Abstract
Background
Use of video research visits in neurologic conditions is rising, but their utility has not been assessed in atypical parkinsonian syndromes. We sought to evaluate the diagnostic concordance between video-based vs self-reported diagnoses of multiple system atrophy, progressive supranuclear palsy, dementia with Lewy bodies, and corticobasal syndrome. We also assessed patient satisfaction with video-based visits.
Methods
We conducted a study of video-based research visits in individuals with an atypical parkinsonian syndrome enrolled in The Michael J. Fox Foundation's Fox Trial Finder. Participants completed a recorded real-time video visit with a remote evaluator who was blinded to the participant's self-reported diagnosis. The investigator conducted a structured interview and performed standard assessments of motor function. Following the visit, the investigator selected the most likely diagnosis. The recorded visit was reviewed by a second blinded investigator who also selected the most likely diagnosis. We evaluated diagnostic concordance between the 2 independent investigators and assessed concordance between investigator consensus diagnosis and self-reported diagnosis using Cohen's kappa. We assessed participant satisfaction with a survey.
Results
We enrolled 45 individuals with atypical parkinsonian syndromes, and 44 completed the investigator-performed video assessment. We demonstrated excellent concordance in diagnosis between the investigators (κ = 0.83) and good reliability of self-reported diagnosis (κ = 0.73). More than 90% of participants were satisfied or very satisfied with the convenience, comfort, and overall visit.
Conclusions
Video research visits are feasible and reliable in those with an atypical parkinsonian syndrome. These visits represent a promising option for reducing burden and extending the reach of clinical research to individuals with these rare and disabling conditions.
Participation in clinical research is challenging for individuals with neurodegenerative conditions. These challenges are particularly apparent among the atypical parkinsonian syndromes1: multiple system atrophy (MSA), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB), and corticobasal syndrome (CBS). Poor mobility, frequent falls, cognitive impairment, and rapid progression are common to the conditions, limiting research participation and driving high dropout rates.2 In addition, the conditions are rare, with research specific to each diagnosis often limited to those who live near specialized centers.
Video-based research visits could reduce burden, extend reach, and increase research participation.3 Previous work has explored the feasibility of video research visits for recruitment,4 replacing interim trial visits,5 and long-term follow-up of trial cohorts in Parkinson disease (PD), Batten disease, and migraine, among others.6–10 However, video visits have not been evaluated in the atypical parkinsonian syndromes.11
The Michael J. Fox Foundation's Fox Trial Finder (foxtrialfinder.org/) is an online tool used to match individuals with PD and healthy controls to clinical research. The system recently expanded its functionality to include individuals with a self-reported atypical parkinsonian syndrome, but the veracity of self-reported diagnosis has not been assessed. Here, we present the results of a feasibility study of video research visits in Fox Trial Finder participants with atypical parkinsonism.
Methods
We identified Fox Trial Finder users who self-reported a diagnosis of MSA, PSP, DLB, or CBS and invited them to participate through a site posting and directed messaging to all users reporting one of these diagnoses via the site's messaging system. Inclusion criteria included age 18 or older, fluency in English, access to an internet-enabled device, and home internet connection. We aimed to enroll 40–50 individuals and attempted to enroll approximately equal numbers of individuals with each diagnosis.
A study coordinator contacted interested participants by phone to discuss study procedures and obtain informed consent. We provided enrolled participants with a portable blood pressure cuff (Omron 5 series; Omron, Kyoto, Japan) and web camera (Microsoft LifeCam HD-3000; Microsoft, Redmond, Washington) if needed. Video visits were conducted using the video-conferencing software Zoom (California). A study coordinator conducted a video visit to record demographics, confirm diagnosis, complete a medication log, and remotely administer the Montreal Cognitive Assessment (MoCA).
A movement disorder–trained neurologist (C.G.T., investigator 1) blinded to the self-reported diagnosis subsequently conducted a recorded real-time video visit between 0 and 14 days of the coordinator-completed visit. The neurologist conducted a structured interview with standardized questions and open-ended history taking (appendix e-1, links.lww.com/CPJ/A106). Investigator 1 completed the Movement Disorder Society–Unified Parkinson's Disease Rating (MDS-UPDRS) parts Ia and III, Unified Multiple System Atrophy Rating Scale (UMSARS), Progressive Supranuclear Palsy Rating Scale (PSPRS), and sitting and standing vital signs if able to stand safely. Assessments of reflexes, rigidity, swallowing, and postural stability were not performed or rated. The investigator was trained and certified to perform the MDS-UPDRS. Following the visit, investigator 1 selected the most likely diagnosis based on clinical impression and review of diagnostic criteria.
A second movement disorder–trained neurologist (J.L.A., investigator 2), blinded to both self-reported diagnosis and investigator-selected diagnosis, reviewed the recorded investigator-performed visit. Following review, investigator 2 selected the most likely diagnosis. Concordant diagnoses between investigators 1 and 2 were recorded as the consensus diagnosis. Discordant diagnoses were reviewed in conference by the investigators, and a consensus diagnosis was selected. Consensus diagnoses were compared with self-reported diagnosis.
We analyzed demographics, interview responses, and motor assessments descriptively. We evaluated feasibility by calculating the proportion of participants who completed all assessments during both visits, defining 80% as the acceptable cutoff; participants who were unable to stand safely were not required to complete assessments of gait or standing vital sign measurement. We recorded the number of individuals who were able to complete sitting and standing vital sign measurements and calculated the proportion of participants who met the criteria for orthostatic hypotension.12 We assessed the significance of between-group differences in disease duration and performance on the clinical outcome measures (MoCA, MDS-UPDRS, UMSARS, and PSPRS) using analysis of variance with α = 0.05. Significant results were assessed with post hoc testing using the Tukey method.
We calculated concordance between initial diagnoses selected by investigators 1 and 2 using Cohen's kappa, setting a threshold of ≥0.6. We also calculated concordance between consensus and self-reported diagnoses using Cohen's kappa, setting a threshold of ≥0.6. We assessed whether there was a statistically significant difference in concordance between the self-reported diagnoses using the χ2 test for independence. Using α = 0.05, we defined a χ2 value of ≥7.81 as sufficient to reject the null hypothesis. We measured participant satisfaction with the video visit with a satisfaction survey (appendix e-2, links.lww.com/CPJ/A106). Free-text responses on the survey were analyzed qualitatively with overall themes reported.
Standard protocol approvals, registrations, and patient consents
The Research Subjects Review Board at the University of Rochester approved all study procedures. All participants provided electronic informed consent by telephone before study participation. We provided a copy of the consent documentation to participants. Consent documents were stored in the University of Rochester's Health Insurance Portability and Accountability Act-compliant database.
Data availability
All data were recorded in the University of Rochester's Health Insurance Portability and Accountability Act-compliant database. Deidentified data not presented are available to qualified investigators by request for purposes of replicating procedures and results.
Results
A total of 588 individuals with a self-reported atypical parkinsonian syndrome were identified on Fox Trial Finder. Fifty-six individuals expressed interest, and 45 participants (23 with a self-reported diagnosis of MSA, 14 with PSP, 3 with DLB, and 5 with CBS) from 23 states and 2 Canadian provinces (3 Canadian participants) were enrolled (table e-1 and figure e-1, links.lww.com/CPJ/A106). Baseline characteristics are presented in table 1. Participant recruitment took 9 months with slow recruitment over 7 months (n = 27) and an increase following shortening and clarification of the recruitment message over the remaining 2 months (n = 28).
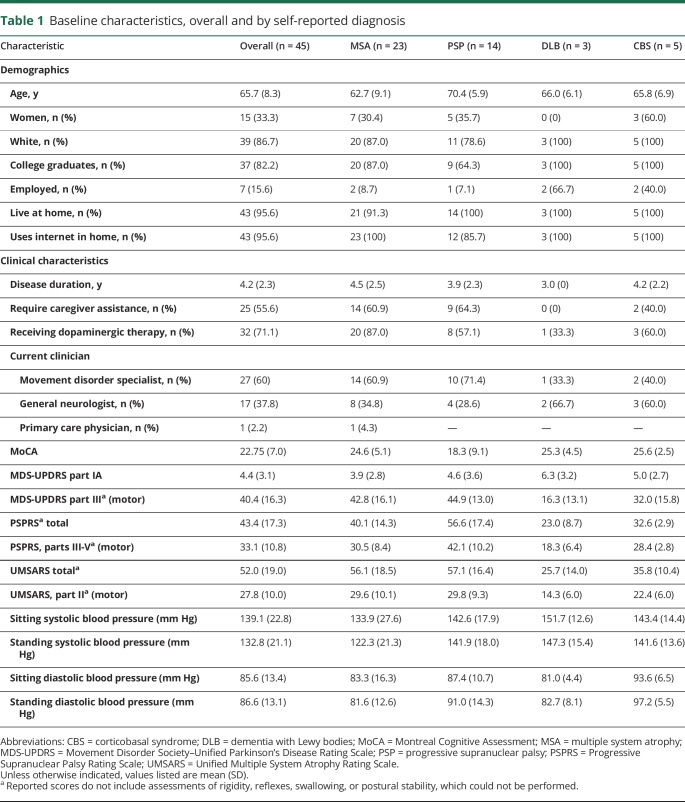
Table 1.
Baseline characteristics, overall and by self-reported diagnosis
Among enrolled participants, 44 (98%) completed all assessments during both video visits; a single participant with MSA had a clinical decline and could not complete the second visit. Twenty-four (55%) participants required caregiver assistance with the investigator visit. We were able to perform sitting and standing vital sign measurement in 35 participants; the remaining 9 participants were unable to perform standing measurements because of severe postural instability (n = 8) or reported severe symptomatic orthostasis (n = 1). Among participants who completed sitting and standing vital sign measurements, 6 met the criteria for orthostatic hypotension; all 6 self-reported a diagnosis of MSA. There was no statistical difference in disease duration between each self-reported diagnosis (F = 0.46, p = 0.71). Individuals with PSP had more severely affected scores than those in other groups on all clinical measures except the MDS-UPDRS IA (table 1). We identified significant between-group differences on the MoCA (F = 3.30, p = 0.03), MDS-UPDRS III (F = 3.717, p = 0.02), PSPRS total (F = 7.18, p < 0.001) and motor subscore (F = 9.20, p < 0.001), and UMSARS total (F = 4.79, p = 0.006) and motor subscore (F = 3.11, p = 0.04). Results of post hoc testing are reported in the Supplement (table e-2, links.lww.com/CPJ/A106).
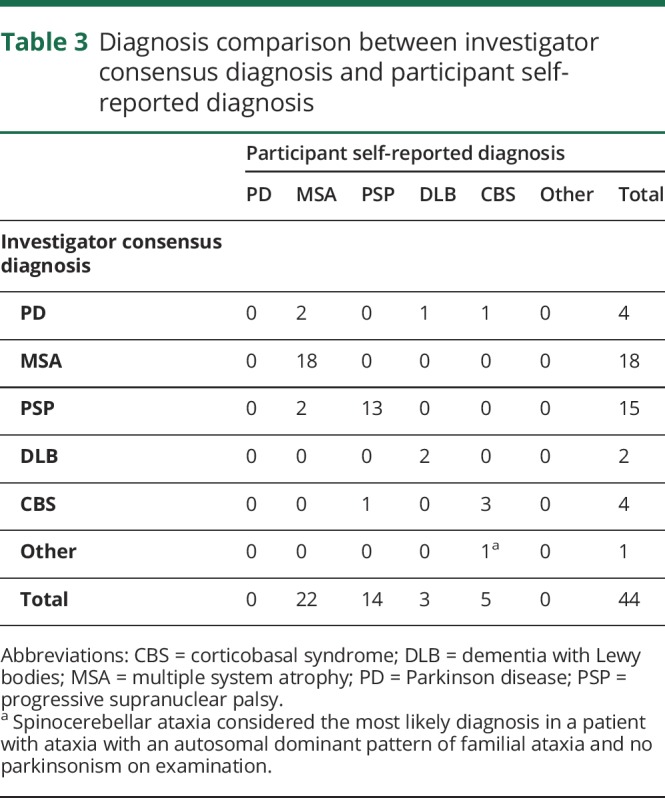
Investigators 1 and 2 reached a concordant initial diagnosis in 88.6% (κ = 0.83) of assessments (table 2). The most common disagreement (n = 3, 60%) was PD vs an atypical parkinsonian syndrome; PD was selected as the consensus diagnosis in all 3 cases. There were also 2 disagreements between PSP vs MSA; PSP was selected in one case, and MSA was selected in the other. Investigator consensus diagnosis agreed with self-reported diagnosis in 81.8% (κ = 0.73) of participants (table 3). Agreement was highest among those with a self-reported diagnosis of PSP (13/15, 93%) and lowest among those with a self-reported diagnosis of CBS (3/5, 60%). There was no significant difference in concordance rates between the diagnoses (χ2 = 3.2, p = 0.36).
Table 2.
Diagnosis comparison between investigator 1 and investigator 2
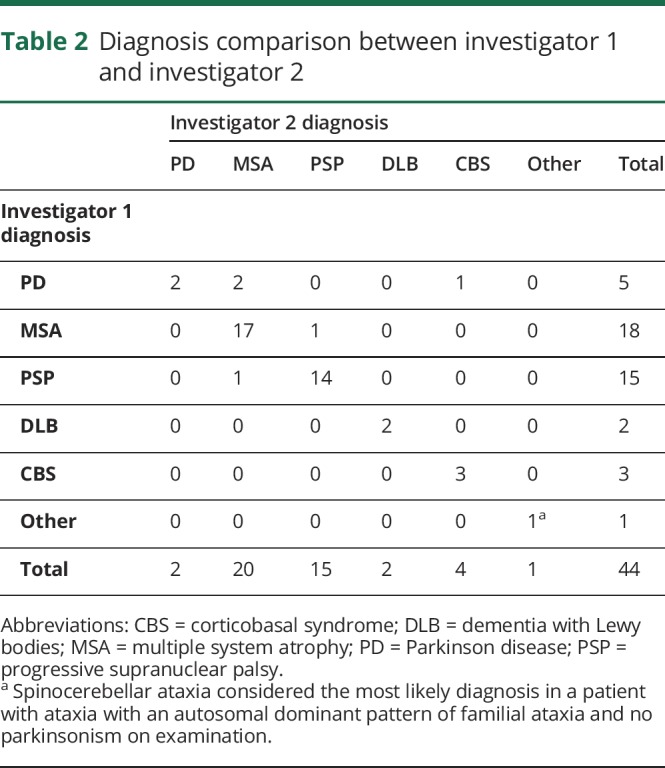
Table 3.
Diagnosis comparison between investigator consensus diagnosis and participant self-reported diagnosis
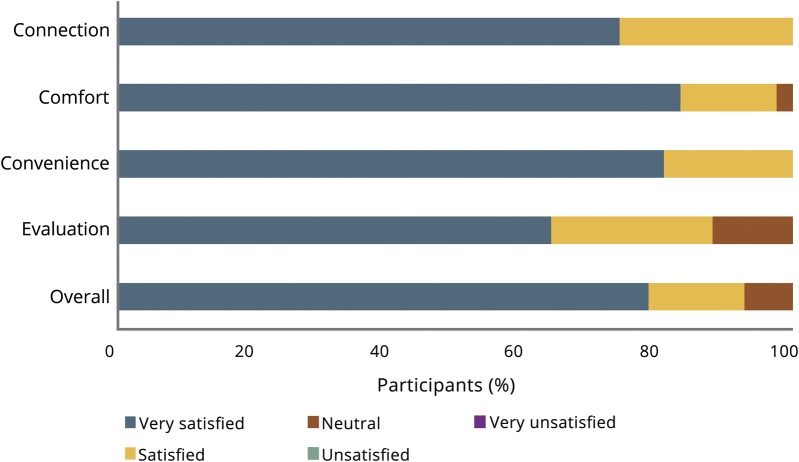
More than 90% of participants were satisfied or very satisfied with the video visit overall, and 100% of participants were satisfied or very satisfied with the convenience (figure 1). Participants specifically described appreciating the visit at home, comfort with the investigator, and ease of conducting the visit. All respondents would recommend video visits to others with an atypical parkinsonian syndrome, and 93.0% would be likely or very likely to participate in future virtual visits. Although dissatisfaction rates were low, 2 participants (4.5%) identified difficulty with the long duration of the visit (around 1 hour), and 3 participants (6.8%) described a general discomfort with technology.
Figure 1. Participant satisfaction with the video research visits.
Discussion
In this study, we successfully conducted video-based research visits in individuals with atypical parkinsonian syndromes, demonstrated the ability to reach a consensus diagnosis via video visit, and demonstrated the reliability of self-reported diagnosis in Fox Trial Finder. Participants were overwhelmingly satisfied with the visits, felt comfortable with the investigator and method of assessment, and appreciated the convenience of assessment in their own home.
The study had some limitations. First, the sample is small and cannot capture the breadth of disease severity seen among the atypical parkinsonian syndromes. In addition, the sample was overwhelmingly white and highly educated with all participants having high-speed internet access, which may limit applicability to the broader population with these conditions. Recruitment was slow with fewer than 10% of eligible subjects expressing interest. The improvement in recruitment with simplification of the distributed message suggests that the method of recruitment primarily accounts for the low interest and slow enrollment. However, disease burden, discomfort with technology, or the absence of an intervention may have also contributed. There was unequal representation of the diagnoses with fewer individuals and lower symptom severity among those with DLB or CBS. This recruitment differential is out of proportion to the number of individuals with each diagnosis in Fox Trial Finder; however, it is unclear whether disease-specific features or methods specific to this study drove lower recruitment among these groups. The examination was limited to visual assessment with investigators unable to assess findings (e.g., reflexes) that may be key to diagnosis. In addition, the second movement disorder specialist's diagnosis was based on review of a recorded video rather than an independent assessment. This may have biased the results toward greater investigator concordance. Finally, the study relied on participant self-reported diagnosis without in-person diagnostic concordance or confirmation with the participant's clinician.
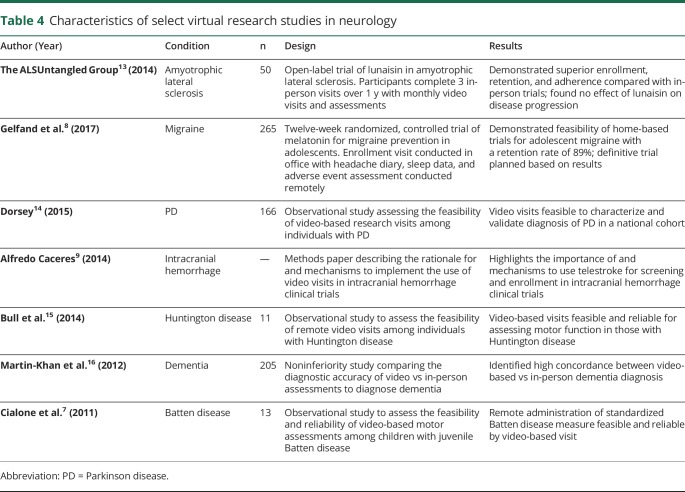
Despite these limitations, this work is the largest study to date of video visits among those with the atypical parkinsonian syndromes. This is particularly relevant, given the rising recognition of the use of telemedicine and video research visits in natural history studies and clinical trials among those with PD, dementia, and migraine, among others (table 4).4,6,8–10 Our data indicate that similar studies are feasible among those with an atypical parkinsonian syndrome. The use of remote video-based visits could substantially enhance research participation among this population by reducing burden and expanding access.
Table 4.
Characteristics of select virtual research studies in neurology
The study also furthers our understanding of the utility and limitations of video research visits in neurodegenerative conditions more broadly. The results are relevant to numerous other diseases, as the majority of patients with neurodegenerative conditions have similar demographics and clinical characteristics. Our data suggest that reliance on self-reported diagnosis is feasible and reliable as demonstrated by the high concordance between investigator consensus diagnosis and self-reported diagnosis. Still, concerns remain, particularly among those with conditions that require in-person examination or prolonged follow-up. Partnering with local clinicians may allow further diagnostic validation, confirmation of testing performed and natural history, and clarification of examination features unable to be performed by video visit. This can likely be done without compromising recruitment. Reassuringly, despite advanced age and high disability among many of our participants, the use of technology was not a major concern; the improved convenience was in fact a driver of satisfaction among the cohort.
We have taken the first step in demonstrating the feasibility of video research visits in those with an atypical parkinsonian syndrome. Although video-based assessment may be insufficient to make an initial diagnosis, virtual visits can be used to screen participants, replace interim trial visits, or follow study cohorts for the long term. Still, further work exploring these methods, particularly related to tracking disease progression over time, is necessary. Video visits could reduce burden, enhance recruitment and retention, and expand access to research for individuals with the atypical parkinsonian syndromes and other neurodegenerative diseases. Ultimately, this has the potential to broaden our phenotypic characterization of these conditions, enhance our understanding of their natural history, and streamline the evaluation of novel experimental therapeutics.
Appendix. Authors

Study funding
This study was funded by The Michael J. Fox Foundation for Parkinson's Research (GR 503832).
Disclosure
C. G. Tarolli, G.A. Zimmerman, S. Goldenthal, B. Feldman, E.R. Dorsey, and J.L. Adams received salary support from The Michael J. Fox Foundation for Parkinson's Research, which funded this work. S. Berk, B. Siddiqi, C.M. Kopil, and S. Chowdhury are employees of The Michael J. Fox Foundation for Parkinson's Research, which funded this work. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ There is limited evidence for the use of video-based research visits among the atypical parkinsonian syndromes
→ This study evaluated the diagnostic concordance between video-based vs self-reported diagnoses of these conditions and assessed participant satisfaction with the visits
→ There was excellent agreement between providers and good agreement between provider consensus diagnosis and self-reported diagnosis
→ Participants were overwhelmingly satisfied with the video-based visits, particularly the improved convenience
→ Video-based visits could reduce burden and extend the reach of clinical care and research among those with the atypical parkinsonian syndromes
References
- 1.Tsai RM, Boxer AL. Clinical trials: past, current, and future for atypical Parkinsonian syndromes. Semin Neurol 2014;34:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boxer AL, Lang AE, Grossman M, et al. Davunetide for progressive supranuclear palsy: a multicenter, randomized, double-blind, placebo controlled trial. Lancet Neurol 2014;13:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey ER, Venuto C, Venkataraman V, Harris DA, Kieburtz K. Novel methods and technologies for 21st-century clinical trials: a review. JAMA Neurol 2015;72:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsey ER, Wagner JD, Bull MT, et al. Feasibility of virtual research visits in Fox trial finder. J Parkinsons Dis 2015;5:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarolli CG, Zimmerman G, Bull MT, et al. Virtual Research Visits in a Phase 3 Clinical Trial of Individuals with Parkinson Disease: REACT-PD. Los Angeles, CA: American Academy of Neurology Annual Meeting; 2018. [Google Scholar]
- 6.Tanner CM, Meng CC, Ravina B, et al. A practical approach to remote longitudinal follow-up of Parkinson's disease: the FOUND study. Mov Disord 2014;29:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cialone J, Augustine EF, Newhouse N, Vierhile A, Marshall FJ, Mink JW. Quantitative telemedicine ratings in Batten disease: implications for rare disease research. Neurology 2011;77:1808–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelfand AA, Qubty W, Patniyot I, et al. Home-based trials in adolescent migraine: a randomized clinical trial. JAMA Neurol 2017;74:744–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfredo Caceres J, Greer DM, Goldstein JN, et al. Enrollment of research subjects through telemedicine networks in a multicenter acute intracerebral hemorrhage clinical trial: design and methods. J Vasc Interv Neurol 2014;7:34–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Bossen AL, Kim H, Williams KN, Steinhoff AE, Strieker M. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol Telehealth 2015;3:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Pazi H, Browne P, Chan P, et al. The promise of telemedicine for movement disorders: an interdisciplinary approach. Curr Neurol Neurosci Rep 2018;18:26. [DOI] [PubMed] [Google Scholar]
- 12.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ALSUntangled Group. ALSUntangled no. 26: Lunasin, Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2014;15:622–626. [DOI] [PubMed] [Google Scholar]
- 14.Dorsey ER, Wagner JD, Bull MT, et al. Feasibility of virtual research visits in Fox Trial Finder. J Parkinsons Dis 2015;5:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull MT, Darwin K, Venkataraman V, et al. A pilot study of virtual visits in Huntington disease. J Huntingtons Dis 2014;3:189–195. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Khan M, Flicker L, Wootton R, et al. The diagnostic accuracy of telegeriatrics for the diagnosis of dementia via video conferencing. J Am Med Dir Assoc 2012;13:487.e19–487.e24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were recorded in the University of Rochester's Health Insurance Portability and Accountability Act-compliant database. Deidentified data not presented are available to qualified investigators by request for purposes of replicating procedures and results.