 pathway in cardiac cells has been poorly investigated but the finding that p53 controls the expression of genes related to mitochondrial activity and biogenesis, oxidative phosphorylation and FAO, cardiac architecture, and excitation-contraction coupling in adult cardiomyocytes raises important questions regarding its physiological role in heart and potential implication in cardiovascular diseases. Consistent with this notion, p53 inactivation in adult murine cardiomyocytes results in cardiac hypertrophy and increased expression of markers of heart failure [202]. Nevertheless, p53 can shift from a death promoting function in cardiomyocytes to a pro-survival activity upon reoxygenation in a model of infarcted heart, an effect that was linked to its acetylation on K118 by TIP60 and differential binding to Bax and Nos3 promoters [203].
4.2. Role of P53-Associated metabolic functions in liver
Another tissue in which p53-metabolic functions play an important physiological role is the liver [204]. p53 is strongly induced in hepatocytes during fasting in an AMPK-dependent manner, but p53 is also activated in liver in conditions of nutrient excess [[205], [206], [207]]. p53 has been linked to several key metabolic pathways in hepatocytes, but conflicting results preclude a definitive conclusion about its exact functions in liver. In contrast to its positive role on mitochondrial respiration in most cell types, a peculiarity of liver-p53 is to inhibit pyruvate transport in the mitochondria and to decrease OXPHOS, a process implicating the binding of its well-described target, PUMA, with the pyruvate transporter MPC2 [208]. Other data support both positive and negative effects of p53 on gluconeogenesis. On one hand, p53 was proposed to control the expression of SIRT6, interfering with the nuclear localization and transcriptional activity of FOXO1, a key regulator of a gluconeogenic program that includes phosphoenolpyruvate carboxykinase 1 (PCK1) and Glucose-6-phosphatase (G6PC). Accordingly, these investigators showed that Trp53 KO animals display an improved response to the pyruvate tolerance test that is commonly used to measure the gluconeogenic ability of hepatocytes. Moreover, fasted mice expressing ectopic p53 in liver exhibit impaired gluconeogenesis after injection of a bolus of pyruvate [209]. On the other hand, several groups have shown that gluconeogenesis is altered in Trp53 KO mice under conditions of nutrient starvation [196,210]. In agreement with the role of p53 in promoting gluconeogenesis, the Rotter laboratory reported that pharmacological stabilization of p53 by Nutlin3A in human hepatocarcinoma HepG2 cells induces a gluconeogenic program that includes PCK2, G6PC, Glycerol kinase (GK), Aquaporins 3 and 9. The same authors also showed that primary hepatocytes isolated from p53-deficient mice exhibit impaired gluconeogenesis ex vivo [211]. In addition, acute inactivation of Tp53 in hepatocytes of adult mice has been shown to decrease glycogen storage and to impair gluconeogenesis, although the latter phenotype was attributed to altered amino-acid catabolism, an important gluconeogenic process, rather than to decreased PCK/G6PC expression [205].
Other investigators have linked changes in p53 activity in hepatocytes to other aspects of glucose metabolism. Thus, knock-in mice expressing the p53 S18A mutant exhibit glucose intolerance and insulin resistance in a hyperinsulinemic-euglycemic clamp test. These phenotypes, that were only observed in 6-month-old mice but not in younger animals, were rescued upon administration of an anti-oxidant, suggesting that this defect is an indirect consequence of chronic oxidative stress in these animals [151]. Consistent with this notion, mice with increased gene dosage of Trp53 display improved glucose tolerance, an effect that is possibly associated to the enhanced expression of anti-oxidant genes regulated by p53 in this animal model [111,212].
One common phenotype found in animal models with perturbed p53 activity is hepatosteatosis, a process that results from abnormal accumulation of triglycerides in hepatocytes. Paradoxically, both p53 activation and inactivation result in liver steatosis through different mechanisms, and nutrient state influences this phenotype. Thus, animals harboring a germline deletion of Trp53 display liver steatosis when fed a high fat diet (HFD) [213]. This phenotype is due, at least in part, to the impaired regulation of the p53-target gene Aromatase which encodes a key enzyme involved in the conversion of testosterone into 17β-estradiol, leading to an increase in testosterone over estradiol levels and triglyceride accumulation [213]. Although expression of ectopic aromatase was shown to rescue hepatosteatosis of Trp53 KO males under HFD, it is likely that other metabolic activities of p53, such as p53-mediated repression of fatty acid synthesis, also contributed to this phenotype. Using Trp53 conditional KO mice, it was also shown that acute inactivation of p53 in adult hepatocytes rapidly induces liver steatosis in fed animals, indicating that this phenotype is not a long-term consequence of p53 loss [205]. Paradoxically, pharmacological inhibition of p53 by pifithrin α p-nitro (PFTα) limits HFD-induced hepatosteatosis by promoting FAO. This metabolic effect was associated to the down-regulation of the p53-responsive miR34a that targets SIRT1 mRNA. PFTα-induced stabilization of SIRT1 in turn facilitates Malonyl-Coenzyme A Decarboxylase (MCD) transcription by activating PGC1α-PPARα and inhibits AcetylCoA Carboxylase (ACC) activity through the LKB1-AMPK cascade, thereby leading to decreased amount of Malonyl-CoA and, consequently, to the activation of CPT1, a mitochondrial fatty acid transporter that fuels FAO [214]. Interestingly, p53 function in lipid metabolism also plays an important role in hepatocytes in conditions where nutrients are limiting. Indeed, hepatocytes respond to nutrient starvation by limiting ribosome assembly and function, one of the most energy consuming biological processes in cells, in part through the activation of the Impaired Ribosome Biogenesis Checkpoint (IRBC). During this physiological response to nutrient deprivation, non-assembled ribosomal proteins bind to MDM2 and inhibit its interaction with p53, thereby unleashing p53 activity. Using an elegant mouse model in which MDM2 function in the ribosomal stress response was compromised, p53 was shown to promote FAO during nutrient starvation by stimulating MCD transcription [22].
A recent observation has linked WT-p53 to the regulation of the mevalonate pathway in hepatocytes through the transcription of ABCA1, a gene encoding a retrograde cholesterol transporter that influences SREBP2 maturation and its nuclear translocation [45]. p53 can also influence reverse cholesterol transport through the transcriptional control of Caveolin1 (CAV1), which gene product directly interacts with ABCA1 [215]. This p53-ABCA1-SREBP2 cascade was initially identified in the context of hepatocellular carcinoma (HCC) initiation [45]. However, given the important ramifications of this key metabolic pathway in cholesterol metabolism, and in steroid hormones, non-sterol isoprenoids, and Coenzyme Q synthesis, it is likely to be implicated in some of the metabolic changes associated with perturbed p53 activity in vivo. Finally, as already mentioned, p53 modulates ureagenesis through repression of CPS1, OTC and ARG1, three key enzymes of the urea cycle, a major detoxifying process that takes place in liver. In agreement with these findings, p53-deficient animals display increased urea levels in liver, serum and urine upon administration of NH4Cl [102].
More work is needed to further understand the importance of p53 in the regulation of each of these key liver functions and evaluate their role in human pathologies beyond cancer. Nevertheless, these data indicate that the contribution of p53 to proper liver homeostasis involves multiple metabolic pathways and is highly dependent on the nutritional status.
4.3. Role of P53-associated metabolic functions in adipose tissue
Converging evidence supports the notion that p53 is an important regulator of adipose tissue (AT) function. The roles of p53 in AT are multiple, including the control of whole body energy metabolism, thermo-regulation, inflammation, senescence, protection against lipotoxicity, and the regulation of insulin sensitivity. Some of these activities have been extensively documented in previous reviews, so we focus in this section on the role of p53 in adipocyte differentiation and on its metabolic activities, which represent an important facet of p53 functions in AT [216,217]. Several reports suggest that p53 plays different roles in white versus beige/brown adipocytes, which are distinct populations of adipocytes with specialized activities in fatty acid storage or lipid catabolism and heat generation, respectively. Independent laboratories have shown that p53 restricts differentiation into the white adipocyte lineage through several mechanisms including the regulation of C/EBPα and PPARγ, two major regulators of adipocyte differentiation. In line with this notion, p53-deficient Mouse Embryonic Fibroblasts (MEFs) and pre-adipocytes differentiate more efficiently into mature adipocytes in vitro, whereas expression of ectopic p53 impairs adipocyte differentiation [197,[218], [219], [220]]. However, p53 functions in mature adipocytes extend beyond the regulation of differentiation. Indeed, p53-associated metabolic activities, including the control of NADPH production by malic enzymes or by the PPP, and the inhibition of SREBP-mediated regulation of genes involved in de novo fatty acid synthesis, likely synergize to limit lipid anabolism in adipocytes [42,221]. Despite these consistent findings based on in vitro experiments, the in vivo function of p53 in adipocytes remains confusing. Indeed, phenotypic analysis of Trp53 KO animals have led to very distinct conclusions, in particular when these animals were challenged on a HFD. Thus, although two groups reported an increased tendency of Trp53 whole-body KO animals to accumulate more fat mass when fed a HFD [213,222], others teams showed that these mice gain less weight under HFD [197,223]. In the latter studies, the resistance of Trp53 KO mice to HFD-induced obesity was linked to increased energy expenditure, an effect correlated with enhanced expression of the uncoupling protein 1 (UCP1) that promotes the thermogenic potential of brown and beige adipocytes [197,223]. Although these results appear contradictory, it is plausible that housing conditions of these animals (at room temperature versus at thermoneutrality) dramatically influenced the outcome of p53 deficiency. In contrast to the above described data suggesting an inhibitory role of p53 on brown/beige adipocytes, several lines of evidence support the notion that p53 enhances their activity. Thus, p53 was proposed to promote brown adipocyte differentiation and function during embryonic development, in part through the direct transcriptional induction of key BAT regulators, including Prdm16 and Elovl3. Thus, Rotter and colleagues showed that Trp53 KO E18.5 embryos display altered expression of BAT markers, including PRDM16 and UCP1, and brown adipocyte differentiation in vitro was impaired upon p53 knock-down [222]. The role of p53 in promoting brown adipocyte function and the browning of white adipocytes is also supported by the analysis of a GEMM in which inactivation of Mdm4 was used to activate a p53-acetylation mutant (p533KR) that is defective for the induction of its senescence, cell death and cell cycle programs, but still proficient for the regulation of some of its metabolic target genes. These compound animals exhibit increased energy expenditure and a marked protection against HFD-induced obesity, a phenotype associated with the ability of p533KR to promote the expression of brown adipocyte markers, including Elovl3, an elongase involved in the elongation of long-chain fatty acids, and genes involved in FAO and mitochondrial respiration uncoupling (Cpt-1b, Ucp1, Pgc1α) [224]. Furthermore, viral delivery of WT-p53 in adult brown adipocytes increases the thermogenic capacity of mice and limits weight gain under HFD. Conversely, inhibition of p53 transcriptional activity leads to decreased expression of several thermogenic markers, including UCP1, and results in increased body weight [223]. In conclusion, although these data point to an important role for p53 in adipocytes, further work is needed to fully delineate the complex activities of p53 in adipocytes and the control of whole body energy homeostasis.
4.4. p53 functions in pancreas
p53 tumor suppressor functions in pancreatic cancer have been extensively studied in pancreatic cancer, but much less is known about the metabolic functions of p53 in the endocrine pancreas. p53 activity is induced in pancreatic β-cells in diabetic patients as well as in rodent animal models of type II diabetes, and p53 was shown to be important for β-cell proliferation and survival [[225], [226], [227], [228], [229]]. In contrast to its recognized role in promoting OXPHOS in many cell types, p53 inhibits mitochondrial respiration and calcium signalling in pancreatic β-cells, thereby limiting glucose-stimulated insulin secretion and leading to glucose intolerance. This effect was linked to its ability to repress the expression of pyruvate carboxylase (PC) that resulted in reduced oxalo-acetate (OAA) levels [230].
5. p53 functions in obesity, type II diabetes and NAFLD
Many of the aforementioned studies support the idea that perturbations of p53-associated metabolic functions play a significant role in obesity, as well as in two of its common consequences, type 2 diabetes (T2D) and non-alcoholic fatty liver disease (NAFLD). Consistent with its role in obesity, p53 protein levels increase in rodent models of obesity as well as in human obese individuals [207,221,231,232]. The notion that p53 is implicated in metabolic disorders independently of its tumor suppressive functions is supported by epidemiological studies showing that the single nucleotide polymorphism (SNP) located at position 72 of the human TP53 gene associates with increased risk of T2D, but not with cancer predisposition [233,234]. The metabolic dysfunctions associated with this SNP were confirmed in a humanized mouse model that was used to study the differential effects of the P72 versus the R72 alleles. These authors showed that knock-in mice expressing the p53 R72 allele exhibit impaired regulation of a subset of p53 target genes (including Tnf, Pck1, Ccl2 and Npc1l1), and were more susceptible than mice expressing the P72 allele to obesity and glucose intolerance when fed a HFD [235].
Although there is currently no clear clinical evidence that p53 directly contributes to type I diabetes, perturbations of p53-associated functions in experimental models can lead to several key biological processes that underlie diabetes, including loss of pancreatic β-cells, pertubations of glucose homeostasis at the organismal level, and insulin resistance in peripheral tissues. Several groups have reported that p53 activation in response to various insults, including oxidative stress and increased free-fatty-acids levels, leads to cell death of pancreatic β-cells [[225], [226], [227], [228], [229],236]. Moreover, by directly regulating glucose uptake, glycolysis, and gluconeogenesis, p53 impacts glucose metabolism in peripheral tissues. p53-mediated alteration of glucose homeostasis can lead to increased levels of circulating glucose, a hallmark of T2D. Finally, as already mentioned, p53 activity and the insulin-PI3K-AKT signalling pathway are intertwined in various cell types, in particular in peripheral tissues that respond to insulin to regulate glucose metabolism such as skeletal muscles, heart, WAT, liver and kidneys [152]. Other aspects of p53 functions influence insulin sensitivity in a cell-autonomous manner as well as through autocrine loops. Thus, the pro-inflammatory activities of p53 in AT that are stimulated by nutrient excess impair insulin sensitivity [231,237]. However, the relationship between p53, the immune system and diabetes is complex since administration of Nutlin3A, which results in p53 activation, was shown to ameliorate the severity of streptozotocin-induced diabetes through a mechanism implicating IL12p40 secretion and the regulation of the immune system [238]. Interestingly, the stabilization of p53 in AT under HFD can reprogram distantly liver gluconeogenesis, thereby amplifying glucose homeostasis defects that ultimately lead to insulino resistance [231]. Another interesting aspect by which p53 influences glucose homeostasis involves the crosstalk between angiogenic cells and skeletal muscles. Indeed, endothelial cell-specific inactivation of Trp53 limits the impaired production of nitric oxide (NO) that occurs under HFD, leading to improved glucose consumption in surrounding muscle cells, a process that was linked to increased expression of PGC1α and mitochondrial biogenesis in these cells. Conversely, p53 activation resulting from Mdm4 inactivation in endothelial cells impaired insulin sensitivity and glucose tolerance [239]. Finally, p53-mediated control of the orixegenic hormone ghrelin in the central nervous system can influence feeding behaviors, thereby contributing to obesity and its related metabolic disorders [240].
NAFLD is a common manifestation of obesity and metabolic syndrome. NAFLD includes a large spectrum of pathological changes, ranging from benign hepatosteatosis (fatty liver) to its more severe manifestation, non-alcoholic steatohepatitis (NASH), a recognized risk factor for hepatocellular carcinomas (HCCs). Many stresses associated with chronic liver injury such as metabolic disturbances, iron deposition, or high saturated fatty acid concentration can induce p53 activity. Conversely, many perturbations of p53 activities can also contribute to each of these liver defects. Consistent with its role in liver disease, p53 protein levels in hepatocytes correlate with the severity of steatosis in NAFLD patients and p53 induction has been reported in several mouse models of NAFLD [206,[241], [242], [243]]. One of the key questions in this field relates to the potential clinical interest of therapeutic strategies aiming at modulating p53 activities for NAFLD and diabetes. As already described, different studies based on genetic inactivation of Trp53 in mice fed a HFD led to very different conclusions regarding the accumulation of fat mass. Nevertheless, p53 deficiency improves various symptoms associated with liver injury in animal models of NAFLD, including fibrosis [206,242,244]. Although these data have interesting clinical perspectives, long-term inhibition of p53 in patients is not an option given its potential dramatic side effects on tumor predisposition. Nevertheless, pharmacological inhibition of p53 using PFTα, even for a short period of time, has been shown to improve liver steatosis and insulin sensitivity in rodent models fed a HFD [207,214]. Paradoxically, moderate activation of p53 in hepatocytes obtained by administration of low doses of the DNA damaging agent doxorubicin improved diet-induced non-alcoholic steatosis and steatohepatitis in mice [245]. In addition, administration of Nutlin improves insulin sensitivity in an experimental model of T2D [238].
Altogether, these studies provide clear experimental evidence that p53 plays an important role in NAFLD and diabetes. However, they do not come with a definitive conclusion regarding which strategy (p53 activation or p53 inactivation) would provide the best clinical benefit to NAFLD and diabetic patients. Translating such strategies to the clinic will require a much deeper understanding of the mechanisms that fine tune p53 activities in vivo in order to design efficient, but also safe, therapies.
6. Conclusions
An impressive amount of data collected in the past twenty years have shed light on the importance of both WT-p53 and mutated forms of p53 in metabolism and showed that perturbations of these metabolic networks influence cancer progression but also contribute to various metabolic disorders. However, these studies have also underscored the complexity of p53-associated metabolic functions, leading sometimes to contradictory results that preclude definitive conclusions. Many parameters could explain these discrepancies, and it is noteworthy that a significant number of these studies have been performed using different cancer cell lines that have undergone multiple metabolic adaptations during the process of transformation, many of which impinge on p53-associated metabolic activities. In addition, as illustrated extensively in this review, p53 is a key sensor of metabolic changes and therefore, a particular attention should be payed to in vitro cell culture conditions when performing metabolism-related experiments because they can dramatically influence p53 activities. More suprisingly, conflicting results have also been reported when the metabolic functions of p53 were assessed in vivo, even sometime using the same animal models. One intriguing illustration of such opposite conclusions relates to the role of p53 in animals fed a HFD, a situation during which Trp53 inactivation was shown to either promote or limit weight gain [197,213,222,223]. The in vivo function of p53 in gluconeogenesis is also a matter of debate [196,205,[209], [210], [211]]. More work is needed to understand these contrasting results, but it is plausible that different housing conditions of these animals (temperature, inverted light cycle, etc), specific genetic backgrounds, or distinct microbiota, influenced the control of key metabolic activities by p53. In light of these studies, it is becoming obvious that p53 functions in metabolism display some degree of cell-type specificity that is poorly understood but which is likely influenced by a number of factors, including the tissue-specific expression pattern of p53 regulators, as well as that of other p53 family members (p63, p73) that display important metabolic activities [[246], [247], [248]]. Moreover, the differential role of p53 isoforms in metabolism remains an unsolved question that requires more investigations. Finally, although in this review we mainly focused on the metabolic activities of WT-p53, the roles of p53 mutants in metabolism are also gaining momentum but require further investigation. Another aspect of p53 function in metabolism that requires clarification is the mechanism by which p53, and other components of the p53 pathway, coordinate their metabolic activities in different tissues to maintain energy homeostasis at the organismal level. Whether this level of coordination involves specific neurological signals, circulating metabolites, or secreted proteins regulated by p53 remains to be determined, but there is little doubt that the p53 pathway is playing an important role in coordinating whole body metabolism. Hence, in addition to its well-documented function in cancer and its recognition as the “guardian of the genome”, it may be time to consider p53 as a “guardian of energy homeostasis."
Despite the large amount of work already performed in this field we still lack an integrated vision of p53-associated metabolic activities at the cellular level. Nevertheless, some general traits are emerging from past studies. First, in most cell types, p53 appears to limit glycolytic flux and coordinate it with branched anabolic pathways such as the PPP and the serine synthesis pathway, in part to maintain a proper redox state. p53 negatively regulates several anabolic pathways, including de novo fatty-acid synthesis, and concomitantly stimulates lipid catabolism (FAO). It is noteworthy that p53 is highly connected to lipid metabolism both at the systemic and cellular levels through multiple activities controlling lipid transport, storage, modification, and utilization [43]. As a general mechanism to limit proliferation, it is possible that the overall function of p53 in amino-acid metabolism is to divert these building blocks away from protein synthesis. Another important function by which p53 influences metabolism as well as cell fate is through the control of iron metabolism [75]. p53 functions in these multiple metabolic pathways involve transcriptional as well as post-transcriptional mechanisms implicating different pools of the p53 protein exhibiting distinct subcellular localizations. The direct control of metabolic enzymes by p53 through protein–protein interactions raises puzzling questions given the relative stochiometry of these different proteins. A strong body of evidence also suggests that p53 promotes mitochondrial respiration in many cell types, with the exception of pancreatic β cells and hepatocytes [205,208,230] (Figure 3).
Figure 3.
Role of the p53 network in adaptive and stress responses. The molecular mechanisms underlying p53's implication in adaptive versus stress responses are poorly understood. In the proposed model, multiple negative feed-back loops contribute to maintain the p53 response within a range of activity that favors cell survival and ensure proper cell homeostasis. When these stresses increase in intensity and/or duration, p53 gets recruited to a subset of its target genes that leads to the elimination of damaged cells.
It is interesting to think about the links between p53 and metabolism from an evolutionary standpoint. The importance of p53 in nutrient storage and utilization is particularly intriguing in light of the natural selection processes that led to the emergence of gene networks that guarantee proper energy homeostasis during periods when food was limiting. The gene networks regulated by p53 play a pivotal role in adaptive responses to nutrient deprivation by promoting cell survival and restricting various biological processes that are intensively consuming energy such as DNA replication or protein translation. By definition, such adaptive responses are highly dynamic and must be tightly controlled by regulatory mechanisms that are distinct from those involved in stress responses that usually lead to cell demise. Numerous studies have already delineated the role of p53 in acute stress responses using various experimental models, but much less is known about p53 functions during short-term adaptive responses. It is important to note that the majority of our experimental models, in particular those used in vitro, do not faithfully mimic physiological situations faced by normal cells, and are not adapted to precisely evaluate p53 activities during adaptive responses. Finally, it is tempting to speculate that the multiple feedback loops in which p53 is involved define a finely tuned network that maintains p53 functions within a range of activity that is compatible with cell survival (See Figure 3). We propose that the genes contributing to the numerous feedback loops in which p53 plays a central role collectively define a genetic buffer that is instrumental to ensure energy and redox homeostasis. Perturbations of this network might lead to human diseases including cancer as well as some of the metabolic disorders that have been highlighted in this review. Given the worldwide increased prevalence of metabolic syndrome and its associated consequences, pharmacological manipulation of the p53 pathway appears as a promising strategy to improve the health of patients with such symptoms. However, we still need to improve our understanding of p53-associated metabolic networks in order to avoid harmful side effects of such strategies that could influence tumor incidence. This is particularly true for chronic diseases, such as type 2 diabetes or liver disease, which require long-term treatment.
Authors' contributions
LLC, RR, LKL, GA, ML wrote the manuscript. LLC and ML prepared the figures.
Acknowledgements
We thank members of the LLC team, S. Pattingre, N. Loiseau, I. Ben-Sarah and S. Dixon for critical reading of the manuscript. G.A. is supported by the French National Cancer Institute and R.R by the Damon Runyon Foundation (DRG2326-18). LLC group is funded by the Institut National de la Santé et de la Recherche Médicale, the ARC Foundation, the Ligue Nationale contre le Cancer, INCa, the National Agency for Research (ANR), the cancéropôle Grand Sud Ouest, the Laboratory of Excellence EpiGenMed (grant ANR-10-LABX-12-01), SIRIC Montpellier Cancer (Grant INCa_Inserm_DGOS_12553) and the région Occitanie.
Conflict of interest
The authors declare no competing interests.
References
- 1.Kruiswijk F., Labuschagne C.F., Vousden K.H. p53 in survival, death and metabolichealth: a lifeguard with a licence to kill. Nature Reviews Molecular Cell Biology. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 2.Labuschagne C.F., Zani F., Vousden K.H. Control of metabolism by p53- Cancer and beyond. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2018;1870(1):32–42. doi: 10.1016/j.bbcan.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flöter J., Kaymak I., Schulze A. Regulation of metabolic activity by p53. Metabolites. 2017;7(2):21. doi: 10.3390/metabo7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Zhang C., Hu W., Feng Z. Tumor suppressor p53 and metabolism. Journal of Molecular Cell Biology. 2018;11(4):284–292. doi: 10.1093/jmcb/mjy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt V., Nagar R., Martinez L. Control of nucleotide metabolism enables mutant p53's oncogenic gain-of-function activity. International Journal of Molecular Sciences. 2017;18(12):2759. doi: 10.3390/ijms18122759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blandino G., Valenti F., Sacconi A., Di Agostino S. Wild-type and mutant p53 protein in mitochondrial dysfunction: emerging insights in cancer disease. Seminars in Cell & Developmental Biology. 2019;18:30163–30170. doi: 10.1016/j.semcdb.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 7.D'Orazi G., Cirone M. Mutant p53 and cellular stress pathways: a criminal alliance that promotes cancer progression. Cancers. 2019;11(5):614. doi: 10.3390/cancers11050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamp W.M., Wang P.-Y., Hwang P.M. TP53 mutation, mitochondria and cancer. Current Opinion in Genetics & Development. 2016;38:16–22. doi: 10.1016/j.gde.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartzenberg-Bar-Yoseph F. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Research. 2004;64(7):2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 10.Kawauchi K., Araki K., Tobiume K., Tanaka N. p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nature Cell Biology. 2008;10(5):611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 11.Boidot R., Vegran F., Meulle A., Le Breton A., Dessy C., Sonveaux P. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Research. 2012;72(4):939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 12.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N.C., Nakano K., Bartrons R. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C., Liu J., Wu R., Liang Y., Lin M., Liu J. Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget. 2014;5(14):5535–5546. doi: 10.18632/oncotarget.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ros S., Flöter J., Kaymak I., Da Costa C., Houddane A., Dubuis S. 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 is essential for p53-null cancer cells. Oncogene. 2017;36(23):3287–3299. doi: 10.1038/onc.2016.477. [DOI] [PubMed] [Google Scholar]
- 15.Franklin D.A., He Y., Leslie P.L., Tikunov A.P., Fenger N., Macdonald J.M. p53 coordinates DNA repair with nucleotide synthesis by suppressing PFKFB3 expression and promoting the pentose phosphate pathway. Scientific Reports. 2016;6:38067. doi: 10.1038/srep38067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.-R., Roe J.-S., Lee J.-E., Cho E.-J., Youn H.-D. p53 regulates glucose metabolism by miR-34a. Biochemical and Biophysical Research Communications. 2013;437(2):225–231. doi: 10.1016/j.bbrc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Kondoh H., Lleonart M.E., Gil J., Wang J., Degan P., Peters G. Glycolytic enzymes can modulate cellular life span. Cancer Research. 2005;65(1):177–185. [PubMed] [Google Scholar]
- 18.Contractor T., Harris C.R. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Research. 2012;72(2):560–567. doi: 10.1158/0008-5472.CAN-11-1215. [DOI] [PubMed] [Google Scholar]
- 19.Morris J.P., Yashinskie J.J., Koche R., Chandwani R., Tian S., Chen C.-C. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019:1–31. doi: 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S., Tanaka T., Poyurovsky M.V., Nagano H., Mayama T., Ohkubo S. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proceedings of the National Academy of Sciences. 2010;107(16):7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu W., Zhang C., Wu R., Sun Y., Levine A., Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proceedings of the National Academy of Sciences. 2010;107(16):7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., He Y., Jin A., Tikunov A.P., Zhou L., Tollini L.A. Ribosomal protein-Mdm2-p53 pathway coordinates nutrient stress with lipid metabolism by regulating MCD and promoting fatty acid oxidation. Proceedings of the National Academy of Sciences. 2014;11(23):E2414–E2422. doi: 10.1073/pnas.1315605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assaily W., Rubinger D.A., Wheaton K., Lin Y., Ma W., Xuan W. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Molecular Cell. 2011;44(3):491–501. doi: 10.1016/j.molcel.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Macedo N., Feng J., Faubert B., Chang N., Elia A., Rushing E.J. Depletion of the novel p53-target gene carnitine palmitoyltransferase 1C delays tumor growth in the neurofibromatosis type I tumor model. Cell Death & Differentiation. 2013;20(4):659–668. doi: 10.1038/cdd.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang D., LaGory E.L., Brož D.K., Bieging K.T., Brady C.A., Link N. Analysis of p53 transactivation domain mutants reveals Acad11 as a metabolic target important for p53 pro-survival function. Cell Reports. 2015;10(7):1096–1109. doi: 10.1016/j.celrep.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matoba S., Kang J.G., Patino W.D., Wragg A., Boehm M., Gavrilova O. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 27.Park J.Y., Wang P.Y., Matsumoto T., Sung H.J., Ma W., Choi J.W. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circulation Research. 2009;105(7):705–712. doi: 10.1161/CIRCRESAHA.109.205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stambolsky P., Weisz L., Shats I., Klein Y., Goldfinger N., Oren M. Regulation of AIF expression by p53. Cell Death & Differentiation. 2006;13(12):2140–2149. doi: 10.1038/sj.cdd.4401965. [DOI] [PubMed] [Google Scholar]
- 29.Achanta G., Sasaki R., Feng L., Carew J.S., Lu W., Pelicano H. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. The EMBO Journal. 2005;24(19):3482–3492. doi: 10.1038/sj.emboj.7600819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong T.S., Rajagopalan S., Townsley F.M., Freund S.M., Petrovich M., Loakes D. Physical and functional interactions between human mitochondrial single-stranded DNA-binding protein and tumour suppressor p53. Nucleic Acids Research. 2008;37(2):568–581. doi: 10.1093/nar/gkn974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida Y., Izumi H., Torigoe T., Ishiguchi H., Itoh H., Kang D. P53 physically interacts with mitochondrial transcription factor A and differentially regulates binding to damaged DNA. Cancer Research. 2003;63(13):3729–3734. [PubMed] [Google Scholar]
- 32.Bakhanashvili M., Grinberg S., Bonda E., Simon A.J., Moshitch-Moshkovitz S., Rahav G. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death & Differentiation. 2008;15(12):1865–1874. doi: 10.1038/cdd.2008.122. [DOI] [PubMed] [Google Scholar]
- 33.Bergeaud M., Mathieu L., Guillaume A., Moll U., Mignotte B., Le Floch N. Mitochondrial p53 mediates a transcription-independent regulation of cell respiration and interacts with the mitochondrial F₁F₀-ATP synthase. Cell Cycle. 2014;12(17):2781–2793. doi: 10.4161/cc.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem A., Iqbal S., Zhang Y., Hood D.A. Effect of p53 on mitochondrial morphology, import, and assembly in skeletal muscle. The Australian Journal of Pharmacy: Cell Physiology. 2015;308(4):C319–C329. doi: 10.1152/ajpcell.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong B., Wang Q., Fung E., Xue K., Tsang B.K. p53 is required for cisplatin-induced processing of the mitochondrial fusion protein L-opa1 that is mediated by the mitochondrial metallopeptidase Oma1 in gynecologic cancers. Journal of Biological Chemistry. 2014;289(39):27134–27145. doi: 10.1074/jbc.M114.594812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Donath S., Li Y., Qin D., Prabhakar B.S., Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genetics. 2010;6(1):e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.-X., Jiao J.-Q., Li Q., Long B., Wang K., Liu J.-P. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nature Medicine. 2010;17(1):71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura N., Nakamura Y., Miyamoto Y., Miyamoto T., Kabu K., Yoshida M. Mieap, a p53-inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenzelmann Broz D., Spano Mello S., Bieging K.T., Jiang D., Dusek R.L., Brady C.A. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes & Development. 2013;27(9):1016–1031. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C., Lin M., Wu R., Wang X., Yang B., Levine A.J. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proceedings of the National Academy of Sciences. 2011;108(39):16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto Y., Kitamura N., Nakamura Y., Futamura M., Miyamoto T., Yoshida M. Possible existence of lysosome-like organella within mitochondria and its role in mitochondrial quality control. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.J Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nature Cell Biology. 2011;13(3):310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein I., Rotter V. Regulation of lipid metabolism by p53 – fighting two villains with one sword. Trends in Endocrinology and Metabolism. 2012;23(11):567–575. doi: 10.1016/j.tem.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Parrales A., Iwakuma T. p53 as a regulator of lipid metabolism in cancer. International Journal of Molecular Sciences. 2016;17(12):2074. doi: 10.3390/ijms17122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon S.-H., Huang C.-H., Houlihan S.L., Regunath K., Freed-Pastor W.A., Morris J.P., 4th p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2018;176(3):564–580. doi: 10.1016/j.cell.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang P., Du W., Mancuso A., Wellen K.E., Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493(7434):689–693. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deisenroth C., Itahana Y., Tollini L., Jin A., Zhang Y. p53-inducible DHRS3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. Journal of Biological Chemistry. 2011;286(32):28343–28356. doi: 10.1074/jbc.M111.254227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirschner R.D., Rother K., Müller G.A., Engeland K. The retinal dehydrogenase/reductase retSDR1/DHRS3gene is activated by p53 and p63 but not by mutants derived from tumors or EEC/ADULT malformation syndromes. Cell Cycle. 2014;9(11):2177–2188. doi: 10.4161/cc.9.11.11844. [DOI] [PubMed] [Google Scholar]
- 49.Mirza A., Wu Q., Wang L., McClanahan T., Bishop W.R., Gheyas F. Global transcriptional program of p53 target genes during the process of apoptosis and cell cycle progression. Oncogene. 2003;22(23):3645–3654. doi: 10.1038/sj.onc.1206477. [DOI] [PubMed] [Google Scholar]
- 50.Rueda-Rincon N., Bloch K., Derua R., Vyas R., Harms A., Hankemeier T. p53 attenuates AKT signaling by modulating membrane phospholipid composition. Oncotarget. 2015;6(25):21240–21254. doi: 10.18632/oncotarget.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirschner K., Samarajiwa S.A., Cairns J.M., Menon S., Pérez-Mancera P.A., Tomimatsu K. Phenotype specific analyses reveal distinct regulatory mechanism for chronically activated p53. PLoS Genetics. 2015;11(3):e1005053. doi: 10.1371/journal.pgen.1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moiseeva O., Bourdeau V., Roux A., Deschênes-Simard X., Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Molecular and Cellular Biology. 2009;29(16):4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quijano C., Cao L., Fergusson M.M., Romero H., Liu J., Gutkind S. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11(7):1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein I., Ezra O., Rivlin N., Molchadsky A., Madar S., Goldfinger N. p53, a novel regulator of lipid metabolism pathways. Journal of Hepatology. 2012;56(3):656–662. doi: 10.1016/j.jhep.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Ide T., Brown-Endres L., Chu K., Ongusaha P.P., Ohtsuka T., El-Deiry W.S. GAMT, a p53-inducible modulator of apoptosis, is critical for the adaptive response to nutrient stress. Molecular Cell. 2009;36(3):379–392. doi: 10.1016/j.molcel.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Jeffries K.A., Krupenko N.I. Ceramide signaling and p53 pathways. Advances in Cancer Research. 2018;140:191–215. doi: 10.1016/bs.acr.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taha T.A., Osta W., Kozhaya L., Bielawski J., Johnson K.R., Gillanders W.E. Down-regulation of sphingosine kinase-1 by DNA damage. Journal of Biological Chemistry. 2004;279(19):20546–20554. doi: 10.1074/jbc.M401259200. [DOI] [PubMed] [Google Scholar]
- 58.Heffernan-Stroud L.A., Helke K.L., Jenkins R.W., De Costa A.-M., Hannun Y.A., Obeid L.M. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene. 2011;31(9):1166–1175. doi: 10.1038/onc.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fekry B., Jeffries K.A., Esmaeilniakooshkghazi A., Ogretmen B., Krupenko S.A., Krupenko N.I. CerS6Is a novel transcriptional target of p53 protein activated by non-genotoxic stress. Journal of Biological Chemistry. 2016;291(32):16586–16596. doi: 10.1074/jbc.M116.716902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shamseddine A.A., Clarke C.J., Carroll B., Airola M.V., Mohammed S., Rella A. P53-dependent upregulation of neutral sphingomyelinase-2: role in doxorubicin-induced growth arrest. Cell Death & Disease. 2015;6(10):e1947. doi: 10.1038/cddis.2015.268. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu R., Garcia-Barros M., Wen S., Li F., Lin C.-L., Hannun Y.A. Tumor suppressor p53 links ceramide metabolism to DNA damage response through alkaline ceramidase 2. Cell Death & Differentiation. 2018;25(5):841–856. doi: 10.1038/s41418-017-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fekry B., Jeffries K.A., Esmaeilniakooshkghazi A., Szulc Z.M., Knagge K.J., Kirchner D.R. -ceramide is a natural regulatory ligand of p53 in cellular stress response. Nature Communications. 2018:1–12. doi: 10.1038/s41467-018-06650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoeferlin L.A., Fekry B., Ogretmen B., Krupenko S.A., Krupenko N.I. Folate stress induces apoptosis via p53-dependent de Novo ceramide synthesis and up-regulation of ceramide synthase 6. Journal of Biological Chemistry. 2013;288(18):12880–12890. doi: 10.1074/jbc.M113.461798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torti S.V., Torti F.M. Iron and cancer: more ore to be mined. Nature Reviews Cancer. 2013;13(5):342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weizer-Stern O., Adamsky K., Margalit O., Ashur-Fabian O., Givol D., Amariglio N. Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. British Journal of Haematology. 2007;138(2):253–262. doi: 10.1111/j.1365-2141.2007.06638.x. [DOI] [PubMed] [Google Scholar]
- 66.Funauchi Y., Tanikawa C., Lo P.H.Y., Mori J., Daigo Y., Takano A. Regulation of iron homeostasis bythe p53-ISCU pathway. Scientific Reports. 2015;2(5):16497. doi: 10.1038/srep16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang P.M., Bunz F., Yu J., Rago C., Chan T.A., Murphy M.P. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nature Medicine. 2001;7(10):1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G., Chen X. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene. 2002;21(47):7195–7204. doi: 10.1038/sj.onc.1205862. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu R., Lan N.N., Tai T.T., Adachi Y., Kawazoe A., Mu A. p53 directly regulates the transcription of the human frataxin gene and its lack of regulation in tumor cells decreases the utilization of mitochondrial iron. Gene. 2014;551(1):79–85. doi: 10.1016/j.gene.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Dongiovanni P., Fracanzani A.L., Cairo G., Megazzini C.P., Gatti S., Rametta R. Iron-dependent regulation of MDM2 influences p53 activity and hepatic carcinogenesis. American Journal Of Pathology. 2010;176(2):1006–1017. doi: 10.2353/ajpath.2010.090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An W.G., Kanekal M., Simon M.C., Maltepe E., Blagosklonny M.V., Neckers L.M. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392(6674):405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 72.Saletta F., Suryo Rahmanto Y., Noulsri E., Richardson D.R. Iron chelator-mediated alterations in gene expression: identification of novel iron-regulated molecules that are molecular targets of hypoxia-inducible factor-1 and p53. Molecular Pharmacology. 2010;77(3):443–458. doi: 10.1124/mol.109.061028. [DOI] [PubMed] [Google Scholar]
- 73.Shen J., Sheng X., Chang Z., Wu Q., Wang S., Xuan Z. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulationof p53 localization, stability, and function. Cell Reports. 2014;7(1):180–193. doi: 10.1016/j.celrep.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J.-H., Jang H., Cho E.-J., Youn H.-D. Ferritin binds and activates p53 under oxidative stress. Biochemical and Biophysical Research Communications. 2009;389(3):399–404. doi: 10.1016/j.bbrc.2009.08.125. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J., Chen X. p53 tumor suppressor and iron homeostasis. FEBS Journal. 2018;286(4):620–629. doi: 10.1111/febs.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Qian Y., Zhang J., Yan W., Jung Y.-S., Chen M. Ferredoxin reductase is critical for p53-dependent tumor suppression via iron regulatory protein 2. Genes & Development. 2017;31(12):1243–1256. doi: 10.1101/gad.299388.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palomo G.M., Cerrato T., Gargini R., Diaz-Nido J. Silencing of frataxin gene expression triggers p53-dependent apoptosis in human neuron-like cells. Human Molecular Genetics. 2011;20(14):2807–2822. doi: 10.1093/hmg/ddr187. [DOI] [PubMed] [Google Scholar]
- 78.Li T., Kon N., Jiang Le, Tan M., Ludwig T., Zhao Y. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang Le, Kon N., Li T., Wang S.-J., Su T., Hibshoosh H. Ferroptosis as a p53-mediated activity during tumor suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S.-J., Li D., Ou Y., Jiang Le, Chen Y., Zhao Y. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Reports. 2016;17(2):366–373. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Yang L., Zhang X., Cui W., Liu Y., Sun Q.R. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Reports. 2019 doi: 10.15252/embr.201847563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu B., Kon N., Chen D., Li T., Liu T., Jiang Le. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nature Cell Biology. 2019;21(5):579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ou Y., Wang S.-J., Li D., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proceedings of the National Academy of Sciences. 2016;113(44):E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jennis M., Kung C.-P., Basu S., Budina-Kolomets A., Leu J.I.-J., Khaku S. An African-specific polymorphism in the TP53gene impairs p53 tumor suppressor function in a mouse model. Genes & Development. 2016;30(8):918–930. doi: 10.1101/gad.275891.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Reports. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 86.Tarangelo A., Magtanong L., Bieging-Rolett K.T., Li Y., Ye J., Attardi L.D. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Reports. 2018;22(3):569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones R.G., Plas D.R., Kubek S., Buzzai M., Mu J., Xu Y. AMP-Activated Protein Kinase induces a p53-dependent Metabolic checkpoint. Molecular Cell. 2005;18(3):283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 88.Reid M.A., Wang W.-I., Rosales K.R., Welliver M.X., Pan M., Kong M. The B55a subunit of PP2A Drivesa p53-dependent metabolic adaptation to glutamine deprivation. Molecular Cell. 2013;50(2):200–211. doi: 10.1016/j.molcel.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Maddocks O.D.K., Berkers C.R., Mason S.M., Zheng L., Blyth K., Gottlieb E. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493(7433):542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tajan M., Hock A.K., Blagih J., Robertson N.A., Labuschagne C.F., Kruiswijk F. A role for p53 in the adaptation to glutamine starvation through the expression of SLC1A3. Cell Metabolism. 2018;28(5):721–736. doi: 10.1016/j.cmet.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowman X.H., Hanse E.A., Yang Y., Gabra M.B.I., Tran T.Q., Li H. p53 promotes cancer cell adaptation to glutamine deprivation by upregulating Slc7a3 to increase arginine uptake. Cell Reports. 2019;26(11):3051–3054. doi: 10.1016/j.celrep.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou Y., Wang S.-J., Jiang L., Zheng B., Gu W. p53 protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. Journal of Biological Chemistry. 2015;290(1):457–466. doi: 10.1074/jbc.M114.616359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Riscal R., Schrepfer E., Arena G., Cissé M.Y., Bellvert F., Heuillet M. Chromatin-bound MDM2 regulates serine metabolism and redox homeostasis independently of p53. Molecular Cell. 2016;62(6):890–902. doi: 10.1016/j.molcel.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 94.Phang J.M. Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxidants and Redox Signaling. 2019;30(4):635–649. doi: 10.1089/ars.2017.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoon K.-A., Nakamura Y., Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. Journal of Human Genetics. 2004;49(3):134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]
- 96.Polyak K., Xia Y., Zweier J.L., Kinzler K.W., Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389(6648):300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 97.Raimondi I., Ciribilli Y., Monti P., Bisio A., Pollegioni L., Fronza G. P53 family members modulate the expression of PRODH, but not PRODH2, via intronic p53 response elements. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei C.-L., Wu Q., Vega V.B., Chiu K.P., Ng P., Zhang T. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 99.Donald S.P., Sun X.Y., Hu C.A., Yu J., Mei J.M., Valle D. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Research. 2001;61(5):1810–1815. [PubMed] [Google Scholar]
- 100.Nagano T., Nakashima A., Onishi K., Kawai K., Awai Y., Kinugasa M. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. Journal of Cell Science. 2017;130(8):1413–1420. doi: 10.1242/jcs.196469. [DOI] [PubMed] [Google Scholar]
- 101.Pang S., Lynn D.A., Lo J.Y., Paek J., Curran S.P. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nature Communications. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Li, Mao Y., Zhao L., Li L., Wu J., Zhao M. p53 regulation of ammonia metabolism throughurea cycle controls polyamine biosynthesis. Nature. 2019;567(7747):253–256. doi: 10.1038/s41586-019-0996-7. [DOI] [PubMed] [Google Scholar]
- 103.Kim H.-R., Roe J.-S., Lee J.-E., Hwang I.-Y., Cho E.-J., Youn H.-D. A p53-inducible microRNA-34a downregulates Ras signaling by targeting IMPDH. Biochemical and Biophysical Research Communications. 2012;418(4):682–688. doi: 10.1016/j.bbrc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 104.Holzer K., Drucker E., Roessler S., Dauch D., Heinzmann F., Waldburger N. Proteomic Analysis Reveals GMP Synthetase as p53 repression taarget in liver cancer. American Journal Of Pathology. 2017;187(2):228–235. doi: 10.1016/j.ajpath.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 105.Reddy B.A., van der Knaap J.A., Bot A.G.M., Mohd-Sarip A., Dekkers D.H.W., Timmermans M.A. Nucleotide biosynthetic enzyme GMP synthase is a TRIM21-controlled relay of p53 stabilization. Molecular Cell. 2014;53(3):458–470. doi: 10.1016/j.molcel.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 106.Wilson P.M., Fazzone W., LaBonte M.J., Lenz H.-J., Ladner R.D. Regulation of human dUTPase gene expression and p53-mediated transcriptional repression in response to oxaliplatin-induced DNA damage. Nucleic Acids Research. 2008;37(1):78–95. doi: 10.1093/nar/gkn910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanaka H., Arakawa H., Yamaguchi T., Shiraishi K., Fukuda S., Matsui K. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404(6773):42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 108.Nakano K., Bálint E., Ashcroft M., Vousden K.H. A ribonucleotide reductase gene is a transcriptional target of p53 and p73. Oncogene. 2000;19(37):4283–4289. doi: 10.1038/sj.onc.1203774. [DOI] [PubMed] [Google Scholar]
- 109.He Z., Hu X., Liu W., Dorrance A., Garzon R., Houghton P.J. P53 suppresses ribonucleotide reductase via inhibiting mTORC1. Oncotarget. 2017;8(25):41422–41431. doi: 10.18632/oncotarget.17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sablina A.A., Budanov A.V., Ilyinskaya G.V., Agapova L.S., Kravchenko J.E., Chumakov P.M. The antioxidant function of the p53 tumor suppressor. Nature Medicine. 2005;11(12):1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matheu A., Maraver A., Klatt P., Flores I., Garcia-Cao I., Borras C. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448(7151):375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 112.Forrester K., Ambs S., Lupold S.E., Kapust R.B., Spillare E.A., Weinberg W.C. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(6):2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hussain S.P., Amstad P., He P., Robles A., Lupold S., Kaneko I. p53-Induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Research. 2004;64(7):2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 114.Kang M.Y., Kim H.-B., Piao C., Lee K.H., Hyun J.W., Chang I.-Y. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death & Differentiation. 2012;20(1):117–129. doi: 10.1038/cdd.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O'Connor J.C., Wallace D.M., O'Brien C.J., Cotter T.G. A novel antioxidant function for the tumor-suppressor gene p53 in the retinal ganglion cell. Investigative Ophthalmology & Visual Science. 2008;49(10):4237–4244. doi: 10.1167/iovs.08-1963. [DOI] [PubMed] [Google Scholar]
- 116.Velasco-Miguel S., Buckbinder L., Jean P., Gelbert L., Talbott R., Laidlaw J. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18(1):127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- 117.Budanov A.V., Shoshani T., Faerman A., Zelin E., Kamer I., Kalinski H. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21(39):6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 118.Budanov A.V., Karin M. p53 target genes Sestrin1 and Sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bae S.H., Sung S.H., Oh S.Y., Lim J.M., Lee S.K., Park Y.N. Sestrins activate Nrf2 by promoting p62-dependent autophagic Degradationof Keap1 and prevent oxidative liver damage. Cell Metabolism. 2013;17(1):73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Okamura S., Arakawa H., Tanaka T., Nakanishi H., Ng C.C., Taya Y. p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis. Molecular Cell. 2001;8(1):85–94. doi: 10.1016/s1097-2765(01)00284-2. [DOI] [PubMed] [Google Scholar]
- 121.Tomasini R., Samir A.A., Pebusque M.-J., Calvo E.L., Totaro S., Dagorn J.C. P53-dependent expression of the stress-induced protein (SIP) European Journal of Cell Biology. 2002;81(5):294–301. doi: 10.1078/0171-9335-00248. [DOI] [PubMed] [Google Scholar]
- 122.N'guessan P., Pouyet L., Gosset G., Hamlaoui S., Seillier M., Cano C.E. Absence of tumor suppressor tumor protein 53-induced nuclear protein 1 (TP53INP1) sensitizes mouse thymocytes and embryonic fibroblasts to redox-driven apoptosis. Antioxidants and Redox Signaling. 2011;15(6):1639–1653. doi: 10.1089/ars.2010.3553. [DOI] [PubMed] [Google Scholar]
- 123.Itahana Y., Han R., Barbier S., Lei Z., Rozen S., Itahana K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. 2014;34(14):1799–1810. doi: 10.1038/onc.2014.119. [DOI] [PubMed] [Google Scholar]
- 124.Italiano D., Lena A.M., Melino G., Candi E. Identification of NCF2/p67phox as a novel p53 target gene. Cell Cycle. 2014;11(24):4589–4596. doi: 10.4161/cc.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dhar S.K., Xu Y., Chen Y., St Clair D.K. Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. Journal of Biological Chemistry. 2006;281(31):21698–21709. doi: 10.1074/jbc.M601083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y., Chaiswing L., Velez J.M., Batinic-Haberle I., Colburn N.H., Oberley T.D. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Research. 2005;65(9):3745–3750. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- 127.Trinei M., Giorgio M., Cicalese A., Barozzi S., Ventura A., Migliaccio E. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21(24):3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 128.Guo X., Disatnik M.-H., Monbureau M., Shamloo M., Mochly-Rosen D., Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington's disease–associated neurodegeneration. Journal of Clinical Investigation. 2013;123(12):5371–5388. doi: 10.1172/JCI70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marchenko N.D., Zaika A., Moll U.M. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. Journal of Biological Chemistry. 2000;275(21):16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 130.Leu J.I.-J., Dumont P., Hafey M., Murphy M.E., George D.L. Mitochondrial p53 activates Bak and causes disruption of a Bak–Mcl1 complex. Nature Cell Biology. 2004;6(5):443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 131.Eriksson S.E., Ceder S., Bykov V.J.N., Wiman K.G. p53 as a hub in cellular redox regulation and therapeutic target in cancer. Journal of Molecular Cell Biology. 2019;11(4):330–341. doi: 10.1093/jmcb/mjz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hafsi H., Hainaut P. Redox control and interplay between p53 isoforms: roles in the regulation of basal p53 levels, cell fate, and senescence. Antioxidants and Redox Signaling. 2011;15(6):1655–1667. doi: 10.1089/ars.2010.3771. [DOI] [PubMed] [Google Scholar]
- 133.Maillet A., Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxidants and Redox Signaling. 2012;16(11):1285–1294. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 134.Niwa-Kawakita M., Ferhi O., Soilihi H., Le Bras M., Lallemand-Breitenbach V., de Thé H. PML is a ROS sensor activating p53 upon oxidative stress. Journal of Experimental Medicine. 2017;214(11):3197–3206. doi: 10.1084/jem.20160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tessier S., Martin-Martin N., de Thé H., Carracedo A., Lallemand-Breitenbach V. Promyelocytic leukemia protein, a protein at the crossroad of oxidative stress and metabolism. Antioxidants and Redox Signaling. 2017;26(9):432–444. doi: 10.1089/ars.2016.6898. [DOI] [PubMed] [Google Scholar]
- 136.De Stanchina E., Querido E., Narita M., Davuluri R.V., Pandolfi P.P., Ferbeyre G. PML is a direct p53 target that modulates p53 effector functions. Molecular Cell. 2004;13(4):523–535. doi: 10.1016/s1097-2765(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 137.Vaseva A.V., Marchenko N.D., Ji K., Tsirka S.E., Holzmann S., Moll U.M. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149(7):1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eby K.G., Rosenbluth J.M., Mays D.J., Marshall C.B., Barton C.E., Sinha S. ISG20L1 is a p53 family target gene that modulates genotoxic stress-induced autophagy. Molecular Cancer. 2010;9(1):95. doi: 10.1186/1476-4598-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao W., Shen Z., Shang L., Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death & Differentiation. 2011;18(10):1598–1607. doi: 10.1038/cdd.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crighton D., Wilkinson S., O'Prey J., Syed N., Smith P., Harrison P.R. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 141.Martoriati A., Doumont G., Alcalay M., Bellefroid E., Pelicci P.G., Marine J.-C. dapk1, encoding an activator of a p19ARF-p53-mediated apoptotic checkpoint, is a transcription target of p53. Oncogene. 2004;24(8):1461–1466. doi: 10.1038/sj.onc.1208256. [DOI] [PubMed] [Google Scholar]
- 142.Feng Z., Zhang H., Levine A.J., Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tasdemir E., Maiuri M.C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D'Amelio M. Regulation of autophagy by cytoplasmic p53. Nature Cell Biology. 2008;10(6):676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tavernarakis N., Pasparaki A., Tasdemir E., Maiuri M.C., Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2014;4(7):870–873. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]
- 145.Lee I.H., Kawai Y., Fergusson M.M., Rovira I.I., Bishop A.J.R., Motoyama N. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science. 2012;336(6078):225–228. doi: 10.1126/science.1218395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huo Y., Cai H., Teplova I., Bowman-Colin C., Chen G., Price S. Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer. Cancer Discovery. 2013;3(8):894–907. doi: 10.1158/2159-8290.CD-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo J.Y., Karsli-Uzunbas G., Mathew R., Aisner S.C., Kamphorst J.J., Strohecker A.M. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes & Development. 2013;27(13):1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosenfeldt M.T., O'Prey J., Morton J.P., Nixon C., Mackay G., Mrowinska A. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504(7479):296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 149.Okoshi R., Ozaki T., Yamamoto H., Ando K., Koida N., Ono S. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. Journal of Biological Chemistry. 2008;283(7):3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 150.Imamura K., Ogura T., Kishimoto A., Kaminishi M., Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-Carboxamide-1-β--Ribofuranoside, in a human hepatocellular carcinoma cell line. Biochemical and Biophysical Research Communications. 2001;287(2):562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 151.Armata H.L., Golebiowski D., Jung D.Y., Ko H.J., Kim J.K., Sluss H.K. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Molecular and Cellular Biology. 2010;30(24):5787–5794. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feng Z., Hu W., de Stanchina E., Teresky A.K., Jin S., Lowe S. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Research. 2007;67(7):3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 153.Co N.N., Iglesias D., Celestino J., Kwan S.Y., Mok S.C., Schmandt R. Loss of LKB1 in high-grade endometrial carcinoma: LKB1 is a novel transcriptional target of p53. Cancer. 2014;120(22):3457–3468. doi: 10.1002/cncr.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pappas K., Xu J., Zairis S., Resnick-Silverman L., Abate F., Steinbach N. p53 maintains baseline expression of multiple tumor suppressor genes. Molecular Cancer Research. 2017;15(8):1051–1062. doi: 10.1158/1541-7786.MCR-17-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cam M., Bid H.K., Xiao L., Zambetti G.P., Houghton P.J., Cam H. p53/TAp63 and AKT regulate mammalian target of rapamycin complex 1 (mTORC1) signaling through two independent parallel pathways in the presence of DNA damage. Journal of Biological Chemistry. 2014;289(7):4083–4094. doi: 10.1074/jbc.M113.530303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ellisen L.W., Ramsayer K.D., Johannessen C.M., Yang A., Beppu H., Minda K. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Molecular Cell. 2002;10(5):995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 157.Stambolic V., MacPherson D., Sas D., Lin Y., Snow B., Jang Y. Regulation of PTEN transcription by p53. Molecular Cell. 2001;8(2):317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 158.Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B.R. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377(6550):646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 159.Kawase T., Ohki R., Shibata T., Tsutsumi S., Kamimura N., Inazawa J. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136(3):535–550. doi: 10.1016/j.cell.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 160.Webster N.J., Resnik J.L., Reichart D.B., Strauss B., Haas M., Seely B.L. Repression of the insulin receptor promoter by the tumor suppressor gene product p53: a possible mechanism for receptor overexpression in breast cancer. Cancer Research. 1996;56(12):2781–2788. [PubMed] [Google Scholar]
- 161.Ashcroft M., Ludwig R.L., Woods D.B., Copeland T.D., Weber H.O., MacRae E.J. Phosphorylation of HDM2 by Akt. Oncogene. 2002;21(13):1955–1962. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- 162.Zhou B.P., Liao Y., Xia W., Zou Y., Spohn B., Hung M.C. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nature Cell Biology. 2001;3(11):973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 163.Chen D., Li M., Luo J., Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. Journal of Biological Chemistry. 2003;278(16):13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 164.Alarcon R., Koumenis C., Geyer R.K., Maki C.G., Giaccia A.J. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Research. 1999;59(24):6046–6051. [PubMed] [Google Scholar]
- 165.Zhu Y., Mao X.O., Sun Y., Xia Z., Greenberg D.A. p38 mitogen-activated protein kinase mediates hypoxic regulation of Mdm2 and p53 in Neurons. Journal of Biological Chemistry. 2002;277(25):22909–22914. doi: 10.1074/jbc.M200042200. [DOI] [PubMed] [Google Scholar]
- 166.Lee S.-J., Lim C.-J., Min J.-K., Lee J.-K., Kim Y.-M., Lee J.-Y. Protein phosphatase 1 nuclear targeting subunit is a hypoxia inducible gene: its role in post-translational modification of p53 and MDM2. Cell Death & Differentiation. 2007;14(6):1106–1116. doi: 10.1038/sj.cdd.4402111. [DOI] [PubMed] [Google Scholar]
- 167.Galban S., Martindale J.L., Mazan-Mamczarz K., Lopez de Silanes I., Fan J., Wang W. Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Molecular and Cellular Biology. 2003;23(20):7083–7095. doi: 10.1128/MCB.23.20.7083-7095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hammond E.M., Denko N.C., Dorie M.J., Abraham R.T., Giaccia A.J. Hypoxia links ATR and p53 through replication arrest. Molecular and Cellular Biology. 2002;22(6):1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chandel N.S., Vander Heiden M.G., Thompson C.B., Schumacker P.T. Redox regulation of p53 during hypoxia. Oncogene. 2000;19(34):3840–3848. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- 170.Schmaltz C., Hardenbergh P.H., Wells A., Fisher D.E. Regulation of proliferation-survival decisions during tumor cell hypoxia. Molecular and Cellular Biology. 1998;18(5):2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thomas L.W., Ashcroft M. Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cellular and Molecular Life Sciences. 2019;76(9):1759–1777. doi: 10.1007/s00018-019-03039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sermeus A., Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death & Disease. 2011;2(5):e164. doi: 10.1038/cddis.2011.48. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Humpton T.J., Vousden K.H. Regulation of cellular metabolism and hypoxia by p53. Cold Spring Harbor Perspectives in Medicine. 2016;6(7):a026146. doi: 10.1101/cshperspect.a026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Al Tameemi W., Dale T.P., Al-Jumaily R.M.K., Forsyth N.R. Hypoxia-modified cancer cell metabolism. Frontiers of Cell & Developmental Biology. 2019;7:4. doi: 10.3389/fcell.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hammond E.M., Mandell D.J., Salim A., Krieg A.J., Johnson T.M., Shirazi H.A. Genome-wide analysis of p53 under hypoxic conditions. Molecular and Cellular Biology. 2006;26(9):3492–3504. doi: 10.1128/MCB.26.9.3492-3504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xenaki G., Ontikatze T., Rajendran R., Stratford I.J., Dive C., Krstic-Demonacos M. PCAF is an HIF-1α cofactor that regulates p53 transcriptional activity in hypoxia. Oncogene. 2008;27(44):5785–5796. doi: 10.1038/onc.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koumenis C., Alarcon R., Hammond E., Sutphin P., Hoffman W., Murphy M. Regulation of p53 by hypoxia: Dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Molecular and Cellular Biology. 2001;21(4):1297–1310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang E.Y., Gang H., Aviv Y., Dhingra R., Margulets V., Kirshenbaum L.A. p53 mediates autophagy and cell death by a mechanism contingent on Bnip3. Hypertension. 2013;62(1):70–77. doi: 10.1161/HYPERTENSIONAHA.113.01028. [DOI] [PubMed] [Google Scholar]
- 179.Fei P., Wang W., Kim S.-H., Wang S., Burns T.F., Sax J.K. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell. 2004;6(6):597–609. doi: 10.1016/j.ccr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 180.Ito A., Kawaguchi Y., Lai C.-H., Kovacs J.J., Higashimoto Y., Appella E. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. The EMBO Journal. 2002;21(22):6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barlev N.A., Liu L., Chehab N.H., Mansfield K., Harris K.G., Halazonetis T.D. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Molecular Cell. 2001;8(6):1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 182.Hua W.-K., Qi J., Cai Q., Carnahan E., Ayala Ramirez M., Li L. HDAC8 regulates long-term hematopoietic stem-cell maintenance under stress by modulating p53 activity. Blood. 2017;130(24):2619–2630. doi: 10.1182/blood-2017-03-771386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yan W., Liu S., Xu E., Zhang J., Zhang Y., Chen X. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene. 2012;32(5):599–609. doi: 10.1038/onc.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim S.C., Sprung R., Chen Y., Xu Y., Ball H., Pei J. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Molecular Cell. 2006;23(4):607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 186.McLure K.G., Takagi M., Kastan M.B. NAD+ Modulates p53 DNA Binding Specificity and cellular reprogramming. Molecular and Cellular Biology. 2004;24(22):9958–9967. doi: 10.1128/MCB.24.22.9958-9967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ong A.L.C., Ramasamy T.S. Role of Sirtuin 1-p53 regulatory axis in aging, cancer and Ageing. Ageing Research Reviews. 2018;43:64–80. doi: 10.1016/j.arr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 188.Cheng H.-L., Mostoslavsky R., Saito S., Manis J.P., Gu Y., Patel P. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Han M.-K., Song E.-K., Guo Y., Ou X., Mantel C., Broxmeyer H.E. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2(3):241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Q., Zhang Y., Yang C., Xiong H., Lin Y., Yao J. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Proceedings of the National Academy of Sciences of the United States of America. 2010;103(5968):10230–10235. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nemoto S., Fergusson M.M., Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 192.Naqvi A., Hoffman T.A., DeRicco J., Kumar A., Kim C.-S., Jung S.-B. A single-nucleotide variation in a p53-binding site affects nutrient-sensitive human SIRT1 expression. Human Molecular Genetics. 2010;19(21):4123–4133. doi: 10.1093/hmg/ddq331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamakuchi M., Ferlito M., Lowenstein C.J. miR-34a repression of SIRT1 regulates apoptosis. Proceedings of the National Academy of Sciences. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Amano H., Chaudhury A., Rodriguez-Aguayo C., Lu L., Akhanov V., Catic A. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metabolism. 2019;29(6):1274–1279. doi: 10.1016/j.cmet.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sahin E., Colla S., Liesa M., Moslehi J., Müller F.L., Guo M. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sen N., Satija Y.K., Das S. PGC-1α, a key modulator of p53, promotes cell survival upon metabolic stress. Molecular Cell. 2011;44(4):621–634. doi: 10.1016/j.molcel.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 197.Hallenborg P., Fjære E., Liaset B., Petersen R.K., Murano I., Sonne S.B. p53 regulates expression of uncoupling protein 1 through binding and repression of PPARγ coactivator-1α. American Journal of Physiology-Endocrinology and Metabolism. 2016;310(2):E116–E128. doi: 10.1152/ajpendo.00119.2015. [DOI] [PubMed] [Google Scholar]
- 198.Saleem A., Adhihetty P.J., Hood D.A. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiological Genomics. 2009;37(1):58–66. doi: 10.1152/physiolgenomics.90346.2008. [DOI] [PubMed] [Google Scholar]
- 199.Saleem A., Hood D.A. Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. The Journal of Physiology. 2013;591(14):3625–3636. doi: 10.1113/jphysiol.2013.252791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Safdar A., Khrapko K., Flynn J.M., Saleem A., Lisio M., Johnston A.P.W. Exercise-induced mitochondrial p53 repairs mtDNA mutations in mutator mice. Skeletal Muscle. 2016:1–18. doi: 10.1186/s13395-016-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 201.Beyfuss K., Hood D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Report. 2018;23(1):100–117. doi: 10.1080/13510002.2017.1416773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mak T.W., Hauck L., Grothe D., Billia F. p53 regulates the cardiac transcriptome. Proceedings of the National Academy of Sciences. 2017;114(9):2331–2336. doi: 10.1073/pnas.1621436114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gogna R., Madan E., Khan M., Pati U., Kuppusamy P. p53's choice of myocardial death or survival: oxygen protects infarct myocardium by recruiting p53 on NOS3 promoter through regulation of p53-Lys 118acetylation. EMBO Molecular Medicine. 2013;5(11):1662–1683. doi: 10.1002/emmm.201202055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Krstic J., Galhuber M., Schulz T., Schupp M., Prokesch A. p53 as a Dichotomous regulator of liver disease: the dose makes the medicine. International Journal of Molecular Sciences. 2018;19(3):921. doi: 10.3390/ijms19030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Prokesch A., Graef F.A., Madl T., Kahlhofer J., Heidenreich S., Schumann A. Liver p53 is stabilized upon starvation and required for amino acid catabolism and gluconeogenesis. The FASEB Journal. 2017;31(2):732–742. doi: 10.1096/fj.201600845R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yahagi N., Shimano H., Matsuzaka T., Sekiya M., Najima Y., Okazaki S. p53 involvement in the pathogenesis of fatty liver disease. Journal of Biological Chemistry. 2004;279(20):20571–20575. doi: 10.1074/jbc.M400884200. [DOI] [PubMed] [Google Scholar]
- 207.Homayounfar R., Jeddi-Tehrani M., Cheraghpour M., Ghorbani A., Zand H. Relationship of p53 accumulation in peripheral tissues of high-fat diet-induced obese rats with decrease in metabolic and oncogenic signaling of insulin. General and Comparative Endocrinology. 2015;214(C):134–139. doi: 10.1016/j.ygcen.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 208.Kim J., Yu L., Chen W., Xu Y., Wu M., Todorova D. Wild-type p53 promotes cancer metabolic switch by inducing PUMA-dependent suppression of oxidative phosphorylation. Cancer Cell. 2019;35(2):191–198. doi: 10.1016/j.ccell.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 209.Zhang P., Tu B., Wang H., Cao Z., Tang M., Zhang C. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proceedings of the National Academy of Sciences. 2014;111(29):10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang S.-J., Yu G., Jiang L., Li T., Lin Q., Tang Y. p53-dependent regulation of metabolic function through transcriptional activation of pantothenate kinase-1 gene. Cell Cycle. 2014;12(5):753–761. doi: 10.4161/cc.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goldstein I., Yizhak K., Madar S., Goldfinger N., Ruppin E., Rotter V. p53 promotes the expression of gluconeogenesis- related genes and enhances hepatic glucose production. Cancer & Metabolism. 2013;1(1):1. doi: 10.1186/2049-3002-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Franck D., Tracy L., Armata H.L., Delaney C.L., Jung D.Y., Ko H.J. Glucose tolerance in mice is linked to the dose of the p53 transactivation domain. Endocrine Research. 2012;(3):139–150. doi: 10.3109/07435800.2012.735735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang X., Zhao X., Gao X., Mei Y., Wu M. A new role of p53 in regulating lipid metabolism. Journal of Molecular Cell Biology. 2013;5(2):147–150. doi: 10.1093/jmcb/mjs064. [DOI] [PubMed] [Google Scholar]
- 214.Derdak Z., Villegas K.A., Harb R., Wu A.M., Sousa A., Wands J.R. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. Journal of Hepatology. 2013;58(4):785–791. doi: 10.1016/j.jhep.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bist A., Fielding C.J., Fielding P.E. p53 regulates Caveolin gene transcription, cell cholesterol, and growth by a novel mechanism. Biochemistry. 2000;39(8):1966–1972. doi: 10.1021/bi991721h. [DOI] [PubMed] [Google Scholar]
- 216.Krstic J., Reinisch I., Schupp M., Schulz T., Prokesch A. p53 functions in adipose tissue metabolism and homeostasis. International Journal of Molecular Sciences. 2018;19(9):2622. doi: 10.3390/ijms19092622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bazuine M., Stenkula K.G., Cam M., Arroyo M., Cushman S.W. Guardian of corpulence: a hypothesis on p53 signaling in the fat cell. Clinical Lipidology. 2009;4(2):231–243. doi: 10.2217/clp.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Molchadsky A., Shats I., Goldfinger N., Pevsner-Fischer M., Olson M., Rinon A. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS One. 2008;3(11):e3707. doi: 10.1371/journal.pone.0003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang Q., Liu M., Du X., Zhang R., Xue Y., Zhang Y. Role of p53 in preadipocyte differentiation. Cell Biology International. 2014;38(12):1384–1393. doi: 10.1002/cbin.10334. [DOI] [PubMed] [Google Scholar]
- 220.Okita N., Ishikawa N., Mizunoe Y., Oku M., Nagai W., Suzuki Y. Inhibitory effect of p53 on mitochondrial content and function during adipogenesis. Biochemical and Biophysical Research Communications. 2014;446(1):91–97. doi: 10.1016/j.bbrc.2014.02.059. [DOI] [PubMed] [Google Scholar]
- 221.Yahagi N., Shimano H., Matsuzaka T., Najima Y., Sekiya M., Nakagawa Y. p53 activation in adipocytes of obese mice. Journal of Biological Chemistry. 2003;278(28):25395–25400. doi: 10.1074/jbc.M302364200. [DOI] [PubMed] [Google Scholar]
- 222.Molchadsky A., Ezra O., Amendola P.G., Krantz D., Kogan-Sakin I., Buganim Y. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death & Differentiation. 2013;20(5):774–783. doi: 10.1038/cdd.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Al-Massadi O., Porteiro B., Kuhlow D., Köhler M., Gonzalez-Rellan M.J., Garcia-Lavandeira M. Pharmacological and genetic manipulation of p53 in Brown fat at adult but not embryonic stages regulates thermogenesis and body weight in male mice. Endocrinology. 2016;157(7):2735–2749. doi: 10.1210/en.2016-1209. [DOI] [PubMed] [Google Scholar]
- 224.Kon N., Wang D., Li T., Jiang L., Qiang L., Gu W. Inhibition of Mdmx (Mdm4) in vivo induces anti-obesity effects. Oncotarget. 2018;9(7):7282–7297. doi: 10.18632/oncotarget.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang Y., Zeng S.X., Hao Q., Lu H. Monitoring p53 by MDM2 and MDMX is required for endocrine pancreas development and function in a spatio-temporal manner. Developmental Biology. 2017;423(1):34–45. doi: 10.1016/j.ydbio.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tornovsky-Babeay S., Dadon D., Ziv O., Tzipilevich E., Kadosh T., Haroush R.S.-B. Type 2 diabetes and congenital hyperinsulinism cause DNA Double-strand breaksand p53 activity in b cells. Cell Metabolism. 2014;19(1):109–121. doi: 10.1016/j.cmet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 227.Hoshino A., Ariyoshi M., Okawa Y., Kaimoto S., Uchihashi M., Fukai K. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic-cell function in diabetes. Proceedings of the National Academy of Sciences. 2014;111(8):3116–3121. doi: 10.1073/pnas.1318951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kon N., Zhong J., Qiang L., Accili D., Gu W. Inactivation of arf-bp1 induces p53 activation and diabetic phenotypes in mice. Journal of Biological Chemistry. 2012;287(7):5102–5111. doi: 10.1074/jbc.M111.322867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hinault C., Kawamori D., Liew C.W., Maier B., Hu J., Keller S.R. Δ40 isoform of p53 controls cell proliferation and glucose homeostasis in mice. Diabetes. 2011;60(4):1210–1222. doi: 10.2337/db09-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li X., Liu Z., Yang J.-K., Wang B., Jiang X., Zhou Y. The MDM2–p53–pyruvate carboxylase signalling axis couples mitochondrial metabolism to glucose-stimulated insulin secretion in pancreatic. Nature Communications. 2016;7:1–14. doi: 10.1038/ncomms11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Medicine. 2009;15(9):1–7. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 232.Ortega F.J., Moreno-Navarrete J.M., Mayas D., Serino M., Rodriguez-Hermosa J.I., Ricart W. Inflammation and insulin resistance exert dual effects on adipose tissue tumor protein 53 expression. International Journal of Obesity. 2014;38(5):737–745. doi: 10.1038/ijo.2013.163. [DOI] [PubMed] [Google Scholar]
- 233.Gaulton K.J., Willer C.J., Li Y., Scott L.J., Conneely K.N., Jackson A.U. Comprehensive association study of type 2 diabetes and related quantitative traits with 222 candidate genes. Diabetes. 2008;57(11):3136–3144. doi: 10.2337/db07-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Burgdorf K.S., Grarup N., Justesen J.M., Harder M.N., Witte D.R., Jørgensen T. Studies of the association of Arg72Pro of tumor suppressor protein p53 with type 2 diabetes in a combined analysis of 55,521 Europeans. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0015813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kung C.-P., Leu J.I.-J., Basu S., Khaku S., Anokye-Danso F., Liu Q. The P72R polymorphism of p53 predisposes to obesity and metabolic dysfunction. Cell Reports. 2016;14(10):2413–2425. doi: 10.1016/j.celrep.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yuan H., Zhang X., Huang X., Lu Y., Tang W., Man Y. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vergoni B., Cornejo P.-J., Gilleron J., Djedaini M., Ceppo F., Jacquel A. DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes. 2016;65(10):3062–3074. doi: 10.2337/db16-0014. [DOI] [PubMed] [Google Scholar]
- 238.Secchiero P., Toffoli B., Melloni E., Agnoletto C., Monasta L., Zauli G. The MDM2 inhibitor Nutlin-3 attenuates streptozotocin-induced diabetes mellitus and increases serum level of IL-12p40. Acta Diabetologica. 2013;50(6):899–906. doi: 10.1007/s00592-013-0476-8. [DOI] [PubMed] [Google Scholar]
- 239.Yokoyama M., Okada S., Nakagomi A., Moriya J., Shimizu I., Nojima A. Inhibition of endothelial p53 ImprovesMetabolic abnormalities related to dietary obesity. Cell Reports. 2014;7(5):1691–1703. doi: 10.1016/j.celrep.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 240.Velasquez D.A., Martinez G., Romero A., Vazquez M.J., Boit K.D., Dopeso-Reyes I.G. The central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes. 2011;60(4):1177–1185. doi: 10.2337/db10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Farrell G.C., Larter C.Z., Hou J.Y., Zhang R.H., Yeh M.M., Williams J. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. Journal of Gastroenterology and Hepatology. 2009;24(3):443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 242.Tomita K., Teratani T., Suzuki T., Oshikawa T., Yokoyama H., Shimamura K. p53/p66Shc-mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. Journal of Hepatology. 2012;57(4):837–843. doi: 10.1016/j.jhep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 243.Panasiuk A., Dzieciol J., Panasiuk B., Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World Journal of Gastroenterology. 2006;12(38):6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Tsunematsu H. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. Journal of Clinical Investigation. 2011;121(8):3343–3356. doi: 10.1172/JCI44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Porteiro B., Fondevila M.F., Buque X., Gonzalez-Rellan M.J., Fernandez U., Mora A. Pharmacological stimulation of p53 with low-dose doxorubicin ameliorates diet-induced nonalcoholic steatosis and steatohepatitis. Molecular Metabolism. 2018;8:132–143. doi: 10.1016/j.molmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Itahana Y., Itahana K. Emerging roles of p53 family members in glucose metabolism. International Journal of Molecular Sciences. 2018;19(3):776. doi: 10.3390/ijms19030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Candi E., Smirnov A., Panatta E., Lena A.M., Novelli F., Mancini M. Metabolic pathways regulated by p63. Biochem Biophysic Res Commun. 2017;482(3):440–444. doi: 10.1016/j.bbrc.2016.10.094. [DOI] [PubMed] [Google Scholar]
- 248.Napoli M., Flores E.R. The p53 family orchestrates the regulation of metabolism: physiological regulation and implications for cancer therapy. British Journal of Cancer. 2016;116(2):149–155. doi: 10.1038/bjc.2016.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ruiz-Lozano P., Hixon M.L., Wagner M.W., Flores A.I., Ikawa S., Badwin A.S. p53 is a transcriptional activator of the muscle-specific phosphoglycerate mutase gene and contributes in vivo to the control of its cardiac expression. Cell Growth & Differentiation. 1999;10(5):295–306. [PubMed] [Google Scholar]
- 250.Mathupala S.P., Heese C., Pedersen P.L. The type II of hehokinase promoter contains funcionally active response elements for the tumor suppressor p53. Journal of Biological Chemistry. 1997;272(36):22776–22780. doi: 10.1074/jbc.272.36.22776. [DOI] [PubMed] [Google Scholar]
- 251.Andrysik Z., Galbraith M.D., Guarnieri A.L., Zaccara S., Sullivan K.D., Pandey A. Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity. Genome Research. 2017;27(10):1645–1657. doi: 10.1101/gr.220533.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tan M., Li S., Swaroop M., Guan K., Oberley L.W., Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. Journal of Biological Chemistry. 1999;274(17):12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 253.Lehar S.M., Nacht M., Jacks T., Vater C.A., Chittenden T., Guild B.C. Identification and cloning of EI24, a gene induced by p53 in etoposide-treated cells. Oncogene. 1996;12(6):1181–1187. [PubMed] [Google Scholar]