Abstract
Objective
To determine if the concentration and saturation of natalizumab (NTZ) administration at extended interval dosing (EID; every 5–8 weeks) over 18 months is able to be maintained in the range considered adequate to sustain the clinical efficacy of NTZ.
Methods
In a cross-sectional assessment of patients with multiple sclerosis (MS) who received standard interval dosing (every 4 weeks) or EID, serum NTZ concentrations were measured using ELISA, and α4-integrin receptor saturations were analyzed via cytometry, in blood samples obtained at trough timepoints.
Results
Trough serum concentration was above the “therapeutic” concentration of 2.0 μg/mL in 72% of EID patients. Trough saturation was above the “therapeutic” 50% threshold in 79% of EID-treated patients. Our model predicted that at least 9 NTZ infusions/year are required to maintain adequate trough saturation and concentration levels. Higher body mass index (BMI) was a predictor of suboptimal trough saturation on EID NTZ.
Conclusions
Trough α4-integrin receptor saturation >50% correlated with high clinical efficacy of NTZ in previous studies. A continual treatment with EID maintains receptor saturation and concentration that are in the “therapeutic range” for most patients. This finding provides biological plausibility for the clinical efficacy of NTZ EID. Patients with higher BMI may require closer clinical and MRI follow-up.
Natalizumab (NTZ) administered at extended interval dosing (EID, 5–8 weeks) instead of the approved standard interval dosing (SID, every 4 weeks) demonstrates reduction of risk of NTZ-associated progressive multifocal leukoencephalopathy (PML) in patients with multiple sclerosis (MS).1 This finding raises the important question of whether NTZ should be infused at EID. However, high-level evidence that supports the efficacy of NTZ EID is presently lacking.
The biological plausibility of EID efficacy depends on its pharmacodynamic properties. Previous studies indicated that clinical and radiologic disease activity correlate with the level of NTZ saturation of lymphocyte α4-integrin receptor sites.2 The standard 4-week dosing (SID) was chosen to ensure continuous “maximal” (defined as >80%) α4-integrin receptor saturation.3 This strategy achieves adequate blockade of autoreactive lymphocytes from entering the CNS but may compromise immune surveillance against JC virus infection of glial cells in the CNS, thereby placing patients at risk of PML, a well-known complication of NTZ. By 8 weeks postadministration, saturation generally declines to “submaximal levels” of 50%–80%.4 “Receptor desaturation”, defined as saturation <50%, is usually observed only after 8 weeks postinfusion when NTZ serum concentrations fall below 1 μg/mL.5 The serum concentration of 2 μg/mL has been postulated to be adequate to maintain efficacy in most NTZ-treated patients because it corresponds to α4-integrin receptor saturation in the 70%–100% range.6 These data are consistent with the observation that the return of MS clinical and radiologic activity occurs approximately 10 weeks following NTZ withdrawal.7 Dosing of NTZ in the intermediate range—less frequently than every 4 weeks, but more frequently than every 10 weeks—may result in acceptable reduction of trough concentration and saturation of NTZ.8
The objective of this study was to determine whether the steady-state pharmacologic parameters of NTZ EID, determined after at least 18 months of continuous EID treatment, would maintain α4-integrin saturation in the submaximal but “therapeutic” (>50%) range and serum concentration ≥2 μg/mL. Our secondary objective was to define the minimal number of infusions per year (NI) that is required to maintain NTZ concentration/saturation levels that are consistent with NTZ efficacy, based on thresholds inferred from findings of previous studies.4–9
Methods
NTZ-treated patients with MS from the NYU MS Care Center (New York, NY) and Rocky Mountain MS Clinic (Salt Lake City, UT) were offered enrollment if their NTZ infusion history satisfied requirements for EID and SID as defined below. This study was approved by the respective institutional review boards.
EID was defined as having received ≤15 infusions in the previous 18 months of treatment (548 days), with the EID schedule maintained for at least 12 months. Patients received SID for ≥6 months before receiving EID. SID was defined as ≥16 infusions in the previous 18 months. All patients had more than 18 months of NTZ except for 14 patients in the SID cohort who were treated for more than 12 months with frequency of infusions >0.833/y. Patients with “dosing gaps” (infusions >12 weeks apart) or “overdoses” (infusions <3 weeks apart) were excluded.
In a cross-sectional assessment, the blood was collected on the day of and before the scheduled NTZ infusion. Serum NTZ concentrations were measured using ELISA (Covance, Princeton, NJ), and α4-integrin saturation on circulating lymphocytes was analyzed via flow cytometry of whole blood (LabCorp, Burlington, NC).
Demographic and clinical characteristics were summarized using descriptive statistics. Means of concentration and saturation were compared between the EID and SID groups using 2-sided t tests with a Bonferroni adjustment for the 2 comparisons (significance level 0.025). Multivariable linear regression was used to compare concentration and saturation levels between 2 groups with adjustments for age, sex, and body mass index (BMI). Linear regressions were also used to investigate the relationships of concentration and saturation vs BMI for the 2 groups. Polynomial regressions for concentration and saturation vs the NI were used to determine the minimum number of EID infusions per year that yields concentration higher than 2 μg/mL and α4-integrin saturation higher than 50%.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Two hundred fourteen NTZ-treated patients with MS from the NYU MS Care Center (n = 19) and Rocky Mountain MS Clinic (n = 195) were available for analysis. The average age of patients was 47.7 (SD = 11.2) years; 71% were female. No differences between the 2 dosing groups were observed with respect to age, sex, and BMI. The mean duration of treatment for EID was 4.3 years (SD = 2.1; range 1.8–9.2 years) and for SID was 3.44 years (SD = 1.2; range 1.0–10.3 years), p = 0.08.
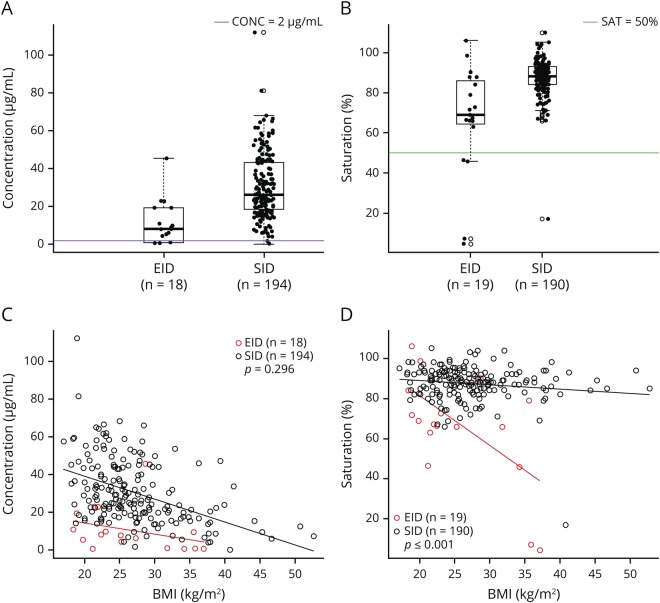
Trough concentration and saturation for the SID and EID groups are presented in figure 1, A and B. Trough concentration and saturation were significantly higher in the SID group compared with the EID group. The mean concentration in SID was 30.5 μg/mL (SD = 17) and in EID was 10.8 μg/mL (SD = 11.5), p < 0.001. The mean saturation in SID was 87.5% (SD = 8.9) and in EID was 67.3% (SD = 26.7), p = 0.004. NTZ trough concentrations >2.0 μg/mL were observed in 99% of SID patients and 72% of EID patients. α4-integrin saturation levels of ≥50% were observed in 99% of SID and 79% of EID patients.
Figure 1. Concentration and saturation in patients on SID and EID schedules.
Distribution of (A) serum CONC (μg/mL) and (B) α4-integrin SAT (%) at dosing trough in patients on EID and SID. (C) CONC vs BMI (EID group equation: 26.0 − 0.58 * BMI; SID equation: 63.2 − 1.20 * BMI); (D) SAT vs BMI (EID group equation: 130.5 − 2.47 * BMI; SID equation: 93.2 − 0.20 * BMI) in SID and EID patients. BMI = body mass index; CONC = concentration; EID = extended interval dosing; SAT = saturation; SID = standard interval dosing.
Concentration levels inversely correlated with BMI for each group, but the slope of the decline did not differ between the 2 groups (difference between slopes of 0.62, 95% CI: −0.54 to 1.79). Saturation levels inversely correlated with BMI for both groups, but the slope of decline was significantly lower in the EID group compared with the SID group (differences in slope of −2.25, 95% CI: −3.05 to −1.44; p < 0.001) (figure 1, C and D). In multivariable regression analyses with adjustment for age, sex, and BMI, only BMI was a significant predictor of NTZ concentration and saturation (BMI: p < 0.001).
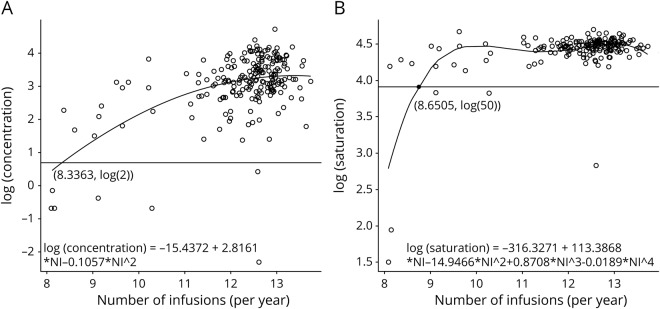
We also performed stepwise regression on log (concentration) vs polynomial functions of the NI to model the minimum NI needed to achieve the desired NTZ concentration (≥2.0 μg/mL). The best-fitting model selected by the stepwise procedure was log (concentration) = 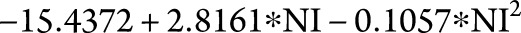 with adj-R2 = 0.29. Based on this model, patients require at least 8.3363 infusions/year (1 infusion every 6.2 weeks) to ensure NTZ trough concentration levels >2 μg/mL (figure 2A). A similar procedure was used to model saturation as a function of NI. The best-fitting model was log (saturation) =
with adj-R2 = 0.29. Based on this model, patients require at least 8.3363 infusions/year (1 infusion every 6.2 weeks) to ensure NTZ trough concentration levels >2 μg/mL (figure 2A). A similar procedure was used to model saturation as a function of NI. The best-fitting model was log (saturation) = 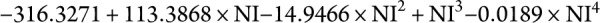 with adj-R2 = 0.46. Based on this model, patients require at least 8.6505 infusions/year (1 infusion every 6.0 weeks) to ensure saturation levels of greater than 50% (figure 2B). Therefore, we recommend that at least 9 NTZ infusions per year are required to maintain adequate trough saturation and concentration level.
with adj-R2 = 0.46. Based on this model, patients require at least 8.6505 infusions/year (1 infusion every 6.0 weeks) to ensure saturation levels of greater than 50% (figure 2B). Therefore, we recommend that at least 9 NTZ infusions per year are required to maintain adequate trough saturation and concentration level.
Figure 2. Modeling of minimum frequency of infusions per year.
(A) Polynomial regression of log of concentration vs NI. (B) Polynomial regression of log of saturation vs NI. NI = number of infusions/year.
Discussion
Findings from previous studies suggest an NTZ concentration of at least 2.0 μg/mL, and an α4-integrin saturation of at least 50%, to maintain clinical and MRI efficacy.4–9 Using these thresholds, we have shown that 72% of patients on continual EID maintained NTZ concentration above the “therapeutic” threshold of 2.0 μg/mL, and 79% of EID patients maintained “therapeutic” α4-integrin saturation higher than 50%. These results provide pharmacodynamic support for the clinical efficacy of EID NTZ and are consistent with previous retrospective observational studies of EID NTZ efficacy.10
Another conclusion of our study is that approximately 8.5 infusions per year are needed to maintain “therapeutic” NTZ concentration and saturation. This dosing frequency, 1 infusion every 6 weeks, fits well with the chosen frequency of EID in the ongoing clinical trial, in which the efficacy of every 4-week NTZ dosing is compared with every 6-week dosing (NCT03689972). However, because our multivariable regression analysis identified higher BMI as a predictor if subtherapeutic concentration and saturation, overweight patients may require personalized dosing regimen and closer clinical and MRI follow-up to ensure efficacy.
Limitations of our study include cross-sectional rather than longitudinal design and relatively small number of patients on continuous EID treatment schedule. Despite these limitations, the data provide biological plausibility for the clinical efficacy of EID NTZ, which is being tested in the ongoing randomized clinical trial.
Acknowledgment
The authors acknowledge Zuniga-Estrada G and Jacobs A for their help in running the study.
Glossary
- BMI
body mass index
- EID
extended interval dosing
- NI
number of infusions per year
- NTZ
natalizumab
- PML
progressive multifocal leukoencephalopathy
- SID
standard interval dosing
Appendix. Authors
Study funding
This work was supported by an unrestricted investigator-initiated grant from Biogen.
Disclosure
L. Zhovtis Ryerson received personal compensation for serving on speaker bureau and advisory committees for Biogen, Genentech, Teva, and Celgene and has received research support from Biogen. X. Li, J.D. Goldberg, T. Hoyt, and A. Christensen report no disclosures. R. Metzger's spouse is an employee of Sanofi and owns Biogen stock. G. Zuniga-Estrada and A. Jacobs report no disclosures. I. Kister has served on the scientific advisory boards of Biogen-Idec and Genentech and received consulting fees for Roche and research support from the Guthy-Jackson Charitable Foundation, National Multiple Sclerosis Society, Biogen-Idec, Serono, Genzyme, and Novartis. J. Foley sits on the scientific advisory boards of Biogen, Genentech-Roche, Genzyme, Celgene, and EMD Serono. He is also on the speakers' bureau of Biogen and Genentech-Roche. J. Foley receives research support from Biogen, Genentech-Roche, Novartis, Genzyme, Mallinckrodt, and Adamas and has equity interest in Abreos Biosciences. Go to Neurology.org/NN for full disclosures.
References
- 1.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019;93:e1452–e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wipfler P, Harrer A, Pilz G, et al. Natalizumab saturation: biomarker for individual treatment holiday after natalizumab withdrawal? Acta Neurol Scand 2014;129:e12–e15. [DOI] [PubMed] [Google Scholar]
- 3.Rudick R, Sandrock A. Natalizumab: α4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004;4:571–580. [DOI] [PubMed] [Google Scholar]
- 4.Plavina T, Muralidharan KK, Kuesters G, et al. Reversibility of the effects of natalizumab on peripheral immune cell dynamics in MS patients. Neurology 2017;89:1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Kempen ZL, Leurs CE, Witte BI, et al. The majority of natalizumab-treated MS patients have high natalizumab concentrations at time of re-dosing. Mult Scler J 2018;24:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derfuss T, Kovarik JM, Kappos L, et al. α4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol Neuroimmunol Neuroinflamm 2017;4:e388 doi: 10.1212/NXI.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley J, Goelz S, Hoyt T, et al. Evaluation of natalizumab pharmacokinetics and pharmacodynamics with standard and extended interval dosing. Mult Scler Relat Disord 2019;31:65–71. [DOI] [PubMed] [Google Scholar]
- 9.Fox RJ, Cree BA, De Sèze J, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology 2014;82:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:885–889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.