Abstract
Background
Obesity has become the main health issue in developed countries as it impacts life expectancy and increases mortality of cerebrovascular or cardiovascular diseases. The leptin is one of the adipokines which presents in the serum in proportion to the amount of adipose tissue and is translated from LEP gene. It involves in energy homeostasis, lipid and glucose metabolisms, modulation of immune systems, and thermogenesis. Many previous studies have revealed controversial results between LEP polymorphisms and leptin levels in different ages and ethnicities. Herein, we investigated the impacts of LEP polymorphism against leptin levels in Taiwanese subjects.
Methods
In 599 Taiwanese subjects, excluding clinically overt systemic disease, age below 18 years old, and C‐reactive protein (CRP) level of above 10 mg/L, few of LEP polymorphisms were genotyped with TaqMan SNP genotyping assays, were further analyzed for association with leptin level in univariate and multivariate linear regression analyses with Bonferroni correction for multiple tests in stratified groups. The univariate and stepwise multivariate linear regression analyses were performed to determine the coefficient of determinant of LEP polymorphisms over leptin level.
Results
Significant associations were found between LEP polymorphisms and leptin levels in obese women. Circulating leptin level was positively correlated with inflammatory, insulin resistance markers, and visceral obesity markers in all subjects. Furthermore, stratified and interaction analyses revealed that LEP polymorphisms, rs7799039 and rs2167270, were significantly associated with leptin levels in obese women—8%–10% of which could be explained by LEP polymorphisms.
Conclusion
The LEP polymorphisms are independently associated with leptin levels in Taiwanese obese women. Further, the genetic determinants for leptin levels may be different between obese and nonobese, and in different sex individuals. The obesity status and female sex may exert modification effect on transcription of LEP, particularly in obese women.
Keywords: LEP, leptin, obesity, single‐nucleotide polymorphism
The LEP polymorphisms are independently associated with leptin levels in Taiwanese obese women. Furthermore, the genetic determinants for leptin levels may differ between obese and nonobese individuals and between sexes. Obesity status and female sex may exert modifying effects on LEP transcription, particularly in obese women.
1. INTRODUCTION
Obesity, a prevalent condition in both developing and developed countries, results from both genetic and environmental factors. When body mass index (BMI) exceeds ≥20% of the ideal value—an indicator of obesity—life expectancy decreases. Leptin is an adipocyte‐derived hormone that suppresses food intake and increases energy expenditure by binding to and activating its specific receptor in the hypothalamus (Tartaglia et al., 1995; Woods & Stock, 1996). Leptin is released from white adipose tissue, and thus, leptin levels are positively correlated with body fat and body mass (Bribiescas & Hickey, 2006; Kelesidis, Kelesidis, Chou, & Mantzoros, 2010). Leptin levels increase with age and are higher in females than in males (Fulda et al., 2010). Several studies on the association of LEP (OMIM: 164160; GenBank: NC_000007.14) polymorphisms with BMI or obesity have been conducted. Although the LEP promoter polymorphism G‐2548A (rs7799039) has been studied intensively (De Silva et al., 1999; Duarte, Colagiuri, Palu, Wang, & Wilcken, 2003; Furusawa et al., 2011; Paracchini, Pedotti, & Taioli, 2005; Wang et al., 2006), the results for the associated single‐nucleotide polymorphism (SNP) and their alleles, which may indicate the roles of genetic and environmental factors in obesity, have been controversial. In a BMI‐adjusted meta‐analysis of genome‐wide association studies including both men and women, association of LEP SNPs reached genome‐wide significance in both sexes (p < 5 × 10−8) (Kilpeläinen et al., 2016). The LEP SNPs are associated with circulating leptin levels predominantly in obese subjects, especially in obese women and girls (Ma et al., 2009). Moreover, leptin levels increase with age and are higher in females than in males (Fulda et al., 2010); however, a study demonstrated a decline in leptin levels in women after menopause (Rosenbaum et al., 1996). In the present study, we investigated the genetic and environmental effects of LEP SNPs on obesity, obesity‐related metabolic traits, and leptin level.
2. METHODS
2.1. Ethical compliance
All individuals provided written informed consent. The study was approved by the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation Institutional Review Board according to the guidelines of ICH‐GCP.
2.2. Study population
We recruited 599 Taiwanese individuals (men: n = 315, mean age = 46.1 ± 10.0 years; women: n = 284, mean age = 46.8 ± 9.9 years) during routine health examinations between October 2003 and September 2005 in the Chang Gung Memorial Hospital, Taiwan. Their self‐reported medical history and lifestyle characteristics were recorded; next, in their physical examination, we measured their height, weight, waist and hip circumferences, and blood pressure in the sitting position after 15 min of rest. Fasting blood samples were obtained from each individual. Exclusion criteria included age <18 years; C‐reactive protein (CRP) level >10 mg/L; and history of myocardial infarction, stroke, or transient ischemic attack, cancer, and current renal or liver disease. Obesity was defined as BMI ≥25 kg/m2, according to the Asian criteria (WHO Expert Consultation, 2004). Current smokers were defined as those who smoked at least one cigarette per day at the time of the survey. The clinical characteristics and biometrics of the study population are summarized in Table 1.
Table 1.
Clinical and biochemical characteristics of participants
| Obese | Nonobese | p value | |||
|---|---|---|---|---|---|
| Women | Men | Women | Men | — | |
| Number, n (%) | 86 | 147 | 198 | 168 | |
| Age (years) | 49.66 ± 9.41 | 45.41 ± 9.59 | 45.55 ± 9.88 | 45.65 ± 10.3 | .005 |
| Body mass index (kg/m2) | 27.90 ± 2.60 | 27.52 ± 2.23 | 21.65 ± 1.90 | 22.71 ± 1.75 | 2.00 × 10–130 |
| Waist–hip ratio | 0.88 ± 0.07 | 0.89 ± 0.04 | 0.83 ± 0.06 | 0.87 ± 0.05 | 1.46 × 10–26 |
| Waist circumference (cm) | 91.81 ± 9.75 | 93.23 ± 6.16 | 77.61 ± 7.08 | 83.45 ± 5.59 | 8.38 × 10–81 |
| Systolic BP (mm Hg) | 120.22 ± 18.19 | 118.76 ± 16.45 | 111.18 ± 18.45 | 114.05 ± 15.89 | .00001 |
| Diastolic BP (mm Hg) | 77.33 ± 10.16 | 79.80 ± 10.24 | 72.19 ± 9.97 | 76.38 ± 10.05 | 1.47 × 10–10 |
| Glucose AC (mg/dl) | 100.94 ± 26.64 | 99.58 ± 21.05 | 91.69 ± 16.19 | 99.63 ± 30.16 | .001 |
| Insulin | 10.42 ± 3.85 | 12.06 ± 6.78 | 7.72 ± 3.38 | 7.81 ± 3.05 | 5.91 × 10–21 |
| HOMA‐IR index | 2.68 ± 1.59 | 3.01 ± 1.93 | 1.75 ± 0.83 | 1.92 ± 1.06 | 7.06 × 10–24 |
| QUICKI | 0.33 ± 0.02 | 0.33 ± 0.025 | 0.35 ± 0.02 | 0.35 ± 0.02 | 1.83 × 10–23 |
| Total cholesterol (mg/dl) | 197.46 ± 35.76 | 204.68 ± 36.77 | 196.19 ± 35.77 | 197.16 ± 36.73 | .149 |
| HDL cholesterol (mg/dl) | 55.17 ± 12.12 | 48.42 ± 10.32 | 63.93 ± 14.24 | 51.01 ± 13.05 | 2.38 × 10–23 |
| LDL cholesterol (mg/dl) | 115.53 ± 30.92 | 120.31 ± 32.72 | 112.29 ± 31.67 | 116.58 ± 34.80 | .161 |
| Triglyceride (mg/dl) | 132.69 ± 63.72 | 192.34 ± 150.28 | 100.63 ± 57.84 | 154.95 ± 142.79 | 6.68 × 10–12 |
| Creatinine (mg/dl) | 0.90 ± 0.76 | 1.13 ± 0.27 | 0.80 ± 0.12 | 1.11 ± 0.55 | 4.83 × 10–11 |
| Current smokers (%) | 3 (3.5%) | 58 (39.5%) | 8 (4%) | 48 (28.6%) | .079 |
| C‐reactive protein (mg/L) | 1.58 ± 1.37 | 1.20 ± 1.30 | 0.84 ± 1.34 | 1.01 ± 1.45 | 3.38 × 10–10 |
| Leptin (g/L) | 44.92 ± 26.49 | 15.15 ± 9.5 | 21.81 ± 17.11 | 7.31 ± 5.08 | 2.14 × 10–93 |
Data are presented as mean ± standard deviation or percentage as appropriate. Obesity was defined as BMI ≥25 kg/m2 according to the Asian criteria (WHO Expert Consultation, 2004).
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL, low‐density lipoprotein; QUICKI, quantitative insulin sensitivity check index.
2.3. Genomic DNA extraction and genotyping
Genomic DNA was extracted as reported previously (Teng et al., 2013). Two SNPs around LEP, rs7799039 (HGVS nomenclature: NC_000007.13:g.127878783A>G) and rs2167270 (HGVS nomenclature: NM_000230.2:c.‐39G>A), were selected. Genotyping was performed using TaqMan SNP with genotyping assays (Applied Biosystems).
2.4. Laboratory examinations and assays
The laboratory examinations and assays were performed as described below. Fasting plasma glucose levels and lipid profiles were obtained, and the homeostatic model assessment of insulin resistance (HOMA‐IR) index and quantitative insulin sensitivity check index (QUICKI) were calculated as reported previously (Wu et al., 2014). Most markers, including serum CRP, soluble E‐selectin (sE‐selectin), soluble P‐selectin (sP‐selectin), and adiponectin, were measured using a sandwich enzyme‐linked immunosorbent assay (ELISA) developed in‐house. All in‐house kits demonstrated good correlation compared with commercially available ELISA kits. Circulating lipocalin‐2 and sP‐selectin were measured using commercially available ELISA kits from R&D Systems. Furthermore, serum insulin levels were measured using an immunoradiometric assay kit from BioSource. ELISA and genotyping were performed by laboratory personnel blinded to the clinical status of the participants.
2.5. Statistical analysis
The statistical analysis was performed on IBM SPSS Statistics (version 22; IBM) unless otherwise specified. The independent samples t test was performed for categorical variables. The continuous variables, expressed as mean ± standard deviation, were tested using one‐way analysis of variance (ANOVA) with Bonferroni correction for multiple tests. Tests of normality were conducted for all quantitative traits. Moreover, the HOMA‐IR index and leptin, insulin, triglyceride (TG), sE‐selectin, sP‐selectin, adiponectin, and CRP levels were logarithmically transformed before statistical analysis to adhere to a normality assumption. A p of < .05 through a two‐sided test was considered statistically significant. Linear regression coefficients with 95% confidence intervals were calculated for leptin levels and the predicted confounders. Allelic frequencies for each SNP were estimated through gene counting, and the polymorphism distribution was tested for Hardy–Weinberg equilibrium using the Chi‐square test. The stratified association analysis according to sex and obesity was performed using one‐way ANOVA with Bonferroni correction for multiple tests in additive and dominant genetic models. Furthermore, we investigated the sex‐ and obesity‐specific effects of leptin level variants. The resultant significant polymorphisms were included in interaction analysis using a linear regression model of SVS Win32 (version 7.3.1; Golden Helix) to determine the impact of dependent variables. The univariate and stepwise multivariate linear regression analyses were performed to detect the coefficients of determination of genetic and traditional predictors against leptin level in both all subjects and obese women groups. The statistical power of >0.8 was obtained in the univariate analyses of LEP polymorphisms and leptin levels in the dominant model using alpha of 0.05. Linkage disequilibrium (LD) was analyzed by D′ and r 2 measures.
3. RESULTS
3.1. Clinical and biochemical characteristics
The participants were stratified into four groups according to sex and obesity status; the demographic features, clinical profiles, and levels of biomarkers are listed in Table 1. The obese women were older and demonstrated significantly higher metabolic traits, including BMI, waist circumference, waist‐to‐hip ratio (WHR), systolic and diastolic blood pressures, and leptin level. The obese men and women had significantly higher HOMA‐IR index, insulin, and CRP levels but lower QUICKI levels than did their nonobese counterparts.
3.2. Correlations between leptin levels and clinical parameters and biomarker levels
In the general population, circulating leptin level was significantly and positively correlated with BMI, waist circumference, systolic blood pressure, insulin, HOMA‐IR index, and CRP levels but negatively with QUICKI in all individuals. Similar correlations, except those for waist circumference, were found in obese women. In addition, diastolic blood pressure and sE‐selectin level were significantly and positively correlated with leptin level in obese women (Table 2).
Table 2.
Association between leptin levels and measurable risk factors in Taiwanese individuals
| All subjects | Obese women | |||
|---|---|---|---|---|
| r | r | p value | ||
| Age | 0.064 | 0.060 | −0.031 | .386 |
| Body mass index | 0.350 | 2.72 × 10–18 | 0.323 | .001 |
| Waist circumference | 0.205 | 3.85 × 10–7 | 0.044 | .345 |
| Waist–hip ratio | −0.006 | 0.437 | −0.113 | .152 |
| Systolic BP | 0.125 | 0.001 | 0.249 | .011 |
| Diastolic BP | 0.039 | 0.174 | 0.327 | .001 |
| Fasting plasma glucose | −0.058 | 0.082 | −0.164 | .066 |
| Fasting serum insulin | 0.371 | 1.92 × 10–20 | 0.350 | .0005 |
| HOMA‐IR index | 0.311 | 1.39 × 10–14 | 0.201 | .033 |
| QUICKI | −0.320 | 2.11 × 10–15 | −0.219 | .022 |
| Total cholesterol | −0.008 | 0.418 | −0.055 | .307 |
| LDL cholesterol | −0.045 | 0.137 | −0.066 | .272 |
| HDL cholesterol | 0.095 | 0.011 | 0.098 | .185 |
| Triglyceride | 0.013 | 0.375 | −0.083 | .223 |
| CRP | 0.205 | 3.61 × 10–7 | 0.261 | .008 |
| sE‐selectin | 0.051 | 0.107 | 0.237 | .014 |
| sP‐selectin | −0.093 | 0.012 | −0.128 | .122 |
| Lipocalin2 | 0.031 | 0.225 | 0.094 | .195 |
| Adiponectin | 0.078 | 0.029 | 0.086 | .217 |
3.3. Associations of LEP polymorphisms with respective circulation levels in different sexes and obesity status
The allele frequencies at all polymorphisms satisfied the Hardy–Weinberg equilibrium and agreed closely with the allele frequencies described at these polymorphisms in the study population (Table 3). The association analyses in additive and dominant models were adjusted for age and smoking status. In the additive model, minor alleles of rs7799039 and rs2167270 were associated with higher leptin levels in obese individuals (p = .003 and .001, respectively; Table 4) and women (p = .025 and .031, respectively). This association also persisted in the dominant model for rs7799039 and rs2167270 in obese individuals (p = .003 and .028, respectively) and women (p = .015 and .040, respectively; Table 4). We stratified the individuals into four groups to analyze the associations between LEP polymorphisms and leptin levels with respect to sex and obesity status. In the dominant model, the associations between LEP polymorphisms and leptin level were significant only in obese women (rs7799039; p = .008; rs2167270, p = .006; Table S1).
Table 3.
The allele frequency distributions of LEP SNPs and Hardy–Weinberg equilibrium (HWE) tests
| SNP | Chr | Position | Minor allele | Allele frequency (CHS) |
HWE p value |
||
|---|---|---|---|---|---|---|---|
| rs7799039 | 7 | 127,878,783 | intergenic variant | G | 0.286 | .580 | |
| rs2167270 | 7 | 127,881,349 | 5′ UTR | A | 0.219 | .254 | |
p adjusted for sex, age, BMI, and smoking status.
Abbreviations: Allele frequency, data from 1,000 Genome project Phase 3; Chr, chromosome; CHS, southern Han in China; LEP GenBank Ref Seq, NC_000007.14.
Table 4.
Associations of LEP SNPs with leptin levels in relation to sex and obesity status
| Genotype | MM | Mm | mm | β (95% CI) | p | MM | Mm+mm | p |
|---|---|---|---|---|---|---|---|---|
| rs7799039 | AA | AG | GG | AA | AG + GG | |||
| Total | 17.56 ± 16.62 (301) | 21.41 ± 21.88 (251) | 23.15 ± 19.36 (37) | 0.051 (0.001–0.102) | .045 | 17.56 ± 16.62 (301) | 21.64 ± 21.55 (288) | .115 |
| Obese | 20.61 ± 13.04 (117) | 32.07 ± 29.19 (100) | 31.31 ± 26.97 (14) | 0.103 (0.035–0.170) | .003 | 20.61 ± 13.04 (117) | 31.98 ± 28.82 (114) | .003 |
| Nonobese | 15.62 ± 18.32 (184) | 14.36 ± 10.48 (151) | 18.19 ± 10.75 (23) | 0.015 (−0.049 to 0.078) | .652 | 15.62 ± 18.32 (184) | 14.86 ± 10.56 (174) | .849 |
| Male | 10.99 ± 7.41 (168) | 11.00 ± 10.05 (127) | 10.62 ± 6.30 (14) | −0.023 (−0.089 to 0.044) | .505 | 10.99 ± 7.41 (168) | 10.96 ± 9.73 (141) | .439 |
| Female | 25.86 ± 20.84 (133) | 32.08 ± 25.36 (124) | 30.79 ± 20.72 (23) | 0.059 (0.007–0.111) | .025 | 25.86 ± 20.84 (133) | 31.88 ± 24.63 (147) | .028 |
| rs2167270 | GG | GA | AA | GG | GA + AA | |||
| Total | 17.91 ± 17.09 (331) | 21.45 ± 21.71 (229) | 24.91 ± 22.87 (23) | 0.041 (−0.013 to 0.095) | .137 | 17.91 ± 17.09 (331) | 21.77 ± 21.79 (252) | .256 |
| Obese | 21.61 ± 15.30 (133) | 32.02 ± 29.07 (90) | 37.61 ± 33.91 (8) | 0.096 (0.022–0.169) | .011 | 21.61 ± 15.30 (133) | 32.47 ± 29.34 (98) | .015 |
| Nonobese | 15.43 ± 17.81 (198) | 14.61 ± 10.66 (139) | 18.14 ± 10.29 (15) | 0.010 (−0.057 to 0.078) | .761 | 15.43 ± 17.81 (198) | 14.95 ± 10.64 (154) | .912 |
| Male | 11.52 ± 9.30 (186) | 10.25 ± 7.23 (112) | 10.63 ± 7.42 (8) | −0.052 (−0.123–0.020) | .157 | 11.52 ± 9.30 (186) | 10.27 ± 7.21 (120) | .139 |
| Female | 26.12 ± 20.92 (145) | 32.18 ± 25.28 (117) | 32.53 ± 24.81 (15) | 0.061 (0.006–0.116) | .031 | 26.12 ± 20.92 (145) | 32.22 ± 25.13 (132) | .040 |
Leptin levels, Means ± SD (N). p adjusted for age and smoking status.
Abbreviations: m, minor allele; M, major allele.
3.4. Interaction analysis
Regarding the association of LEP polymorphisms with leptin levels, the interaction with obesity existed in the dominant model of rs7799039 in obese individuals and that of rs2167270 in women (interaction p = .020 and .018, respectively; Figure 1a,b). Nevertheless, the interaction with obesity existed in the dominant model of rs7799039 and rs2167270 in the subgroup analysis for obese women (interaction p = .030 and .024, respectively; Figure 2a,b). In addition, strong LD was detected between rs7799039 and rs2167270 (r 2 = .728; D′ = 0.999).
Figure 1.
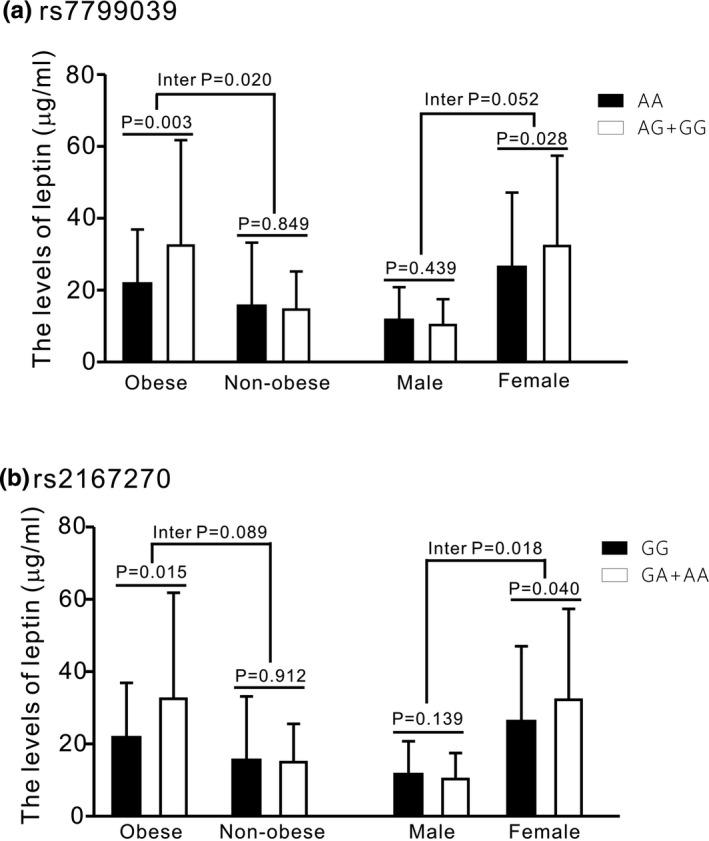
Association and interaction analysis between LEP polymorphisms and leptin levels in different groups (P adjusted for age and smoking status)
Figure 2.
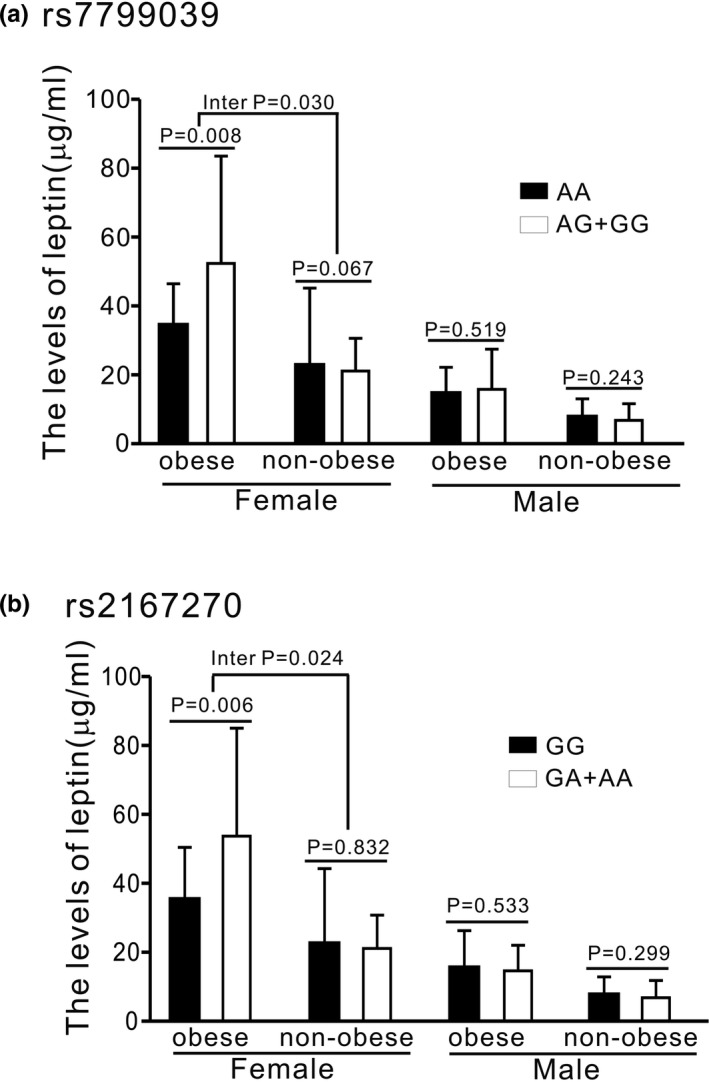
Association and interaction analysis between LEP polymorphisms and leptin levels in different groups (P adjusted for age and smoking status)
3.5. Coefficient of determinant of polymorphisms over leptin level
Both the univariate and stepwise multivariate linear regression revealed significant determinants of dominant models of both rs7799039 and rs2167270 over substantial variation in circulating leptin levels exclusively in obese women (p = .003 and .002 for univariate analyses and p = .005 and .016 for multivariate analyses, respectively), 8%–10% of which could be explained by LEP polymorphisms (Table 5). In contrast, traditional predictors, not LEP polymorphisms, conferred significant determinants in all subjects group (Table S2).
Table 5.
Coefficient of determination of genetic and traditional predictors over leptin levels in obese women
| Predictors | Univariate analysis | Stepwise multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | R2 | p value | Beta (SE) | R2 | p value* | Beta (SE) | R2 | p value* | |
|
rs7799039 AG + GG |
0.138 (0.308) | 0.095 | .003 | 0.118 (0.041) | 0.092 | .005 | — | — | — |
|
rs2167270 GA + AA |
0.142 (0.319) | 0.102 | .002 | — | — | — | 0.104 (0.042) | 0.080 | .016 |
| Insulin | 0.514 (0.145) | 0.128 | .0006 | 0.505 (0.133) | 0.152 | .0002 | 0.482 (0.135) | 0.152 | .0006 |
| DBP | 1.209 (0.397) | 0.099 | .003 | 0.836 (0.360) | 0.047 | .360 | 0.795 (0.369) | 0.042 | .034 |
| BMI | 1.937 (0.596) | 0.111 | .0016 | — | — | — | — | — | — |
| SBP | 0.811 (0.361) | 0.056 | .027 | — | — | — | — | — | — |
| CRP | 0.105 (0.052) | 0.045 | .049 | — | — | — | — | — | — |
| sE‐selectin | 0.262 (0.119) | 0.054 | .031 | — | — | — | — | — | — |
adjusted for BMI, systolic and diastolic pressures, insulin levels, CRP, and sE‐selectin level.
4. DISCUSSION
Leptin is an adipokine derived from adipocytes in the white adipose tissue; its serum levels are in general in proportion with the amount of adipose tissue. It is translated from LEP and is involved in energy homeostasis, lipid and glucose metabolisms, immune system modulation, and thermogenesis. In the present study, leptin levels were significantly and positively correlated with BMI, waist circumference, high blood pressure, and insulin resistance and inflammatory marker levels. The highest leptin level and a similar correlation were noted in obese women.
Several studies have demonstrated the correlation of leptin with metabolic syndrome, including visceral obesity, hypertension, insulin resistance, and dyslipidemia. In Taiwanese school‐aged children, high leptin levels were correlated with high body weight, BMI, WHR, and blood pressure (Chu, Wang, & Shieh, 2001). The higher leptin level increased the risk of metabolic syndrome in Taiwanese individuals (Li et al., 2011). In the Copenhagen City Heart Study, leptin was significantly associated with new‐onset hypertension with an odds ratio of 1.28 (Asferg et al., 2010). In addition, the leptin level is positively correlated with blood pressure independent of body weight (Agata et al., 1997). A similar result was reported in a US adult population after adjustments for age, sex, and BMI (Shankar & Xiao, 2010). In a Polish study, postmenopausal hypertensive women had the highest leptin level among their counterparts, independent of their BMI (Olszanecka, Posnik‐Urbanska, Kawecka‐Jaszcz, Czarnecka, & Fedak, 2010). During pregnancy, leptin‐treated Sprague Dawley rats showed significantly lower angiotensin‐converting enzyme 2 levels, which cleaved angiotensin I and II into angiotensin 1–9 and 1–7, respectively; both of these products have vasodilatory, hypotensive effects, countering the effects of angiotensin II, finally leading to increase in blood pressure (Ibrahim, Froemming, Omar, & Singh, 2014). The chronic elevation of plasma leptin levels increases renal Na+, K+‐ATPase activity and subsequently disrupts natriuresis, elevating blood pressure (Beltowski, 2010). Our results showed that increased leptin levels may be associated with insulin resistance because leptin levels were positively correlated with fasting serum insulin levels and HOMA‐IR index and negatively with QUICKI. In the Brazilian study, higher leptin levels were positively correlated with insulin resistance (HOMA‐IR index) in children and adolescents (Gonzaga, Medeiros, de Carvalho, & Alves, 2014). In addition, the fasting leptin level is negatively associated with insulin sensitivity in women with a previous history of gestational diabetes mellitus (Madarász et al., 2009). Leptin also potentially exerts its function through an inflammatory pathway. CRP levels are positively correlated with leptin levels in women with preeclampsia (Molvarec et al., 2011).
LEP, a candidate gene of obesity mapped in chromosome 7q31.3, comprises three exons spanning approximately 20 kb and encodes a 16‐kDa protein, leptin. In our study, after adjustments for age and smoking status, both LEP SNPs rs7799039 and rs2167270 were significantly associated with leptin levels in obese individuals and women in either the dominant or additive model. These significant associations persisted in the dominant models of LEP polymorphisms in obese women. Obesity status and female sex interact with LEP polymorphisms, leading to higher leptin level either in all individuals or in obese women. Although several studies have reported controversial results regarding the associations of rs7799039 and rs2167270 with leptin levels, however, most results were consistent with our current results. In the association study of three Tunisian consanguineous families, the minor allele of LEP rs2167270 (A), but not rs7799039, was positively and significantly associated with leptin level (Fourati et al., 2013). The rs2167270 was predicted to modify the transcription factor binding sites (Jiang et al., 2004). This SNP may affect gene expression at a transcriptional level, leading to disrupted leptin production. However, the rs7799039 GA/AA polymorphisms were also not associated with leptin level in Egyptian individuals (Motawi, Salman, Shaker, & Abdelhamid, 2015). Different polymorphism frequencies in different populations may affect leptin levels. In addition, the association between rs7799039 and either plasma leptin level or gestational diabetes mellitus risk was not found in Chinese population (Yang et al., 2016). A Polish study reported no significant association of rs7799039 and rs12672770 with either relative mRNA level in subcutaneous adipose tissue or serum leptin levels in children and adolescents (Cieslak et al., 2012). The individuals who were exposed to prenatal famine in the Netherlands were investigated, the study revealed that LEP methylation was higher in the experimental groups, this could be explained by LEP methylation differences, which may depend on sex and gestational timing (Tobi et al., 2009). The correlation of LEP rs2167270 polymorphisms with increased BMI and waist circumference in women has been previously reported (Fourati et al., 2013; Friedlander et al., 2010). In our present study, rs1267270 was associated with BMI in additive or dominant models in obese women (p = .048 and p = .019, respectively).
In the interaction analysis, obesity status and female sex had a significant interactive effect on LEP polymorphisms related to leptin level in rs7799039 and rs2167270, respectively. When we compared the obese and nonobese women, we found that obesity status had an interaction effect on leptin level. Fat distribution, particularly subcutaneous abdominal fat as a determinant of leptin level, contributes to the variability in leptin level in obese individuals (Minocci et al., 2000). The sex difference in circulating leptin levels can be due to at least two mechanisms: higher proportion of adipose tissue and increased production rate of leptin per unit mass of adipose tissue (Hellström, Wahrenberg, Hruska, Reynisdottir, & Arner, 2000). Thus, obesity status may modify leptin levels directly or indirectly through gene transcription. rs7799039 (–2548G/A) influences leptin expression, possibly at the transcriptional level, and therefore, also adipose secretion levels of the hormone (Hoffstedt, Eriksson, Mottagui‐Tabar, & Arner, 2002). The polymorphism located in the promoter region of the leptin gene appears to be associated with large variations in leptin level in obese humans (given their wide range of adiposity) (Le Stunff, Le Bihan, Schork, & Bougnères, 2000). In univariate and multivariate analyses, the significant coefficient of determination of LEP polymorphisms, dominant models of both rs7799039 and rs2167270, over substantial variation in circulating leptin levels in obese women was observed, 8%–10% of which was explained by genetic predictors. As abovementioned, we supposed that LEP polymorphisms are closely correlated with leptin levels exclusively in obese women, and obesity status and female sex may exert modifying effects on LEP transcription.
5. CONCLUSION
The LEP polymorphisms are independently associated with leptin levels in Taiwanese obese women. Furthermore, the genetic determinants for leptin levels may differ between obese and nonobese individuals and between sexes. Obesity status and female sex may exert modifying effects on LEP transcription, particularly in obese women.
CONFLICT OF INTEREST
There are no conflict to declare.
AUTHOR CONTRIBUTIONS
DMD and JYJ participated in genotyping, performed statistical analysis, and drafted the manuscript. MST prepared the DNA samples and participated in genotyping. LAH participated in sample collection and prepared the DNA samples. DMD and JYJ performed and corrected statistical analysis. YLK and SW supervised the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We greatly appreciate technical support from the Core Laboratory of the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, and expert statistical analysis assistance from Tsung‐Han Hsieh. This research was sponsored by allocation from the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD‐TPE‐106‐C1‐1, TCRD‐TPE‐106‐RT‐3), Buddhist Tzu Chi Medical Foundation Academic Advancement (TCMF‐EP 108‐05) to Y. L. Ko and the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD‐TPE‐104‐21) to D. M. Duan.
Duan D‐M, Jhang J‐Y, Wu S, Teng M‐S, Hsu L‐A, Ko Y‐L. Modification effect of sex and obesity on the correlation of LEP polymorphisms with leptin levels in Taiwanese obese women. Mol Genet Genomic Med. 2020;8:e1113 10.1002/mgg3.1113
De‐Min Duan and Jing‐Yi Jhang contributed equally to this work, so they shared first authorship for this manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Agata, J. , Masuda, A. , Takada, M. , Higashiura, K. , Murakami, H. , Miyazaki, Y. , & Shimamoto, K. (1997). High plasma immunoreactive leptin level in essential hypertension. American Journal of Hypertension, 10(10), 1171–1174. 10.1016/S0895-7061(97)00310-5 [DOI] [PubMed] [Google Scholar]
- Asferg, C. , Mogelvang, R. , Flyvbjerg, A. , Frystyk, J. , Jensen, J. S. , Marott, J. L. , … Jeppesen, J. (2010). Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. American Journal of Hypertension, 23(3), 327–333. 10.1038/ajh.2009.244 [DOI] [PubMed] [Google Scholar]
- Beltowski, J. (2010). Leptin and the regulation of renal sodium handling and renal Na+‐transporting ATPases: Role in the pathogenesis of arterial hypertension. Current Cardiology Reviews, 6(1), 31–40. 10.2174/157340310790231644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bribiescas, R. G. , & Hickey, M. S. (2006). Population variation and differences in serum leptin independent of adiposity: A comparison of Ache Amerindian men of Paraguay and lean American male distance runners. Nutrition & Metabolism, 3(1), 34 10.1186/1743-7075-3-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, N.‐F. , Wang, D.‐J. , & Shieh, S.‐M. (2001). Obesity, leptin and blood pressure among children in Taiwan: The Taipei Children's Heart Study. American Journal of Hypertension, 14(2), 135–140. 10.1016/S0895-7061(00)01243-7 [DOI] [PubMed] [Google Scholar]
- Cieslak, J. , Bartz, M. , Stachowiak, M. , Skowronska, B. , Majewska, K. A. , Harasymczuk, J. , … Switonski, M. (2012). Effect of three common SNPs in 5′‐flanking region of LEP and ADIPOQ genes on their expression in Polish obese children and adolescents. Molecular Biology Reports, 39(4), 3951–3955. 10.1007/s11033-011-1174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva, A. , Walder, K. R. , Aitman, T. J. , Gotoda, T. , Goldstone, A. P. , Hodge, A. M. , … Collier, G. R. (1999). Combination of polymorphisms in OB‐R and the OB gene associated with insulin resistance in Nauruan males. International Journal of Obesity, 23(8), 816–822. 10.1038/sj.ijo.0800931 [DOI] [PubMed] [Google Scholar]
- Duarte, N. L. , Colagiuri, S. , Palu, T. , Wang, X. L. , & Wilcken, D. E. (2003). Obesity, Type II diabetes and the Ala54Thr polymorphism of fatty acid binding protein 2 in the Tongan population. Molecular Genetics and Metabolism, 79(3), 183–188. 10.1016/S1096-7192(03)00088-X [DOI] [PubMed] [Google Scholar]
- Fourati, M. , Mnif, M. , Kharrat, N. , Charfi, N. , Kammoun, M. , Fendri, N. , … Fakhfakh, F. (2013). Association between Leptin gene polymorphisms and plasma leptin level in three consanguineous families with obesity. Gene, 527(1), 75–81. 10.1016/j.gene.2013.05.064 [DOI] [PubMed] [Google Scholar]
- Friedlander, Y. , Li, G. , Fornage, M. , Williams, O. D. , Lewis, C. E. , Schreiner, P. , … Siscovick, D. S. (2010). Candidate molecular pathway genes related to appetite regulatory neural network, adipocyte homeostasis and obesity: Results from the CARDIA Study. Annals of Human Genetics, 74(5), 387–398. 10.1111/j.1469-1809.2010.00596.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, S. , Linseisen, J. , Wolfram, G. , Himmerich, S. , Gedrich, K. , Pollmacher, T. , & Himmerich, H. (2010). Leptin plasma levels in the general population: Influence of age, gender, body weight and medical history. Protein and Peptide Letters, 17(11), 1436–1440. 10.2174/0929866511009011436 [DOI] [PubMed] [Google Scholar]
- Furusawa, T. , Naka, I. , Yamauchi, T. , Natsuhara, K. , Kimura, R. , Nakazawa, M. , … Ohashi, J. (2011). The serum leptin level and body mass index in Melanesian and Micronesian Solomon Islanders: Focus on genetic factors and urbanization. American Journal of Human Biology, 23(4), 435–444. 10.1002/ajhb.21124 [DOI] [PubMed] [Google Scholar]
- Gonzaga, N. C. , Medeiros, C. C. , de Carvalho, D. F. , & Alves, J. G. (2014). Leptin and cardiometabolic risk factors in obese children and adolescents. Journal of Paediatrics and Child Health, 50(9), 707–712. 10.1111/jpc.12610 [DOI] [PubMed] [Google Scholar]
- Hellström, L. , Wahrenberg, H. , Hruska, K. , Reynisdottir, S. , & Arner, P. (2000). Mechanisms behind gender differences in circulating leptin levels. Journal of Internal Medicine, 247(4), 457–462. 10.1046/j.1365-2796.2000.00678.x [DOI] [PubMed] [Google Scholar]
- Hoffstedt, J. , Eriksson, P. , Mottagui‐Tabar, S. , & Arner, P. (2002). A polymorphism in the leptin promoter region (‐2548 G/A) influences gene expression and adipose tissue secretion of leptin. Hormone and Metabolic Research, 34(07), 355–359. 10.1055/s-2002-33466 [DOI] [PubMed] [Google Scholar]
- Ibrahim, H. S. , Froemming, G. R. A. , Omar, E. , & Singh, H. J. (2014). ACE2 activation by xanthenone prevents leptin‐induced increases in blood pressure and proteinuria during pregnancy in Sprague‐Dawley rats. Reproductive Toxicology, 49, 155–161. 10.1016/j.reprotox.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Jiang, Y. , Wilk, J. B. , Borecki, I. , Williamson, S. , DeStefano, A. L. , Xu, G. , … Myers, R. H. (2004). Common variants in the 5′ region of the leptin gene are associated with body mass index in men from the National Heart, Lung, and Blood Institute Family Heart Study. The American Journal of Human Genetics, 75(2), 220–230. 10.1086/422699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis, T. , Kelesidis, I. , Chou, S. , & Mantzoros, C. S. (2010). Narrative review: The role of leptin in human physiology: Emerging clinical applications. Annals of Internal Medicine, 152(2), 93–100. 10.7326/0003-4819-152-2-201001190-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpeläinen, T. O. , Carli, J. F. M. , Skowronski, A. A. , Sun, Q. I. , Kriebel, J. , Feitosa, M. F. , … Loos, R. J. F. (2016). Genome‐wide meta‐analysis uncovers novel loci influencing circulating leptin levels. Nature Communications, 7, 10494 10.1038/ncomms10494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff, C. , Le Bihan, C. , Schork, N. J. , & Bougnères, P. (2000). A common promoter variant of the leptin gene is associated with changes in the relationship between serum leptin and fat mass in obese girls. Diabetes, 49(12), 2196–2200. 10.2337/diabetes.49.12.2196 [DOI] [PubMed] [Google Scholar]
- Li, W.‐C. , Hsiao, K.‐Y. , Chen, I.‐C. , Chang, Y.‐C. , Wang, S.‐H. , & Wu, K.‐H. (2011). Serum leptin is associated with cardiometabolic risk and predicts metabolic syndrome in Taiwanese adults. Cardiovascular Diabetology, 10(1), 36 10.1186/1475-2840-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, D. , Feitosa, M. F. , Wilk, J. B. , Laramie, J. M. , Yu, K. , Leiendecker‐Foster, C. , … Borecki, I. B. (2009). Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension, 53(3), 473–479. 10.1161/HYPERTENSIONAHA.108.118133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madarász, E. , Tabák, Á. G. , Speer, G. , Lakatos, P. , Kerényi, Z. , & Tamás, G. (2009). Abnormal glucose tolerance is associated with diminished postload change in leptin levels in women. Diabetes/metabolism Research and Reviews, 25(7), 632–638. 10.1002/dmrr.1001 [DOI] [PubMed] [Google Scholar]
- Minocci, A. , Savia, G. , Lucantoni, R. , Berselli, M. E. , Tagliaferri, M. , Calò, G. , … Liuzzi, A. (2000). Leptin plasma concentrations are dependent on body fat distribution in obese patients. International Journal of Obesity, 24(9), 1139–1144. 10.1038/sj.ijo.0801385 [DOI] [PubMed] [Google Scholar]
- Molvarec, A. , Szarka, A. , Walentin, S. , Bekő, G. , Karádi, I. , Prohászka, Z. , & Rigó, J. (2011). Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reproductive Biology and Endocrinology, 9(1), 124 10.1186/1477-7827-9-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motawi, T. , Salman, T. , Shaker, O. , & Abdelhamid, A. (2015). Association of polymorphism in adiponectin (+ 45 T/G) and leptin (–2548 G/A) genes with type 2 diabetes mellitus in male Egyptians. Archives of Medical Science: AMS, 11(5), 937–944. 10.5114/aoms.2015.54848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanecka, A. , Posnik‐Urbanska, A. , Kawecka‐Jaszcz, K. , Czarnecka, D. , & Fedak, D. (2010). Adipocytokines and blood pressure, lipids and glucose metabolism in hypertensive perimenopausal women. Kardiologia Polska, 68(7), 753–760. PMID: 20648430. [PubMed] [Google Scholar]
- Paracchini, V. , Pedotti, P. , & Taioli, E. (2005). Genetics of leptin and obesity: A HuGE review. American Journal of Epidemiology, 162(2), 101–114. 10.1093/aje/kwi174 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, M. , Nicolson, M. , Hirsch, J. , Heymsfield, S. B. , Gallagher, D. , Chu, F. , & Leibel, R. L. (1996). Effects of gender, body composition, and menopause on plasma concentrations of leptin. The Journal of Clinical Endocrinology & Metabolism, 81(9), 3424–3427. 10.1210/jcem.81.9.8784109 [DOI] [PubMed] [Google Scholar]
- Shankar, A. , & Xiao, J. (2010). Positive relationship between plasma leptin level and hypertension. Hypertension, 56(4), 623–628. 10.1161/HYPERTENSIONAHA.109.148213 [DOI] [PubMed] [Google Scholar]
- Tartaglia, L. A. , Dembski, M. , Weng, X. , Deng, N. , Culpepper, J. , Devos, R. , … Tepper, R. I. (1995). Identification and expression cloning of a leptin receptor, OB‐R. Cell, 83(7), 1263–1271. 10.1016/0092-8674(95)90151-5 [DOI] [PubMed] [Google Scholar]
- Teng, M.‐S. , Hsu, L.‐A. , Wu, S. , Chou, H.‐H. , Chang, C.‐J. , Sun, Y.‐Z. , … Ko, Y.‐L. (2013). Mediation analysis reveals a sex‐dependent association between ABO gene variants and TG/HDL‐C ratio that is suppressed by sE‐selectin level. Atherosclerosis, 228(2), 406–412. 10.1016/j.atherosclerosis.2013.03.032 [DOI] [PubMed] [Google Scholar]
- Tobi, E. W. , Lumey, L. H. , Talens, R. P. , Kremer, D. , Putter, H. , Stein, A. D. , … Heijmans, B. T. (2009). DNA methylation differences after exposure to prenatal famine are common and timing‐and sex‐specific. Human Molecular Genetics, 18(21), 4046–4053. 10.1093/hmg/ddp353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.‐N. , Huang, M.‐C. , Chang, W.‐T. , Ko, A.‐S. , Tsai, E.‐M. , Liu, C.‐S. , … Ko, Y.‐C. (2006). G‐2548A polymorphism of the leptin gene is correlated with extreme obesity in Taiwanese aborigines. Obesity, 14(2), 183–187. 10.1038/oby.2006.23 [DOI] [PubMed] [Google Scholar]
- WHO Expert Consultation (2004). Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England), 363(9403), 157 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- Woods, A. J. , & Stock, M. J. (1996). Leptin activation in hypothalamus. Nature, 381(6585), 745 10.1038/381745a0 [DOI] [PubMed] [Google Scholar]
- Wu, S. , Hsu, L.‐A. , Cheng, S.‐T. , Teng, M.‐S. , Yeh, C.‐H. , Sun, Y.‐C. , … Ko, Y.‐L. (2014). Circulating YKL‐40 level, but not CHI3L1 gene variants, is associated with atherosclerosis‐related quantitative traits and the risk of peripheral artery disease. International Journal of Molecular Sciences, 15(12), 22421–22437. 10.3390/ijms151222421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Peng, S. , Li, W. , Wan, Z. , Fan, L. , & Du, Y. (2016). Relationships between plasma leptin levels, leptin G2548A, leptin receptor Gln223Arg polymorphisms and gestational diabetes mellitus in Chinese population. Scientific Reports, 6, 23948 10.1038/srep23948 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


