Abstract
Objective
To explore disease burden in Parkinson disease (PD) by evaluating the prevalence of symptoms and key disease milestones (critical events, e.g., hospitalization or frequent falls) and their association with quality of life (QOL) in those with PD.
Methods
We created and pretested an online needs assessment survey to evaluate the clinical characteristics, QOL, symptom prevalence, and critical event frequency among those with PD. We recruited individuals with self-reported Hoehn and Yahr stage II–V PD through online postings and email through the Davis Phinney Foundation. We used logistic regression to evaluate the association between a large number of uncontrolled symptoms and events on QOL.
Results
A total of 612 individuals (mean age 70.1 years, 49.8% women) completed the survey. Among respondents, 13.6% reported poor QOL. Nearly 20% of respondents reported >3 falls, and 15% of respondents had been hospitalized over the previous 6 months. Participants had an average of 5.1 uncontrolled symptoms, with 86.1% of respondents reporting at least 1 uncontrolled symptom; more than 10% of respondents reported >10 uncontrolled symptoms. Depression, confusion, pain, and bothersome hallucinations were associated with poor QOL among the cohort.
Conclusions
In this national survey of individuals with PD, we identified poor QOL, frequent critical events, and numerous uncontrolled symptoms among a substantial proportion of respondents. Although motor symptoms were common, only nonmotor symptoms were associated with poor QOL. Many of these symptoms and events are treatable or preventable, highlighting the need for better identification and management to improve QOL among those with PD.
Parkinson disease (PD) is a chronic neurodegenerative condition associated with an increased risk of mortality and substantial morbidity.1,2 Motor symptoms including tremor, stiffness, and loss of mobility are well recognized over the course of the disease. Nonmotor symptoms including pain, comorbid psychiatric disease, and sleep disturbances are also common3–6; however, many remain underrecognized and undertreated with a negative effect on quality of life (QOL).7–12 In addition, key disease milestones (critical events) that herald advancing disease, like frequent falls, hospitalizations, and the development of hallucinations, increase disease burden.13–15 Previous work has evaluated the effect of isolated symptoms on QOL, but few have systematically evaluated a large number of symptoms and their relative contribution to poor QOL across disease stages. To address these questions, we conducted a national needs assessment of individuals with PD in the United States to (1) identify the prevalence of symptoms and critical events among those with PD; (2) evaluate the relationship between symptoms and critical events with QOL; and (3) characterize differences in symptoms and QOL between those with early vs advanced disease.
Methods
Survey creation
We created an online survey to evaluate the interaction between PD features and QOL using multiple strategies. The survey consists of novel items and questions derived from previously validated questionnaires (full survey in appendix e-1, links.lww.com/CPJ/A141).16,17 We selected symptoms and critical events for inclusion based on literature review, expert opinion (palliative care specialists and movement disorder neurologists), and patient interviews. The developing survey drafts were pretested with content and survey experts and iteratively improved to enhance quality control for content, readability, survey navigation, and item clarity.
We conducted key informant interviews with 6 individuals with Hoehn and Yahr stage II–IV PD to optimize the survey before broader distribution. Four interviews were conducted with the participant alone, and 2 were conducted with the assistance of a care partner. Key informant interview participants completed the online survey in the office. Following survey completion, participants completed a short, recorded interview with the study investigator consisting of open-ended questioning about general opinions on the survey followed by closed-ended questions on preferences on survey items.
During and following each interview, the investigator recorded and collated responses in a spreadsheet. Simple changes (e.g., syntax clarification) were made to the survey following each key informant interview. More substantive changes (e.g., item removal) were delayed until reaching majority participant consensus. Key informant interviews continued until reaching majority consensus on all survey items. We made 12 changes to the needs assessment survey based on participant feedback with the majority (n = 8) being nonsubstantive clarification of survey language. The remainder involved selection of one question format over another (e.g., check box vs yes/no on symptom questionnaire) based on consensus participant preference.
National needs assessment survey
The final survey was administered using Research Electronic Data Capture, a secure web application that provides a toolset for effective data collection and management.18,19 Participants were recruited through postings in the movement disorders clinic at the University of Rochester and on the Michael J. Fox Foundation Fox Trial Finder20 and through direct email communication by the Davis Phinney Foundation; the email was sent to 37,743 recipients (includes individuals with and without PD) with 9,296 individuals opening the message and 2,384 clicks on the survey link over 2 emails. Participants accessed the survey via weblink. We aimed to recruit at least 500 individuals to complete the survey. To increase recruitment of those with more advanced disease, care partners were allowed to assist with survey completion or complete the survey on behalf of the individual with PD.
Participants completed a 4-item screening survey (appendix e-1, links.lww.com/CPJ/A141) to confirm age, PD diagnosis, and ambulatory status corresponding to Hoehn and Yahr stages I–V. Participants meeting the inclusion criteria (English-speaking adults with self-reported Hoehn and Yahr state II–V PD17 of at least 5-year duration) were directed to the needs assessment survey.
The needs assessment survey included questions on demographics, disease stage, overall QOL, symptoms (present and uncontrolled), critical events, care utilization, advance directive completion, goals of current PD care, and knowledge and opinions about palliative care (appendix e-1, links.lww.com/CPJ/A141). The QOL question was derived from the McGill Quality of Life Questionnaire Single-Item Subscore,21 a validated single-item score found to correlate with more detailed assessments of QOL.22,23 The score is scaled from 0 to 10, with 0 indicating very poor QOL and 10 indicating excellent QOL.
Survey data analysis
We analyzed demographic data, disease characteristics, care utilization, and QOL descriptively. We assessed the prevalence of symptoms (overall and uncontrolled) and critical events among respondents. We also evaluated the correlation between the number of reported uncontrolled symptoms and QOL using a Spearman correlation (α = 0.05).
We evaluated which uncontrolled symptoms were most associated with poor QOL. As defined in previous work, we used a score of ≤4 on the McGill Quality of Life Single-Item Subscore as corresponding with poor QOL.16 We first evaluated which uncontrolled symptoms were associated with poor QOL creating individual multivariate logistic regression models for each symptom using α = 0.002 to account for multiple comparisons. We included ambulatory status, disease duration, age, sex, and the 4 most prevalent uncontrolled symptoms among the cohort as covariates in the models.
We subsequently evaluated the association between uncontrolled symptom and critical events on QOL accounting for other relevant uncontrolled symptoms in multivariate logistic regression analysis. We initially evaluated which of the 21 uncontrolled symptoms and 9 critical events were associated with poor QOL in χ2 analyses, using p ≤ 0.2. Features significantly associated with poor QOL in bivariate analyses were subsequently evaluated for multicollinearity, and none were identified. We included the 16 identified symptoms and 4 identified events in a forward selection multivariate logistic regression model (α to enter = 0.2, α to remain = 0.05) to determine which were associated with poor QOL. We included participant ambulatory status, disease duration, age, and sex as covariates in the model regardless of statistical significance. We performed the analysis among respondents overall and in subgroups of those with early/midstage disease defined as Hoehn and Yahr stages II/III and late disease defined as Hoehn and Yahr stages IV/V. Ambulatory status was not included in the early and late regression models, as ambulatory status is the defining characteristic of these subgroups. Data from portions of the survey regarding opinions and knowledge of palliative care will be presented in a separate article.
Standard protocol approvals, registrations, and patient consents
The Research Subjects Review Board at the University of Rochester approved all study procedures. Key informant interview participants provided informed consent before study participation. Survey respondents were directed to an information sheet before accessing the survey.
Data availability
The study protocol and deidentified data not presented in the article will be made available to qualified investigators by request for purposes of replicating procedures and confirming results.
Results
A total of 1,706 individuals accessed the survey with 691 individuals completing the screening survey and meeting the eligibility criteria. The principal reasons for ineligibility included being in Hoehn and Yahr stages 0 or 1 (n = 412) and disease duration <5 years (n = 480). We received 612 completed surveys over approximately 2 months; we did not assess data from incomplete surveys and identified no demographic or survey-specific trends in those who did not complete the survey. Among respondents, 543 completed the survey alone, 36 completed the survey with a care partner, and 33 were completed by a care partner alone. Baseline characteristics are presented in table 1.
Table 1.
Baseline characteristics (n = 612)
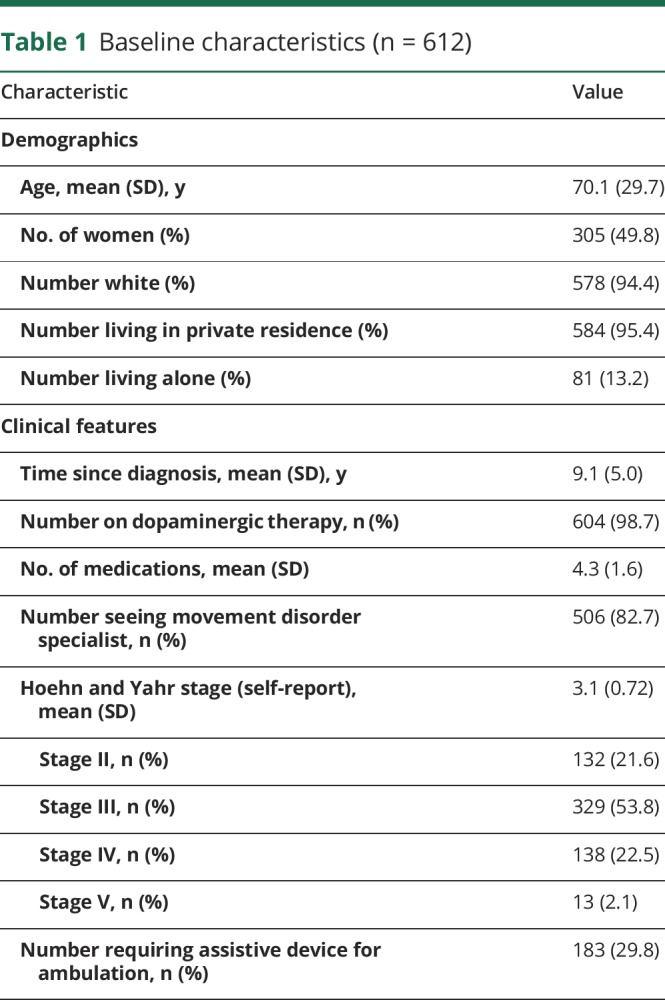
The majority of participants (n = 604 [98.7%]) received care from a neurologist within the previous year, with 506 (82.7%) participants seeing a movement disorder specialist. Respondents endorsed seeing an average of 3.5 different providers for symptoms related to their PD over the previous year (range 1–10).
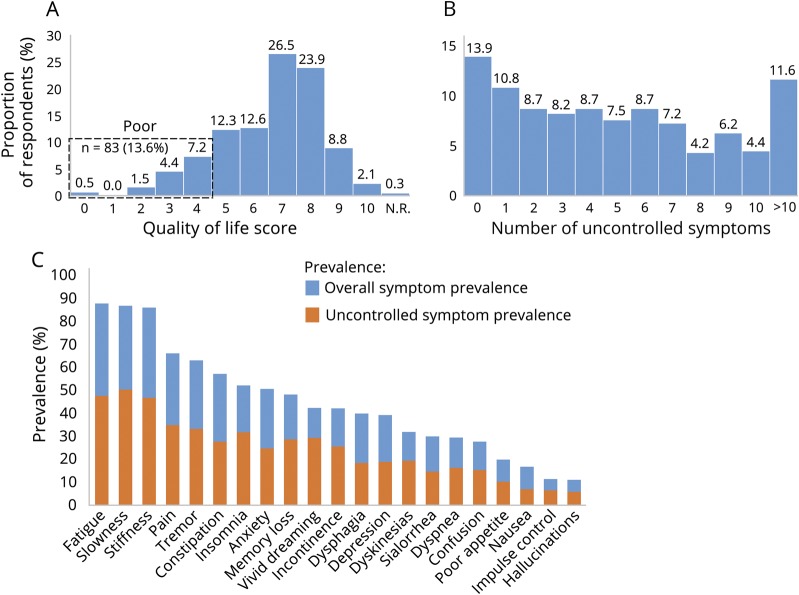
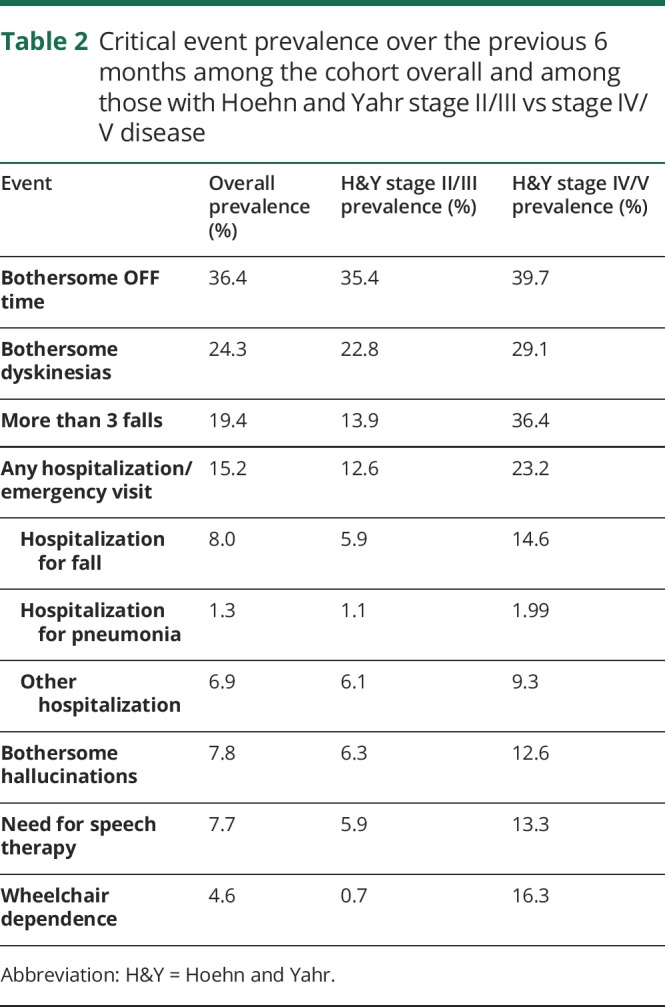
The mean (SD) QOL score on the Single-Item Subscore was 6.6 (1.8), with 83 (13.6%) participants selecting a score corresponding to poor QOL (0–4). A histogram showing the distribution of QOL ratings is presented in figure 1A. The prevalence of each critical event over the previous 6 months among the cohort overall and among those with early vs late disease is shown in table 2.
Figure 1. Quality of life and symptom burden among the cohort.
(A) Quality of life distribution as measured by the McGill Quality of Life Single-Item Subscore. N.R. = not rated. (B) Number of uncontrolled symptoms among the cohort. (C) Overall (blue) and uncontrolled (orange) symptom prevalence among the cohort.
Table 2.
Critical event prevalence over the previous 6 months among the cohort overall and among those with Hoehn and Yahr stage II/III vs stage IV/V disease
A histogram showing the distribution of number of uncontrolled symptoms among the cohort is presented in figure 1B. Participants had an average of 5.1 uncontrolled symptoms, with 86.1% of respondents reporting at least 1 uncontrolled symptom; 11.6% of respondents reported >10 uncontrolled symptoms. There was a modest inverse correlation between the number of uncontrolled symptoms and QOL (rs = −0.38, p < 0.001); a scatter plot of the relationship is shown in figure e-1 (links.lww.com/CPJ/A140). The prevalence of overall and uncontrolled symptoms among the cohort is shown in figure 1C. Fatigue, slowness, and stiffness were the most prevalent symptoms overall (87.6%, 86.6%, and 85.8%, respectively); among the entire cohort, these symptoms were uncontrolled in 47.5%, 50.3%, and 46.7%, respectively.
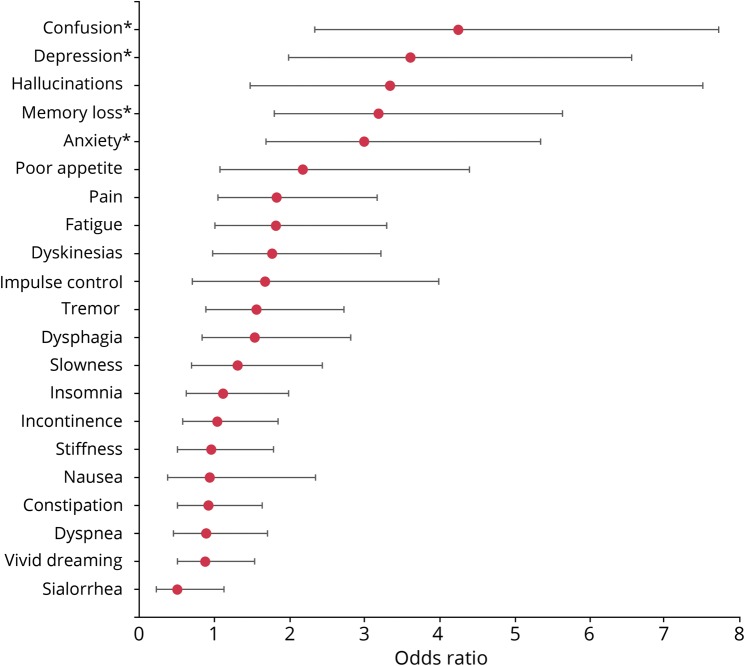
We identified 4 uncontrolled symptoms that were associated (p < 0.002) with poor QOL in the individual regression models. These include confusion (odds ratio [OR] 4.3, 95% confidence interval [CI] 2.3–7.7), depression (OR 3.6, 95% CI 2.0–6.6), memory loss (OR 3.2, 95% CI 1.8–5.6), and anxiety (OR 3.0, 95% CI 1.7–5.4). Figure 2 shows the ORs for each symptom predicting poor QOL in the individual models with 95% CIs.
Figure 2. Odds ratios of each uncontrolled symptom predicting poor quality of life in independent regression models.
*Statistically significant variable; α = 0.002 to account for multiple comparison.
Results of the stepwise regression models accounting for relevant uncontrolled symptoms in the cohort overall, those with Hoehn and Yahr stage II/III disease, and those with Hoehn and Yahr stage IV/V disease are shown in table 3. Among the cohort overall, uncontrolled pain, depression, and confusion as well as bothersome hallucinations and wheelchair dependence were associated with poor QOL. Other variables associated with poor QOL in the model included requiring an assistive device for walking ≤50% of the time and requiring an assistive device for walking >50% of the time or being a wheelchair user.
Table 3.
Multivariate regression models evaluating the relationship between respondent characteristics and poor quality of life among the cohort overall; those with early motor disease (Hoehn and Yahr stages II/III); and those with late motor disease (Hoehn and Yahr stages IV/V)
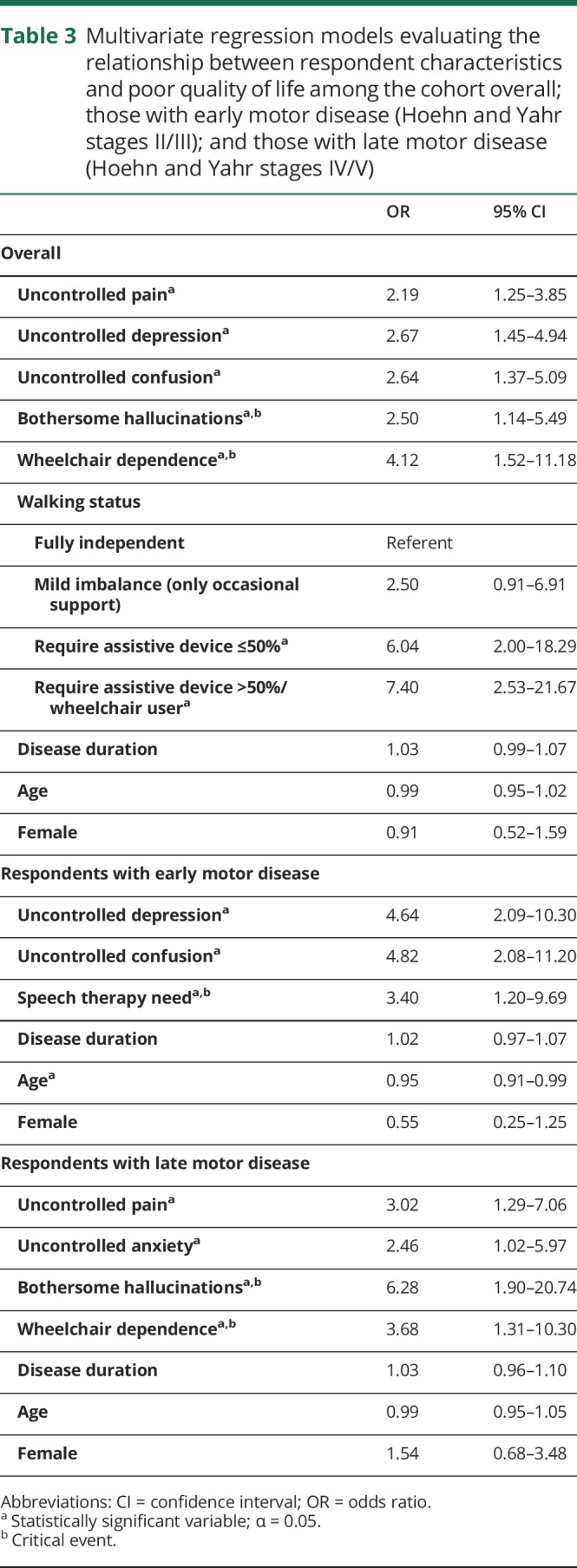
Among those with Hoehn and Yahr stage II/III disease, uncontrolled depression, uncontrolled confusion, and the need for speech therapy were associated with poor QOL. Older age was identified as being protective against poor QOL in those with Hoehn and Yahr stage II/III disease with a modest effect size. Among those with advanced disease, uncontrolled pain, uncontrolled anxiety, bothersome hallucinations, and wheelchair dependence were associated with poor QOL.
Discussion
In this national study, individuals with PD had high disease burden with frequent critical events, numerous uncontrolled symptoms, and a substantial proportion of respondents reporting poor QOL. Uncontrolled nonmotor symptoms had a negative association with QOL. This is particularly relevant as many of these symptoms including pain, depression, and hallucinations may be treatable. In addition, given the selective nature of online surveys and a majority of respondents receiving subspecialty care for their PD, burden and suffering could be even higher among the broader PD population.
Symptom and critical event prevalence were similar to previous reports for pain,24 depression,11,25 decreased appetite,26 and hallucinations,27 among others. However, unlike previous studies, we evaluated the association between a large number of uncontrolled symptoms and QOL to understand the relative effect of each.2 Although motor symptoms including slowness, stiffness, and tremor were some of the most prevalent among the cohort, we found that uncontrolled neuropsychiatric (depression, confusion, and hallucinations) and somatic (pain) symptoms were more highly associated with poor QOL. In fact, except those with impaired ambulatory status, we identified only nonmotor symptoms as being associated with poor QOL in both individual and stepwise regression analyses.
Our survey identified high symptom burden in those with both early and late motor disease; however, we found important differences in specific symptoms that had the greatest relationship with QOL between disease stages. Depression was a major predictor of poor QOL among the cohort overall, although the effect was driven by its high association with poor QOL in those with early motor disease. In contrast, the presence of uncontrolled pain, anxiety, and hallucinations were predictive of poor QOL exclusively among those with late motor disease.
The study has limitations. First, we relied on self-report of PD diagnosis and motor status, and around 5% of surveys were completed by care partners alone with a higher representation among those with more advanced disease (46% of those with H&Y stage V). This may limit the reliability of QOL and symptom report among these respondents. Second, we limited recruitment to those with a disease duration of ≥5 years to limit inclusion of those with other neurodegenerative conditions. This may have reduced the variability in motor severity and decreased our ability to identify a relationship between motor symptoms and QOL. Third, nearly 95% of respondents were white and lived in a private residence, and the survey was only available online, which limits applicability to the broader PD population. Given the differential profile and course of PD in patients from underrepresented populations, additional research is needed to understand the different burden of disease in these different populations. However, previous work suggests that minority (nonwhite) patients are diagnosed at later disease stages, less likely to be treated by a neurologist, and less likely to receive symptomatic therapy for motor and nonmotor symptoms, suggesting that burden may be even higher among these groups.28–30 Finally, given the cross-sectional design of the study, we cannot confirm a causal link between symptom burden and QOL.
Despite these limitations, this study demonstrates the high symptom burden among those with PD and adds further evidence for the influence of nonmotor symptoms on QOL over the course of the condition. These results highlight the importance of identifying nonmotor symptoms in those with PD, particularly because many of these symptoms and events may be treatable or preventable. Unfortunately, commonly used symptom assessment tools, QOL measures, and current care models in PD place a strong emphasis on motor symptoms and mobility.31–33 Instead, we must consider a broader picture of disease burden in PD that prioritizes individual patient disease stage and needs. Future work should focus on the development of novel needs assessment tools that are exhaustive and patient centered to explore the multitude of symptoms associated with the condition. This approach will allow us to more effectively identify and manage symptoms to reduce disease burden in those with PD.
Acknowledgment
The authors thank the Davis Phinney Foundation for their assistance in distributing the study survey.
Appendix. Authors
Footnotes
Podcast: NPub.org/NCP/podcast10-1
Study funding
Study funded by the American Academy of Neurology Institute (GR 503960).
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Hassan A, Wu SS, Schmidt P, et al. What are the issues facing Parkinson's disease patients at ten years of disease and beyond? Data from the NPF-QII study. Parkinsonism Relat Disord 2012;18(suppl 3):S10–S14. [DOI] [PubMed] [Google Scholar]
- 2.Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 2000;15:1112–1118. [DOI] [PubMed] [Google Scholar]
- 3.Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson's disease: a controlled cross-sectional study. Parkinsonism Relat Disord 2004;10:129–136. [DOI] [PubMed] [Google Scholar]
- 4.Ha AD, Jankovic J. Pain in Parkinson's disease. Mov Disord 2012;27:485–491. [DOI] [PubMed] [Google Scholar]
- 5.Richard IH, Kurlan R. The under-recognition of depression in Parkinson's disease. Neuropsychiatr Dis Treat 2006;2:349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravina B, Camicioli R, Como PG, et al. The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–510. [DOI] [PubMed] [Google Scholar]
- 8.Higginson IJ, Gao W, Saleem TZ, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLoS One 2012;7:e46327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash KM, Nadkarni NV, Lye WK, Yong MH, Tan EK. The impact of non-motor symptoms on the quality of life of Parkinson's disease patients: a longitudinal study. Eur J Neurol 2016;23:854–860. [DOI] [PubMed] [Google Scholar]
- 10.Hu M, Cooper J, Beamish R, et al. How well do we recognise non-motor symptoms in a British Parkinson's disease population? J Neurol 2011;258:1513–1517. [DOI] [PubMed] [Google Scholar]
- 11.Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci 2006;248:151–157. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–245. [DOI] [PubMed] [Google Scholar]
- 13.Bryant MS, Rintala DH, Hou JG, Protas EJ. Relationship of falls and fear of falling to activity limitations and physical inactivity in Parkinson's disease. J Aging Phys Act 2015;23:187–193. [DOI] [PubMed] [Google Scholar]
- 14.Schneider RB, Iourinets J, Richard IH. Parkinson's disease psychosis: presentation, diagnosis and management. Neurodegener Dis Manag 2017;7:365–376. [DOI] [PubMed] [Google Scholar]
- 15.Hassan A, Wu SS, Schmidt P, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson's disease. Parkinsonism Relat Disord 2013;19:949–954. [DOI] [PubMed] [Google Scholar]
- 16.Gofton TE, Kumar H, Roberts-South A, Speechley M, Jog MS. Validity, reliability, and insights from applying the McGill Quality of Life Questionnaire to People Living with Parkinson's Disease (MQoL-PD). J Palliat Care 2015;31:213–220. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.University of Rochester Medical Center. REDCap. 2019. Available at: redcap.urmc.rochester.edu/redcap/. Accessed April 1, 2019.
- 20.Foundation MJF. Michael J Fox Foundation Fox Trial Finder. 2017. Available at: foxtrialfinder.michaeljfox.org/. Accessed November 11, 2017.
- 21.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 1995;9:207–219. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 1997;11:3–20. [DOI] [PubMed] [Google Scholar]
- 23.Lo RS, Woo J, Zhoc KC, et al. Cross-cultural validation of the McGill quality of life questionnaire in Hong Kong Chinese. Palliat Med 2001;15:387–397. [DOI] [PubMed] [Google Scholar]
- 24.Broen MP, Braaksma MM, Patijn J, Weber WE. Prevalence of pain in Parkinson's disease: a systematic review using the modified QUADAS tool. Mov Disord 2012;27:480–484. [DOI] [PubMed] [Google Scholar]
- 25.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 2008;23:183–189; quiz 313. [DOI] [PubMed] [Google Scholar]
- 26.Cersosimo MG, Raina GB, Pellene LA, Micheli FE, Calandra CR, Maiola R. Weight loss in Parkinson's disease: the relationship with motor symptoms and disease progression. Biomed Res Int 2018;2018:9642524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Shen B, Lu L, et al. Prevalence and risk factors for visual hallucinations in Chinese patients with Parkinson's disease. J Neurol Sci 2017;372:471–476. [DOI] [PubMed] [Google Scholar]
- 28.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson's disease. Ann Neurol 2009;66:142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng EM, Siderowf AD, Swarztrauber K, et al. Disparities of care in veterans with Parkinson's disease. Parkinsonism Relat Disord 2008;14:8–14. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 32.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4:241–248. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhuri KR, Yates L, Martinez-Martin P. The non-motor symptom complex of Parkinson's disease: a comprehensive assessment is essential. Curr Neurol Neurosci Rep 2005;5:275–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol and deidentified data not presented in the article will be made available to qualified investigators by request for purposes of replicating procedures and confirming results.