Abstract
Background
Achondroplasia (ACH), the most common form of short‐limbed skeletal dysplasia, is caused by gain‐of‐function mutations in the fibroblast growth factor receptor 3 (FGFR3) gene. More than 97% of patients result from a heterozygous p.G380R mutation in the FGFR3 gene. We present here a child who had two de novo variants in the FGFR3 on the same allele, a common p.G380R mutation and a novel p.S378N variant.
Methods
A 3‐year‐old Japanese girl born from non‐consanguineous healthy parents showed more severe clinical and radiological phenotypes than classic ACH, including severe short‐limbed short stature with marked ossification defects in the metaphysis and epiphysis, hydrocephalus and cervicomedullary compression due to foramen magnum stenosis, prolonged pulmonary hypoplasia, and significant delay in the gross motor development. Genomic DNA was extracted from the proband and whole‐exome sequencing was performed. The variants were subsequently confirmed by Sanger sequencing.
Results
Mutation analysis demonstrated that the proband had p.S378N (c.1133G>A) and p.G380R (c.1138G>A) variants in the FGFR3 gene. Both variants were not detected in her parents and therefore considered de novo. An allele‐specific PCR was developed in order to determine whether these mutations were on the same allele (cis) or on different alleles (trans). The c.1138G>A mutation was found in the PCR product generated with the primer for the mutant 1133A, but it was not detected in the product with the wild‐type 1133G, confirming that p.S378N and p.G380R variants were located on the same allele (cis).
Conclusion
This is the second case who had two FGFR3 variants in the transmembrane domain on the same allele. The p.S378N variant may provide an additive effect on the activating receptor with the p.G380R mutation and alter the protein function, which could be responsible for the severe phenotype of the present case.
Keywords: achondroplasia, allele‐specific PCR, exome sequencing, FGFR3, skeletal dysplasia
We present a case who had two de novo variants in the FGFR3 on the same allele, a common p.G380R mutation and a novel p.S378N variant. The p.S378N variant may provide an additive effect on the activating receptor with the p.G380R mutation and alter the protein function, which could be responsible for severe phenotype of the present case.
1. INTRODUCTION
Achondroplasia (ACH) is the most common form of short‐limbed skeletal dysplasia, with an estimated prevalence of one in 16,000–26,000 live births (Waller et al., 2008). ACH is caused by activating mutations in the fibroblast growth factor receptor 3 (FGFR3) gene, which is a negative regulator of longitudinal bone growth (Rousseau et al., 1994; Shiang et al., 1994). Approximately 98% of patients harbor a heterozygous p.G380R mutation that maps to the transmembrane domain of the FGFR3 gene (Bonaventure et al., 1996). Infants with ACH, thus, present relatively homogeneous skeletal features with macrocephaly with frontal bossing, midface hypoplasia, increased lumbar lordosis, limitation of elbow extension, and trident hands (Horton, Hall, & Hecht, 2007). Recently, phase 2 clinical trial using vosoritide, which is a biological analog of C‐type natriuretic peptide, demonstrated increased growth velocity with minimal side effects in children with ACH (Savarirayan et al., 2019).
Homozygous p.G380R mutations lead to a lethal skeletal phenotype with a narrow thoracic cage, neurologic deficits from cervicomedullary stenosis, and stillbirth due to severe respiratory insufficiency (Aterman, Welch, & Taylor, 1983). Several patients with two different FGFR3 mutations have been reported. The majority of these patients were compound heterozygous for the classic ACH mutation (p.G380R) and the p.N540K mutation associated with hypochondroplasia (HCH), and they showed a milder skeletal phenotype than homozygous ACH (Chitayat et al., 1999; Flynn & Pauli, 2003; Huggins et al., 1999; Prinos, Costa, Sommer, Kilpatrick, & Tsipouras, 1995; Sommer, Young‐Wee, & Frye, 1987). Generally, these patients inherited both mutations from their affected parents.
Here, we report on a Japanese girl manifesting a more severe skeletal dysplasia than classic ACH, with two de novo FGFR3 variants, the common p.G380R mutation and the p.S378N variant, both of which were located on the same allele. To the best of our knowledge, this is the second case with two FGFR3 mutations on the same allele.
2. CLINICAL REPORT
The proband, a 3‐year‐old girl, was the first child of non‐consanguineous Japanese parents. Their younger sister was normal. Screening ultrasound at 33 weeks of trimester showed increased biparietal diameter (85.9 mm: +1.2 SD) and shortening of the femur length (40.9 mm: −5.8 SD). The infant was delivered at 37 weeks of gestation with a birth weight of 2.690 g (+0.3 SD), length of 40.0 cm (−3.1 SD), and head circumference of 33.6 cm (+0.6 SD), from a 33‐year‐old mother and a 34‐year‐old father. The Apgar scores were four and six at 1 and 5 min, respectively. She showed severe shortening of the upper and lower extremities with redundant skin folds, a small chest, frontal bossing, midface hypoplasia, and trident hands. Neonatal radiographs showed a small thorax with short ribs, abdominal distension (Figure 1a), hypoplastic ilium with narrow sciatic notch, proximal femoral radiolucencies, and metaphyseal cupping of the long tubular bones (Figure 1b). Based on these clinical and radiological characteristics, she was tentatively diagnosed as ACH.
Figure 1.
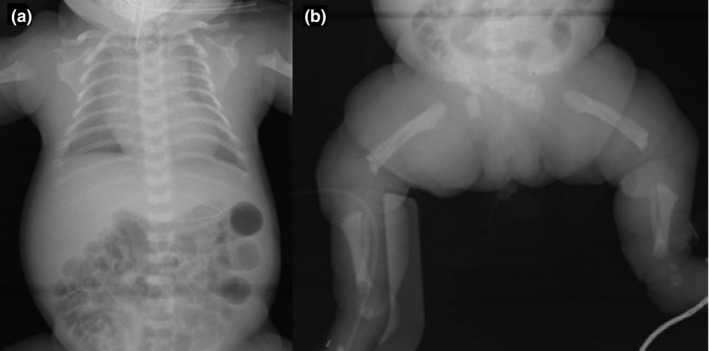
Neonatal radiographs. Anteroposterior radiograph of the thorax and vertebrae demonstrated narrow rib cage, platyspondyly, and narrow interpediculate distance of the lumbar spine (a). Anteroposterior radiograph of the pelvis and lower limb showed squared iliac wings, narrow sacrosciatic notches, shortened long bones with metaphyseal irregularities, proximal femoral translucencies, and bowed fibulae (b)
She showed evident signs of respiratory distress and required intubation with an artificial respirator at a neonatal intensive care unit. Craniocervical magnetic resonance imaging (MRI) demonstrated hydrocephalus and foramen magnum stenosis with cervicomedullary compression (Figure 2a). She underwent a surgical decompression of craniocervical junction at the age of 3 months for central respiratory insufficiency. However, she needed all‐day continuous positive airway pressure (CPAP) until 6 months of age and night‐time CPAP, thereafter, due to persistent pulmonary hypoplasia. In addition to respiratory problems, the linear growth was severely delayed with a weight of 6.2 kg (−2.5 SD) and a height of 56.3 cm (−6.8 SD) at 1 year of age, and a weight of 8.8 kg (−2.6 SD) and a height of 64.7 cm (−7.8 SD) at 2 year 9 months of age. According to the growth chart of Japanese ACH children (Tachibana, Suwa, Nishiyama, & Matsuda, 1997), her height was −3.7 SD at 1 year of age and −4.6 SD at 2 year 9 months of age (Figure 3). Her gross motor development was markedly delayed, as she was not sitting independently at 3 years of age. Cognitive and language development were also delayed.
Figure 2.
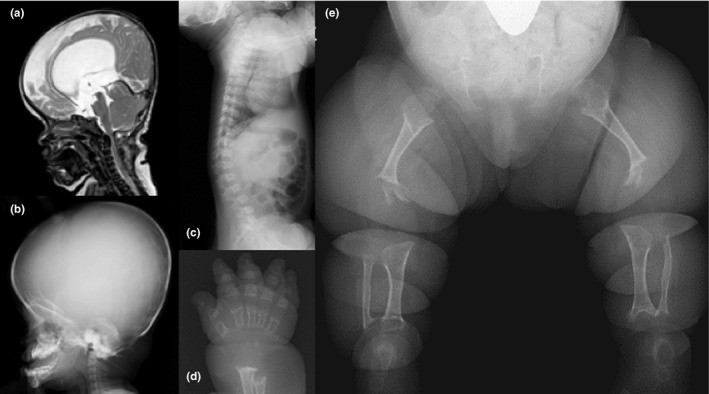
Image findings during infancy. Magnetic resonance imaging of the head and cervical spine at the age of 3 months indicated hydrocephalus and foramen magnum stenosis (a). Lateral radiograph of the skull at the age of 1 year showed a large skull with relatively small cranial base, hypoplastic maxilla, and protruded mandible (b). Lateral radiograph of the spine at the age of 1 year demonstrated mild platyspondyly with tongue‐like protrusion of the lumbar spine and extremely shortened ribs with metaphyseal flaring (c). Anteroposterior radiograph of the right hand at the age of 1 year showed generalized brachydactyly with marked metaphyseal irregularities (d). Anteroposterior radiograph of the lower limbs at the age of 2.7 years demonstrated extremely shortened and thin long tubular bones with deficient ossifications at the metaphysis and epiphysis (e)
Figure 3.
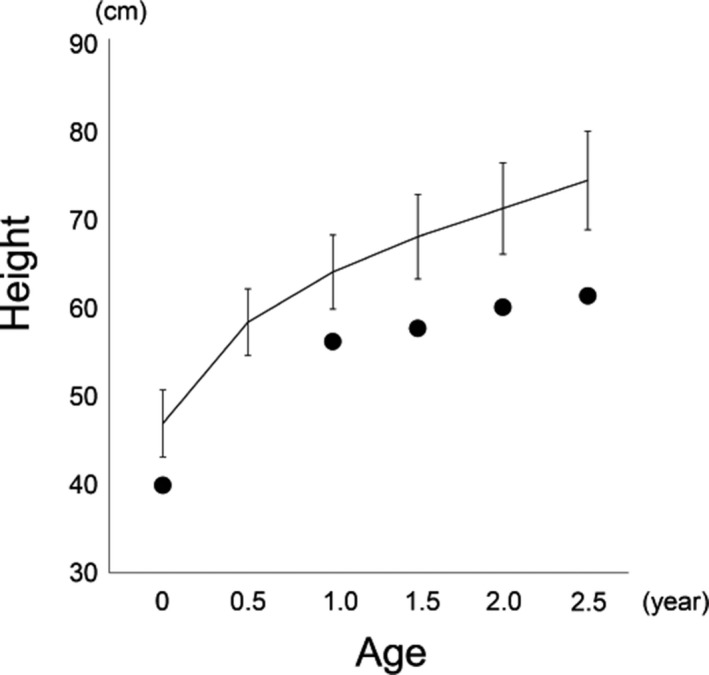
Growth curves of the proband and standard Achondroplasia (ACH) patients. The dots and line showed the height of proband and the mean ± 2 SD of standard ACH patients (Tachibana et al., 1997), respectively
Craniofacial dysmorphisms including frontal bossing, hypoplastic maxilla, and short cranial base were remarkable (Figure 2b). Thoracolumbar kyphosis and mild platyspondyly with central tongue‐like protrusions were observed on the lateral spine radiograph (Figure 2c). Brachydactyly with metaphyseal cupping of phalangeal bones was severe (Figure 2d). In the long tubular bones of the lower limbs, there were not only shortening but also poor epiphyseal and metaphyseal ossifications, proximal femoral radiolucencies, and slender diaphysis (Figure 2e).
3. MUTATION ANALYSIS
After informed consent was obtained from her parents, genomic DNAs were purified from peripheral blood of the proband and saliva of her parents using the QIAamp DNA Blood Midi kit (Qiagen, Inc.). Considering the possibility of other skeletal dysplasia than ACH from her severe phenotype, whole‐exome sequencing was performed on the proband using the Sure Select v.6 whole exon target enrichment system (Agilent) and HiSeq2500 platform (Illumina). Variant annotation revealed that the proband had p.S378N (c.1133G>A) and p.G380R (c.1138G>A) in the FGFR3 gene. Next, we performed Sanger sequencing using the forward primer (5'‐cctcaacgcccatgtcttt‐3') and the reverse primer (5'‐aggcagctcagaacctggta‐3') located in exon‐intron boundaries using the Applied Biosystems 3730xl DNA Analyzer (Thermo Fisher Scientific). The two FGFR3 variants were confirmed in the proband (Figure 4a), while there were no mutations in her parents.
Figure 4.
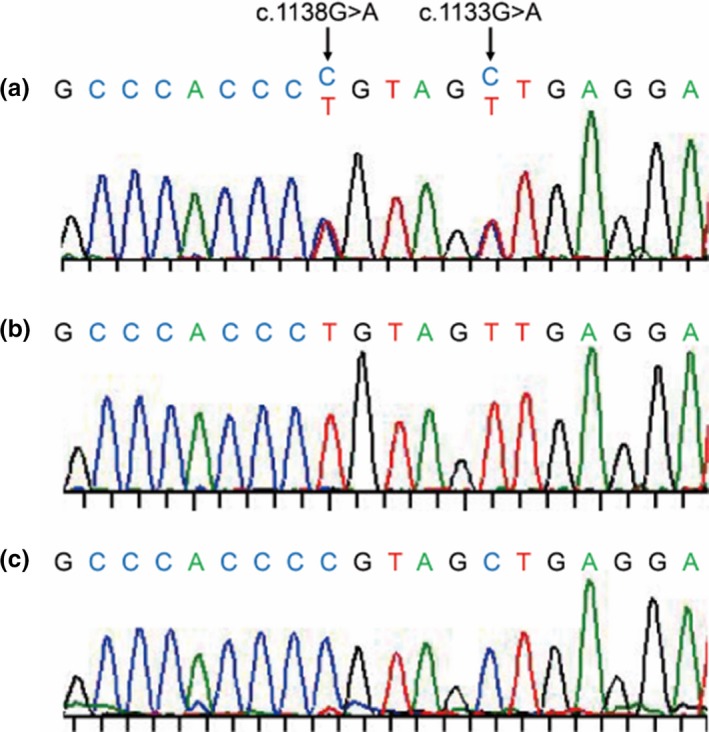
Sanger sequencing of the proband in the region about the mutations in the FGFR3 gene. There were two heterozygous mutations of the c.1133G>A and the c.1138G>A (a). The allele‐specific PCR revealed that the c.1133G>A and the c.1138G>A were in the same allele (b), while no mutations were detected in another allele (c)
The alternative forward primers for the allele‐specific PCR were created with 3'‐ends overlapping position 1133 of the FGFR3 sequence (5'‐tgtgtatgcaggcatcctcag‐3' for wild‐type 1133G and 5'‐tgtgtatgcaggcatcctcaa‐3' for mutant 1133A). The PCR products were obtained by these alternative forward primers and the reverse primer (5'‐aggcagctcagaacctggta‐3') in the exon‐intron boundary with sequencing by the reverse primer. The c.1138G>A mutation was found in the PCR product generated with the primer for the mutant 1133A (Figure 4b), but it was not detected in the product with wild‐type 1133G (Figure 4c), confirming that p.S378N and p.G380R variants were located on the same allele.
4. DISCUSSION
Here, we report on a child who had p.S378N and p.G380R variants on the same allele in the FGFR3 gene. Clinical and radiological findings of the current subject were more severe than classic ACH children but milder than homozygous ACH or thanatophoric dysplasia. Actually, she could survive irrespective of having moderate respiratory distress, but her height was apparently shorter than the standard of ACH children. Shortening was more marked in the lower limbs rather than in the trunk, with distinct findings of secondary ossification defects. In addition to epi‐metaphyseal involvements, the shafts of the long tubular bones were extremely thin, suggesting the impairment of intramembranous ossification. The motor milestones of the current subject were too delayed compared to those of standard ACH children, which are usually delayed due to muscular hypotonia (Trotter, Hall, & American Academy of Pediatrics Committee on Genetics, 2005). Moreover, she had severe complications, including hydrocephalus, pulmonary hypoplasia, and cervicomedullary compression. Rump et al. (Rump et al., 2006) reported a severe ACH infant with a similar degree of severity and complications to our subject, who had p.L377R and p.G380R variants on the same allele. Although skeletal phenotypes were more severe in our subject, these two children may have the same molecular mechanism of FGFR3 activation.
The FGFR3 receptor with p.G380R was activated via a delay in the down‐regulation of the receptor (Monsonego‐Ornan, Adar, Feferman, Segev, & Yayon, 2000). Patients with this common mutation show relatively homogeneous skeletal phenotypes, although the rate of associated complications varies. Since there have been no case reports describing the patients who had the p.L377R or the p.S378N variant independently, we do not know whether these variants are pathogenic or not. A previous in vitro study by ToxR plasmids carrying the FGFR3 transmembrane domain demonstrated that the substitutions of the p.S378 to glycine, alanine, cysteine, or isoleucine showed no or very little changes in the dimerization of the receptor (Mudumbi, Julius, Herrmann, & Li, 2013), although the effect of the p.S378N was not examined. They also demonstrated that p.S378I significantly altered the dimerization propensity of the receptor with the p.A391E, which is a pathogenic mutation causing Crouzon syndrome with acanthosis nigricans. Considering a severe phenotype of the current subject, we speculate that the p.S378N may provide an additive effect on the activating receptor with the p.G380R mutation and alter the protein function.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was supported by AMED under Grant Number JP17ek0109281 (TO).
Nagata T, Matsushita M, Mishima K, et al. Severe achondroplasia due to two de novo variants in the transmembrane domain of FGFR3 on the same allele: A case report. Mol Genet Genomic Med. 2020;8:e1148 10.1002/mgg3.1148
REFERENCES
- Aterman, K. , Welch, J. P. , & Taylor, P. G. (1983). Presumed homozygous achondroplasia. A review and report of a further case. Pathology, Research and Practice, 178(1), 27–39. 10.1016/S0344-0338(83)80082-X [DOI] [PubMed] [Google Scholar]
- Bonaventure, J. , Rousseau, F. , Legeai‐Mallet, L. , Le Merrer, M. , Munnich, A. , & Maroteaux, P. (1996). Common mutations in the fibroblast growth factor receptor 3 (FGFR 3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. American Journal of Medical Genetics, 63(1), 148–154. [DOI] [PubMed] [Google Scholar]
- Chitayat, D. , Fernandez, B. , Gardner, A. , Moore, L. , Glance, P. , Dunn, M. , … Allingham‐Hawkins, D. (1999). Compound heterozygosity for the achondroplasia‐hypochondroplasia FGFR3 mutations: Prenatal diagnosis and postnatal outcome. American Journal of Medical Genetics, 84(5), 401–405. [DOI] [PubMed] [Google Scholar]
- Flynn, M. A. , & Pauli, R. M. (2003). Double heterozygosity in bone growth disorders: Four new observations and review. American Journal of Medical Genetics: Part A, 121A(3), 193–208. 10.1002/ajmg.a.20143 [DOI] [PubMed] [Google Scholar]
- Horton, W. A. , Hall, J. G. , & Hecht, J. T. (2007). Achondroplasia. The Lancet, 370(9582), 162–172. 10.1016/S0140-6736(07)61090-3 [DOI] [PubMed] [Google Scholar]
- Huggins, M. J. , Smith, J. R. , Chun, K. , Ray, P. N. , Shah, J. K. , & Whelan, D. T. (1999). Achondroplasia‐hypochondroplasia complex in a newborn infant. American Journal of Medical Genetics, 84(5), 396–400. [DOI] [PubMed] [Google Scholar]
- Monsonego‐Ornan, E. , Adar, R. , Feferman, T. , Segev, O. , & Yayon, A. (2000). The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand‐mediated receptor activation from down‐regulation. Molecular and Cellular Biology, 20(2), 516–522. 10.1128/MCB.20.2.516-522.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumbi, K. C. , Julius, A. , Herrmann, J. , & Li, E. (2013). The pathogenic A391E mutation in FGFR3 induces a structural change in the transmembrane domain dimer. Journal of Membrane Biology, 246(6), 487–493. 10.1007/s00232-013-9563-6 [DOI] [PubMed] [Google Scholar]
- Prinos, P. , Costa, T. , Sommer, A. , Kilpatrick, M. W. , & Tsipouras, P. (1995). A common FGFR3 gene mutation in hypochondroplasia. Human Molecular Genetics, 4(11), 2097–2101. 10.1093/hmg/4.11.2097 [DOI] [PubMed] [Google Scholar]
- Rousseau, F. , Bonaventure, J. , Legeai‐Mallet, L. , Pelet, A. , Rozet, J.‐M. , Maroteaux, P. , … Munnich, A. (1994). Mutations in the gene encoding fibroblast growth factor receptor‐3 in achondroplasia. Nature, 371(6494), 252–254. 10.1038/371252a0 [DOI] [PubMed] [Google Scholar]
- Rump, P. , Letteboer, T. , Gille, J. , Torringa, M. , Baerts, W. , van Gestel, J. , … van Essen, A. J. (2006). Severe complications in a child with achondroplasia and two FGFR3 mutations on the same allele. American Journal of Medical Genetics: Part A, 140(3), 284–290. 10.1002/ajmg.a.31084 [DOI] [PubMed] [Google Scholar]
- Savarirayan, R. , Irving, M. , Bacino, C. A. , Bostwick, B. , Charrow, J. , Cormier‐Daire, V. , … Hoover‐Fong, J. (2019). C‐type natriuretic peptide analogue therapy in children with achondroplasia. New England Journal of Medicine, 381(1), 25–35. 10.1056/NEJMoa1813446 [DOI] [PubMed] [Google Scholar]
- Shiang, R. , Thompson, L. M. , Zhu, Y.‐Z. , Church, D. M. , Fielder, T. J. , Bocian, M. , … Wasmuth, J. J. (1994). Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell, 78(2), 335–342. 10.1016/0092-8674(94)90302-6 [DOI] [PubMed] [Google Scholar]
- Sommer, A. , Young‐Wee, T. , Frye, T. , & Reynolds, J. F. (1987). Achondroplasia‐hypochondroplasia complex. American Journal of Medical Genetics, 26(4), 949–957. 10.1002/ajmg.1320260426 [DOI] [PubMed] [Google Scholar]
- Tachibana, K. , Suwa, S. , Nishiyama, S. , & Matsuda, I. (1997). Height of Japanese achondroplasia patients based on a nationwide investigation. Syounikashinryou, 60, 1363–1369 (Japanese). [Google Scholar]
- Trotter, T. L. , Hall, J. G. , & American Academy of Pediatrics Committee on Genetics . (2005). Health supervision for children with achondroplasia. Pediatrics, 116(3), 771–783. 10.1542/peds.2005-1440 [DOI] [PubMed] [Google Scholar]
- Waller, D. K. , Correa, A. , Vo, T. M. , Wang, Y. , Hobbs, C. , Langlois, P. H. , … Hecht, J. T. (2008). The population‐based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. American Journal of Medical Genetics: Part A, 146A(18), 2385–2389. 10.1002/ajmg.a.32485 [DOI] [PMC free article] [PubMed] [Google Scholar]


