Abstract
Background
Ulceration of the feet, which can result in loss of limbs and even death, is one of the major health problems for people with diabetes mellitus.
Objectives
To assess the effects of patient education on the prevention of foot ulcers in patients with diabetes mellitus.
Search methods
We searched The Cochrane Wounds Group Specialised Register (searched 03 September 2014); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8).
Selection criteria
Prospective randomised controlled trials (RCTs) that evaluated educational programmes for preventing foot ulcers in people with diabetes mellitus.
Data collection and analysis
Two review authors independently undertook data extraction and assessment of risk of bias. Primary end points were foot ulceration or ulcer recurrence and amputation.
Main results
Of the 12 RCTs included, the effect of patient education on primary end points was reported in only five. Pooling of outcome data was precluded by marked, mainly clinical, heterogeneity. One of the RCTs showed reduced incidence of foot ulceration (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.14 to 0.66) and amputation (RR 0.33, 95% CI 0.15 to 0.76) during one‐year follow‐up of diabetes patients at high risk of foot ulceration after a one‐hour group education session. However, one similar study, with lower risk of bias, did not confirm this finding (RR amputation 0.98, 95% CI 0.41 to 2.34; RR ulceration 1.00, 95% CI 0.70 to 1.44). Three other studies, also did not demonstrate any effect of education on the primary end points, but were most likely underpowered. Patients' foot care knowledge was improved in the short term in five of eight RCTs in which this outcome was assessed, as was patients' self‐reported self‐care behaviour in the short term in seven of nine RCTs. Callus, nail problems and fungal infections improved in only one of five RCTs. Only one of the included RCTs was at low risk of bias.
Authors' conclusions
In some trials, foot care knowledge and self reported patient behaviour seem to be positively influenced by education in the short term. Yet, based on the only two sufficiently powered studies reporting the effect of patient education on primary end points, we conclude that there is insufficient robust evidence that limited patient education alone is effective in achieving clinically relevant reductions in ulcer and amputation incidence.
Keywords: Humans; Patient Education as Topic; Amputation, Surgical; Amputation, Surgical/statistics & numerical data; Diabetic Foot; Diabetic Foot/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Educating people with diabetes about foot care to help reduce foot ulcers and amputations
Foot ulcers (open sores) are common in people with diabetes, especially those with problems in the nerves (peripheral neuropathy), the blood supply to their legs (peripheral vascular disease (PVD)), or both. People with ulcers due to diabetes sometimes need an amputation (surgical removal of part of the limb). Foot ulcers not only lead to physical disability and loss of quality of life but also to economic burden (healthcare costs, industrial disability). The aim is therefore to prevent foot ulcers occurring. This review of high‐level studies found that educating people with diabetes about the need to look after their feet seems to improve people's foot care knowledge and behaviour in the short term. There is insufficient evidence that education alone, without any additional preventive measures, will effectively reduce the occurrence of ulcers and amputations.
Background
Ulceration of the foot is one of the major health problems for people with diabetes mellitus. It is estimated to affect 15% to 25% of people with diabetes at some time in their lives (Singh 2005). Foot ulceration can result in marked physical disability and reduction of quality of life (Nabuurs‐Franssen 2005; Vileikyte 2001), not to mention limb loss and even death (Robbins 2008). Diabetic foot ulcers precede 25% to 90% of all amputations (Global Lower Extremity Amputation Study Group 2000; Pecoraro 1990). The risk of a lower extremity amputation in people with diabetes is therefore much higher than in people without diabetes (Canavan 2008; Icks 2009).
Several factors are involved in the development of foot ulcers, including peripheral neuropathy, PVD, limited joint mobility and repeated trauma from abnormal load distribution on the foot (Dinh 2005; Edmonds 2006). The underlying causes of foot ulcers are usually irreversible and chronically progressive. Therefore, 70% of healed foot ulcers recur within five years (Apelqvist 1993). Moreover, treatment itself is very challenging and often needs to be long lasting. It requires not only expert interference, orthopaedic appliances and antimicrobial drugs but also costly topical dressings and inpatient care (Boulton 2004; Cavanagh 2005; Edmonds 2006; Jeffcoate 2003; Singh 2005). Not surprisingly, this leads to substantial economic burden. Healing of a single ulcer costs approximately USD17,500 (1998 US dollars) (Ragnarson Tennvall 2004). In cases where lower extremity amputation is required, health care is even more expensive: USD30,000 to USD33,500 (Ragnarson Tennvall 2004). These costs do not even represent the total economic burden, since costs related to loss of productivity, preventive efforts, rehabilitation and home care should also be considered. When all this is taken into account, 7% to 20% of total expenditure on diabetes in North America and Europe might be attributable to diabetic foot ulceration (Boulton 2005).
In 1989 one of the main five‐year targets of the European Declaration of St. Vincent was to reduce amputations caused by diabetes mellitus by 50% (St Vincent Declaration 1989). In order to reach these goals, international guidelines underline the need to reduce the incidence of foot ulceration by preventive efforts. This not only includes optimising metabolic control and identification and screening of people at high risk for diabetic foot ulceration, but also patient education in order to promote foot self‐examination and foot care knowledge (American Diabetes Association 2007; Frykberg 2006; IDF clinical guidelines task force 2005).
Population‐based research suggests that a meaningful reduction in the incidence of amputations caused by diabetes mellitus has already been achieved. Before the European Assembly in St. Vincent, the risk ratio (RR) of a lower extremity amputation was still 15 times higher in people with diabetes mellitus than in people without diabetes mellitus (Most 1983). This RR has since dropped to 8.8 (95% CI 7.3 to 10.7) in men and 5.7 (95% CI 4.3 to 7.6) in women in one study (Icks 2009) and to 7.7 (95% CI 5.0 to 12.9) in another (Canavan 2008).
However, it cannot be inferred from these figures that current preventive efforts are effective, since the reduction in amputation incidence may also have resulted from improvements in ulcer treatment. In this review of randomised controlled trials (RCTs) we, therefore, evaluate the effect of education of people with diabetes aiming to promote foot self‐care and to prevent the occurrence of foot lesions. Although this type of prevention is nowadays widely advocated and implemented in standard practice, the evidence for the effectiveness is still scarce. Several review articles on the diabetic foot, which include education among the prevention strategies discussed, have been published (Armstrong 1998; Assal 1985; Bild 1989; Boulton 1995; Bowering 2001; Edmonds 1996; Larsson 1995; Levin 1995; Majid 2000; Mason 1999; Mayfield 1998; Rith‐Najarian 2000; Singh 2005; Wu 2007). However, only three of these reviews were systematic (Majid 2000; Mason 1999; Singh 2005) and most of these reviews dealt primarily with uncontrolled studies. Furthermore, only two of these reviews assessed the methodological quality of the included studies (Majid 2000; Mason 1999). The overall conclusion of these review articles was that education is effective for the prevention of diabetic foot ulceration, but consequently this conclusion must be treated with care; especially since previous systematic reviews of patient education for adults with, for example, asthma and neck pain, have suggested that health outcomes were unlikely to be improved by limited patient education (Gibson 2002; Haines 2009).
Thus, after reviewing the available evidence, we decided to perform a systematic review of the effectiveness of (components of) education programmes targeted at people with diabetes with the aim of preventing foot ulceration.
Objectives
To assess the effects of patient education on the prevention of foot ulcers in patients with diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Prospective RCTs evaluating educational programmes for the prevention of foot ulcers in people with diabetes mellitus. We excluded studies that were solely aimed at optimising blood glucose concentration. An explicit focus on foot care was required.
Types of participants
Studies involving people aged 18 years or older with type 1 or type 2 diabetes mellitus in any healthcare setting.
Types of interventions
Studies of educational programmes (or programmes that include education) aiming to reduce the incidence of foot ulceration in people with diabetes mellitus.
Studies where foot‐care education was part of a larger educational programme (e.g. on diabetes in general) were eligible, but education on foot care had to contrast with the control intervention. The foot care education could also be part of a more comprehensive diabetic foot programme, but in these cases patient education on foot care had to be the main contrast with the control intervention. All types of control intervention were considered for inclusion in the review.
Types of outcome measures
Primary outcomes
Foot ulceration or ulcer recurrence.
Amputation.
Secondary outcomes
Clinical outcomes:
callus development,
resolution of callus,
fungal infection,
number and duration of hospital admissions for diabetic foot problems.
Process outcomes:
foot care knowledge scores,
patients' behaviour assessment scores.
Trials were included if secondary outcomes only were reported.
Search methods for identification of studies
Electronic searches
The search methods section of the third update of this review can be found in Appendix 2
For this fourth update we searched the following electronic databases:
The Cochrane Wounds Group Specialised Register, comprising references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings (searched 03 September 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8).
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Education] explode all trees #2 patient near/3 education*:ti,ab,kw #3 diabetes near/3 education*:ti,ab,kw #4 patient near/3 information:ti,ab,kw #5 education* near/2 program*:ti,ab,kw #6 (foot next care) or footcare:ti,ab,kw #7 leaflet* or booklet* or pamphlet* or "poster" or "posters":ti,ab,kw #8 (written or printed or oral) near/3 information:ti,ab,kw #9 academic next detailing:ti,ab,kw #10 training next program*:ti,ab,kw #11 algorithm* or (decision next tree*):ti,ab,kw #12 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13 MeSH descriptor: [Foot Ulcer] explode all trees #14 MeSH descriptor: [Diabetic Foot] explode all trees #15 diabet* near/3 ulcer*:ti,ab,kw #16 diabet* near/3 (foot or feet):ti,ab,kw #17 diabet* near/3 amputat*:ti,ab,kw #18 diabet* near/3 wound*:ti,ab,kw #19 diabet* near/3 defect*:ti,ab,kw #20 (#13 or #14 or #15 or #16 or #17 or #18 or #19) #21 (#12 and #20)
No date or language restrictions were applied.
Searching other resources
The bibliographies of all retrieved and relevant publications identified by these strategies and the list of articles that cited previous versions of this review were searched for further studies.
Data collection and analysis
Selection of studies
Full copies of potentially eligible studies were obtained and two review authors (GV and DK or GV and JD), acting independently, decided on inclusion or exclusion. In case of disagreement, consensus was reached by discussion between three review authors (GV, DK and JD).
Data extraction and management
We extracted details of eligible studies and summarised them using a data extraction sheet. We recorded the content of the educational package, plus the content of the total programme, if education was merely one component. Data from multiple reports of individual studies were extracted and the primary reference identified (Borges 2004; Rönnemaa 1997). We recorded all outcomes if different but relevant outcomes were available from different publications of the same RCT. Data regarding the interventions studied, type of outcome measures, duration of follow‐up, loss to follow‐up and outcomes were extracted by two review authors (GV and DK or GV and JD) independently. In case of disagreement, consensus was reached by discussion between three review authors (GV, DK and JD).
Assessment of risk of bias in included studies
We assigned two review authors (JD and DK or GV) to assess each study independently using The Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 1 for details of criteria on which the judgement was based). Because blinding of patients and healthcare providers does not appear to be feasible considering the nature of the interventions studied, judgement was solely based on the information provided about blinding of outcome assessors. Blinding and completeness of outcome data were assessed for each outcome separately. Any disagreements were discussed in a consensus meeting. We completed a 'Risk of bias' table for each eligible study (see Characteristics of included studies).
We assessed risk of bias using a 'Risk of bias summary' figure (see Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity may guide the weight the reader may give to the results of the particular studies.
1.
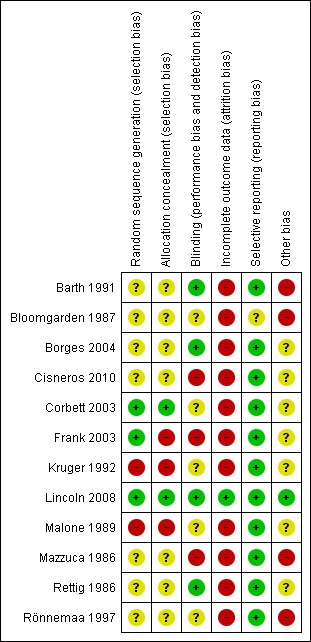
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
We reported separately for each study. Depending on the available data we aimed to present the results for binary outcomes (e.g. ulceration or amputation) as RR with corresponding 95% confidence intervals (CI) and the results for continuous data (e.g. callus diameter) as mean differences with corresponding 95% CIs.
Dealing with missing data
If data were missing from reports, we then attempted to contact the study authors. We were successful in contacting the authors of Corbett 2003; Rönnemaa 1997 and Mazzuca 1986, and additional data on effect sizes were obtained.
Data synthesis
Because substantial statistical and methodological heterogeneity between studies was observed, all results were presented in a qualitative summary (Reed 2005).
Subgroup analysis and investigation of heterogeneity
Possible sources of variation among studies that would require pre‐planned stratified analysis were:
character of patient groups (e.g. patients at high risk for foot ulceration compared with patients at low risk; patients with a history of foot ulceration compared with patients without, etc.),
health care setting,
risk of bias of studies,
outcome measures used,
type of intervention (e.g. brief compared with intensive programmes; education tailored to the individual needs compared with standardised education programmes),
nature of contrast (e.g. intervention compared with control intervention, patient education plus co‐intervention A compared with intervention A alone, intervention compared with no intervention).
Results
Description of studies
Results of the search
For this fourth update of the original review article (Other published versions of this review) no additional RCTs were identified. One study (Zhenghua 2011) previously placed in the Studies awaiting classification was moved to the Excluded studies, because no full text report was published. One other study (Gershater 2011) remained in the Studies awaiting classification, because the study report is still pending.
Included studies
Twelve RCTs are included in this review and are described in the Characteristics of included studies and summarised below.
Healthcare settings
Four RCTs were performed in a community‐based care setting (Cisneros 2010; Corbett 2003; Rettig 1986; Rönnemaa 1997): patients were recruited from a home care organisation register (Corbett 2003), hospital records (Rettig 1986), the national drug imbursement register (Rönnemaa 1997) or the National Health System database (Cisneros 2010). The care settings of three studies were classified as primary care (Bloomgarden 1987; Frank 2003; Mazzuca 1986): one was performed in a diabetes outpatient clinic in the USA (Bloomgarden 1987), one in a country hospital's outpatient clinic in the USA (Frank 2003) and one in an academic general medicine outpatient clinic in the USA. Four studies were performed in a secondary care setting: one in an outpatient clinic in Australia (Barth 1991), two in secondary outpatient care in the USA (Kruger 1992; Malone 1989) and one in secondary outpatient care in the UK (Lincoln 2008). Finally, one study setting could not be categorised with any of the above, because it was performed in the emergency departments of two community hospitals in the USA (Borges 2004).
Participants' risk of foot ulceration
In four of the 12 included RCTs, patients were at high risk of foot ulceration (Cisneros 2010; Corbett 2003; Lincoln 2008; Malone 1989). In Malone 1989 all patients were referred to podiatry or vascular surgery prior to study entry and had foot infection, ulceration or prior amputation. In Lincoln 2008 all participants had newly healed diabetic foot ulcers. In Corbett 2003 patients were excluded if they had a history of foot amputation, but it follows from the presence of important risk factors at baseline that the studied population was at high risk for foot ulceration: loss of protective sensation was present in 70%, impaired lower extremity circulation in 67% and foot deformity in 50%. Finally, in Cisneros 2010 all patients had diabetic neuropathy and most had additional risk factors such as plantar overpressure, deformity and previous foot ulceration. In four RCTs patients were at low or medium risk of foot ulceration (Barth 1991; Bloomgarden 1987; Frank 2003; Rönnemaa 1997). In Rönnemaa 1997 patients were even excluded if they had any need for podiatry. Finally in four RCTs, the level of risk for ulceration or amputation could not be determined (Borges 2004; Kruger 1992; Mazzuca 1986; Rettig 1986).
Interventions
Three RCTs compared the effectiveness of a patient education programme on diabetes in general (which included a component of foot care education) compared with usual care (Bloomgarden 1987; Mazzuca 1986; Rettig 1986). In one of these studies, the content of the educational programme was preset and consisted of nine group patient education sessions, one of which was about foot care and skin hygiene (Bloomgarden 1987). In the other two studies, the content of the educational programme was tailored to patients' individual needs (Mazzuca 1986; Rettig 1986). In Mazzuca 1986 those educational needs were identified using a set of safety‐level objectives selected by a multidisciplinary group of healthcare professionals. These objectives covered seven areas of patient education, of which foot care was one. In the other study areas of diabetes self‐management, patients most in need of improvement were determined using an assessment instrument (100 short answer and yes‐no questions, brief patient demonstrations of urine testing and insulin injection techniques) (Rettig 1986). In both studies, participants received a variable number of educational sessions.
In addition to Mazzuca 1986 and Rettig 1986, there were two more RCTs that adopted the concept of education tailored to patients' individual needs, but in these studies the educational interventions were only directed at improving foot care (Borges 2004; Corbett 2003) and they were less intensive than in Mazzuca 1986 and Rettig 1986. In Corbett 2003 a single 10‐ to 20‐minute educational session, combined with written instructions, was compared with no intervention, and in Borges 2004 a 15‐minute educational session after a risk assessment for foot ulceration was compared with risk assessment alone and with no intervention at all.
In the remaining six RCTs, an intensive foot care education programme was compared with a less proactive intervention (Barth 1991; Cisneros 2010; Frank 2003; Kruger 1992; Lincoln 2008; Malone 1989; Rönnemaa 1997). This, however, does not mean that the interventions in these studies were all similar. In two studies the intervention was only one hour of patient education, reinforced by hand‐outs (Lincoln 2008; Malone 1989). In Malone 1989, this was compared with routine patient education, and in Lincoln 2008 with written instructions only. In Rönnemaa 1997, patients in the intervention group also received 45 minutes of foot care education, but this was combined with a variable number of follow‐up visits at a podiatry clinic. The control intervention consisted of written instructions on foot care only. In the other studies, the educational interventions were more comprehensive, more intensive or both. In Frank 2003, for example, the intervention comprised viewing a videotape about proper foot care, a 30‐ to 40‐minute individualised educational session, a foot ulceration risk assessment, hand‐outs, a foot care checklist, a bag of foot care supplies and weekly reminder telephone calls. Control group patients received a foot ulceration risk assessment only. In Cisneros 2010 people in the intervention group received four 90‐minute therapeutic education sessions on the complications of diabetes, disease treatment, inspection and foot hygiene and choice and use of proper footwear. In addition, participants received footwear after completion of the educational programme. This was compared with routine care. In two other studies a basic educational programme on diabetes in general was provided to all study participants and supplemented by specific foot care education in the intervention group (Barth 1991; Kruger 1992). In Barth 1991, the general diabetes education consisted of 14 hours of group education and included a one‐hour lecture on foot care. In addition to that, the intervention group followed nine hours of group education about the diabetic foot (Barth 1991). In Kruger 1992, the general diabetes education consisted of a one‐week course, which included viewing an instructional videotape on foot care. In the intervention group this was supplemented with a 'hands‐on' foot care approach, a patient education kit and daily foot care sheets (Kruger 1992).
Duration of follow‐up
The median time to follow‐up was six months (Barth 1991; Kruger 1992; Rettig 1986), ranging from only four weeks (Borges 2004; Frank 2003) to seven years (Rönnemaa 1997).
Excluded studies
Eighteen studies are excluded from the review. Six studies are not randomised controlled studies (Dargis 1999; Davidson 2000; Litzelman 1997; Pieber 1995; Ward 1999; Wooldridge 1996). Four studies did not report an educational programme that includes patient education aimed at reducing diabetic foot ulcers (De Weerdt 1991; Donohoe 2000; Reichard 1993; Vinicor 1985). Foot care education was not a unique intervention in five studies (Litzelman 1993; McCabe 1998; McMurray 2002; Nesari 2010; Plank 2003), one study investigated education initially directed at wound healing (Fresenius 2009) and one study (Glasgow 1992) did not report any outcomes relevant to this review.
Risk of bias in included studies
The risk of bias of most included studies was high, except for one RCT (Lincoln 2008). Details are presented in a 'Risk of bias' table for each eligible study (see Characteristics of included studies) and in a 'Risk of bias summary' figure (see Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. Judgements on the six items were made by two review authors independently for each of the 12 studies. There was initial disagreement on six items (percentage of agreement 92%). All disagreements were resolved by discussion without needing to consult the third review author.
Allocation
True randomisation with allocation concealment was evident in only two of the included RCTs. In Corbett 2003 the sequence was determined by drawing labelled consent forms that were covered with opaque stickers and shuffled. In Lincoln 2008, the sequence was based on a computer‐generated list held by an independent randomisation centre that was contacted by telephone each time a person was randomised. Randomisation was inadequate in three studies. In Frank 2003 group allocation was determined by drawing papers from an envelope, but this envelope was not sealed. Moreover, in Kruger 1992 and Malone 1989, people were allocated to the experimental and control group on alternating weeks and the last digit of their Social Security number respectively, which are both considered non‐random. Insufficient information on randomisation and allocation concealment was provided in the remaining studies.
Blinding
Outcome assessment was blinded in four RCTs (Barth 1991; Borges 2004; Lincoln 2008; Rettig 1986), unblinded in three (Cisneros 2010; Frank 2003; Mazzuca 1986) and not described in the remaining studies. Inadequate blinding may affect subjectively measured secondary outcomes more than foot ulcer and amputation incidence.
Incomplete outcome data
The withdrawal/dropout rate was unacceptably high in six RCTs (Borges 2004; Cisneros 2010; Corbett 2003; Kruger 1992; Mazzuca 1986; Rettig 1986). In only one of the RCTs an intention‐to‐treat (ITT) analysis was performed for primary outcomes (Lincoln 2008).
Selective reporting
Study protocols were not sought and therefore not available for review, but most trial reports listed all expected outcomes in both the methods and the results section. Only Bloomgarden 1987 reported additional outcomes in the results section.
Other potential sources of bias
Co‐interventions were avoided or similar in two studies (Borges 2004; Lincoln 2008). The adherence to the interventions reached an acceptable level in five RCTs (Borges 2004; Cisneros 2010; Corbett 2003; Frank 2003; Lincoln 2008). The most important baseline prognostic indicators were clearly incomparable in two RCTs (Barth 1991; Bloomgarden 1987), sufficiently similar in two others (Frank 2003; Lincoln 2008) and inadequately described in the remaining RCTs.
The eligibility criteria with regard to risk for foot ulceration were sufficiently described in only five RCTs (Borges 2004; Corbett 2003; Frank 2003; Lincoln 2008; Malone 1989).
Effects of interventions
Additional data are presented in Table 1. Results of studies are summarised below in a study‐by‐study qualitative synthesis.
1. Results from trials.
| Study ID | Primary outcomes | Secondary outcomes |
| Barth 1991 | No primary outcomes reported |
Foot problems requiring treatment:
Significant reduction in intervention after 1 month (P < 0.001), maintained until final follow‐up at 6 months
Reduction was significantly smaller in control than in intervention after 1 month (P < 0.006), but not after 6 months (P = 0.216) Foot care knowledge: Significant increase in both groups at 1 month (P < 0.001), but more in intervention than in control (P < 0.001). Changes were maintained until final follow‐up at 6 months Foot care routine compliance: Significant increase in intervention after 1 month (P < 0.001), maintained until final follow‐up Increase was significantly greater in intervention than in control after 1 month (P = 0.012) |
| Bloomgarden 1987 |
Ulcer or amputation:
people with no foot lesions at baseline:
intervention 2/83 vs control 2/63 people with callus, nail dystrophy or fungal infection at baseline: intervention 2/37 vs control 3/63 people with an ulcer or amputation at baseline: intervention 6/7 vs control 11/13 |
Callus, nail dystrophy and fungal infection:
people with no foot lesions at baseline: intervention 31/83 vs control 28/63 (ns)
people with callus, nail dystrophy or fungal infection at baseline: intervention 24/37 vs control 46/63 (ns)
people with an ulcer or amputation at baseline: intervention 1/7 vs control 1/13 (ns) Behaviour assessment scores: intervention from 3.4 to 4.3. Control from 3.6 to 4.1 (P = 0.10). Separate data for foot care not provided |
| Borges 2004 | No primary outcomes reported |
Patients' self‐reported behaviour assessment scores:
intervention from 4.7 to 5.6 (P < 0.01). RA from 4.8 to 5.2 (P = 0.06). C from 5.1 to 5.4 (P < 0.05) Observed self‐care behaviour: 4 of 16 items significantly (P < 0.05) more observed in intervention than in control Foot care knowledge scores: Increased within the control group, but not in the intervention or RA groups |
| Cisneros 2010 |
Ulcer incidence: people without a history of foot ulceration: intervention 8/21 vs control 8/14 (P = 0.317) Patient with a history of foot ulceration: intervention 1/8 vs control 5/8 (P = 0.119) All people: difference between the survival curves of intervention and control (P = 0.362) (HR not reported) |
No secondary outcomes reported |
| Corbett 2003 | No primary outcomes reported |
Foot care knowledge scores:
intervention from 4.9 to 6.1 vs control from 4.6 to 5.2 (P = 0.03) Foot care practice scores: intervention from 4.3 to 5.6 vs control from 4.1 to 4.3 (P = 0.007) |
| Frank 2003 | No primary outcomes reported |
Foot care knowledge scores:
Means: intervention 20.98 (SD 2.46) vs control 18.60 (SD 2.93), (P < 0.001)
Mean differences: intervention 2.33 (SD 2.49) vs control 1.10 (SD 2.89), (P = 0.028) Patients' behaviour assessment: (mean number of days per week) Checking feet: intervention 6.33 vs control 5.88 (P = 0.203). Mean differences: intervention 1.13 vs control 1.35 (P = 0.708) Washing feet: intervention 5.75 vs control 5.94 (P = 0.573). Mean differences: intervention 0.58 vs control 0.52 (P = 0.863) Applying lotion: intervention 5.96 vs control 4.94 (P = 0.044). Mean differences: intervention 1.42 vs control 0.75 (,P = 0.191) Wearing shoes and socks: intervention 5.60 vs control 5.42 (P = 0.705). Mean differences: intervention 1.90 vs control 0.50 (P = 0.036) |
| Kruger 1992 | No primary outcomes reported |
Foot status:
No significant difference. Foot care knowledge scores: intervention from 9.1 to 10.0 vs control from 8.66 to 9.86, statistically significant increase in control group (P = 0.02), but not in the intervention group (P = 0.078) Behaviour assessment: Daily foot inspection: intervention from 52.5% to 66.7% vs control from 34.8% to 66.7% (ns) Daily foot washing: intervention from 82.6% to 86.7% vs control from 74.1% to 73.3% (statistically significant increase in intervention group) Use of pumice stones for corns: intervention from 4.3% to 26.7% vs control from 3.7% to 26.7% (ns) Trimming toenails regularly: intervention from 34.8% to 80.0% vs control from 66.7% to 66.7% (statistically significant increase in intervention group) Improvement in keeping toenails shorter: intervention from 30.4% to 80.0% vs control from 66.7% to 86.7% (ns) |
| Lincoln 2008 |
Ulcer incidence:
After 6 months: intervention 26 vs control 18, RR 1.41 (95% CI 0.84 to 2.38)
After 12 months: intervention 36 vs control 35, RR 1.00 (95% CI 0.70 to 1.44) Amputation rate: After 6 months: intervention 3 vs control 0, RR not estimable After 12 months: intervention 9 vs control 9, RR 0.98 (95% CI 0.41 to 2.34) |
Behaviour assessment scores: intervention 42.0 vs control 38.7 (P = 0.03) |
| Malone 1989 |
Ulcer incidence:
intervention 8 vs control 26; significantly lower in intervention group (P ≤ 0.005) Amputation rate: intervention 7 vs control 21; significantly lower in intervention group (P < 0.025) |
No secondary outcomes reported |
| Mazzuca 1986 | No primary outcomes reported | Foot care knowledge scores: No significant difference |
| Rettig 1986 | No primary outcomes reported |
Foot appearance scores (mean ±standard error):
intervention 70.2 ± 0.7 vs control 68.8 ±0.7 (ns) Foot care knowledge scores: intervention 62.2 ±1.7 vs control 53.1 ± 1.8 (P = 0.001). Significant increase in intervention group Foot care skills scores: intervention 71.8 ±2.0 vs control 68.9 ± 1.8 (ns) |
| Rönnemaa 1997 |
Amputation:
1‐year follow‐up: intervention 0 vs control 0 7‐year follow‐up: intervention 1 vs control 0 Foot ulceration: 1‐year follow‐up: intervention 1 vs control 0 7‐year follow‐up: intervention 1 vs control 1 |
Callus development:
1‐year follow‐up: Calcaneal region:
Other regions:
7‐year follow‐up: Calcaneal region:
Other regions:
Foot care knowledge scores: 1‐year follow‐up:
7‐year follow‐up:
Patients' behaviour assessment scores: 1‐year follow‐up:
7‐year follow‐up:
|
| Abbreviations: CI = confidence interval, ns = no statistical significance, RA = group that received risk assessment only, RR = risk ratio, SD = standard deviation. | ||
We attempted to estimate pooled effect sizes of the primary outcomes of two seemingly similar studies (Lincoln 2008; Malone 1989), but this was precluded by inconsistencies in the unit of analysis (one analysed the number of limbs and the other the number of people), unequal methodological quality and considerable statistical heterogeneity. Pooling of the remaining studies was not attempted because of considerable clinical heterogeneity.
1. Foot care education as part of general diabetes education compared with usual care (3 RCTs)
Primary outcomes
The incidence of foot ulceration or amputation was only reported in Bloomgarden 1987: 146 patients had no foot lesion at the initial evaluation and since only two severe foot lesions (ulceration or amputation) were observed in both the intervention and the control group during follow‐up of approximately 1.5 years, the effect was not significant. Also in the subgroup of patients with callus, nail dystrophy or fungal infection at baseline (n = 100) and in the subgroup of patients with a previous ulcer or amputation at baseline (n = 20), no significant effects of the intervention were observed.
Secondary outcomes
After six months' follow‐up, Rettig 1986 reported that foot care knowledge scores were significantly higher in the intervention group (mean score: 62 ± 1.7 SE) compared with the control group (mean score: 53 ± 1.8 SE) (P = 0.001), but this had not resulted in positive effects on foot appearance and foot care skills score. Mazzuca 1986 reported no significant improvements in foot care knowledge, and Bloomgarden 1987 found no significant improvements in behaviour assessment scores, neither in the occurrence of callus, fungal infection and nail dystrophy.
It should be noted that in both Mazzuca 1986 and Bloomgarden 1987, adherence to the intervention and follow‐up were poor. In Mazzuca 1986, only 52% of patients were followed up, and only 67% of patients requiring foot care completed treatment. Bloomgarden 1987 reported that 77% of patients who gave consent completed follow‐up, but only 50% of patients in the intervention group adhered to the intervention. Rettig 1986 did not report adherence to the intervention.
2. Foot care education tailored to educational needs compared with no intervention (2 RCTs)
Primary outcomes
Not reported in any of the included RCTs.
Secondary outcomes
After a 10‐ to 20‐minute individualised foot care education session at home, Corbett 2003 found that participants in the intervention group (n = 19) had significantly greater foot care knowledge (P = 0.03) and improved self‐care practices (P = 0.007) compared with participants in the control group (n = 16). This study, however, was limited by a small sample size and short duration of follow‐up (six weeks). In Borges 2004, self‐reported behaviour assessment scores were significantly improved after one month of follow‐up in both the intervention group, who received a 15‐minute education session (P < 0.01), and in the control group, who received no intervention at all (P < 0.05). Four of 16 foot self‐care behaviours were significantly more frequently observed in the intervention group compared with the control group. Paradoxically, foot care knowledge increased only in the control group but not in the intervention group.
3. Intensive compared with brief educational interventions (6 RCTs)
Primary outcomes
In Malone 1989, 34 foot ulcers, 28 lower‐extremity amputations and 4 foot infections were observed during one year of follow‐up of 182 patients. A marked reduction in ulcer incidence (intervention 8, control 26) and amputation rate (intervention 7, control 21) was observed in the intervention group. It should be noted, however, that outcomes were reported per limb (n = 354) instead of per person (n = 182). Therefore, a single person could have had two events, although multiple events on the same limb were reported as one. This may have resulted in overestimation of the effect sizes (RR foot ulceration 0.31, 95% CI 0.14 to 0.66; 354 limbs, one trial, Analysis 1.1, and RR amputation 0.33, 95% CI 0.15 to 0.76, 354 limbs, one trial, Analysis 1.2).
1.1. Analysis.
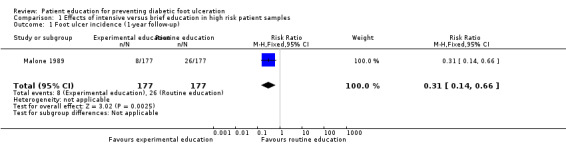
Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 1 Foot ulcer incidence (1‐year follow‐up).
1.2. Analysis.
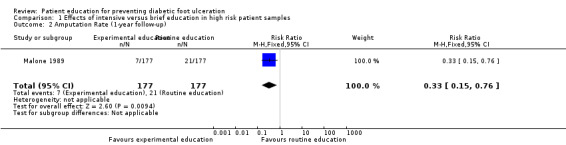
Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 2 Amputation Rate (1‐year follow‐up).
These findings were not reproduced in the study by Lincoln 2008, in which 71 foot ulcers and 18 lower‐extremity amputations were observed during one‐year follow‐up of 172 participants. No effects of the intervention on primary outcomes were observed. The RR for foot ulceration was 1.00 (172 participants, one trial, Analysis 1.3) and the RR for lower extremity amputation was 0.98 (172 participants, one trial, Analysis 1.4).
1.3. Analysis.
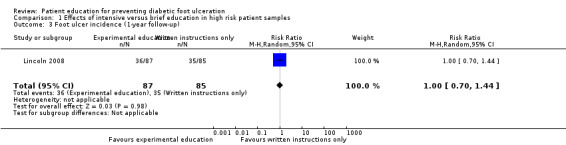
Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 3 Foot ulcer incidence (1‐year follow‐up).
1.4. Analysis.
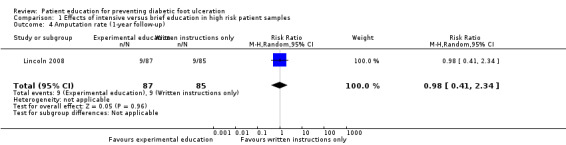
Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 4 Amputation rate (1‐year follow‐up).
In Cisneros 2010, 22 foot ulcers were observed in 51 people. The accompanying survival curve in the trial report showed a trend towards longer event‐free survival in intervention‐group participants, but this was not statistically significant (P = 0.362; hazard ratio (HR) not reported). In Rönnemaa 1997 ulcerations and amputations were reported, but these occurred too infrequently to be evaluated conclusively.
Secondary outcomes
In Lincoln 2008 a statistically significant increase in the persons behaviour assessment scores was reported in the intervention group (P = 0.03), but for this outcome only 72% of people were followed up. Moreover, the authors questioned the reliability of their assessment tool.
Rönnemaa 1997 found a significant increase in foot care knowledge in the intervention group after one year of follow‐up (P = 0.004). Also the persons behaviour assessment scores (5.4 increased to 7.0), the mean diameter of callosities (calcaneal region 40.5 mm decreased to 25.5 mm, P = 0.065; other regions 16.6 mm decreased to 11.4 mm, P < 0.001) and the presence of callus in other regions than the calcaneal region (54.4% decreased to 39.5%, P < 0.009) initially improved after the intervention. However, after seven years of follow‐up, the control group had made up all these arrears.
In Frank 2003, foot care knowledge was marginally but significantly better in the intervention than in the control group four weeks after the educational intervention. Adherence to one of the four daily foot care behaviours studied (wearing shoes and socks) was significantly more improved in the intervention group than in the control group, but this did not account for adherence to the other three foot care behaviours studied (checking feet, washing and drying feet, applying lotion).
In Barth 1991, already after one month of follow‐up a significant reduction of foot problems requiring treatment (P < 0.001) and an increase in foot care routine compliance (P < 0.001) was seen in the intervention group. Also foot care knowledge improved in both groups, but most in the intervention group (P < 0.001). Improvements were maintained until after six months of follow‐up, but the differences between intervention and control group diminished. Kruger 1992 also found almost similar improvements of foot care knowledge scores in both the intervention and control group (intervention 9.1 increased to 10.0, control 8.7 increased to 9.9) and no significant differences in the status of the participants' feet after six months of follow‐up. Some (daily foot washing, trimming of toenails), but not all foot care behaviours (daily foot inspection, use of pumice stones, keeping toenails shorter) improved in the intervention group. However, this study dealt with small groups (23 people in the intervention group and 27 in the control group), and also had a relatively high dropout rate (40%).
Discussion
Summary of main results
A wide range and combinations of patient educational interventions have been evaluated for the prevention of diabetic foot ulceration. These interventions varied from brief patient education to intensive patient education including demonstration and 'hands‐on' teaching.
The ultimate aim of foot care education for people with diabetes is to prevent foot ulceration and amputations. However, these end points were assessed in only five of the 12 RCTs (Table 1). The results of this review are presented in a study‐by‐study qualitative synthesis. Pooling of the results was precluded by marked heterogeneity (mainly clinical), because participants, types of interventions, types of control interventions, outcome measures, outcome assessment tools, duration of follow‐up and risk of bias varied widely between studies.
Only one RCT showed that, after one‐year follow‐up, the incidence of foot ulcers and amputations was lower in the group who received one hour of group education on the diabetic foot by a podiatrist compared with the group who received routine foot care education (Malone 1989). In this RCT, the number of legs instead of the number of people was taken as the unit of analysis (so‐called 'unit of analysis error') leading to an overestimation of the precision of the study and thus the ability to reach statistical significance. Moreover, an inadequate quasi‐randomised method was used for group allocation and it was not clear if baseline variables were comparable. Also, blinding of the outcome assessor and co‐interventions were not reported and ITT analysis was not attempted (Malone 1989). More importantly, the positive findings of this study are contradicted by the results of a more recent study that was included in this review (Lincoln 2008), which also studied a population at high risk for foot ulceration. Most other characteristics of this study were also similar to Malone 1989, although regular care, which was available to both the intervention and the control groups of both studies might have improved between 1989 and 2008 when the latter study was published. It can be argued that in Lincoln 2008, the educational intervention and the control intervention did not contrast enough to result in significantly different outcomes. Nevertheless, the risk of bias in Lincoln 2008 was very low. This study concluded that one hour of patient education compared with written instructions only had no beneficial effects on the incidence of foot ulceration and amputation rate after 12 months of follow‐up. The other three RCTs that reported the effect of patient education on foot ulceration and amputation demonstrated no effect either (Bloomgarden 1987; Cisneros 2010; Rönnemaa 1997). However, the overall event rate in the populations studied showed that these two studies were underpowered to show any effect on these primary outcome measures.
The present review demonstrates a positive short‐term effect of education on patients' foot care knowledge, which improved in five of the eight RCTs in which this outcome was assessed (Barth 1991; Corbett 2003; Frank 2003; Rettig 1986; Rönnemaa 1997). However, in the one RCT with longer follow‐up, the difference in foot care knowledge between intervention and control group had disappeared at seven years (Rönnemaa 1997). Similarly patients' behaviour at 6 to 18 months improved in seven of the nine RCTs in which this outcome was assessed (Barth 1991; Borges 2004; Corbett 2003; Frank 2003; Kruger 1992; Lincoln 2008; Rönnemaa 1997). Although behaviour assessment scores were still improved after seven years of follow‐up compared with the baseline measurements, the control group had made up arrears (Rönnemaa 1997). The assessment tools for measuring foot care knowledge and self‐care behaviour varied between studies, because there is currently no single standardised validated tool widely used for these purposes. We were therefore unable to evaluate the importance (clinical relevance) of the reported statistically significant improvements in foot care knowledge scores and self‐care behaviour assessment scores.
The effects on callus, nail problems and/or fungal infections were described in five studies. In only two of them were some positive effects at short‐term follow‐up reported (Barth 1991; Rönnemaa 1997), but the differences between intervention and control group were not maintained until final follow‐up. In three additional studies no benefit on these outcomes was achieved (Bloomgarden 1987; Kruger 1992; Rettig 1986). It may be possible that co‐interventions, such as podiatry care, influenced these outcomes. In Rönnemaa 1997 podiatry care was part of the experimental intervention. Therefore, people in the intervention group were reported to have seen a podiatrist more often (mean number of visits 4.7) than people in the control group (mean number of visits 0.4) after one year of follow‐up, and consequently an improvement on callus, nail problems and fungal infections was reported in the intervention group. During the last year of follow‐up, only 30.8% of the people in the intervention group still visited a podiatrist compared with 25.2% of the control group. The initial advantage of the intervention group had disappeared. A similar explanation accounts for the initial benefit of the intervention group in Barth 1991: after one month of follow‐up, 17 people in the intervention group compared with seven in the control group had consulted a podiatrist. Between the third and sixth month of follow‐up, these figures levelled to seven and eight people in each group, respectively. These examples show the importance of adequate reporting of co‐interventions, which was one of the shortcomings of the other three studies that reported these outcomes.
Overall completeness and applicability of evidence
The studies in this review that included people with diabetes at low or medium risk for foot ulceration recruited too few participants and followed them up for too short a period of time to be able to detect clinically important differences in primary outcomes. For example, in order to detect a 50% reduction in the incidence of diabetic foot ulceration, 430 to 870 participants would be required per treatment group (based on an annual incidence of foot ulceration in the general diabetes population of 2% to 4% per year or 4% to 8% over two years) (De Sonnaville 1997; Reenders 1993). The mean size of studies in this review that included people at low or medium risk for foot ulceration was 138 people per treatment group, ranging from 25 (Kruger 1992) to 266 (Mazzuca 1986) and with six months median time to follow‐up, indicating that none of these studies were actually sufficiently powered to detect differences in the long term on one of the primary outcomes. Unfortunately, the trials included in this review do not share a common set of characteristics (participants, educational methods, intensity of education to intervention and the control group, outcome measures, duration of follow‐up), thereby hindering present and future pooling.
Of the studies that reported the effect of foot care education on primary outcomes in a population at high risk, two were well‐powered (Lincoln 2008; Malone 1989). However, in these studies the educational programme under study was very limited, comprising only a single one‐hour educational session, reinforced by hand‐outs, compared with either routine patient education (Malone 1989) or written instructions only (Lincoln 2008). The conclusions of these studies were contradictory, but because the risk of bias was lower in Lincoln 2008, more weight should be given to the outcomes of this study. It shows that a limited educational intervention is not likely to result in improvement of ulcer incidence and amputation incidence. This, however, does not rule out effectiveness of more comprehensive and/or more intensive educational strategies, but these strategies were not studied.
In summary, evidence on the effectiveness of comprehensive and intensive patient education programmes to prevent diabetic foot ulceration is still needed. Below we make suggestions for future research (Implications for research).
Quality of the evidence
One of the most important findings of the present review is the high or unclear risk of bias in all but one of the included RCTs. This was mainly caused by insufficient reporting. Usually methodological flaws lead to an overestimation of the effect size. Therefore, the few positive effects that were found should be interpreted with caution. In addition, unknown and unregistered co‐interventions in the control groups of the included trials (e.g. podiatry care, unstructured patient education by the care provider) could have led to reductions in the effects of the experimental educational interventions. Finally, it must be stressed that foot care knowledge and patient behaviour were measured using subjective outcome measures and are therefore also prone to bias.
Potential biases in the review process
The clinical heterogeneity of the RCTs meant it was not possible to produce a funnel plot to assess the presence of publication bias. However, in general, publication bias would be likely to lead to an overestimation of the effects. In this review, most of the RCTs identified reported non‐significant findings and it is therefore unlikely that we overestimated any effect.
The availability of co‐interventions to those participating in the studies may have influenced the outcomes of the trials in this review. For example, in Rönnemaa 1997 and Barth 1991 the incidence of callus, nail problems and fungal infection were probably influenced by the availability of podiatry care (see Summary of main results). In most other studies, it is not reported which co‐interventions were available and whether they have influenced the outcomes. Furthermore, 'care as usual' has greatly improved and must no longer be mistaken for 'doing nothing'. Therefore, a limited educational intervention may add little to the existing knowledge of patients to result in any beneficial effects. This especially accounts for more recent studies like Lincoln 2008.
The studies included in this review used different tools to assess care knowledge and self‐care behaviour. Therefore, it was not possible to evaluate the importance (clinical relevance) of the reported statistically significant improvements in foot care knowledge scores and self‐care behaviour assessment scores. Foot care knowledge improved to some extent in five of eight studies, but consequently, this does not provide proof of effectiveness.
Agreements and disagreements with other studies or reviews
The conclusions of this systematic review contrast with those in earlier reviews on this topic (Armstrong 1998; Assal 1985; Bild 1989; Boulton 1995; Bowering 2001; Edmonds 1996; Larsson 1995; Levin 1995; Majid 2000; Mason 1999; Mayfield 1998; Rith‐Najarian 2000; Singh 2005; Wu 2007). According to these review articles, there was enough evidence to support the effectiveness of patient education for the prevention of diabetic foot ulceration. This systematic review of RCTs, however, provides a more complete and well‐considered overview of the available evidence, incorporating an evaluation of the risk of bias of the included studies.
This review was written in close conjunction with another review on the effectiveness of complex interventions for preventing diabetic foot ulceration (New Reference). That review included only five RCTs that were at high or unclear risk of bias. In agreement with this review, it was concluded that there is also no evidence to support the effectiveness of a multilevel integrated care approach for the prevention of diabetic foot ulceration (complex intervention, some of which included patient education), although this should be interpreted as a lack of evidence rather than evidence of no effect.
The RCTs included in this review show the same shortcomings as the RCTs included in a systematic review about the effectiveness of individual patient education for improvement of general metabolic control of people with diabetes mellitus (Duke 2009). The RCTs in that review were also small and had too many methodological flaws from which to draw firm conclusions.
Authors' conclusions
Implications for practice.
Overall, it appears that little evidence is available to support the effectiveness of patient education for the prevention of diabetic foot ulceration or amputations. The RCTs that have been conducted on the topic of patient education for preventing diabetic foot ulceration are generally underpowered and at high or unclear risk of bias. Consequently, while some results are suggestive of positive effects, this must be viewed with caution. Foot care knowledge and patient behaviour seem to be positively influenced by education in the short term, but the ultimate goal of educational interventions (improving knowledge and behaviour) is preventing foot ulceration and amputation. One RCT with good methodological quality showed that limited patient education did not result in any beneficial effect on these primary outcomes. The effectiveness of more comprehensive and/or more intensive educational programmes, however, remains to be further investigated.
Implications for research.
If future randomised trials are conducted, the focus should be on comparing comprehensive and intensive educational interventions with usual care. Because standard care nowadays usually comprises basic and unstructured patient education on the diabetic foot, limited education is unlikely to result in marked improvement of clinical outcome. Thus, experimental educational interventions should clearly contrast with standard education and the content of 'usual care' that is provided to the control group should be explicitly described. Furthermore, the main shortcomings of the studies included in this review that need to be avoided in future trials are: (1) insufficient power and duration of follow‐up to detect clinically relevant improvements in foot ulceration and amputation incidence, (2) marked clinical heterogeneity and (3) high risk of bias.
First, the ultimate aim of preventive strategies is to reduce the incidence of foot ulceration. This means that randomised trials that include people with diabetes at average risk for foot ulceration need at least 430 to 870 people per treatment arm in order to detect a 50% reduction in the incidence of foot ulceration (based on an annual incidence of foot ulceration in the general diabetes population of 2% to 4% per year or 4% to 8% over two years) (De Sonnaville 1997; Reenders 1993). For studies including people at high risk for foot ulceration, fewer participants are needed. Most RCTs included in this review were underpowered to show any effects of the intervention on amputation and/or foot ulceration incidence.
Second, to facilitate meta‐analysis of studies evaluating the effects of patient education programmes, more homogeneity of study characteristics and study reporting is desirable. To facilitate pooling of similar studies, future RCTs need to be reproducible. This could, for example, be achieved by standardising patient education on the diabetic foot by formulating clear and commonly accepted learning objectives is recommended (Colagiuri 2009). Furthermore, future RCTs studying the effect of patient education for preventing diabetic foot ulceration should at least report the incidence of foot ulceration and amputation. An outline of the costs associated with each intervention is vital to assess cost‐effectiveness. If changes in foot care knowledge and self‐care behaviour are reported, these should be measured with standardised and validated tools. However, such standard sets of outcomes, like those available in rheumatology research (OMERACT) and low back pain research (Deyo 1998), still need to be developed for research on the diabetic foot.
Third, efforts must be made to reduce risk of bias of future studies. Methods of randomisation and allocation concealment, for example, should be explicitly described. Blinding of participants and healthcare providers is often not possible due to the nature of the intervention, but blinding of outcome assessors must be ensured. Also, more pragmatic study design options like the Zelen's design (in which the control group is not informed) might be an option (Schellings 2005). Co‐interventions need to be registered and reported accurately. Furthermore, loss to follow‐up should be avoided because this may lead to underestimation of the intervention results. If loss to follow‐up is notable, reasons for study withdrawal should be reported in order to reveal any causality. Finally, RCTs must be reported in accordance with CONSORT guidelines (Schultz 2010) and its extension to cluster randomised trials (Campbell 2004).
We realise that trials of this magnitude are costly, but the benefits in terms of potential reduction in costs associated with effective treatment are potentially significant. Still, since most of the RCTs in this review with low risk of bias did not find any positive effects or only marginal improvements, it may be that patient education alone is not sufficient for achieving clinically relevant risk reductions. Therefore above all, we recommend the study of the effect of educational interventions when combined with other interventions for the prevention of diabetic foot ulceration (complex interventions). In Dorresteijn 2010a, an overview is provided of the existing RCTs on complex interventions for the prevention of diabetic foot ulceration.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2014 | New citation required but conclusions have not changed | New searches, no new studies identified. |
| 3 September 2014 | New search has been performed | Fourth update. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 1 August 2012 | New citation required but conclusions have not changed | Three new studies were identified. Of these, one was included in the review (Cisneros 2010) and two were excluded (Fresenius 2009; Nesari 2010). In addition two studies are awaiting assessment whilst we seek clarification from the trial authors (Gershater 2011; Zhenghua 2011a). No change to conclusions. |
| 1 August 2012 | New search has been performed | For this third update new searches were carried out in August 2012. |
| 29 March 2010 | New citation required and conclusions have changed | Three adjustments were made to the review protocol: we have excluded studies in which the intervention consisted of multiple combined strategies for the prevention of diabetic foot ulceration, where patient education was not the main comparator with the control intervention (Litzelman 1993) this study is now included in a Cochrane review of complex interventions (New Reference). In addition we have redefined the previously used primary outcome 'infection' into the secondary outcome 'fungal infection'. Thirdly, we have completed a 'Risk of bias' assessment based on guidance from the Cochrane Handbook for Systematic Reviews of Interventions. |
| 29 March 2010 | New search has been performed | For this second update, new searches were carried out in December 2009. Four new studies were identified. Of these, three (Frank 2003; Borges 2004; Lincoln 2008) were included in the review and one (Schiel 2004) was excluded. The background section was updated and the review authors' conclusions amended. |
| 25 March 2008 | Amended | Converted to new review format. |
| 10 September 2004 | New search has been performed | This review was originally published in the Cochrane Library, Issue 4, 2001. For this first update, new searches were carried out in September 2004. Six new studies were identified. Of these, one study (Corbett 2003) was included in the review and five studies (Dargis 1999, Davidson, 2000, Donohoe, 2000, McMurray, 2002, Plank 2003) were excluded. The reviewers' conclusions remain unchanged. |
| 20 August 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank:
Nicky Cullum for putting her existing work on education for the diabetic foot at our disposal,
The Cochrane Wounds Group referees (Neil Baker, Althea Foster, Sue O'Meara, Jude Smith) and editors (Nicky Cullum, Andrew Jull) who commented on the original review
Anne Lawson, copy editor.
Appendices
Appendix 1. 'Risk of bias' table judgement criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 2. Electronic searches
For the third update the search strings were run in the following electronic databases:
The Cochrane Wounds Group Specialised Register (searched 1 August 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 7);
Ovid MEDLINE (2009 to July Week 3 2012);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, July 31, 2012);
Ovid EMBASE (2009 to 2012 Week 30);
EBSCO CINAHL (2009 to 26 July 2012).
The following search strategy was used for CENTRAL:
#1 MeSH descriptor Education explode all trees #2 patient NEAR/3 education* #3 diabetes NEAR/3 education* #4 patient NEAR/3 information #5 education* NEAR/2 program* #6 (foot NEXT care) or footcare #7 leaflet* or booklet* or pamphlet* or "poster" or "posters" #8 (written or printed or oral) NEAR/3 information #9 academic NEXT detailing #10 training NEXT program* #11 algorithm* or (decision NEXT tree*) #12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 MeSH descriptor Foot Ulcer explode all trees #14 MeSH descriptor Diabetic Foot explode all trees #15 diabet* NEAR/3 ulcer* #16 diabet* NEAR/3 (foot or feet) #17 diabet* NEAR/3 infection* #18 diabet* NEAR/3 wound* #19 (#13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#12 AND #19)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN). No date or language restrictions were applied.
Appendix 3. Ovid MEDLINE search strategy
1 exp Education/ 2 (patient adj3 education).ti,ab. 3 (diabetes adj3 education).ti,ab. 4 (patient adj3 information).ti,ab. 5 (education adj2 program$).ti,ab. 6 (foot care or footcare).ti,ab. 7 (leaflet$ or booklet$ or pamphlet$ or poster$).ti,ab. 8 ((written or printed or oral) adj3 information).ti,ab. 9 academic detailing.ti,ab. 10 training program$.ti,ab. 11 (algorithm$ or decision tree$).ti,ab. 12 or/1‐11 13 exp Foot Ulcer/ 14 exp Diabetic Foot/ 15 (diabet$ adj3 ulcer$).ti,ab. 16 (diabet$ adj3 (foot or feet)).ti,ab. 17 or/13‐16 18 12 and 17
Appendix 4. Ovid EMBASE search strategy
1 exp Education/ 2 (patient adj3 education).ti,ab. 3 (diabetes adj3 education).ti,ab. 4 (patient adj3 information).ti,ab. 5 (education adj2 program$).ti,ab. 6 (foot care or footcare).ti,ab. 7 (leaflet$ or booklet$ or pamphlet$ or poster$).ti,ab. 8 ((written or printed or oral) adj3 information).ti,ab. 9 academic detailing.ti,ab. 10 training program$.ti,ab. 11 (algorithm$ or decision tree$).ti,ab. 12 or/1‐11 13 exp Foot Ulcer/ 14 exp Diabetic Foot/ 15 (diabet$ adj3 ulcer$).ti,ab. 16 (diabet$ adj3 (foot or feet)).ti,ab. 17 or/13‐16 18 12 and 17
Appendix 5. EBSCO CINAHL search strategy
S21 S13 and S20 S20 S14 or S15 or S16 or S17 or S18 or S19 S19 TI diabet* N3 wound* or AB diabet* N3 wound* S18 TI diabet* N3 infection* or AB diabet* N3 infection* S17 TI ( diabet* N3 foot or diabet* N3 feet ) or AB ( diabet* N3 foot or diabet* N3 feet ) S16 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S15 (MH "Diabetic Foot") S14 (MH "Foot Ulcer+") S13 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 S12 TI ( algorithm* or decision tree* ) or AB ( algorithm* or decision tree* ) S11 TI training program* or AB training program* S10 TI academic detailing or AB academic detailing S9 TI ( written N3 information or printed N3 information or oral N3 information ) or AB ( written N3 information or printed N3 information or oral N3 information ) S8 TI ( leaflet* or booklet* or pamphlet* or poster or posters ) or AB ( leaflet* or booklet* or pamphlet* or poster or posters ) S7 TI ( foot care or footcare ) or AB ( foot care or footcare ) S6 (MH "Foot Care") S5 TI education* N3 program* or AB education* N3 program* S4 TI diabetes N3 information or AB diabetes N3 information S3 TI diabetes N3 education* or AB diabetes N3 education* S2 TI patient N3 education* or AB patient N3 education* S1 (MH "Patient Education+")
Data and analyses
Comparison 1. Effects of intensive versus brief education in high risk patient samples.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Foot ulcer incidence (1‐year follow‐up) | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.66] |
| 2 Amputation Rate (1‐year follow‐up) | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.76] |
| 3 Foot ulcer incidence (1‐year follow‐up) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.70, 1.44] |
| 4 Amputation rate (1‐year follow‐up) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.41, 2.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barth 1991.
| Methods | RCT | |
| Participants | 70 patients with type 2 diabetes mellitus ‐ randomised (intervention 38 vs control 32) Baseline risk for foot ulceration: PVD, intervention 19 vs control 6. Number of foot problems: 'no significant difference between groups' Baseline outcome measures: 'No significant difference between groups' Study setting: secondary outpatient care, outpatient clinic in Australia Inclusion criteria: people with type 2 diabetes mellitus > 3 months and current treatment > 1 month, sub optimal glucose control, BMI ≥ 25, energy fat intake at least 35%, no education in previous 6 months, competence in English language | |
| Interventions | Intervention group:
Normal patient education programme, consisting of 14 hours group patient education (over 3 consecutive days; groups of 8 to 10 people) including 1‐hour lecture and discussion by podiatrist. Content: standard diabetes education, 1 hour on foot care and footwear
4 weekly group patient education sessions of 1.5 to 2.5 hours (total 9 hours), 3 by podiatrist, 1 by psychologist on the base of cognitive motivation theory. Content: recommendations and foot care education and demonstration and practicing foot care procedures Control group: Normal patient education programme, consisting of 14 hours group patient education (over 3 consecutive days; groups of 8 to 10 people) including 1‐hour lecture and discussion by podiatrist. Content: standard diabetes education, 1 hour on foot care and footwear Adherence: not described |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: foot care knowledge, behaviour assessment score, foot problems requiring treatment | |
| Duration and completion of follow‐up | 6 months; 62 people completed follow‐up (intervention 33 vs control 29) | |
| Types of assessment | Outcomes measured by multiple choice questions on knowledge and compliance. Number of questions and range of outcomes not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Low risk | Foot care knowledge and foot care routine compliance were assessed with a questionnaire using multiple choice answers. Foot problems were scored by an independent podiatrist, who was not aware of the patients' experimental conditions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 62 of 70 people completed 6 months' follow‐up (intervention 33 vs control 29). Reasons for missing outcome data are described and are unlikely to be related to the outcome No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | High risk | Baseline risk for foot ulceration: PVD, intervention 19 vs control 6 (P < 0.05)
Number of foot problems: 'No significant difference between groups' Co‐interventions were not described Adherence to the intervention was not described |
Bloomgarden 1987.
| Methods | RCT | |
| Participants | 749 insulin‐treated people with diabetes mellitus randomised: 345 consented to participate:
intervention 165 vs control 180 Baseline risk for foot ulceration: 146 people had no foot lesion at initial evaluation, intervention 83 vs control 63 100 people had callus, nail dystrophy or fungal infection at initial evaluation, intervention 37 vs control 63 20 people had an ulcer or amputation at initial evaluation, intervention 7 vs control 13 Study setting: primary care, diabetes clinic in the USA Inclusion criteria: insulin‐treated diabetes mellitus (unclear which type of diabetes) |
|
| Interventions | I: 9 group patient education sessions by nurse educator and nutritionist using film and card games and individual instruction. Content: 1 group session of education on foot care and skin hygiene, the other sessions on understanding diabetes, basic nutrition, weight loss, food purchasing, meal planning, insulin administration, emergencies, risk factors for macrovascular disease and individual diet instruction C: usual care. Content: not specified Adherence: 82 (50%) intervention group people completed 7 or more educational group sessions |
|
| Outcomes | Primary outcomes: ulcer or amputations Secondary outcomes: callus, nail dystrophy or fungal infection, behaviour assessment score | |
| Duration and completion of follow‐up | Intervention 1.6 ± 0.3 years vs control 1.5 ± 0.3 years; 266 people completed follow‐up: intervention 127 vs control 139 | |
| Types of assessment | Behaviour assessment score: 7 questions of which 1 on foot care | |
| Notes | The reported outcome data on knowledge scores are not included in this review, because the assessment tool only included questions about diabetes in general, but not on foot care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 266 of 345 people completed follow‐up (intervention 127 vs control 139). Reasons for missing outcome data are described and are unlikely to be related to the outcome. Note: 749 people were originally randomised, but 193 did not attend the clinic during the period of the study and 211 of those who did attend to the clinic declined to participate, leaving only 345 study subjects No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Unclear risk | Unclear. No study protocol available. The outcomes ulcer and amputation incidence, callus, nail dystrophy, fungal infection and behaviour assessment score were not prespecified in the methods section of the study report, but this is more likely to be a result of insufficient rather than selective reporting |
| Other bias | High risk | Baseline risk for foot ulceration:
146 people had no foot lesion at initial evaluation, intervention 83 vs control 63
100 people had callus, nail dystrophy or fungal infection at initial evaluation, intervention 37 vs control 63
20 people had an ulcer or amputation at initial evaluation, intervention 7 vs control 13 Co‐interventions were not described Adherence: 82 (50%) intervention group people completed 7 or more educational group sessions |
Borges 2004.
| Methods | RCT | |
| Participants | 167 people with type 2 diabetes randomised: intervention 55, only RA 55, control 57 Baseline risk for foot ulceration: no data provided Baseline outcome measures: patients' behaviour assessment scores: intervention 4.7, RA 4.8, control 5.1. Foot care knowledge scores: no significant differences. Self‐efficacy scores: no significant differences Study setting: 2 community hospital emergency departments near the USA‐Mexico border Inclusion criteria: people with type 2 diabetes, age 40 years or older, residing within the country, presenting at the emergency department with non‐emergent health problems, not having active foot ulceration or other foot pathology, able to communicate verbally, agreeing on a home visit |
|
| Interventions | Intervention group:
Lower extremity amputation risk assessment. Content: use of a monofilament
15‐minute foot self‐care education session by the researcher. Content: information about the patients' risk assessment score, recommendations for foot self‐care based on the individual risk score, a discussion about the barriers to optimal self‐care and an outline of the importance of daily foot self‐care Risk assessment group: lower extremity amputation risk assessment. Content: use of a monofilament Control group: No intervention Adherence: no data provided, but likely that all intervention group people received the single brief educational session directly after randomisation |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: foot care knowledge scores, patients' behaviour assessment scores (self‐reported and observed) | |
| Duration and completion of follow‐up | 1 month; 141 people completed follow‐up: intervention 47, RA 48, control 46 | |
| Types of assessment | Foot care knowledge scores: 5‐item foot care subscale from the Diabetes Knowledge Questionnaire 24 Self‐reported self‐care behaviour: SDSCA questionnaire Observed self‐care behaviour: self developed 16‐item observation guide | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Low risk | The research assistant, who was the outcome assessor, was masked to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 141 of 167 people completed 1‐month follow‐up (intervention 47, RA 48, control 46). Dropout was balanced in numbers across intervention groups, but reasons for missing data were not reported No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration: no data provided There were no co‐interventions Adherence: no data provided, but likely that all intervention group people received the single brief educational session directly after randomisation |
Cisneros 2010.
| Methods | RCT | |
| Participants | 53 people with type 1 and 2 diabetes randomised: intervention 30 vs control 23 Baseline risk for foot ulceration: 35 people had no history of foot ulceration, intervention 21 vs control 14. 16 people had risk 1 (only insensitivity), intervention 6 vs control 10 22 people had risk 2 (insensitivity and plantar overpressure or deformity), intervention 15 vs control 7 6 people had risk 3 (insensitivity and previous ulcers), intervention 3 vs control 3 9 people had risk 4 (insensitivity, previous ulcers and plantar overpressure), intervention 6 vs control 3 Baseline outcome measures: no data provided. Study setting: a unit of the National Health System (SUS) in Porto Alegre, Rio Grande do Sul, Brazil Study setting: community‐based care. Participants were selected from the National Health System (SUS) database Inclusion criteria: diabetes type 1 or 2, presence of neuropathy caused exclusively by diabetes mellitus as evidenced by inability to feel a Semmes‐Weinstein 5.07 monofilament in 2 of 3 of the following sites: digital pulp of the hallux, the head of the first metatarsal and the head of the fifth metatarsal |
|
| Interventions | Intervention group:
Control group:
Adherence: of the 30 intervention group participants, 1 withdrew before completion of the education programme and 29 completed the education programme and received protective shoes. Of the 29 participants, 34.5% wore them daily up to 6 hours, 37.9% wore them daily for more than 6 hours and 27.6% did not wear the shoes daily |
|
| Outcomes | Primary outcomes: foot ulceration, foot ulcer recurrence Secondary outcomes: not reported | |
| Duration and completion of follow‐up | 2 years. 35 participants completed follow‐up: intervention 21 vs control 14 | |
| Types of assessment | Inspection of the feet for the occurrence of neuropathic injury during individual consultations with the researcher, held quarterly in the first 18 months and after 2 years' follow‐up | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods are not described |
| Allocation concealment (selection bias) | Unclear risk | Methods are not described |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | High risk | Both participants and the outcome assessor were not blinded due to the nature of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of people lost to follow‐up, although balanced between groups, could have induced clinically relevant bias. In I 9 out of 30 participants were censored (30%) and in C 9 out of 23 (39%) |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration: the intervention group consisted of more men (intervention 70% vs control 52%), was older (mean age intervention 64.4 years vs control 59.8 years) and appeared to be at higher risk for foot ulceration (risk categories 1 to 4, intervention 20%, 50%, 10%, 20% vs control 43%, 30%, 13%, 13%). Baseline imbalances were not statistically significant and were not adjusted for Co‐interventions: not described Adherence: of the 30 participants, 1 withdrew before completion of the education programme and 29 completed the education programme and received protective shoes |
Corbett 2003.
| Methods | RCT | |
| Participants | 40 people with type 2 diabetes mellitus randomised: intervention 20 vs control 20 Baseline risk for foot ulceration: 70% had loss of protective sensation 67% had impaired lower extremity circulation 50% had a foot deformity Foot risk assessment: no significant differences between groups Baseline outcome measures: no significant differences between groups Study setting: community‐based care, people with type 2 diabetes mellitus admitted to home care in the USA Inclusion criteria: physically and mentally able to participate, able to read and understand English, age 18 years or older, no lower‐extremity ulcer, no history of lower‐extremity amputation |
|
| Interventions | Intervention group:
10 to 20 minutes' individualised patient education including verbal and written instructions according to participants' risk factors and foot care knowledge, self‐efficacy and reported self‐care behaviour by research nurse. Content: foot care education topics: individual risk factors, washing and drying feet, toenail care, footwear, moisturising feet, reportable foot problems. If desired: demonstration of nail trimming and problem‐solving discussion to discover alternative care solutions Control group: No intervention Adherence: 19 of 20 intervention group people attended the single education session |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: foot care knowledge score, foot care practice score, patients' self confidence scores | |
| Duration and completion of follow‐up | 6 weeks after the intervention (people were enrolled in the study already 6 weeks prior to the intervention to ensure proper baseline measurements); 35 people completed follow‐up intervention 19 vs control 16 | |
| Types of assessment | Foot care knowledge assessment: 7 questions with 4 choices; foot care practice assessment: 7 questions with 4 choices; patients' self confidence assessment: 7 aspects of foot care rated on a 6‐point scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly drawing labelled consent forms, the sequence having been generated by shuffling |
| Allocation concealment (selection bias) | Low risk | Consent form labels were covered by opaque stickers and randomly shuffled |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35 of 40 people completed follow‐up (intervention 19 vs control 16). Reasons for missing data were not reported No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline foot risk assessment: 'no significant differences between groups' Co‐interventions were not described Adherence: 19 of 20 intervention group people attended the single education session |
Frank 2003.
| Methods | RCT | |
| Participants | 100 people with type 2 diabetes mellitus randomised: intervention 50 vs control 50 Baseline risk for foot ulceration: Current smoking. intervention 12.5% vs control 16.7% Mean HbA1c: intervention 7.44 vs control 7.66, P = 0.559 Mean score neuropathy screening questionnaire (0 to 13): intervention 2.46 vs control 2.46, P = 1.00 Mean number of positive sensations of a monofilament on prespecified locations on the foot (0 to 8): intervention 6.06 vs control 5.38, P = 0.215 Baseline outcome measures: Foot care knowledge scores: intervention 18.65 (SD 2.65) vs control 17.50 (SD 3.14), P = 0.056 Patients' behaviour assessment:
Study setting: primary care (mostly indigent) people with type 2 diabetes visiting a podiatrist in 1 of 2 designated community health centres associated with the Indiana University School of Medicine in Indianapolis, Indiana Inclusion criteria: > 65 years of age, no previous foot or leg amputation, access to a working telephone, able to understand English |
|
| Interventions | Intervention group:
Lower extremity amputation risk assessment. Content: use of a monofilament
Foot care videotape. Content: people demonstrating proper foot care
Bag of foot supplies. Content: soap, towel, socks, mirror, toenail clippers, lotion samples, information on smoking cessation and exercise
Hand‐out. Content: foot care instructions
30‐ to 40‐minute individualised education session by research nurse. Content: persuasion to perform foot care + demonstration of content of bag of foot supplies
Reminder checklist. Content: instructions for daily foot care
Weekly reminder telephone calls. Content: persuasion to perform foot care
Care as usual by a podiatrist Control group: Lower extremity amputation risk assessment. Content: use of a monofilament Weekly telephone calls. Content: only outcome assessment Care as usual by a podiatrist Adherence: no data provided, but likely that all intervention group people received the single brief educational session directly after randomisation |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: foot care knowledge scores, patients' self‐reported foot care behaviour scores | |
| Duration and completion of follow‐up | 4 weeks; 96 people completed follow‐up intervention 48 vs control 48 | |
| Types of assessment | Foot care knowledge: 26 items, with a ''true'', ''false'' or ''don't know'' answer (range 0 to 26) Behaviour scores: retrospectively self‐reported. Data collection during weekly telephone calls. Foot care behaviours of interest were checking feet daily for injury, washing and drying feet daily, applying lotion to the feet daily, wear socks and shoes, trimming toenails weekly. Results for each item were presented by the mean number of days per week that people adhered to the desired behaviour (range 0 to 7 per item) | |
| Notes | It was originally intended to report changes in 'weekly trimming of toenails', but this was abandoned, as all people were seen by a podiatrist for trimming of their toenails | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Papers with either 'group A' or 'group B' were drawn from an envelope |
| Allocation concealment (selection bias) | High risk | The envelope was not sealed |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | High risk | Outcomes were assessed by the research nurse, who also performed the educational intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 96 of 100 people completed 4 weeks' follow‐up (intervention 48 vs control 48) No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration:
Current smoking. intervention 12.5% vs control 16.7%
Mean HbA1c: intervention 7.44 vs control 7.66, P = 0.559
Mean score neuropathy screening questionnaire (0 to 13): intervention 2.46 vs control 2.46, P = 1.00
Mean number of positive sensations of a monofilament on prespecified locations on the foot (0 to 8): intervention 6.06 vs control 5.38, P = 0.215 Co‐interventions were not described Adherence: no data provided, but likely that all intervention group people received the single brief educational session directly after randomisation |
Kruger 1992.
| Methods | RCT | |
| Participants | 50 people with diabetes mellitus randomised: intervention 23 vs control 27
Baseline risk for foot ulceration: no data provided
Study setting: secondary outpatient care in the USA Inclusion criteria: diabetes duration at least 5 years (unclear which type of diabetes), no frank pathology, entering weekly hospital diabetes programme |
|
| Interventions | Intervention group:
1‐week patient education session. Content: education and guidance (unclear by whom) to assist people in achieving higher levels of general diabetes control
Instructional videotape with supplementary explanation from an instructor. Content: usual teaching on foot care
Additional hands‐on learning sessions during the same week. Content: actual foot washing, inspection, assessment, demonstration of care of corns and callus, toenail cutting, identification of potential foot problems, evaluation foot care
Patient education kit. Content: buff pads and mirror
Daily foot check sheets. Content: encouragement to perform daily foot inspection Control group: 1‐week patient education session. Content: education and guidance (unclear by whom) to assist people in achieving higher levels of general diabetes control Instructional videotape with supplementary explanation from an instructor. Content: usual teaching on foot care Daily foot check sheets. Content: encouragement to daily foot inspection Adherence: no data provided |
|
| Outcomes | Primary outcomes: none reported Secondary outcomes: foot status, foot care knowledge scores, behaviour assessment | |
| Duration and completion of follow‐up | 6 months; 30 people completed follow‐up: intervention 15 vs control 15 | |
| Types of assessment | Foot status assessment: 67 items Foot care knowledge assessment: 12‐item test Behaviour assessment: daily foot check sheets | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | People were allocated to intervention or control on the basis of the week that they entered the diabetes program. The experimental intervention group was developed similarly from the control group on alternate weeks Quote: "A random selection process determined whether the control or the experimental [intervention] group would begin the study" |
| Allocation concealment (selection bias) | High risk | Alternation is not an adequate method of allocation concealment |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 of 50 people completed 6 months' follow‐up (intervention 15 vs control 15). Reasons for dropping out were death (n = 2), not wanting to make an appointment at the scheduled time and moving without leaving a forwarding address (numbers not reported) No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | Yes. No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration: no data provided Co‐interventions were not described Adherence: no data provided |
Lincoln 2008.
| Methods | RCT | |
| Participants | 178 people with diabetes and a newly healed foot ulcer randomised. Intervention 87 vs control 85. Excluded after randomisation: 6 Baseline risk for foot ulceration: Loss of 10 g monofilament stimulus perception: intervention 47% vs control 42% Loss of neuro tip perception: intervention 35% vs control 36% Loss of vibration perception: intervention 68% vs control 62% Absent foot pulses: intervention 20% vs control 28% Baseline outcome measures: History of foot ulcer: all participants Site of previous foot ulcer: fore‐foot: intervention 81% vs control 80%. Mid‐ and hind‐foot: intervention 19% vs control 20% Amputation rate: previous amputation same leg: intervention 20% vs control 12%. Previous amputation other leg: intervention 7% minor, 3% major vs control 6% minor, 3% major No baseline behaviour assessment scores provided Study setting: secondary outpatient care: specialist foot clinic in Nottingham, UK Inclusion criteria: people with newly healed diabetic foot ulcers (ulcer free for 28 days or more), not living in institutions, no history of dementia, no serious medical problems, English speaking or having an English speaking carer, living < 50 miles from the clinic, not included in any other study |
|
| Interventions | Intervention group:
Single 1‐hour structured foot care education session by 1 of the researchers during a home visit. Content: explanation of the principal causes of foot ulcers, illustrations of foot lesions, advises on avoiding accidental damage, identification personal risk factors, evaluation of footwear
Hand‐outs. Content: information about the causes of foot ulcers, foot care and ways to reduce the likelihood of accidents
Telephone call 4 weeks after the education session. Content: assessment of the need for clarification and reinforcement of the educational session content Control group: Hand‐outs. Content: information about the causes of foot ulcers, foot care and ways to reduce the likelihood of accidents Adherence: no data provided, but likely that all intervention group people received the 1 hour at home education session |
|
| Outcomes | Primary outcomes: ulcer incidence (recurrence), amputation rate Secondary outcomes: patients' behaviour assessment scores |
|
| Duration and completion of follow‐up | 6 and 12 months; 168 people completed 12 months' follow‐up for primary outcomes. 138 people completed 12 months' follow‐up for secondary outcomes | |
| Types of assessment | Occurrence of new ulcers and amputation rate: hospital and foot clinic records, supported by questionnaires sent to the patient. At 12 months also corroborated by writing to participants' general practitioners Patients' behaviour assessment: questionnaire with 29 items (Nottingham Assessment of Functional Footcare), posted to the patient with a reply‐paid envelope | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was randomly generated by a computer in advance of the study |
| Allocation concealment (selection bias) | Low risk | People were allocated after telephoning an independent randomisation centre which held the sequence list |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Low risk | Scoring of ulcer incidence and amputation rate was based on hospital and foot clinic records, supported by questionnaires sent to the patient. In case of discrepancy, the records were rechecked by a blinded observer. The success of blinding was not assessed but it was believed to be complete Patients' behaviour assessment score was based on questionnaires that were posted to participants and scored by a researcher who was blinded to participants' group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 168 of 178 people completed 12 months' follow‐up for primary outcomes. 138 of 178 people completed 12 months' follow‐up for secondary outcomes. Reasons for dropping out were death (n = 10), illness (n = 2), erroneous double‐recruitment (n = 1), withdrawal of consent (n = 1), not fitting the eligibility criteria (n = 1), not returning questionnaires (n = 22) and incompleteness of the questionnaire answers (n = 2) An ITT analysis was performed for primary outcomes only |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Low risk | Baseline risk for foot ulceration: Loss of 10 g monofilament stimulus perception: intervention 47% vs control 42% Loss of neuro tip perception: intervention 35% vs control 36% Loss of vibration perception: intervention 68% vs control 62% Absent foot pulses: intervention 20% vs control 28% Co‐interventions included regular podiatry and suitable orthoses when appropriate, but no structured education. The clinical care of people in both groups was unaffected by the study Adherence: no data provided, but likely that all intervention group people received the 1 hour at home education session |
Malone 1989.
| Methods | RCT | |
| Participants | 227 people with diabetes mellitus and foot infection, ulceration or prior amputation ‐ randomised. 203 people included: intervention 103 vs control 100. Baseline risk for foot ulceration: although described as 'not significant', prior vascular reconstruction higher in control and incidence of foot callus higher in intervention (P < 0.05). No significant differences in foot deformities, neuropathy, gangrene, prior amputation or ulcer and level of distal pulses Study setting: secondary outpatient care, podiatric or vascular surgery care in the USA Inclusion criteria: people with diabetes (unclear which type) with foot infection, ulceration or prior amputation referred for podiatry or vascular surgery |
|
| Interventions | Intervention group:
1‐hour group patient education with slides given by podiatrist and set of patient instructions. Content: slides of infected diabetic feet and amputated diabetic limbs, simple set of patient instructions for diabetic foot care
Routine patient education. Content: routine diabetic teaching on diet, weight, exercise and medication Control group: Routine patient education. Content: routine diabetic teaching on diet, weight, exercise and medication Adherence: no data provided |
|
| Outcomes | Primary outcomes: ulcer incidence, incidence of infections, amputation rate Secondary outcomes: none | |
| Duration and completion of follow‐up | Intervention mean 12 months, median 13.2 months (range 1 to 26 months) vs control mean 8 months, median 9.2 months (range 1 to 26 months); 182 people completed follow‐up: intervention 90 vs control 92 | |
| Types of assessment | No information provided | |
| Notes | Unit of randomisation: individual people. Unit of analyses: separate limbs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomised into two groups based upon the odd or even last digit of their Social Security number" |
| Allocation concealment (selection bias) | High risk | Sequence generation was based upon the last digit of the persons social security number |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 182 of 227 people completed follow‐up (intervention 90 vs control 92). Reasons for dropping out were not fitting the eligibility criteria (n = 24), death (n = 13) and unspecified (n = 8) No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report listed the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration: although described as 'not significant', prior vascular reconstruction higher in control and incidence of foot callus higher in intervention (P < 0.05). No significant differences in foot deformities, neuropathy, gangrene, prior amputation or ulcer and level of distal pulses Co‐interventions were not described Adherence: no data provided |
Mazzuca 1986.
| Methods | RCT | |
| Participants | 532 people with diabetes mellitus randomised intervention 263 vs control 269 Baseline risk for foot ulceration: no data provided Study setting: primary care, academic general medicine clinic in the USA Inclusion criteria: either 2 fasting blood glucose levels > 130 mg/dL or 1 > 150 mg/dL or 2‐hour value > 250 mg/dL, able to perform 2 basic self‐care tasks, no psychiatric or terminal illness, under care of an internal medicine resident, informed consent |
|
| Interventions | Intervention group:
Diagnosis of educational needs according to protocol
Patient education in appropriate modules of instruction by nurses and dieticians by group education using lecture, discussion and/or audio‐visual materials, demonstration, return demonstration and feedback, goal setting, and written contract on goals. Content (depending on individual educational needs): understanding diabetes, acute complications, antidiabetic medication, antihypertensive medication, diet and activity, foot care and urine testing
Reinforcement by phone contact 2 and 6 weeks after instruction Control group: Usual care. Content: including routine education Adherence: 139 of 208 (67%) people needing instruction on foot care completed this |
|
| Outcomes | Primary outcomes: none reported Secondary outcomes: level of foot care knowledge | |
| Duration and completion of follow‐up | Median interval between instruction and follow‐up measurement 11.8 to 14.3 months; 275 people completed follow‐up: intervention 135 vs control 140 | |
| Types of assessment | Level of foot care knowledge: nurse‐administered patient history following predefined learning objectives | |
| Notes | Knowledge objectives unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | High risk | Assessments were not conducted by personnel who were blind to subjects' experimental condition |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 275 of 532 people completed follow‐up (intervention 135 vs control 140). Reasons for dropping out were death (n = 30), physical or psychological incapacitation (n = 43), transfer to a senior staff physician (n = 32), relocation (n = 13), work conflict (n = 24), personal reasons (n = 45), failure to keep appointments (n = 11) and lost contact (n = 58) No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | High risk | Baseline risk for foot ulceration: no data provided Co‐interventions were not described Adherence: 139 of 208 (67%) people needing instruction on foot care completed this |
Rettig 1986.
| Methods | RCT | |
| Participants | 471 people with diabetes mellitus randomised intervention 228 vs control 243 Baseline risk for foot ulceration: no data provided Study setting: community‐based care Inclusion criteria: identified as diabetic inpatient of participating hospitals (unclear which type of diabetes), age < 65 years (at begin of study), no terminal illness, physician approval |
|
| Interventions | Intervention group:
Up to 12 home patient education sessions, provided by nurses, who attended special 4‐day intensive course in diabetes self care. Content: according to judgement of nurse, tailored to patient self management needs, which were defined with 100 short answer and yes/no questions Control group: Usual care. Content: not specified Adherence: no data provided |
|
| Outcomes | Primary outcomes: none Secondary outcomes: foot appearance score, foot care knowledge, behaviour assessment score | |
| Duration and completion of follow‐up | 6 months; 373 people completed follow‐up: intervention 180 vs control 193 | |
| Types of assessment | Foot appearance assessment: nurse scored a 16‐item checklist Foot care knowledge assessment: 70 multiple‐choice questions, covering 4 areas of which foot care was 1 Behaviour assessment: skills assessed by nurse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Low risk | The staff nurses, who were the outcome assessors, were not aware of subject assignment at the time of the follow‐up visit |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 373 of 471 people completed follow‐up (intervention 180 vs control 193). Reasons for dropping out were deaths, violations of the protocol (such as an excessive number of home visits) and unspecified No ITT analyses were undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Baseline risk for foot ulceration: no data provided Co‐interventions were not described Adherence: no data provided |
Rönnemaa 1997.
| Methods | RCT | |
| Participants | 530 people with diabetes mellitus randomised, intervention 267 vs control 263Baseline risk for foot ulceration: no data provided Baseline outcome measures: Foot care knowledge score: intervention 26.7 (SD 11.4) vs control 26.1 (SD 11.8) Self‐care behaviour assessment score: intervention 5.4 (SD 2.8) vs control 5.3 (SD 2.6) Callosities: intervention 18.5% calcaneal region, 54.5% other regions; control 16.8% calcaneal region, 51.3% other regions Diameter of greatest callosity: intervention calcaneal region (n = 49) 40.5 mm (SD 30.8 mm), other regions (n = 141) 16.6 mm (SD 10.2 mm); control calcaneal region (n = 55) 30.6 mm (SD 28.5 mm), other regions (n = 138) 15.2 mm (SD 9.8 mm) Podiatrist visit: intervention 12.4% in previous year, 73.4% never before vs control 10.4% in previous year, 76.1% never before Foot examination by physician in previous year: intervention 36.7% routinely, 9.5% following complaints vs control 46.4% routinely, 12.3% following complaints Study setting: community‐based care in the vicinity of Turku, Finland Inclusion criteria: included in the national drug imbursement register for receiving antidiabetic treatment, no obvious need for podiatry, no visit with podiatrist in previous 6 months, age between 10 to 79 years |
|
| Interventions | Intervention group:
45 minutes' individual patient education. Content: education on use of proper footwear, daily hygiene, cutting of toenails, use of emollient cream, avoidance of high‐risk situations and foot gymnastics
Podiatric care visits (to 1 of 3 participating podiatrists) of 30 to 60 minutes' duration as necessary. Content: preventive podiatric care as debridement of callus, preparation of insoles, treatment of ingrowing toenails and guidance for foot gymnastics Control group: Written information. Content: instructions on foot care Adherence: intervention mean number of podiatry visits 4.7 in first year. After first and before seventh follow‐up year at least 1 podiatry visit in 82.3% of people in intervention and in 49.7% in control |
|
| Outcomes | Primary outcomes: amputation rate, ulcer incidence Secondary outcomes: callus development, foot care knowledge, behaviour assessment scores | |
| Duration and completion of follow‐up | 1 and 7 years; 459 completed 1 year of follow‐up: intervention 233 vs control 226 332 completed 7 years of follow‐up: intervention 169 vs control 163 | |
| Types of assessment | Callus diameter in millimetres. Knowledge score: 19 three‐choice questions of which 1 or 2 correct answers: correct = 1, unknown = 0, incorrect = 1 (total score range 0 to 57) Behaviour assessment score: range 0 to 12 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed separately for men and women and for people below and above 20 years of age. Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Blinding of outcome assessors | Unclear risk | The outcome assessor was blinded to the baseline characteristics, but no further information on blinding to the group allocation is provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up was completed by only 63% of people in the intervention group and 62% of people in the control group at 7 years No ITT analysis undertaken |
| Selective reporting (reporting bias) | Low risk | No study protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | High risk | Baseline risk for foot ulceration: no data provided Adherence: intervention mean number of podiatry visits 4.7 in first year. After first and before seventh follow‐up year at least 1 podiatry visit in 82.3% of people in intervention and in 49.7% in control Co‐interventions: podiatry care was provided to intervention group people only |
BMI = body mass index, CI = confidence interval, HbA1c = glycated haemoglobin, HR = hazard ratio, ITT = intention to treat, PVD = peripheral vascular disease, RA = risk assessment, RCT = randomised controlled trial, RR = risk ratio, SD = standard deviation, SDSCA = Summary of Diabetes Self‐Care Activities.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dargis 1999 | Not a randomised controlled study design |
| Davidson 2000 | Not a randomised controlled study design and no educational programme that includes patient education aimed at reducing diabetic foot ulcers |
| De Weerdt 1991 | No educational programme that included patient education aimed at reducing diabetic foot ulcers, and no relevant outcomes reported |
| Donohoe 2000 | No educational programme targeted at people that includes patient education aimed at reducing diabetic foot ulcers |
| Fresenius 2009 | Education initially directed at wound healing. Patient education for preventing ulcer recurrence only offered to participants with healed index lesions |
| Glasgow 1992 | No relevant outcomes reported |
| Litzelman 1993 | Foot care education is part of the comprehensive intervention on foot ulceration but not the main contrast with the control |
| Litzelman 1997 | Not a randomised controlled study design |
| McCabe 1998 | Foot care education is not the main contrast with the control |
| McMurray 2002 | Foot care education is not the main contrast with the control |
| Nesari 2010 | Foot care education is not the main contrast with the control |
| Pieber 1995 | Not a randomised controlled study design |
| Plank 2003 | No educational programme targeted at people that includes patient education aimed at reducing diabetic foot ulcers, and education not the main contrast with the control |
| Reichard 1993 | No educational programme that includes patient education aimed at reducing diabetic foot ulcers |
| Schiel 2004 | Foot care education is not the main contrast with the control |
| Vinicor 1985 | No educational programme that includes patient education aimed at reducing diabetic foot ulcers, and no relevant outcomes reported |
| Ward 1999 | Not a randomised controlled study design |
| Wooldridge 1996 | Not a randomised controlled study design |
| Zhenghua 2011 | Only conference abstract. No full‐text article available. |
Characteristics of studies awaiting assessment [ordered by study ID]
Gershater 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The main result is planned to be presented at the Diabetic Foot Meeting in Haag 20‐23 May 2015, and a manuscript is submitted. |
Differences between protocol and review
In this updated review, we have excluded studies in which the intervention consisted of multiple combined strategies for the prevention of diabetic foot ulceration, where patient education was not the main comparator with the control intervention (Litzelman 1993). We have developed and published a further Cochrane review that provides a more comprehensive overview of the effects of such integrated prevention strategies (complex interventions) (Dorresteijn 2010a). In addition, we have redefined the previously used primary outcome 'infection' into the secondary outcome 'fungal infection'. Furthermore, while the Amsterdam‐Maastricht consensus list was used to score risk of bias in previous versions of this review (van Tulder 1997), we have adopted The Cochrane Collaboration's recommended tool for assessing risk of bias in this update (Higgins 2011).
Contributions of authors
JAN Dorresteijn extracted, checked and analysed data, undertook and checked quality assessment, completed the first draft of the review update, made an intellectual contribution to the review update, approved review update prior to submission and performed previous work that was the foundation of the current review. DMW Kriegsman performed part of the writing and editing of the review update, made an intellectual contribution, approved final review update prior to submission and performed previous work that was the foundation of the current review. WJJ Assendelft performed part of the writing and editing of the review update, made an intellectual contribution, approved final review update prior to submission and performed previous work that was the foundation of the current review. GD Valk coordinated the review update, extracted, checked and analysed data, undertook and checked quality assessment, performed part of the writing and editing of the review update, made an intellectual contribution, approved final review update prior to submission, performed previous work that was the foundation of the current update and is guarantor of the review update.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content; approved the final review and review updates prior to submission. Sally Bell‐Syer: coordinated the editorial process, advised on methodology, interpretation and content; edited the updates of the review. Ruth Foxlee and Amanda Briant: designed the search strategy, ran the searches and edited the search methods section for the updates.
Sources of support
Internal sources
Dutch Cochrane Centre, Netherlands.
Leiden University Medical Center, Department of Public Health and Primary Care, Netherlands.
University Medical Center Utrecht, Department of Internal Medicine, Netherlands.
External sources
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barth 1991 {published data only}
- Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance and foot problems in type 2 diabetes. Diabetic Medicine 1991;8:111‐17. [DOI] [PubMed] [Google Scholar]
Bloomgarden 1987 {published data only}
- Bloomgarden ZT, Karmally W, Metzger MJ, Brothers M, Nechemias C, Bookman J, et al. Randomized controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10(3):263‐72. [DOI] [PubMed] [Google Scholar]
Borges 2004 {published data only}
- Borges WJ. The impact of a brief foot care intervention for persons with diabetes [PhD thesis]. University of Texas Health Science Center at Houston School of Nursing, doctoral dissertation, 2004. [Google Scholar]
- Borges WJ, Ostwald SK. Improving foot self‐care behaviors with Pies Sanos. Western Journal of Nursing Research 2008;30(3):325‐41. [DOI] [PubMed] [Google Scholar]
Cisneros 2010 {published data only}
- Cisneros LL. Evaluation of a neuropathic ulcers prevention program for patients with diabetes [Avaliação de um programa para prevenção de úlceras neuropáticas em portadores de diabetes]. Revista brasileira de fisioterapia 2010;14(1):31‐7. [PubMed] [Google Scholar]
Corbett 2003 {published and unpublished data}
- Corbett CF. A randomized pilot study of improving foot care in home health patients with diabetes. The Diabetes Educator 2003;29:273‐82. [DOI] [PubMed] [Google Scholar]
Frank 2003 {published data only}
- Frank KI. Self‐management of foot care for patients 65 years of age or older with diabetes. Dissertation Abstracts International 2003; Vol. 64‐10, issue B:4863.
Kruger 1992 {published data only}
- Kruger S, Guthrie D. Foot care: knowledge retention and self‐care practices. The Diabetes Educator 1992;18(6):487‐90. [DOI] [PubMed] [Google Scholar]
Lincoln 2008 {published data only}
- Lincoln NB, Radford KA, Game FL, Jeffcoate WJ. Education for secondary prevention of foot ulcers in people with diabetes: a randomised controlled trial. Diabetologia 2008;51(11):1954‐61. [DOI] [PubMed] [Google Scholar]
Malone 1989 {published data only}
- Malone JM, Snyder M, Anderson G, Bernhard VM, Holloway GA, Bunt TJ. Prevention of amputation by diabetic education. The American Journal of Surgery 1989;158:520‐4. [DOI] [PubMed] [Google Scholar]
Mazzuca 1986 {published and unpublished data}
- Mazzuca SA, Moorman NH, Wheeler ML, Norton JA, Fineberg NS, Vinicor F, et al. The diabetes education study: a controlled trial of the effects of diabetes patient education. Diabetes Care 1986;9(1):1‐10. [DOI] [PubMed] [Google Scholar]
Rettig 1986 {published data only}
- Rettig BA, Shrauger DG, Recker RR, Gallagher TF, Wiltse H. A randomized study of the effects of a home diabetes education program. Diabetes Care 1986;9(2):173‐8. [DOI] [PubMed] [Google Scholar]
Rönnemaa 1997 {published and unpublished data}
- Hämäläinen H, Rönnemaa T, Toikka T, Liukkonen I. Long‐term effects of one year of intensified podiatric activities on foot‐care knowledge and self‐care habits in patients with diabetes. The Diabetes Educator 1998;24(6):734‐40. [DOI] [PubMed] [Google Scholar]
- Rönnemaa T, Hämäläinen H, Toikka T, Liukkonen I. Evaluation of the impact of podiatrist care in the primary prevention of foot problems in diabetic subjects. Diabetes Care 1997;20(12):1833‐7. [DOI] [PubMed] [Google Scholar]
- Rönnemaa T, Liukkonen I, Knuts L‐R, Seppälä P, Kallio V. Prevalence of foot problems and need for foot care in an unselected diabetic population. The Journal of British Podiatric Medicine 1993;48(12):185‐90. [Google Scholar]
References to studies excluded from this review
Dargis 1999 {published data only}
- Dargis V, Pantelejeva O, Jonushaite A, Vileikyte L, Boulton AJM. Benefits of a multidisciplinary approach in the management of recurrent diabetic foot ulceration in Lithuania ‐ a prospective study. Diabetes Care 1999;22:1428‐31. [DOI] [PubMed] [Google Scholar]
Davidson 2000 {published data only}
- Davidson MB, Karlan VJ, Hair TL. Effect of a pharmacist‐managed diabetes care program in a free medical clinic. American Journal of Medical Quality 2000;15:137‐41. [DOI] [PubMed] [Google Scholar]
De Weerdt 1991 {published data only}
- Weerdt I de, Visser AP, Kok GJ, Weerdt O de, Veen EA van der. Randomized controlled multicentre evaluation of an education programme for insulin‐treated diabetic patients: effects on metabolic control, quality of life, and costs of therapy. Diabetic Medicine 1990;8:338‐45. [DOI] [PubMed] [Google Scholar]
Donohoe 2000 {published data only}
- Donohoe ME, Fletton JA, Hook A, Powell R, Robinson I, Stead JW, et al. Improving foot care for people with diabetes mellitus ‐ a randomized controlled trial of an integrated care approach. Diabetic Medicine 2000;17:581‐7. [DOI] [PubMed] [Google Scholar]
Fresenius 2009 {published data only}
- Fresenius K, Kramer I. Implementation and evaluation of pharmaceutical care on the outcomes of patients suffering from diabetic foot syndrome. Krankenhauspharmazie 2009;30(1):2‐10. [Google Scholar]
Glasgow 1992 {published data only}
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self‐care among older patients with type II diabetes: the 'sixty something...' study. Patient Education and Counseling 1992;19:61‐74. [DOI] [PubMed] [Google Scholar]
Litzelman 1993 {published data only}
- Litzelman DK, Slemenda CW, Langeveld CD, Hays LM, Welch MA, Bild DE, et al. Reduction of lower extremity clinical abnormalities in patients with non‐insulin‐dependent diabetes mellitus. Annals of Internal Medicine 1993;119(1):36‐41. [DOI] [PubMed] [Google Scholar]
Litzelman 1997 {published data only}
- Litzelman DK, Marriot DJ, Vinicor F. The role of footwear in the prevention of foot lesions in patients with NIDDM. Diabetes Care 1997;20(2):156‐62. [DOI] [PubMed] [Google Scholar]
McCabe 1998 {published data only}
- McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabetic Medicine 1998;15:80‐4. [DOI] [PubMed] [Google Scholar]
McMurray 2002 {published data only}
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. American Journal of Kidney Diseases 2002;40:566‐75. [DOI] [PubMed] [Google Scholar]
- McMurray SD, McDougall K. Improving diabetes foot care in the dialysis facility. Nephrology News & Issues 2003;17(10):57,60‐1,65‐6. [PubMed] [Google Scholar]
Nesari 2010 {published data only}
- Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow‐up on adherence to a diabetes therapeutic regimen. Japan Journal of Nursing Science 2010;7(2):121‐8. [DOI] [PubMed] [Google Scholar]
Pieber 1995 {published data only}
- Pieber TR, Holler A, Siebenhofer A, Brunner GA, Semlitsch B, Schattenberg S, et al. Evaluation of a structured teaching and treatment programme for type 2 diabetes in general practice in a rural area of Austria. Diabetic Medicine 1995;12:349‐54. [DOI] [PubMed] [Google Scholar]
Plank 2003 {published data only}
- Plank J, Haas W, Rakovac I, Gorzer E, Sommer R, Siebenhofer A, et al. Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects. Diabetes Care 2003;26:1691‐5. [DOI] [PubMed] [Google Scholar]
Reichard 1993 {published data only}
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. New England Journal of Medicine 1993;329(5):304‐9. [DOI] [PubMed] [Google Scholar]
Schiel 2004 {published data only}
- Schiel R, Braun A, Müller R, Helbich C, Siefke S, Franke I, et al. A structured treatment and educational program for patients with type 2 diabetes mellitus, insulin therapy and impaired cognitive function (DikoL) [Ein strukturiertes behandlung‐ und schulungsprogramm für patienten mit typ‐2‐diabetes mellitus, insulintherapie und verminderten kognitiver leistungsfähigkeit (DikoL)]. Medizinische Klinik 2004;99(6):285‐92. [DOI] [PubMed] [Google Scholar]
Vinicor 1985 {published data only}
- Vinicor F, Cohen SJ, Mazzuca SA, Moorman N, Wheeler M, Kuebler T, et al. DIABEDS: a randomized trial of the effects of physician and/or patient education on diabetes patient outcomes. Journal of Chronic Disease 1987;40(4):345‐56. [DOI] [PubMed] [Google Scholar]
Ward 1999 {published data only}
- Ward A, Metz L, Oddone EZ, Edelman D. Foot education improves knowledge and satisfaction among patients at high risk for diabetic foot ulcer. Diabetes Educator 1999;25(4):560‐7. [DOI] [PubMed] [Google Scholar]
Wooldridge 1996 {published data only}
- Wooldridge J, Bergeron J, Thornton C. Preventing diabetic foot disease: lessons from the Medicare therapeutic shoe demonstration. American Journal of Public Health 1996;86(7):935‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhenghua 2011 {published data only}
- Zhenghua X, Dingyu C, Qiling Y, Qian Z, Jin X, Chunling H, et al. Individualised diabetic education can contribute to decrease the incidence of diabetic foot and avoid amputation: Results of a 9‐year prospective study. Diabetologia 2011;54:S32. [Google Scholar]
References to studies awaiting assessment
Gershater 2011 {published data only}
- Gershater MA, Pilhammar E, Apelqvist J, Alm‐Roijer C. Patient education for the prevention of diabetic foot ulcers. Interim analysis of a randomised controlled trial due to morbidity and mortality of participants. European Diabetes Nursing 2011;8(3):102‐107b. [Google Scholar]
Additional references
American Diabetes Association 2007
- American Diabetes Association. Standards of medical care in diabetes ‐ 2007. Diabetes Care 2007;30(Suppl 1):S4‐S41. [DOI] [PubMed] [Google Scholar]
Apelqvist 1993
- Apelqvist J, Larsson J, Agardh CD. Long‐term prognosis for diabetic patients with foot ulcers. Journal of Internal Medicine 1993;233:485‐91. [DOI] [PubMed] [Google Scholar]
Armstrong 1998
- Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. American Family Physician 1998;57:1325‐32. [PubMed] [Google Scholar]
Assal 1985
- Assal JP, Mühlhauser I, Pernet A, Gfeller R, Jörgens V, Berger M. Patient education as the basis for diabetes care in clinical practice and research. Diabetologia 1985;28:602‐13. [DOI] [PubMed] [Google Scholar]
Bild 1989
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower‐extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989;12:24‐31. [DOI] [PubMed] [Google Scholar]
Boulton 1995
- Boulton AJM. Why bother educating the multi‐disciplinary team and the patient ‐ the example of prevention of lower extremity amputation in diabetes. Patient Education and Counseling 1995;26:183‐8. [DOI] [PubMed] [Google Scholar]
Boulton 2004
- Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. New England Journal of Medicine 2004;351(1):48‐55. [DOI] [PubMed] [Google Scholar]
Boulton 2005
- Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719‐24. [DOI] [PubMed] [Google Scholar]
Bowering 2001
- Bowering CK. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Canadian Family Physician 2001;47:1007‐16. [PMC free article] [PubMed] [Google Scholar]
Campbell 2004
- Campbell MK, Elbourne DR, Altman DG, CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328(7441):702‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canavan 2008
- Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes‐ and nondiabetes‐related lower extremity amputation incidence before and after the introduction of better organized diabetes foot care: continuous longitudinal monitoring using a standard method. Diabetes Care 2008;31(3):459‐63. [DOI] [PubMed] [Google Scholar]
Cavanagh 2005
- Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet 2005;366(9498):1725‐35. [DOI] [PubMed] [Google Scholar]
Colagiuri 2009
- Colagiuri R, Eigenmann CA. A national consensus on outcomes and indicators for diabetes patient education. Diabetic Medicine 2009;26(4):442‐6. [DOI] [PubMed] [Google Scholar]
De Sonnaville 1997
- Sonnaville JJ, Colly LP, Wijkel D, Heine RJ. The prevalence and determinants of foot ulceration in type 2 diabetic patients in a primary health care setting. Diabetes Research and Clinical Practice 1997;35:149‐56. [DOI] [PubMed] [Google Scholar]
Deyo 1998
- Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine 1998;23(18):2003‐13. [DOI] [PubMed] [Google Scholar]
Dinh 2005
- Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. International Journal of Lower Extremity Wounds 2005;4(3):154‐9. [DOI] [PubMed] [Google Scholar]
Dorresteijn 2010a
- Dorresteijn JAN, Kriegsman DMW, Valk GD. Complex interventions for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007610.pub2] [DOI] [PubMed] [Google Scholar]
Duke 2009
- Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005268.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmonds 1996
- Edmonds ME, Acker K, Foster AVM. Education and the diabetic foot. Diabetic Medicine 1996;13:S61‐S64. [PubMed] [Google Scholar]
Edmonds 2006
- Edmonds ME, Foster AV. Diabetic foot ulcers. BMJ 2006;332(7538):407‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frykberg 2006
- Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). The Journal of Foot and Ankle Surgery 2006;45(5 Suppl):S1‐66. [DOI] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, Bauman A, et al. The effects of limited (information only) patient education programs on the health outcomes of adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001005] [DOI] [PubMed] [Google Scholar]
Global Lower Extremity Amputation Study Group 2000
- Global Lower Extremity Amputation Study Group. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. The British Journal of Surgery 2000;87(3):328‐37. [DOI] [PubMed] [Google Scholar]
Haines 2009
- Haines T, Gross A, Burnie SJ, Goldsmith CH, Perry L. Patient education for mechanical neck disorders. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005106.pub3] [DOI] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org The Cochrane Collaboration, 2011.
Icks 2009
- Icks A, Haastert B, Trautner C, Giani G, Glaeske G, Hoffmann F. Incidence of lower‐limb amputations in the diabetic compared to the non‐diabetic population. Findings from nationwide insurance data, Germany, 2005‐2007. Experimental and Clinical Endocrinology and Diabetes 2009;117(9):500‐4. [DOI] [PubMed] [Google Scholar]
IDF clinical guidelines task force 2005
- IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation 2005.
Jeffcoate 2003
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361(9368):1545‐51. [DOI] [PubMed] [Google Scholar]
Larsson 1995
- Larsson J, Apelqvist J. Towards less amputations in diabetic patients. Incidence, causes, cost, treatment, and prevention ‐ a review. Acta Orthopaedica Scandinavica 1995;66:181‐92. [DOI] [PubMed] [Google Scholar]
Levin 1995
- Levin ME. Preventing amputation in the patient with diabetes. Diabetes Care 1995;18:1383‐94. [DOI] [PubMed] [Google Scholar]
Majid 2000
- Majid M, Cullum N, O'Meara S Sheldon T. Systematic reviews of wound care management: (4) diabetic foot ulceration. Health Technology Assessment 2000;21:113‐238. [PubMed] [Google Scholar]
Mason 1999
- Mason J, O'Keeffe C, McIntosh A, Hutchinson A, Booth A, Young RJ. A systematic review of foot ulcer in patients with type 2 diabetes mellitus. I: prevention. Diabetic Medicine 1999;16:801‐12. [DOI] [PubMed] [Google Scholar]
Mayfield 1998
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care 1998;21:2161‐77. [DOI] [PubMed] [Google Scholar]
Most 1983
- Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care 1983;6:87‐91. [DOI] [PubMed] [Google Scholar]
Nabuurs‐Franssen 2005
- Nabuurs‐Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health‐related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48(9):1906‐10. [DOI] [PubMed] [Google Scholar]
Pecoraro 1990
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513‐21. [DOI] [PubMed] [Google Scholar]
Ragnarson Tennvall 2004
- Ragnarson Tennvall G, Apelqvist J. Health‐economic consequences of diabetic foot lesions. Clinical Infectious Diseases 2004;39(Suppl 2):S132‐9. [DOI] [PubMed] [Google Scholar]
Reed 2005
- Reed D, Price EG, Windish DM, Wright SM, Gozu A, Hsu EB, et al. Challenges in systematic reviews of educational intervention studies. Annals of Internal Medicine 2005;142(12 Pt 2):1080‐9. [DOI] [PubMed] [Google Scholar]
Reenders 1993
- Reenders K, Nobel E, Hoogen HJM, Rutten GEHM, Weel C. Diabetes and its long‐term complications in general practice: a survey in a well‐defined population. Family Practice 1993;10:169‐72. [DOI] [PubMed] [Google Scholar]
Rith‐Najarian 2000
- Rith‐Najarian SJ, Reiber GE. Prevention of foot problems in persons with diabetes. Journal of Family Practice 2000;49(11 Suppl):S30‐9. [PubMed] [Google Scholar]
Robbins 2008
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration?. Journal of the American Podiatric Medical Association 2008;98(6):489‐93. [DOI] [PubMed] [Google Scholar]
Schellings 2005
- Schellings R, Kessels AG, Ter Riet G, Kleijnen J, Leffers P, Knottnerus JA, et al. Members of research ethics committees accepted a modification of the randomized consent design. Journal of Clinical Epidemiology 2005;58(6):589‐94. [DOI] [PubMed] [Google Scholar]
Schultz 2010
- Schulz KF, Altman D, Moher D, for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):1‐7. [DOI] [PubMed] [Google Scholar]
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217‐28. [DOI] [PubMed] [Google Scholar]
St Vincent Declaration 1989
- World Health Organization (Europe) and International Diabetes Federation (Europe). Diabetes care and research in Europe: the Saint Vincent Declaration. Diabetic Medicine 1990;7(4):360. [PubMed] [Google Scholar]
van Tulder 1997
- Tulder van MW, Assendelft WJJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine 1997;22:2323‐30. [DOI] [PubMed] [Google Scholar]
Vileikyte 2001
- Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes/Metabolism Research and Reviews 2001;17(4):246‐9. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vascular Health and Risk Management 2007;3(1):65‐76. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Dorresteijn 2010b
- Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD001488.pub3] [DOI] [Google Scholar]
Dorresteijn 2012
- Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001488.pub4] [DOI] [PubMed] [Google Scholar]
Valk 2001
- Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001488] [DOI] [PubMed] [Google Scholar]
Valk 2005
- Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001488.pub2] [DOI] [PubMed] [Google Scholar]


