Abstract
Background: Impaired lung function has been detected in up to 65% of all childhood cancer survivors. It is often caused by exposure to radiation therapy and various chemotherapeutics. The first cytotoxic drug ever identified as a causative agent of lung injury was busulfan, reported in the early 1960s. Signs and symptoms of busulfan lung are nonspecific and it is therefore difficult to differentiate the condition from pulmonary impairment caused by other pulmotoxic agents, infections, pulmonary metastases, graft-versus-host disease, or other noninfectious post-transplant complications involving the lungs.
Methods: A case example is provided to illustrate the difficulties in management of busulfan-induced lung injury in children. A retrospective review of cases of busulfan-induced lung injury indexed in PubMed until March 2019 was performed. Inclusion criteria for articles was available in full text in English.
Results: Impaired lung function caused by busulfan may become an increasing problem for young survivors.
Conclusion: Newly developed dyspnea or subclinical damage detected on pulmonary function tests, indicating primarily restrictive disease, should always arouse suspicion of busulfan-induced lung injury in a child conditioned with busulfan, especially after excluding other leading culprits of pulmonary damage affecting oncology patients.
Keywords: busulfan, lung injury, interstitial lung disease
Introduction
New multimodal treatment regimens, cooperative group trials, and pharmacological advances have led to a remarkable decline in mortality for all childhood cancers combined, which is now almost 50% less compared with 1975.1 Despite the dramatic improvement in survival rates, treatment-related toxicities cause significant nonrelapse morbidity, especially those attributed to chemotherapy, a cornerstone for curing most cancers. Since pulmonary tissue is particularly sensitive to drug-induced injury, both acutely and in the long term, pulmonary adverse reactions are becoming a matter of great concern.2–17 Impaired lung function has been detected in up to 65% of childhood cancer survivors, and although the majority are asymptomatic, the consequences may be fatal.11
Diverse etiopathogeneses of chemotherapy-induced lung injury (CILI) result in a heterogeneous group of clinical syndromes. The major types include interstitial pneumonitis/fibrosis, hypersensitivity syndrome, and capillary leak syndrome, while less common toxic drug effects are alveolar hemorrhage, acute interstitial pneumonia, bronchiolitis obliterans with organizing pneumonia, pleural effusions, bronchospasm, hilar adenopathy, and veno-occlusive disease. Symptoms usually comprise dyspnea, fatigue, fever, weight loss, and nonproductive cough and may appear anytime from minutes to years after treatment initiation.2–10
Pulmonary function tests (PFTs) are abnormal in virtually every patient with CILI, when compared with pretreatment testing, and generally reveal a restrictive pattern.4,18 Diffusing capacity for carbon monoxide (DLCO) often decreases before spirometry or radiographic changes are detected.4 Owing to its nonspecific clinical presentation and radiographic abnormalities, CILI is hardly differentiated from other competing causes of lung damage in pediatric oncology patients.
In addition, children undergoing allogeneic hematopoietic stem cell transplantation (HSCT) are additionally exposed to acute and chronic graft-versus-host disease (GvHD), both associated with pulmonary function deterioration. Acute GvHD strongly correlates with a clinically significant decrease in forced expiratory volume at the end of the 1st second (FEV1), forced vital capacity (FVC), FEV1/FVC, and DLCO, marking combined obstructive and restrictive ventilatory impairment. Chronic GvHD is also associated with a decrease in FEV1 and FVC, while reduction in FEV1/FVC did not reach statistical significance in a study conducted by Uhlving et al.13 Therefore, the diagnosis of CILI is even harder to make in children who underwent allogeneic HSCT as drug-induced toxicity and lung GvHD may overlap.
To summarize, universal criteria for diagnosis of CILI are not available, and there is no single diagnostic test or biopsy finding that definitely confirms the diagnosis of drug-induced disease. Rather, it is a diagnosis of exclusion based generally on appropriate history of drug exposure, histological evidence of lung injury, and, most importantly, exclusion of other causes of lung damage. In addition, it is difficult to identify a specific culprit as patients are often treated with multidrug regimens. Therapeutic maneuvers are limited and based on anecdotal evidence; the first step is withdrawal of the offending agent if the patient is still undergoing treatment, while systemic corticosteroids are introduced depending on the severity of symptoms and progressiveness of pulmonary function decline.2–10
The first cytotoxic drug ever identified as a causative agent of lung injury was busulfan. The entity was described in the early 1960s in 2 adult patients treated for chronic myelogenous leukemia (CML).19 Since then, further reports of busulfan lungs have been published, mostly cases in adult patients,20–24 while literature on the topic concerning the pediatric population remained relatively scarce.25,26
Illustrative Case
A 16-year-old boy was admitted to the Division of Cardiology of the University Children's Hospital, Zagreb, due to progressive exertional dyspnea. A year before the admission, at the age of 15, he was diagnosed with metastatic alveolar rhabdomyosarcoma (RMS) of the pelvis and right inguinal region. At diagnosis, the disease had spread to regional lymph nodes, multiple distant bones, and bone marrow, while the lungs were unaffected.
He was treated for months at our clinic according to the guidelines of the European pediatric Soft tissue sarcoma Study Group (EpSSG) RMS 2005 protocol. The treatment included various chemotherapeutics, including vincristine, ifosfamide, doxorubicin, actinomycin-D, etoposide, epirubicin, and carboplatin, along with zoledronic acid for bone metastases, which resulted in significant metabolic and morphological regression of the disease. His cardiac function was regularly monitored due to the use of anthracyclines and all the obtained echocardiographic parameters, including shortening fraction, were within normal values.
Due to multiple distant metastases, radiotherapists decided that the patient was not suitable for radiation therapy. However, as the boy demonstrated an excellent response to induction chemotherapy, he ultimately underwent high-dose chemotherapy (HDCT), followed by HSCT, although the procedure is not regularly used for RMS. Before the procedure, spirometry confirmed normal lung function and the chest radiograph was without pathological findings. The pretransplant high-dose conditioning regimen consisted of busulfan and melphalan; he altogether received 1216 mg (633 mg/m2) of busulfan (1 mg/kg/dose orally, 4 times a day, from day −6 to day −3) and 269 mg of melphalan (140 mg/m2 intravenously on day −2).
The engraftment for neutrophils was achieved on day +16 and for thrombocytes on day +18. He was released from hospital 26 days post-transplant in good clinical condition and was eupneic, with a normal auscultatory lung finding and peripheral capillary oxygen saturation (SpO2).
At admission to the Division of Cardiology, which was 3 months post-HSCT, the boy was conscious, afebrile, tachycardic, and with decreased SpO2—88%. On chest auscultation, bibasilar crackles and a fixed split-second heart sound, with an accentuated P2, were heard. Laboratory workup revealed pancytopenia, along with elevated C-reactive protein, fibrinogen, and D-dimer values (Table 1).
Table 1.
Laboratory Workup Upon Admission
| Laboratory finding | Results | Reference values |
|---|---|---|
| Complete blood count | ||
| WBC | 4.02 × 109/L | 4.4–11.6 × 109/L |
| Neutrophils | 76.7% | 34%–69% |
| Lymphocytes | 7.2% | 19%–52% |
| Monocytes | 13.9% | 5%–13% |
| Eosinophils | 1.7% | 0%–9% |
| Basophils | 0.5% | 0%–3% |
| RBC | 2.66 × 1012/L | 4.43–5.88 × 1012/L |
| HB | 103 g/L | 129–166 g/L |
| MCV | 116.5 fL | 76.5–92.1 fL |
| Platelet count | 112 × 109/L | 178–420 × 109/L |
| Acid–base status | ||
| pH | 7.467 | 7.350–7.450 |
| pCO2 | 4.24 kPa | 4.90–6.70 |
| pO2 | 10.8 kPa | 4.8–10.6 |
| HCO3 | 24 mM | 22–26 mM |
| Proteins and enzymes | ||
| CRP | 60 mg/L | 0.0–5.0 |
| CK | 29 U/L | 70–285 U/L |
| Troponin I | <10 ng/L | <10 ng/L |
| Fibrinogen | 3.8 g/L | 1.8–3.5 g/L |
| D-dimers | 1.08 mg/L | <0.50 mg/L |
CK, creatinine kinase; CRP, C-reactive protein; HB, hemoglobin; HCO3, bicarbonates; MCV, mean corpuscular volume; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; RBC, red blood cells; WBC, white blood cells.
Echocardiography verified tricuspid and pulmonary valve insufficiency velocities, correspondent to pulmonary systolic hypertension of 40–55 mmHg. As pulmonary hypertension was thought to be secondary due to a lung disease, further diagnostic procedures were performed to identify the cause.
Empirical antimicrobial therapy was introduced, along with sildenafil, low-molecular-weight heparin, and prednisone. No possible pathogen, as a causative agent of a pulmonary infection, was isolated in the obtained cultures (blood, nasopharyngeal swabs for viruses, and bacteria). Serology for cytomegalovirus and Epstein–Barr virus and the galactomannan test excluded these types of recent viral or fungal infections. The chest radiograph, as well as ultrasound of the thorax, was without any pathological findings, while color Doppler ultrasonography of the lower extremity veins and perfusion scintigraphy of the lungs showed no signs of thromboembolism (Fig. 1).
FIG. 1.
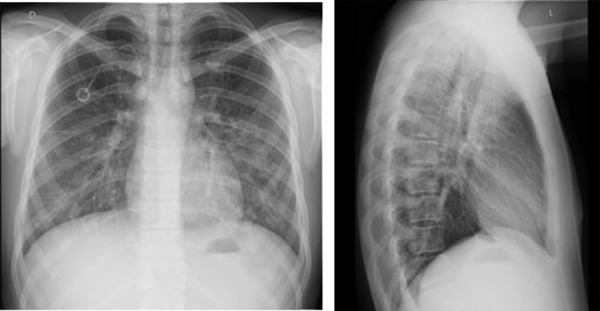
Chest radiographs upon admission revealed a completely normal finding.
Spirometry, however, detected significant ventilatory impairment. In comparison with results obtained before introduction of chemotherapy, he had markedly decreased FVC by almost 50% of the initial value, along with a less prominent fall in FEV1. FEV1/FVC increased compared with initial values. The changes were specific to a restrictive pattern. The patient had a reduction of DLCO, which was 60% when corrected to the hemoglobin value. We did not measure DLCO before the myeloablative regimen and therefore had no initial value for comparison.
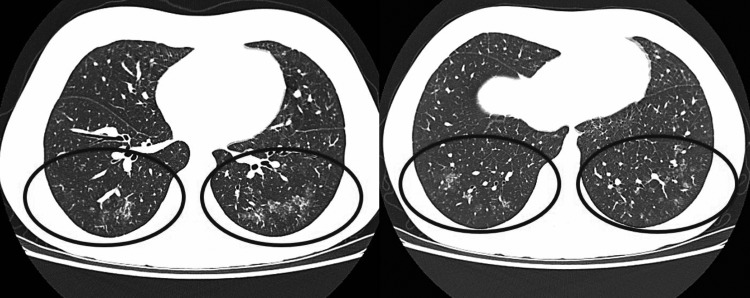
High-resolution computed tomography (HRCT) of lungs was performed, revealing characteristic ground-glass opacities (Fig. 2).
FIG. 2.
HRCT of the lung showing ground-glass opacities of the parenchyma, predominantly in the lower lobes, without signs of lymphadenopathy or pleural effusion. The black circles represent parts of the lung parenchyma where ground glass opacities can be seen. HRCT, high-resolution computed tomography.
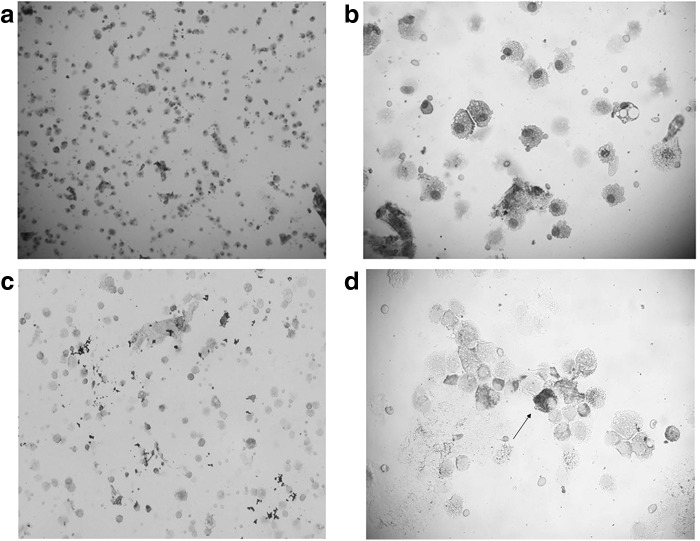
In the end, the patient underwent bronchoscopy. Cytological analysis of the bronchoalveolar lavage (BAL) did not detect any malignant cells, but numerous alveolar macrophages, siderophages, and lymphocytes. Immunophenotyping of BAL lymphocytes provided a pattern highly indicative of CILI (a high count of cytotoxic CD8+ lymphocytes—78% and a low CD4/CD8 ratio—0.3) (Fig. 3).
FIG. 3.
BAL cytology. (a, b) Showing numerous alveolar macrophages and lymphocytes in BAL. (c, d) Showing siderophages on iron staining. Arrow shows a stained siderophage on an enlarged microscopic image. BAL, bronchoalveolar lavage.
In addition, no acid-resistant bacilli were found in the BAL microscopic smear, while the polymerized chain reaction for Pneumocystis jiroveci was negative.
With the exclusion of pulmonary infection, thromboembolism, and metastatic spread of the primary neoplasm, along with the time of onset of symptoms, typical findings on HRCT and BAL lymphocyte immunology, busulfan-induced lung injury was diagnosed. The patient was treated with corticosteroids for 4 months (prednisone 0.75/mg/kg per day for the first 6 weeks, after which the dose was tapered by 5 mg each week). This resulted in prompt cessation of dyspnea, normalization of PFTs, and complete resolution of opacities on the control HRCT, obtained 4 months after initiation of corticosteroid treatment (Table 2).
Table 2.
Spirometry Findings
| Before initiation of chemotherapy | At the time dyspnea occurred | 2 Weeks of corticosteroid treatment | 1 Month of corticosteroid treatment | 3 Months of corticosteroid treatment | End of treatment | |
|---|---|---|---|---|---|---|
| FVC (L) | 4.48 | 2.41 | 2.39 | 2.47 | 3.16 | 4.45 |
| FEV1 (L) | 3.77 | 2.30 | 2.29 | 2.33 | 2.73 | 3.89 |
| FEV1/FVC | 83 | 92 | 90 | 89 | 83 | 84 |
| MEF25-75 (L) | 4.09 | 7.60 | 4.04 | 3.83 | 3.22 | 4.07 |
FEV1, forced expiratory volume at the end of the 1st second; FVC, forced vital capacity; MEF, maximal (mid-) expiratory flow.
Unfortunately, despite the successfully treated lung disease, the patient had RMS relapse and ultimately succumbed to his illness.
Discussion
Busulfan is an alkylating agent that was initially used for treating CML. Nowadays it is exclusively encountered as a component of different conditioning regimens preceding HSCT. It acts by creating cross-links between guanine–adenine and guanine–guanine nucleobases, preventing deoxyribonucleic acid replication, and promoting cell apoptosis.2–8
Historically, it was known as the first cytotoxic drug associated with pulmonary toxicity. In 1961, Oliner et al. described the development of diffuse interstitial pulmonary fibrosis in 2 adult patients treated with busulfan monotherapy for CML. Respiratory symptoms were observed within a year of chemotherapy initiation and showed a striking resolution on high-dose prednisone treatment. At the time, hypersensitivity to busulfan was considered the most likely pathogenic mechanism.19
Subsequent histological examination of busulfan lung revealed not only interstitial involvement but also the presence of intra-alveolar fibrinous edema, as well as atypical, enlarged, alveolar epithelial cells, with abundant cytoplasm and prominent hyperchromatic nuclei. The idea was that increased alveolar wall permeability results in fibrin leakage, stimulating alveolar lining cells to enlarge and undergo bizarre changes. In addition, it was hypothesized that these changes in type II alveolar cells cause aberrant surfactant secretion, all leading to increased surface tension and suction pressure, again promoting pulmonary edema.21
Yet, the precise mechanism of busulfan-induced lung injury remains unknown since adequate animal models do not exist. Besides pulmonary edema, interstitial pneumonitis, fibrosis, organizing pneumonia, and diffuse alveolar damage with acute respiratory distress syndrome, cases of pulmonary alveolar proteinosis and alveolar hemorrhage were also associated with busulfan administration (Fig. 4).2–10,19–27
FIG. 4.
Schematic diagram representing pathophysiology of the busulfan-induced lung injury.
Based on initial case reports and small single-center studies, symptomatic pulmonary injury was estimated to occur in up to 8% of patients who received busulfan.5 At present, when busulfan utility is limited to HDCT regimens preceding HSCT, the exact incidence of busulfan-induced lung injury is more difficult to examine as patients are exposed to other pulmotoxic agents. In a large prospective study conducted on 1483 patients who underwent allogeneic HSCT, the cumulative incidence of interstitial pneumonitis at 100 days post-transplant was 4% among those treated with intravenous busulfan.28 The results obtained from other similar studies vary from 2.5%29 to 8.3%.30 These reports, however, reflect symptomatic pulmonary toxicity while subclinical lung damage probably develops in a considerably higher number of those exposed to the drug.
Symptoms attributed to the condition are nonspecific and include dyspnea, fatigue, dry cough, and weight loss, while pulmonary examination usually reveals basilar crackles.2–10,19–30 The average time from initiation of therapy to onset of respiratory symptoms was ∼3.5 years,4 although symptoms occurred insidiously after only 6 weeks following busulfan exposure.24 Among patients receiving busulfan as a component of the conditioning regimen for HSCT, pulmonary toxicity usually manifests itself between 30 days and 1 year post-transplant.31
Risk factors for pulmonary toxicity include a cumulative dose of more than 500 mg and concomitant administration of additional chemotherapeutics associated with lung toxicity or lung irradiation.5,7,8 However, some initial research observed no correlation between the busulfan dosage and duration of therapy in the development of fibrosing alveolitis, hence relating the development of pulmonary injury to the genetic or immunological constitution of the patient.9
The diagnosis of CILI is one of exclusion, established on a high index of suspicion in the context of a compatible clinical picture and a positive history of busulfan exposure. The differential diagnosis includes pulmonary infection, radiation-induced lung injury, lung involvement by the underlying malignancy, pulmonary thromboembolism, congestive heart failure, GvHD, idiopathic pneumonia syndrome, and periengraftment respiratory distress syndrome.32
Findings on chest radiographs may be normal or, if altered, most frequently demonstrate bibasilar reticular opacities. HRCT is a more sensitive imaging method. It commonly reveals ground-glass opacities, but it is not specific for a causative agent in patients who underwent multidrug regimens.9 PFTs show a reduction in DLCO and eventually a restrictive pattern of ventilatory impairment on spirometry.11,31,33
Even BAL findings are not diagnostic, but may help narrow the differential diagnosis. BAL cytology in patients with busulfan lung usually shows both lymphocytosis and neutrophilia, along with the presence of atypical and hyperplastic pneumocytes. Furthermore, immunophenotyping of BAL lymphocytes can additionally divide CILI, typically having a decreased CD4:CD8 ratio, from conditions of an elevated CD4:CD8 ratio or a normal CD4:CD8 ratio (eg, tuberculosis). The predominance of CD8+ cells in CILI, as described in various types of hypersensitivity pneumonitides, results from a delayed hypersensitivity (type IV) reaction in which numerous chemokines and proinflammatory cytokines mediate a sustained CD8 cytotoxic T cell response, resulting in tissue damage.7,34,35
The therapeutic approach is mainly supportive, including supplemental oxygen and pulmonary rehabilitation. First case reports demonstrated excellent clinical response to corticosteroid treatment.4,19 In patients who are believed to have busulfan toxicity following HSCT, glucocorticoids are initiated in cases of rapid deterioration in lung function. Still, the evidence to support the benefit of glucocorticoids is largely observational as no controlled clinical trial has ever been performed.10 Additionally, some spontaneous improvement may occur. In a prospective study conducted on adult patients receiving busulfan and cyclophosphamide before allogeneic HSCT, baseline pulmonary function values were restored 5 years post-transplant in almost all survivors. Therefore, the authors recommended an observation period longer than a year before diagnosing permanent reduction in lung function.31
The first case of busulfan lung in children was that of a 16-month-old male infant treated with busulfan for CML, published in 1977. The patient had progressive radiographic changes in the sense of a diffuse interstitial and intra-alveolar pattern and died only 4 days upon the onset of respiratory symptoms. At autopsy, histological examination showed extensive proliferation of alveolar and bronchiolar lining cells with nuclear atypia, along with alveolar exudate, including fibrin and inflammatory cells.25 Afterward, the literature on busulfan lung in children remained scarce as the drug was predominantly used for curing CML, rarely diagnosed in the pediatric population.26
With HSCT becoming a mainstay of treatment for various hematological and solid pediatric malignancies, impaired lung function caused by busulfan may become an increasing problem for young survivors. However, most of the studies are being conducted on children undergoing allogeneic HSCT, which has significantly higher rates of pulmonary complications overall compared with autologous HSCT, as the decline in pulmonary function is additionally associated with GvHD.13,14
Therefore, we thought it was important to present the case of our patient as he developed severe pulmonary function impairment after autologous HSCT, emphasizing the need for regular pulmonary monitoring in pediatric patients who underwent autologous HSCT. Although not endangered by GvHD, they may as well develop severe pulmonary sequelae due to high-dose busulfan treatment. We suggest conducting DLCO and spirometry at diagnosis, before HDCT treatment, and then monthly during the first post-transplant year as there is a high possibility of detecting pulmonary impairment at a subclinical level. Further examinations, if needed, include HRCT and ultimately bronchoscopy with BAL.
Author Disclosure Statement
Drs. Nusa Matijasic, Aleksandra Bonevski, Visnja Tokic Pivac, and Ivan Pavic have no conflicts of interest or financial ties to disclose.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017
- 2. Batist G, Andrews JL. Pulmonary Toxicity of Antineoplastic Drugs. JAMA 1981; 246:1449–1453 [PubMed] [Google Scholar]
- 3. Snyder LS, Hertz MI. Cytotoxic drug-induced lung injury. Semin Respir Infect 1988; 3:217–228 [PubMed] [Google Scholar]
- 4. Limper AH. Chemotherapy-induced lung disease. Clin Chest Med 2004; 25:53–64 [DOI] [PubMed] [Google Scholar]
- 5. Leger P, Limper AH, Maldonado F. Pulmonary toxicities from conventional chemotherapy. Clin Chest Med 2017; 38:209–222 [DOI] [PubMed] [Google Scholar]
- 6. Ishioka S. [Strategy of therapy for interstitial lung disease due to chemotherapeutic drugs or radiation]. Gan To Kagaku Ryoho 1997; 24 Suppl 3:432–438 [PubMed] [Google Scholar]
- 7. Ginsberg SJ, Comis RL. The pulmonary toxicity of antineoplastic agents. Semin Oncol 1982; 9:34–51 [PubMed] [Google Scholar]
- 8. Sostman HD, Matthay RA, Putman CE. Cytotoxic drug-induced lung disease. Am J Med 1977; 62:608–615 [DOI] [PubMed] [Google Scholar]
- 9. Cleverley JR, Screaton NJ, Hiorns MP, et al. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol 2002; 57:292–299 [DOI] [PubMed] [Google Scholar]
- 10. Camus P, Bonniaud P, Fanton A, et al. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med 2004; 25:479–519 [DOI] [PubMed] [Google Scholar]
- 11. Record E, Williamson R, Wasilewski-Masker K, et al. Analysis of risk factors for abnormal pulmonary function in pediatric cancer survivors. Pediatr Blood Cancer 2016; 63:1264–1271 [DOI] [PubMed] [Google Scholar]
- 12. Nagasawa M, Mitsuiki N, Aoki Y, et al. Effect of reduced-intensity conditioning and the risk of late-onset non-infectious pulmonary complications in pediatric patients. Eur J Haematol 2017; 99:525–531 [DOI] [PubMed] [Google Scholar]
- 13. Uhlving HH, Bang CL, Christensen IJ, et al. Lung function after allogeneic hematopoietic stem cell transplantation in children: a longitudinal study in a population-based cohort. Biol Blood Marrow Transplant 2013; 19:1348–1354 [DOI] [PubMed] [Google Scholar]
- 14. Hierlmeier S, Eyrich M, Wölfl M, et al. Early and late complications following hematopoietic stem cell transplantation in pediatric patients - A retrospective analysis over 11 years. PLoS One 2018; 13:e0204914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson PA, Lim A, Panek-Hudson Y, et al. Screening with spirometry is a useful predictor of later development of noninfectious pulmonary syndromes in patients undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20:781–786 [DOI] [PubMed] [Google Scholar]
- 16. Kasteler R, Weiss A, Schindler M, et al. Long-term pulmonary disease among Swiss childhood cancer survivors. Pediatr Blood Cancer 2018; 65:e26749. [DOI] [PubMed] [Google Scholar]
- 17. Dietz AC, Chen Y, Yasui Y, et al. Risk and impact of pulmonary complications in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 2016; 122:3687–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fauroux B, Meyer-Milsztain A, Boccon-Gibod L, et al. Cytotoxic drug-induced pulmonary disease in infants and children. Pediatr Pulmonol 1994; 18:347–355 [DOI] [PubMed] [Google Scholar]
- 19. Oliner H, Schwartz R, Rubio F, et al. Interstitial pulmonary fibrosis following busulphan therapy. Am J Med 1961; 31:134–139 [DOI] [PubMed] [Google Scholar]
- 20. Smalley RV, Wall RL. Two cases of busulfan toxicity. Ann Intern Med 1966; 64:154–164 [DOI] [PubMed] [Google Scholar]
- 21. Heard BE, Cooke RA. Busulphan lung. Thorax 1968; 23:187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burns WA, McFarland W, Matthews MJ. Busulfan-induced pulmonary disease. Report of a case and review of the literature. Am Rev Respir Dis 1970; 101:408–413 [DOI] [PubMed] [Google Scholar]
- 23. Podoll LN, Winkler SS. Busulfan lung. Report of two cases and review of the literature. Am J Roentgenol Radium Ther Nucl Med 1974; 120:151–156 [DOI] [PubMed] [Google Scholar]
- 24. Hankins DG, Sanders S, MacDonald FM, et al. Pulmonary toxicity recurring after a six week course of busulfan therapy and after subsequent therapy with uracil mustard. Chest 1978; 73:415–416 [DOI] [PubMed] [Google Scholar]
- 25. Pearl M. Busulfan lung. Am J Dis Child 1977; 131:650–652 [PubMed] [Google Scholar]
- 26. Oakhill A, Green ID, Knowlson GT, et al. Busulphan lung in childhood. J Clin Pathol 1981; 34:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vergnon JM, Boucheron S, Riffat J, et al. [Interstitial pneumopathies caused by busulfan. Histologic, developmental and bronchoalveolar lavage analysis of 3 cases]. Rev Med Interne 1988; 9:377–383 [DOI] [PubMed] [Google Scholar]
- 28. Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood 2013; 122:3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ulrickson M, Aldridge J, Kim HT, et al. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant 2009; 15:1447–1454 [DOI] [PubMed] [Google Scholar]
- 30. Crilley P, Topolsky D, Styler MJ, et al. Extramedullary toxicity of a conditioning regimen containing busulphan, cyclophosphamide and etoposide in 84 patients undergoing autologous and allogenic bone marrow transplantation. Bone Marrow Transplant 1995; 15:361–365 [PubMed] [Google Scholar]
- 31. Lund MB, Brinch L, Kongerud J, et al. Lung function 5 yrs after allogeneic bone marrow transplantation conditioned with busulphan and cyclophosphamide. Eur Respir J 2004; 23:901–905 [DOI] [PubMed] [Google Scholar]
- 32. Cengiz Seval G, Topçuoğlu P, Demirer T. Current Approach to Non-Infectious Pulmonary Complications of Hematopoietic Stem Cell Transplantation. Balkan Med J 2018; 35:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Littler WA, Ogilvie C. Lung function in patients receiving busulphan. Br Med J 1970; 4:530–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Costabel U, Uzaslan E, Guzman J. Bronchoalveolar lavage in drug-induced lung disease. Clin Chest Med 2004; 25:25–35 [DOI] [PubMed] [Google Scholar]
- 35. Miller R, Allen TC, Barrios RJ, et al. Hypersensitivity pneumonitis a perspective from members of the pulmonary pathology society. Arch Pathol Lab Med 2018; 142:120–126 [DOI] [PubMed] [Google Scholar]