Abstract
Object. Results on the associations of fruit and vegetable intake with risk of chronic obstructive pulmonary disease (COPD) are still in conflict. Hence, we conducted a meta-analysis to quantitatively evaluate the association between fruit and vegetable intake and the risk of COPD.
Methods
PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) were searched for relevant studies published up to September 2019. Combined relative risks (RRs) and 95% confidence intervals (CIs) were calculated with the random effects model (REM). Dose-response relationship was assessed by the restricted cubic spline model.
Results
There are 8 studies involving 5,787 COPD cases among 244,154 participants included in this meta-analysis. For the highest versus the lowest level, the pooled RR of COPD was 0.75 (95% CI, 0.68–0.84; I2 = 46.7%) for fruits plus vegetables, 0.72 (95% CI, 0.66–0.79; I2 = 46.7%) for fruits plus vegetables, 0.72 (95% CI, 0.66–0.79; I2 = 46.7%) for fruits plus vegetables, 0.72 (95% CI, 0.66–0.79; Pnon−linearity < 0.01).
Conclusions
This meta-analysis indicates that fruit and vegetable intake might be related to a lower risk of COPD.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease characterized by persistent respiratory symptoms and airflow limitation due to airway inflammation and remodeling [1]. Commonly, airway inflammation and remodeling could promote parenchymal destruction and the development of emphysema, resulting in impaired functional capacity, airway obstruction, and dyspnoea [2]. Furthermore, impaired lung function is associated with an increased risk of serious concomitant diseases [3]. COPD together with its comorbidities causes a heavy economic and social burden around the world, and it is expected to be the third leading cause of death worldwide by 2020 [1]. As mentioned above, COPD is a major public health problem at present.
COPD is a complex disease caused by the interaction of genetic factors with environmental factors, and cigarette smoking is the leading environmental risk factor. However, only 15–20% of ex or current smokers develop COPD during their lifetime [4], suggesting that there are other factors that prevent or promote the development of COPD. Therefore, it is not enough in the prevention of COPD simply by smoking prevention and smoking cessation efforts. Since oxidative stress and inflammation are considered as the main pathogenesis in COPD development and progression [5–7], and fruits and vegetables are foods rich in antioxidants; thus, they have been hypothesized to have a protective effect on COPD. In a 3 yr randomized controlled trial [8], a dietary shift to high fruit and vegetable consumption has been demonstrated to improve lung function in patients with COPD. Some observational studies have been conducted to explore the potential protective effects of fruits and vegetables on the risk of COPD, but the results showed inconsistencies. In terms of fruit intake, reduced risk of COPD was found in four articles [9–12], whereas others revealed no significant relationship [13–15]. In terms of vegetable intake, there are also four articles that showed an inverse association [9, 11, 12, 14]. Given that, we carried out a meta-analysis to evaluate the potential protective effects of fruits and vegetables on COPD, and the dose-response relationship between fruit and vegetable intake and COPD risk.
2. Materials and Methods
This meta-analysis was conducted following the guidelines of Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [16].
2.1. Search Strategy
Relevant English or Chinese publications were searched in PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) up to September 2019. The following search strategy was used: (diet OR dietary pattern OR vegetable OR vegetables OR fruit OR fruits) AND (chronic obstructive pulmonary disease OR COPD). The reference lists of retrieved articles were reviewed to identify additional relevant articles. The specific search strategy of PubMed is shown in Supplementary Table S1.
2.2. Inclusion Criteria
Study inclusion criteria were as follows: (1) observational studies published in English or Chinese; (2) the exposure of interest was fruit or vegetable intake; (3) the outcome of interest was chronic obstructive pulmonary disease; (4) relative risk (RR), odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (CI) after multivariate adjustment was reported. For dose-response analysis, RR (95% CI) for at least three quantitative categories of fruit or vegetable intake, person-years, and the number of cases for each category of fruit or vegetable intake was provided.
2.3. Exclusion Criteria
(1) Review articles, meeting abstracts, and studies written in other languages instead of English or Chinese; (2) unpublished studies; (3) no results reported on the relationship between fruit or vegetable intake and COPD; (4) multivariate adjusted RR and 95% CI not reported, or not possible to calculate; (5) duplicated articles.
Two investigators searched all relevant studies independently. If data from the same population had been published more than once, the most comprehensive one was chosen. If two investigators could not reach an agreement about the eligibility of an article, it was resolved by reaching consensus.
2.4. Data Extraction
The following information was extracted from each identified study by two investigators: (1) name of the first author; (2) publication year; (3) continent and country; (4) gender, age range, or mean age of participants; (5) study design; (6) fruit and vegetable intake measurement; (7) measurement method of COPD; (8) sample size; (9) RRs (we presented all results with RR for simplicity) with their 95% CIs for the highest versus lowest level; (10) adjusted covariates. For dose-response analysis, the number of cases, person-years, and the RR (95% CI) for each category of fruit and vegetable intake were extracted. The median or mean level of fruit and vegetable intake for each category was assigned to the corresponding RR for every study. For the unbounded category, we assumed that the boundary had the same amplitude as the adjacent category data [17]. We used servings/day as a standard unit of measurement for fruit and vegetable intake, and a standard portion size of 106 g was used when fruit or vegetable intake was reported by g/day [18].
The Agency for Healthcare Research and Quality (AHRQ) methodology checklist for cross-sectional studies and the Newcastle-Ottawa Scale (NOS) for cohort and case-control studies were used to assess the quality of the included articles [19].
2.5. Statistical Analysis
For the pooled measure, we use the inverse variance weighted mean of the logarithm of RRs to calculate the pooled RR with corresponding 95% CIs. Statistical heterogeneity was assessed by the I2 statistics, I2 values of 0%, 25%, 50%, and 75% represent no, low, moderate, and high heterogeneity, respectively [20]. Regardless of the value of I2, random effects model was adopted in all analyses. Random effects model was more conservative than the fixed effects model, and the use of random effects model in all analyses could maintain the consistency of the meta-analysis [21]. Subgroup analysis was conducted by study design, continent where the study conducted, gender, and exposure measures. Metaregression with restricted maximum likelihood estimation was performed to assess the potential covariates that may exert substantial impacts on between-study heterogeneity [22]. Leave-one-out sensitivity analysis was performed if I2 ≥ 50%, to find which study influences between-study heterogeneity [23]. The influence analysis was conducted by removing one study at a time whether the results would be significantly affected by a single study. The small-study effect was estimated by funnel plot and Egger regression asymmetry test [24]. All statistical analyses were performed using STATA Version 12.0.
For dose-response analysis, a two-stage random effects dose-response meta-analysis [25] was performed. First, a restricted cubic spline model with three knots at the 10th, 50th, and 90th percentiles of levels of fruit and vegetable intake was estimated using generalized least-square regression, taking into account the correlation within each set of published RR. Then the restricted maximum likelihood method was used to combine the study-specific estimates in multivariate random effects meta-analysis [26]. A P value is calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0.
3. Results
3.1. Search Results and Study Characteristics
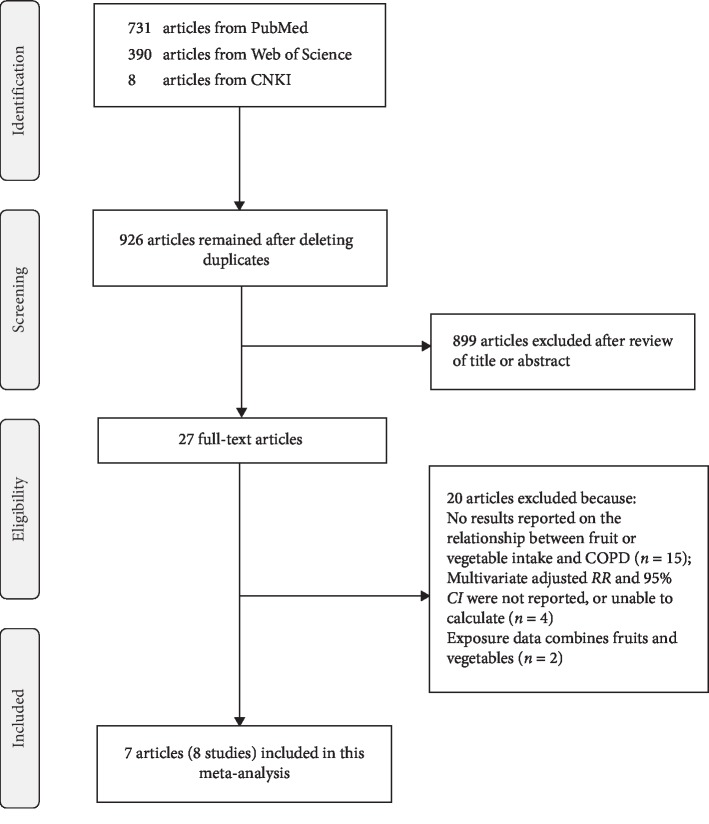
Literature search identified 731 articles from PubMed, 390 articles from Web of Science, 8 articles from China National Knowledge Infrastructure. A total of 926 articles remained after deleting duplicates. After reviewing the title and abstract, 899 articles were excluded. We further excluded 20 articles after reviewing full-text. At last, a total of seven articles [9–15] (8 studies) were involved in this meta-analysis. The two investigators had no disagreement about the eligibility of all included articles. The flow chart of the literature search is shown in Figure 1. The detailed processes of full-text reviewed articles exclusion are demonstrated in Supplementary Table S2.
Figure 1.
Flow diagram of the literature search for studies.
Overall, all of these seven articles [9–15] reported results for fruits and vegetables separately. One [13] of the articles contained two independent cohorts with different genders. As a result, eight studies were included, four of which were cohort studies [9, 10, 13], two were case-control studies [14, 15], and two were cross-sectional studies [11, 12].
Contrary to other studies, one study [12] used the highest exposure level as a reference group, so we made a data transformation to use the lowest level as a reference group. In this study [12], vegetable exposure provided two data on raw and cooked vegetables; however, there was no special description on whether the vegetables have been processed in other studies. Considering the local dietary habits of the participants, raw vegetables data were chosen.
In all eight studies, COPD patients were accurately identified by clinical diagnosis. Five studies [12–15] identified COPD patients through physician's diagnosis and spirometric tests; the diagnostic criteria of spirometric tests were FEV1/FVC < 0.7; one [12] of these five studies defined airway obstruction as FEV1/FVC-ratio below lower limit of normal; z-score for FEV1/FVC-ratio was −1.96. Two studies [9, 10] identified COPD patients through linkage with the national database and the remaining one [11] by self-reported physician's diagnosis; neither method reported detailed criteria.
For exposure measurement, fruit and vegetable consumption was collected by food frequency questionnaire(FFQ) in six studies [9, 10, 13–15]. Standardized questionnaires were used in one study [11], and a self-designed questionnaire was used in another study [12]. The above questionnaires were designed to collect the frequency of intake of vegetables and fruits over a longer period of time. Specifically, in terms of frequency categories, all types of FFQ [9, 10, 13–15] included eight to nine predefined frequency categories, the self-designed questionnaire [12] included eight categories similarly, and the standardized questionnaire [11] included three categories. Essentially, there was no significant difference in the collection process of exposure information between these seven questionnaires.
The potential confounding factors in the original studies were fully taken into account. As the most important confounding factors, smoking and age were adjusted for in all included studies. Other confounders such as BMI, physical activity, and intake of energy also were adjusted in most studies. The quality assessment showed that the Newcastle-Ottawa score of the cohort studies ranged from seven to nine. For the case-control studies, the Newcastle-Ottawa scores were eight. The quality score of the Agency for Healthcare Research and Quality (AHRQ) methodology checklist ranged from eight to nine for cross-sectional studies. Detailed baseline characteristics of these studies are shown in Table 1. Quality assessment of all included studies is shown in Supplementary Tables S3–S5.
Table 1.
Characteristics of included studies on fruit and vegetable intake and COPD risk.
| Author, year [Ref.] | Country | Age range or mean age (gender) | Study design | Sample size (cases) | Exposure measurement | Exposure definition | Outcome assessment | RR (95% CI) for the highest vs. lowest level | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Watson et al. 2002 [14] | UK | ≥45 (both) | C-C-S | 266 (150) | HEA3 Dietary Food Frequency Questionnaire | 0–22 VS 220 for fruits (g); 0–49 VS 93 for vegetables (g) | General practitioner's diagnosis and spirometric tests | 0.45 (0.19–1.06) for fruits; 0.46 (0.23–0.94) for vegetables | Age, gender, smoking (matched), social class, body mass index, and vegetable intake (when analyzing fruits) |
| Hirayama et al. 2009 [15] | Japan | 50–75 (both) | C-C-S | 618 (278) | 138-Item food questionnaire | ≤141.0 VS ≥ 391.4 for fruits (g/day); ≤110.8 VS ≥ 252.0 for vegetables (g/day) | Physician's diagnosis and spirometric tests | 0.82 (0.43–1.54) for fruits; 0.62 (0.32–1.2) for vegetables | Age, gender, BMI (5 years ago), education level (high school or below; college or university), alcohol drinking (nondrinker; drinker), cigarette smoking (never smoker; ex-smoker; current smoker), smoking pack-years, life-long physical activity involvement (never to not any more involved; always been involved), and daily intake of red meat, chicken, and fresh fish |
| Kaluza et al. 2017 [9] | Sweden | 45–79 (male) | C-S | 44335 (1918) | FFQ | <0.5 VS ≥ 1.9 for fruits (servings/day)< 1.2 VS ≥ 3.6 for vegetables (servings/day) | Swedish patient register and cause of death register | 0.73 (0.62–0.85) for fruits; 0.82 (0.7–0.97) for vegetables | Age (years, continuous), education (less than high school, high school, or university), body mass index (<18.5, 18.5–24.9, 25–29.9 or ≥30 kg/m2), total physical activity (MET × hour/day, quintiles), smoking status and pack-years of smoking (never; past <20, 20–39 or ≥40 pack-years; or current < 20, 20–39 or ≥40 pack-years), intake of energy (kcal/day, quintiles), alcohol consumption (g/day, quintiles), and modified recommended food score (scores, continuous) and nonrecommended food score (scores, continuous) |
| Kaluza et al. 2018 [10] | Sweden | 48–83 (female) | C-S | 34739 (1512) | FFQ | <0.8 VS ≥ 2.5 for fruits (servings/day); <1.3 VS ≥ 3.1 for vegetables (servings/day) | Swedish Patient Register And Cause Of Death Register | 0.63 (0.52–0.75) for fruits; 0.94 (0.79–1.13) for vegetables | Age (years, continuous), education (less than high school, high school or university), BMI (<18.5, 18.5–24.9, 25–29.9 or ≥30 kg/m2), total physical activity (MET h/d, quintiles), smoking status and pack-years of smoking (never; past <20, 20–39 or40 pack-years; or current < 20, 20–39 or ≥40 pack-years), dietary supplement use (regular, nonregular or no use), intake of energy (kcal/day, quintiles), alcohol consumption (g/day, quintiles), modified recommended food score (score, continuous) and nonrecommended food score (score, continuous) |
| Varraso et al. 2015 [13] | USA | 40–75 (male) | C-S | 47026 (167) | FFQ | Fruit and vegetable score lowest fifth VS highest fifth | Self-reported physician's diagnosis and clinical test | 0.79 (0.44–1.39) for fruits; 0.92 (0.52–1.59) for vegetables | Age, physical activity, BMI, total energy intake, smoking status, pack-years of smoking, pack-years of smoking, race/ethnicity, physician visits, US region, and the other AHEI-2010 components |
| 30–55 (female) | C-S | 73228 (723) | FFQ | Fruit and vegetable score lowest fifth VS highest fifth | Self-reported physician's diagnosis and clinical test | 0.82 (0.61–1.09) for fruits; 1.06 (0.8–1.4) for vegetables | Age, physical activity, BMI, total energy intake, smoking status, pack-years of smoking, pack-years of smoking, second hand tobacco exposure race/ethnicity, physician visits, US region, spouse's highest educational attainment menopausal status, and the other AHEI-2010 components | ||
| Yin et al. 2011 [11] | China | 15–69 (both) | C-S-S | 32484 (750) | Standardized questionnaire | <2 VS 5–7 for fruits (d/week); <4 VS 6–7 for vegetables (d/week) | Self-reported physician's diagnosis | 0.8 (0.66–0.98) for fruit 0.65 (0.48–0.89) for vegetables | Age, gender, urban/rural areas, smoking status, passive smoking exposure and family history |
| Meteran et al. 2018 [12] | Denmark | 58.9 (both) | C-S-S | 11458 (289) | Questionnaire | Never VS 1–3 times/week for fruits and raw vegetables | Medical history and the prebronchodilator lung function test | 0.4 (0.17–0.97) for fruits; 0.48 (0.31–0.73) for raw vegetables | Age, gender, BMI, smoking, alcohol consumption, and physical activity |
COPD: chronic obstructive pulmonary disease; C-C-S: case-control study; C-S: cohort study; C-S-S: cross-sectional study; RR: relative risk; CI: confidence interval; HEA: Health Education Authority in Oxford; FFQ: Food Frequency Questionnaire; AHEI-2010: Alternate Healthy Eating Index 2010. Standardized questionnaire: the frequency of fruit/vegetable intake was divided into three categories according to the number of days normally consumed in a week (<2, 2–4, and 5–7 d/week for fruit; <4, 4-5, 6-7 d/week for vegetables). Questionnaire: based on self-reported frequency of consuming fruits and vegetables on an 8-point scale ranging from 0 to 8 (never to ≥ 4 times/daily) for each category.
3.2. Quantitative Synthesis
The main results are generalized in Table 2.
Table 2.
Summary risk estimates of COPD for fruit and vegetable intake by study characteristics.
| No. of studies | Pooled RR | 95% CI | I 2 statistic (%) | P value for heterogeneity | ||
|---|---|---|---|---|---|---|
| Fruits plus vegetables | ||||||
| All studies | 8 | 0.75 | 0.68–0.84 | 46.7 | 0.021 | |
| Study design | ||||||
| Cohort | 4 | 0.81 | 0.72–0.92 | 53.9 | 0.034 | |
| Cross-sectional | 2 | 0.63 | 0.48–0.83 | 54.1 | 0.088 | |
| Case-control | 2 | 0.59 | 0.42–0.84 | 0 | 0.592 | |
| Continent | ||||||
| Europe | 4 | 0.69 | 0.58–0.82 | 65.4 | 0.005 | |
| Asia | 2 | 0.75 | 0.64–0.88 | 0 | 0.653 | |
| America | 2 | 0.92 | 0.77–1.10 | 0 | 0.602 | |
| Gender | ||||||
| Both | 4 | 0.64 | 0.53–0.77 | 23.3 | 0.244 | |
| Male | 2 | 0.78 | 0.70–0.87 | 0 | 0.712 | |
| Female | 2 | 0.84 | 0.66–1.07 | 77.7 | 0.004 | |
| Exposure measure | ||||||
| FFQ | 6 | 0.79 | 0.70–0.88 | 44.1 | 0.050 | |
| Others | 2 | 0.63 | 0.48–0.83 | 54.1 | 0.088 | |
|
| ||||||
| Fruits | ||||||
| All studies | 8 | 0.72 | 0.66–0.79 | 1.3 | 0.419 | |
| Study design | ||||||
| Cohort | 4 | 0.71 | 0.63–0.79 | 0 | 0.419 | |
| Cross-sectional | 2 | 0.65 | 0.35–1.21 | 56.8 | 0.128 | |
| Case-control | 2 | 0.66 | 0.40–1.11 | 17.2 | 0.272 | |
| Continent | ||||||
| Europe | 4 | 0.67 | 0.60–0.76 | 19.6 | 0.292 | |
| Asia | 2 | 0.80 | 0.66–0.97 | 0 | 0.942 | |
| America | 2 | 0.81 | 0.63–1.05 | 0 | 0.910 | |
| Gender | ||||||
| Both | 4 | 0.76 | 0.63–0.91 | 21.6 | 0.281 | |
| Male | 2 | 0.73 | 0.63–0.85 | 0 | 0.795 | |
| Female | 2 | 0.70 | 0.54–0.90 | 55.9 | 0.132 | |
| Exposure measure | ||||||
| FFQ | 6 | 0.70 | 0.63–0.78 | 0 | 0.536 | |
| Others | 2 | 0.65 | 0.35–1.21 | 56.8 | 0.128 | |
|
| ||||||
| Vegetables | ||||||
| All studies | 8 | 0.76 | 0.63–0.92 | 66.7 | 0.009 | |
| Study design | ||||||
| Cohort | 4 | 0.90 | 0.81–1.00 | 0 | 0.424 | |
| Cross-sectional | 2 | 0.54 | 0.36–0.83 | 56.4 | 0.130 | |
| Case-control | 2 | 0.54 | 0.33–0.87 | 0 | 0.545 | |
| Continent | ||||||
| Europe | 4 | 0.70 | 0.53–0.94 | 76 | 0.006 | |
| Asia | 2 | 0.64 | 0.49–0.85 | 0 | 0.899 | |
| America | 2 | 1.03 | 0.80–1.32 | 0 | 0.657 | |
| Gender | ||||||
| Both | 4 | 0.56 | 0.45–0.71 | 0 | 0.631 | |
| Male | 2 | 0.83 | 0.71–0.97 | 0 | 0.698 | |
| Female | 2 | 0.97 | 0.84–1.13 | 0 | 0.478 | |
| Exposure measure | ||||||
| FFQ | 6 | 0.88 | 0.79–0.98 | 31.6 | 0.199 | |
| Others | 2 | 0.54 | 0.36–0.83 | 56.4 | 0.130 | |
COPD: chronic obstructive pulmonary disease; RR: relative risk; CI, confidence interval.
3.3. Fruit plus Vegetable Intake and Risk of COPD
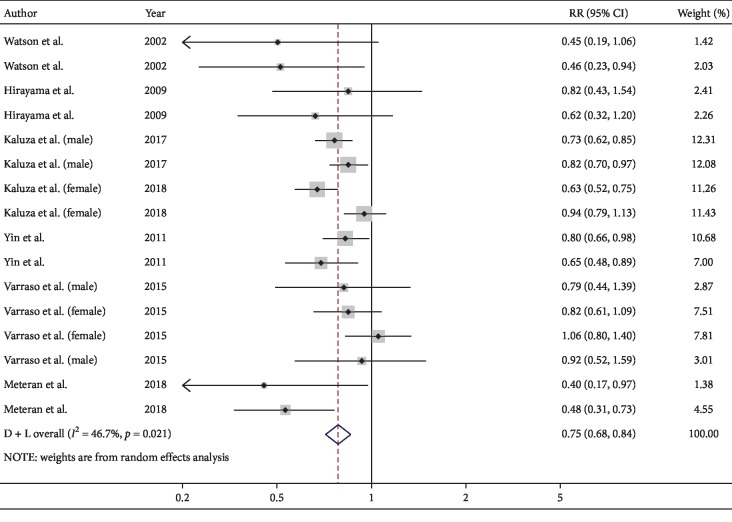
For the highest versus the lowest level of fruit plus vegetable (FV) intake, the pooled RR of COPD was 0.75 (95% CI, 0.68–0.84; I2 = 46.7%, P=0.021; REM; Figure 2). Raw vegetable was included as vegetable intake in one study [12]; the RR (0.77, 95% CI, 0.70–0.85) was consistent with the main result after excluding this article.
Figure 2.
Forest plot for the pooled RR and 95% CI of studies on FV intake and COPD. The size of grey box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
The protective effect of FV was observed in all three study designs. No significant results were found in America (RR, 0.92; 95% CI, 0.77–1.10; I2 = 0%) and female (RR, 0.84; 95% CI, 0.66–1.07; I2 = 0%). In the subgroup analysis by exposure measures, the beneficial effect of FV intake was found both in FFQ (RR, 0.79; 95% CI, 0.70–0.88; I2 = 44.1%) and other types of questionnaires (RR, 0.63; 95% CI, 0.48–0.83; I2 = 54.1%).
3.4. Fruit Intake and Risk of COPD
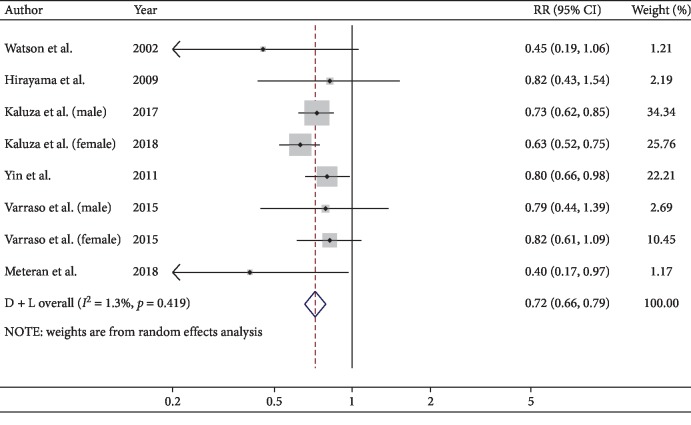
Data from eight studies including a total of 87,364 participants were used to analyze the association between fruit intake and COPD. For the highest versus the lowest level of fruit intake, the pooled RR was 0.72 (95% CI, 0.66–0.79; I2 = 1.3%, P=0.419; REM; Figure 3). That is, fruit intake might reduce the risk of COPD by 28%.
Figure 3.
Forest plot for the pooled RR and 95% CI of studies on fruit intake and COPD. The size of grey box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
As for the types of fruits, two studies provided specific fruit types. The inverse association was found in apple or pears (RR, 0.67; 95% CI, 0.55–0.83; I2 = 0%), while not in banana (RR, 0.87; 95% CI, 0.50–1.51; I2 = 83.9%) and citrus fruits (RR, 1.05; 95% CI, 0.85–1.28; I2 = 0%). Three studies reported the RRs of smoking status, the protective effect of fruit was observed in both nonsmokers (RR, 0.78; 95% CI, 0.66–0.93; I2 = 0%) and ever-smokers (RR, 0.72; 95% CI, 0.64–0.81; I2 = 17.7%).
Subgroup analyses were conducted by study design, continent where the study conducted, gender, and exposure measures. Fruit intake indicated a beneficial effect on lower the risk of COPD in cohort design (RR, 0.71; 95% CI, 0.63–0.79; I2 = 0%), while not in cross-sectional design (RR, 0.65; 95% CI, 0.35–1.21; I2 = 56.8%), and case-control design (RR, 0.66; 95% CI, 0.40–1.11; I2 = 17.2%). In terms of continent, an inverse association was found in Europe (RR, 0.67; 95% CI, 0.60–0.76; I2 = 19.6%) and Asia (RR, 0.80; 95% CI, 0.66–0.97; I2 = 0%), but not in America (RR, 0.81; 95% CI, 0.63–1.05; I2 = 0%). The beneficial effects of fruit intake were found in male (RR, 0.73; 95% CI, 0.63–0.85; I2 = 0%), female (RR, 0.70; 95% CI, 0.54–0.90; I2 = 55.9%) and both genders (RR, 0.76; 95% CI, 0.63–0.91; I2 = 21.6%). The results used by different types of questionnaires were inconsistent, the beneficial effect was found in FFQ (RR, 0.70; 95% CI, 0.63–0.78; I2 = 0%), not in other types of questionnaires (RR, 0.65; 95% CI, 0.35–1.21; I2 = 56.8%).
In dose-response analysis, data from four studies [9, 10, 14, 15] were used. Detailed characteristics of studies and participants included in the dose-response analysis are shown in the Supplementary Material Table S6. A nonlinear relationship was found between fruit intake and COPD risk (Pnon‐linearity < 0.01). Compared with the lowest category of fruit intake, the RRs with 95% CIs of COPD risk were 0.78 (95% CI, 0.67–0.92), 0.70 (95% CI, 0.59–0.82), 0.69 (95% CI, 0.59–0.81), and 0.68 (95% CI, 0.48–0.97) for 1, 2, 3, and 4 servings/day, respectively (Figure 4).
Figure 4.
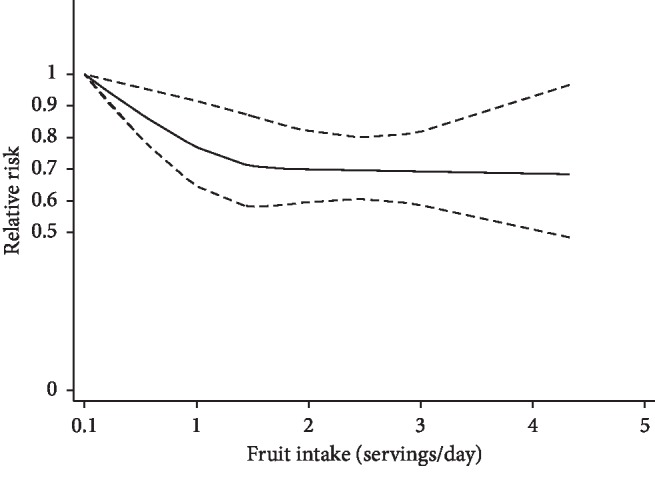
The dose-response analysis of fruit intake and the risk of COPD. The solid line and the long dashed line represent the estimated relative risks and their 95% CIs. The short dashed line represents the linear relationship.
3.5. Vegetable Intake and Risk of COPD
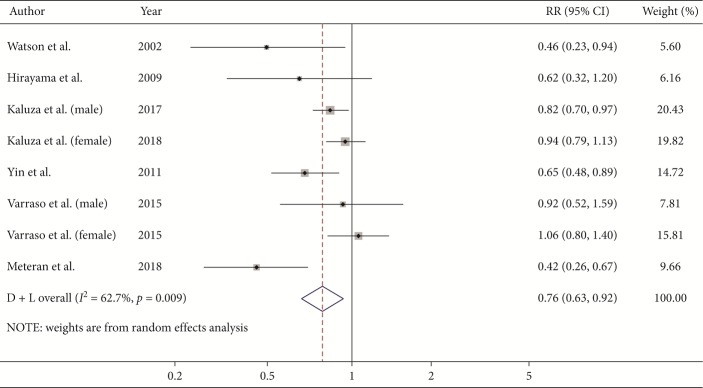
Data from eight studies including a total of 246,154 participants were used to analyze the association between vegetable intake and COPD risk. For the highest versus the lowest level of vegetable intake, the pooled RR was 0.76 (95% CI, 0.63–0.92; I2 = 62.7%, P=0.009; Figure 5; REM), which indicates a 24% reduction in the risk of COPD. Since one of the articles [12] was raw vegetables, the pooled result (RR, 0.83; 95% CI, 0.71–0.97) was still consistent with the main result when this article was removed.
Figure 5.
Forest plot for the pooled RR and 95% CI of studies on vegetable intake and COPD. The size of grey box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
In the analysis of vegetable types, two studies provided the specific vegetable types. Green leafy vegetable (RR, 0.75; 95% CI, 0.52–1.09; I2 = 80.2%), cruciferous vegetable (RR, 1.22; 95% CI, 1.00–1.19; I2 = 0%), and root vegetable (RR, 1.00; 95% CI, 0.86–1.15; I2 = 0%) all showed no statistically significant results. Three studies reported the RRs of smoking status; the beneficial effect of vegetable intake was found in ever-smokers (RR, 0.82; 95% CI, 0.72–0.93; I2 = 0%), but not in nonsmokers (RR, 0.82; 95% CI, 0.53–1.26; I2 = 63.6%).
In the subgroup analysis by study design, vegetable intake was associated with a lower risk of COPD in case-control design (RR, 0.54; 95% CI, 0.33–0.87; I2 = 0%) and cross-sectional design (RR, 0.54; 95% CI, 0.36–0.83; I2 = 56.4%), but not in cohort design (RR, 0.90; 95% CI, 0.81–1.00; I2 = 0%). In the subgroup analysis by continent, the inverse association was found in Europe (RR, 0.70; 95% CI, 0.53–0.94; I2 = 76%), and Asia (RR, 0.64; 95% CI, 0.49–0.85; I2 = 0%), while not in America (RR, 1.03; 95% CI, 0.80–1.32; I2 = 0%). This protective effect was observed both in male (RR, 0.83; 95% CI, 0.71–0.97; I2 = 0%) and in both genders (RR, 0.56; 95% CI, 0.45–0.71; I2 = 0%), but not in female (RR, 0.97; 95% CI, 0.84–1.13; I2 = 0%). In terms of the questionnaire types, the beneficial effects of vegetable intake were found both in FFQ (RR, 0.88; 95% CI, 0.79–0.98; I2 = 31.6%) and other types of questionnaires (RR, 0.54; 95% CI, 0.36–0.83; I2 = 56.4%).
For dose-response analysis of vegetable intake, data from four studies [9, 10, 14, 15] were included. Detailed characteristics of studies and participants included in the dose-response analysis are shown in the Supplementary Material Table S7. A linear trend was found between vegetable intake and COPD risk (Pnon‐linearity=0.597), but the results showed no statistical significance.
3.6. Metaregression
Moderate heterogeneity (I2 = 62.7%; P=0.009) was found in the analysis of vegetable intake and COPD risk. The metaregression analysis was conducted with the covariates of publication year (P=0.290), measurement method of COPD (P=0.842), continent (P=0.298), exposure measures (P=0.052), study design (P=0.017), gender (P=0.009), and quality score (P=0.482) to explore the potential sources of heterogeneity. Study design and gender were found to influence the between-study heterogeneity. We further included study design and gender into the multiple covariates metaregression analysis. The result showed that the estimate of between-study variance Tau-squared was decreased from 0.0331 to 0.
3.7. Sensitivity Analysis and Influence Analysis
In the analysis of FV (I2 = 46.7%; P=0.021) and fruit (I2 = 1.3%; P=0.419) intake, low heterogeneity limiting us to perform leave-one-out sensitivity analysis. In vegetable intake and COPD risk, we performed the leave-one-out sensitivity analysis, after excluding one study [12], I2 was decreased from 62.7% to 43.2% (P=0.103), and the result is still significant (RR, 0.83; 95% CI, 0.71–0.97; I2 = 43.2%, REM). Influence analysis showed that no individual study had an excessive impact on the final results of FV, fruits, and vegetables (Supplementary Figures S1–S3).
3.8. Publication Bias
The visual inspection of funnel plot (Supplementary Figures S4–S6) appears to be symmetrical in analysis of FV, fruits, and vegetables. Egger test illustrated that there was no significant publication bias detected in the analysis of FV (t = −1.42, P=0.178), fruit intake (t = −0.65, P=0.538) and vegetable intake (t = −1.69, P=0.142).
4. Discussion
In this meta-analysis, seven articles including eight studies were quantitatively summarized to assess the potential protective effect of fruit and vegetable intake on reduction of COPD risk. We found that FV, fruit, and vegetable consumption related to a significantly decreased risk of COPD. In subgroup analysis of FV intake and COPD risk, the inverse association exists in all three study designs. A nonlinear dose-response relationship (Pnon‐linearity < 0.01) was found between fruit intake and COPD risk; the RRs with 95% CIs of COPD risk were 0.78 (95% CI, 0.67–0.92), 0.70 (95% CI, 0.59–0.82) for 1, 2 servings/day of fruits, respectively. For more than 2 servings/day, the risk of COPD was decreased slightly.
The potential mechanism for the protective effect of fruit and vegetable intake on the COPD risk has been proposed as follows. Oxidative stress and inflammation play a major role in the occurrence of COPD [27]. Long-term exposure to tobacco smoke, biomass smoke, and other environmental factors is major causes of oxidative stress in the lung [28–30]. The existence of reactive oxygen species (ROS) impairs endogenous antioxidant defenses [27]. Thus a large amount of ROS may cause proinflammatory gene overexpression and oxidative tissue damage, resulting in inflammation [31]. Fruits and vegetables are rich in vitamin C [32–34], minerals, β-carotene [35], dietary fiber [36, 37], and other antioxidants and played a protective role in the development of COPD. It has been observed that dietary antioxidant has the ability to capture exogenous and endogenous free radicals, thereby preventing lipid peroxidation caused by free radicals; for instance, vitamin C can protect α1-proteinase inhibitors from oxidative damage; therefore, it can maintain the balance between protease and antiprotease [38]. All those antioxidant ingredients can not only ameliorate oxidative stress but also inversely associate with inflammatory biomarkers. Research has shown that vitamins and dietary fibers reduce serum levels of C reactive protein [39].
Between-study heterogeneity is an important part in meta-analysis. Our meta-analysis showed moderate between-study heterogeneity in the analysis of vegetable intake and COPD risk. Study design and gender were found to contribute to the heterogeneity. In multiple covariates metaregression analysis of study design and gender, the estimate of between-study variance Tau-squared was decreased from 0.0331 to 0, which means that study design and gender could explain all sources of heterogeneity [40]. In the leave-one-out sensitivity analysis, one study [12] was identified as a significant impact on the between-study heterogeneity. After excluding that cross-sectional study, heterogeneity was lower than 50% and the result was still statistically significant. In this study [12], cross-sectional survey was performed on two twin study cohorts which included two different twin populations. So it was different from other studies in population selection.
The advantages of our article are in the following aspects. First, a restricted cubic spline model was used in dose-response analysis, and a nonlinear dose-response relationship was found for the COPD risk with fruits. Second, potential confounding factors in original studies were fully taken into account. As the most important confounding factors, smoking and age have been adjusted in all included studies. In addition, BMI, physical activity, intake of energy, and other confounders also have been adjusted in most studies. Third, the quality assessment of included studies was performed, and each of the studies scored more than 7 points, indicating that the articles included were reliable.
There also exist some limitations. First, although results from all 8 studies were adjusted for age, gender, smoking status, and other confounders, the effects of residual or unknown confounding factors on the observed findings could not be ruled out completely. Second, the protective effect of FV was observed in all three study designs, but the results of the three study designs were inconsistent in the analysis of fruits and vegetables. In FV and fruits, inverse association was found in cohort design. Cohort design meets the criteria of temporality and provides stronger evidence for the hypothesis. In the analysis of vegetables, protective effects were found in case-control and cross-sectional design, while cohort design showed an inverse but not significant result. Thus, the protective effects of vegetables on COPD need to be investigated further. Third, the assessment methods of fruit and vegetable consumption were different, which may have a certain impact on the results. In the analysis of fruits and COPD, a significant association was found in studies using FFQ (RR, 0.70; 95% CI, 0.63–0.78), but not in studies using other types of questionnaires (RR, 0.65; 95% CI, 0.35–1.21), so the pooled result (RR, 0.72; 95% CI, 0.66–0.79) was underestimated. In the analysis of vegetables, the pooled result (RR, 0.76; 95% CI, 0.63–0.92) was overestimated by 13.6% due to the use of other questionnaires. Fourth, there were no uniform diagnostic criteria for COPD and no detailed disease information in the included studies. Therefore, the status (Mild, Moderate, Severe) of COPD patients was unclear, and we were unable to perform a pooled analysis.
5. Conclusion
The results of this meta-analysis suggest that fruit and vegetable intake may be associated with a reduction of COPD risk. Further research is needed to confirm these results and to explore whether there are gender differences in the protective effects of fruits and vegetables.
Conflicts of Interest
The authors declare no conflicts of interest.
Supplementary Materials
Table S1: the search strategy of fruit and vegetable consumption and COPD in PubMed. Table S2: detailed list of the number of excluded full-text reviewed articles. Table S3: quality assessment of included case-control studies. Table S4: quality assessment of included cross-sectional studies. Table S5: quality assessment of included cohort studies. Table S6: characteristics of studies and participants included in the dose-response analysis of the association between fruit intake and COPD risk. Table S7: characteristics of studies and participants included in the dose-response analysis of the association between vegetable intake and COPD risk. Figure S1: influence analysis of 8 studies on FV consumption and COPD. Figure S2: influence analysis of 8 studies on fruit consumption and COPD. Figure S3: influence analysis of 8 studies on vegetable consumption and COPD. Figure S4: funnel plot of the relative risk of 8 studies on FV consumption and COPD. Each dot represents a different study. Figure S5: funnel plot of the relative risk of 8 studies on fruit consumption and COPD. Each dot represents a different study. Figure S6: funnel plot of the relative risk of 8 studies on vegetable consumption and COPD. Each dot represents a different study. A checklist of MOOSE.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Fontana-on-Geneva Lake, WI, USA: Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2019. [Google Scholar]
- 2.Barnes P. J., Celli B. R. Systemic manifestations and comorbidities of COPD. European Respiratory Journal. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 3.Mannino D. M., Thorn D., Swensen A., Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. European Respiratory Journal. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 4.Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clinics in Chest Medicine. 2014;35(1):17–27. doi: 10.1016/j.ccm.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P. J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Mannino D. M., Buist A. S. Global burden of COPD: risk factors, prevalence, and future trends. The Lancet. 2007;370(9589):765–773. doi: 10.1016/s0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 7.Domej W., Oetll K., Renner W. Oxidative stress and free radicals in COPD – implications and relevance for treatment. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:1207–1224. doi: 10.2147/copd.s51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keranis E., Makris D., Rodopoulou P., et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. European Respiratory Journal. 2010;36(4):774–780. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- 9.Kaluza J., Larsson S. C., Orsini N., Linden A., Wolk A. Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax. 2017;72(6):500–509. doi: 10.1136/thoraxjnl-2015-207851. [DOI] [PubMed] [Google Scholar]
- 10.Kaluza J., Harris H. R., Linden A., Wolk A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. International Journal of Epidemiology. 2018;47:1897–1909. doi: 10.1093/ije/dyy178. [DOI] [PubMed] [Google Scholar]
- 11.Yin P., Jiang Y., Zhang M., Li Y. C., Wang L. M., Zhao W. H. Association between frequency of fruit and vegetable intake and chronic obstructive pulmonary disease. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(8):707–710. [PubMed] [Google Scholar]
- 12.Meteran H., Thomsen S. F., Miller M. R., Hjelmborg J., Sigsgaard T., Backer V. Self-reported intake of fruit and vegetables and risk of chronic obstructive pulmonary disease: a nation-wide twin study. Respiratory Medicine. 2018;144:16–21. doi: 10.1016/j.rmed.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Varraso R., Chiuve S. E., Fung T. T., et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015;350(7):p. h286. doi: 10.1136/bmj.h286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson L., Margetts B., Howarth P., Dorward M., Thompson R., Little P. The association between diet and chronic obstructive pulmonary disease in subjects selected from general practice. European Respiratory Journal. 2002;20(2):313–318. doi: 10.1183/09031936.02.00256402. [DOI] [PubMed] [Google Scholar]
- 15.Hirayama F., Lee A. H., Binns C. W., et al. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case-control study in Japan. Preventive Medicine. 2009;49(2-3):184–189. doi: 10.1016/j.ypmed.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in Epidemiology. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Bekkering G. E., Harris R. J., Thomas S., et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? American Journal of Epidemiology. 2008;167(9):1017–1026. doi: 10.1093/aje/kwn005. [DOI] [PubMed] [Google Scholar]
- 18.Dauchet L., Amouyel P., Hercberg S., Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. The Journal of Nutrition. 2006;136(10):2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X., Zhang Y., Kwong J. S. W., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of Evidence-Based Medicine. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J. P. T., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Brockwell S. E., Gordon I. R. A comparison of statistical methods for meta-analysis. Statistics in Medicine. 2001;20(6):825–840. doi: 10.1002/sim.650. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J. P. T., Thompson S. G. Controlling the risk of spurious findings from meta-regression. Statistics in Medicine. 2004;23(11):1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 23.Patsopoulos N. A., Evangelou E., Ioannidis J. P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American Journal of Epidemiology. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson D., White I. R., Thompson S. G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Statistics in Medicine. 2010;29(12):1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 27.Kirkham P. A., Barnes P. J. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels R. A., Buist A. S., Calverley P. M. A., Jenkins C. R., Hurd S. S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 29.Hu G., Zhou Y., Tian J., et al. Risk of COPD from exposure to biomass smoke. Chest. 2010;138(1):20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 30.Hogg J. C., Chu F., Utokaparch S., et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. New England Journal of Medicine. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 31.Drost E. M., Skwarski K. M., Sauleda J., et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60(4):293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly F. J. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proceedings of the Nutrition Society. 2005;64(4):510–526. doi: 10.1079/pns2005457. [DOI] [PubMed] [Google Scholar]
- 33.Joshi P., Kim W. J., Lee S. A. The effect of dietary antioxidant on the COPD risk: the community-based KoGES (Ansan-Anseong) cohort. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10:2159–2168. doi: 10.2147/copd.s91877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz J., Weiss S. T. Relationship between dietary vitamin C intake and pulmonary function in the first national health and nutrition examination survey (NHANES I) The American Journal of Clinical Nutrition. 1994;59(1):110–114. doi: 10.1093/ajcn/59.1.110. [DOI] [PubMed] [Google Scholar]
- 35.Hu G., Cassano P. A. Antioxidant nutrients and pulmonary function: the third national health and nutrition examination survey (NHANES III) American Journal of Epidemiology. 2000;151(10):975–981. doi: 10.1093/oxfordjournals.aje.a010141. [DOI] [PubMed] [Google Scholar]
- 36.Kaluza J., Harris H., Wallin A., Linden A., Wolk A. Dietary fiber intake and risk of chronic obstructive pulmonary disease. Epidemiology. 2018;29(2):254–260. doi: 10.1097/ede.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 37.Varraso R., Willett W. C., Camargo C. A., Jr. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. American Journal of Epidemiology. 2010;171(7):776–784. doi: 10.1093/aje/kwp455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu G., Zhang X., Chen J., Peto R., Campbell T. C., Cassano P. A. Dietary vitamin C intake and lung function in rural China. American Journal of Epidemiology. 1998;148(6):594–599. doi: 10.1093/oxfordjournals.aje.a009685. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y., Griffith J. A., Chasan-Taber L., et al. Association between dietary fiber and serum C-reactive protein. The American Journal of Clinical Nutrition. 2006;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Liu J., Zhong W. Application of stata in exploring sources of heterogeneity:meta-regression analysis. The Journal of Evidence-Based Medicine. 2009;9:48–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: the search strategy of fruit and vegetable consumption and COPD in PubMed. Table S2: detailed list of the number of excluded full-text reviewed articles. Table S3: quality assessment of included case-control studies. Table S4: quality assessment of included cross-sectional studies. Table S5: quality assessment of included cohort studies. Table S6: characteristics of studies and participants included in the dose-response analysis of the association between fruit intake and COPD risk. Table S7: characteristics of studies and participants included in the dose-response analysis of the association between vegetable intake and COPD risk. Figure S1: influence analysis of 8 studies on FV consumption and COPD. Figure S2: influence analysis of 8 studies on fruit consumption and COPD. Figure S3: influence analysis of 8 studies on vegetable consumption and COPD. Figure S4: funnel plot of the relative risk of 8 studies on FV consumption and COPD. Each dot represents a different study. Figure S5: funnel plot of the relative risk of 8 studies on fruit consumption and COPD. Each dot represents a different study. Figure S6: funnel plot of the relative risk of 8 studies on vegetable consumption and COPD. Each dot represents a different study. A checklist of MOOSE.