Abstract
Background
The role of meteorin (METRN) in colorectal cancer has not been reported previously. We aimed to explore the relationship between METRN and colorectal cancer (CRC) prognosis.
Methods
Data were retrieved from the Gene Expression Omnibus database. Gene expression values were log2 transformed and normalized by quantile normalization. Missing values were imputed with the R impute package. Differentially expressed genes were analyzed using the R limma package. METRN expression was compared between normal and CRC tissues and among different stages and subtypes of CRC. We assessed the relationship between METRN and KRAS/BRAF mutations in CRC. Five‐year overall (OS), disease‐free (DFS), and disease‐specific survival (DSS) rates were determined by Kaplan–Meier analysis and analyzed by log‐rank test.
Results
METRN was expressed at a higher level in CRC (p = .0011) than in normal tissues, especially in advanced stages (p = .0343). METRN expression levels were higher in the MSI (dMMR) subtype (p < .001) and usually with BRAF mutations (p < .0001). METRN overexpression was associated with poor prognosis and low OS (p = .01014), DFS (p = .0146), and DSS (p < .0001) rates.
Conclusion
METRN overexpression is a predictive factor for poor prognosis in patients with CRC.
Keywords: colorectal cancer, GEO, Meteorin, prognosis
Overexpression of meteorin (METRN) is associated with colorectal cancer and can predict poor prognosis of colorectal cancer.

1. INTRODUCTION
Colorectal cancer (CRC) is the third most common malignant tumor globally, and more than 1.3 million people are diagnosed with CRC each year (Allemani et al., 2018). It is well known that uncontrolled replication of medial colorectal epithelial cells is an important risk factor for CRC (Dziki et al., 2015). The symptoms of patients with CRC may vary from abnormal weight reduction to changes in defecation habits, due to the different stage, location, and size of tumors. Despite improvements in the diagnosis and treatment of CRC in recent years, the 5‐year survival rates of patients with CRC remain unsatisfactory. For advanced CRC, 5‐year survival rates have been reported to be less than 50% (Liu et al., 2019; Magaji, Moy, Roslani, & Law, 2017). Although an increasing number of biomarkers have been reported to predict CRC, prognostic indicators for patients with CRC remain limited.
Meteorin (METRN, OMIM number: 610,998) is a neurotrophic factor with angiogenic properties. It was first described in 2004 (Nishino et al., 2004). Previous studies have shown that METRN is mainly expressed in the nervous system, where it plays an important protective role (Jorgensen et al., 2009; Lee, Han, Lee, Park, & Kim, 2010; Wang et al., 2012). METRN prevents striatal neurons from becoming excitotoxic, and it reverses motor deficits (Jorgensen et al., 2011). It also plays an antihyperalgesia role in a chronic constriction injury rat model (Xie, Qu, Munro, Petersen, & Porreca, 2019). In some studies, however, METRN has been shown to be associated with adverse effects in humans. For example, the serum METRN levels are significantly upregulated in pregnant women and can help predict pre‐eclampsia (Garces et al., 2015). In addition, METRN has been reported to exert a regulatory function on the progression of various diseases. In recent years, METRN and the meteorin‐like (METRNL) protein, which is homologous to METRN, have been implicated as biomarkers of hematological and endocrine diseases. Nevertheless, there are few studies on the role of METRN in CRC, and the relationship between METRN expression and CRC patients’ prognosis remains unclear. Therefore, to explore the role of METRN in CRC, we performed a retrospective analysis using data from the Gene Expression Omnibus (GEO) database. We found that METRN expression was upregulated in CRC and the overexpression of METRN was associated with poor prognosis of patients with CRC. Based on these results, we suggest that METRN may be used as a biomarker for the prognosis of CRC.
2. MATERIALS AND METHODS
2.1. Editorial policies and ethical considerations
This study was approved by the Institutional Ethical Review Board of Shanghai Ninth People's Hospital, School of Medicine, Shanghai Jiao Tong University.
2.2. Data acquisition and collection
CRC patient data and RNA‐seq expression data were downloaded from the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/). The following search strategy was used: (metrn OR meteorin) AND (colorectal OR cancer OR tumor OR tumour OR carcinoma). We downloaded the original data from the GEO database datasets, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23878, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE33113, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17536, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14333, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13067, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13294, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE39582, and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE37892.
The GenBank reference sequence of METRN is Chromosome 16—NC_000016.10, and the version number is 109.20190905.
2.3. Statistical analysis
R software was used for data analysis. We used the affy package (R language) to read and process these data. Data were log2 transformed, and the missing values were filled in with the impute package. Differentially expressed genes were filtered out using the limma package. Probes were matched to their corresponding genes using annotation files. We compared the expression levels of METRN between CRC tissues and normal tissues in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23878 dataset. METRN expression was evaluated in different Dukes’ stages in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14333 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17536 datasets. METRN expression levels were also determined in the microsatellite instability (MSI) and microsatellite stability (MSS) subtypes in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13067 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13294 datasets, respectively. The association of METRN expression level with the histopathological grading of tumors was also analyzed in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17536 dataset. Moreover, the relationship between METRN expression level and BRAF and KRAS mutations was analyzed in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE39582 dataset. In addition, overall survival (OS), disease‐free survival (DFS), and disease‐specific survival (DSS) curves were generated using the Kaplan–Meier method, and these data were analyzed for statistical significance using a log‐rank test in the http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17536 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE37892 datasets. In the above analyses, p‐values less than 0.05 indicated statistical significance.
3. RESULTS
3.1. METRN was expressed at a high level in CRC tissues
We compared the expression of METRN between CRC tissues and normal tissues and found that METRN was expressed at significantly higher levels in CRC tissues (Figure 1a, b).
Figure 1.
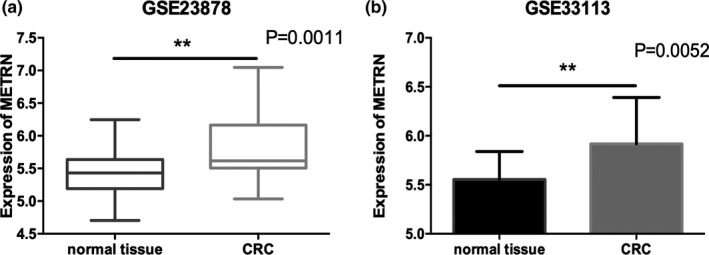
METRN expression in normal tissues and colorectal cancer tissues (a‐b). METRN GenBank reference sequence: Chromosome 16—NC_000016.10, version number: 109.20190905
3.2. METRN expression was higher in advanced colorectal cancer
We compared the expression of METRN in different stages of CRC. We found that METRN expression levels were higher in Dukes’ stage C and D tumors, compared with Dukes’ stage A and B tumors (Figure 2a). METRN expression levels were not significantly different between CRC stages A and B or between stages C and D. Therefore, we further divided the data into two groups and compared the differences between stage A/B and stage C/D. We found a significant increase in METRN expression in the latter group compared with the former group (Figure 2b).
Figure 2.
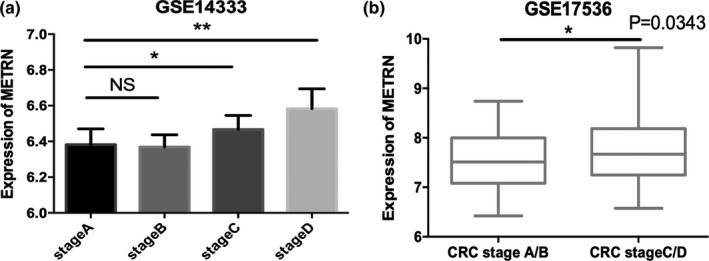
Comparison of METRN expression according to Dukes’ stage (a) and early and advanced colorectal cancer (b). NS, not significant. METRN GenBank reference sequence: Chromosome 16—NC_000016.10, version number: 109.20190905
3.3. METRN was upregulated in the MSI subtype group
We compared the expression level of METRN in MSI and MSS CRC subtypes. We found that METRN expression was higher in the MSI subtype than the MSS subtype (Figure 3a, b). METRN expression was upregulated to a greater extent in deficient mismatch repair (dMMR) CRC than in proficient mismatch repair (pMMR) CRC (Figure 3c). Moreover, METRN expression levels were higher in grade III CRC than in grade I or grade II CRC (Figure 3d).
Figure 3.
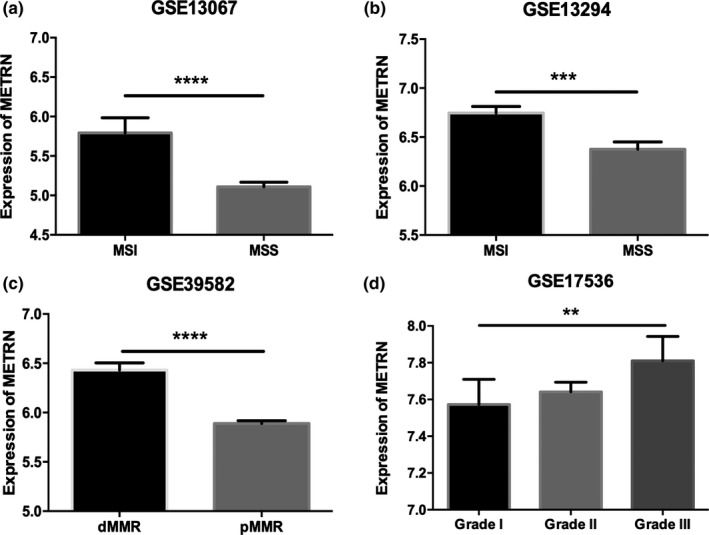
Comparison of METRN expression between microsatellite instability and microsatellite stability subtypes (a‐c) and different colorectal cancer grades (d). METRN GenBank reference sequence: Chromosome 16—NC_000016.10, version number: 109.20190905
3.4. METRN overexpression was associated with BRAF mutations
The BRAF mutation rate was significantly higher in the group with high METRN expression levels than in the group with low METRN expression levels (Figure 4a), while the mutation rate of KRAS was not significantly correlated with METRN expression level (Figure 4b). METRN was expressed at a high level in CRC with BRAF mutations (Figure 4c), but not in CRC with KRAS mutations (Figure 4d).
Figure 4.
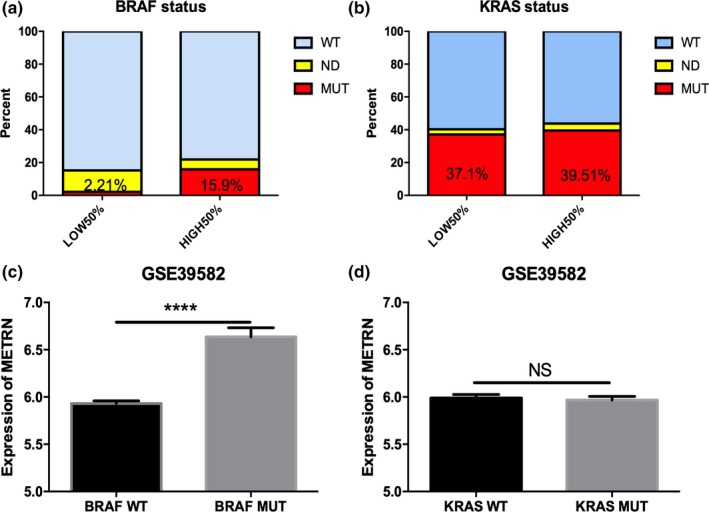
The relationship between METRN expression and BRAF/KRAS mutations (a and b), the expression of METRN in the BRAF and KRAS mutation‐containing CRC groups (c and d). NS, not significant. WT, wild‐type. MUT, mutation. ND, not determined. METRN GenBank reference sequence: Chromosome 16—NC_000016.10, version number: 109.20190905
3.5. METRN was associated with poor prognosis in patients with CRC
A Kaplan–Meier curve was plotted to visualize the 5‐year OS, DFS, and DSS rates of patients with CRC. High levels of METRN expression were associated with worse 5‐year OS, DFS, and DSS rates in patients with CRC (Figure 5a‐d).
Figure 5.
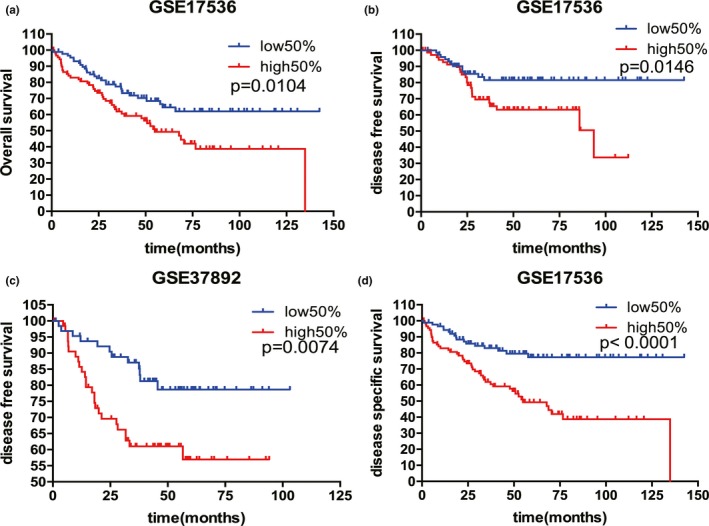
METRN expression was associated with overall, disease‐free, and disease‐specific survival (a‐d). METRN GenBank reference sequence: Chromosome 16—NC_000016.10, version number: 109.20190905
4. DISCUSSION
In this study, we found that METRN played a critical role in CRC and can be used as a prognostic indicator in patients with CRC. We found that METRN was expressed at a high level in CRC tissues compared with normal colorectal tissues. Park et al. previously showed that METRN is involved in the regulation of cerebral angiogenesis (Park et al., 2008). Angiogenesis is important for the growth of tumor cells. Advanced tumors often have a rich vascular base. Blocking angiogenesis pathways has been shown to be an effective strategy to improve prognosis in several types of cancer patients (Chan, 2016). METRN may be able to block the angiogenic activity of microvascular endothelial cells by inducing the expression of thrombin‐sensitive protein‐1/‐2 in astrocytes (Park et al., 2008). In addition, we found that METRN was mainly expressed in Dukes’ stages C and D, which are stages that are usually associated with poor prognosis and low survival rates. To the best of our knowledge, most previous studies on METRN have focused on its effects on the central nervous system (Jorgensen et al., 2009; Kim et al., 2014; Wang et al., 2012), and it has rarely been studied in CRC. This is the first study showing the significance of METRN in human CRC, indicating that METRN may perform a critical role in the prognosis of patients with CRC.
CRC can be classified into the MSI and MSS subtypes, according to the microsatellite stability. The extent of mismatch repair (MMR) can be used as the basis of risk stratification. There is ample evidence that patients with dMMR have a lower risk of recurrence and longer survival time than those with pMMR (Sargent et al., 2010). MSI is the molecular marker for dMMR, and it is often associated with improved overall survival rates in early‐stage CRC. High‐level MSI (MSI‐high) occurs in approximately 15% of patients with CRC and is associated with improved survival rates (Popat, Hubner, & Houlston, 2005; Roth et al., 2012; Vilar & Tabernero, 2013). MSI CRC is more prone to somatic mutations than the MSS or pMMR subtypes of CRC (Chong et al., 2019). In this study, we found that METRN expression was upregulated in the MSI CRC group. This result may indicate that a higher incidence of somatic mutations occurs in CRC with high METRN expression. It is interesting to note that METRN was expressed at a higher level in the CRC group with BRAF mutations than in the group with KRAS mutations. KRAS and BRAF are common sites of somatic mutations in many types of human cancers, especially CRC (Yuen et al., 2002). Mutations in both these genes can increase mortality in CRC patients with both MSS‐ and MSI‐type tumors (Lochhead et al., 2013; Phipps et al., 2015). Recent studies have shown that MSI‐type colorectal tumors exhibit a relatively low frequency of BRAF and KARS mutations (Febbo et al., 2011; Funkhouser et al., 2012; Ogino, Kawasaki, Kirkner, Loda, & Fuchs, 2006). Yuen et al. showed that BRAF mutations are associated with early Dukes’ tumor stages in CRC (Yuen et al., 2002). However, Garcia et al. reported that BRAF mutations are associated with poorer differentiation, mucinous histology, MSI, and larger primary tumors (Sanz‐Garcia, Argiles, Elez, & Tabernero, 2017). Yaeger et al. found that metastatic CRCs with BRAF mutations were prone to progress from stage III disease, and T4 disease was more common in patients with stage III disease (Yaeger et al., 2014). Consequently, we are more inclined to conclude that BRAF mutations indicate poor prognosis. Further histopathological analysis of METRN expression in CRC confirmed our conclusion.
In survival analysis, METRN was found to be a significant predictor of OS, DSS, and DFS, with rates of survival being negatively correlated with METRN expression. This suggested that METRN expression is a significant risk factor in patients with CRC. A previous study reported that METRNL could alleviate lipid‐induced inflammation and insulin resistance via AMPK or PPARδ‐dependent pathways (Jung et al., 2018). Some studies have shown that the AMPK and PPARδ signaling pathways play important roles in CRC. AMPK has an important autophagy‐inducing effect during the treatment of tumors, including CRC tumors, and this can be regulated by redox modification under oxidative stress (Kim, Kundu, Viollet, & Guan, 2011; Yan et al., 2019). Furthermore, it has been reported that PPARδ induces CRC metastasis and increases the activity of CRC cells through various pathways (Wang, Fu, Wei, Xiong, & DuBois, 2019; Zhou, Jin, Liu, Shi, & Hou, 2019). Therefore, we propose that METRN may be involved in regulating CRC prognosis through the above mechanisms.
In addition, it has been reported that low serum METRNL levels may be associated with endothelial dysfunction (El‐Ashmawy, Selim, Hosny, & Almassry, 2019). Therefore, we propose that METRN expression may be involved in regulating the function of colorectal endothelial cells. Another study found that METRN is highly expressed in early embryos during gastrulation and is crucial for mesoendoderm development (Kim et al., 2014). This further increases the evidence that METRN regulates the function of colorectal endothelial cells.
Although our study revealed that METRN might be a prognostic indicator in patients with CRC, we did not explore the possible mechanism of METRN’s involvement in CRC regulation. Therefore, we cannot completely confirm the specific role of METRN in CRC. Additional studies are recommended to explore the role of METRN in CRC. Since there are no previous reports on the relationship between METRN and CRC, our results will play a guiding role for future studies.
In conclusion, overexpression of METRN was closely associated with advanced CRC stage and predicted poor clinical outcomes. Therefore, our results may have a more detailed reference value for the prognosis of patients with CRC in the future clinical practice.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGMENT
The authors thank the financial support for this study from the Grant of Clinical Research Promotion Program of Shanghai Ninth People's Hospital, School of Medical, Shanghai Jiao Tong University (JYLJ201822).
Xu X, Zhang C, Xia Y, Yu J. Over expression of METRN predicts poor clinical prognosis in colorectal cancer. Mol Genet Genomic Med. 2020;8:e1102 10.1002/mgg3.1102
Xin Xu and Chihao Zhang contributed equally to this study.
Funding information
This work was supported by the Grant of Clinical Research Promotion Program of Shanghai Ninth People's Hospital, School of Medical, Shanghai Jiao Tong University (JYLJ201822).
Contributor Information
Yan Xia, Email: 13761388807@163.com.
Jiwei Yu, Email: jenniferyu919@126.com.
REFERENCES
- Allemani, C. , Matsuda, T. , Di Carlo, V. , Harewood, R. , Matz, M. , Niksic, M. , … Coleman, M. P. (2018). Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet, 391(10125), 1023–1075. 10.1016/s0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, E. (2016). Angiogenesis in colorectal cancer: Antibodies. Cancer Journal, 22(3), 179–181. 10.1097/ppo.0000000000000200 [DOI] [PubMed] [Google Scholar]
- Chong, L. C. , Townsend, A. R. , Young, J. , Roy, A. , Piantadosi, C. , Hardingham, J. E. , … Price, T. J. (2019). Outcomes for metastatic colorectal cancer based on microsatellite instability: Results from the South Australian metastatic colorectal cancer registry. Targeted Oncology, 14(1), 85–91. 10.1007/s11523-018-0615-9 [DOI] [PubMed] [Google Scholar]
- Dziki, L. , Pula, A. , Stawiski, K. , Mudza, B. , Wlodarczyk, M. , & Dziki, A. (2015). Patients' awareness of the prevention and treatment of colorectal cancer. Polish Journal of Surgery, 87(9), 459–463. 10.1515/pjs-2015-0088 [DOI] [PubMed] [Google Scholar]
- El‐Ashmawy, H. M. , Selim, F. O. , Hosny, T. A. M. , & Almassry, H. N. (2019). Association of low serum meteorin like (metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Research and Clinical Practice, 150, 57–63. 10.1016/j.diabres.2019.02.026 [DOI] [PubMed] [Google Scholar]
- Febbo, P. G. , Ladanyi, M. , Aldape, K. D. , De Marzo, A. M. , Hammond, M. E. , Hayes, D. F. , … Birkeland, M. L. (2011). NCCN task force report: Evaluating the clinical utility of tumor markers in oncology. Journal of the National Comprehensive Cancer Network, 9(Suppl_5), S‐1–S‐32. 10.6004/jnccn.2011.0137 [DOI] [PubMed] [Google Scholar]
- Funkhouser, W. K. Jr , Lubin, I. M. , Monzon, F. A. , Zehnbauer, B. A. , Evans, J. P. , Ogino, S. , & Nowak, J. A. (2012). Relevance, pathogenesis, and testing algorithm for mismatch repair‐defective colorectal carcinomas: A report of the association for molecular pathology. The Journal of Molecular Diagnostics, 14(2), 91–103. 10.1016/j.jmoldx.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Garcés, M. F. , Sanchez, E. , Cardona, L. F. , Simanca, E. L. , González, I. , Leal, L. G. , … Caminos, J. E. (2015). Maternal Serum meteorin levels and the risk of preeclampsia. PLOS ONE, 10(6), e0131013 10.1371/journal.pone.0131013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, J. R. , Emerich, D. F. , Thanos, C. , Thompson, L. H. , Torp, M. , Bintz, B. , … Wahlberg, L. U. (2011). Lentiviral delivery of meteorin protects striatal neurons against excitotoxicity and reverses motor deficits in the quinolinic acid rat model. Neurobiology of Diseases, 41(1), 160–168. 10.1016/j.nbd.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Jørgensen, J. R. , Thompson, L. , Fjord‐Larsen, L. , Krabbe, C. , Torp, M. , Kalkkinen, N. , … Wahlberg, L. (2009). Characterization of meteorin–an evolutionary conserved neurotrophic factor. Journal of Molecular Neuroscience, 39(1–2), 104–116. 10.1007/s12031-009-9189-4 [DOI] [PubMed] [Google Scholar]
- Jung, T. W. , Lee, S. H. , Kim, H. C. , Bang, J. S. , Abd El‐Aty, A. M. , Hacımüftüoğlu, A. , … Jeong, J. H. (2018). METRNL attenuates lipid‐induced inflammation and insulin resistance via AMPK or PPARδ‐dependent pathways in skeletal muscle of mice. Experimental & Molecular Medicine, 50(9), 122 10.1038/s12276-018-0147-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Kundu, M. , Viollet, B. , & Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13(2), 132–141. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. Y. , Moon, J. S. , Kwon, M. C. , Shin, J. , Im, S. K. , Kim, H. A. , … Kong, Y. Y. (2014). Meteorin regulates mesendoderm development by enhancing nodal expression. PLOS ONE, 9(2), e88811 10.1371/journal.pone.0088811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. , Han, J. , Lee, S. H. , Park, J. A. , & Kim, K. W. (2010). Meteorin promotes the formation of GFAP‐positive glia via activation of the Jak‐STAT3 pathway. Journal of Cell Science, 123(11), 1959–1968. 10.1242/jcs.063784 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Wang, H. J. , Meng, T. , Lei, C. , Yang, X. H. , Wang, Q. S. , … Zhu, J. F. (2019). lncRNA GAS5 inhibits cell migration and invasion and promotes autophagy by targeting miR‐222‐3p via the GAS5/PTEN‐signaling pathway in CRC. Molecular Therapy ‐ Nucleic Acids, 17, 644–656. 10.1016/j.omtn.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lochhead, P. , Kuchiba, A. , Imamura, Y. U. , Liao, X. , Yamauchi, M. , Nishihara, R. , … Ogino, S. (2013). Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. JNCI: Journal of the National Cancer Institute, 105(15), 1151–1156. 10.1093/jnci/djt173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaji, B. A. , Moy, F. M. , Roslani, A. C. , & Law, C. W. (2017). Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer, 17(1), 339 10.1186/s12885-017-3336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino, J. , Yamashita, K. , Hashiguchi, H. , Fujii, H. , Shimazaki, T. , & Hamada, H. (2004). Meteorin: A secreted protein that regulates glial cell differentiation and promotes axonal extension. The EMBO Journal, 23(9), 1998–2008. 10.1038/sj.emboj.7600202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino, S. , Kawasaki, T. , Kirkner, G. J. , Loda, M. , & Fuchs, C. S. (2006). CpG island methylator phenotype‐low (CIMP‐low) in colorectal cancer: Possible associations with male sex and KRAS mutations. The Journal of Molecular Diagnostics, 8(5), 582–588. 10.2353/jmoldx.2006.060082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. A. , Lee, H. S. , Ko, K. J. , Park, S. Y. , Kim, J. H. , Choe, G. , … Kim, K. W. (2008). Meteorin regulates angiogenesis at the gliovascular interface. Glia, 56(3), 247–258. 10.1002/glia.20600 [DOI] [PubMed] [Google Scholar]
- Phipps, A. I. , Limburg, P. J. , Baron, J. A. , Burnett‐Hartman, A. N. , Weisenberger, D. J. , Laird, P. W. , … Newcomb, P. A. (2015). Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology, 148(1), 77–87.e72. 10.1053/j.gastro.2014.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat, S. , Hubner, R. , & Houlston, R. S. (2005). Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of Clinical Oncology, 23(3), 609–618. 10.1200/jco.2005.01.086 [DOI] [PubMed] [Google Scholar]
- Roth, A. D. , Delorenzi, M. , Tejpar, S. , Yan, P. , Klingbiel, D. , Fiocca, R. , … Van Cutsem, E. (2012). Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. JNCI Journal of the National Cancer Institute, 104(21), 1635–1646. 10.1093/jnci/djs427 [DOI] [PubMed] [Google Scholar]
- Sanz‐Garcia, E. , Argiles, G. , Elez, E. , & Tabernero, J. (2017). BRAF mutant colorectal cancer: Prognosis, treatment, and new perspectives. Annals of Oncology, 28(11), 2648–2657. 10.1093/annonc/mdx401 [DOI] [PubMed] [Google Scholar]
- Sargent, D. J. , Marsoni, S. , Monges, G. , Thibodeau, S. N. , Labianca, R. , Hamilton, S. R. , … Gallinger, S. (2010). Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. Journal of Clinical Oncology, 28(20), 3219–3226. 10.1200/jco.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar, E. , & Tabernero, J. (2013). Molecular dissection of microsatellite instable colorectal cancer. Cancer Discovery, 3(5), 502–511. 10.1158/2159-8290.Cd-12-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Fu, L. , Wei, J. , Xiong, Y. , & DuBois, R. N. (2019). PPARδ mediates the effect of dietary fat in promoting colorectal cancer metastasis. Cancer Research, 79(17), 4480–4490. 10.1158/0008-5472.Can-19-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Andrade, N. , Torp, M. , Wattananit, S. , Arvidsson, A. , Kokaia, Z. , … Lindvall, O. (2012). Meteorin is a chemokinetic factor in neuroblast migration and promotes stroke‐induced striatal neurogenesis. Journal of Cerebral Blood Flow and Metabolism, 32(2), 387–398. 10.1038/jcbfm.2011.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. Y. , Qu, C. , Munro, G. , Petersen, K. A. , & Porreca, F. (2019). Antihyperalgesic effects of meteorin in the rat chronic constriction injury model: A replication study. Pain, 160(8), 1847–1855. 10.1097/j.pain.0000000000001569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger, R. , Cercek, A. , Chou, J. F. , Sylvester, B. E. , Kemeny, N. E. , Hechtman, J. F. , … Saltz, L. B. (2014). BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer, 120(15), 2316–2324. 10.1002/cncr.28729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. , Dou, X. , Zhou, J. , Xiong, Y. , Mo, L. , Li, L. , & Lei, Y. (2019). Tubeimoside‐I sensitizes colorectal cancer cells to chemotherapy by inducing ROS‐mediated impaired autophagolysosomes accumulation. Journal of Experimental & Clinical Cancer Research, 38(1), 353 10.1186/s13046-019-1355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen, S. T. , Davies, H. , Chan, T. L. , Ho, J. W. , Bignell, G. R. , Cox, C. , … Leung, S. Y. (2002). Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Research, 62(22), 6451–6455. [PubMed] [Google Scholar]
- Zhou, D. , Jin, J. , Liu, Q. , Shi, J. , & Hou, Y. (2019). PPARδ agonist enhances colitis‐associated colorectal cancer. European Journal of Pharmacology, 842, 248–254. 10.1016/j.ejphar.2018.10.050 [DOI] [PubMed] [Google Scholar]


